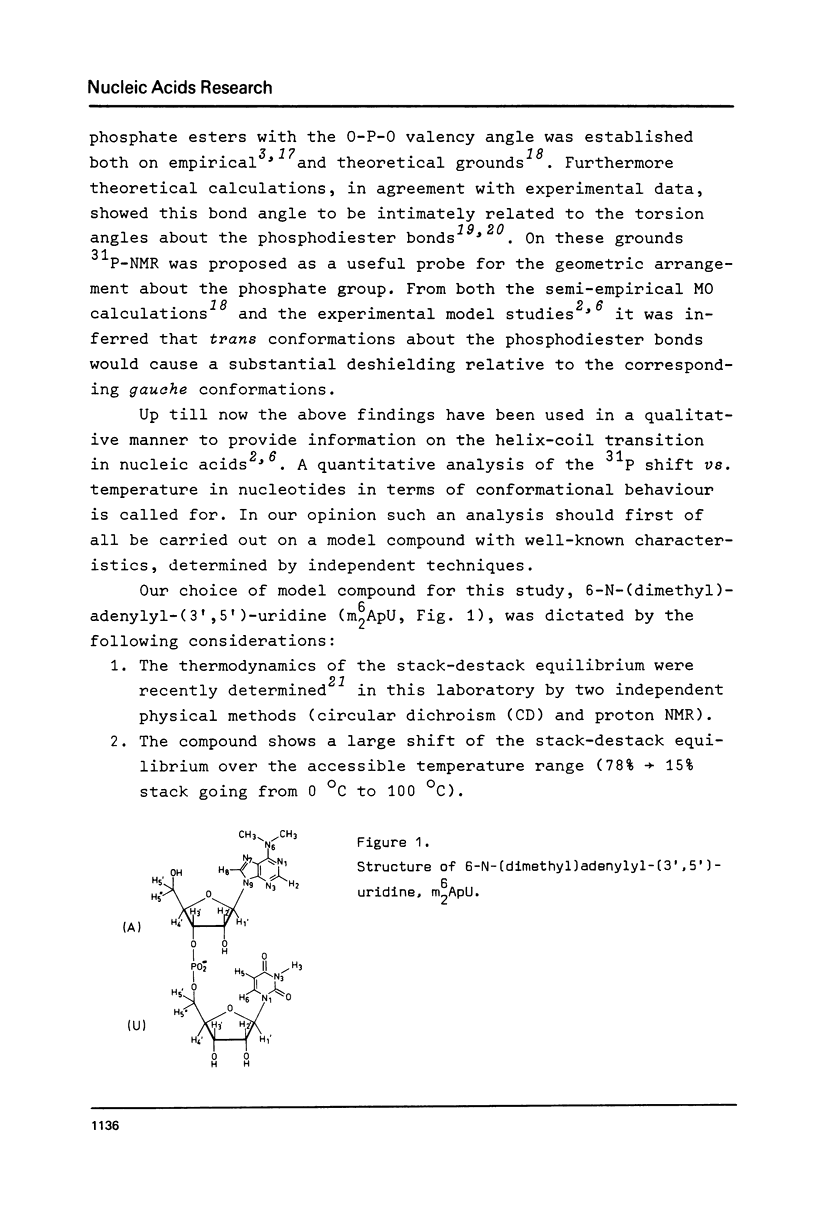
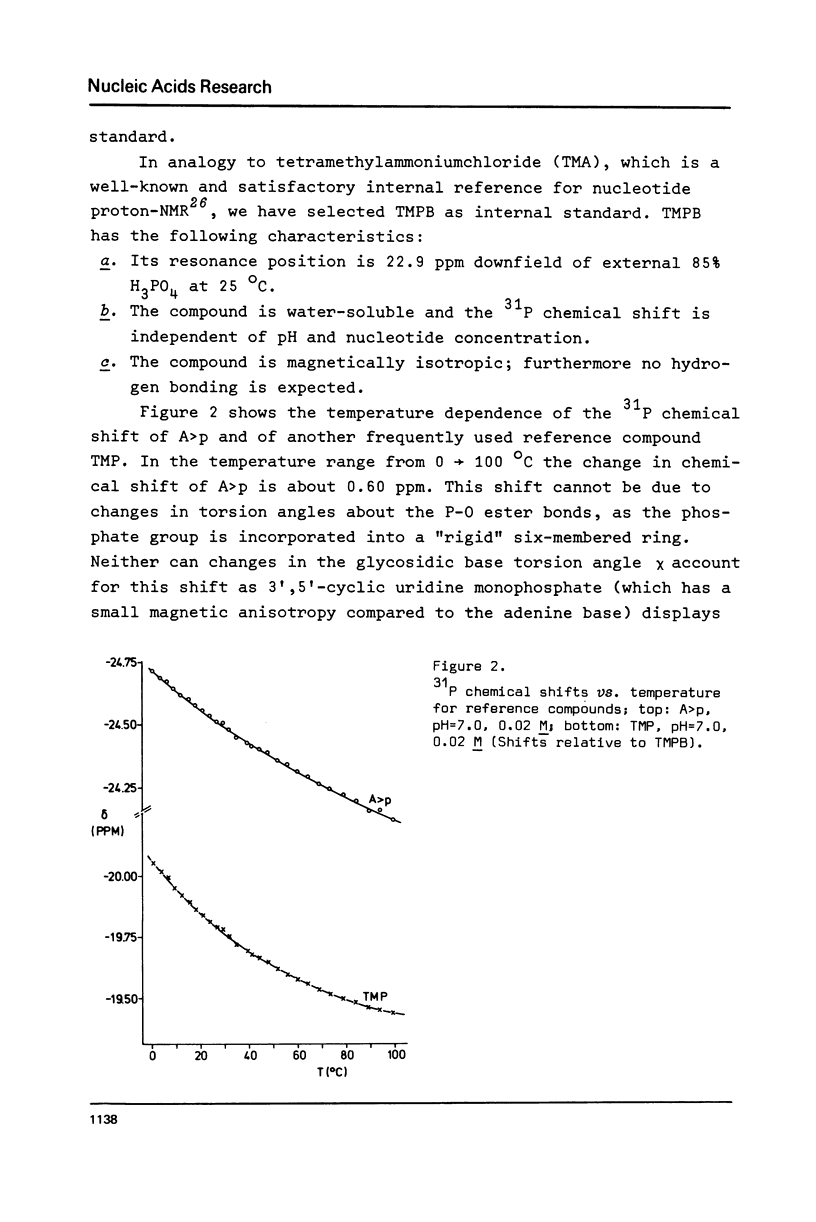
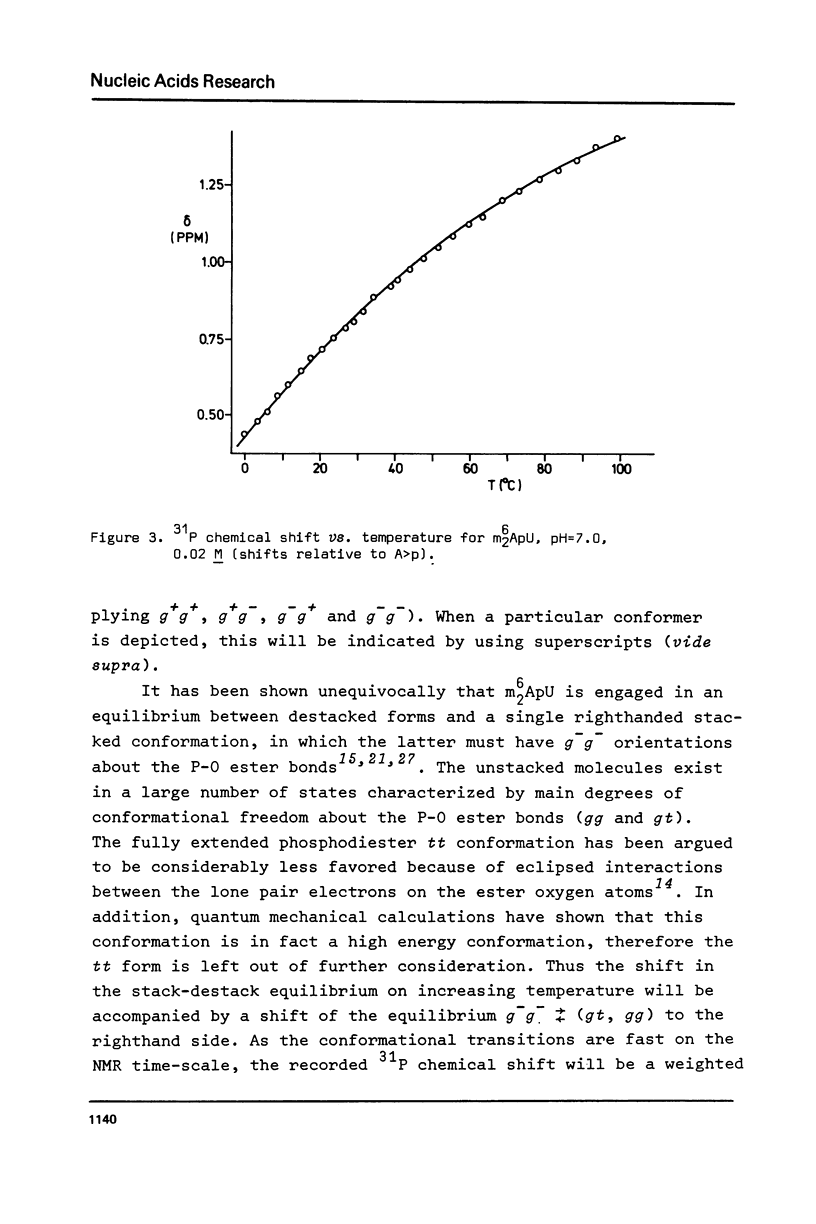
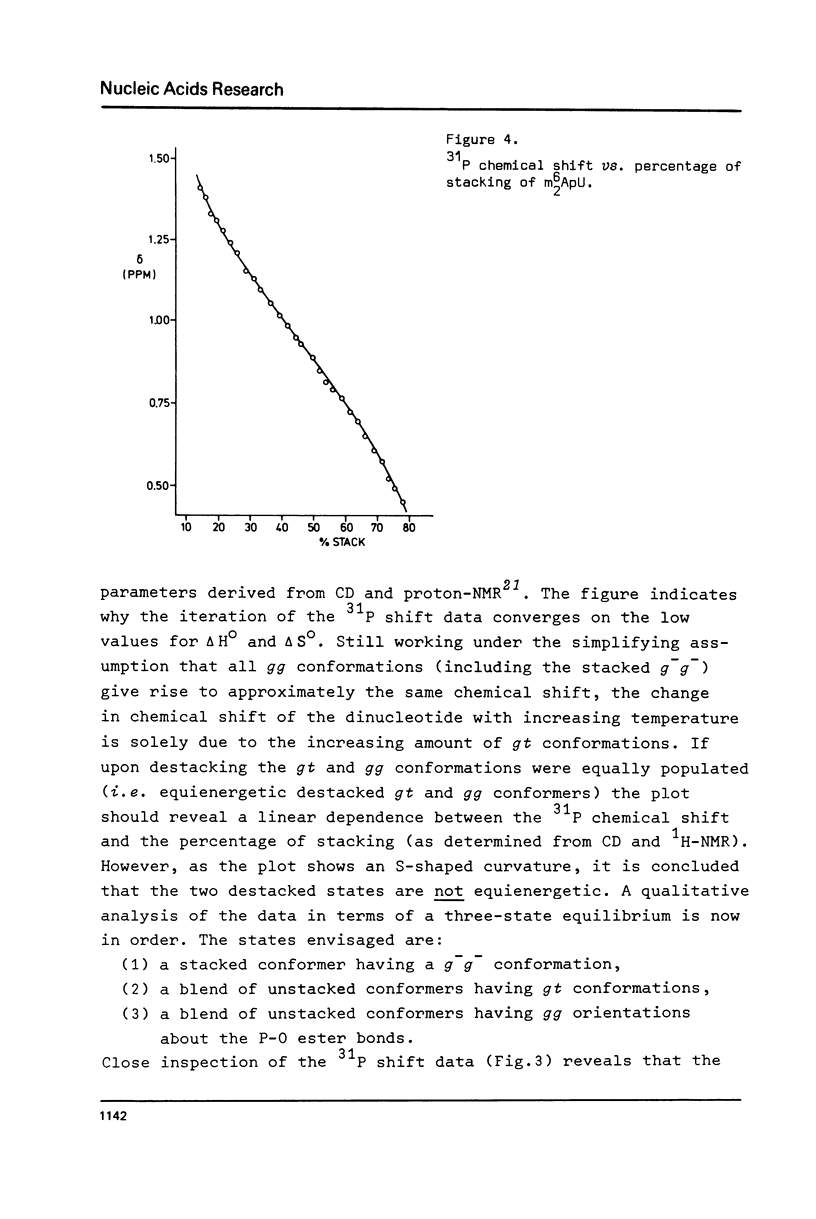
Abstract
A systematic phosphorus-31 nuclear magnetic resonance study of some nucleic acid constituents (6-N-(dimethyl)adenylyl-(3',5')-uridine and some nucleotide methyl esters) is presented. The temperature dependent phosphorus-31 chemical shifts were analyzed by standard thermodynamic procedures. It is shown that gt conformations about the P-O ester bonds have a lower free energy content relative to gg conformers.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akasaka K., Yamada A., Hatano H. 31PH dependence of P magnetic resonance spectra of homopolyribonucleotides. FEBS Lett. 1975 May 15;53(3):339–341. doi: 10.1016/0014-5793(75)80050-0. [DOI] [PubMed] [Google Scholar]
- Altona C., van Boom J. H., de Jager J., Koeners H. J., Van Binst G. Conformational analysis of N6-methyladenylyl-uridine. Nature. 1974 Feb 22;247(5442):558–561. doi: 10.1038/247558a0. [DOI] [PubMed] [Google Scholar]
