Abstract
The management of castration-resistant prostate cancer (CRPC) presents a clinical challenge because of limitations in efficacy of current therapies. Novel therapeutic strategies for the treatment of CRPC are needed. Antagonists of hypothalamic growth hormone-releasing hormone (GHRH) inhibit growth of various malignancies, including androgen-dependent and independent prostate cancer, by suppressing diverse tumoral growth factors, especially GHRH itself, which acts as a potent autocrine/paracrine growth factor in many tumors. We evaluated the effects of the GHRH antagonist, JMR-132, on PC-3 human androgen-independent prostate cancer cells in vitro and in vivo. JMR-132 suppressed the proliferation of PC-3 cells in vitro in a dose-dependent manner and significantly inhibited growth of PC-3 tumors by 61% (P < 0.05). The expression of GHRH, GHRH receptors, and their main splice variant, SV1, in PC-3 cells and tumor xenografts was demonstrated by RT-PCR and Western blot. The content of GHRH protein in PC-3 xenografts was lowered markedly, by 66.3% (P < 0.01), after treatment with JMR-132. GHRH induced a significant increase in levels of ERK, but JMR-132 abolished this outcome. Our findings indicate that inhibition of PC-3 prostate cancer by JMR-132 involves inactivation of Akt and ERK. The inhibitory effect produced by GHRH antagonist can result in part from inactivation of the PI3K/Akt/mammalian target of rapamycin and Raf/MEK/ERK pathways and from the reduction in GHRH produced by cancer cells. Our findings support the role of GHRH as an autocrine growth factor in prostate cancer and suggest that antagonists of GHRH should be considered for further development as therapy for CRPC.
Keywords: advanced prostate cancer, hormone refractory prostate cancer, novel treatment, prostatic cell death, targeted therapy
Prostate cancer is the most common noncutaneous malignancy and is the second leading cause of death from cancer in men in most Western countries (1). Prostatic adenocarcinomas are dependent on serum androgen for proliferation and survival, and androgen deprivation provides an effective therapy for patients with advanced prostate cancer. Although most patients with advanced prostate cancer show an initial response to androgen-deprivation therapy, nearly all patients eventually progress to a castration-resistant state (2). No effective treatment exists for advanced castration-resistant prostate cancer (CRPC). For the advanced disease, chemotherapy with docetaxel, inhibition of cytochrome P-450c17α (CYP 17) with abiraterone, and immunotherapy based on sipuleucel-T can improve overall survival. However, even docetaxel, the most active chemotherapeutic agent (3), provides only a modest survival advantage, and most patients eventually progress because of drug resistance (4, 5). Thus, there is a clear need for novel therapeutic strategies for the treatment of CRPC.
Growth hormone-releasing hormone (GHRH) is a hypothalamic neuropeptide that stimulates the secretion of growth hormone (GH) from the anterior pituitary gland upon binding to its receptor (GHRH-R) (6). In turn, GH stimulates the production of hepatic insulin-like growth factor I (IGF-I), which is a potent mitogen for many cancers (7). GHRH and its pituitary-type receptor, as well as truncated splice variants (SV) of GHRH receptors, are expressed in various normal human tissues including prostate, kidney, lung, and liver (8), and on many human cancer cell lines and tumors (6, 9). Pituitary-type GHRH-R and SV1 appear to mediate the direct effects of GHRH and its antagonistic analogs on tumors (10). GHRH itself acts as an autocrine/paracrine growth factor in human cancers (6, 11), including prostate (12).
For about two decades, our laboratory has been engaged in the synthesis of GHRH antagonists for therapeutic use in the management of various cancers and for investigation of the pathophysiological role of GHRH in various malignancies (6, 9, 13). The inhibitory effects of GHRH antagonists on tumors are exerted in part by an indirect endocrine mechanism through the suppression of GHRH-evoked release of GH from the pituitary, in turn resulting in the decrease of the hepatic production of IGF-I (9). Direct mechanisms involved in the main antitumor effects of GHRH antagonists appear to be based on blocking the action of autocrine GHRH on tumors and inhibition of autocrine IGF-I/II production (6, 9). We have demonstrated previously that GHRH antagonists inhibit the growth of diverse human tumors xenografted into nude mice, including androgen-dependent and -independent prostate cancers, and also suppress the tumoral growth factors EGF, FGF2, IGF-I, IGF-II, and VEGF-A (6, 14–20).
The PI3K/Akt and Raf/MEK/ERK pathways are implicated in the progression of prostate cancer to androgen independence (21–23). Activation of Akt, a serine/threonine protein kinase, results in protection from apoptosis in response to growth factors, cytokines, c-myc overexpression, and matrix detachment (24). For complete activation, Akt requires phosphorylation at Ser-473 and Thr-308 sites. In the phosphorylated state, Akt promotes cell survival by inactivating the proapoptotic protein B-cell chronic lymphocytic leukemia/lymphoma 2 (BCL2)-antagonist of cell death (Bad), thereby causing the release of the antiapoptotic protein Bcl-2 (24). Akt also stimulates cell-cycle progression by phosphorylating glycogen synthase kinase 3, which in turn stimulates cyclin D1 transcription (24).
The ERK pathway is triggered mainly by mitogens and cytokines, acting through G protein-coupled receptors (GPCRs), receptor tyrosine kinases (RTKs), and nonnuclear steroid hormone receptors (25). Eventually, the signaling activity results in phosphorylation of ERK1/2, a potent MAPK (26). Stimulated ERK1/2 (p42/44 MAPK) mediates diverse biological processes by activating or inactivating a wide variety of proteins involved in proliferation, apoptosis, and invasion of tumor cells (25, 26).
