Abstract
Progress in gene therapy has hinted at the potential misuse of gene transfer in sports to achieve better athletic performance, while escaping from traditional doping detection methods. Suitable animal models are therefore required in order to better define the potential effects and risks of gene doping. Here we describe a mouse model of gene doping based on adeno-associated virus (AAV)-mediated delivery of the insulin-like growth factor-I (IGF-I) cDNA to multiple muscles. This treatment determined marked muscle hypertrophy, neovascularization, and fast-to-slow fiber type transition, similar to endurance exercise. In functional terms, treated mice showed impressive endurance gain, as determined by an exhaustive swimming test. The proteomic profile of the transduced muscles at 15 and 30 days after gene delivery revealed induction of key proteins controlling energy metabolism. At the earlier time point, enzymes controlling glycogen mobilization and anaerobic glycolysis were induced, whereas they were later replaced by proteins required for aerobic metabolism, including enzymes related to the Krebs cycle and oxidative phosphorylation. These modifications coincided with the induction of several structural and contractile proteins, in agreement with the observed histological and functional changes. Collectively, these results give important insights into the biological response of muscles to continuous IGF-I expression in vivo and warn against the potential misuse of AAV-IGF1 as a doping agent.
Macedo and colleagues use adeno-associated virus type 2 (AAV2) to examine the long-term impact of human insulin-like growth factor (IGF)-I gene transfer in muscle. They find that overexpressing IGF-I in mice induces skeletal muscle hypertrophy and angiogenesis and leads to profound changes in skeletal muscle structure and metabolism. In functional terms, treated mice show impressive endurance gain, as determined by an exhaustive swimming test.
Introduction
The successful development of gene therapy has provided the concepts, tools, opportunities, and, arguably, justifications for genetic modification of functions that affect normal human traits, including athletic performance. As a consequence, gene doping, which is defined by the World Anti-Doping Agency (WADA) as “the nontherapeutic use of cells, genes, genetic elements, or of the modulation of gene expression, having the capacity to improve athletic performance” (http://www.wada-ama.org/en/Science-Medicine/Prohibited-List/), is perceived as a coming threat and a prime concern to the antidoping community.
Several genes, when overexpressed, have the capacity to induce muscle hypertrophy and improve muscle function; among these gene are those encoding insulin-like growth factor-I (IGF-I) (Barton-Davis et al., 1998), growth hormone (GH), erythropoietin (Epo; Zhou et al., 1998), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and endorphins or enkephalins (Lee et al., 2004; Kota et al., 2009) or myostatin blockers, such as follistatin (Lee and McPherron, 2001). A few studies have also specifically investigated the potential of genetic modification to enhance athletic performance, including the generation of peroxisome proliferator-activated receptor-δ (PPARδ; Wang et al., 2004) and phosphoenolpyruvate carboxykinase (PEPCK; Hakimi et al., 2007) transgenic mice and the delivery of the IGF-I gene to the anterior muscle mass of the hind limb (Lee et al., 2004). In particular, IGF-I promotes the proliferation and differentiation of satellite cells into myoblasts (Jennische et al., 1987). Consistent with this function, this factor is known to play key roles in normal muscle development in the embryo (Benito et al., 1996), to be important for muscle regeneration and reinnervation after injury in adult muscles (Keller et al., 1999; Musaro et al., 1999, 2004; Takahashi et al., 2003), and to be critical for the maintenance of the muscle mass during aging (Goldspink and Yang, 2004). Once overexpressed in the skeletal muscle, IGF-I increases muscle mass and mediates adaptation to strength training (White and Esser, 1989).
Although a large body of information is available on the effects of IGF-I at the cellular level, assessment of the performance of animals treated with the factor, as well as the molecular and biochemical modifications induced by IGF-I over time in vivo, are poorly elucidated. To fill this gap, here we exploited the property of adeno-associated viral (AAV) vectors to transduce muscle fibers in vivo at high efficiency and to express their transgenes for prolonged periods of time in the absence of detectable inflammation or major immune response (Kessler et al., 1996; Fisher et al., 1997; Mueller and Flotte, 2008). Although all these characteristics are appealing in the gene therapy arena, nevertheless they also appear to encourage the use of these vectors to enhance muscle performance for doping purposes.
By delivering the IGF-I cDNA to the major muscle mass of the four limbs, using an AAV serotype 2 (AAV2) vector, here we describe a mouse model of gene doping in which we assessed the molecular modifications induced by IGF- I overexpression over time by quantitative differential in-gel electrophoresis (2D-DIGE) and mass spectrometry (MS) (Alban et al., 2003) and correlated the detected changes with structural and functional modifications.
Materials and Methods
Production, purification, and characterization of rAAV vectors
The human hepatic IGF1-IA (ref. seq. NM_000618.3) was amplified by PCR and cloned into the pZac recombinant AAV expression vector, generating the pAAV-IGF1 vector. Viral particles were produced by the AAV Vector Unit at the International Centre for Genetic Engineering and Biotechnology (ICGEB, Trieste, Italy; http://www.icgeb.org/avu-core-facility.html). Methods for production and purification were previously described (Arsic et al., 2003). AAV titers were in the range of 1×1012 genome copies per milliliter.
Animals and experimental protocols
Animal care and treatment were conducted in conformity with institutional guidelines in compliance with national and international laws and policies (EEC Council Directive 86/609, OJL 358, December 12, 1987). All experiments were performed in male CD1 mice, 4–6 weeks of age.
For biochemical and histological analyses, tibialis anterior and gastrocnemius muscles were injected with 50 μl of either phosphate-buffered saline (PBS) or a viral suspension containing 1011 viral particles of AAV2-IGF1, and harvested after the indicated periods of time.
For the evaluation of swimming performance, mice were injected via their tibialis anterior, gastrocnemius, quadriceps femoris, biceps brachii, and triceps brachii muscles with either a control AAV vector or AAV-IGF1 in order to transduce the major muscle groups of the body (50 μl/injection, corresponding to 1×1011 viral particles). To assess swimming performance, a swimming apparatus was developed, particularly suited for both exercise training and execution of exhaustive stress tests to analyze and quantify physical endurance in mice. The system is composed of a 200-liter water plastic tank, equipped with a mobile shovel to produce artificial waves, in order to prevent the animals from floating during swimming sessions. The water temperature was kept between 30 and 32°C during each entire swimming session. Thirty days after AAV delivery, each mouse (n=8 per group) was trained to swim for 15 min, every second day for 2 weeks. This training period helped the mice to become adapted to the swimming sessions before challenging them to an exhaustive test. By the end of that period, mice were overloaded with metallic wires attached to their tails, corresponding to 10% of their body weight, and then subjected to a swimming session until exhaustion. The maximal swimming time before submersion was determined for each mouse, after which mice were rapidly rescued, warmed, and returned back to their cages.
