Abstract
Background
Evidence suggests that excess mortality among African-American cancer patients is explained in part by health care setting. Our objective was to compare mortality among African-American and Caucasian cancer patients and to evaluate the influence of NCI-Cancer Center attendance.
Methods
We conducted a retrospective cohort analysis of Medicare beneficiaries with an incident diagnosis of lung, breast, colorectal, or prostate cancer from 1998–2002, as identified in SEER. Multivariate logistic regression models assessed the impact of NCI-Cancer Center attendance and race on all-cause and cancer-specific mortality at one and three years from diagnosis.
Results
Likelihoods of one- and three-year all-cause and cancer-specific mortality were higher for African-Americans than for Caucasians in crude and adjusted models (cancer-specific adjusted: Caucasian referent, 1year: OR=1.13; 95% CI 1.07–1.19, 3-year OR=1.23; 95% CI 1.17–1.30). By cancer site, cancer-specific mortality was higher among African-Americans at one year for breast and colorectal cancers and for all cancers at three years. NCI-Cancer Center attendance was associated with significantly lower odds of mortality for African-Americans (1-year: OR=0.63; 95% CI 0.56–0.76, 3-years: OR=0.71; 95% CI 0.62–0.81). The excess mortality risk among African-Americans was no longer observed for all-cause or cancer-specific mortality risk among patients attending NCI-Cancer Centers (Caucasian referent, cancer-specific mortality at:1-year: OR=0.95; 95% CI 0.76–1.19, 3-years: OR=1.00; 95% CI 0.82–1.21).
Conclusions
African-American Medicare beneficiaries with lung, breast, colorectal, and prostate cancers have higher mortality compared to their Caucasian counterparts; however, there were no significant mortality differences by race among those attending NCI-Cancer Centers. This study suggests that place of service may explain some of the cancer mortality excess observed in African Americans.
Keywords: Healthcare Disparities, Quality of Healthcare, Mortality, Health facilities
INTRODUCTION
Recent efforts to understand racial and ethnic health disparities have examined the extent to which individual, social, and health care factors contribute to observed disparities. The Institute of Medicine reviewed a large number of studies and reported that racial/ethnic disparities exist, but diminish somewhat when accounting for variation in health insurance coverage and system attributes that influence access to, and quality of, health care 1. The role of these factors in health disparities are still not well understood, and even less so for cancer care.
Cancer-related mortality in African American cancer patients has repeatedly been shown to be higher than that in Caucasians, although the causal factors are not clear. A 2007 Robert Wood Johnson Foundation review and synthesis focused on disparities in access to and quality of health care and drew several cancer-specific conclusions: breast cancer screening was similar for blacks and whites when adjusting for other factors; and, cancer treatment differences between blacks and whites are significant, with blacks less likely to undergo newer treatments and invasive treatments 2. At the same time, other evidence demonstrates that blacks and whites treated in the same health care setting receive similar cancer care 3–5. Other studies 6, 7 not confined to cancer care, reported that blacks and whites in Medicare managed care plans were comparable in receipt of recommended processes of care, but differed significantly in intermediate outcomes, such as blood pressure control in hypertensive patients.
While treatment and mortality differences have been examined for African American and Caucasian cancer patients, very little effort has focused on racial/ethnic differences in where cancer care is received. Our objective was to assess whether place of serviceis associated with differences in mortality for African American and Caucasian patients with lung, breast, colorectal, or prostate cancer in the Medicare population.
MATERIALS AND METHODS
Study Population and Data
We used SEER-Medicare data to identify incident primary cases of lung, breast, colorectal, and prostate cancers from 1998–2002, with linked claims through 2003. The 14 SEER registries represent ~26% of the U.S. population8. For the cancer sites included – lung, breast, colorectal, and prostate -- the majority occur in individuals over age 65 9.
We did not include individuals from Hawaii since the restricted variable, patient zip code, was not available for this state. Washington State residents were excluded due to missing data from the NCI Cancer Center in the Seattle/Puget Sound registry (N=15,661). From a total of 558,663 cancer cases, we excluded 329,520 based on several criteria, including: unequal parts A and B Medicare enrollment (n=24,657) which can limit ascertainment of services, any enrollment in a Medicare risk-bearing HMO in 12 months prior to diagnosis (n=113,854), <66 years of age at diagnosis (121,411), indeterminate month of diagnosis (n=2,357), initial entitlement due to end stage renal disease (n=164), cancer diagnosis prior to 1998 (23,789), death within one month of diagnosis (n=20,247), and absence of MedPAR or Outpatient claims in the first 12 months following diagnosis (n=7,380). Patients with only one hospital-based claim (MEDPAR or Outpatient) in the first year following diagnosis (n=3,974) were further excluded for the main analysis, since assignment to a care setting based on one claim did not meet our threshold; however, these patients were included in subsequent sensitivity analyses (described below). Due to small numbers individuals reporting race other than Caucasian or African American were excluded from further analysis (n=9,743).
Variables
The main variables of interest were race, NCI Cancer Center attendance, and overall and cancer-specific mortality at one and three years. Racial categories included Caucasian and African American and were mutually exclusive. Race was self-designated during the application processing at the Social Security Administration (SSA). We excluded other racial/ethnic categories based on previous studies using this cohort that demonstrated a lack of statistical power.
During our study period (1998–2003), 47 institutions held continuous designation as an NCI-designated comprehensive or clinical cancer center (hereafter referred to as “NCI-Cancer Centers”). Of these, 15 were located within SEER areas corresponding to the most recent complete data. NCI Cancer Center attendance was defined as two or more claim-days for inpatient or outpatient procedural care occurring at an NCI Cancer Center within 12 months of the index cancer diagnosis as recorded by SEER 10. A claim-day was defined as one calendar date on which one or more of the above claims occurred; inpatient stays were considered as one claim, and Outpatient claims (those from institutional outpatient providers) occurring during an inpatient stay were not counted. An index cancer was defined as the first primary cancer of the breast, lung, colon/rectum, or lung within the study period. Claims were identified as occurring at an NCI Cancer Center through the SEER-Medicare Hospital file 11.
