Abstract
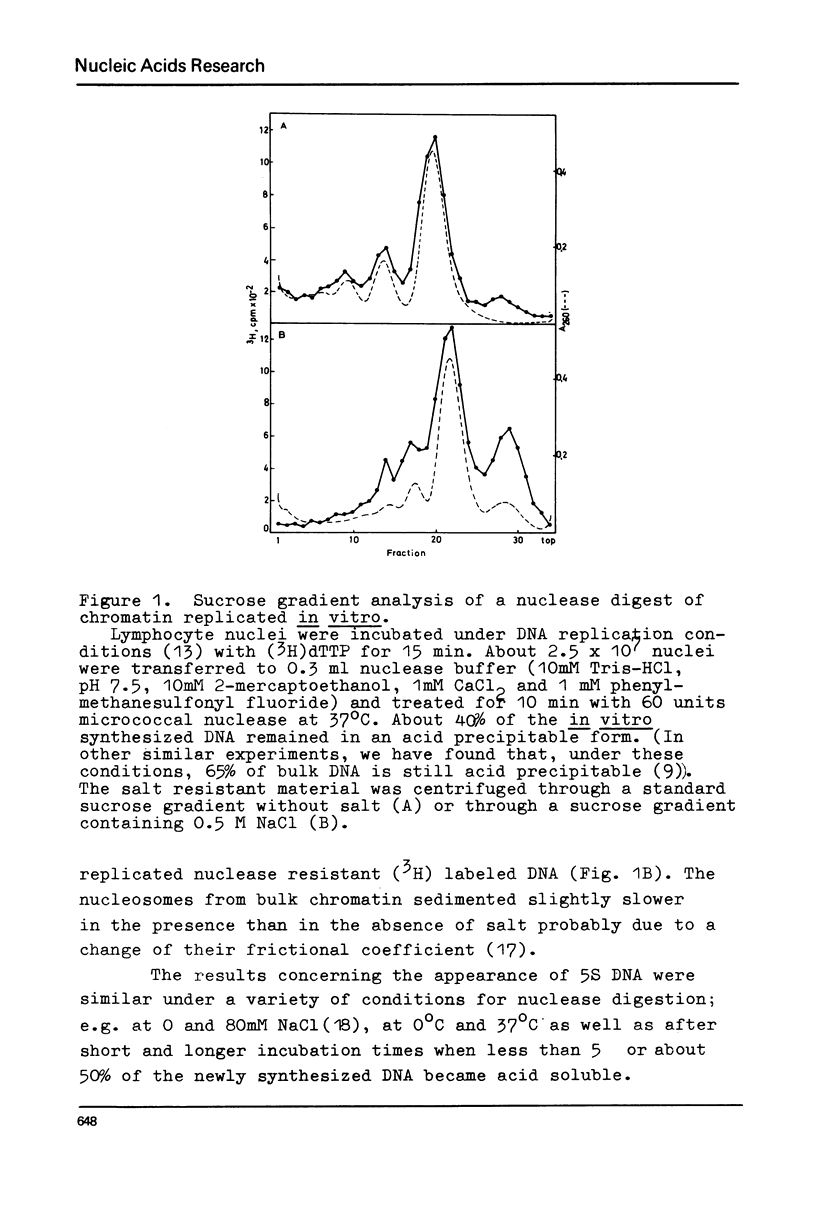
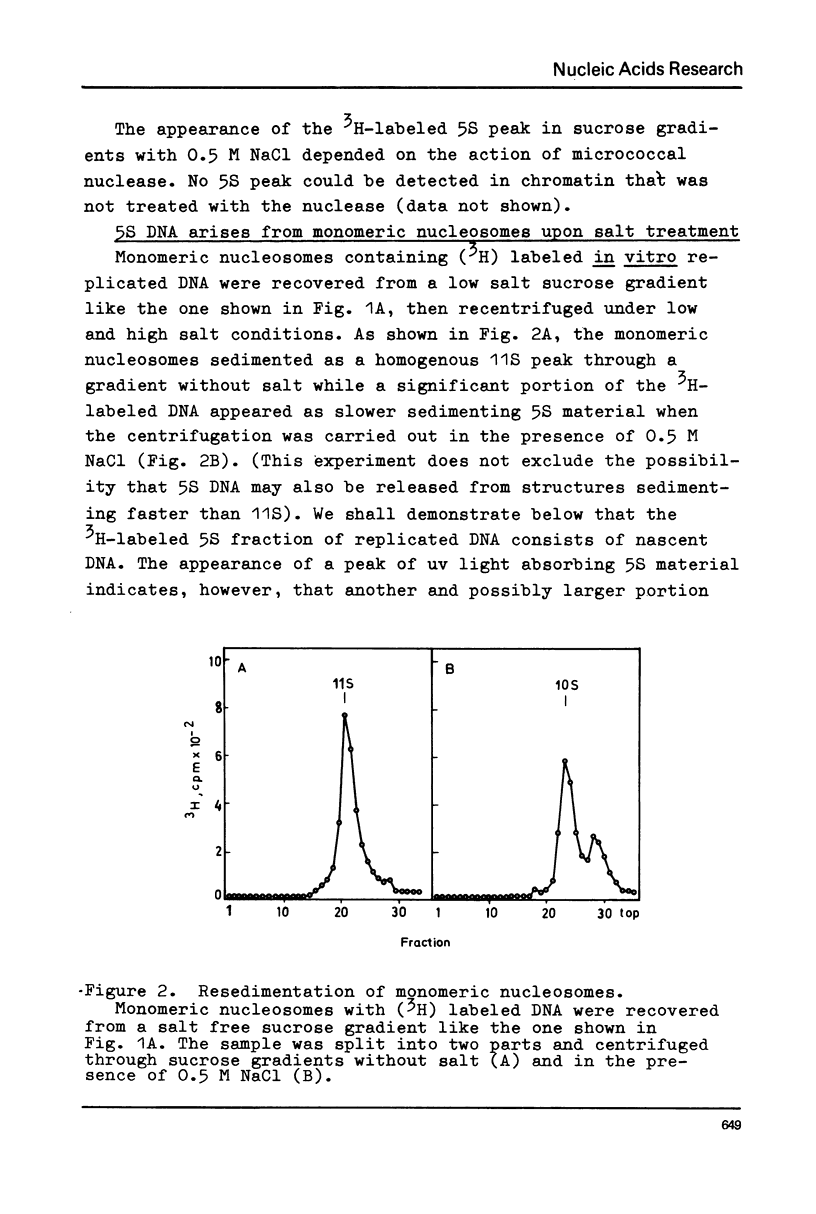
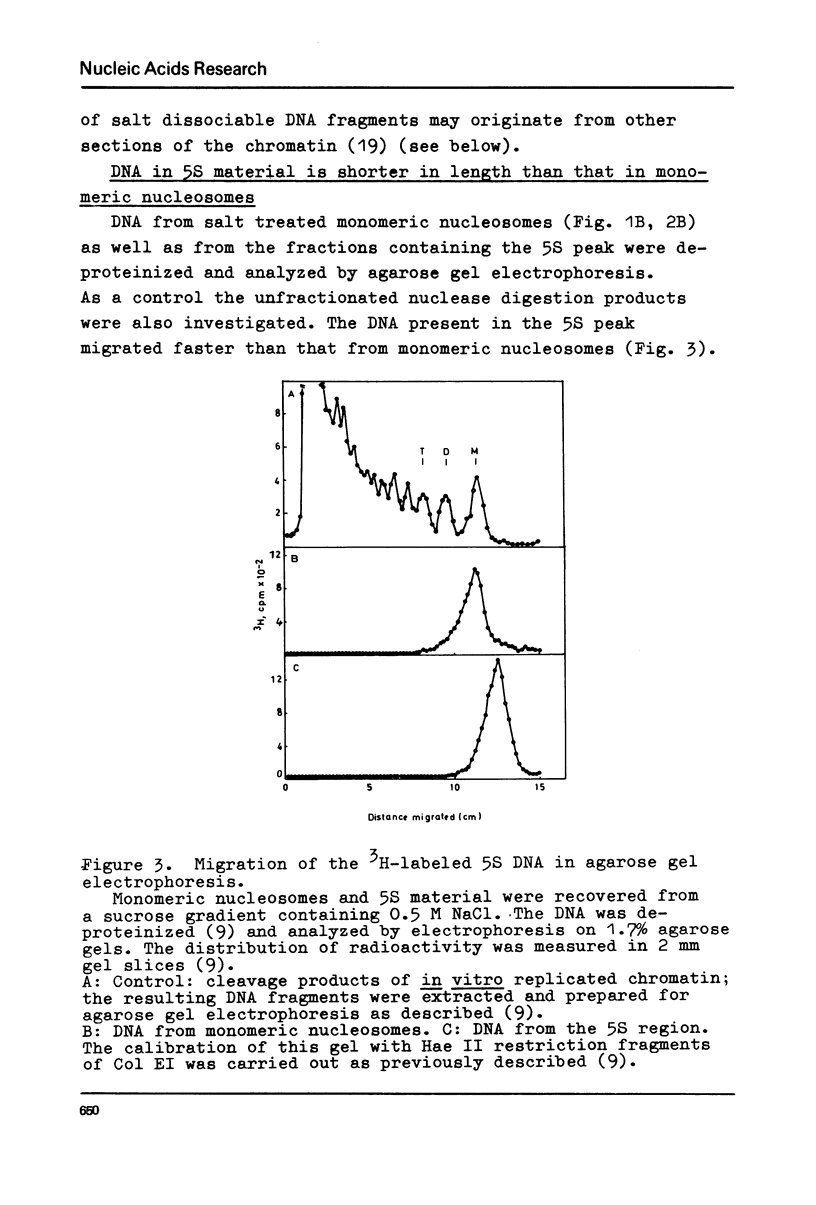
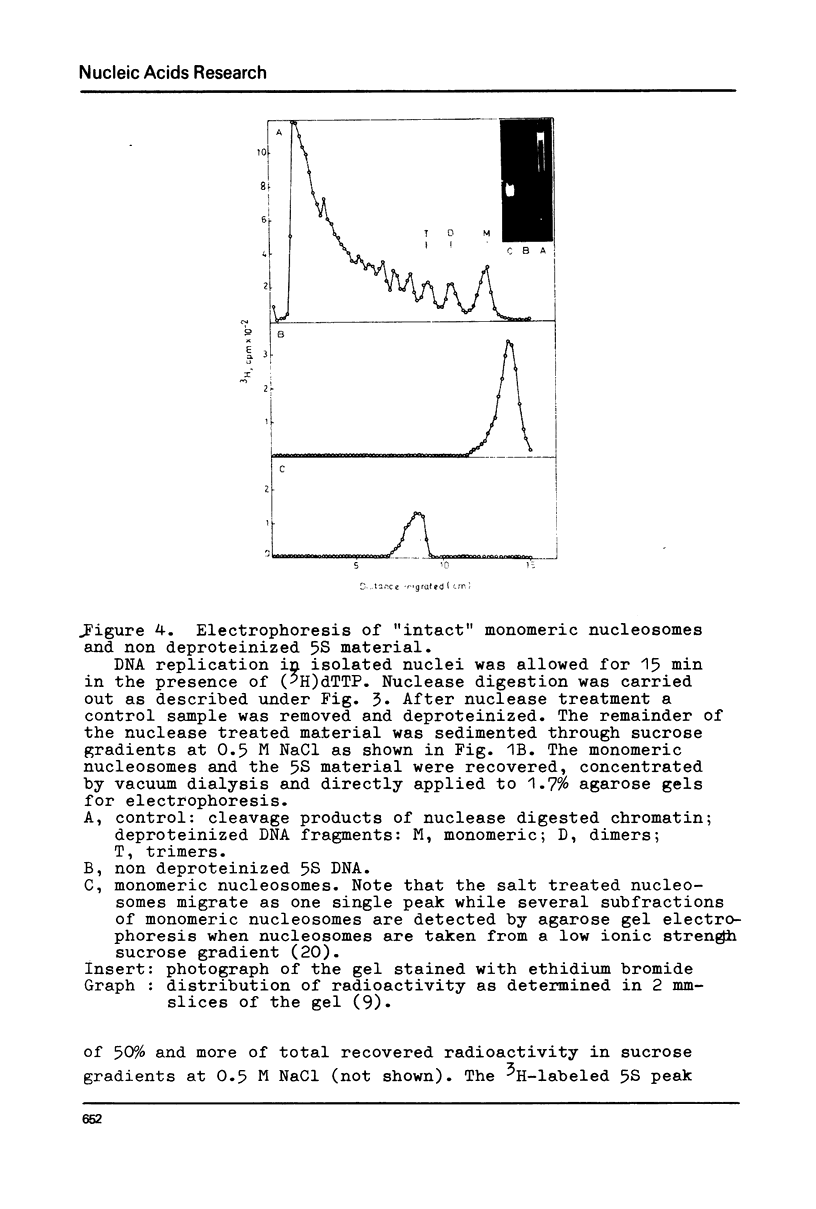
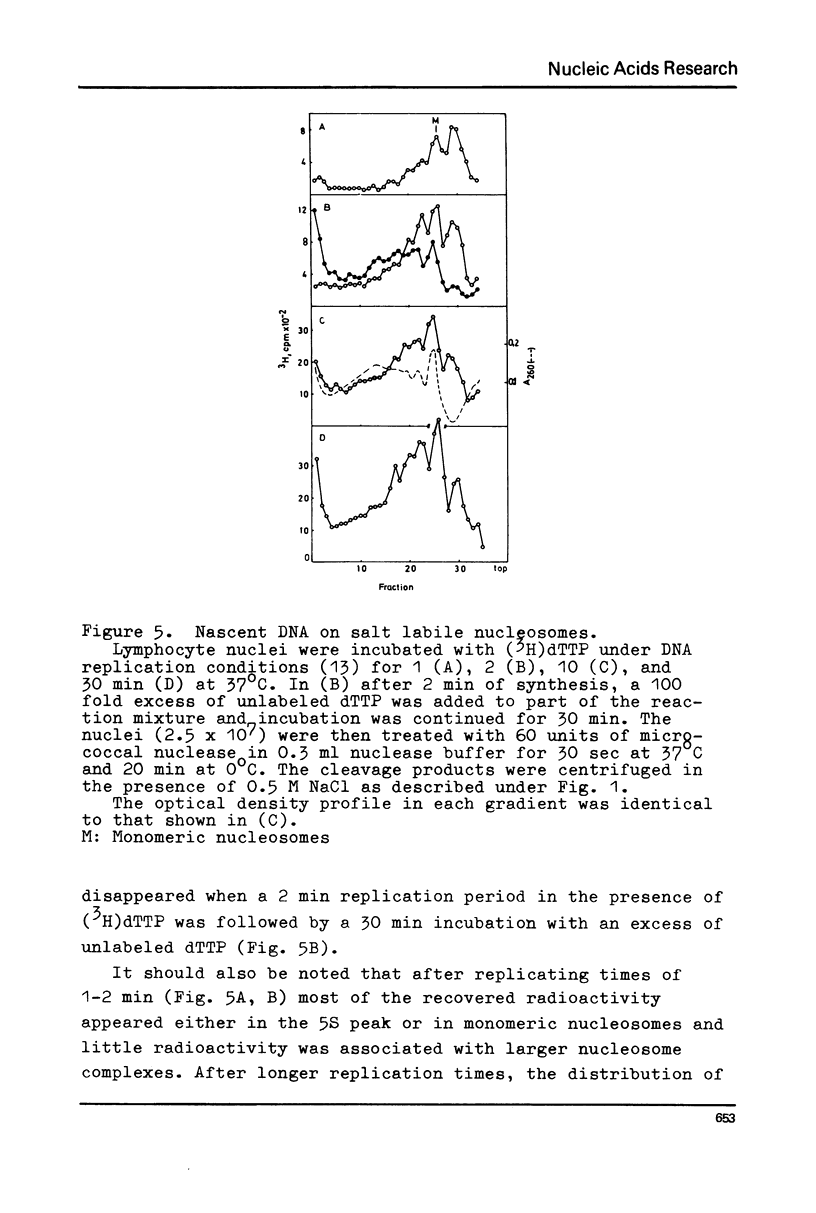
Chromatin replication was studied in isolated nuclei from Concanavalin A activated lymphocytes. Digestion with micrococcal nuclease revealed that the resistant fraction of in vitro replicated DNA is associated with nucleosomes. Earlier experiments had shown that the nuclease resistant fraction of nascent DNA is composed of fragments which are shorter than the nuclease resistant fragments of bulk DNA. In this communication we demonstrate that the short fragments of nascent DNA are differently bound to nucleosome like structures compared to bulk DNA. At 0.5 M NaCl a fraction of pulse labeled labeled DNA is released from these structures and appears as free double stranded DNA of about 140 base pair length (5S DNA) while the 185 pair fragments of mature replicated DNA remain attached to nucleosomes under these conditions. The experiments may indicate that the interaction of a fraction of replicating DNA with histones differs from that of bulk DNA.