Real-time PCR
Viral genome quantification was performed by extracting total DNA from transduced muscles, using a DNeasy blood & tissue kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions. Viral genome was detected by quantitative real-time PCR (Q-PCR), using a cytomegalovirus (CMV) promoter-specific TaqMan probe (Applied Biosystems/Invitrogen, Foster City, CA). The number of viral DNA molecules was calculated on the basis of a standard curve, obtained from serial dilutions of the pAAV-IGF1 plasmid, and further normalized to muscle tissue weight. mRNA levels of endogenously and ectopically expressed genes were also assessed by Q-PCR. Briefly, total RNA was extracted from transduced muscles on days 7, 15, 30, and 60 postinjection, using TRIzol reagent (Invitrogen, Carlsbad, CA). After reverse transcription, specific transcripts were quantified with the following commercial TaqMan probes: human IGF-I (hIGF-I; Hs00153126_m1), murine IGF-I (mIGF-I; Mm01233960_m1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 4352339E). The levels of murine and human IGF-I were normalized by the levels of GAPDH. PCRs were carried out in a CFX96 real-time system (Bio-Rad, Hercules, CA).
Histological evaluation and immunostaining
For histological analysis, muscles were collected after 15 or 30 days of IGF-I gene transfer, fixed in 2% formaldehyde, and embedded in paraffin. After hematoxylin–eosin staining, 5-μm muscle histological sections were analyzed with an Olympus CX40 microscope (Olympus, Tokyo, Japan). Fiber cross-sectional area was quantified with ImageJ software. For immunofluorescence staining, muscles were frozen in liquid nitrogen-cooled isopentane and kept at −80°C. Sections were fixed in 4% paraformaldehyde and permeabilized in 1% Triton X. For assessing new blood vessels formation and fast fiber content, anti-CD31 antibodies (550274; BD Biosciences, San Jose, CA) and anti-fast myosin heavy chain (clone F18 from the Developmental Studies Hybridoma Bank [DSHB], Iowa City, IA), were used followed by fluorescently labeled secondary antibodies (Molecular Probes/Invitrogen, Eugene, OR). Images were acquired with a DMLC upright fluorescence microscope (Leica Microsystems, Wetzlar, Germany) equipped with a charge-coupled device camera (CoolSNAP CF; Roper Scientific, Tucson, AZ) using MetaView 4.6 quantitative analysis software (MDS Analytical Technologies, Sunnyvale, CA).
Western blotting and ELISA
To quantify the levels of IGF-I overexpression in vivo, transduced muscles were harvested, snap frozen in liquid nitrogen, and stored at −80°C before lysis in radioimmunoprecipitation assay (RIPA) buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 5 mM EDTA, 1% sodium deoxycholate [NaDOC], 1% Triton X-100, and 0.1% sodium dodecyl sulfate [SDS]) containing protease inhibitor cocktail tablets (Roche Applied Science, Indianapolis, IN) and 100 μM NaVO4. Protein concentration was determined by the bicinchoninic acid method (BCA protein assay reagent; Thermo Fisher Scientific, Waltham, MA). Fifty micrograms of total protein extract was resolved by SDS–polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes (GE Healthcare, Piscataway, NJ), which were incubated overnight with the following antibodies: anti-hIGF-I (AF-291-NA; R&D Systems, Minneapolis, MN) and anti-α-tubulin (B-5-1-2; Sigma-Aldrich, St. Louis, MO). Both murine and human IGF-I were also quantified by ELISA, according to the manufacturer's recommendations (mouse/rat IGF-I immunoassay [MG100] and human IGF-I immunoassay [DG100] from R&D Systems). For this purpose, muscles were homogenized in PBS and subjected to five freeze–thaw cycles: After centrifugation at 5000×g, clarified protein supernatants were quantified as described previously and used for the immunoassays.
Muscle differential proteome profile
Gastrocnemius muscle biopsies from three sets of mice (control, AAV-IGF1 15 days, and AAV-IGF1 30 days) were analyzed according to a quantitative 2D-DIGE protocol. 2D-DIGE results were obtained and inserted in an MIAPE–GE (minimal information about a proteomics experiment–gel electrophoresis) compliant form (Taylor et al., 2007), as detailed in Supplementary Table S3 (supplementary data are available online at www.liebertonline.com/hum).
For protein extraction, frozen muscles were ground in a frozen mortar, suspended in lysis buffer (7 M urea, 2 M thiourea, 4% 3-[(3-cholamidopropyl)-dimethyl-ammonio]-1-propanesulfonate [CHAPS], 30 mM Tris, 1 mM phenylmethylsulfonyl fluoride [PMSF]), and solubilized by sonication on ice. Proteins were selectively precipitated with a PlusOne 2-D clean-up kit (GE Healthcare) in order to remove nonprotein impurities, and resuspended in lysis buffer. The pH of the protein extracts were adjusted to pH 8.5 by addition of 1 M NaOH. Protein concentrations of three different pools were determined with a PlusOne 2-D quant kit (GE Healthcare).
Protein minimal labeling with cyanine dyes (Cy3 and Cy5) was performed according to the manufacturer's recommendations (CyDye DIGE fluor minimal dye; GE Healthcare). Proteins extracted from each group of mice were labeled with Cy5, whereas an internal standard, generated by pooling an aliquot of all muscle samples, was labeled with Cy3. The inclusion of an internal standard improves the matching of intra- and intergel images and allows normalization across all gels (Alban et al., 2003), using a double laser beam scanner (Tonge et al., 2001; Karp et al., 2004; Karp and Lilley, 2005).
Before isoelectric focusing, labeled samples were diluted in an equal volume of 2× sample buffer containing 130 mM dithiothreitol (DTT) and 2% (v/v) IPG buffer (GE Healthcare). Samples from each group (40 μg) were combined with an equal amount of internal standard, and 80 μg of total protein was loaded on the gel; rehydration buffer (7 M urea, 2 M thiourea, 2% CHAPS, 65 mM DTT, 0.5% IPG buffer [pH 3.5–9.5], and bromophenol blue [BBF] in traces) was added to a final volume of 450 μl. Samples were separated on 24-cm, 3–10 nonlinear pH gradient IPG strips, with a voltage gradient ranging from 200 to 8000 V, for a total of 70,000 VhT (VhT, total volt hours accumulated during the entire run at the current time), using an Ettan IPGphor electrophoresis unit (GE Healthcare). Each sample type was run in triplicate to minimize the intergel variability and to increase the reliability of the results. After focusing, IPG strips were equilibrated in an SDS reducing buffer (6 M urea, 2% SDS, 20% glycerol, 375 mM Tris-HCl [pH 8.8], 65 mM DTT) for 15 min, and then alkylated for 8 min in the same buffer containing 135 mM iodoacetamide instead of DTT. The second dimension was carried out in 20×25 cm2, 12% T, 2.5% C constant concentration polyacrylamide gels at 20°C and 15 mA per gel, using an Ettan DALT II system (GE Healthcare).