We used date of death as recorded in the Medicare Denominator File, in conjunction with date of diagnosis, to determine all-cause and cancer-specific mortality at one and three years. Cause of death was obtained from SEER records. To account for possible referral and/or health care encounter patterns that might influence mortality we determined the dominant physician care type in the 6 months prior to diagnosis. This measure was derived by tabulating physician encounters as recorded in Carrier claims according to physician type, generalist or specialist, and assigning primary care predominance to those with ≥ 50% generalist care. We also adjusted for comorbid conditions identified by ICD-9 codes for all hospital and physician encounters within 12 months preceding the date of diagnosis, then calculated a Charlson score, modified to exclude solid tumors 12–15. Stage at diagnosis was based on TNM Staging Classification 1–4, as recorded by SEER registries. Receipt of cancer-directed surgery in the first year following diagnosis was determined using procedure codes from the International Classification of Diseases (ICD-9) and Current Procedural Terminology (CPT).
Group-level variables included median household income and educational attainment for the ZIP code of residence, and travel time to the nearest NCI-Cancer Center, travel time to nearest academic-based care, and per-capita oncologist supply, as described previously16. We found median income and median education by ZIP to be 99% correlated, thus we included only median income in subsequent analyses.
Statistical Analysis
We evaluated 1- and 3-year all-cause and cancer-specific mortality for African Americans and Caucasians by modeling mortality as a logit function of NCI-Cancer Center attendance and race. We evaluated models of 1- and 3-year mortality for African Americans compared to Caucasians (referent), with and without adjusted for NCI Cancer Center attendance, age, sex, stage at diagnosis, travel time to nearest NCI Cancer Center, cancer site, predominance of primary care prior to diagnosis, median household income quintile for ZIP code of residence, and SEER registry at diagnosis. We evaluated models stratified by cancer site and for all cancers combined. We also examined the effect of NCI Cancer Center attendance on 1- and 3-year mortality stratifying by race. The logistic regression models of mortality were then stratified by NCI-Cancer Center attendance to assess potential interactions between race and place of cancer care. We performed post hoc analyses to examine differences in baseline characteristics such as comorbidities, stage, and number of primaries, and also in the likelihood of receiving cancer-directed surgery. To account for potential correlation of unmeasured factors, we also applied random effects models with clustering at the level of the hospital referral region, and separately for SEER registry. Because variances were exceedingly low for residuals from these random effects models (0.04), here we report only multivariate logistic regression models.
All analyses were performed using Stata statistical software v.9.2 (Stata Corporation, College Station, TX). This study was approved by the Committee for Protection of Human Subjects at Dartmouth Medical School.
RESULTS
The analytic sample consisted of 201,305 Medicare beneficiaries, of whom 18,008 (8.9%) were African American (Table 1). A higher proportion of African Americans attended an NCI Cancer Center than Caucasians (11.1% vs. 6.9%). Some of the notable characteristics which differed between African Americans and Caucasians who attended NCI Cancer Centers included: 1. predominance of primary care prior to diagnosis (NCI Cancer Center attendees: Caucasian, 40.5%, African American, 49.6%; non-NCI Cancer Center attendees: Caucasian, 46.5%, African American, 55.8%), 2. number of comorbidities (5 or more comorbidities -- NCI Cancer Center attendees: Caucasian, 2.5%, African American, 3.9%; non-NCI Cancer Center attendees: Caucasian, 3.8%, African American, 6.0%), and receipt of cancer-directed surgery (NCI Cancer Center attendees: Caucasian, 60.4%, African American, 53.8%; non-NCI Cancer Center attendees: Caucasian, 58.2%, African American, 49.9%) (Table 1). These factors were included in subsequent adjusted models.
Table 1.
Characteristics of African-American and Caucasian Medicare Beneficiaries With an Incident Diagnosis of Breast Cancer as Recorded in the Surveillance, Epidemiology, and End Results Program From 1998 to 2002 (n=201,305)
| NCI Cancer Center Attendance: No. of Patients (%)a |
||||
|---|---|---|---|---|
| Caucasians | African Americans | |||
| Variable | Yes | No | Yes | No |
| Total | 12,690 (6.9) | 170,607 (93.1) | 1993 (11.1) | 16,015 (88.9) |
| Woman | 5631 (44.4) | 82,768 (48.5) | 867 (43.5) | 6944 (43.4) |
| Predominant primary careb | 5136 (40.5) | 79,391 (46.5) | 988 (49.6) | 8930 (55.8) |
| Rurality | ||||
| Urban/suburban | 10,339 (81.4) | 132,361 (77.6) | 1968 (98.8) | 14,117 (88.2) |
| Large town/rural | 2351 (18.6) | 38,246 (22.4) | 25 (1.2) | 1898 (11.8) |
| Cancer Site | ||||
| Breast | 2933 (23.1) | 41,662 (24.4) | 465 (23.3) | 3126 (19.5) |
| Lung | 3178 (25) | 39,644 (23.2) | 383 (19.2) | 3632 (22.7) |
| Colon/rectum | 2407 (19) | 40,662 (23.8) | 336 (16.9) | 3673 (22.9) |
| Prostate | 4172 (32.9) | 48,639 (32.9) | 809 (40.6) | 5584 (34.9) |
| Charlson Score | ||||
| 0 | 8338 (65.7) | 104,210 (61.1) | 1171 (58.8) | 8436 (52.7) |
| 1–2 | 3041 (24) | 43,371 (25.4) | 528 (26.5) | 4610 (28.8) |
| 3–4 | 993 (7.8) | 16,604 (9.7) | 216 (10.8) | 2005 (12.5) |
| ≥5 | 318 (2.5) | 6422 (3.8) | 78 (3.9) | 964 (6) |
| Cancer-directed surgery | 7660 (60.4) | 99,213 (58.2) | 1072 (53.8) | 7985 (49.9) |
| Stage | ||||
| I | 2817 (22.2) | 42,024 (24.6) | 419 (21) | 2852 (17.8) |
| II | 1697 (15.7) | 27,107 (15.9) | 224 (11.2) | 2390 (14.9) |
| III | 1814 (14.3) | 23,189 (13.6) | 249 (12.5) | 2283 (14.3) |
| IV | 1943 (15.3) | 22,189 (13) | 286 (14.3) | 2608 (16.3) |
| Unknown | 4419 (34.8) | 56,158 (32.9) | 815 (40.9) | 5882 (36.7) |
| Age at diagnosis: Median [interquartile range], y | 73 [69–77] | 75 [70–80] | 73 [69–77] | 74 [69–79] |
| Travel time to nearest: Median [interquartile range], min | ||||
| NCI cancer center | 28 [16–64] | 55 [24–137] | 10 [7–15] | 29 [15–152] |
| Academic-based care | 19 [11–50] | 25 [13–70] | 7 [5–8] | 10 [6–24] |
| Income of zip code in $1000: Median [interquartile range] | 48.6 [36.1–60.4] | 46.4 [33.5–59.1] | 31.8 [24.6–41.0] | 32.2 [25.1–44.3] |
| Physician supply by hospital referral region: Median [interquartile range] | ||||
| Primary care, per 1000 | 1.3 [1.0–1.9] | 1.2 [0.9–1.6] | 1.0 [0.8–2.3] | 1.2 [0.8–1.5] |
| Oncologists, per 100,000 | 3.4 [2.3–4.3] | 2.5 [2.0–3.8] | 3.8 [3.8–3.8] | 3.7 [2.4–3.8] |
NCI indicates National Cancer Institute.
