Abstract
The core pathology of sickle cell disease (SCD) starts with the erythrocyte (RBC). Aberration in MAPK/ERK1/2 signaling, which can regulate cell adhesion, occurs in diverse pathologies. Because RBCs contain abundant ERK1/2, we predicted that ERK1/2 is functional in sickle (SS) RBCs and promotes adherence, a hallmark of SCD. ERK1/2 remained active in SS but not normal RBCs. β2-adrenergic receptor stimulation by epinephrine can enhance ERK1/2 activity only in SS RBCs via PKA- and tyrosine kinase p72syk-dependent pathways. ERK signaling is implicated in RBC ICAM-4 phosphorylation, promoting SS RBC adhesion to the endothelium. SS RBC adhesion and phosphorylation of both ERK and ICAM-4 all decreased with continued cell exposure to epinephrine, implying that activation of ICAM-4–mediated SS RBC adhesion is temporally associated with ERK1/2 activation. Furthermore, recombinant ERK2 phosphorylated α- and β-adducins and dematin at the ERK consensus motif. Cytoskeletal protein 4.1 also showed dynamic phosphorylation but not at the ERK consensus motif. These results demonstrate that ERK activation induces phosphorylation of cytoskeletal proteins and the adhesion molecule ICAM-4, promoting SS RBC adhesion to the endothelium. Thus, blocking RBC ERK1/2 activation, such as that promoted by catecholamine stress hormones, could ameliorate SCD pathophysiology.
Introduction
Sickle (homozygous hemoglobin S, SS) RBC-based adhesion and vaso-occlusive events likely initiate and/or exacerbate the profound vasculopathy present in patients with sickle cell disease (SCD).1,2 SS RBCs possess unusually active signaling pathways that contribute to a panoply of abnormalities, including RBC adhesion to the endothelium and vaso-occlusion.2–4
Cell adhesion is a multistep cellular process that is regulated by complex extracellular and intracellular signals that may differ from one cell type to another. We have previously shown that abnormal SS RBC interaction with the endothelium and with leukocytes can be induced via β2 adrenergic receptor (β2AR) activation by the stress hormone epinephrine.4–6 Such stimulation activates the intracellular cAMP/protein kinase A (PKA) pathway.4 β2ARs are prototypic G-coupled receptors whose signaling properties are in part mediated by the activation of stimulatory GTP-binding proteins (Gs proteins), which in turn activate adenylate cyclase (AC), leading to the generation of cAMP and the subsequent activation of PKA. The cAMP/PKA pathway can modulate the MAPK/ERKs cascade both directly and indirectly.7–9 PKA has been reported to stimulate B-Raf, while inhibiting c-Raf. Therefore, the activity of downstream signaling proteins, such as MEKs and ERKs, could be either enhanced or inhibited depending on the balance of c-Raf and B-Raf activation.10,11 The cellular functions mediated by β2ARs can also be independent of adenylyl cyclase activation and involve other mediators instead.12–15
The functions attributed to ERK1/2 at both the cellular and physiologic levels are diverse, including modulation of proliferation, differentiation, apoptosis, migration, and cell adhesion.16–19 Physiologically, ERK1/2 is required for immune system development, homeostasis and antigen activation, memory formation, development of the heart, and responses to many hormones, growth factors, and insulin. Most of these previous studies have involved only nucleated cells, including erythroid cells, in which erythropoietin is the primary regulatory cytokine of this pathway.20 However, aberrations in ERK1/2 signaling are known to occur in a wide range of pathologies, including cancer, diabetes, viral infection, and cardiovascular disease.21,22 In preliminary studies, authors have indicated that ERK1/2 is highly abundant in both SS and normal RBCs. Yet, whether this kinase remains functional in normal or SS RBCs is unknown, and an extremely critical question in the study of SCD pathophysiology. Such a mechanism of action could represent a novel target for the treatment of SCD.
Methods
Endothelial cells
Primary HUVECs were grown as monolayers in EBM2 medium (Lonza Walkersville) supplemented with EGM2 (Lonza Walkersville) as described previously.4 All experiments were approved by the Duke University institutional review board.
Antibodies
Abs used included the following monoclonal and polyclonal Abs (as purified Ig unless otherwise noted): BS46 (mouse IgG1 anti–ICAM-4, generously provided by Dr Jean-Pierre Cartron, Inserm Unité 665)23; mouse anti–phospho-myelin basic protein (anti–phospho-MBP; Millipore); mouse anti–human transferrin receptor (BD Biosciences); and mouse anti–human glycophorin C, produced in our laboratory.24 Rabbit anti–human ERK1/2 (Upstate Biotechnology); rabbit anti–human phospho-ERK1/2 (Cell Signaling Technology); and rabbit anti–human MEK1/2 (Sigma-Aldrich) were used. The murine myeloma protein P3 × 63/Ag8 (P3 ascitic fluid, diluted 1:500) was used as a nonreactive control murine IgG1 for mAbs.25 In all studies, Abs were used at saturating dilutions unless otherwise indicated.
Collection, preparation, and treatment of RBCs
Patients with SCD homozygous for hemoglobin S had not undergone transfusion for at least 3 months, had not experienced vaso-occlusion for 3 weeks, and were not on hydroxyurea. Blood samples from patients with SCD and healthy donors homozygous for hemoglobin A (AA), collected into citrate tubes, were used within 24 hours of collection. Packed SS and AA RBCs were separated as previously described.5
Packed RBCs were treated with various reagents to affect cAMP signaling or protein phosphorylation. Sham-treated RBCs were incubated with the same buffer and vehicle but without the active agent. RBCs were treated at 37°C with one or more of the following reagents: 20nM epinephrine (Sigma-Aldrich) for 1 or 30 minutes; 2mM phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX; Sigma-Aldrich) for 2 hours; 80μM forskolin (Sigma-Aldrich) for 30 minutes; 1 or 2 μg/mL Pertussis toxin (PTx; Calbiochem); 5μM MEK1/2 inhibitor (MEKI) U0126 (Calbiochem); 30nM protein kinase A inhibitor (PKAI) 14-22 amide (Calbiochem); 10μM damnacanthal (Enzo Life Sciences International); or 10μM piceatannol (Enzo Life Sciences International) for 1 hour. Treated RBCs were then washed 5 times with 4 mL of PBS with Ca2+ and Mg2+. Before adhesion assays, treated RBCs were labeled with PKH 26 red fluorescent cell linker kit (Sigma-Aldrich), following the manufacturer's instructions.
Western blot
Packed RBCs were lysed with hypotonic buffer (5mM Na2HPO4 + 1mM EDTA + 0.1% NaN3, pH 8) containing 2mM PMSF (Sigma-Aldrich), phosphatase inhibitor cocktail (Sigma-Aldrich), and protease inhibitor cocktail (Sigma-Aldrich). Protein separation by PAGE used equal amounts of total RBC membrane ghost proteins per lane, after we corrected total protein measurements for residual hemoglobin content. Western blots,26 using the appropriate Ab, were then performed. Mouse 3T3/A31 fibroblast lysate was used as an ERK1/2-positive control. For total ERK1/2, membranes blotted with anti-phosphoERK Ab were stripped and reexposed to anti-ERK1/2 Ab. Bands were analyzed densitometrically with the use of ImageJ 64 software downloaded from the National Institutes of Health Web site. PhosphoERK1/2 data were normalized according to total ERK1/2 and are presented as fold change in ERK phosphorylation.
ERK activity assay
Treated packed normal and SS RBCs were lysed at 4°C with lysis buffer (10mM EDTA, 20mM Tris, 110mM NaCl, pH 7.5) containing 2mM PMSF, 1% Triton X-100, phosphatase inhibitor cocktail (Sigma-Aldrich), and protease inhibitor cocktail (Sigma-Aldrich). ERK1/2 was immunoprecipitated with anti-ERK1/2 antibody at 4°C, and immune complexes were obtained with protein A-agarose (Amersham Biosciences). ERK1/2 immunocomplex was examined for ERK1/2 activity via the use of MBP at 2 mg/mL (Millipore) as a substrate, and ATP as a phosphate donor with equal protein amounts per assay condition. Commercial active recombinant human ERK2 was used (Sigma-Aldrich) as a positive control. The reaction mixture was incubated for 20 minutes at 30°C, followed by protein separation and immunoblotting with anti–phospho-MBP mAb (Millipore).