CyDye-labeled gels were visualized and acquired with a Typhoon 9200 imager (GE Healthcare). Excitation and emission wavelengths were chosen according to the manufacturer's recommendations (Cy3: excitation, 532 nm; emission, 580 nm; bandpass filter [BP], 30; Cy5: excitation, 633 nm, emission, 670 nm; BP, 30). Resolution was at 100 μm and the photomultiplier voltage was set to a level at which the most abundant spots in the gels were slightly beneath saturation. Image analysis was performed with DeCyder version 6.5 software (GE Healthcare). All gel images were imported into individual Differential In-gel Analysis (DIA) workspaces. Using the Batch Processor tool, automated detection of protein spots was performed with the following filter settings: estimated number of spots, 10,000; exclusion slope, >1.2; minimal area cutoff, <200; and peak height, <14 or >100,000 (proteins with a value of peak height <14 or >100000 were excluded from the analysis). DIA workspaces were then manually edited to eliminate gel artifacts (e.g., plate scratches, dust specks) and to reinclude any incorrectly excluded spots. The resulting spot maps (containing the spot identifiers, locations, and normalized volumes for all protein spots in each channel of each gel) were further processed in the Biological Variation Analysis (BVA) module. Individual DIA workspaces for all analytical gels were imported into the BVA module. The BVA workspace was used for intergel protein spot matching. Statistical analysis was performed with DeCyder EDA module version 1.0 (Extended Data Analysis). Protein filters were set to select only those protein spots that matched at least on 90% of the gel images; these protein spots were included in data analysis. Proteins of interest were identified by matrix-assisted laser desorption/ionization-time of flight peptide mass fingerprinting (MALDI-ToF PMF).
Protein identification by MALDI-ToF/ToF
To provide a proper amount of peptides for mass spectrometry analysis, semipreparative gels, containing 400 μg of total protein extract per strip, were loaded; electrophoretic conditions were the same as for 2D-DIGE, except that gels were stained with a protein fluorescent stain, as recommended by the manufacturer (deep purple total protein stain, 5 ml/liter; GE Healthcare). Image acquisition was performed with a Typhoon 9200 laser scanner. Protein identification methods are described below and in an MIAPE-MS compliant form in Supplementary Table S3 (Taylor et al., 2007). Spots of interest were excised from each gel, using an Ettan spot picker robotic system (GE Healthcare), destained in 50% methanol–50 mM ammonium bicarbonate (AMBIC), and incubated with 30 μl of trypsin (4 ng/μl) (Promega, Madison, WI) dissolved in 10 mM AMBIC for 16 hr at 37°C. Released peptides were subjected to reversed-phase chromatography (Zip-Tip C18 micro; Millipore, Bedford, MA), eluted with 50% acetonitrile–1% formic acid. One microliter of peptide mixture was spotted onto the sample plate of an Ultraflex III MALDI-ToF/ToF (Bruker Daltonics, Billerica, MA) mass spectrometer; an equal volume of 10-mg/ml α-cyano-4-hydroxycinnamic acid (CHCA) matrix dissolved in 70% acetonitrile–30% 50 mM citric acid was applied and spots were air dried at room temperature. MS proceeded at an accelerating voltage of 25 kV and spectra were externally calibrated with a peptide calibration standard mixture (Bruker Daltonics); 1000 laser shots were taken per spectrum. Proteins were identified by comparing the digest peaks with a computer-generated database of tryptic peptides from known proteins, using MASCOT, which uses a robust probabilistic scoring algorithm. Search was carried out by correlation of uninterpreted spectra to Rodentia entries in the National Center for Biotechnology Information (NCBI) nonredundant (nr) database. With regard to MASCOT parameters, one missed cleavage per peptide was allowed and carbamidomethylation, as fixed modification, and methionine oxidation, as variable modification, were set. Peptide mass tolerance was set at 30 ppm.
Myosin heavy chain isoform composition
SDS electrophoresis was performed on muscle extracts, using a discontinuous buffer system with a 4% stacking gel (pH 6.8) and 37% glycerol, 6% T constant concentration running gel (pH 8.8) (Danieli Betto et al., 1986). Samples were separated at 100 V, overnight. Gels were stained with SYPRO orange (Molecular Probes/Invitrogen) and scanned with a 570 nm emission filter on Typhoon 9200 imager (GE Healthcare). Protein band quantification was achieved with ImageQuant software (Molecular Dynamics/GE Healthcare, Sunnyvale, CA); for each lane, myosin heavy chain (MyHC) bands were normalized against the total MyHC content. Individual samples (1 μg) were run in triplicate. Molecular weight markers containing MyHC from rabbit (212 kDa) and bovine α2-macroglobulin (170 kDa) were run in a separate lane. The significance of differences between control/AAV-IGF1 15 days and control/AAV-IGF1 30 days was computed by Student t test, the significance level being set at p<0.01. A two-tailed F test was applied in order to verify the homoscedasticity of variances.
Statistical analysis
One-way analysis of variance (ANOVA) and Bonferroni/Dunn's post hoc test were used to compare multiple groups. Pairwise comparison between groups was performed by Student t test.
For proteomic experiments, statistically significant differences were computed by independent one-way ANOVA coupled to Turkey's multiple group comparison test; the significance level was set at p<0.01. To minimize inclusion of false-positive protein spot changes, protein expression data were filtered according to the following criteria: independent one-way ANOVA coupled to Turkey's multiple group comparison test (p<0.01), 1.15-fold difference in abundance and false discovery rate (FDR). A change of 1.15-fold and above in protein abundance was considered for the present analysis, taking into account the power of the DIGE method to detect reliable difference in protein abundance down to 15% (Marouga et al., 2005; Viswanathan et al., 2006). FDR correction was applied as the multiple testing correction method to keep the overall error rate as low as possible.