In a previous study, we estimated changes in protein expression of phosphorylated p42/p44 MAPK after treatment of PC-3 and DU-145 tumors with antagonists of GHRH (15). However, the expression of phosphorylated MAPK in PC-3 tumor cells as well as in PC-3 cells grown in vitro was very low. Therefore, the effects of therapies on the MAPK levels in PC-3 tumors could not be investigated.
In the present study, using cell cultures of PC-3 human androgen-independent prostate cancer and PC-3 cells xenografted into nude mice, we investigated the inhibitory effects of the GHRH antagonist JMR-132 in vitro and in vivo. Activation of ERK in cultured PC-3 cells and in PC-3 tumors also was evaluated in view of evidence from earlier studies that ERK signaling plays a role in several steps of tumor development. In addition, we investigated further the mechanisms of action of the GHRH antagonist by quantitative real-time PCR array for cell proliferation, apoptosis, cell cycle, angiogenesis, invasion, and metastasis.
Results
Presence of GHRH Ligand, GHRH-R, and SV1 in PC-3 Human Prostate Cancer Cells and PC-3 Tumor Xenografts.
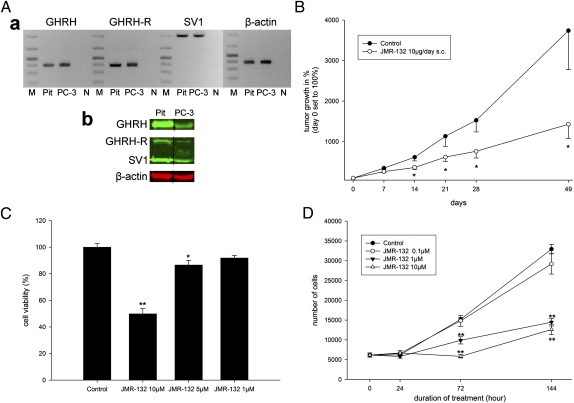
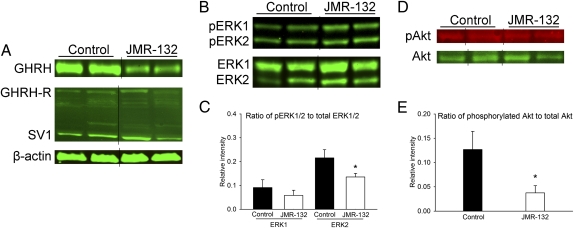
Reverse-transcribed mRNA from PC-3 cells was subjected to real-time RT-PCR to determine the expression of GHRH, pituitary-type GHRH-R (pGHRH-R), and its main splice variant, SV1. The amplicons for GHRH (150 bp), pGHRH-R (145 bp), and SV1 (523 bp) were detected in PC-3 cells (Fig. 1Aa) by their respective specific primers and probes. The antibody used to detect the expression of the receptor could identify both pGHRH-R and SV1. The expression of protein for GHRH, pGHRH-R, and SV1 in PC-3 cells (Fig. 1Ab) and tumor xenografts (Fig. 2A) was confirmed by Western blot. The expression of GHRH protein in PC-3 tumor xenografts was lowered markedly, by 66.3% (P < 0.01), after treatment with the GHRH antagonist JMR-132 (Fig. 2A). No significant changes in protein levels of GHRH-R and SV1 occurred in PC-3 tumor xenografts after JMR-132 treatment (Fig. 2A).
Fig. 1.
Expression of target receptors GHRH-R, SV1, and GHRH ligand and inhibition of growth of PC-3 human androgen-independent prostate cancer in vivo and in vitro. (A) Real-time RT-PCR. (a) Analysis of GHRH, GHRH-R, SV1, and β-actin in cultured PC-3 cells. Human pituitary (Pit) was used as positive control. DNA molecular weight marker is shown in lane M. Negative controls with no template in real-time PCR are shown in lane N. (b) Western blot analysis of GHRH, GHRH-R, and SV1 in cultured PC-3 cells. Representative blots of three independent experiments are presented and include β-actin as an internal standard. Grouping of representative bands for each experimental group was performed digitally. (B) The GHRH antagonist JMR-132 inhibits the growth of s.c. PC-3 prostate cancer xenografted into nude mice. Statistical analysis was performed by one-way ANOVA, followed by Bonferroni t test. Asterisks indicate a significant difference (P < 0.05 compared with control). (C) MTS cell-viability assay performed on PC-3 prostate cancer cells treated with different concentrations of the GHRH antagonist JMR-132 reveals a dose-dependent inhibitory effect. *P < 0.05 and **P < 0.01 compared with control by Student's t test. (D) Cell proliferation (absolute cell-number counts) of the PC-3 prostate cancer cell line treated with different doses of the GHRH antagonist JMR-132. **P < 0.01 compared with control by Student's t test. Data are shown as means ± SEM.
Fig. 2.
Effects of the GHRH antagonist JMR-132 on the expression of GHRH and its receptors, activation of ERK1/2, and activation of Akt in tumor samples of PC-3 human androgen-independent prostate cancer xenografted into nude mice. (A) Western blot analysis of GHRH, GHRH-R, and SV1 in PC-3 tumor samples. Representative blots of three independent experiments are presented and include β-actin as an internal standard. Representative bands for each experimental group were grouped digitally. (B) Western blot analysis of pERK1/2 and total ERK1/2. Representative blots of three independent experiments are presented. Representative bands for each experimental group were grouped digitally. (C) Relative expression of pERK1/2. Bars represent average relative intensity values of pERK1/2 compared with total ERK1/2. *P < 0.05 compared with control by Student's t test. (D) Western blot analysis of phosphorylated Akt (pAkt) and total Akt. Representative blots of three independent experiments are presented. Representative bands for each experimental group were grouped digitally. (E) Relative expression of pAkt. Bars represent average relative intensity values of pAkt compared with total Akt. Data are shown as means ± SEM. *P < 0.05 by Student's t test.
