Abstract
Interleukin-33 (IL-33) (NF-HEV) is a chromatin-associated nuclear cytokine from the IL-1 family, which has been linked to important diseases, including asthma, rheumatoid arthritis, ulcerative colitis, and cardiovascular diseases. IL-33 signals through the ST2 receptor and drives cytokine production in type 2 innate lymphoid cells (ILCs) (natural helper cells, nuocytes), T-helper (Th)2 lymphocytes, mast cells, basophils, eosinophils, invariant natural killer T (iNKT), and natural killer (NK) cells. We and others recently reported that, unlike IL-1β and IL-18, full-length IL-33 is biologically active independently of caspase-1 cleavage and that processing by caspases results in IL-33 inactivation. We suggested that IL-33, which is released upon cellular damage, may function as an endogenous danger signal or alarmin, similar to IL-1α or high-mobility group box 1 protein (HMGB1). Here, we investigated the possibility that IL-33 activity may be regulated by proteases released during inflammation. Using a combination of in vitro and in vivo approaches, we demonstrate that neutrophil serine proteases cathepsin G and elastase can cleave full-length human IL-331–270 and generate mature forms IL-3395–270, IL-3399–270, and IL-33109–270. These forms are produced by activated human neutrophils ex vivo, are biologically active in vivo, and have a ∼10-fold higher activity than full-length IL-33 in cellular assays. Murine IL-33 is also cleaved by neutrophil cathepsin G and elastase, and both full-length and cleaved endogenous IL-33 could be detected in the bronchoalveolar lavage fluid in an in vivo model of acute lung injury associated with neutrophil infiltration. We propose that the inflammatory microenvironment may exacerbate disease-associated functions of IL-33 through the generation of highly active mature forms.
Keywords: innate immunity, inflammatory protease, serine protease inhibitor, alveolar epithelium
Cytokines of the IL-1 family (IL-1α, IL-1β, IL-18) play a major role in inflammatory, infectious, and autoimmune diseases (1–3). IL-33 [previously known as nuclear factor from high endothelial venule or NF-HEV (4, 5)], is a chromatin-associated nuclear cytokine from the IL-1 family (6, 7), which has been linked to important diseases (8–10), including asthma (11), rheumatoid arthritis (12, 13), ulcerative colitis (14), and cardiovascular diseases (15).
IL-33 signals through the ST2 receptor (4), a member of the IL-1 receptor family, which is expressed (or induced) on various immune cell types, including mast cells, basophils, eosinophils, T-helper (Th)2 lymphocytes, invariant natural killer T (iNKT) and natural killer (NK) cells, macrophages, dendritic cells, and neutrophils (8–10). IL-33 stimulation of ST2 on Th2 cells induces secretion of the Th2 cytokines IL-5 and IL-13 (4, 16). Recently, IL-33 has been shown to drive production of extremely high amounts of these Th2 cytokines by type 2 innate lymphoid cells (ILCs) (natural helper cells, nuocytes, innate helper 2 cells), which play important roles in innate immune responses, after helminth infection in the intestine (17–19) or influenza virus infection in the lungs (20, 21). An important role of IL-33 in innate rather than acquired immunity is also supported by observations in IL-33-deficient mice (22).
It was initially believed that, like IL-1β and IL-18, processing of IL-33 by caspase-1 to a mature form was required for biological activity (4). However, we (23) and others (24–26) demonstrated that full-length IL-33 is biologically active and that processing of IL-33 by caspases results in its inactivation, rather than its activation. Further analyses revealed that IL-33 is constitutively expressed to high levels in the nuclei of endothelial and epithelial cells in vivo (27) and that it can be released in the extracellular space after cellular damage (23, 24). IL-33 was, thus, proposed (23, 24, 27) to function as an endogenous danger signal or alarmin, similar to IL-1α and high-mobility group box 1 protein (HMGB1) (28–32), to alert cells of the innate immune system of tissue damage during trauma or infection.
An important question that has not yet been resolved is whether full-length IL-33 is the unique bioactive form of the cytokine or whether mature bioactive forms are also generated in vivo. Because IL-33 plays important roles in inflammatory diseases, we hypothesized that IL-33 could be cleaved by proteases released from innate effector cells during inflammation. In this report, we show, by using both in vitro and in vivo approaches, that IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Interestingly, the IL-33 mature forms generated by neutrophil serine proteases, IL-3395–270, IL-3399–270, and IL-33109–270, have an increased biological activity compared with the full-length IL-331–270 protein. Proteolytic processing may, thus, play an important role in the regulation of IL-33 activity during inflammation.
Results
Biological Activity of Full-Length IL-33 Is Increased by Neutrophil Elastase and Cathepsin G.