- Cozzone P. J., Jardetzky O. Phosphorus-31 Fourier transform nuclear magnetic resonance study of mononucleotides and dinucleotides. 1. Chemical shifts. Biochemistry. 1976 Nov 2;15(22):4853–4859. doi: 10.1021/bi00667a016. [DOI] [PubMed] [Google Scholar]
- Garssen G. J., Hilbers C. W., Schoenmakers J. G., van Boom J. H. Studies on DNA unwinding. Proton and phosphorus nuclear-magnetic-resonance studies of gene V protein from bacteriophage M13, interacting with d(pC-G-C-G). Eur J Biochem. 1977 Dec;81(3):453–463. doi: 10.1111/j.1432-1033.1977.tb11970.x. [DOI] [PubMed] [Google Scholar]
- Gorenstein D. G., Findlay J. B., Momii R. K., Luxon B. A., Kar D. Temperature dependence of the 31P chemical shifts of nucleic acids. A prode of phosphate ester torsional conformations. Biochemistry. 1976 Aug 24;15(17):3796–3803. doi: 10.1021/bi00662a023. [DOI] [PubMed] [Google Scholar]
- Gorenstein D. G., Kar D. 31-P chemical shifts in phosphate diester monoanions. Bond angle and torsional angle effects. Biochem Biophys Res Commun. 1975 Aug 4;65(3):1073–1080. doi: 10.1016/s0006-291x(75)80495-5. [DOI] [PubMed] [Google Scholar]
