Abstract
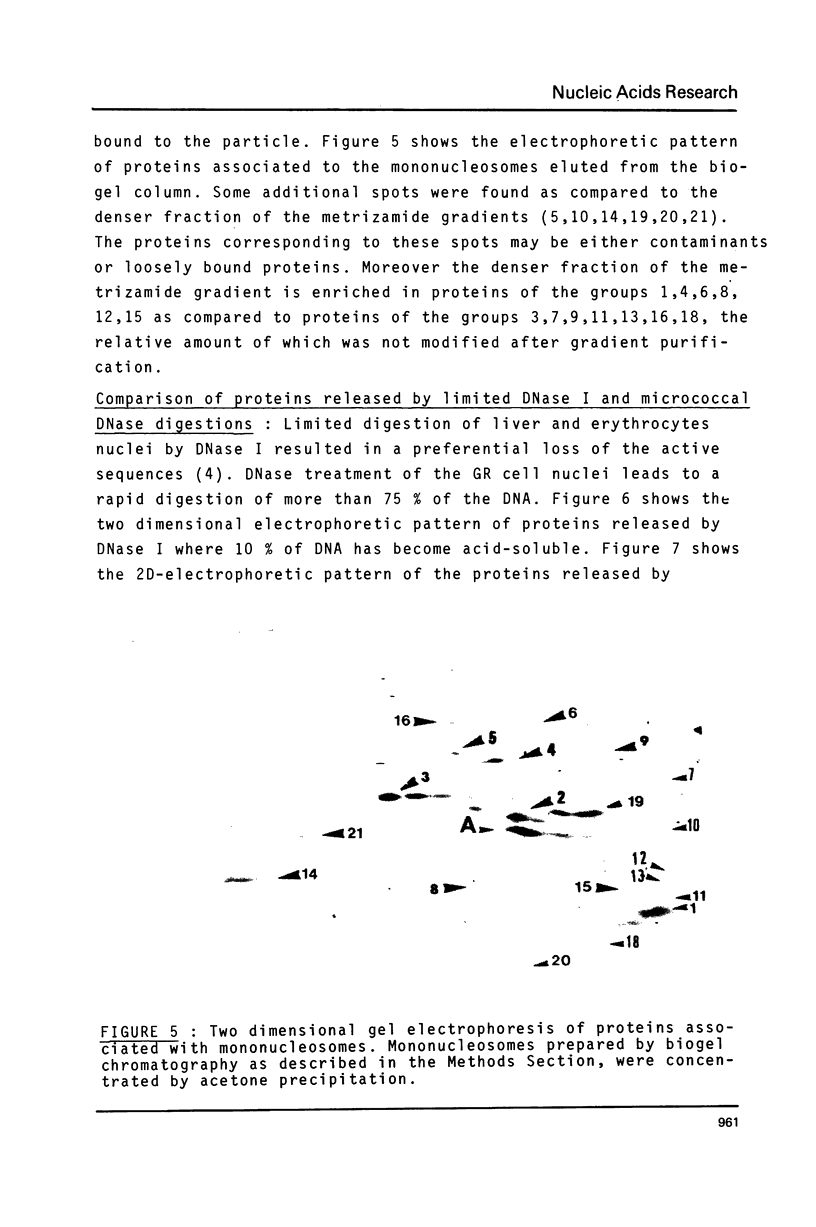
Cultured mammary cells from GR mouse were used to analyse proteins associated with the mononucleosomes and released by a short micrococcal DNase treatment of nuclei. On metrizamide density gradients, mononucleosomes appear to be heterogeneous according to their content of associated non-histone proteins. Proteins associated with the denser fraction (1.22 - 1.24 g/ml) were analysed by two dimensional electrophoresis and compared to the proteins released by DNase I treatment. All the proteins associated with mononucleosomes were also released by DNase I treatment. It could then be assumed that these proteins are associated with the active part of the genome. Additional proteins were released by micrococcal DNase treatment of the nuclei. They could be involved in a higher order organization of chromatin.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axel R., Cedar H., Felsenfield G. The structure of the globin genes in chromatin. Biochemistry. 1975 Jun 3;14(11):2489–2495. doi: 10.1021/bi00682a031. [DOI] [PubMed] [Google Scholar]
- Bakayev V. V., Bakayeva T. G., Varshavsky A. J. Nucleosomes and subnucleosomes: heterogeneity and composition. Cell. 1977 Jul;11(3):619–629. doi: 10.1016/0092-8674(77)90079-4. [DOI] [PubMed] [Google Scholar]
- Defer N., Kitzis A., Levy F., Tichonicky L., Sabatier M. M., Kruh J. Presence of non-histone proteins in nucleosomes. Eur J Biochem. 1978 Aug 1;88(2):583–591. doi: 10.1111/j.1432-1033.1978.tb12484.x. [DOI] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattoni R., Stevenin J., Jacob M. Metrizamide dissociates nuclear particles containing heterogeneous nuclear RNA. Nucleic Acids Res. 1977 Nov;4(11):3931–3941. doi: 10.1093/nar/4.11.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G. H., Woodhead L., Johns E. W. The presence of high mobility group non-histone chromatin proteins in isolated nucleosomes. FEBS Lett. 1977 Jan 15;73(1):85–88. [PubMed] [Google Scholar]
- Johnson E. M., Littau V. C., Allfrey V. G., Bradbury E. M., Matthews H. R. The subunit structure of chromatin from Physarum polycephalum. Nucleic Acids Res. 1976 Dec;3(12):3313–3329. doi: 10.1093/nar/3.12.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg R. D. Chromatin structure: a repeating unit of histones and DNA. Science. 1974 May 24;184(4139):868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- Lacy E., Axel R. Analysis of DNA of isolated chromatin subunits. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3978–3982. doi: 10.1073/pnas.72.10.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew C. C., Chan P. K. Identification of nonhistone chromatin proteins in chromatin subunits. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3458–3462. doi: 10.1073/pnas.73.10.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr D., Van Holde K. E. Yeast chromatin subunit structure. Science. 1975 Apr 11;188(4184):165–166. doi: 10.1126/science.1090006. [DOI] [PubMed] [Google Scholar]