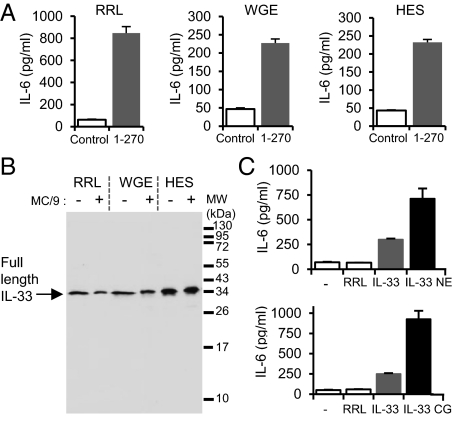
In situ processing of full-length IL-33 has been observed in some biological assays (25), and it has been questioned whether the full-length IL-33 form itself or one of its cleavage products represented the truly bioactive species. To address this issue, we tested the biological activity of full-length IL-331–270, produced in three different expression systems, using a cellular bioassay validated in our previous studies, IL-33-dependent secretion of IL-6 by MC/9 mast cells (23). As shown in Fig. 1A, full-length IL-33 proteins produced in rabbit reticulocyte lysate (RRL), wheat germ extract (WGE), or human in vitro protein expression system (HES) induced IL-6 secretion in mast cells. We then determined the size of the IL-33 proteins at the beginning and at the end of the assay (24-h incubation) by Western blot analysis (Fig. 1B). Whatever the expression system used, we found no evidence for IL-33 processing during the bioassay. We concluded that full-length IL-33 does not require processing for biological activity.
Fig. 1.
The biological activity of full-length IL-331–270 is increased by neutrophil elastase and cathepsin G. (A) Capacity of full-length IL-331–270 produced in RRL, WGE, or HES (5 μL of lysate) to activate the IL-33-responsive mast cell line MC/9 (2 × 105 cells/well) was analyzed by determining IL-6 levels in supernatants using an ELISA. (B) Western blot analysis (305B mAb) of full-length IL-331–270 produced in RRL, WGE, or HES before or after incubation with MC/9 cells for 24 h. (C) Full-length IL-331–270 produced in RRL (5 μL of lysate) was preincubated with neutrophil elastase (NE) (30 mU/μL; 30 min at 37 °C) or cathepsin G (CG) (0.09 mU/μL; 1 h at 37 °C) before incubation with MC/9 cells (105 cells/well) for 24 h. IL-6 levels in supernatants were detected by ELISA. Results in A and C are shown as means and SD of three separate data points and are representative of two independent experiments.
The observation that full-length IL-33 is biologically active did not exclude the possibility that some proteases released during inflammation may cleave IL-33 and generate mature forms with increased biological activity. Because neutrophil serine proteases have been shown to play key roles in inflammatory processes and maturation of IL-1 family cytokines (1, 3, 33–37), we tested the possibility that neutrophil serine proteases may be involved in the processing and regulation of IL-33. We found that incubation of full-length IL-33 with neutrophil elastase and cathepsin G resulted in an increase of IL-6 secretion by mast cells, compared with cells treated with full-length IL-33 alone (Fig. 1C). These later results indicated that neutrophil elastase and cathepsin G can regulate IL-33 bioactivity.
Neutrophil Elastase and Cathepsin G Cleave IL-33 and Generate Mature Forms IL-3395–270, IL-3399–270, and IL-33109–270.
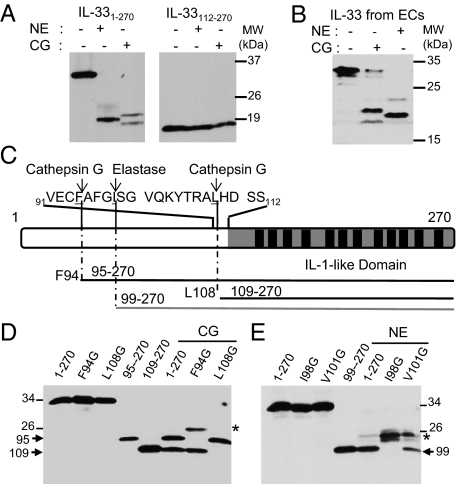
We then asked whether full-length IL-331–270 is cleaved by neutrophil serine proteases. Western blot analysis revealed that neutrophil elastase and cathepsin G process in vitro-translated full-length human IL-33 and generate cleavage products of ∼18–21 kDa (Fig. 2A). In contrast, the C-terminal IL-1-like domain of IL-33 (IL-33112–270) is not cleaved by neutrophil serine proteases. Importantly, neutrophil elastase and cathepsin G also cleaved endogenous native IL-33 released from human necrotic endothelial cells and generated similar cleavage products, one major product of ∼20 kDa for elastase and two major products of ∼21 and 18 kDa for cathepsin G (Fig. 2B). Based on size of the cleavage products, MS analyses (Fig. S1 and Table S1) and site-directed mutagenesis experiments (Fig. 2 C–E), we mapped the cathepsin G cleavage sites at F94 and L108 and the elastase cleavage site at I98 in the human IL-33 sequence. As shown in Fig. 2D, replacement of residue F94 by a glycine abrogated the formation of the larger cathepsin G cleavage product, whereas mutagenesis of residue L108 prevented formation of the smaller product. In addition, the cathepsin G cleavage products comigrated on SDS/PAGE with in vitro-translated IL-3395–270 and IL-33109–270 proteins. Similarly, the elastase cleavage product comigrated on gels with in vitro-translated IL-3399–270, and this product was no longer observed when residue I98 was replaced by a glycine (Fig. 2E). Cleavage products of higher molecular mass were observed with the F94G and I98G mutants (Fig. 2 D and E), indicating that cathepsin G and elastase can cleave IL-33 at additional secondary sites, further upstream in the N-terminal part. Together, these findings indicated that IL-33 is a substrate for neutrophil elastase and cathepsin G, which generate mature forms, IL-3395–270, IL-3399–270, and IL-33109–270.