NCI cancer Center attendance was defined as having two or more claim-days in the first 12 months following diagnosis.
Predominant primary care was defined as having the same number or equal numbers of primary care visits and specialist visits in the 6 months before diagnosis.
Overall cancer-specific mortality occurred in a higher proportion of African American patients compared to Caucasian at both one and three years (one year: 18.0% vs. 14.7%, respectively; three years: 25.0% vs. 20.0%, respectively) (Table 2). These mortality differences were nearly the same for patients not attending NCI Cancer Centers. Among NCI Cancer Center attendees, no material differences in mortality was seen by race: cancer-specific mortality at one year: 12.3% (African Americans), 12.4% (Caucasians); at three years: 19.9% (African American), 20.0% (Caucasian).
Table 2.
Characteristics of African-American (n=18,008) and Caucasian (n=183,297) Medicare Beneficiaries With Breast, Lung, Colorectal, or Prostate Cancer at 1-Year or 3-Years After Diagnosis
| Mortality: No. of Patients(%) | ||||||
|---|---|---|---|---|---|---|
| Variable | None | Caucasians Cancer-Specific |
Other Cause | None | African Americans Cancer-Specific |
Other Cause |
| 1-Year mortality | ||||||
| Overall | 148,86 (81) | 27,008 (14.7) | 7453 (4) | 13,865 (77) | 3164 (18) | 979 (5) |
| NCI cancer center attendance | ||||||
| No | 138,084 (80.9) | 25,430 (14.9) | 7093 (4.2) | 12,190 (76.1) | 2918 (18.2) | 907 (5.7) |
| Yes | 10,752 (84.7) | 1578 (12.4) | 360 (2.8) | 1675 (84) | 246 (12.3) | 72 (3.6) |
| Cancer site | ||||||
| Breast | 41,827 (93.8) | 1622 (3.6) | 1146 (2.6) | 3205 (89.2) | 252 (7) | 134 (3.7) |
| Lung | 22,172 (51.8) | 18,195 (42.5) | 2455 (5.7) | 1807 (45) | 1915 (47.7) | 293 (7.3) |
| Colorectal | 35,106 (81.5) | 5479 (12.7) | 2484 (5.8) | 3019 (75.3) | 672 (16.8) | 318 (7.9) |
| Prostate | 49,731 (94.2) | 1712 (3.2) | 1368 (2.6) | 5834 (91.3) | 325(5.1) | 234 (3.6) |
| Stage | ||||||
| I or II | 68,545 (93.1) | 2567 (3.5) | 2533 (3.4) | 5325 (90.5) | 280 (4.8) | 280 (4.8) |
| III or IV | 27,819 (56.7) | 18,801 (38.3) | 2455 (5) | 2805 (51.7) | 2268 (41.8) | 353 (6.5) |
| Unknown | 52,472 (82.6) | 5640 (9.3) | 2465 (4.1) | 5735 (85.6) | 616 (9.2) | 346 (5.2) |
| No. of comorbidities | ||||||
| 0 | 96,797 (86) | 12,942 (11.5) | 2809 (2.5) | 7868 (81.9) | 1401 (14.6) | 338 (3.5) |
| 1–2 | 35,945 (77.4) | 8222 (17.7) | 2245 (4.8) | 3918 (76.3) | 946 (18.4) | 274 (5.3) |
| 3–4 | 12,028 (68.3) | 4131 (23.5) | 1438 (8.2) | 1490 (67.1) | 533 (24) | 198 (8.9) |
| ≥5 | 4066 (60.3) | 1713 (25.4) | 961 (14.3) | 589 (56.5) | 284 (27.3) | 169 (16.2) |
| 3-Year mortality | ||||||
| Overall | 12,217 (73) | 37,523 (20) | 13,557 (7) | 11,745 (66) | 4546 (25) | 1717 (9) |
| NCI cancer center attendance | ||||||
| No | 122,733 (71.9) | 35,003 (20.5) | 12,871 (7.5) | 10,293 (64.3) | 4150 (25.9) | 1572 (9.8) |
| Yes | 9484 (74.5) | 2520 (20) | 686 (5.4) | 1452 (72.8) | 396 (19.9) | 145 (7.3) |
| Cancer site | ||||||
| Breast | 38,893 (87.2) | 3014 (6.8) | 2688 (6) | 2846 (79.2) | 459 (12.8) | 286 (8) |
| Lung | 16,469 (38.5) | 22,902 (53.5) | 3451 (8) | 1216 (30.3) | 2413 (60.1) | 386 (9.6) |
| Colorectal | 30,651 (71.2) | 8256 (19.2) | 4162 (9.6) | 2446 (61) | 1059 (26.4) | 504 (12.6) |
| Prostate | 46,204 (87.5) | 3351 (6.4) | 3256 (6.1) | 5237 (81.9) | 615 (9.6) | 541 (8.5) |
| Stage | ||||||
| I or II | 63,134 (85.7) | 5272 (7.2) | 5239 (7.1) | 4773 (81.2) | 566 (9.5) | 546 (9.3) |
| III or IV | 21,645 (44.1) | 23,930 (48.8) | 3500 (7.1) | 1950 (35.9) | 2983 (55) | 493 (9.1) |
| Unknown | 47,438 (78.3) | 8321 (13.7) | 4818 (8) | 5022 (75) | 997 (14.9) | 678 (10.1) |
| No. of comorbidities | ||||||
| 0 | 88,359 (78.5) | 18,588 (16.5) | 5601 (5) | 6867 (71.5) | 2098 (21.8) | 642 (6.7) |
| 1–2 | 31,008 (66.8) | 11,311 (24.4) | 4093 (8.8) | 3274 (63.7) | 1366 (26.6) | 498 (9.7) |
| 3–4 | 9793 (55.6) | 5410 (30.7) | 2394 (13.6) | 1180 (53.2) | 719 (32.3) | 322 (14.5) |
| ≥5 | 3057 (45.4) | 2214 (32.8) | 1469 (21.8) | 424 (40.7) | 363 (34.8) | 255 (24.5) |
NCI indicates National Cancer Institute.