Nonradiolabeled treated RBC ghosts were separated by mass spectrometry and then subjected to label-free quantitative phosphoproteomic analysis after phosphopeptide enrichment (see supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Reticulocyte enrichment
Reticulocytes were separated from mature SS RBCs by the use of anti–transferrin receptor mAb and goat anti–mouse IgG-coated microbead affinity columns (MACS; Miltenyi Biotec), following the manufacturer's instructions.
Flow chamber assays
Graduated height flow chambers were used to quantify RBC adhesion to HUVECs as previously described.4,27
ICAM-4 phosphorylation and immunoprecipitation
Packed RBCs 32P-labeled as previously described28 were incubated with phosphatase inhibitor cocktail (Sigma-Aldrich) in the presence or absence of MEKI, PKAI, or a combination of MEKI and PKAI before 1 or 30 minutes of treatment with epinephrine. Cells were then washed 4 times. ICAM-4 immunoprecipitation and total and phospho-ICAM-4 detection were performed as previously described in detail.4 To confirm that the immunoprecipitates were specific for ICAM-4, anti–ICAM-4 mAb and the negative control Ig P3 were used to immunoprecipitate ICAM-4 from nonradiolabeled treated SS RBCs. Blots were immunostained with anti–ICAM-4 mAb.
Whole-cell cAMP accumulation
Washed, packed RBCs were pretreated with IBMX to define basal cAMP accumulation, followed by treatment with epinephrine for 1 minute or 30 minutes, or with forskolin. Samples were placed on ice, stimulation was halted, and cells fixed by the addition of 12.5mM EDTA. Cell samples were boiled, clarified by centrifugation, and assayed for cAMP content by radioimmunoassay as described previously.9 Basal cAMP production was subtracted from the total cAMP produced by the cells. The amounts of cAMP were then normalized as fmol cAMP/108 RBCs.
Statistical analysis
Data were compared with the use of parametric analyses (GraphPad Prism 4 software), including repeated and nonrepeated measures of ANOVA. One-way ANOVA analyses were followed by Bonferroni corrections for multiple comparisons (multiplying the P value by the number of comparisons). A P value less than .05 was considered significant.
Results
ERK1/2 is active in SS RBCs and undergoes increased activation by epinephrine
The cAMP/PKA pathway is known to both promote the abnormal adhesion of SS RBCs to endothelial cells (ECs)4 and modulate the MAPK/ERK cascade. Given the importance of abnormal SS RBC adherence in SCD pathophysiology, we investigated the possibility that ERK is present and active in SS RBCs and can be induced by epinephrine. RBC ghosts consisting of membrane fragments prepared from SS and normal (AA) RBCs were first analyzed to confirm the presence of ERK1/2 and MEK1/2, the upstream kinase of ERK1/2 activation. MEK1/2 was abundant in both SS and AA RBCs, whereas ERK1/2 was expressed at greater levels in SS versus AA RBCs (P < .05, Figure 1A-B). Because ERK1/2 is also well expressed by platelets and leukocytes, we examined our RBC suspensions for contamination by other blood cells. Our SS RBC preparations (0.13 ± 0.01 × 106/mL RBCs) showed no contamination by platelets, but a very low level of contamination by leukocytes (0.2 ± 0.06 × 103/mL) was sometimes detected. However, when similar numbers of isolated sickle cell patient leukocytes were examined for the presence of ERK1/2, no detectable signal was observed (data not shown), demonstrating that the observed ERK signal was in fact derived from SS RBCs.
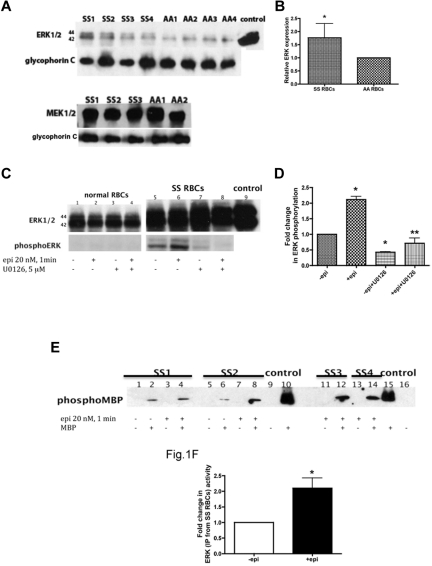
Figure 1.
ERK undergoes activation in SS but not normal RBCs. (A-B) Fifty micrograms of membrane protein ghosts (SS RBC ghosts, n = 4, lanes: SS1, SS2, SS3, and SS4; and normal RBC ghosts, n = 4, lanes: AA1, AA2, AA3, and AA4) were used per lane. Western blots of protein ghosts were stained with antibodies against ERK1/2, glycophorin C as a loading control, and MEK1/2 (n = 3 for SS RBC ghosts, lanes: SS1, SS2, and SS3; and n = 2 for normal RBC ghosts, lanes: AA1 and AA2). (A) ERK1/2 and MEK1/2 are highly expressed in both SS and normal RBCs and are bound to the RBC plasma membrane. (B) Quantitative analysis of the data (normalized according to glycophorin C expression) presented as relative ERK1/2 expression compared with normal RBCs (P < .05 for SS vs normal RBCs, n = 4 for each). (C-D) Normal RBCs (n = 3, lanes: 1, 2, 3, and 4) and SS RBCs (n = 3, lanes: 5, 6, 7, and 8) were sham-treated (lanes 1 and 5), incubated for 1 minute with 20nM epinephrine (epi; lanes 2 and 6), pretreated with the MEKI, U0126, followed by epi treatment (lanes 4 and 8), or treated with U0126 alone (lanes 3 and 7). Mouse 3T3/A31 fibroblast lysate was used as a positive control (lane 9). One hundred micrograms of SS and normal RBC ghost proteins were used per lane. Western blots were stained with antibodies against ERK and phosphoERK. (C) ERK1/2 is phosphorylated at baseline in SS RBCs and undergoes increased phosphorylation by epi stimulation. ERK in normal RBCs was not phosphorylated and completely failed to undergo increased phosphorylation after epi stimulation. (D) Quantitative analysis of the data is presented as fold change in ERK phosphorylation. *P < .01 compared with untreated cells. **P < .001 compared with epi-treated SS RBCs. (E-F) ERK immunoprecipitated from sham-treated (lanes: 1, 2, 5, and 6) and epi-treated (lanes: 3, 4, 7, 8, 11, 12, 13, and 14) SS RBCs was incubated without MBP (lanes: 1, 3, 5, 7, 11 and 13) or with MBP (lanes: 2, 4, 6, 8, 12, and 14) as a substrate for ERK, with equal protein amounts per assay condition. Commercial active recombinant human ERK2 was incubated without MBP (lanes: 9 and 16) or with MBP (lanes: 10 and 15) as negative and positive controls, respectively. (E) Immunoblots indicate that the activity of ERK is conserved and functional in SS RBCs and epi can intensify its activity. SS RBCs obtained from 4 different patients (SS1, SS2, SS3, and SS4) were tested. (F) Quantitative analysis of the data are presented as fold change in ERK phosphorylation (n = 4). *P = .0286 compared with nontreated cells.
Our data also indicated that ERK1/2 is phosphorylated at baseline in SS RBCs and that the use of epinephrine at a physiologic “stress” dose (20nM)29 promoted a 2.1- ± 0.1-fold increase in ERK phosphorylation within 1 minute (n = 3; P < .001; Figure 1C-D). Incubation of SS RBCs with the MEKI U0126, which specifically inhibits MEK1/2, before epinephrine treatment significantly inhibited the effect of epinephrine on ERK1/2 phosphorylation (P < .001; Figure 1C-D). Because our previous data indicated that the degree of adhesive response to epinephrine stimulation varied from patient to patient,4 the effect of epinephrine on ERK phosphorylation was measured in samples obtained from a larger group of patients (n = 19). Although a statistically significant increase (2- ± 0.17-fold) in ERK phosphorylation above basal levels was observed (P < .05), SS RBCs from only 40% of patients exhibited more than 1.5-fold elevation in ERK phosphorylation by epinephrine. These patients were classified as responders. These data suggest that not all patients with SCD are susceptible to epinephrine-stimulated increased ERK phosphorylation. In contrast, ERK1/2 was never found phosphorylated in AA RBCs and failed to undergo phosphorylation by epinephrine (Figure 1C).