Fig. 2.
Neutrophil elastase and cathepsin G generate IL-33 mature forms IL-3395–270, IL-3399–270, and IL-33109–270. (A and B) IL-33 is a substrate for neutrophil elastase and cathepsin G. In vitro-translated IL-331–270 and IL-33112–270 proteins (A) or endogenous native IL-33 isolated from necrotic endothelial cells (B) were incubated with purified neutrophil elastase (NE) (30 mU/μL; 30 min at 37 °C) or cathepsin G (CG) (0.09 mU/μL; 1 h at 37 °C). (C) Primary structure of human IL-33. The IL-1-like domain with its 12 β-strands (black boxes) is indicated. The sequence surrounding the cathepsin G and elastase cleavage sites is shown. (D and E) Identification of the cathepsin G and elastase cleavage sites using IL-33 single-point and deletion mutants. Mutation of F94 and L108 to glycine abrogates formation of the 21- and 18-kDa CG (0.06 mU/μL; 1 h at 37 °C) cleavage products, respectively (D). Mutation of I98 to glycine abrogates formation of the 20 kDa elastase (19 mU/μL; 30 min at 37 °C) cleavage product (E). In vitro-translated proteins IL-3395–270, IL-33109–270, and IL-3399–270 comigrate on SDS/PAGE with the 21- and 18-kDa cathepsin G cleavage products (D) and the 20-kDa elastase cleavage product (E), respectively. *, secondary cleavage products. Proteins were separated by SDS/PAGE and revealed by Western blot with anti-IL-33-Cter mAb 305B (A–E). Blots are representative of at least three independent experiments.
IL-33 Mature Forms Are Generated by Activated Human Neutrophils ex Vivo.
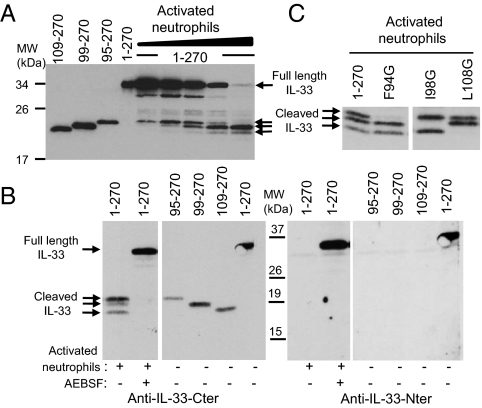
We observed that PMA-activated human neutrophils can process full-length IL-331–270 and generate three cleavage products, which comigrate on SDS/PAGE with IL-3395–270, IL-3399–270, and IL-33109–270 (Fig. 3A). Cleavage of IL-331–270 was prevented when neutrophils were treated with the serine protease inhibitor 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF) (Fig. 3B). Western blot analysis revealed that the three cleavage products are detected with anti-IL-33-Cter antibodies directed against the IL-1-like domain but not detected with anti-IL-33-Nter antibodies recognizing the first 15 amino-terminal residues of IL-33, indicating that the cleaved forms contain the C-terminal IL-1-like domain of IL-33. Incubation of IL-331–270 single point mutants with activated neutrophils (Fig. 3C) indicated that: (i) the larger cleavage product corresponds to the IL-3395–270 form, because this form is not observed in the Phe94 mutant; (ii) the second cleavage product corresponds to the IL-3399–270 form, which is eliminated in the Ile98 mutant; (iii) the smaller cleavage product corresponds to the IL-33109–270 form, which is not generated in the Leu108 mutant. These results thus demonstrate that activated human neutrophils can process full-length IL-33 into mature forms IL-3395–270, IL-3399–270, and IL-33109–270.
Fig. 3.