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakayev V. V., Bakayeva T. G., Varshavsky A. J. Nucleosomes and subnucleosomes: heterogeneity and composition. Cell. 1977 Jul;11(3):619–629. doi: 10.1016/0092-8674(77)90079-4. [DOI] [PubMed] [Google Scholar]
- Benz W. C., Strominger J. L. Viral and cellular DNA synthesis in nuclei from human lymphocytes transformed by Epstein-Barr virus. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2413–2417. doi: 10.1073/pnas.72.6.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Hildebrand C. E., Walters R. A. Rapid assembly of newly synthesized DNA into chromatin subunits prior to joining to small DNA replication intermediates. Biochem Biophys Res Commun. 1976 Nov 8;73(1):157–163. doi: 10.1016/0006-291x(76)90510-6. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Allfrey V. G., Bradbury E. M., Matthews H. R. Altered nucleosome structure containing DNA sequences complementary to 19S and 26S ribosomal RNA in Physarum polycephalum. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1116–1120. doi: 10.1073/pnas.75.3.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg R. D. Structure of chromatin. Annu Rev Biochem. 1977;46:931–954. doi: 10.1146/annurev.bi.46.070177.004435. [DOI] [PubMed] [Google Scholar]
- Levy A., Jakob K. M. Nascent DNA in nucleosome like structures from chromatin. Cell. 1978 Jun;14(2):259–267. doi: 10.1016/0092-8674(78)90112-5. [DOI] [PubMed] [Google Scholar]
- Noll M., Kornberg R. D. Action of micrococcal nuclease on chromatin and the location of histone H1. J Mol Biol. 1977 Jan 25;109(3):393–404. doi: 10.1016/s0022-2836(77)80019-3. [DOI] [PubMed] [Google Scholar]
- Peters J. H. Preparation of large quantities of pure bovine lymphocytes and a monolayer technique for lymphocyte cultivation. Methods Cell Biol. 1975;9(0):1–23. doi: 10.1016/s0091-679x(08)60065-5. [DOI] [PubMed] [Google Scholar]