Results
IGF1 overexpression by AAV vectors induces skeletal muscle hypertrophy and angiogenesis
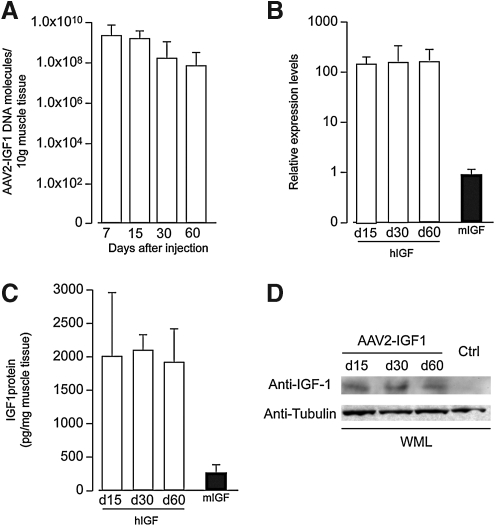
We generated an AAV2 vector carrying the human IGF-I cDNA under the control of the constitutive cytomegalovirus (CMV) promoter. To evaluate vector persistence and transgene expression in vivo, we injected 1×1011 viral genomes (VG) into the tibialis anterior muscle of mice (n=10). On days 7, 15, 30, and 60 postinjection, we harvested both the AAV-injected and contralateral PBS-injected muscles and extracted DNA, RNA, and proteins. Viral DNA was found to persist in the injected muscles up to 60 days after transduction, yet with a net tendency to decrease over time (Fig. 1A), most likely as a consequence of the loss of nonnuclear DNA in the transduced cells, in accordance with previous observations (Tafuro et al., 2009). However, transgene expression remained stable from day 15 to day 60, as determined by measuring human IGF-I mRNA levels by quantitative real-time PCR (Fig. 1B), as well as by detecting the human IGF-I protein by both ELISA (Fig. 1C) and Western blotting (Fig. 1D), in whole muscle lysates. By these measurements, the levels of expression of transduced IGF-I exceeded by more than 100- and 10-fold those of the endogenous mouse mRNA and protein, respectively.
FIG. 1.
AAV2-IGF1 efficiently transduces mouse skeletal muscle, resulting in long-lasting expression of the IGF-I protein. (A) Quantification of the number of AAV2-IGF1 DNA molecules in the injected tibialis anterior muscle at the indicated time points, as quantified by real-time PCR. (B) Real-time RT-PCR quantification of human IGF mRNA in the tibialis anterior muscle of mice injected with AAV2-IGF1 at the indicated time points. The solid column represents the quantification of endogenous IGF mRNA. Results are expressed on normalization for GAPDH mRNA. (C) ELISA assessing IGF-I protein concentration in the skeletal muscle after transduction with AAV2-IGF1. The solid column represents the concentration of murine IGF-I. (D) Representative Western blot analysis of tibialis anterior muscles transduced with AAV2-IGF1, showing constant levels of IGF-I protein expression at the indicated time points. In all panels, data are shown as means and standard deviation. WML, whole muscle lysate.
To start assessing the biological effect of IGF-I on muscle fibers, morphometric analysis was performed on sections from AAV2-IGF1-injected tibialis anterior, as well as from three control groups, including (1) the contralateral, PBS-injected muscle from AAV2-IGF1-treated mice, (2) the tibialis anterior muscle from mice injected with an AAV vector lacking an insert, and (3) the same muscle from untreated mice. No differences were ever observed for any of the analyzed parameters between the three control groups (data not shown); for this reason, only controls from the contralateral muscles, injected with PBS, are included in the figures.
By hematoxylin staining, several fibers with a central nucleus, a known hallmark of muscle regeneration, were specifically detected in IGF-I-expressing muscles at both 15 and 30 days after vector injection (Fig. 2A), suggesting that IGF-I stimulated the growth of skeletal muscle cells. This effect, which persisted for at least 3 months after transduction, was not present in any of the controls and was never elicited by any of the several control vectors used in our laboratory (data not shown). To better quantify this effect, we measured fiber cross-sectional areas in both control and AAV2-IGF1-injected muscles. Whereas the distribution of these values in control muscles was relatively narrow, with about 90% of the fibers in the 2- to 5-μm2 range, the fiber area distribution on IGF-I overexpression was much broader and included more than 10% of fibers with a size less than 2 μm2 and 40% of fibers with a size greater than 5 μm2, consistent with the coexistence of newly formed (small) and hypertrophic (large) fibers, respectively (Fig. 2B).
FIG. 2.
Persistent IGF-I overexpression induces skeletal muscle growth and neoangiogenesis. (A) Hematoxylin and eosin (HE)-stained sections of normoperfused muscle tibialis anterior from untreated and AAV2-IGF1-injected mice, 15 and 30 days after transduction. Long-term expression of IGF1 induced the appearance of small fibers with a central nucleus (arrows), a hallmark of an ongoing regeneration process. Scale bars: 100 μm. (B) Fiber size analysis of muscles injected with PBS or AAV2-IGF1. The histograms show the distribution of the fiber cross-sectional areas with a normal distribution curve superimposed. Data were obtained from the analysis of 20 cross-sections from 6 different animals per group. (C) Immunofluorescence staining of muscle sections of animals treated with either PBS or AAV2-IGF1, as indicated, 1 month after injection, using an antibody against the endothelial cell marker CD31. An increase in the number of CD31+ endothelial cells (green) is visible in IGF-I-expressing muscles. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). (D) Number of capillaries in injected muscles 30 days after transduction. Data are shown as means and standard deviation of counts. Values were analyzed by setting statistical significance at p<0.01. Scale bars: 100 μm. C, control; I, IGF-I injected.
Muscle hypertrophy and regeneration, as well as IGF-I expression itself (Su et al., 2003; Lopez-Lopez et al., 2004; Rabinovsky and Draghia-Akli, 2004), are known to be accompanied by new blood vessel formation. To assess the vascularization in our gene transfer model, we stained both control and AAV2-IGF1-injected muscle sections with anti-CD31 antibodies, to selectively label endothelial cells, and found that IGF-I expression was accompanied by a modest, but significant, increase in the number of vessels, as shown in Fig. 2C and quantified in Fig. 2D. Whether the local increase in blood vessel number is a direct effect of IGF-I or a consequence of muscle cell proliferation and hypertrophy remains to be established.
Chronic IGF-I overexpression leads to profound changes in skeletal muscle structure and metabolism
To start assessing the molecular pathways underlying the morphological and functional features induced by IGF-I overexpression, we wanted to identify and characterize the proteins differentially expressed by IGF-I-transduced muscles. For this purpose, a new set of mice was injected via the gastrocnemius with either an AAV2 vector lacking insert or AAV2-IGF1 (n=16 per group). Half the mice were killed at 15 days postinjection, whereas the other half were killed on day 30 after transduction; the harvested muscles were pooled into three experimental groups according to the time of transduction (control, IGF15, and IGF30).
Initially, to characterize myosin heavy chain (MyHC) isoform distribution in the gastrocnemius, we performed SDS gel analysis followed by band intensity quantification, as shown in Fig. 3A–C. We found that IGF-I overexpression resulted in a marked downregulation of the classic fast-twitch, type IIB MyHC (9 and 15% decrease on days 15 and 30 compared with control, respectively), paralleled by upregulation of the slow, type I MyHC (4% increase on day 15 and 1% increase on day 30 compared with control), indicating a transition toward the slow-type fiber phenotype on AAV-IGF1 gene transfer (see also below).
FIG. 3.