Activated human neutrophils generate IL-33 mature forms IL-3395–270, IL-3399–270, and IL-33109–270. (A and B) In vitro-translated full-length human IL-331–270 was incubated with PMA-activated human neutrophils. Proteins were comigrated on SDS/PAGE with in vitro-translated IL-3395–270, IL-3399–270, and IL-33109–270 proteins and revealed by Western blot with anti-IL-33-Cter mAb 305B (A and B, Left) or anti-IL-33-Nter antibodies (B, Right). IL-33 was incubated with increasing amounts of supernatants from activated neutrophils (6 × 104–106 neutrophils; 15 min at 37 °C) (A). Cleavage of IL-33 by activated neutrophils (105 neutrophils; 2 h at 37 °C) was inhibited by serine protease inhibitor AEBSF (1 mM) (B). (C) In vitro-translated IL-331–270 or single-point mutants IL-33F94G, IL-33I98G, and IL-33L108G were incubated with PMA-activated human neutrophils (105 neutrophils; 2 h at 37 °C). Proteins were separated on SDS/PAGE and revealed by Western blot with anti-IL-33-Cter mAb 305B. Blots are representative of three independent experiments, with neutrophils isolated from three different donors.
IL-33 Mature Forms Have Increased Biological Activity Compared with the Full-Length IL-331–270 Protein.
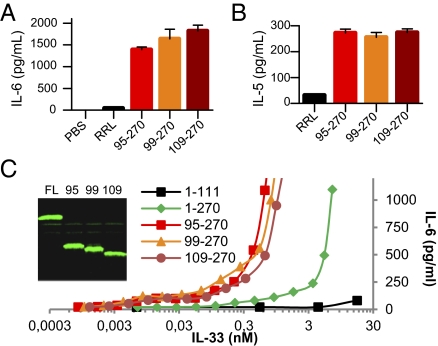
We tested the biological activity of IL-33 mature forms in two cellular bioassays (23, 38) and found that in vitro-translated human IL-3395–270, IL-3399–270, and IL-33109–270 induced IL-6 secretion by MC/9 mast cells (Fig. 4A) and IL-5 secretion by KU812 basophil-like cells (Fig. 4B). We then compared the biological activity of the IL-33 mature forms to that of the full-length IL-331–270 protein. The four IL-33 forms were quantified by fluorescence (Fig. 4C), and secretion of IL-6 by MC/9 cells in response to different concentrations of the proteins was analyzed. These experiments revealed that ∼10-fold higher concentrations of the full-length IL-33 protein were required to obtain similar levels of IL-6 secretion by MC/9 cells (Fig. 4C). We concluded that mature forms IL-3395–270, IL-3399–270, and IL-33109–270 have a higher biological activity (∼10-fold) than the full-length IL-33 protein.
Fig. 4.
IL-33 mature forms IL-3395–270, IL-3399–270, and IL-33109–270 are biologically active. (A and B) The capacity of IL-33 mature forms produced in RRL (5 μL of lysate) to activate the IL-33-responsive MC/9 mast cells (105 cells/well; 24-h stimulation) (A) and KU812 basophil-like chronic myelogenous leukemia cells (5 × 105 cells/well; 24-h stimulation) (B) was analyzed by determining cytokine levels in supernatants [IL-6 (A); IL-5 (B)] using ELISAs. Results are shown as means and SD of three separate data points. (C) Comparison of the biological activity of IL-33 full-length and mature forms. The different proteins were quantified by fluorescence and various concentrations were used to stimulate MC/9 mast cells (105 cells/well; 24-h stimulation). IL-6 protein levels in supernatants were detected by ELISA. Results are representative of at least two independent experiments.
IL-33 Mature Forms Are Biologically Active in Vivo.
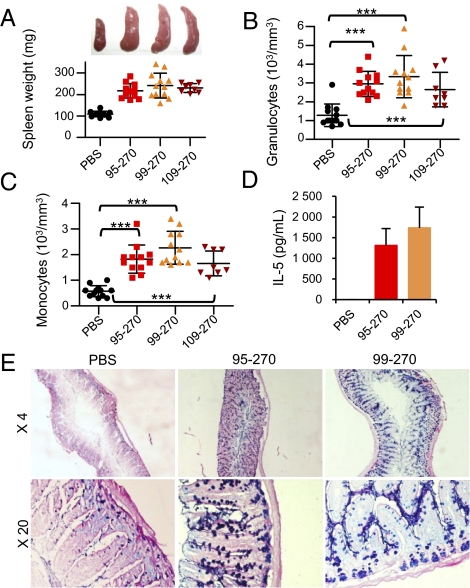
To determine whether IL-3395–270, IL-3399–270, and IL-33109–270 are biologically active in vivo, we produced recombinant proteins corresponding to these mature forms in Escherichia coli. We injected the IL-3395–270, IL-3399–270, and IL-33109–270 recombinant proteins into wild-type mice, intraperitoneally (i.p.) every day over a 1-wk period and observed a striking increase in the size of the spleen (Fig. 5A), as previously observed with the artificially truncated form IL-33112–270 (4, 24). Spleen weight increased from ∼100 mg in PBS-treated mice to >200 mg in mice treated with IL-3395–270, IL-3399–270, or IL-33109–270 recombinant proteins (Fig. 5A). Injection of IL-33 mature forms in vivo also increased the numbers of blood granulocytes and monocytes (Fig. 5 B and C) and the serum concentrations of IL-5 (Fig. 5D), a Th2 cytokine known to be produced by natural helper cells in vivo in response to IL-33 (17). In the intestine, IL-33 has previously been shown to induce goblet cell hyperplasia and mucus secretion, an effect which is mediated through up-regulation of the cytokine IL-13 (4). Periodic acid Schiff and alcian blue staining revealed that mucus secretion was highly increased in the jejunum of mice treated with the IL-3395–270 and IL-3399–270 recombinant proteins, compared with PBS-treated mice (Fig. 5E). Together, these results indicated that the mature forms IL-3395–270, IL-3399–270, and IL-33109–270 are biologically active in vivo. As previously reported by another group (24), we had difficulties to obtain correctly folded recombinant full-length IL-331–270 in quantities compatible with in vivo assays, and this precluded the comparison of the bioactivity of full-length and mature IL-33 forms in vivo.