To confirm that phosphorylation was indeed an indicator of ERK activation, we tested the activity of ERK1/2 isolated from both sham-treated and epinephrine-treated SS and normal RBCs using MBP as a substrate for ERK in the presence of inhibitors of PKA, PKC, Ca2+/calmodulin-dependent kinase, and p34cdc2 kinase to prevent nonspecific MBP phosphorylation by these enzymes.30 ERK1/2 immunoprecipitated from sham-treated SS RBCs was capable of phosphorylating MBP (Figure 1E-F). MBP phosphorylation by ERK1/2 immunoprecipitated from epinephrine-treated SS RBCs increased 2.1- ± 0.3-fold compared with MPB phosphorylation induced by ERK1/2 isolated from sham-treated cells (n = 4; P = .0286; Figure 1E-F). In contrast, ERK1/2 immunoprecipitated from either sham-treated or epinephrine-treated normal RBCs completely failed to phosphorylate MBP (n = 4; data not shown). These data indicate that ERK1/2 is active at baseline in SS RBCs and that epinephrine can augment this activity.
ERK1/2 activation in SS RBCs acts downstream of the cAMP/PKA signaling pathway
Forskolin, which directly activates AC to produce cAMP, increased ERK1/2 phosphorylation in SS RBCs, which was in turn prevented by U0126. These data suggest that cAMP-stimulated ERK activation is MEK dependent in the sickle cell samples tested (Figure 2A).
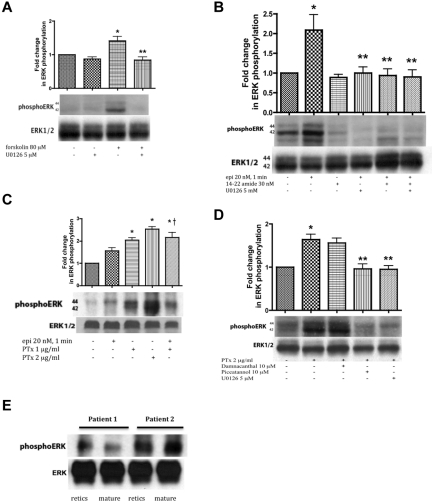
Figure 2.
ERK activation in SS RBCs involves the cAMP/PKA pathway and the tyrosine kinase p72syk and is sensitive to the effect of Gαi protein. SS RBCs (A-D), and reticulocyte-enriched and -depleted (mature) SS RBCs (E) were sham-treated, treated with forskolin (A), epi (B-C), PKAI, 14-22 amide, (B), or PTx (C-D) in the presence or absence of the MEKI U0126 (A,B,D), piceatannol (D) or damnacanthal (D). RBC proteins were blotted with antibodies against ERK and phosphoERK. Quantitative analysis of the blots is presented as fold change in ERK phosphorylation. (A-B) ERK undergoes phosphorylation via the cAMP/PKA pathway. (A) ERK undergoes increased phosphorylation after RBC incubation with forskolin, which is inhibited by U0126 (n = 3). *P < .05 compared with untreated cells. **P < .01 compared with forskolin-treated SS RBCs. (B) Phosphorylation of ERK is increased by epi, and this increase was abrogated by either 14-22 amide or U0126 (n = 3). *P < .01 compared with untreated cells. **P < .01 compared with epi-treated SS RBCs. (C) ERK phosphorylation in SS RBCs is enhanced by inactivation of the Gαi protein. PTx at either 1 or 2 μg/mL increased basal ERK phosphorylation (n = 9). *P < .001 compared with nontreated cells; †P < .05 compared with epi-treated SS RBCs. (D) The tyrosine kinase p72syk is implicated in ERK phosphorylation. PTx at 2 μg/mL up-regulated ERK phosphorylation, an effect that was blocked by piceatannol. Conversely, damnacanthal failed to block ERK phosphorylation induced by PTx (n = 3). *P < .01 compared with untreated cells. **P < .01 compared with PTx-treated SS RBCs. (E) ERK1/2 is phosphorylated at baseline in both reticulocyte-enriched and reticulocyte-depleted (mature) SS RBCs (n = 2).
PKA, which acts downstream of cAMP, was also involved in ERK phosphorylation. Treatment of SS RBCs with the PKA-specific inhibitor (PKAI), 14-22 amide at 30nM, a concentration known to optimally inhibit PKA in SS RBCs, completely blocked the effect of epinephrine on ERK phosphorylation (P < .01, n = 3; Figure 2B). PKAI and the MEKI U0126 combined also completely blocked ERK phosphorylation in response to epinephrine stimulation (P < .01; Figure 2B). These data suggest that ERK1/2 activation in SS RBCs is dependent on the cAMP/PKA pathway.
In some instances, β2AR activation uses a Gαi (or Gαo) pathway to stimulate ERK activity.7–9 We investigated whether epinephrine-stimulated β2AR-mediated ERK activation in SS RBCs also involved the Gαi using PTx, which inhibits Gαi-signaling. Inhibition of Gαi with 1 or 2 μg/mL PTx alone significantly increased basal phosphorylation of ERK1/2 by 2.04- ± 0.1-fold and 2.53- ± 0.11-fold, respectively, and combining PTx with epinephrine produced no additional effect (Figure 2C, P < .001). These results suggest that increased ERK1/2 phosphorylation in these SCD patient samples is negatively affected by Gαi activation or because of the direct actions of PTx.
Because direct or indirect involvement of cytoplasmic tyrosine kinases in activation of MEK cascades has also been demonstrated,31,32 we evaluated the contribution of tyrosine kinase-induced signaling to RBC ERK1/2 phosphorylation. ERK1/2 was phosphorylated at baseline in sham-treated SS RBCs (Figure 2D). Treatment with 2 μg/mL PTx markedly increased ERK1/2 phosphorylation. Damnacanthal, a highly potent and selective inhibitor of the tyrosine kinase p56lck,33 did not abrogate ERK phosphorylation in response to PTx (Figure 2D). However, piceatannol, which preferentially inhibits the tyrosine kinase p72syk versus p56lyn, completely blocked the effect of PTx on ERK phosphorylation. Once more, U0126 blocked the effect of PTx on ERK1/2 phosphorylation. These data suggest that the piceatannol-sensitive tyrosine kinase p72syk also plays a role in SS RBC ERK1/2 activation.
To determine whether ERK1/2 is active only in the youngest cell population (reticulocytes), reticulocyte-enriched and -depleted (mature) SS RBCs were analyzed for kinase phosphorylation. Flow cytometric analysis showed that up to 15% of unseparated SS RBCs expressed the transferrin receptor, a reticulocyte marker. After separation, > 95% of the reticulocyte-enriched cells expressed the transferrin receptor, whereas the reticulocyte-depleted population reacted with the anti–transferrin receptor antibody no more strongly than with the negative control Ig (data not shown). ERK1/2 was strongly phosphorylated in both reticulocyte-enriched and reticulocyte-depleted cells (n = 2; Figure 2E), suggesting that ERK activity is preserved in both reticulocytes and mature SS RBCs.
ERK1/2 is involved in SS RBC adhesion to endothelial cells and is implicated in phosphorylation of the RBC adhesion receptor ICAM-4 (Landsteiner-Wiener blood group antigen)
Because the pharmacologic agents epinephrine and forskolin can modulate both SS RBC adhesion to ECs4 and ERK activation, we determined the contribution of MEK/ERK signaling to RBC adhesion. Epinephrine significantly up-regulated SS RBC adhesion to HUVECs at a shear stress of 2 dynes/cm2 in intermittent flow condition assays (P < .001; Figure 3A). However, U0126 completely inhibited the effect of epinephrine on SS RBC adhesion (P < .001). Treatment of SS RBCs with U0126 alone also blocked SS RBC adhesion to HUVECs (91% ± 4.6% inhibition) compared with adhesion of sham-treated SS RBCs (P < .01).