- Renz M., Nehls P., Hozier J. Involvement of histone H1 in the organization of the chromosome fiber. Proc Natl Acad Sci U S A. 1977 May;74(5):1879–1883. doi: 10.1073/pnas.74.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. M., Hsu J. T. Fractionation of purified nucleosomes on the basis of aggregation properties. Biochemistry. 1977 Apr 19;16(8):1690–1695. doi: 10.1021/bi00627a026. [DOI] [PubMed] [Google Scholar]
- Schlaeger E. J. Chromatin replication in isolated nuclei from bovine lymphocytes. Biochem Biophys Res Commun. 1978 Mar 15;81(1):8–18. doi: 10.1016/0006-291x(78)91623-6. [DOI] [PubMed] [Google Scholar]
- Schlaeger E. J., Klempnauer K. H. The structure of chromatin replicated in vitro. Eur J Biochem. 1978 Sep 1;89(2):567–574. doi: 10.1111/j.1432-1033.1978.tb12561.x. [DOI] [PubMed] [Google Scholar]
- Schlaeger E. J., van Telgen H. J., Klempnauer K. H., Knippers R. Association of DNA polymerase with nucleosomes from mammalian cell chromatin. Eur J Biochem. 1978 Mar;84(1):95–102. doi: 10.1111/j.1432-1033.1978.tb12145.x. [DOI] [PubMed] [Google Scholar]
- Seale R. L. Nucleosomes associated with newly replicated DNA have an altered conformation. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2717–2721. doi: 10.1073/pnas.75.6.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale R. L. Studies on the mode of segregation of histone nu bodies during replication in HeLa cells. Cell. 1976 Nov;9(3):423–429. doi: 10.1016/0092-8674(76)90087-8. [DOI] [PubMed] [Google Scholar]
- Sheinin R., Humbert J. Some aspects of eukaryotic DNA replication. Annu Rev Biochem. 1978;47:277–316. doi: 10.1146/annurev.bi.47.070178.001425. [DOI] [PubMed] [Google Scholar]
- Shelton E. R., Kang J., Wassarman P. M., DePamphilis M. L. Chromatin assembly in isolated mammalian nuclei. Nucleic Acids Res. 1978 Feb;5(2):349–362. doi: 10.1093/nar/5.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sons W., Unsöld H. J., Knippers R. Increase of chromatin-bound protein kinase after stimulation of lymphocytes by concanavalin A. Eur J Biochem. 1976 May 17;65(1):263–269. doi: 10.1111/j.1432-1033.1976.tb10413.x. [DOI] [PubMed] [Google Scholar]