Fig. 5.
In vivo activity of IL-33 mature forms IL-3395–270, IL-3399–270, and IL-33109–270. (A–E) BALB/c mice were injected (i.p.) with recombinant proteins (4 μg) every day during 1 wk, and spleens (A), peripheral blood (B and C), serum (D), and jejunum (E) were collected at day 8 (n = 12/3 experiments for PBS, IL-3395–270, and IL-3399–270; n = 8/2 experiments for IL-33109–270). Spleen weights and representative photographs for each group of mice are shown (A). Numbers of peripheral blood granulocytes (B), monocytes (C), and serum concentrations of IL-5 (D) were determined. ***P < 0.001 (Student unpaired and two-tailed t test). Epithelial changes in the jejunum are shown (E). Mucus was stained with periodic acid Schiff and alcian blue.
Full-Length and Cleaved Endogenous IL-33 Are Detected in Bronchoalveolar Lavage Fluid in an in Vivo Model of Acute Lung Injury Associated with Neutrophil Infiltration.
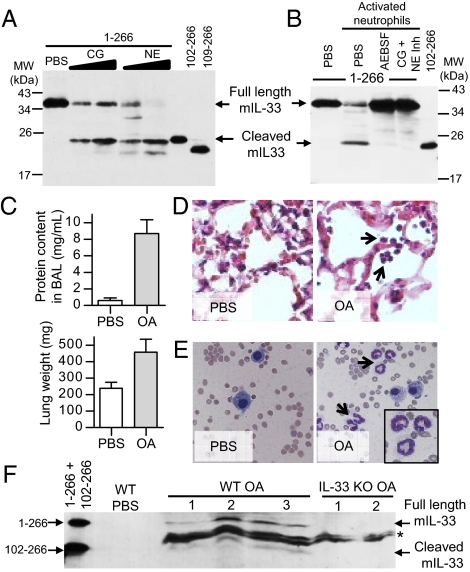
We then determined whether cleavage of IL-33 by neutrophil elastase and cathepsin G also occurs in mice. Western blot analysis revealed that neutrophil cathepsin G processes in vitro-translated full-length murine IL-331–266 and generates one major cleavage product of ∼20 kDa (Fig. 6A). This product comigrated on SDS/PAGE with in vitro-translated murine IL-33102–266, indicating that cleavage by cathepsin G may occur after Phe101. Neutrophil elastase generated a similar major cleavage product of ∼20 kDa and a second minor product, which comigrated with in vitro-translated murine IL-33102–266 and IL-33109–266, respectively. A single major cleavage product of ∼20 kDa comigrating with IL-33102–266 was also generated when full-length murine IL-331–266 was incubated with supernatant of activated neutrophils prepared from murine bone marrow (Fig. 6B). Cleavage was abrogated in the presence of serine protease inhibitor AEBSF or neutrophil elastase and cathepsin G inhibitors. To determine whether cleaved IL-33 is generated in vivo, we used a mouse model of acute lung injury induced by i.v. administration of oleic acid (OA), which results in damage to the lung alveolar epithelium and recruitment of neutrophils in the injured lung (39). We selected this murine model of human ARDS (acute respiratory distress syndrome) because endogenous IL-33 is constitutively expressed in the alveolar epithelium (40) and can be released in the extracellular space after cellular damage (23, 24). Two hours after i.v. injection of OA, we observed an increase in lung weight and protein content in bronchoalveolar lavage (BAL) fluid (Fig. 6C) and an accumulation of neutrophils in the alveolar wall and in BAL fluid (Fig. 6 D and E). Western blot analysis with anti-IL-33-Cter antibodies revealed that full-length endogenous IL-33 is released in BAL fluid following acute lung injury (Fig. 6F). Interestingly, a cleaved IL-33 form comigrating with in vitro-translated murine IL-33102–266 was also detected in BAL fluid from the injured lung. Full-length and cleaved IL-33 forms were detected with anti-IL-33 antibodies in OA-treated wild-type mice but not in OA-treated IL-33−/− mice (Fig. 6F), indicating they correspond to bona fide endogenous IL-33 forms. We concluded that full-length and cleaved endogenous IL-33 are detected in BAL fluid after alveolar epithelium damage and neutrophil recruitment in the alveolar wall.