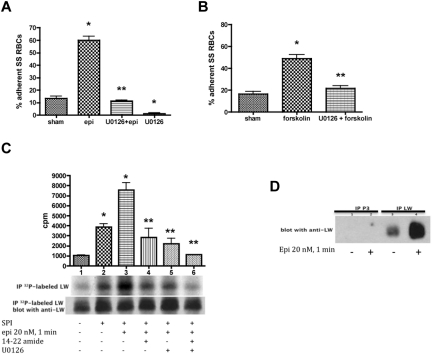
Figure 3.
ERK signaling modulates both SS RBC adhesion to endothelial cells and ICAM-4 phosphorylation. (A-B) Activation of ERK signaling up-regulates SS RBC adhesion to the endothelium. SS RBCs were sham-treated, stimulated with epi for 1 minute or forskolin, preincubated with U0126 followed by epi or forskolin, or treated with U0126 alone. Adhesion of SS RBCs to HUVECs was tested in intermittent flow condition assays. Results are presented as percent adherent SS RBCs at a shear stress of 2 dynes/cm2. Error bars show SEM of 4 different experiments. (A) *P < .001 compared with sham-treated; **P < .001 compared with epi-treated. (B) *P < .001 compared with sham-treated; **P < .001 compared with forskolin-treated. (C-D) The MEK/ERK signaling cascade is involved in ICAM-4 (LW) serine phosphorylation. (C) Inorganic 32P-radiolabeled intact SS RBCs were incubated in the absence (lane 1) or presence (lanes 2, 3, 4, 5, and 6) of serine/threonine protein phosphatase inhibitors (SPI), followed or not (lanes 1 and 2) by treatment with epi (lanes 3, 4, 5, and 6). In lanes 4, 5, and 6, SS RBCs were preincubated with SPI in presence of PKAI, MEKI, or PKAI + MEKI followed by epi treatment, respectively. The counts per minute (cpm) are representative of 3 different experiments, calculated by subtraction of cpm present in a lane (not shown) containing immunoprecipitates using immunoglobulin P3 from cpm obtained using anti-LW (ICAM-4) mAb for immunoprecipitation under each set of conditions indicated. *P < .05 and *P < .001 for SPI-treated and SPI + epi-treated vs sham-treated, respectively; **P < .001 compared with SPI + epi-treated SS RBCs. Total LW loaded in each lane was detected with the use of nitrocellulose membranes of phosphorylated LW blotted with anti-LW mAb. (D). SS RBCs were incubated without (lanes 1 and 3) or with epi (lanes 2 and 4). Lanes 1 and 2 were immunoprecipitated with P3. Lanes 3 and 4 were immunoprecipitated with anti-LW mAb; all lanes for panel D were immunostained with anti-LW mAb.
Similarly, forskolin also enhanced SS RBC adhesion to HUVECs at a shear stress of 2 dynes/cm2 (P < .001, n = 3; Figure 3B), and this effect was blocked by U0126 (83% ± 4% inhibition, compared with increased adhesion by forskolin alone; P < .01). This finding suggests that the MEK/ERK pathway contributes to the up-regulation of SS RBC adhesive function to ECs.
We further explored the possibility that ERK signaling is involved in ICAM-4 (Landsteiner-Wiener blood group antigen; LW) phosphorylation, which mediates RBC adhesion to ECs.4 Up-regulation of SS RBC adhesion to nonactivated ECs requires serine phosphorylation of the ICAM-4 receptor.4 PhosphorImager analysis of immunoprecipitated 32P-radiolabeled ICAM-4 and negative control immune complexes demonstrated that ICAM-4 of nonstimulated SS RBCs (Figure 3C, lane 1) is modestly phosphorylated as previously shown. Treatment of SS RBCs with serine phosphatase inhibitors (SPIs; lane 2) increased ICAM-4 phosphorylation by 3.7- ± 0.46-fold (P < .05, n = 3), suggesting that increased ICAM-4 phosphorylation is a result of serine phosphorylation, as tyrosine phosphatase inhibitors were not present. These findings were similar to the effects of epinephrine; although SPI stimulation induced a significant increase (2.62- ± 0.6-fold) in ICAM-4 phosphorylation above baseline in a larger group of patients (n = 8; P < .05), only one-half of all SS RBC samples exhibited ≥ 2-fold elevation in ICAM-4 phosphorylation in response to SPI. Epinephrine in the presence of SPI had a stronger effect on ICAM-4 phosphorylation (7.4- ± 1.07-fold increase over sham-treated SS RBCs; P < .001; lane 3). Treatment of SS RBCs with either the PKAI or U0126 (lanes 4 and 5, respectively) significantly decreased the combined effect of epinephrine and SPI on ICAM-4 phosphorylation compared with cells treated with epinephrine alone (P < .001; lane 3). Treatment of SS RBCs with both PKAI and MEKI completely blocked epinephrine and SPI from up-regulating phosphorylation of ICAM-4 (P < .001; Figure 3C lane 6).
Immunoblots of 32P-radiolabeled ICAM-4 immunoprecipitates from stimulated and nonstimulated SS RBCs (Figure 3C) indicated that a similar amount of ICAM-4 was immunoprecipitated from these cells. Control immunoblots of immunoprecipitated ICAM-4 and the negative control complexes immunoprecipitated with P3 from stimulated and nonstimulated SS RBCs are shown in Figure 3D.
To define whether ICAM-4 is a substrate for ERK, we used nontreated packed normal RBCs as a source of ICAM-4 because ERK is inactive in these cells (Figure 1A) and ICAM-4 is not phosphorylated at baseline.4 Exposure of immunoprecipitated ICAM-4 to active recombinant ERK2 did not cause ICAM-4 phosphorylation, indicating that ICAM-4 is not a substrate for ERK (data not shown). Together, our data demonstrate that ICAM-4 in SS RBCs undergoes serine phosphorylation by a yet unknown kinase and that this process is PKA and MEK/ERK1/2 dependent.
SS RBC adhesion is strictly related to the inception of ERK activation
Epinephrine significantly increased SS RBC adhesion to HUVECs under both intermittent and constant flow conditions after 1 minute of exposure (P < .001 for each condition), whereas adhesion decreased after 30 minutes' cell exposure to epinephrine (mean decrease of all samples = 56% ± 1.5% and 73% ± 4.7% for intermittent and constant flow conditions, respectively; P < .001 for each; Figure 4A-B). In contrast, epinephrine treatment for either 1 or 30 minutes, had minimal effect on normal RBC adhesion to HUVECs under either intermittent or constant flow conditions (Figure 4A-B).
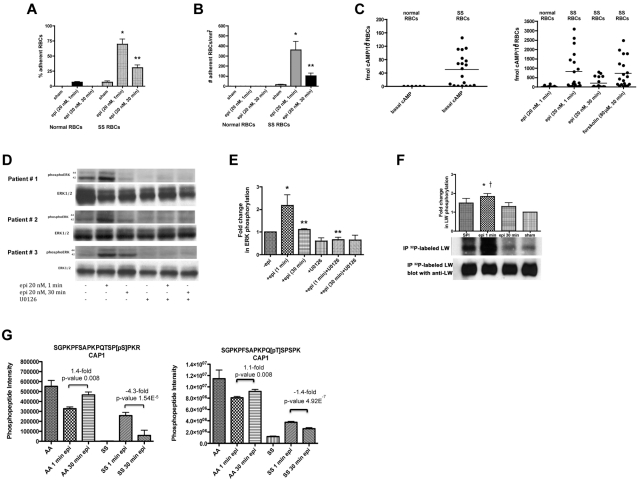
Figure 4.