To examine the observed differences in mortality by race and NCI Cancer Center attendance while accounting for covariates, we developed logistic regression models. We first compared likelihood of 1- and 3- year mortality between Caucasians and African Americans in our study population. Crude models demonstrated the expected greater odds of mortality at both one and three years for African Americans relative to Caucasians (Table 3). Higher odds for 1- and 3-year all-cause mortality among African Americans compared to Caucasians persisted, although attenuated in models adjusted for age at diagnosis, sex, travel time to the nearest NCI Cancer Center, attendance at an NCI Cancer Center, predominance of primary care prior to diagnosis, stage at diagnosis, cancer site, rurality, comorbidities, median household income for ZIP code of residence, and SEER registry of residence, higher odds (Table 3). Likelihoods of cancer-specific mortality were similar to those of all-cause mortality (Table 3). To account more fully for differing risks of mortality based on cancer site, we performed logistic regression models stratified by cancer site. Excess risk of mortality for African Americans was seen for all four cancers, with the strongest effect at three years (Figure 1). (Caucasian referent – 1-year mortality: Breast:OR=1.19; 95% CI 1.03–1.37, Lung:OR=1.10; 95% CI 1.02–1.19, Colorectal:OR=1.19; 95% CI 1.08–1.31, Prostate:OR= 1.12; 95% CI 0.99–1.26. 3-year mortality: Breast:OR=1.22; 95% CI 1.10–1.36, Lung:OR=1.15; 95% CI 1.06–1.24, Colorectal:OR=1.30; 95% CI 1.20–1.41, Prostate:OR=1.18; 95% CI 1.08–1.28).
Table 3.
Comparison of Crude and Adjusted Predictive Models of Mortality for African Americans Relative to Caucasians at 1 Year and 3 Years After Diagnosis Among Medicare Beneficiaries (n=201,305) With an Incident Diagnosis of Breast, Lung, Colon/Rectal, or Prostate Cancer as Identified in Surveillance, Epidemiology, and End Results-Medicare Data From 1998–2002
| Mortality: OR (95% CI) | |||
|---|---|---|---|
| Race/Ethnicity | Crude | Adjusted Overalla | Adjusted Cancer-Specific |
| 1-Year mortality | |||
| Caucasian | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| African American | 1.29 (1.24–1.34) | 1.16 (1.10–1.22) | 1.13 (1.07–1.19) |
| 3-Year mortality | |||
| Caucasian | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| African American | 1.38 (1.34–1.43) | 1.22 (1.17–1.28) | 1.23 (1.17–1.30) |
OR indicates odds ratio; CI, confidence interval.
Adjusted models included the covariates age at diagnosis; sex; travel time to the nearest National Cancer Institute cancer center; predominance of primary care before diagnosis; cancer site; rurality; comorbidities; Surveillance, Epidemiology, and End Results registry of residence at diagnosis; median household income of zip code at diagnosis; and year of diagnosis.
Figure 1.
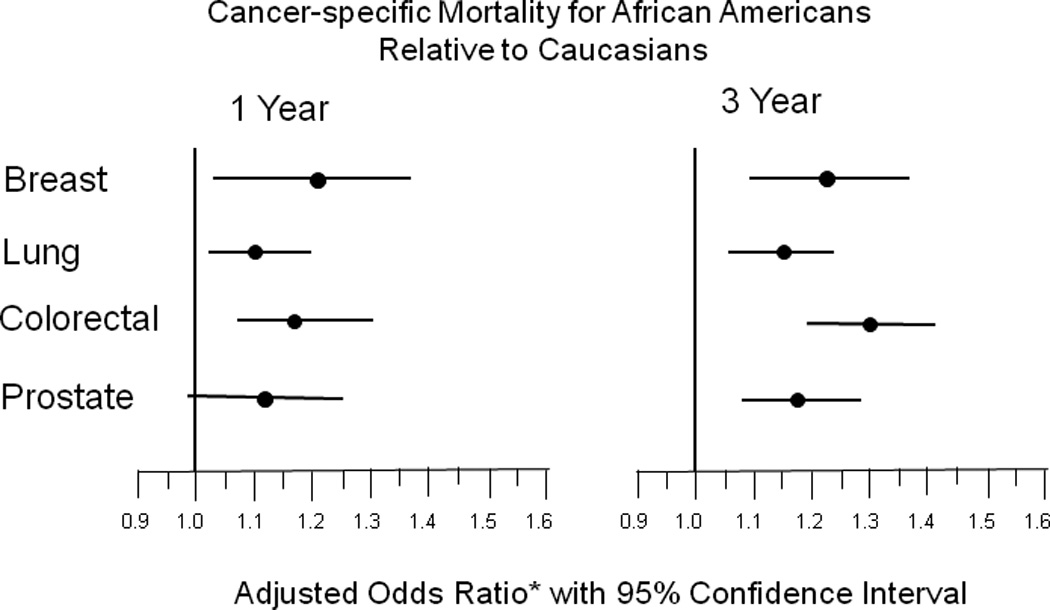
Predictive models of 1- and 3-year cancer-specific mortality as a function of cancer site in African American Medicare beneficiaries compared to Caucasian beneficiaries with an incident diagnosis of breast, lung, colon/rectal, or prostate cancer as identified in SEER-Medicare data from 1998–2002.