Fig. 6.
Murine IL-33 is processed by neutrophil elastase and cathepsin G ex vivo, and cleaved endogenous IL-33 is detected in vivo in a model of acute lung injury associated with neutrophil infiltration. (A and B) In vitro-translated full-length murine IL-331–266 (2.5 μL) was incubated with purified neutrophil cathepsin G (CG) (14.7 and 29.4 μU/μL) or elastase (NE) (7.3 and 14.7 mU/μL) (A) or with supernatants of activated mouse bone marrow neutrophils (3 × 104 neutrophils) (B) for 1 h at 37 °C. Activated neutrophils were pretreated with PBS, serine protease inhibitor AEBSF (8 mM), or cathepsin G and elastase inhibitors (50 μM) for 1 h at 37 °C. Proteins were comigrated on SDS/PAGE with in vitro-translated murine IL-33102–266 and IL-33109–266 and revealed by Western blot with goat anti-mouse IL-33-Cter antibodies. Blots are representative of at least three independent experiments. (C–F) Full-length and cleaved endogenous IL-33 are released in BAL fluid following OA-induced acute lung injury. Lung injury was assessed by measuring lung weight and protein content in BAL fluid two hours after i.v. OA administration (C). Accumulation of neutrophils (black arrows) was analyzed by hematoxylin/eosin staining of lung tissue sections (D) and May–Grunwald–Giemsa staining of BAL fluids cytospins (E). Proteins from cell-free BAL fluids (30 μL) of OA-treated wild-type (three mice) or IL-33−/− mice (two mice) were comigrated on SDS/PAGE with in vitro-translated murine IL-331–266 and IL-33102–266 and revealed by Western blot with goat anti-mouse IL-33-Cter antibodies (F). *Nonspecific bands detected in both wild-type and IL-33−/− mice.
Discussion
In this report, we describe mature bioactive forms of the IL-1 family cytokine IL-33. Because full-length IL-33 is biologically active and cleavage by caspases results in its inactivation, rather than its activation, it remained unclear whether mature bioactive forms of the cytokine could be generated. We now demonstrate that the bioactivity of human IL-33 is increased by neutrophil serine proteases cathepsin G and elastase and that full-length IL-331–270 is processed by these purified proteases or activated neutrophils, into mature forms IL-3395–270, IL-3399–270, and IL-33109–270. These IL-33 mature forms contain an intact IL-1-like cytokine domain and exhibit a ∼10-fold higher activity than the full-length protein in cellular bioassays. They are very potent in vivo and induce striking increases in spleen weight, blood granulocyte and monocyte numbers, and serum concentrations of IL-5, as well as profound changes in the intestine. Murine IL-331–266 can also be processed by neutrophil elastase and cathepsin G or activated neutrophils, and both full-length and cleaved endogenous IL-33 were detected in vivo in BAL fluid in a mouse model of acute lung injury associated with high levels of neutrophil recruitment. Together, these findings bring important insights into the molecular mechanisms regulating the activity of IL-33. They provide experimental evidence that proteolytic processing of IL-33 and removal of the N-terminal part can greatly increase IL-33 bioactivity. These results, thus, support the possibility that proteolytic processing of IL-33 may be required for the extracellular generation of highly active cytokine in vivo. In addition, they suggest that the inflammatory microenvironment may exacerbate disease-associated functions of IL-33 through the generation of these highly active mature forms.
We have previously proposed (23, 27) that IL-33 may function as an endogenous danger signal or alarmin, similar to IL-1α and HMGB1 (28–32), to alert the immune system of cell or tissue damage during trauma or infection. In support of this model, we have shown that IL-33 is constitutively expressed to high levels in the nuclei of endothelial and epithelial cells in normal human tissues (27) and that it can be released in the extracellular space after cellular damage (23). Neutrophils are rapidly recruited into injured tissues during infection or in the absence of infection during “sterile inflammation,” following the release of major alarmin molecules such as IL-1α and HMGB1 (28–32). After activation, they rapidly release serine proteases from cytoplasmic granules into the extracellular space (33). Neutrophil elastase and cathepsin G may, thus, process IL-33 released from damaged cells into highly active mature forms, soon after the tissue injury, in the early stages of inflammation. Regulation of IL-33 bioactivity by neutrophil serine proteases may be particularly important in sterile neutrophilic inflammation, which is thought to contribute to the pathogenesis of acute lung and liver injuries, acute ischemia-induced injuries, and chronic diseases affecting the lung, bowel, and joints (29). IL-33 has been shown to play important roles in mouse models of rheumatoid arthritis (12, 13) and to orchestrate neutrophil migration into the joints (41). Neutrophil serine proteases are critical for IL-1β processing in the acute phase of arthritis, characterized by a strong neutrophilic infiltrate (36, 37), and could also mediate IL-33 processing into mature bioactive forms in inflammatory arthritis. Finally, processing of IL-33 by neutrophils proteases may also occur during bacterial, fungal or viral infections. For instance, generation of IL-33 mature forms by airway neutrophils following influenza virus infection could modulate the activity of type 2 innate lymphoid cells in the lungs (20, 21).