Inhibition of Cell Proliferation in the PC-3 Cell Line.
In assays, in vitro, treatment with the GHRH antagonist JMR-132 resulted in a significant inhibition of cell proliferation of the PC-3 human androgen-independent prostate cancer cell line in a dose-dependent manner. In the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assays, the growth of PC-3 cells was inhibited significantly by JMR-132 at doses of 10 μM (P < 0.01) and 5 μM (P < 0.05) after 72 h, as compared with control (Fig. 1C). In the experiments using absolute cell counts, markedly fewer cells were observed after 72 h and 144 h of exposure to JMR-132 at doses of 10 μM (P < 0.01) and 1 μM (P < 0.01) (Fig. 1D). No inhibition of cell proliferation was observed with a dose of 0.1 μM of JMR-132 in PC-3 cells (Fig. 1D).
In Vivo Effects of the GHRH Antagonist JMR-132 on the Growth of PC-3 Human Androgen-Independent Prostate Cancer Xenografts in Nude Mice.
Treatment of nude mice bearing xenografts of PC-3 human prostate cancer with the GHRH antagonist JMR-132 inhibited tumor growth significantly, by 41.2, 45.4, and 50.1% on days 14, 21, and 28 of the study, respectively, compared with the control group (P < 0.05 for all; Fig. 1B). The inhibition of tumor growth remained significant until the end of study (day 49). The final tumor growth in animals treated with JMR-132 was increased by only 1,428 ± 348% compared with the control group, which showed an increase of 3,739 ± 960%. This result corresponded to a tumor inhibition of 61.8% (P < 0.05). The tumor doubling time was extended significantly, to 11.4 ± 0.7 d, for the JMR-132 group, whereas the control group had a tumor doubling time of 8.1 ± 0.6 d (P < 0.05). In addition, the DNA content of tumors in animals treated with JMR-132 was 16.6% less (1.69 ± 0.08 μg DNA/mg tissue) than that of the controls (2.03 ± 0.03 μg DNA per mg tissue; P < 0.05).
GHRH Antagonist JMR-132 Inhibits Cell Division and Induces Apoptosis.
The assessment of Ki67-labeling indices revealed that the number of mitoses was reduced significantly, by 44.3%, in PC-3 tumors of animals treated with GHRH antagonists (P < 0.05; Table S1 and Fig. S1 A–D). Histological evaluation on H&E slides of PC-3 tumor xenografts revealed that mitotic indices were reduced significantly, by 59.2%, in the group treated with JMR-132 (P < 0.001; Table S1). The number of apoptotic cells was 30% higher in JMR-132–treated tumors than in controls (P < 0.05; Fig. S1 E and F Table S1).We also found that proliferating cell nuclear antigen protein in tumor xenografts was reduced significantly, by 56.5%, after treatment JMR-132 (P < 0.05; Fig. S1G).
Effect of IGF-I, EGF, GHRH, and GHRH Antagonist JMR-132 on Phosphorylation of ERK in PC-3 Cells.
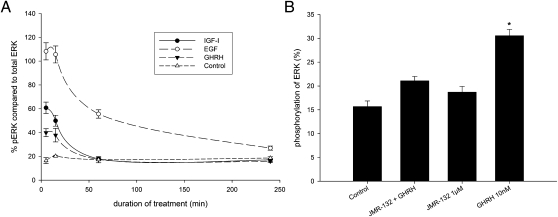
ELISA showed that treatment with 10 nM of IGF-I, EGF, and GHRH caused a significant (P < 0.001), increase in levels of phosphorylated ERK (pERK) compared with control. This activation was highest after cells had been stimulated for 5 min and remained significantly elevated at 15 min (Fig. 3A). The activation levels at 5 min and 15 min were highest for EGF, followed by IGF-I and GHRH. The activation of ERK faded over time and reached the level in controls 60 min after treatment with IGF-I or GHRH. Treatment with EGF resulted in a prolonged activated level of ERK, and the pERK level did not reach the level of control in the observed time period of 4 h (P < 0.01).
Fig. 3.
Activation of ERK in PC-3 human androgen-independent prostate cancer cells. (A) Time course of the phosphorylation of ERK related to total ERK protein in PC-3 prostate carcinoma cell line. Cells were treated with 10 nM IGF-I, EGF, and GHRH, which caused a significant increase in pERK after 5 min and 15 min compared with control (P < 0.001). EGF remained significantly elevated even after 4 h (P < 0.001). Statistical analysis was performed by Student's t test. (B) Phosphorylation of ERK in the PC-3 prostate cancer cell line related to total ERK protein. Stimulation of PC-3 cells with 10 nM GHRH caused a significant increase in p ERK (*P < 0.01) compared with control, whereas treatment with 1 μM of the GHRH antagonist JMR-132 showed no activation of ERK. Pretreatment for 30 min with 1 μM of JMR-132 almost completely abolished the activation of ERK by 10 nM of GHRH. Statistical analysis was performed by Student's t test.
Stimulation of PC-3 cells with 10 nM GHRH caused a significant increase in pERK (P < 0.01) compared with control, whereas treatment with the GHRH antagonist JMR-132 did not cause activation of ERK (Fig. 3B). Pretreatment with 1 μM JMR-132 for 30 min almost completely abolished the activation of ERK in response to 10 nM of GHRH (Fig. 3B).
Effect of GHRH Antagonist JMR-132 on Phosphorylation of ERK1/2 and Akt in PC-3 Tumors.