SS RBC adhesion is associated with the extent of ERK activation. (A-B) Adhesion of SS RBCs to endothelial cells is related to the duration of cell stimulation with epinephrine. Adhesion of RBCs to HUVECs was tested in both intermittent flow and flowing condition assays, and results are presented as precent adherent RBCs at a shear stress of 2 dynes/cm2 and number of adherent RBCs/mm2, respectively. Normal and SS RBCs were sham-treated, or stimulated with epi for 1 minute or 30 minutes. *P < .001 compared with sham-treated SS RBCs; **P < .001 compared with epi-treated SS RBCs. Error bars show SEM of 4 different experiments. (C) cAMP production in SS RBCs is associated with the time of cell stimulation with epinehprine. RBCs were treated with IBMX (for basal cAMP levels), followed or not with epi (for 1 minute or 30 minutes) or forskolin. The specific effect of epi and forskolin on cAMP accumulation was obtained by subtracting basal cAMP levels from the total cAMP levels. The basal cAMP production and specific amounts of cAMP because of epi or forskolin stimulation were normalized as fmol cAMP/108 RBCs. (D-E) ERK phosphorylation is dependent on the time of SS RBC exposure to epinephrine. SS RBCs were sham-treated or treated with epi for 1 or 30 minutes, U0126, or U0126 followed by epi for 1 or 30 minutes. Immunoblots of RBC proteins with antibodies against ERK and phosphoERK (D) and quantitative analysis of the data presented as fold change in ERK phosphorylation (E) are shown. ERK underwent increased phosphorylation after 1 minute exposure to epi, whereas phosphorylation decreased with longer (30 minutes) cell exposure to epi (n = 4). *P < .01 compared with nontreated cells; **P < .01 and **P < .001 for epi-treated for 30 minutes and U0126+epi-treated for 1 minute versus cells treated with epi for 1 minute, respectively (E). (F) Inorganic 32P radiolabeled intact SS RBCs were incubated in the presence (lanes 1, 2, and 3) or absence (lane 4) of SPI, followed (lanes 2 and 3) or not (lane 1) by treatment with epi for 1 minute (lane 2) or 30 minutes (lane 3). The cpm are representative of 3 different experiments, calculated by subtraction of cpm present in a lane (not shown) containing immunoprecipitates using immunoglobulin P3 from cpm obtained using anti-LW (ICAM-4) mAb for immunoprecipitation under each set of conditions indicated. *P < .01 compared with sham-treated; †P < .05 compared with SPI + epi (30 minutes)–treated SS RBCs. (G) Prolonged cell exposure to epinephrine negatively affects phosphorylation of adenylate cyclase-associated protein 1. RBC ghosts isolated from SS and normal RBCs treated with epi for 1 and 30 minutes were enriched in phosphopeptides and then subjected to a label-free quantitative phosphoproteomics analysis. Phosphorylation of both serine and threonine on CAP1 in SS RBCs decreased with increased time (1 minute vs 30 minutes) of cell exposure to epi, whereas an increase in the abundance of these phosphopeptides was observed in normal (AA) RBCs after 30 minutes exposure to epi. Each data point is an average of 3 analytical replicate measurements with error bars indicating SD.
We also examined the effect of exposure time of SS RBCs to epinephrine on cAMP production, which appears to act upstream of ERK1/2. Basal cAMP in normal RBCs from healthy donors was significantly lower than basal cAMP in SS RBCs (P = .0187; Figure 4C). In approximately 50% of the samples examined (n = 19), incubation of SS RBCs with epinephrine for 1 minute resulted in accumulation of high levels of intracellular cAMP comparable with the levels of cAMP induced by forskolin treatment for 30 minutes. Although the cAMP response to epinephrine (1-minute exposure time) varied among patients, as previously described,34 cAMP levels uniformly declined with 30 minutes exposure time of SS RBCs to epinephrine (P < .05; Figure 4C). Epinephrine exposure for 1 minute had lower effect on cAMP accumulation in normal RBCs (n = 12) than in SS RBCs (Figure 4C) as previously shown.
In addition, although a 1-minute exposure of SS RBCs to epinephrine markedly increased ERK phosphorylation (P < .01 for epinephrine-treated for 1 minute vs sham-treated), ERK phosphorylation decreased after a 30-minute exposure (P < .01 for epinephrine-treated for 1 minute vs 30 minutes) to levels observed in sham-treated cells (P > .05 for epinephrine-treated for 30 minutes vs sham-treated; Figure 4D-E).
ICAM-4 phosphorylation also decreased with longer exposure time (30 minutes vs 1 minute) of SS RBCs to epinephrine (Figure 4F). PhosphorImager analysis of immunoprecipitated 32P-radiolabeled ICAM-4 and negative control immune complexes showed that treatment of SS RBCs with epinephrine for 1 minute in the presence of SPI (lane 2) enhanced ICAM-4 phosphorylation by 1.84- ± 0.15-fold over sham-treated cells (lane 4, P < .01). Thirty minutes exposure of SS RBCs to epinephrine in the presence of SPI (lane 3) significantly diminished the effect of epinephrine and SPI on ICAM-4 phosphorylation compared with cells treated with epinephrine for 1 minute (lane 2, P < .05). Altogether, these data indicate that exposure time to epinephrine influences all these downstream effects—SS RBC adhesion, cAMP levels and phosphorylation of both ERK1/2 and ICAM-4—in a parallel fashion, suggesting that the time course of up-regulation of ICAM-4–mediated SS RBC adhesion is closely associated with the extent of ERK1/2 activation.
To identify potential proteins involved in regulation of the ERK pathway, a label-free quantitative phosphoproteomics analysis of RBC ghosts isolated from SS and normal RBCs treated with epinephrine for 1 and 30 minutes was undertaken. SS RBCs treated with epinephrine for 30 minutes showed a dramatic decrease in phosphorylation of serine 310 within adenylate cyclase-associated protein 1 (CAP1) compared with cells stimulated with epinephrine for 1 minute (−4.3-fold, P = 1.54 × 10−5; Figure 4G). Conversely, AA RBCs exposed to epinephrine for 30 minutes showed an enhancement in phosphorylation of the CAP1 serine versus 1-minute epinephrine exposure (+1.4-fold, P = .008). Threonine 307 within CAP1 also underwent a smaller yet statistically significant decrease in phosphorylation in SS RBCs exposed to epinephrine for 30 minutes versus 1 minute epinephrine exposure (−1.4-fold, P = 4.92 × 10−7). Our data indicate that exposure to epinephrine for a prolonged period of time negatively affects phosphorylation of CAP1 in SS but not in AA RBCs. Adenylate cyclase-associated proteins (CAPs) are known to regulate AC activation to increase cAMP levels under specific environmental conditions. We therefore suggest that a decrease in CAP1 phosphorylation in SS RBCs might down-regulate AC activity in these cells, negatively affecting signaling downstream of ERK.
ERK signaling is implicated in phosphorylation of protein 4.1
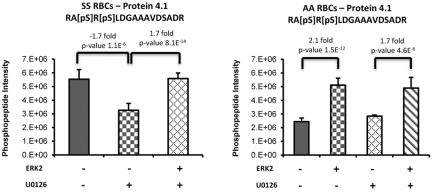
A label-free quantitative phosphoproteomics analysis was also performed to identify additional putative downstream targets of ERK by adding recombinant active ERK2 to RBC ghosts isolated from SS and normal RBCs. Because endogenous ERK is active at baseline in SS but not normal RBCs (Figure 1), SS RBCs were treated with U0126 before incubation of the ghosts with recombinant ERK2. We found that phosphorylation of protein 4.1 was induced in the presence of recombinant ERK2. Treatment of SS RBCs with U0126 resulted in a significant decrease (−1.7-fold, P = 1.01 × 10−6) of a Ser540/Ser542 doubly phosphorylated peptide within protein 4.1 (Figure 5). The addition of recombinant ERK2 to the U0126-treated SS RBC ghosts increased the abundance of this phosphopeptide (+1.7-fold, P = 8.06 × 10−14) back to levels observed in untreated SS RBCs, indicating the specificity of ERK2 as the upstream kinase. As expected, treatment of AA RBCs with U0126 did not induce a decrease of this doubly phosphorylated peptide within protein 4.1 because endogenous ERK is inactive in these cells. However, the complementary trend for this phosphorylated peptide was observed on the addition of recombinant ERK2 to untreated and U0126-treated AA RBC ghosts, for which an increase of 2.1-fold (P = 1.5 × 10−12 for untreated AA RBCs vs untreated AA RBCs + ERK2) and 1.7-fold (P = 4.6 × 10−6 for U0126-treated AA RBCs vs U0126-treated AA RBCs + ERK2) were measured, respectively.
Figure 5.