* Adjusted models include the covariates: age at diagnosis, sex, travel time to nearest NCI Cancer Center, predominance of primary care prior to diagnosis, stage, rurality, comorbidities, median household income for ZIP of residence at diagnosis, SEER registry of residence at diagnosis, and year of diagnosis.
Based on previous evidence of a mortality benefit among NCI Cancer Center attendees at one and three years from diagnosis 17, we sought to examine whether the benefit was observed for both African Americans and Caucasians. Stratifying our mortality models by race, NCI Cancer Center attendance was associated with a significant decrease in the likelihood of 1- and 3-year mortality for both African Americans and Caucasians, with a somewhat greater decrease for African Americans (1-year mortality – Caucasians: OR=0.77; 95% CI 0.72–0.82, African Americans: OR=0.65; 95% CI 0.55–0.79. 3-year mortality – Caucasians: OR=0.95; 95% CI 0.89–1.00, African Americans: OR=0.74; 95% CI 0.63–0.86) (Figure 2). We further examined the interaction of NCI Cancer Center attendance with race by comparing mortality among African Americans and Caucasians for those patients who attended an NCI Cancer Center and those who did not (Table 4). When stratifying by NCI Cancer Center attendance, we found that the adjusted 1- and 3-year all-cause and cancer-specific mortality excess for African Americans was not evident for attendees (Table 4). Post-hoc analyses to investigate potential explanatory factors for the observed mortality differences revealed a greater likelihood for African Americans to be diagnosed at late stage and a lower likelihood to receive cancer-directed surgery (Data not shown). These differences were largely accounted for by NCI Cancer Center attendance.
Figure 2.
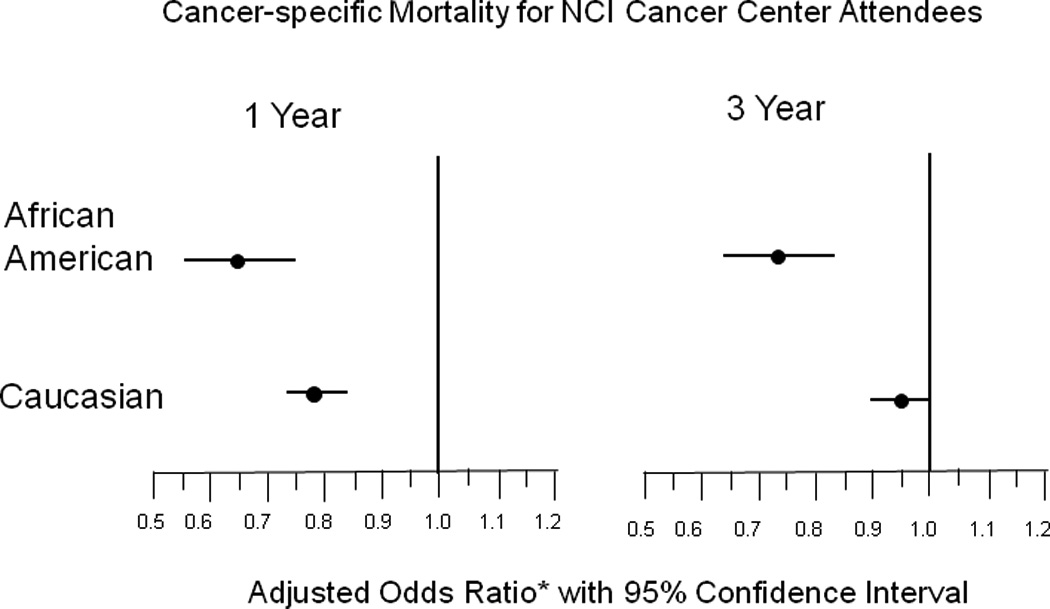
Predictive models of 1- and 3-year cancer-specific mortality as a function of NCI Cancer Center attendance in Medicare beneficiaries with an incident diagnosis of breast, lung, colon/rectal, or prostate cancer as identified in SEER-Medicare data from 1998–2002.
* Adjusted models include the covariates: age at diagnosis, sex, travel time to nearest NCI Cancer Center, predominance of primary care prior to diagnosis, stage, rurality, comorbidities, median household income for ZIP of residence at diagnosis, SEER registry of residence at diagnosis, and year of diagnosis.
Table 4.
Predictive Models of 1-Year and 3-Year Mortality as a Function of National Cancer Center Cancer Center Attendance in African-American Medicare Beneficiaries With an Incident Diagnosis of Breast, Lung, Colon/Rectal, or Prostate Cancer as Identified in Surveillance, Epidemiology, and End Results-Medicare Dataa
| Mortality: OR (95% CI)b | ||||
|---|---|---|---|---|
| Attended an NCI Cancer Center |
Did Not Attend an NCI Cancer Center |
|||
| African Americans vs Caucasians | All-Cause | Cancer-Specific | All Cause | Cancer-Specific |
| 1-Year mortality | 0.95 (0.77–1.17) | 0.95 (0.76–1.19) | 1.15 (1.09–1.21) | 1.14 (1.08–1.21) |
| 3-Year mortality | 0.98 (0.82–1.18) | 1.00 (0.82–1.21) | 1.23 (1.17–1.28) | 1.26 (1.20–1.33) |
OR indicates odds ratio; CI, confidence interval; NCI, National Cancer Institute.
Attendance was defined as 2 or more claim days in the first 12 months after diagnosis.