The relative expression of pERK2 in PC-3 tumor xenografts decreased markedly after treatment with JMR-132 for 7 wk as compared with controls (relative intensity 0.136 and 0.216, respectively; P < 0.05; Fig. 2 B and C). Changes in the relative expression of ERK1 did not reach statistical significance compared with controls because of the high SE (Fig. 2 B and C). Treatment of PC-3 prostate cancers with the GHRH antagonist JMR-132 also significantly diminished the phosphorylation of Akt relative to controls (relative intensity 0.037 and 0.127, respectively; P < 0.05; Fig. 2 D and E).
Effect of GHRH Antagonist JMR-132 on Expression of Genes Related to Cell Proliferation, Apoptosis, Cell Cycle, Angiogenesis, Invasion, and Metastasis.
The Human Cancer Pathway Finder PCR array used in our study provided a simple and sensitive tool for profiling the expression of 84 genes related to cell proliferation, apoptosis, cell cycle, angiogenesis, invasion, and metastasis. We identified important functional molecules affected by treatment with the GHRH antagonist and selected genes potentially related to tumor shrinkage. More than 25 genes in PC-3 xenografts exhibited significant change in mRNA expression after treatment with JMR-132 relative to control (Table S2).
Expression of mRNA for several factors involved in cell-cycle control and DNA damage repair including cyclin-dependent kinase inhibitor 1A (Cdkn1a)/p21 and cyclin-dependent kinase inhibitor 2A (Cdkn2a)/p16 was decreased by the GHRH antagonist JMR-132 (P < 0.05 for all; Table S2). Transcriptional levels of proapoptotic genes such as Bad and BCL2-associated X protein (Bax) were up-regulated (7.46-fold and 1.87-fold increase, respectively; P < 0.05), whereas expression of antiapoptotic Bcl2 was lowered significantly, by 2.30-fold (P < 0.05) by the GHRH antagonist (Table S2). We found that mRNA levels of signal-transduction molecules and transcription factors such as V-akt murine thymoma viral oncogene homolog 1 (Akt1), V-erb-b2 erythroblastic leukemia viral oncogene homolog 2 (Erbb2), TNF receptor superfamily, member 6 (Fas), and nuclear factor of kappa light polypeptide 1 (Nfkb1) were down-regulated (4.00-, 3.73-, 2.14-, and 2.92-fold, respectively), whereas NfkbIa/IκBα was up-regulated by 2.46-fold after treatment with the GHRH antagonist (P < 0.05 for all). Transcriptional suppression of several genes involved in adhesion, including integrins α1, α3, β1, β3, and β5 and metastasis suppressor 1, occurred after treatment with GHRH antagonist (6.50-, 2.83-, 2.02-, 2.46-, 2.85-, and 5.66-fold decrease, respectively; P < 0.05 for all). Significant transcriptional suppression of several factors related to angiogenesis, such as angiopoietin 1, angiopoietin 2, Igf1, thrombospondin 1, and Vegfa, was observed following GHRH antagonist treatment (3.73-, 11.31-, 2.16, 1.87-, and 2.07-fold decrease, respectively; P < 0.05 for all). The mRNA levels for several molecules involved in invasion and metastasis, including matrix metallopeptidase 1, matrix metallopeptidase 9, plasminogen activator, urokinase receptor, and TIMP metallopeptidase inhibitor 1, were lowered by GHRH antagonist treatment (1.82-, 3.03-, 2.30-, and 1.89-fold decrease, respectively; P < 0.05).
Discussion
The main finding of our study is that JMR-132, one of our GHRH antagonists developed for possible tumor therapy, inhibits the growth of human androgen-independent prostate cancer xenografted into nude mice through the suppression of tumoral GHRH and inactivation of ERK and Akt. We demonstrated that JMR-132 inhibits the proliferation of PC-3 cells in vitro and growth of PC-3 tumors in vivo. PCR arrays revealed that GHRH antagonists cause transcriptional up-regulation of proapoptotic genes and suppression of several factors related to angiogenesis, tumor invasion, and metastasis.
Our initial rationale for the use of GHRH antagonists in the treatment of experimental human cancers, including prostatic cancer, was based on the assumption that the blockade of the pituitary GH/hepatic IGF-I axis might inhibit the growth of IGF-I–dependent cancers (6, 13). However, numerous studies revealed that the inhibitory effects of GHRH antagonists on tumor growth also may be produced by suppression of tumoral IGF-I/IGF-II, EGF, and VEGF levels or interference with local GHRH (6, 14, 16). Such effects were thought to be mediated by the pituitary type GHRH-R and its splice variant SV1, which is generated by alternative splicing from the GHRH receptor gene. Our study verifies the expression of protein for both types of receptors in cultured PC-3 cells and PC-3 tumor xenografts. A previous radioligand-binding assay revealed a single class of specific, high-affinity binding sites for GHRH in membrane preparation of PC-3 tumors (16). The presence of GHRH ligand and SV1 of GHRH-R was confirmed in 86 and 65% of surgical specimens of prostate cancer, respectively (27). Specific, high-affinity binding sites for GHRH also were demonstrated by radioligand assays in 60% of prostate cancer specimens (27). Furthermore, the expression of pituitary-type GHRH-R in normal human (8) and rat (28) prostates was reported recently. It appears that SV1 is the predominant type of GHRH-R on certain cancer cells or tumors (6, 16, 28). Thus, SV1-type GHRH receptors could function as the main therapeutic target for the anticancer effect of GHRH antagonists.
