Abstract
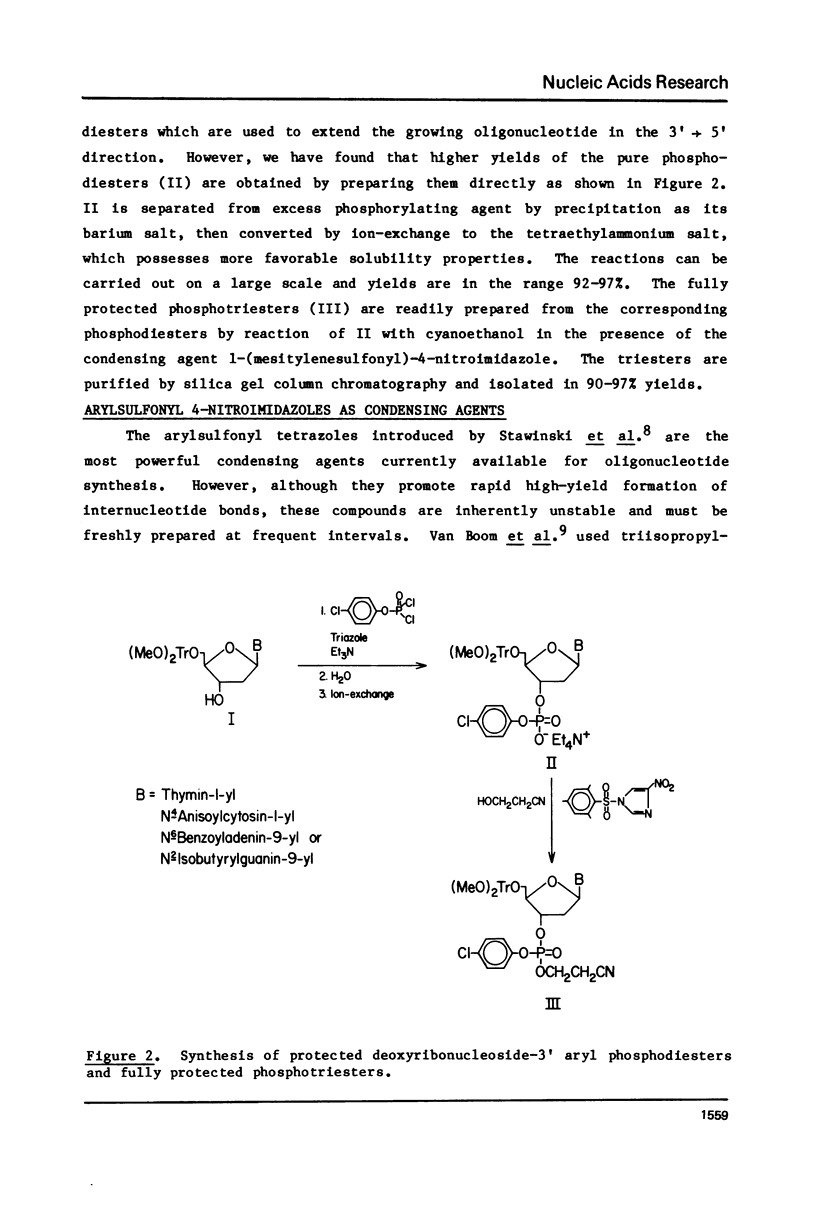
Several modifications have been incorporated into the phosphotriester strategy for chemical synthesis of oligodeoxyribonucleotides. These include high-yield methods of preparation and isolation of O5', N-protected deoxyribonucleoside-3' p-chlorophenyl phosphates which serve as key intermediates, and the elimination of some superfluous manipulation and purification steps commonly used in the process of synthesizing oligonucleotide blocks. In addition, two new arylsulfonyl nitroimidazole derivatives have been prepared and found to be highly effective agents for internucleotide bond formation. These techniques have been applied in construction of the iconsamer d(G-C-C-A-T-T-T-T-A-C-C-A-T-T-C-A-C-C-A)-rC, equivalent to a ribonucleotide sequence located at both the 5' and 3' ends of Rous sarcoma virus 35S RNA.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamiak R. W., Barciszewska M. Z., Biala E., Grzéskowiak K., Kierzek R., Kraszewski A., Markiewicz W. T., Wiewiórowski M. Nucleoside-3'-phosphotriesters as key intermediates for the oligoribonucleotide synthesis. III. An improved preparation of nucleoside 3'-phosphotriesters, their 1H NMR characterization and new conditions for removal of 2-cyanoethyl group. Nucleic Acids Res. 1976 Dec;3(12):3397–3408. doi: 10.1093/nar/3.12.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asteriadis G. T., Armbruster M. A., Gilham P. T. Separation of oligonucleotides, nucleotides, and nucleosides on columns of polystyrene anion-exchangers with solvent systems containing ethanol. Anal Biochem. 1976 Jan;70(1):64–74. doi: 10.1016/s0003-2697(76)80048-6. [DOI] [PubMed] [Google Scholar]
- Büchi H., Khorana H. G. CV. Total synthesis of the structural gene for an alanine transfer ribonucleic acid from yeast. Chemical synthesis of an icosadeoxyribonucleotide corresponding to the nucleotide sequence 31 to 50. J Mol Biol. 1972 Dec 28;72(2):251–288. doi: 10.1016/0022-2836(72)90148-9. [DOI] [PubMed] [Google Scholar]
- Cashion P., Notman H., Sathe G., Cadger T., Tranquilla T. Improved synthesis of N4-anisoyldeoxycytidine using Bio-Rex 5 columns. J Chromatogr. 1977 Jun 1;136(1):159–161. doi: 10.1016/s0021-9673(00)83005-8. [DOI] [PubMed] [Google Scholar]
- Catlin J. C., Cramer F. Deoxy oligonucleotide synthesis via the triester method. J Org Chem. 1973 Jan 26;38(2):245–250. doi: 10.1021/jo00942a011. [DOI] [PubMed] [Google Scholar]