Our results show that mature bioactive forms of IL-33 are generated by caspase-1-independent mechanisms. Interestingly, caspase-1-independent activation of IL-1β and IL-18 has been reported in several studies (1, 3, 34–37, 42, 43). Neutrophil serine proteases cathepsin G, elastase, and proteinase-3 (PR3) have been shown to cleave the IL-1β precursor a few residues upstream the caspase-1 maturation site and to produce mature bioactive forms of the cytokine (1, 3, 34–37). Extracellular processing of IL-33 into mature bioactive forms by neutrophil serine proteases is, thus, a mechanism shared with other IL-1 family members. In addition to cathepsin G and elastase, PR3 may also play a role in the regulation of IL-33 bioactivity because we observed that PR3 can process full-length IL-33 into cleavage products of ∼18–20 kDa (Fig. S2). Moreover, other proteases, including granzymes, matrix metalloprotease 9, and mast cell chymase, have been shown to process IL-1α, IL-1β, and IL-18 precursors into active cytokines (1, 3, 36, 44). It remains to be seen whether these or other proteases may also play a role in the processing of IL-33 into mature bioactive forms. Furthermore, the IL-1α-processing protease calpain has been reported to cleave IL-33 (45), but neither the molecular nature nor the biological activity of the cleavage product has been characterized yet.
In summary, this study provides strong evidence that mature bioactive forms of the IL-1 family cytokine IL-33 can be generated by neutrophil serine proteases cathepsin G and elastase. This report describes a precise mechanism leading to the generation of highly active IL-33 mature forms. Further characterization of IL-33 processing could bring important insights in the regulation of IL-33 activity in inflammatory disease processes.
Materials and Methods
SI Materials and Methods provides details regarding plasmid constructions, protein production, Western blot analysis, histology, and analysis of blood, spleen, lung, and BAL samples.
Protein Cleavage Assays with Neutrophil Proteases and Isolated Neutrophils.
In vitro-translated proteins (2–5 μL of lysate) were incubated with neutrophil elastase (0.3 U; Calbiochem) and cathepsin G (1 mU; Calbiochem) in 15 μL of assay buffer (2–5 μL of RRL lysate plus 10 μL of PBS) for 30 min to 1 h at 37 °C. Cleavage assays with activated neutrophils (6 × 104–106 neutrophils; 15 min to 2 h at 37 °C) were performed using human neutrophils from healthy blood donors (Etablissement Français du sang) or mouse neutrophils isolated from femur and tibia bone marrow. For a full description, see SI Materials and Methods.
IL-33 Activity Assays.
In vitro-translated full-length IL-33 or mature forms (5 μL of lysate/well; 24-h treatment) were used to stimulate IL-33-responsive MC/9 mast cells (ATCC; 105 to 2 × 105 cells/well in 96-well plates) (23) and KU812 basophil-like chronic myelogenous leukemia cells (ATCC; 5 × 105 cells/well in 96-well plates) (38). Cytokine levels in supernatants were determined using DuoSet IL-6 and IL-5 ELISAs (R&D Systems).
Animals.
Female BALB/c mice received daily i.p. injections of 4 μg of recombinant human IL-3395–270, IL-3399–270, IL-33109–270, or saline for 7 d. Blood and histologic analyses were performed on day 8. Acute lung injury was induced in female C57BL/6 wild type or IL-33−/− mice (8–10 wk old) by i.v. injection of OA (0.8 μL/g body weight; Sigma) in a 15% solution with 0.1% BSA. Lung histology and BAL fluid were analyzed 2 h after OA injection. All mice were bred under specific pathogen-free conditions and handled according to institutional guidelines under protocols approved by the Institut de Pharmacologie et de Biologie Structurale (IPBS) and “Région Midi-Pyrénées” animal care committees.
Supplementary Material
Acknowledgments
We thank the Infrastructures en Biologie, Santé et Agronomie (IBiSA) Toulouse Proteomics, Toulouse Réseau Imagerie (TRI)-IPBS and Anexplo-IPBS facilities. We are grateful to members of the J.-P.G. laboratory for help with animal experiments. This work was supported by Ligue Nationale contre le Cancer Equipe Labellisée “LIGUE 2009” (to J.-P.G.), an Association pour la Recherche sur le Cancer fellowship (to E.L.), and an Association pour la Recherche sur le Cancer Programme grant (to J.-P.G.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115884109/-/DCSupplemental.