In addition to its endocrine role, GHRH has been shown to be a pleiotropic hormone, given the identification of various extrahypothalamic sources for GHRH production and the demonstration that GHRH acts directly on several tissues other than the pituitary (29). Extrahypothalamic GHRH has a broad spectrum of activity, exemplified by its ability to modulate cell proliferation and regulate differentiation of some cell types, especially in malignant tissues (29–31). Various studies from several groups, including our own, suggest that GHRH is a locally acting growth factor in various human cancers, including prostate cancer (reviewed in ref. 6). A recent study by Siriwardana et al. (32) demonstrated that autocrine/paracrine GHRH stimulates the proliferation of breast cancer cells in a dose- and receptor-dependent manner, through the Raf/MEK/ERK pathway. Chopin et al. (12) reported the presence of a prostatic autocrine pathway based on GHRH which may stimulate the proliferation of prostate cells. Our study shows that both protein for GHRH and receptors for GHRH are expressed in PC-3 cells, suggesting that an autocrine/paracrine GHRH loop may be present in this cell line. Moreover, we showed that our GHRH antagonist, JMR-132, significantly suppressed tumoral levels of GHRH protein. This reduction in tumoral GHRH is an indirect sign of the blockade of its autocrine production in PC-3 tumors. Treatment with JMR-132 did not affect tumoral levels of GHRH-R and SV1 significantly.
The present study also demonstrates that the GHRH antagonist JMR-132 markedly inhibits the growth of PC-3 tumors. We demonstrated that JMR-132 significantly reduces the DNA content and number of mitoses and induces apoptosis in PC-3 tumor xenografts. Our in vitro and in vivo data also imply that this inhibition of human experimental prostate cancer involves an effect on the activation of PI3K/Akt and Raf/MEK/ERK pathways. Treatment of PC-3 cells with GHRH resulted in increased phosphorylation of ERK. This augmentation in pERK was comparable to that produced by treatment with IGF-I and EGF, both of which are established growth factors and activators of the Raf/MEK/ERK pathway in prostate cancer (7, 33, 34). Furthermore, pretreatment of PC-3 cells with the GHRH antagonist JMR-132 prevented the activation of ERK by GHRH. It is important that the inactivation of ERK and Akt occurred after treatment with antagonist JMR-132 in PC-3 tumors in vivo.
RTKs are cell-surface receptors for many polypeptide growth factors, cytokines, and hormones (35) involved in the development and progression of various cancers (36). The PI3K/Akt/mammalian target of rapamycin (mTOR) pathway is a major signaling pathway for RTKs. Akt, a downstream effector of the PI3K/Akt/mTOR pathway, is associated with androgen-independent prostate cancer (24). Investigations of prostate tumor cell lines have shown that activation of Akt is important for the progression of prostate cancer to an androgen-independent status (21). Studies of human prostate cancer tissues show that there is no Akt gene amplification or enhanced protein expression in prostate cancer compared with normal tissue, but poorly differentiated tumors exhibit increased expression of a phosphorylated (activated) form of Akt compared with normal tissue, prostatic intraepithelial neoplasia, or well-differentiated prostate cancer (37). Moreover, phosphorylation of Akt has been reported to be an excellent predictor of poor clinical outcome in prostate cancer (38).
Mitogen-activated kinases are multifunctional effector proteins that participate in cellular responses to external stimuli. MAPK activity correlates with the growth of prostate cancer in studies in vitro (34), and an overexpression of MAPK is found in patients with advanced and androgen-independent prostate cancers (22). It also was reported that activation of androgen receptor by growth-promoting compounds requires a functional MAPK signaling pathway (39).
The Raf/MEK/ERK pathway, which is controlled by both GPCRs and RTKs (36), has a pivotal role in controlling cell survival, cell-cycle progression, and differentiation, and its dysregulated signaling is a central signature of many epithelial cancers, including prostate (26, 40). The key effectors of this pathway, ERK1/2, are multifunctional protein kinases, which modulate gene expression through the activation of a multitude of transcription factors such as c-fos, c-myc, or NF-κβ and after translocating to the nucleus also can phosphorylate cytoplasmic and nuclear kinases (25). Increased activity of this pathway has been associated with advanced prostate cancer, hormonal independence, and a poor prognosis (22, 41). ERK1/2 phosphorylation often is detected in correlation with increased tumor grade of primary or metastatic prostate cancer and tumor relapse after therapy (23, 42). Several investigators suggest associations between a decline in ERK activity and increasing Gleason grades and differentiation in prostate cancer (37, 43). ERK activation may be involved in mediating cellular differentiation, and ERK inactivation may be one cause of dedifferentiation in cancer (24, 37). The role of the Raf/MEK/ERK pathway in prostate cancer remains controversial.
Previously, we used a site-specific antibody-recognizing phosphorylated form of ERK1/2 to estimate changes in protein expression in DU-145 and PC-3 tumors and in cultured cells as well after treatment of tumors with GHRH antagonists (15). The expression of pERK in PC-3 tumor cells was very low, more than 10-fold lower than in DU-145 tumors. Therefore, the effects of various therapies on the ERK levels in PC-3 tumors could not be investigated. However, high basal levels of ERK found in the DU-145 model permitted the evaluation of the effects of treatments on the ERK expression in this tumor model. The GHRH antagonist MZ-J-7–118 potently decreased the expression of ERK in DU-145 tumors. This difference in constitutive activation of ERK in PC-3 and DU-145 cells is in accord with earlier findings described by others (44, 45). However, Zelivianski et al. (46) found a higher expression of phosphorylated MAPK in PC-3 cells. These differences might be explained by variable ERK expression in different PC-3 cell clones and may be changed by several passages of tumor cells. Contrary to our previous report, the basal pERK expression in PC-3 cells and in tumors passaged in nude mice used in the present study was high. Our data suggest that blockade of GHRH receptors (belonging to GPCRs) by antagonist JMR-132 results in inactivation of both the RTK-linked PI3K/Akt/mTOR pathway and the GPCR-linked Raf/MEK/ERK pathway.