- Miller D. M., Turner P., Nienhuis A. W., Axelrod D. E., Gopalakrishnan T. V. Active conformation of the globin genes in uninduced and induced mouse erythroleukemia cells. Cell. 1978 Jul;14(3):511–521. doi: 10.1016/0092-8674(78)90237-4. [DOI] [PubMed] [Google Scholar]
- Noll M. Subunit structure of chromatin. Nature. 1974 Sep 20;251(5472):249–251. doi: 10.1038/251249a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Olins A. L., Senior M. B., Olins D. E. Ultrastructural features of chromatin nu bodies. J Cell Biol. 1976 Mar;68(3):787–793. doi: 10.1083/jcb.68.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudet P., Gross-Bellard M., Chambon P. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell. 1975 Apr;4(4):281–300. doi: 10.1016/0092-8674(75)90149-x. [DOI] [PubMed] [Google Scholar]
- Paul J., Malcolm S. A class of chromatin particles associated with nonhistone proteins. Biochemistry. 1976 Aug 10;15(16):3510–3515. doi: 10.1021/bi00661a018. [DOI] [PubMed] [Google Scholar]
- Pospelov V. A., Svetlikova S. B., Vorob'ev V. I. Heterogeneity of chromatin subunits. FEBS Lett. 1977 Mar 1;74(2):229–233. doi: 10.1016/0014-5793(77)80852-1. [DOI] [PubMed] [Google Scholar]
- Ringold G., Lasfargues E. Y., Bishop J. M., Varmus H. E. Production of mouse mammary tumor virus by cultured cells in the absence and presence of hormones: assay by molecular hybridization. Virology. 1975 May;65(1):135–147. doi: 10.1016/0042-6822(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Shaw B. R., Corden J. L., Sahasrabuddhe C. G., Van Holde K. E. Chromatographic separation of chromatin subunits. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1193–1198. doi: 10.1016/s0006-291x(74)80410-9. [DOI] [PubMed] [Google Scholar]
- Vidali G., Boffa L. C., Allfrey V. G. Selective release of chromosomal proteins during limited DNAase 1 digestion of avian erythrocyte chromatin. Cell. 1977 Oct;12(2):409–415. doi: 10.1016/0092-8674(77)90117-9. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- de Pomerai D. I., Chesterton C. J., Butterworth P. H. Preparation of chromatin. Variation in the template properties of chromatin dependent on the method of perparation. Eur J Biochem. 1974 Aug 1;46(3):461–471. doi: 10.1111/j.1432-1033.1974.tb03639.x. [DOI] [PubMed] [Google Scholar]