Proteomic profiling reveals profound changes in the expression levels of several proteins in IGF-I-overexpressing muscles. (A) Representative SDS–PAGE images of MyHC fiber type composition of mouse muscle proteins extracts: pooled control (Ctrl), AAV2-IGF1 after 15 days (d15), and AAV2-IGF1 after 30 days (d30). (B) Graphical representation of fiber type IIB composition (%) in the various muscle pools, as indicated. Protein band quantification was performed with ImageQuant (Molecular Dynamics/GE Healthcare) software. Data are shown as means and standard deviation; asterisks denote statistical significance (p<0.01). (C) Graphical representation of fiber type I composition (%) in the various muscle pools, as indicated. Protein band quantification was performed by Image Quant (Molecular Dynamics) software. Data are shown as means and standard deviation; asterisks denote statistical significance (p<0.01). (D) Gastrocnemius muscle protein profiling by 2D-DIGE. Typical 2-D image of gastrocnemius muscle protein extract separated in a pH 3–10 nonlinear IPG strip in the first dimension and SDS gel (12% T, 2.5% C) as the second dimension. The image was acquired with a 532-nm laser beam and 580-nm emission filter. Automated image analysis by DeCyder software detected and matched. Spots found differentially changed by in-gel differential analysis are indicated by spot number and listed in Supplementary Table S1.
Although MyHC isoform distribution provides direct information on adjustments of contractile properties, it can indirectly suggest possible metabolic adaptations. To fully explore this issue, we performed 2D-DIGE, which indicated a number of spots differentially expressed on AAV2-IGF1 gene transfer into the gastrocnemius. After gel excision and trypsin digestion, the spots were identified by mass spectrometry. Figure 3D shows a representative 2D map of the muscle proteome, in which the identified spots are indicated by number. The entire list of proteins differentially expressed in all comparisons, together with statistical analysis, protein accession numbers, molecular mass, and theoretical isoelectric points, are reported in Supplementary Table S1 for each numbered spot. The MS data, referring to the numbered spots indicated in Fig. 3D, are shown in Supplementary Table S2.
Overall, 100 spots were identified as differentially expressed in IGF-I-treated muscles. In particular, 44 spots were differentially expressed after 15 days of IGF-I overexpression (control vs. IGF-I, 15 days), whereas 71 spots were changed in abundance after 30 days of IGF-I overexpression (control vs. IGF-I, 30 days). Statistical comparison among the three investigated groups indicated that 18 spots were commonly changed at both time points after induction (10 spots upregulated, 8 spots downregulated), thus representing possible biomarkers of IGF-I overexpression in muscle tissue. By comparing the differential expression between 15 and 30 days of IGF-I overexpression, we could detect differential expression of 55 proteins (IGF-I, 15 days vs. IGF-I, 30 days), suggesting the occurrence of mechanisms of adaptation to long-term IGF-I overexpression.
The identified proteins were then grouped according to functional categories. Consistent with both the hypertrophic effect observed by histological examination and the shift in MyHC composition, AAV2-IGF1 determined a net increase in the levels of several muscle structural proteins, as well as in protein isoforms involved in muscle force and velocity control (Fig. 4). These included skeletal muscle α-actin (1.19- and 1.15-fold increase on day 15 and day 30, respectively), tropomyosin β chain (two isoforms, 1.32-fold increase on day 15, 1.72- and 1.3-fold increase on day 30), desmin (two isoforms, 1.39-fold increase on day 15, 1.56- and 1.53-fold increase on day 30) and myosin light chain 3 (2.16-fold increase on day 15).
FIG. 4.
Proteomic profiling of IGF-I-overexpressing muscles reveals profound changes in the levels of structural and contractile proteins. Schematic representation showing the major compartments of the sarcomere; proteins known for each compartment are listed. Numbers in parentheses refer to spot numbers, whereas arrows indicate significantly (p<0.05) up- or downregulated spots between the three experimental groups (controls and muscles overexpressing IGF-I for either 15 or 30 days).
Of note, we also found that the prolonged expression of IGF-I markedly enhanced muscle mitochondrial activity, as revealed by the increased levels of several enzymes of the Krebs cycle, such as aconitate hydratase (five isoforms, 1.26- and 1.17-fold increase on day 15; 1.58-, 1.39-, 1.55-, 1.47-, and 1.22-fold increase on day 30), isocitrate dehydrogenase (two isoforms, 1.49- and 1.25-fold increase on day 30), malate dehydrogenase (two isoforms, 1.41- and 1.21-fold increase on day 30), succinate dehydrogenase (1.43-fold increase on day 30) and members of the α-ketoglutarate dehydrogenase complex (2-oxoglutarate dehydrogenase, 1.34-fold increase on day 30; dihydrolipoyl dehydrogenase, 1.25-fold increase on day 30) (Fig. 5).
FIG. 5.
Proteomic profiling of IGF-I-overexpressing muscles reveals profound changes in the levels of metabolic proteins. Shown is the clustering of IGF-I-regulated enzymes involved in the glycolytic pathway and in the Krebs cycle. The specific function of each enzyme is indicated in the schematic diagram of the metabolic pathways. At the side of the schematic representation of the glycolytic pathway and TCA cycle is a list of the proteins differentially expressed between the three experimental groups. Numbers in parentheses refer to the spot numbers, whereas arrows indicate significantly (p<0.05) up- or downregulated spots.
In accordance, numerous proteins involved in the glycolytic pathway (Fig. 5), but mostly in oxidative phosphorylation (Fig. 6), were also clearly upregulated in IGF-I-overexpressing muscles, at both 15 and 30 days. Among the glycolytic enzymes are the following: triosephosphate isomerase (1.16- and 1.15-fold increase on days 15 and 30, respectively), glyceraldehyde-3-phosphate dehydrogenase (1.19-fold increase on day 30), α-enolase (1.42-fold increase on day 30), β-enolase (two isoforms, 1.25-fold increase on day 15, 1.41- and 1.21-fold increase on day 30), and pyruvate kinase (1.17- and 1.19-fold increase on day 15 and day 30, respectively). In contrast, proteins from NADH dehydrogenase complex I, such as succinate dehydrogenase complex II, cytochrome c reductase complex III, and ATP synthase complex V, increased where specifically upregulated on day 30.
FIG. 6.
Proteomic profiling of IGF-I-overexpressing muscles reveals profound changes in the levels of proteins involved in oxidative phosphorylation. Differentially expressed proteins involved in mitochondrial respiration were classified according to their participation in the various enzyme complexes forming the oxidative phosphorylation chain. Shown is how the proton gradient is formed through the inner mitochondrial membrane, leading to ATP production. Below the schematic representation of oxidative phosphorylation is a list of the differentially expressed proteins in the three experimental groups. Numbers in parentheses refer to the spot numbers, whereas arrows indicate significantly (p<0.05) up- or downregulated spots.
On the other hand, transport proteins, such as serum albumin and serotransferrin, which in skeletal muscle are associated mainly with reactive oxygen species (ROS) production, appeared clearly decreased in IGF-I-expressing muscle, indicating a lower level of oxidative stress in these muscles (two isoforms, 1.33- and 1.27-fold decrease on day 15, 1.36- and 1.31-fold decrease on day 30 for serum albumin; two isoforms, 1.38- and 1.28-fold decrease on day 15, 1.36- and 1.21-fold decrease on day 30 for serotransferrin).