Phosphorylation of protein 4.1 is induced via the ERK signaling pathway. Sham-treated or U0126-treated SS and normal (AA) RBCs ghosts coincubated with or without recombinant active ERK2 (ERK2) were enriched in phosphopeptides, followed by a label-free quantitative phosphoproteomics analysis. Treatment of SS RBCs with U0126 caused a significant decrease in doubly phosphorylated peptide within protein 4.1. The addition of ERK2 to the U0126-treated SS RBC ghosts increased the abundance of this phosphopeptide back to levels observed in untreated SS RBCs. The complementary trend for this phosphorylated peptide was also observed on the addition of ERK2 to AA RBCs sham-treated or U0126-treated.
To confirm that the measured changes in phosphorylated peptide levels were not the result of a difference in protein level between these treatment conditions, a nonphosphopeptide-enriched proteomic analysis of AA RBC ghosts and AA RBC ghosts coincubated with recombinant ERK2 was performed and confirmed that protein 4.1 levels were similar between the 2 conditions (supplemental Figure 1A). This finding indicates that the observed changes in phosphopeptide abundance results from upstream kinase activity. Similar results were obtained when SS RBC ghosts and SS RBC ghosts coincubated with recombinant ERK2 were analyzed (supplemental Figure 1B). Collectively, these data further strengthen our findings that ERK is active in SS RBCs, and suggest that activation of the ERK cascade induces phosphorylation of the cytoskeletal protein 4.1.
Recombinant active ERK2 phosphorylates the ERK consensus motif on dematin and adducins α and β
To identify ERK substrates in RBCs, all phosphopeptide sequences within the dataset identified when active recombinant ERK2 was added to RBC ghosts were searched for the known ERK consensus motif, [PV]x[pST]P. Adducin-α and -β, and dematin contained 9, 7, and 1 unique phosphorylated peptide, respectively, with phosphorylation of residues within the ERK consensus motif. Only the statistically significant phosphopeptides with fold-changes of > 1.5 are listed in Table 1. These peptides underwent a significant increase in phosphorylation in AA RBCs when recombinant ERK2 was added to the ghosts, whereas a decrease in phosphorylation of these phosphylated peptides was observed in U0126-pretreated SS RBCs (Table 1). This finding suggests that the cytoskeletal proteins adducins α and β and dematin are substrates for ERK in RBCs.
Table 1.
Motif-specific phosphorylation by active recombinant ERK2
| Protein description | Modified peptide sequence | Phospho- residue | AA + ERK2 vs AA | P | SS + U012 6 vs SS | P |
|---|---|---|---|---|---|---|
| α-adducin | 355-[pS]PG[pS]PVGEGTGSPPK-369 | S355, S358 | −1.71 | .035 | 1.47 | .122 |
| α-adducin | 661-EEEAHRPP[pS]PTEAPTEASP | S669, S707 | −1.69 | .025 | 1.60 | .074 |
| EPAPDPAPVAEEAAPSAVEEGAA | ||||||
| ADPG[pS]DGSPGK-713 | ||||||
| β-adducin | 584-ETAPEEPG[pS]PAK[pS]AP | S592, S596, | −2.36 | 4.21 × 10−06 | 1.91 | 3.87 × 10−09 |
| A[pS]PVQSPAK-607 | S600 | |||||
| β-adducin | 584-ETAPEEPGSPAK[pS]APA[pS]PV | S596, S600 | 1.75 | .020 | 1.82 | .018 |
| QSPAK-607 | ||||||
| β-adducin | 584-ETAPEEPG[pS]PAK[pS]APA | S592, S596 | 1.68 | .037 | 1.68 | .010 |
| SPVQSPAK-607 | ||||||
| Dematin | 90-[pS]TSPPP[pS]PEVWADSR-104 | S90, S96 | −1.76 | 9.46 × 10−09 | 1.48 | 4.98 × 10−04 |
Fold changes in phosphorylation for peptides containing the ERK consensus motif [PV]x[pST]P were presented. Phopshorylation is up-regulated in healthy RBCs (AA) with addition of active ERK2 and down-regulated in SS RBCs (SS) with addition of the MEK inhibitor U0126.
Discussion
Our data show that the extracellular signal-regulated kinase (ERK1/2) is active in enucleated sickle red cells and that triggering this kinase promotes activation of signaling pathways and consequent RBC adhesion to the endothelium. We propose that stimulation of β2ARs on SS RBCs by epinephrine for a brief period of time can increase activation of the ERK1/2 signaling cascade, which we show is involved in phosphorylation of the RBC adhesion receptor ICAM-4 and protein 4.1. We also demonstrate that the ERK consensus motifs on dematin and α- and β-adducins undergo increased serine phosphorylation, indicating that these cytoskeletal proteins are direct substrates for ERK.
ERK has been implicated in erythropoietin-induced erythroid its cell proliferation and survival,35 and we have now demonstrated that the activity of this kinase and its upstream signal are conserved in SS RBCs. ERK1/2 is abnormally functional at baseline in all SS RBCs (unseparated, reticulocyte-enriched, and mature). Activation of this kinase can be enhanced within 1 minute of SS RBC exposure to epinephrine. However, the increase in ERK activity by epinephrine did not occur in all SCD patient samples tested, probably attributable at least in part to the inability of epinephrine to increase cAMP production in all SCD patient samples tested.34 In contrast, despite the abundance of both ERK1/2 and its upstream kinase MEK1/2 in normal RBCs, ERK immunoprecipitated from these cells was never found active at baseline, and epinephrine failed to stimulate its activation, suggesting that ERK activity is lost in normal RBCs. Indeed, investigators have previously described that RBCs undergo maturation-related loss of multiple protein kinase activities, including PKA, PKC, and casein kinases.36 In contrast, although SS RBCs are fully differentiated, preservation of ERK activities and its downstream signaling molecule appears to be necessary for abnormal RBC adhesive function.
Our data further implicate involvement of PKA as an upstream mediator in activation of ERK and its downstream signal transduction pathway. Our findings are consistent with studies by Schmitt and Stork7 in which they demonstrated that isoproterenol stimulation of endogenous β2ARs activated ERK in HEK293 cells via a cAMP-dependent PKA pathway. Interestingly PTx, which inactivates Gαi, increased ERK phosphorylation/activation in SS RBCs, suggesting that Gαi negatively impacts this signal. Furthermore, we also identified a role for the tyrosine kinase p72Syk in activation of ERK in SS RBCs while excluding involvement of p56lck-related Src family tyrosine kinases. Thus, in SS RBCs, PKA and the tyrosine kinase p72Syk appear to be important for ERK activation, acting most likely in concert to regulate the MEK/ERK signaling pathway.
The engagement of epinephrine-stimulated ERK in regulation of SS RBC adhesion to the endothelium implies that the MEK/ERK signal can promote an adhesive, vaso-occlusive pathology. It is also apparent from our data that epinephrine-induced adhesion of SS RBCs to nonactivated endothelial cells requires ICAM-4 phosphorylation, which occurs via the PKA/ERK signaling pathway. Furthermore, the adhesive function of SS RBCs appeared to be related to the extent of ERK and ICAM-4 phosphorylation/activation because all 3 similarly increased or decreased depending on the time of cell exposure to epinephrine. In addition, basal cAMP levels, the upstream effector of MEK/ERK, were much greater in SS RBCs than in normal cells, suggesting that the increased level of cAMP in SS RBCs reflects at least in part the persistence of the abnormal ERK activation and RBC adhesive phenotype. However, although epinephrine increased cAMP levels in only 50% of the SCD patient samples tested, cAMP production, which seems to be needed to activate ERK signaling in these sickle cells, was also influenced by the duration of cell exposure to epinephrine. This may be explained at least in part by the dramatic decrease in the abundance of phosphopeptides within CAP1 in SS RBCs because of continued cell exposure to epinephrine stimulation. PKA might also exert a negative feedback loop through activation of phosphodiesterases, resulting in cAMP hydrolysis switching off downstream signaling because of the extended cell exposure to epinephrine.37
CAPs are not only involved in AC association but in actin binding, SH3 binding, and cell morphology maintenance as well.38,39 Previous observations of increased normal RBC membrane filterability after epinephrine treatment for 20 minutes40 explain the enhanced phosphorylated CAP1 in normal RBCs after 30 minutes of epinephrine exposure. Furthermore, Shain et al suggested that maintenance of altered cell morphology required persistent increased cAMP levels because of continuous βAR stimulation.41 In contrast, our data suggest that when an increase in ERK activation occurs within 1 minute of cell exposure to epinephrine, persistent β2AR stimulation has a negative effect on ERK activation and consequently on RBC adhesive function.