To elucidate further the beneficial effects of the GHRH antagonist JMR-132 in inhibiting PC-3 tumors, we used quantitative PCR arrays. Our observation of the transcriptional suppression of Cdkn1a/p21 and Cdkn2a/p16, factors involved in cell-cycle control and DNA damage repair, in PC-3 tumors after treatment with the GHRH antagonist JMR-132 are in line with our recent findings (17). The mRNA levels of the proapoptotic genes Bad and Bax were up-regulated, whereas the expression of antiapoptotic Bcl2 was lowered significantly, confirming our recent observations (28, 47). We found that transcriptional levels of signal transduction molecules and transcription factors Akt1, Erbb2, Fas, and Nfkb1 were down-regulated after treatment with JMR-132. Transcriptional suppression of integrins involved in adhesion also occurred. In accordance with our recent observations in experimental prostate cancer models treated with GHRH antagonists (14, 16), a significant transcriptional suppression of several factors related to angiogenesis, such as angiopoietins and VEGFA, occurred following GHRH antagonist treatment. The mRNA levels for several molecules involved in invasion and metastasis also were lowered by GHRH antagonist treatment.
In summary, this study demonstrated the efficacy of potent GHRH antagonist, JMR-132, in inhibiting the growth of androgen-independent prostate cancer in vitro and in vivo. The inhibitory effect produced by this GHRH antagonist could be explained in part by inactivation of Akt and ERK and by a reduction in GHRH produced by the cancer cells. Our findings elucidate the mechanisms of action of GHRH antagonists. Our work supports the potential role for GHRH as an autocrine growth factor in prostate cancer. The synthesis of still more potent GHRH antagonists might convert this class of compounds into a therapeutic tool.
Materials and Methods
Additional information is provided in SI Materials and Methods.
Drugs and Chemicals.
The GHRH antagonist JMR-132 was synthesized by solid-phase methodology using Boc-chemistry as described (48). Antagonist JMR-132 had the sequence [PhAc0-Tyr1, d-Arg2, Cpa6, Ala8, Har9, Tyr(Me)10, His11, Abu15, His20, Nle27, d-Arg28, Har29]hGH-RH(1-29)NH2, where Abu is α-aminobutyryl, Cpa is 4-chloro-Phe, Har is homoarginine, Nle is norleucine, PhAc is phenylacetyl, and Tyr(Me) is O-methyl-Tyr. GHRH(1-29) NH2 also was synthesized in our laboratory. Stock solutions of JMR-132 and GHRH(1-29) NH2 were dissolved in DMSO. The final concentration of DMSO diluted with incubation medium was <0.1%, avoiding any effects on cell growth in cell cultures. Human recombinant EGF and IGF-I were purchased from Sigma-Aldrich. For in vivo experiments, JMR-132 was dissolved in 0.1% DMSO in 10% (vol/vol) aqueous propylene glycol solution (vehicle solution).
Cell Lines and Animals.
The PC-3 androgen-independent prostate cancer cell line was obtained from the American Type Culture Collection (ATCC) and was maintained in culture using F12K medium (ATCC) supplemented with 10% FBS and antibiotics (100 U/mL penicillin, 100 μg/mL streptomycin). The cells were grown at 37 °C in a humidified 95% air/5% CO2 atmosphere.
Male athymic (NCr nu/nu) nude mice, ∼5- to 6-wk-old on arrival, were obtained from the National Cancer Institute (Frederick Cancer Center), were housed in laminar airflow cabinets under pathogen-free conditions with a 12-h light/12-h dark schedule, and were fed autoclaved standard chow and water ad libitum.
Proliferation Assays.
For in vitro proliferation assays, 5,000 cells per well were seeded in 96-well plates in 100 μL medium. After 24 h, culture medium was replaced by serum-reduced medium (0.5% FBS) containing different concentrations of JMR-132 (1 μM, 5 μM, and 10 μM), and the cells were incubated for 72 h in a humidified thermostat at 37 °C. After the treatment, the relative number of viable cells was measured using an MTS assay (CellTiter 96 AQueous Assay; Promega) following manufacturer's instructions. Briefly, 20 μL of MTS solution was added to the cells and incubated for 1.5 h; then the absorbance was measured at 550 nm with a Dynax Plate reader. Experiments were performed in hexaplicate and repeated three times.
An absolute cell count was performed also using a Z2 Coulter cell counter (Beckman Coulter). Cells were seeded for 24 h in 24-well plates (5,000 cells per well) in medium supplemented with 10% (vol/vol) FBS. After 24 h culture medium was replaced by serum-reduced medium (0.5% FBS) containing JMR-132. Cells were harvested by incubation with 0.05% trypsin (Invitrogen Life Technologies) for 7 min and counted after 24 h, 72 h, and 144 h of incubation with JMR-132.
In Vivo Studies.
Treatment of nude mice bearing PC-3 human androgen-independent prostate tumor xenografts with the GHRH antagonist JMR-132 was performed as described (SI Materials and Methods). Animal care was in accordance with institutional guidelines and complied with National Institutes of Health policy.
Supplementary Material
Acknowledgments
This study was supported by the Medical Research Service of the Veterans Affairs Department; the Division of Hematology/Oncology of the Miller Medical School, Departments of Pathology and Medicine, University of Miami; the South Florida Veterans Affairs Foundation for Research and Education (all A.V.S.); and the L. Austin Weeks Endowment for Urologic Research (N.L.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120588109/-/DCSupplemental.