References
- 1.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 2.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 3.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Baekkevold ES, et al. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol. 2003;163:69–79. doi: 10.1016/S0002-9440(10)63631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carriere V, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci USA. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roussel L, Erard M, Cayrol C, Girard JP. Molecular mimicry between IL-33 and KSHV for attachment to chromatin through the H2A-H2B acidic pocket. EMBO Rep. 2008;9:1006–1012. doi: 10.1038/embor.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith DE. IL-33: a tissue derived cytokine pathway involved in allergic inflammation and asthma. Clin Exp Allergy. 2010;40:200–208. doi: 10.1111/j.1365-2222.2009.03384.x. [DOI] [PubMed] [Google Scholar]
- 9.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 10.Oboki K, Ohno T, Kajiwara N, Saito H, Nakae S. IL-33 and IL-33 receptors in host defense and diseases. Allergol Int. 2010;59:143–160. doi: 10.2332/allergolint.10-RAI-0186. [DOI] [PubMed] [Google Scholar]
- 11.Moffatt MF, et al. GABRIEL Consortium A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu D, et al. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci USA. 2008;105:10913–10918. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer G, et al. Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis Rheum. 2009;60:738–749. doi: 10.1002/art.24305. [DOI] [PubMed] [Google Scholar]
- 14.Pastorelli L, et al. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci USA. 2010;107:8017–8022. doi: 10.1073/pnas.0912678107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanada S, et al. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo L, et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci USA. 2009;106:13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moro K, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 18.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price AE, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang YJ, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monticelli LA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. 10.1038/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oboki K, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci USA. 2010;107:18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci USA. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lüthi AU, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G. Interleukin-33 is biologically active independently of caspase-1 cleavage. J Biol Chem. 2009;284:19420–19426. doi: 10.1074/jbc.M901744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali S, Nguyen DQ, Falk W, Martin MU. Caspase 3 inactivates biologically active full length interleukin-33 as a classical cytokine but does not prohibit nuclear translocation. Biochem Biophys Res Commun. 2010;391:1512–1516. doi: 10.1016/j.bbrc.2009.12.107. [DOI] [PubMed] [Google Scholar]
- 27.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS ONE. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 29.Chen CJ, et al. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 30.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 31.Cohen I, et al. Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci USA. 2010;107:2574–2579. doi: 10.1073/pnas.0915018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rider P, et al. IL-1α and IL-1β recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187:4835–4843. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- 33.Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 34.Hazuda DJ, Strickler J, Kueppers F, Simon PL, Young PR. Processing of precursor interleukin 1 beta and inflammatory disease. J Biol Chem. 1990;265:6318–6322. [PubMed] [Google Scholar]
- 35.Coeshott C, et al. Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc Natl Acad Sci USA. 1999;96:6261–6266. doi: 10.1073/pnas.96.11.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guma M, et al. Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis Rheum. 2009;60:3642–3650. doi: 10.1002/art.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joosten LA, et al. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum. 2009;60:3651–3662. doi: 10.1002/art.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tare N, et al. KU812 cells provide a novel in vitro model of the human IL-33/ST2L axis: functional responses and identification of signaling pathways. Exp Cell Res. 2010;316:2527–2537. doi: 10.1016/j.yexcr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Z, Kozlowski J, Schuster DP. Physiologic, biochemical, and imaging characterization of acute lung injury in mice. Am J Respir Crit Care Med. 2005;172:344–351. doi: 10.1164/rccm.200503-343OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Louten J, et al. Endogenous IL-33 enhances Th2 cytokine production and T-cell responses during allergic airway inflammation. Int Immunol. 2011;23:307–315. doi: 10.1093/intimm/dxr006. [DOI] [PubMed] [Google Scholar]
- 41.Verri WA, Jr, et al. IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann Rheum Dis. 2010;69:1697–1703. doi: 10.1136/ard.2009.122655. [DOI] [PubMed] [Google Scholar]
- 42.Fantuzzi G, et al. Response to local inflammation of IL-1 beta-converting enzyme- deficient mice. J Immunol. 1997;158:1818–1824. [PubMed] [Google Scholar]
- 43.van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol. 2011;32:110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Afonina IS, et al. Granzyme B-dependent proteolysis acts as a switch to enhance the proinflammatory activity of IL-1α. Mol Cell. 2011;44:265–278. doi: 10.1016/j.molcel.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayakawa M, et al. Mature interleukin-33 is produced by calpain-mediated cleavage in vivo. Biochem Biophys Res Commun. 2009;387:218–222. doi: 10.1016/j.bbrc.2009.07.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.