Abstract
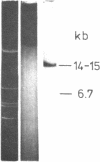
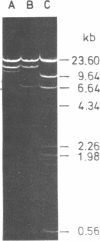
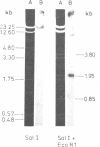
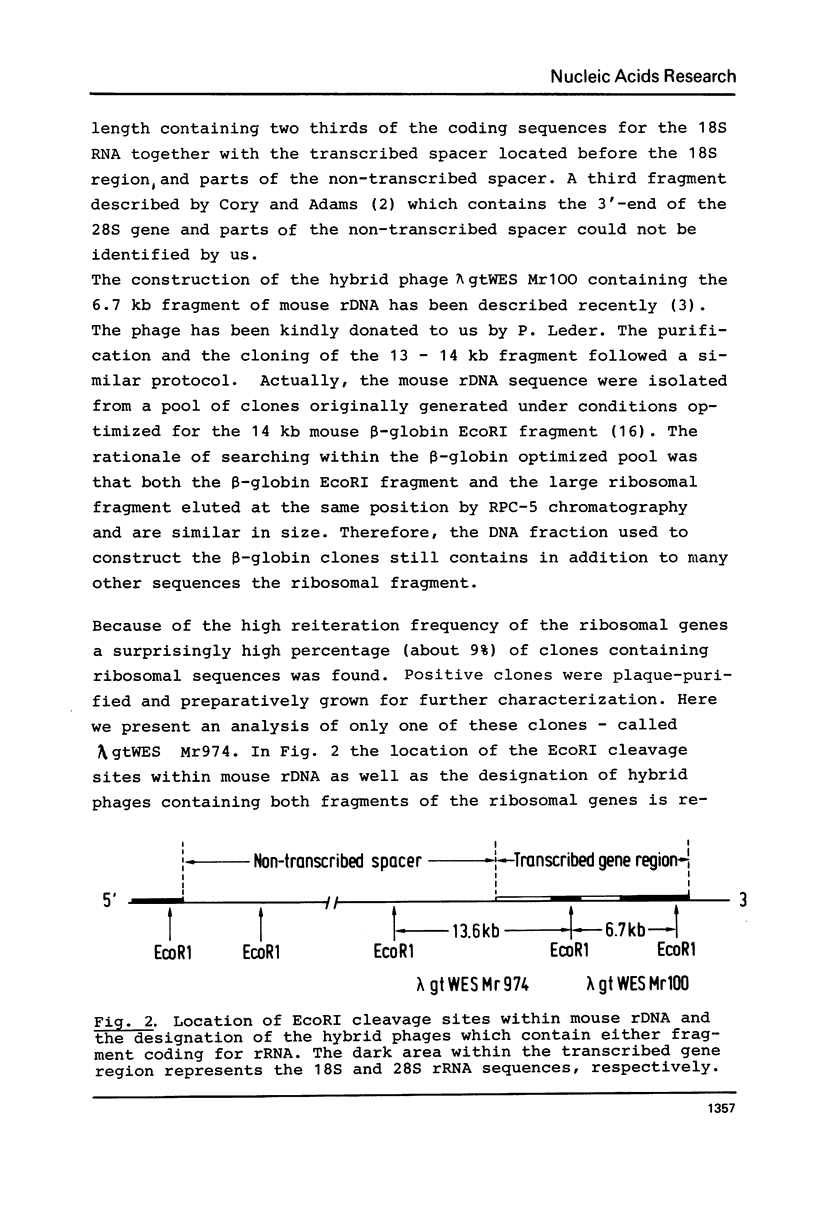
The large EcoRI fragment of mouse ribosomal genes containing parts of the non-transcribed spacer, the external transcribed spacer located at the 5' end of the precursor molecule and about two thirds of the 18S sequence has been cloned in bacteriophage lambda gtWES. A physical map of the DNA was constructed by cleavage with several restriction endonucleases and hybridization of the restriction fragments of the recombinant DNA with labelled 18S and 45S rRNA. The orientation of the inserted fragment as well as the length of the 18S sequence was determined by electron microscopy of R-loop containing molecules. The absence of hybridization of the cloned fragment to other fragments in the genome shows that the non-transcribed spacer does not have a significant length of sequences in common with other sequences in the genome.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Botchan P., Reeder R. H., Dawid I. B. Restriction analysis of the nontranscribed spacers of Xenopus laevis ribosomal DNA. Cell. 1977 Jul;11(3):599–607. doi: 10.1016/0092-8674(77)90077-0. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Cartwright E. M., Brown D. D. Sequence studies of the 5 S DNA of Xenopus laevis. J Mol Biol. 1974 Nov 15;89(4):703–718. doi: 10.1016/0022-2836(74)90046-1. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Fry K., Nelson T., Hung P. Synthesis of hybrid bacterial plasmids containing highly repeated satellite DNA. Cell. 1977 Mar;10(3):509–519. doi: 10.1016/0092-8674(77)90038-1. [DOI] [PubMed] [Google Scholar]
- Chesterton C. J., Coupar B. E., Butterworth P. H., Green M. H. Studies on the control of ribosomal RNA synthesis in HeLa cells. Eur J Biochem. 1975 Sep 1;57(1):79–83. doi: 10.1111/j.1432-1033.1975.tb02278.x. [DOI] [PubMed] [Google Scholar]
- Cohen A., Ram D. Deletions within E. coli plasmids carrying yeast rDNA. Gene. 1978 Apr;3(2):135–147. doi: 10.1016/0378-1119(78)90057-4. [DOI] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J. M. A very large repeating unit of mouse DNA containing the 18S, 28S and 5.8S rRNA genes. Cell. 1977 Aug;11(4):795–805. doi: 10.1016/0092-8674(77)90292-6. [DOI] [PubMed] [Google Scholar]
- Cramer J. H., Farrelly F. W., Barnitz J. T., Rownd R. H. Construction and restriction endonuclease mapping of hybrid plasmids containing Saccharomyces cerevisiae ribosomal DNA. Mol Gen Genet. 1977 Mar 16;151(3):229–244. doi: 10.1007/BF00268786. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Fedoroff N. V., Brown D. D. The nucleotide sequence of oocyte 5S DNA in Xenopus laevis. I. The AT-rich spacer. Cell. 1978 Apr;13(4):701–716. doi: 10.1016/0092-8674(78)90220-9. [DOI] [PubMed] [Google Scholar]