Adjusted models included the covariates age at diagnosis; sex; travel time to the nearest NCI cancer center; predominance of primary care before diagnosis; stage; cancer site; rurality; comorbidities; Surveillance, Epidemiology, and End Results registry of residence at diagnosis; and year of diagnosis.
DISCUSSION
This study suggests a strong role for place of service in cancer mortality disparities by race. We found an overall, all-cause mortality excess for African American Medicare beneficiaries compared to Caucasians that is similar to the range reported in the literature (20–30%) 18–28. These 1- and 3-year mortality disparities were consistently significant across the four major cancers we examined, even while accounting for racial differences in stage at diagnosis, comorbidities, and socioeconomic measures. NCI Cancer Center attendance has been associated with decreased mortality in this population 29. The present study demonstrates that this mortality benefit is similar for African Americans and Caucasians. Specifically, this study provides evidence that when African American and Caucasian cancer patients attend similar types of specialized cancer care settings, all-cause and cancer-specific mortality is similar.
The idea that racial disparities in health outcomes are mediated by different care is well-documented. Most of this evidence stems from studies of differential treatment patterns for minorities. The majority of population-based studies examining cancer treatment differences between African Americans and Caucasians have found African Americans to be less likely to receive surgery, chemotherapy, radiation, and surveillance 1, 28, 30–38. A driving force behind these treatment differences appears to be in location of care, in addition to other factors such as treatment choices. That is, African Americans and Caucasians with comparable baseline characteristics attending the same facilities receive similar treatment. However, much less evidence is available to for the role of facilities/health care systems than for specific treatments. Studies within equal access systems, such as the military, have demonstrated an absence of racial disparities for cancer and acute myocardial infarction 39–41. An examination of facility-level racial disparities in treatment suggests that the effect of the hospital attended was more influential than race in explaining observed population-level racial disparities 42–44.
Focusing on NCI Cancer Centers in assessing the influence of race versus place on mortality is salient for cancer patients given its high degree of specialization for cancer care and research, and the demonstrated clinical benefit associated with these institutions 29, 45. Further, examining cancer settings or health care systems makes sense in racial disparities research since organizations and facilities represent actionable units. That is, identifying differences in treatment patterns can only lead to change through systems that provide that treatment.
Although attendance at an NCI Cancer Center obviated the mortality differences between African Americans and Caucasians in our study, racial disparities in cancer are complex and multifactorial. The oncology health disparities model of Polite et al. illustrates the likely interplay of lifestyle and environment factors, personal health beliefs, health system factors, and biological factors 33. In our sub-analysis of potential intervening variables in the overall relation between race and mortality, we found African Americans more likely to be diagnosed at a later stage, less likely to receive cancer-directed surgery, and, as previously reported by this group --- more likely to attend an NCI Cancer Center 10. African Americans have been shown to present at a later stage for breast cancer 46–48 and colorectal cancer, 24, 49 and lower surgery rates among African Americans have been noted for lung cancer 46, 50 and colon/rectal cancer 30, 49.
As in any observational research, there is the potential for bias related to unmeasured factors differing between the comparison groups. We accounted for this by adjustment, examining clustered correlations, and performing sub-analyses. Given that this study question is not amenable to randomization, we believe these results are important, although interpreted cautiously. Also inherent in observational research is the potential for lead time bias to distort measures of survival/mortality. Our study attempted to minimize this possibility by controlling for stage at diagnosis. Although such a bias is possible, our results for lung cancer are unlikely to be influenced by lead-time bias because lung cancer currently is not screened for routinely and clinical interventions typically yield only a modest benefit. We were unable to examine patients receiving no hospital-based services and those of other racial/ethnic groups (due to insufficient sample size). Finally, we could not ascertain complete provider utilization longitudinally, such as office-based physician visits.
Our results suggest that when care is received in similar, specialized cancer care settings, African American and Caucasian cancer patients have similar risk for mortality. These results extend previous research and have implications for efforts to address racial disparities in cancer outcomes. From a clinical perspective, primary care physicians may wish to focus increased effort on early cancer detection in African American patients to address disparities in stage at diagnosis, and on referral patterns that incorporate performance measures, which may optimize cancer care and outcomes. From a policy standpoint, this study augments the impetus for validated, public reporting of quality measures for cancer care. If these results are found to be generalizable to other cancer care settings, then patients, referring physicians, health care organizations, and policy-makers should place a premium on reliable facility-level quality reporting in order to maximize equal benefits for all patients.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health Grant Number 1 P20 RR018787 from the Institutional Development Award (IDeA) Program of the National Center for Research Resources and by the Agency for Healthcare Research and Quality under Ruth L. Kirschstein National Research Service Award number T32HS000070.
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
There are no financial disclosures from any of the authors.
Contributor Information
Tracy Onega, The Dartmouth Institute for Health Policy and Clinical Practice, Dartmouth Medical School; Department of Community and Family Medicine, Dartmouth Medical School; Norris Cotton Cancer Center.
Eric J. Duell, The Dartmouth Institute for Health Policy and Clinical Practice, Dartmouth Medical School; Department of Community and Family Medicine, Dartmouth Medical School; Norris Cotton Cancer Center; Unit of Nutrition, Environment and Cancer, Cancer Epidemiology Research Programme, Catalan Institute of Oncology (ICO), Barcelona, Spain.
Xun Shi, Department of Geography, Dartmouth College.
Eugene Demidenko, Department of Community and Family Medicine, Dartmouth Medical School; Norris Cotton Cancer Center.
David C. Goodman, The Dartmouth Institute for Health Policy and Clinical Practice, Dartmouth Medical School; Department of Community and Family Medicine, Dartmouth Medical School.