- Gorenstein D. G., Kar D., Luxon B. A., Momii R. K. Conformational study of cyclic and acyclic phosphate esters. CNDO/2 calculations of angle strain and torsional strain. J Am Chem Soc. 1976 Mar 31;98(7):1668–1673. doi: 10.1021/ja00423a005. [DOI] [PubMed] [Google Scholar]
- Gorenstein D. G., Wyrwicz A. M., Bode J. Interaction of uridine and cytidine monophosphates with ribonuclease A. IV. Phosphorus-31 nuclear magnetic resonance studies. J Am Chem Soc. 1976 Apr 14;98(8):2308–2314. doi: 10.1021/ja00424a052. [DOI] [PubMed] [Google Scholar]
- Guéron M., Shulman R. G. 31P magnetic resonance of tRNA. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3482–3485. doi: 10.1073/pnas.72.9.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoult D. I., Busby S. J., Gadian D. G., Radda G. K., Richards R. E., Seeley P. J. Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature. 1974 Nov 22;252(5481):285–287. doi: 10.1038/252285a0. [DOI] [PubMed] [Google Scholar]
- Newton M. D. A model conformational study of nucleic acid phosphate ester bonds. The torsional potential of dimethyl phosphate monoanion. J Am Chem Soc. 1973 Jan 10;95(1):256–258. doi: 10.1021/ja00782a055. [DOI] [PubMed] [Google Scholar]