Label-free quantitative phosphoproteomics analysis implicates protein 4.1, dematin, and α- and β-adducins as putative downstream targets of ERK in RBCs. Dematin is also a known substrate for PKC and PKA, and PKA-induced dematin phosphorylation completely abolishes its actin bundling capability.42,43 Alternatively, rapid phosphorylation of α- and β-adducins by PKC at Ser-726 and Ser-713, respectively,44 leads to decreased F-actin capping and dissociation of spectrin from actin, implicating adducin phosphorylation in cytoskeletal remodeling.45 Investigators have also shown that protein 4.1 phosphorylation, induced by cAMP-dependent kinase at Ser-331 and PKC at Ser-312 documented after 20 minutes of cell stimulation,44 results in a significant reduction both in the ability of protein 4.1 to promote spectrin binding to F-actin and in spectrin-protein 4.1 binding.46 Thus, phosphorylation of cytoskeletal proteins by ERK in SS RBCs may also lead to cytoskeletal disorganization, which in turn, could potentially render ICAM-4 accessible to undergo phosphorylation to mediate adhesion to the endothelium, or affect its adhesiveness via an as yet undetermined kinase. In fact, cell adhesion can be regulated by an intricate network of signaling molecules, which are responsible for guiding their interaction with substrate mainly via cytoskeleton rearrangement.47 A schematic overview of the proposed increased activation of ERK signaling pathway in SS RBCs is shown in Figure 6.
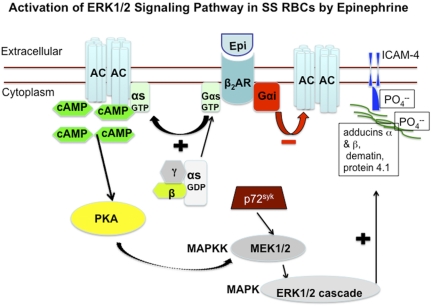
Figure 6.
Schematic depiction of proposed increased activation of ERK signaling pathway in SS RBCs. Epinephrine stimulates β2ARs on SS RBCs. β2ARs are prototypic G-coupled receptors whose signaling is largely mediated by activation of stimulatory GTP-binding proteins (Gs proteins), and inhibited by activation of Gαi protein. Activation of Gs proteins in turn activates AC, leading to generation of cAMP, and the subsequent activation of PKA. The activity of downstream signaling proteins, such as MEKs and ERKs is enhanced by PKA activation. The tyrosine kinase p72Syk acts synergistically with PKA to activate MEK/ERK cascade. Activation of ERK results in phosphorylation of the ERK consensus motif on the cytoskeletal proteins α- and β-adducins, dematin, and protein 4.1, albeit not at the ERK consensus motif. Phosphorylation of cytoskeletal proteins may result in cytoplasmic membrane protein conformational changes, which could render ICAM-4 accessible to phosphorylation.
In summary, although aberrant ERK activation arises in a wide range of pathologies, this report is the first that implicates atypical ERK activation in SS RBCs and its involvement in the abnormal RBC adhesion to the endothelium. Abnormal activation of ERK in SS RBCs could be associated with the pathophysiology of SCD, making the MEK/ERK pathway a potential therapeutic target for preventing and treating vaso-occlusion.
Supplementary Material
Acknowledgments
This work was supported by grants DK065040 to R.Z. from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; a National Blood Foundation award to R.Z.; and HL079915 to R.Z. and M.T. from the National Heart, Lung, and Blood Institute, National Institutes of Health.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.Z. designed and supervised all of the research study, performed most of the experiments and prepared the samples for mass spectrometry separation and label-free phosphopeptide enrichment experiments, analyzed and interpreted all the data, and wrote the manuscript; E.J.W. helped perform cAMP production experiments, participated in the analysis of some of the Western blot data related to ERK phosphorylation, and helped edit the manuscript; E.J.S. performed mass spectrometry separation and label-free phosphopeptide enrichment experiments, participated in the interpretation of the corresponding data, wrote the supplemental Methods section, generated the graphs for proteomic data, and helped edit the manuscript; S.C.A. helped perform some of the Western blot experiments related to ERK phosphorylation; J.W.T. helped to design proteomic experiments, interpret the corresponding data, and edit the manuscript; L.G.D. helped perform mass spectrometry separation and label-free phosphopeptide enrichment experiments; M.A.M. helped edit the proteomic part of the manuscript; and M.J.T. helped interpret some of the data and edit the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for E.J.W. is The Novartis Institutes for Biomedical Research, Cambridge, MA.
Correspondence: Rahima Zennadi, PhD, Box 2615, Duke University Medical Center, Durham, NC 27710; e-mail: zenna001@mc.duke.edu.
References
- 1.Frenette PS, Atweh GF. Sickle cell disease: old discoveries, new concepts, and future promise. J Clin Invest. 2007;117(4):850–858. doi: 10.1172/JCI30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hebbel RP, Boogaerts MA, Eaton JW, Steinberg MH. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med. 1980;302(18):992–995. doi: 10.1056/NEJM198005013021803. [DOI] [PubMed] [Google Scholar]
- 3.Mohandas N, Evans E. Sickle erythrocyte adherence to vascular endothelium. Morphologic correlates and the requirement for divalent cations and collagen-binding plasma proteins. J Clin Invest. 1985;76(4):1605–1612. doi: 10.1172/JCI112144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zennadi R, Hines PC, De Castro LM, Cartron JP, Parise LV, Telen MJ. Epinephrine acts through erythroid signaling pathways to activate sickle cell adhesion to endothelium via LW-alphavbeta3 interactions. Blood. 2004;104(12):3774–3781. doi: 10.1182/blood-2004-01-0042. [DOI] [PubMed] [Google Scholar]
- 5.Zennadi R, Chien A, Xu K, Batchvarova M, Telen MJ. Sickle red cells induce adhesion of lymphocytes and monocytes to endothelium. Blood. 2008;112(8):3474–3483. doi: 10.1182/blood-2008-01-134346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zennadi R, Moeller BJ, Whalen EJ, et al. Epinephrine-induced activation of LW-mediated sickle cell adhesion and vaso-occlusion in vivo. Blood. 2007;110(7):2708–2717. doi: 10.1182/blood-2006-11-056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitt JM, Stork PJ. Beta 2-adrenergic receptor activates extracellular signal-regulated kinases (ERKs) via the small G protein rap1 and the serine/threonine kinase B-Raf. J Biol Chem. 2000;275(33):25342–25350. doi: 10.1074/jbc.M003213200. [DOI] [PubMed] [Google Scholar]
- 8.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390(6655):88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 9.Zamah AM, Delahunty M, Luttrell LM, Lefkowitz RJ. Protein kinase A-mediated phosphorylation of the beta 2-adrenergic receptor regulates its coupling to Gs and Gi. Demonstration in a reconstituted system. J Biol Chem. 2002;277(34):31249–31256. doi: 10.1074/jbc.M202753200. [DOI] [PubMed] [Google Scholar]
- 10.Houslay MD, Kolch W. Cell-type specific integration of cross-talk between extracellular signal-regulated kinase and cAMP signaling. Mol Pharmacol. 2000;58(4):659–668. [PubMed] [Google Scholar]
- 11.Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305. [PMC free article] [PubMed] [Google Scholar]
- 12.Brzostowski JA, Kimmel AR. Signaling at zero G: G-protein–independent functions for 7-TM receptors. Trends Biochem Sci. 2001;26(5):291–297. doi: 10.1016/s0968-0004(01)01804-7. [DOI] [PubMed] [Google Scholar]
- 13.Hall RA, Premont RT, Chow CW, et al. The beta2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature. 1998;392(6676):626–630. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- 14.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9(5):373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17(3):126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung EC, Slack RS. Emerging role for ERK as a key regulator of neuronal apoptosis. Sci STKE. 2004;2004(251):PE45. doi: 10.1126/stke.2512004pe45. [DOI] [PubMed] [Google Scholar]