REFERENCES
- 1.Medicine Io. Unequal Treatment: confronting racial and ethnic disparities in health care. National Academy Press; 2002. [PMC free article] [PubMed] [Google Scholar]
- 2.Escarce JJ. Racial and ethnic disparities in access to and quality of health care. Robert Wood Johnson Foundation. 2007 Research Synthesis Report No. 12(September). [PubMed]
- 3.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. Jama. 2002;287(16):2106–2113. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 4.Hodgson DC, Fuchs CS, Ayanian JZ. Impact of patient and provider characteristics on the treatment and outcomes of colorectal cancer. J Natl Cancer Inst. 2001;93(7):501–515. doi: 10.1093/jnci/93.7.501. [DOI] [PubMed] [Google Scholar]
- 5.Baicker K, Chandra A, Skinner JS, Wennberg JE. Who You Are And Where You Live: How Race And Geography Affect The Treatment Of Medicare Beneficiaries. Health Aff (Millwood) 2004 doi: 10.1377/hlthaff.var.33. [DOI] [PubMed] [Google Scholar]
- 6.Trivedi AN, Zaslavsky AM, Schneider EC, Ayanian JZ. Trends in the quality of care and racial disparities in Medicare managed care. N Engl J Med. 2005;353(7):692–700. doi: 10.1056/NEJMsa051207. [DOI] [PubMed] [Google Scholar]
- 7.Trivedi AN, Zaslavsky AM, Schneider EC, Ayanian JZ. Relationship between quality of care and racial disparities in Medicare health plans. Jama. 2006;296(16):1998–2004. doi: 10.1001/jama.296.16.1998. [DOI] [PubMed] [Google Scholar]
- 8. [accessed 7/24/09]; http://seer.cancer.gov/about/
- 9. [accessed 7/24/09]; http://seer.cancer.gov/csr/1975_2002/results_merged/topic_age_dist.pdf.
- 10.Onega TDE, Shi X, Demidenko E, Goodman D. Determinants of NCI Cancer Center attendance among Medicare beneficiaries with lung, breast, colorectal, or prostate cancer. J Gen Intern Med. 2008;24(2):205–210. doi: 10.1007/s11606-008-0863-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrag D, Bach PB, Dahlman C, Warren JL. Identifying and measuring hospital characteristics using the SEER-Medicare data and other claims-based sources. Med Care. 2002;40(8 Suppl):IV-96–IV-103. doi: 10.1097/00005650-200208001-00013. [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Iezzoni LI, Foley SM, Daley J, Hughes J, Fisher ES, Heeren T. Comorbidities, complications, and coding bias. Does the number of diagnosis codes matter in predicting in-hospital mortality? Jama. 1992;267(16):2197–2203. doi: 10.1001/jama.267.16.2197. [DOI] [PubMed] [Google Scholar]
- 14.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 15.Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care. 2002;40(8 Suppl):IV-26–IV-35. doi: 10.1097/00005650-200208001-00004. [DOI] [PubMed] [Google Scholar]
- 16.Onega T, Duell EJ, Shi X, Wang D, Demidenko E, Goodman D. Geographic access to cancer care in the U.S. Cancer. 2008;112(4):909–918. doi: 10.1002/cncr.23229. [DOI] [PubMed] [Google Scholar]
- 17.Onega TDE, Goodman DC, Demidenko E, Shi X. The influence of NCI Cancer Center attendance on mortality for lung, breast, colorectal, and prostate cancer patients. Medical Care Res and Review. 2009 doi: 10.1177/1077558709335536. In Press: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clegg LX, Li FP, Hankey BF, Chu K, Edwards BK. Cancer survival among US whites and minorities: a SEER (Surveillance, Epidemiology, and End Results) Program population-based study. Arch Intern Med. 2002;162(17):1985–1993. doi: 10.1001/archinte.162.17.1985. [DOI] [PubMed] [Google Scholar]
- 19.Dignam JJ. The ongoing search for the sources of the breast cancer survival disparity. J Clin Oncol. 2006;24(9):1326–1328. doi: 10.1200/JCO.2005.04.8561. [DOI] [PubMed] [Google Scholar]
- 20.Eley JW, Hill HA, Chen VW, Austin DF, Wesley MN, Muss HB, et al. Racial differences in survival from breast cancer. Results of the National Cancer Institute Black/White Cancer Survival Study. Jama. 1994;272(12):947–954. doi: 10.1001/jama.272.12.947. [DOI] [PubMed] [Google Scholar]
- 21.Farkas A, Marcella S, Rhoads GG. Ethnic and racial differences in prostate cancer incidence and mortality. Ethn Dis. 2000;10(1):69–75. [PubMed] [Google Scholar]
- 22.Godley PA, Schenck AP, Amamoo MA, Schoenbach VJ, Peacock S, Manning M, et al. Racial differences in mortality among Medicare recipients after treatment for localized prostate cancer. J Natl Cancer Inst. 2003;95(22):1702–1710. doi: 10.1093/jnci/djg094. [DOI] [PubMed] [Google Scholar]
- 23.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 24.Marcella S, Miller JE. Racial differences in colorectal cancer mortality. The importance of stage and socioeconomic status. J Clin Epidemiol. 2001;54(4):359–366. doi: 10.1016/s0895-4356(00)00316-4. [DOI] [PubMed] [Google Scholar]
- 25.Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97(19):1407–1427. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 26.Roetzheim RG, Pal N, Gonzalez EC, Ferrante JM, Van Durme DJ, Krischer JP. Effects of health insurance and race on colorectal cancer treatments and outcomes. Am J Public Health. 2000;90(11):1746–1754. doi: 10.2105/ajph.90.11.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roetzheim RG, Pal N, van Durme DJ, Wathington D, Ferrante JM, Gonzalez EC, et al. Increasing supplies of dermatologists and family physicians are associated with earlier stage of melanoma detection. J Am Acad Dermatol. 2000;43(2 Pt 1):211–218. doi: 10.1067/mjd.2000.106242. [DOI] [PubMed] [Google Scholar]
- 28.Schrag D, Rifas-Shiman S, Saltz L, Bach PB, Begg CB. Adjuvant chemotherapy use for Medicare beneficiaries with stage II colon cancer. J Clin Oncol. 2002;20(19):3999–4005. doi: 10.1200/JCO.2002.11.084. [DOI] [PubMed] [Google Scholar]