- Gaubatz J., Prashad N., Cutler R. G. Ribosomal RNA gene dosage as a function of tissue and age for mouse and human. Biochim Biophys Acta. 1976 Feb 5;418(3):358–375. doi: 10.1016/0005-2787(76)90297-5. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Gross K. J., Pogo A. O. Control of ribonucleic acid synthesis in eukaryotes. 3. The effect of cycloheximide and edeine on rna synthesis in yeast. Biochemistry. 1976 May 18;15(10):2082–2086. doi: 10.1021/bi00655a008. [DOI] [PubMed] [Google Scholar]
- Grummt I., Smith V. A., Grummt F. Amino acid starvation affects the initiation frequency of nucleolar RNA polymerase. Cell. 1976 Mar;7(3):439–445. doi: 10.1016/0092-8674(76)90174-4. [DOI] [PubMed] [Google Scholar]
- Hershey N. D., Conrad S. E., Sodja A., Yen P. H., Cohen M., Jr, Davidson N., Iigen C., Carbon J. The sequence arrangement of Drosophila melanogaster 5s DNA cloned in recombinant plasmids. Cell. 1977 Jul;11(3):585–598. doi: 10.1016/0092-8674(77)90076-9. [DOI] [PubMed] [Google Scholar]
- KIRBY K. S. ISOLATION AND CHARACTERIZATION OF RIBOSOMAL RIBONUCLEIC ACID. Biochem J. 1965 Jul;96:266–269. doi: 10.1042/bj0960266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert A., Feigelson P. A short lived polypeptide component of one of two discrete functional pools of hepatic nuclear alpha-amanitin resistant RNA polymerases. Biochem Biophys Res Commun. 1974 Jun 18;58(4):1030–1038. doi: 10.1016/s0006-291x(74)80247-0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Philippsen P., Kramer R. A., Davis R. W. Cloning of the yeast ribosomal DNA repeat unit in SstI and HindIII lambda vectors using genetic and physical size selections. J Mol Biol. 1978 Aug 15;123(3):371–386. doi: 10.1016/0022-2836(78)90085-2. [DOI] [PubMed] [Google Scholar]
- Polsky F., Edgell M. H., Seidman J. G., Leder P. High capacity gel preparative electrophoresis for purification of fragments of genomic DNA. Anal Biochem. 1978 Jul 1;87(2):397–410. doi: 10.1016/0003-2697(78)90689-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tiemeier D. C., Tilghman S. M., Leder P. Purification and cloning of a mouse ribosomal gene fragment in coliphage lambda. Gene. 1977;2(3-4):173–191. doi: 10.1016/0378-1119(77)90016-6. [DOI] [PubMed] [Google Scholar]
- Tiemeier D. C., Tilghman S. M., Polsky F. I., Seidman J. G., Leder A., Edgell M. H., Leder P. A comparison of two cloned mouse beta-globin genes and their surrounding and intervening sequences. Cell. 1978 Jun;14(2):237–245. doi: 10.1016/0092-8674(78)90110-1. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B., Brown D. D., Reeder R. H. The molecular basis for length heterogeneity in ribosomal DNA from Xenopus laevis. J Mol Biol. 1976 Aug 25;105(4):461–486. doi: 10.1016/0022-2836(76)90229-1. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. The structural organization of ribosomal DNA in Drosophila melanogaster. Cell. 1977 Feb;10(2):193–212. doi: 10.1016/0092-8674(77)90214-8. [DOI] [PubMed] [Google Scholar]