- 17.Fincham VJ, James M, Frame MC, Winder SJ. Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. EMBO J. 2000;19(12):2911–2923. doi: 10.1093/emboj/19.12.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joslin EJ, Opresko LK, Wells A, Wiley HS, Lauffenburger DA. EGF-receptor-mediated mammary epithelial cell migration is driven by sustained ERK signaling from autocrine stimulation. J Cell Sci. 2007;120(Pt 20):3688–3699. doi: 10.1242/jcs.010488. [DOI] [PubMed] [Google Scholar]
- 19.Park KS, Jeon SH, Kim SE, et al. APC inhibits ERK pathway activation and cellular proliferation induced by RAS. J Cell Sci. 2006;119(Pt 5):819–827. doi: 10.1242/jcs.02779. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt EK, Fichelson S, Feller SM. PI3 kinase is important for Ras, MEK and Erk activation of Epo-stimulated human erythroid progenitors. BMC Biol. 2004;2:7. doi: 10.1186/1741-7007-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai K, Qi D, Hou X, et al. MCP-1 upregulates amylin expression in murine pancreatic beta cells through ERK/JNK-AP1 and NF-kappaB related signaling pathways independent of CCR2. PloS One. 2011;6(5):e19559. doi: 10.1371/journal.pone.0019559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang N, Guan P, Zhang JP, et al. Fasudil hydrochloride hydrate, a Rho-kinase inhibitor, suppresses isoproterenol-induced heart failure in rats via JNK and ERK1/2 pathways. J Cell Biochem. 2011;112(7):1920–1929. doi: 10.1002/jcb.23112. [DOI] [PubMed] [Google Scholar]
- 23.Bloy C, Blanchard D, Hermand P, Kordowicz M, Sonneborn HH, Cartron JP. Properties of the blood group LW glycoprotein and preliminary comparison with Rh proteins. Mol Immunol. 1989;26(11):1013–1019. doi: 10.1016/0161-5890(89)90065-5. [DOI] [PubMed] [Google Scholar]
- 24.Telen MJ, Scearce RM, Haynes BF. Human erythrocyte antigens. III. Characterization of a panel of murine monoclonal antibodies that react with human erythrocyte and erythroid precursor membranes. Vox Sang. 1987;52(3):236–243. doi: 10.1111/j.1423-0410.1987.tb03035.x. [DOI] [PubMed] [Google Scholar]
- 25.Galfre G, Howe SC, Milstein C, Butcher GW, Howard JC. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- 26.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Udani M, Zen Q, Cottman M, et al. Basal cell adhesion molecule/lutheran protein. The receptor critical for sickle cell adhesion to laminin. J Clin Invest. 1998;101(11):2550–2558. doi: 10.1172/JCI1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brunati AM, Bordin L, Clari G, et al. Sequential phosphorylation of protein band 3 by Syk and Lyn tyrosine kinases in intact human erythrocytes: identification of primary and secondary phosphorylation sites. Blood. 2000;96(4):1550–1557. [PubMed] [Google Scholar]
- 29.Oonishi T, Sakashita K, Uyesaka N. Regulation of red blood cell filterability by Ca2+ influx and cAMP-mediated signaling pathways. Am J Physiol. 1997;273(6 Pt 1):C1828–1834. doi: 10.1152/ajpcell.1997.273.6.C1828. [DOI] [PubMed] [Google Scholar]
- 30.Crews CM, Alessandrini A, Erikson RL. Erks: their fifteen minutes has arrived. Cell Growth Differ. 1992;3(2):135–142. [PubMed] [Google Scholar]
- 31.Lee MH, Klein RL, El-Shewy HM, Luttrell DK, Luttrell LM. The adiponectin receptors AdipoR1 and AdipoR2 activate ERK1/2 through a Src/Ras-dependent pathway and stimulate cell growth. Biochemistry. 2008;47(44):11682–11692. doi: 10.1021/bi801451f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon MS, Koo JB, Hwang JH, Lee KS, Han JS. Activation of phospholipase D by 8-Br-cAMP occurs through novel pathway involving Src, Ras, and ERK in human endometrial stromal cells. FEBS Lett. 2005;579(25):5635–5642. doi: 10.1016/j.febslet.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 33.Faltynek CR, Schroeder J, Mauvais P, et al. Damnacanthal is a highly potent, selective inhibitor of p56lck tyrosine kinase activity. Biochemistry. 1995;34(38):12404–12410. doi: 10.1021/bi00038a038. [DOI] [PubMed] [Google Scholar]
- 34.Hines PC, Zen Q, Burney SN, et al. Novel epinephrine and cyclic AMP-mediated activation of BCAM/Lu-dependent sickle (SS) RBC adhesion. Blood. 2003;101(8):3281–3287. doi: 10.1182/blood-2001-12-0289. [DOI] [PubMed] [Google Scholar]
- 35.Fukumoto T, Kubota Y, Kitanaka A, et al. Gab1 transduces PI3K-mediated erythropoietin signals to the Erk pathway and regulates erythropoietin-dependent proliferation and survival of erythroid cells. Cell Signal. 2009;21(12):1775–1783. doi: 10.1016/j.cellsig.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Jindal HK, Ai Z, Gascard P, Horton C, Cohen CM. Specific loss of protein kinase activities in senescent erythrocytes. Blood. 1996;88(4):1479–1487. [PubMed] [Google Scholar]
- 37.Rochais F, Vandecasteele G, Lefebvre F, et al. Negative feedback exerted by cAMP-dependent protein kinase and cAMP phosphodiesterase on subsarcolemmal cAMP signals in intact cardiac myocytes: an in vivo study using adenovirus-mediated expression of CNG channels. J Biol Chem. 2004;279(50):52095–52105. doi: 10.1074/jbc.M405697200. [DOI] [PubMed] [Google Scholar]
- 38.Hubberstey AV, Mottillo EP. Cyclase-associated proteins: CAPacity for linking signal transduction and actin polymerization. FASEB J. 2002;16(6):487–499. doi: 10.1096/fj.01-0659rev. [DOI] [PubMed] [Google Scholar]
- 39.Bertling E, Hotulainen P, Mattila PK, Matilainen T, Salminen M, Lappalainen P. Cyclase-associated protein 1 (CAP1) promotes cofilin-induced actin dynamics in mammalian nonmuscle cells. Mol Biol Cell. 2004;15(5):2324–2334. doi: 10.1091/mbc.E04-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tuvia S, Moses A, Gulayev N, Levin S, Korenstein R. Beta-adrenergic agonists regulate cell membrane fluctuations of human erythrocytes. J Physiol. 1999;516(Pt 3):781–792. doi: 10.1111/j.1469-7793.1999.0781u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shain W, Forman DS, Madelian V, Turner JN. Morphology of astroglial cells is controlled by beta-adrenergic receptors. J Cell Biol. 1987;105(5):2307–2314. doi: 10.1083/jcb.105.5.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Husain-Chishti A, Levin A, Branton D. Abolition of actin-bundling by phosphorylation of human erythrocyte protein 4.9. Nature. 1988;334(6184):718–721. doi: 10.1038/334718a0. [DOI] [PubMed] [Google Scholar]
- 43.Siegel DL, Branton D. Partial purification and characterization of an actin-bundling protein, band 4.9, from human erythrocytes. J Cell Biol. 1985;100(3):775–785. doi: 10.1083/jcb.100.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manno S, Takakuwa Y, Mohandas N. Modulation of erythrocyte membrane mechanical function by protein 4.1 phosphorylation. J Biol Chem. 2005;280(9):7581–7587. doi: 10.1074/jbc.M410650200. [DOI] [PubMed] [Google Scholar]
- 45.George A, Pushkaran S, Li L, et al. Altered phosphorylation of cytoskeleton proteins in sickle red blood cells: the role of protein kinase C, Rac GTPases, and reactive oxygen species. Blood Cells Mol Dis. 2010;45(1):41–45. doi: 10.1016/j.bcmd.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ling E, Danilov YN, Cohen CM. Modulation of red cell band 4.1 function by cAMP-dependent kinase and protein kinase C phosphorylation. J Biol Chem. 1988;263(5):2209–2216. [PubMed] [Google Scholar]
- 47.Zambuzzi WF, Bruni-Cardoso A, Granjeiro JM, et al. On the road to understanding of the osteoblast adhesion: cytoskeleton organization is rearranged by distinct signaling pathways. J Cell Biochem. 2009;108(1):134–144. doi: 10.1002/jcb.22236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.