- Donelson J. E., Barrell B. G., Weith H. L. The use of primed synthesis by DNA polymerase I to study an intercistronic sequence of phiX-174 DNA. Eur J Biochem. 1975 Oct 15;58(2):383–395. doi: 10.1111/j.1432-1033.1975.tb02385.x. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Maxam A. M., Gilbert W. Rous sarcoma virus genome is terminally redundant: the 5' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):989–993. doi: 10.1073/pnas.74.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura K., Hirose T., Crea R., Riggs A. D., Heyneker H. L., Bolivar F., Boyer H. W. Expression in Escherichia coli of a chemically synthesized gene for the hormone somatostatin. Science. 1977 Dec 9;198(4321):1056–1063. doi: 10.1126/science.412251. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Zamecnik P. C., Weith H. L. Rous sarcoma virus genome is terminally redundant: the 3' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):994–998. doi: 10.1073/pnas.74.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sninsky J. J., Last J. A., Gilham P. T. The use of terminal blocking groups for the specific joining of oligonucleotides in RNA ligase reactions containing equimolar concentrations of acceptor and donor molecules. Nucleic Acids Res. 1976 Nov;3(11):3157–3166. doi: 10.1093/nar/3.11.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood A. K., Narang S. A. A rapid and convenient synthesis of poly-thymidylic acid by the modified triester approach. Nucleic Acids Res. 1977 Aug;4(8):2757–2765. doi: 10.1093/nar/4.8.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawinski J., Hozumi T., Narang S. A., Bahl C. P., Wu R. Arylsulfonyltetrazoles, new coupling reagents and further improvements in the triester method for the synthesis of deoxyribooligonucleotides. Nucleic Acids Res. 1977 Feb;4(2):353–371. doi: 10.1093/nar/4.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C., Cameron V. Equimolar addition of oligoribonucleotides with T4 RNA ligase. Nucleic Acids Res. 1977 Jan;4(1):85–98. doi: 10.1093/nar/4.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boom J. H., Burgers P. M., van der Marel G., Verdegaal C. H., Wille G. Synthesis of oligonucleotides with sequences identical with or analogous to the 3'-end of 16S ribosomal RNA of Escherichia coli: preparation of A-C-C-U-C-C via the modified phosphotriester method. Nucleic Acids Res. 1977 Apr;4(4):1047–1063. doi: 10.1093/nar/4.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]