Last, and also in keeping with the induced muscle hypertrophy, a significant increment was also observed for enzymes involved in muscle energy production and transfer, such as adenylate kinase isoenzyme 1 (two isoforms, 1.33- and 1.15-fold increase on day 15, 1.36-fold increase on day 30) and M-type creatine kinase (two isoforms, 1.33- and 1.18-fold increase on day 15, 1.27-fold increase on day 30) (Supplementary Table S1).
AAV-IGF1 remarkably improves muscle performance
To investigate whether the profound changes observed in the proteome of AAV2-IGF1-injected muscles eventually affected muscle performance, we applied an exhaustive stress test to analyze and quantify physical endurance in mice. For this purpose, we used a swimming apparatus, consisting of a 200-liter water plastic tank, equipped with a mobile shovel to produce artificial waves, in order to prevent the animals from floating during the swimming sessions. Mice were injected either with an AAV2 vector lacking insert or with AAV2-IGF1 (n=12 per group), via muscles of all four limbs (tibialis anterior, gastrocnemius, quadriceps femoris, biceps brachii, and triceps brachii), in order to transduce the major muscle groups of the body.
Thirty days after AAV delivery, each mouse was allowed to swim for 15 min, every second day for 2 weeks, in the presence of artificial waves. At the end of this training period, physical endurance was determined by letting the animals swim until exhaustion. As shown in Fig. 7A, the injection of AAV2-IGF1 into the whole limb muscle mass accounted for a >3-fold increase in endurance performance.
FIG. 7.
Persistent IGF-I overexpression induces fast-to-slow fiber type transition. (A) Changes in the swimming time to exhaustion between controls (C) and mice that had received multiple injections of AAV2-IGF1 (I) in all four limbs. Data are shown as means and standard deviation; asterisks denote statistical significance (p<0.01). (B) Representative immunofluorescence staining of fast fibers (green) in control muscles and after injection of AAV2-IGF1. Nuclei are counterstained with DAPI. Scale bars: 100 μm. (C) Quantification of the number of fast fibers in the same experimental groups as in (B). Data are shown as means and standard deviation; asterisks denote statistical significance (p<0.01).
Parallel to this functional test, we also analyzed whether AAV2-IGF1 gene transfer modified the composition of the injected muscles. At the end of the swimming experiment, one tibialis anterior muscle for each animal was snap frozen to histologically analyze fiber type composition, by using an antibody specifically recognizing fast fiber myosin. In complete agreement with the different pattern of myosin heavy chain composition, AAV2-IGF1 transduction resulted in a lower number of fast fibers (1.8-fold; shown in Fig. 7B and C), confirming the capacity of AAV2-IGF1 to induce a switch toward the slow phenotype.
Discussion
In this work we describe the functional and structural effects of IGF-I gene delivery into multiple muscles of adult animals, using AAV vectors. At least three novel features render this mouse model relevant for both gene therapy and gene doping. First, unlike in previous models, we transduced all the major muscle masses of the four limbs, therefore being able to assess not only the strength of a single muscle but the overall performance of the mouse in a novel endurance test. Second, in the same animals we analyzed biochemical, functional, and structural parameters, thereby providing a complete scenario of the muscle response to IGF-I gene transfer over time. Third, to our knowledge, this is the first report of whole-proteome changes occurring in muscle on IGF-I gene transfer over time.
We found that the administration of AAV2-IGF1 to skeletal muscle resulted in a relevant amount of muscle fibers displaying a central nucleus, suggesting an ongoing process of muscle growth, a moderate angiogenic response, and a switch of fiber type composition toward the slow type. The proteomic analysis of IGF-I-overexpressing muscles fully supported these findings, as concluded by the specific increase in the amount of structural proteins involved in muscle hypertrophy, as well as the upregulation of several slow fiber-specific proteins (i.e., tropomyosin-2, troponin T-1, myosin light chain 3, and desmin), paralleled by the downregulation of fast type-specific proteins (i.e., tropomyosin-1, troponin T-3, and MYLPF [myosin light chain, phosphorylatable, fast skeletal muscle]). This remarkable fast-to-slow transition in muscle fiber types and myosin isoforms is similar to the adaptation that occurs in response to increased neuromuscular activity by exercise training or mechanical loading and encompasses increases in enzyme activities of aerobic–oxidative energy supply (Pette and Staron, 2001).
Concomitant with the increase in the number of oxidative, slow fibers, IGF-I markedly impacted the levels of proteins involved in muscle energy metabolism, including several enzymes required for both glycolysis and the Krebs cycle. In particular, the proteomic profile 15 days after AAV2-IGF1 administration showed a significant increase in the levels of glycogen phosphorylase (PYGM) and phosphoglucomutase (PGM2), two key enzymes of glycogenolysis, indicating the mobilization of muscular glycogen to form glucose 6-phosphate to be used as the energy substrate in glycolysis. In addition, the increase in the levels of both triosephosphate isomerase (TPI1) and pyruvate kinase (PKM2) is expected to further stimulate the glycolytic pathway.
The observed upregulation of the B isoform of lactate dehydrogenase (LDHB), with subsequent accumulation of lactate, would tend to suggest that, in the IGF-I-expressing mice, the oxygen availability was not sufficient to cope with the increased production of pyruvate from glycolysis, therefore pushing the muscle toward anaerobic metabolism. This is fully consistent with previous observations, showing that IGF-I treatment increases lactate dehydrogenase activity and lactate production during the hypertrophic response of C2C12 myotubes (Semsarian et al., 1999). This scenario seems to change, at least in part, after an additional 15 days of IGF-I overexpression, when glycogenolytic enzymes are still upregulated but to a lesser extent than at the earlier time point (in particular, we could appreciate a reduction in the levels of PGM2 at 30 days relative to 15 days, indicating less use of glycogen). However, other glycolytic enzymes, such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH), enolase 1 and 3 (ENO1 and ENO3), and PKM2, appeared clearly increased over control, thereby indicating that glycolysis is still fully activated in these muscles and probably relies on glucose rather than glycogen consumption. These results are congruent with previous observations indicating that IGF-I administration to fasted rats induces hypoglycemia by increasing glucose uptake by muscle cells (Lund et al., 1994; Diaz et al., 2009).
In contrast to what we observed at 15 days, 1 month of IGF-I expression resulted in the marked induction of the aerobic metabolism of pyruvate, as the major components of the pyruvate dehydrogenase complex were highly activated, resulting in increased production of acetyl-CoA, ready to be metabolized in the Krebs cycle. Indeed, several of its enzymes, such as aconitase (ACO2), succinate dehydrogenase (SDHA), malate dehydrogenase (MDH), isocitrate dehydrogenase (IDH3A), and components of the α-ketoglutarate dehydrogenase complex (OGDH and DLST), were clearly upregulated, the latter two representing key control points of the cycle.