- Patel D. J. Proton and phosphorus NMR studies of d-CpG(pCpG)n duplexes in solution. Helix-coil transition and complex formation with actinomycin-D. Biopolymers. 1976 Mar;15(3):533–558. doi: 10.1002/bip.1976.360150310. [DOI] [PubMed] [Google Scholar]
- Perahia D., Pullman B. Molecular orbital calculations on the conformation of phosphodiesters. An extended correlation between the geometry and the conformation of the phosphate group. Biochim Biophys Acta. 1976 Jul 2;435(3):282–289. doi: 10.1016/0005-2787(76)90109-x. [DOI] [PubMed] [Google Scholar]
- Perahia D., Pullman B., Saran A. Molecular orbital calculations on the conformation of nucleic acids and their constituents. IX. The geometry of the phosphate group: key to the conformation of polynucleotides? Biochim Biophys Acta. 1974 Mar 27;340(3):299–313. doi: 10.1016/0005-2787(74)90275-5. [DOI] [PubMed] [Google Scholar]
- Powell J. T., Richards E. G., Gratzer W. B. The nature of stacking equilibria in polynucleotides. Biopolymers. 1972 Jan;11(1):235–250. doi: 10.1002/bip.1972.360110118. [DOI] [PubMed] [Google Scholar]
- Reinhardt C. G., Krugh T. R. Phosphorus-31 nuclear magnetic resonance studies of actinomycin D, ethidium bromide, and 9-aminoacridine complexes with dinucleotides. Biochemistry. 1977 Jun 28;16(13):2890–2895. doi: 10.1021/bi00632a014. [DOI] [PubMed] [Google Scholar]
- Salhany J. M., Yamane T., Shulman R. G., Ogawa S. High resolution 31P nuclear magnetic resonance studies of intact yeast cells. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4966–4970. doi: 10.1073/pnas.72.12.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A. R., Yathindra N. A novel representation of the conformational structure of transfer RNAs. Correlation of the folding patterns of the polynucleotide chain with the base sequence and the nucleotide backbone torsions. Nucleic Acids Res. 1977 Nov;4(11):3969–3979. doi: 10.1093/nar/4.11.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner L. M., Backer J. M., Rezvukhin A. I. 31P-NMR studies of tRNA. FEBS Lett. 1974 Apr 15;41(1):40–42. doi: 10.1016/0014-5793(74)80948-8. [DOI] [PubMed] [Google Scholar]