References
- 1.Jemal A, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Garcia JA, Rini BI. Castration-resistant prostate cancer: Many treatments, many options, many challenges ahead. Cancer. 2011 doi: 10.1002/cncr.26582. 10.1002/cncr.26582. [DOI] [PubMed] [Google Scholar]
- 3.O'Neill AJ, et al. Characterisation and manipulation of docetaxel resistant prostate cancer cell lines. Mol Cancer. 2011;10:126. doi: 10.1186/1476-4598-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mostaghel EA, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: Therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 5.Scher HI, et al. Prostate Cancer Clinical Trials Working Group Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schally AV, Varga JL, Engel JB. Antagonists of growth-hormone-releasing hormone: An emerging new therapy for cancer. Nat Clin Pract Endocrinol Metab. 2008;4:33–43. doi: 10.1038/ncpendmet0677. [DOI] [PubMed] [Google Scholar]
- 7.Westley BR, May FE. Insulin-like growth factors: The unrecognised oncogenes. Br J Cancer. 1995;72:1065–1066. doi: 10.1038/bjc.1995.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Havt A, et al. The expression of the pituitary growth hormone-releasing hormone receptor and its splice variants in normal and neoplastic human tissues. Proc Natl Acad Sci USA. 2005;102:17424–17429. doi: 10.1073/pnas.0506844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schally AV. New approaches to the therapy of various tumors based on peptide analogues. Horm Metab Res. 2008;40:315–322. doi: 10.1055/s-2008-1073142. [DOI] [PubMed] [Google Scholar]
- 10.Rekasi Z, Czompoly T, Schally AV, Halmos G. Isolation and sequencing of cDNAs for splice variants of growth hormone-releasing hormone receptors from human cancers. Proc Natl Acad Sci USA. 2000;97:10561–10566. doi: 10.1073/pnas.180313297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiaris H, Schally AV, Varga JL, Groot K, Armatis P. Growth hormone-releasing hormone: An autocrine growth factor for small cell lung carcinoma. Proc Natl Acad Sci USA. 1999;96:14894–14898. doi: 10.1073/pnas.96.26.14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chopin LK, Herington AC. A potential autocrine pathway for growth hormone releasing hormone (GHRH) and its receptor in human prostate cancer cell lines. Prostate. 2001;49:116–121. doi: 10.1002/pros.1125. [DOI] [PubMed] [Google Scholar]
- 13.Schally AV, Varga JL. Antagonists of growth hormone-releasing hormone in oncology. Comb Chem High Throughput Screen. 2006;9:163–170. doi: 10.2174/138620706776055449. [DOI] [PubMed] [Google Scholar]
- 14.Letsch M, Schally AV, Busto R, Bajo AM, Varga JL. Growth hormone-releasing hormone (GHRH) antagonists inhibit the proliferation of androgen-dependent and -independent prostate cancers. Proc Natl Acad Sci USA. 2003;100:1250–1255. doi: 10.1073/pnas.0337496100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stangelberger A, et al. Inhibition of human androgen-independent PC-3 and DU-145 prostate cancers by antagonists of bombesin and growth hormone releasing hormone is linked to PKC, MAPK and c-jun intracellular signalling. Eur J Cancer. 2005;41:2735–2744. doi: 10.1016/j.ejca.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Heinrich E, et al. Dose-dependent growth inhibition in vivo of PC-3 prostate cancer with a reduction in tumoral growth factors after therapy with GHRH antagonist MZ-J-7-138. Prostate. 2008;68:1763–1772. doi: 10.1002/pros.20843. [DOI] [PubMed] [Google Scholar]
- 17.Stangelberger A, et al. Inhibitory effects of antagonists of growth hormone releasing hormone on experimental prostate cancers are associated with upregulation of wild-type p53 and decrease in p21 and mutant p53 proteins. Prostate. 2011 doi: 10.1002/pros.21458. 10.1002/pros.21458. [DOI] [PubMed] [Google Scholar]
- 18.Rick FG, et al. LHRH antagonist Cetrorelix reduces prostate size and gene expression of proinflammatory cytokines and growth factors in a rat model of benign prostatic hyperplasia. Prostate. 2011;71:736–747. doi: 10.1002/pros.21289. [DOI] [PubMed] [Google Scholar]
- 19.Stangelberger A, et al. Inhibitory effect of antagonists of bombesin and growth hormone-releasing hormone on orthotopic and intraosseous growth and invasiveness of PC-3 human prostate cancer in nude mice. Clin Cancer Res. 2005;11:49–57. [PubMed] [Google Scholar]
- 20.Papadia A, et al. Growth hormone-releasing hormone antagonists inhibit growth of human ovarian cancer. Horm Metab Res. 2011;43:816–820. doi: 10.1055/s-0031-1287766. [DOI] [PubMed] [Google Scholar]
- 21.Graff JR. Emerging targets in the AKT pathway for treatment of androgen-independent prostatic adenocarcinoma. Expert Opin Ther Targets. 2002;6:103–113. doi: 10.1517/14728222.6.1.103. [DOI] [PubMed] [Google Scholar]
- 22.Gioeli D, Mandell JW, Petroni GR, Frierson HF, Jr, Weber MJ. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res. 1999;59:279–284. [PubMed] [Google Scholar]
- 23.Uzgare AR, Kaplan PJ, Greenberg NM. Differential expression and/or activation of P38MAPK, erk1/2, and jnk during the initiation and progression of prostate cancer. Prostate. 2003;55:128–139. doi: 10.1002/pros.10212. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh PM, Malik S, Bedolla R, Kreisberg JI. Akt in prostate cancer: Possible role in androgen-independence. Curr Drug Metab. 2003;4:487–496. doi: 10.2174/1389200033489226. [DOI] [PubMed] [Google Scholar]