Why should IGF-I overexpression have different metabolic consequences at 15 and 30 days? A possible, reasonable explanation could stem from the different oxygen availability at the two time points. In fact, the Krebs cycle can work only under aerobic conditions, because the critical electron acceptors NAD+ and FAD+ can be generated in the mitochondrion only by the transfer of electrons to molecular oxygen. Interestingly, we could detect the formation of new capillaries, reasonably resulting in better oxygenation of the muscle, at 30 days, but not at 15 days of IGF-I expression; the same difference was observed for the levels of myoglobin, the main oxygen carrier in the muscle. An additional element further supporting active oxidative phosphorylation at 30 days after AAV-IGF1 delivery is the upregulation of several enzymes participating both in the formation of the proton gradient and ATP synthesis. The accelerated respiratory chain not only provides more NAD+ and FAD+ to the Krebs cycle but also makes higher ATP levels available to the muscle. Of note, creatine kinase, which catalyzes the transfer of a phosphate group from the newly generated ATP to creatine, thereby forming creatine phosphate as an extra energy reservoir, was also upregulated by IGF-I.
Previous studies have exploited either microarray or proteomic approaches to dissect the effect of IGF-I in a variety of cell types, including myoblasts (Bhasker and Friedmann, 2008; King et al., 2009), thyroid cells (Lee et al., 2007), primary cardiomyocytes (Li et al., 2003), and fibroblasts (Toyoshima et al., 2004). Most of these studies concluded that IGF-I regulates the expression of genes related to mitogenesis and differentiation, cell cycle, transcriptional and translational processes, cellular respiration, and mitochondrial function and cell survival. However, a few overlaps exist between these results and ours, and include GAPDH (Dont and Holzenberger, 2003), triosephosphate isomerase and enolase 1 (King et al., 2009), and ATP synthase and aldolase A (ALDOA) (Liu and Yi, 2001) (the latter being induced in cardiomyocytes, while reduced in our model). This wide discrepancy again underlines the relevance of performing genomic and proteomic studies in living animal models, including postmitotic cells.
When the IGF-I-dosed mice were challenged by subjecting them to an exhaustive swimming test, the observed histological and metabolic events were found to eventually result in markedly improved endurance, allowing the mice to swim for a 3-fold longer time than controls. Consistent with previous results showing that IGF-I transgenic mice displayed an enhanced tetanic force (Musaro et al., 2001), this finding clearly shows that diffuse AAV2-IGF1 delivery to both forelimbs and hind limbs confers not only a specific gain in muscle power, but also higher endurance in a complex physical activity such as swimming. Thus, our model fully supports the concept that IGF-I gene delivery can be considered a realistic way to achieve greater athletic performance.
Supplementary Material
Acknowledgments
This work was supported by grant 06B6MG from the World Anti-Doping Agency (WADA) to M.G., and from the Italian Ministry of University and Scientific Research (grant FIRB RBRN07BMCT to C.G.).
Author Disclosure Statement
No competing financial interests exist.
References
- Alban A. David S.O. Bjorkesten L., et al. A novel experimental design for comparative two-dimensional gel analysis: Two-dimensional difference gel electrophoresis incorporating a pooled internal standard. Proteomics. 2003;3:36–44. doi: 10.1002/pmic.200390006. [DOI] [PubMed] [Google Scholar]
- Arsic N. Zentilin L. Zacchigna S., et al. Induction of functional neovascularization by combined VEGF and angiopoietin-1 gene transfer using AAV vectors. Mol. Ther. 2003;7:450–459. doi: 10.1016/s1525-0016(03)00034-0. [DOI] [PubMed] [Google Scholar]
- Barton-Davis E.R. Shoturma D.I. Musaro A., et al. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15603–15607. doi: 10.1073/pnas.95.26.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito M. Valverde A.M. Lorenzo M. IGF-I: A mitogen also involved in differentiation processes in mammalian cells. Int. J. Biochem. Cell Biol. 1996;28:499–510. doi: 10.1016/1357-2725(95)00168-9. [DOI] [PubMed] [Google Scholar]
- Bhasker C.R. Friedmann T. Insulin-like growth factor-1 coordinately induces the expression of fatty acid and cholesterol biosynthetic genes in murine C2C12 myoblasts. BMC Genomics. 2008;9:535. doi: 10.1186/1471-2164-9-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danieli Betto D. Zerbato E. Betto R. Type 1, 2A, and 2B myosin heavy chain electrophoretic analysis of rat muscle fibers. Biochem. Biophys. Res. Commun. 1986;138:981–987. doi: 10.1016/s0006-291x(86)80592-7. [DOI] [PubMed] [Google Scholar]
- Diaz M. Vraskou Y. Gutierrez J. Planas J.V. Expression of rainbow trout glucose transporters GLUT1 and GLUT4 during in vitro muscle cell differentiation and regulation by insulin and IGF-I. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R794–R800. doi: 10.1152/ajpregu.90673.2008. [DOI] [PubMed] [Google Scholar]
- Dont J. Holzenberger M. IGF type 1 receptor: A cell cycle progression factor that regulates aging. Cell Cycle. 2003;2:270–272. [PubMed] [Google Scholar]
- Fisher K.J. Jooss K. Alston J., et al. Recombinant adeno-associated virus for muscle directed gene therapy. Nat. Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- Goldspink G. Yang S.Y. The splicing of the IGF-I gene to yield different muscle growth factors. Adv. Genet. 2004;52:23–49. doi: 10.1016/S0065-2660(04)52002-3. [DOI] [PubMed] [Google Scholar]
- Hakimi P. Yang J. Casadesus G., et al. Overexpression of the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) in skeletal muscle repatterns energy metabolism in the mouse. J. Biol. Chem. 2007;282:32844–32855. doi: 10.1074/jbc.M706127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennische E. Skottner A. Hansson H.A. Satellite cells express the trophic factor IGF-I in regenerating skeletal muscle. Acta Physiol. Scand. 1987;129:9–15. doi: 10.1111/j.1748-1716.1987.tb08034.x. [DOI] [PubMed] [Google Scholar]
- Karp N.A. Lilley K.S. Maximising sensitivity for detecting changes in protein expression: Experimental design using minimal CyDyes. Proteomics. 2005;5:3105–3115. doi: 10.1002/pmic.200500083. [DOI] [PubMed] [Google Scholar]