- 29.Onega TDE, Shi X, Demidenko E, Gottlieb D, Goodman D. The influence of NCI Cancer Center attendance on mortality for lung, breast, colorectal, and prostate cancer patients. J Clin Oncol. 2008 doi: 10.1177/1077558709335536. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demissie K, Oluwole OO, Balasubramanian BA, Osinubi OO, August D, Rhoads GG. Racial differences in the treatment of colorectal cancer: a comparison of surgical and radiation therapy between Whites and Blacks. Ann Epidemiol. 2004;14(3):215–221. doi: 10.1016/j.annepidem.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Ellison GL, Warren JL, Knopf KB, Brown ML. Racial differences in the receipt of bowel surveillance following potentially curative colorectal cancer surgery. Health Serv Res. 2003;38(6 Pt 2):1885–1903. doi: 10.1111/j.1475-6773.2003.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gross CP, Smith BD, Wolf E, Andersen M. Racial disparities in cancer therapy: did the gap narrow between 1992 and 2002? Cancer. 2008;112(4):900–908. doi: 10.1002/cncr.23228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polite BN, Dignam JJ, Olopade OI. Colorectal cancer model of health disparities: understanding mortality differences in minority populations. J Clin Oncol. 2006;24(14):2179–2187. doi: 10.1200/JCO.2005.05.4775. [DOI] [PubMed] [Google Scholar]
- 34.Potosky AL, Harlan LC, Kaplan RS, Johnson KA, Lynch CF. Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer. J Clin Oncol. 2002;20(5):1192–1202. doi: 10.1200/JCO.2002.20.5.1192. [DOI] [PubMed] [Google Scholar]
- 35.Ayanian JZ, Zaslavsky AM, Fuchs CS, Guadagnoli E, Creech CM, Cress RD, et al. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol. 2003;21(7):1293–1300. doi: 10.1200/JCO.2003.06.178. [DOI] [PubMed] [Google Scholar]
- 36.Schrag D, Cramer LD, Bach PB, Begg CB. Age and adjuvant chemotherapy use after surgery for stage III colon cancer. J Natl Cancer Inst. 2001;93(11):850–857. doi: 10.1093/jnci/93.11.850. [DOI] [PubMed] [Google Scholar]
- 37.Schrag D, Gelfand SE, Bach PB, Guillem J, Minsky BD, Begg CB. Who gets adjuvant treatment for stage II and III rectal cancer? Insight from surveillance, epidemiology, and end results--Medicare. J Clin Oncol. 2001;19(17):3712–3718. doi: 10.1200/JCO.2001.19.17.3712. [DOI] [PubMed] [Google Scholar]
- 38.Baldwin LM, Taplin SH, Friedman H, Moe R. Access to multidisciplinary cancer care: is it linked to the use of breast-conserving surgery with radiation for early-stage breast carcinoma? Cancer. 2004;100(4):701–709. doi: 10.1002/cncr.20030. [DOI] [PubMed] [Google Scholar]
- 39.Dominitz JA, Samsa GP, Landsman P, Provenzale D. Race, treatment, and survival among colorectal carcinoma patients in an equal-access medical system. Cancer. 1998;82(12):2312–2320. doi: 10.1002/(sici)1097-0142(19980615)82:12<2312::aid-cncr3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 40.Mulligan CR, Meram AD, Proctor CD, Wu H, Zhu K, Marrogi AJ. Unlimited access to care: effect on racial disparity and prognostic factors in lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(1):25–31. doi: 10.1158/1055-9965.EPI-05-0537. [DOI] [PubMed] [Google Scholar]
- 41.Taylor AJ, Meyer GS, Morse RW, Pearson CE. Can characteristics of a health care system mitigate ethnic bias in access to cardiovascular procedures? Experience from the Military Health Services System. J Am Coll Cardiol. 1997;30(4):901–907. doi: 10.1016/s0735-1097(97)00271-4. [DOI] [PubMed] [Google Scholar]
- 42.Barnato AE, Lucas FL, Staiger D, Wennberg DE, Chandra A. Hospital-level racial disparities in acute myocardial infarction treatment and outcomes. Med Care. 2005;43(4):308–319. doi: 10.1097/01.mlr.0000156848.62086.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Groeneveld PW, Laufer SB, Garber AM. Technology diffusion, hospital variation, and racial disparities among elderly Medicare beneficiaries: 1989–2000. Med Care. 2005;43(4):320–329. doi: 10.1097/01.mlr.0000156849.15166.ec. [DOI] [PubMed] [Google Scholar]
- 44.Skinner J, Chandra A, Staiger D, Lee J, McClellan M. Mortality after acute myocardial infarction in hospitals that disproportionately treat black patients. Circulation. 2005;112(17):2634–2641. doi: 10.1161/CIRCULATIONAHA.105.543231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birkmeyer NJ, Goodney PP, Stukel TA, Hillner BE, Birkmeyer JD. Do cancer centers designated by the National Cancer Institute have better surgical outcomes? Cancer. 2005;103(3):435–441. doi: 10.1002/cncr.20785. [DOI] [PubMed] [Google Scholar]
- 46.Shavers VL, Harlan LC, Stevens JL. Racial/ethnic variation in clinical presentation, treatment, and survival among breast cancer patients under age 35. Cancer. 2003;97(1):134–147. doi: 10.1002/cncr.11051. [DOI] [PubMed] [Google Scholar]
- 47.Chu KC, Lamar CA, Freeman HP. Racial disparities in breast carcinoma survival rates: seperating factors that affect diagnosis from factors that affect treatment. Cancer. 2003;97(11):2853–2860. doi: 10.1002/cncr.11411. [DOI] [PubMed] [Google Scholar]
- 48.Maggard MA, Thompson JE, Ko CY. Why do breast cancer mortality rates vary across states? Am Surg. 2003;69(1):59–62. [PubMed] [Google Scholar]
- 49.Morris AM, Billingsley KG, Baxter NN, Baldwin LM. Racial disparities in rectal cancer treatment: a population-based analysis. Arch Surg. 2004;139(2):151–155. doi: 10.1001/archsurg.139.2.151. discussion 56. [DOI] [PubMed] [Google Scholar]
- 50.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341(16):1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]