- 25.Rodríguez-Berriguete G, et al. MAP kinases and prostate cancer. J Signal Transduct. 2012;2012:169170. doi: 10.1155/2012/169170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong SK, Kim JH, Lin MF, Park JI. The Raf/MEK/extracellular signal-regulated kinase 1/2 pathway can mediate growth inhibitory and differentiation signaling via androgen receptor downregulation in prostate cancer cells. Exp Cell Res. 2011;317:2671–2682. doi: 10.1016/j.yexcr.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halmos G, et al. Expression of growth hormone-releasing hormone and its receptor splice variants in human prostate cancer. J Clin Endocrinol Metab. 2002;87:4707–4714. doi: 10.1210/jc.2002-020347. [DOI] [PubMed] [Google Scholar]
- 28.Rick FG, et al. Antagonists of growth hormone-releasing hormone (GHRH) reduce prostate size in experimental benign prostatic hyperplasia. Proc Natl Acad Sci USA. 2011;108:3755–3760. doi: 10.1073/pnas.1018086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiaris H, Chatzistamou I, Papavassiliou AG, Schally AV. Growth hormone-releasing hormone: Not only a neurohormone. Trends Endocrinol Metab. 2011;22:311–317. doi: 10.1016/j.tem.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Barabutis N, Schally AV. Growth hormone-releasing hormone: Extrapituitary effects in physiology and pathology. Cell Cycle. 2010;9:4110–4116. doi: 10.4161/cc.9.20.13787. [DOI] [PubMed] [Google Scholar]
- 31.Kiaris H, Koutsilieris M, Kalofoutis A, Schally AV. Growth hormone-releasing hormone and extra-pituitary tumorigenesis: Therapeutic and diagnostic applications of growth hormone-releasing hormone antagonists. Expert Opin Investig Drugs. 2003;12:1385–1394. doi: 10.1517/13543784.12.8.1385. [DOI] [PubMed] [Google Scholar]
- 32.Siriwardana G, Bradford A, Coy D, Zeitler P. Autocrine/paracrine regulation of breast cancer cell proliferation by growth hormone releasing hormone via Ras, Raf, and mitogen-activated protein kinase. Mol Endocrinol. 2006;20:2010–2019. doi: 10.1210/me.2005-0001. [DOI] [PubMed] [Google Scholar]
- 33.Culig Z, et al. Regulation of prostatic growth and function by peptide growth factors. Prostate. 1996;28:392–405. doi: 10.1002/(SICI)1097-0045(199606)28:6<392::AID-PROS9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 34.Putz T, et al. Epidermal growth factor (EGF) receptor blockade inhibits the action of EGF, insulin-like growth factor I, and a protein kinase A activator on the mitogen-activated protein kinase pathway in prostate cancer cell lines. Cancer Res. 1999;59:227–233. [PubMed] [Google Scholar]
- 35.Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548–5557. doi: 10.1038/sj.onc.1203957. [DOI] [PubMed] [Google Scholar]
- 36.Zwick E, Bange J, Ullrich A. Receptor tyrosine kinase signalling as a target for cancer intervention strategies. Endocr Relat Cancer. 2001;8:161–173. doi: 10.1677/erc.0.0080161. [DOI] [PubMed] [Google Scholar]
- 37.Malik SN, et al. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin Cancer Res. 2002;8:1168–1171. [PubMed] [Google Scholar]
- 38.Kreisberg JI, et al. Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer Res. 2004;64:5232–5236. doi: 10.1158/0008-5472.CAN-04-0272. [DOI] [PubMed] [Google Scholar]
- 39.Culig Z, Klocker H, Bartsch G, Steiner H, Hobisch A. Androgen receptors in prostate cancer. J Urol. 2003;170:1363–1369. doi: 10.1097/01.ju.0000075099.20662.7f. [DOI] [PubMed] [Google Scholar]
- 40.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 41.Bakin RE, Gioeli D, Sikes RA, Bissonette EA, Weber MJ. Constitutive activation of the Ras/mitogen-activated protein kinase signaling pathway promotes androgen hypersensitivity in LNCaP prostate cancer cells. Cancer Res. 2003;63:1981–1989. [PubMed] [Google Scholar]
- 42.Weber MJ, Gioeli D. Ras signaling in prostate cancer progression. J Cell Biochem. 2004;91:13–25. doi: 10.1002/jcb.10683. [DOI] [PubMed] [Google Scholar]
- 43.Paweletz CP, et al. Reverse phase protein microarrays which capture disease progression show activation of pro-survival pathways at the cancer invasion front. Oncogene. 2001;20:1981–1989. doi: 10.1038/sj.onc.1204265. [DOI] [PubMed] [Google Scholar]
- 44.Hassan S, Dobner PR, Carraway RE. Involvement of MAP-kinase, PI3-kinase and EGF-receptor in the stimulatory effect of Neurotensin on DNA synthesis in PC3 cells. Regul Pept. 2004;120:155–166. doi: 10.1016/j.regpep.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Shimada K, et al. Contributions of mitogen-activated protein kinase and nuclear factor kappa B to N-(4-hydroxyphenyl)retinamide-induced apoptosis in prostate cancer cells. Mol Carcinog. 2002;35:127–137. doi: 10.1002/mc.10084. [DOI] [PubMed] [Google Scholar]
- 46.Zelivianski S, et al. ERK inhibitor PD98059 enhances docetaxel-induced apoptosis of androgen-independent human prostate cancer cells. Int J Cancer. 2003;107:478–485. doi: 10.1002/ijc.11413. [DOI] [PubMed] [Google Scholar]
- 47.Hohla F, et al. GHRH antagonist causes DNA damage leading to p21 mediated cell cycle arrest and apoptosis in human colon cancer cells. Cell Cycle. 2009;8:3149–3156. doi: 10.4161/cc.8.19.9698. [DOI] [PubMed] [Google Scholar]
- 48.Zarandi M, et al. Synthesis and biological activities of highly potent antagonists of growth hormone-releasing hormone. Proc Natl Acad Sci USA. 1994;91:12298–12302. doi: 10.1073/pnas.91.25.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.