- Karp N.A. Kreil D.P. Lilley K.S. Determining a significant change in protein expression with DeCyder during a pair-wise comparison using two-dimensional difference gel electrophoresis. Proteomics. 2004;4:1421–1432. doi: 10.1002/pmic.200300681. [DOI] [PubMed] [Google Scholar]
- Keller H.L. St. Pierre Schneider B. Eppihimer L.A. Cannon J.G. Association of IGF-I and IGF-II with myofiber regeneration in vivo. Muscle Nerve. 1999;22:347–354. doi: 10.1002/(sici)1097-4598(199903)22:3<347::aid-mus7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kessler P.D. Podsakoff G.M. Chen X., et al. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C.C. Bouic K. Friedmann T. A fractionation method to identify qauntitative changes in protein expression mediated by IGF-1 on the proteome of murine C2C12 myoblasts. Proteome Sci. 2009;7:28. doi: 10.1186/1477-5956-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota J. Handy C.R. Haidet A.M., et al. Follistatin gene delivery enhances muscle growth and strength in nonhuman primates. Sci. Transl. Med. 2009;1:6ra15. doi: 10.1126/scitranslmed.3000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Barton E.R. Sweeney H.L. Farrar R.P. Viral expression of insulin-like growth factor-I enhances muscle hypertrophy in resistance-trained rats. J. Appl. Physiol. 2004;96:1097–1104. doi: 10.1152/japplphysiol.00479.2003. [DOI] [PubMed] [Google Scholar]
- Lee S.J. McPherron A.C. Regulation of myostatin activity and muscle growth. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.J. Park D.O.J. Shin C.S., et al. Microarray analysis of thyroid stimulating hormone, insulin-like growth factor-1, and insulin-induced gene expression in FRTL-5 thyroid cells. J. Korean Med. Sci. 2007;22:883–890. doi: 10.3346/jkms.2007.22.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T. Chen Y.H. Liu T.J., et al. Using DNA microarray to identify Sp1 as a transcriptional regulatory element of insulin-like growth factor 1 in cardiac muscle cells. Circ. Res. 2003;93:1202–1209. doi: 10.1161/01.RES.0000104085.76261.02. [DOI] [PubMed] [Google Scholar]
- Liu J.H. Yi Z.W. [Relationship between serum thyroid hormone and GH–IGF axis, growth failure in nephrotic rats] Hunan Yi Ke Da Xue Xue Bao. 2001;26:263–266. [PubMed] [Google Scholar]
- Lopez-Lopez C. Leroith D. Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc. Natl. Acad. Sci. U.S.A. 2004;101:9833–9838. doi: 10.1073/pnas.0400337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund S. Flyvbjerg A. Holman G.D., et al. Comparative effects of IGF-I and insulin on the glucose transporter system in rat muscle. Am. J. Physiol. 1994;267:E461–E466. doi: 10.1152/ajpendo.1994.267.3.E461. [DOI] [PubMed] [Google Scholar]
- Marouga R. David S. Hawkins E. The development of the DIGE system: 2D fluorescence difference gel analysis technology. Anal. Bioanal. Chem. 2005;382:669–678. doi: 10.1007/s00216-005-3126-3. [DOI] [PubMed] [Google Scholar]
- Mueller C. Flotte T.R. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 2008;15:858–863. doi: 10.1038/gt.2008.68. [DOI] [PubMed] [Google Scholar]
- Musaro A. McCullagh K.J. Naya F.J., et al. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature. 1999;400:581–585. doi: 10.1038/23060. [DOI] [PubMed] [Google Scholar]
- Musaro A. McCullagh K. Paul A., et al. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat. Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- Musaro A. Giacinti C. Borsellino G., et al. Stem cell-mediated muscle regeneration is enhanced by local isoform of insulin-like growth factor 1. Proc. Natl. Acad. Sci. U.S.A. 2004;101:1206–1210. doi: 10.1073/pnas.0303792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pette D. Staron R.S. Transitions of muscle fiber phenotypic profiles. Histochem. Cell Biol. 2001;115:359–372. doi: 10.1007/s004180100268. [DOI] [PubMed] [Google Scholar]
- Rabinovsky E.D. Draghia-Akli R. Insulin-like growth factor I plasmid therapy promotes in vivo angiogenesis. Mol. Ther. 2004;9:46–55. doi: 10.1016/j.ymthe.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Semsarian C. Sutrave P. Richmond D.R. Graham R.M. Insulin-like growth factor (IGF-I) induces myotube hypertrophy associated with an increase in anaerobic glycolysis in a clonal skeletal-muscle cell model. Biochem. J. 1999;339:443–451. [PMC free article] [PubMed] [Google Scholar]
- Su E.J. Cioffi C.L. Stefansson S., et al. Gene therapy vector-mediated expression of insulin-like growth factors protects cardiomyocytes from apoptosis and enhances neovascularization. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H1429–H1440. doi: 10.1152/ajpheart.00885.2002. [DOI] [PubMed] [Google Scholar]
- Tafuro S. Ayuso E. Zacchigna S., et al. Inducible adeno-associated virus vectors promote functional angiogenesis in adult organisms via regulated vascular endothelial growth factor expression. Cardiovasc. Res. 2009;83:663–671. doi: 10.1093/cvr/cvp152. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Ishida K. Itoh K., et al. IGF-I gene transfer by electroporation promotes regeneration in a muscle injury model. Gene Ther. 2003;10:612–620. doi: 10.1038/sj.gt.3301900. [DOI] [PubMed] [Google Scholar]
- Taylor C.F. Paton N.W. Lilley K.S., et al. The minimum information about a proteomics experiment (MIAPE) Nat. Biotechnol. 2007;25:887–893. doi: 10.1038/nbt1329. [DOI] [PubMed] [Google Scholar]
- Tonge R. Shaw J. Middleton B., et al. Validation and development of fluorescence two-dimensional differential gel electrophoresis proteomics technology. Proteomics. 2001;1:377–396. doi: 10.1002/1615-9861(200103)1:3<377::AID-PROT377>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Toyoshima Y. Karas M. Yakar S., et al. TDAG51 mediates the effects of insulin-like growth factor I (IGF-I) on cell survival. J. Biol. Chem. 2004;279:25898–25904. doi: 10.1074/jbc.M400661200. [DOI] [PubMed] [Google Scholar]
- Viswanathan S. Unlu M. Minden J.S. Two-dimensional difference gel electrophoresis. Nat. Protoc. 2006;1:1351–1358. doi: 10.1038/nprot.2006.234. [DOI] [PubMed] [Google Scholar]
- Wang Y.X. Zhang C.L. Yu R.T., et al. Regulation of muscle fiber type and running endurance by PPARδ. PLoS Biol. 2004;2:e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T.P. Esser K.A. Satellite cell and growth factor involvement in skeletal muscle growth. Med. Sci. Sports Exerc. 1989;21:S158–S163. [PubMed] [Google Scholar]
- Zhou S. Murphy J.E. Escobedo J.A. Dwarki V.J. Adeno-associated virus-mediated delivery of erythropoietin leads to sustained elevation of hematocrit in nonhuman primates. Gene Ther. 1998;5:665–670. doi: 10.1038/sj.gt.3300648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.