Abstract
The proper timing of flowering is of crucial importance for reproductive success of plants. Regulation of flowering is orchestrated by inputs from both environmental and endogenous signals such as daylength, light quality, temperature and hormones, and key flowering regulators construct several parallel and interactive genetic pathways. This integrative regulatory network has been proposed to create robustness as well as plasticity of the regulation. Although knowledge of key genes and their regulation has been accumulated, there still remains much to learn about how they are organized into an integrative regulatory network. Here, we have analyzed the CRYPTIC PRECOCIOUS (CRP) gene for the Arabidopsis counterpart of the MED12 subunit of the Mediator. A novel dominant mutant, crp-1D, which causes up-regulation of SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1), FRUITFULL (FUL) and APETALA1 (AP1) expression in a FLOWERING LOCUS T (FT)-dependent manner, was identified in an enhancer screen of the early-flowering phenotype of 35S::FT. Genetic and molecular analysis of both crp-1D and crp loss-of-function alleles showed that MED12/CRP is required not only for proper regulation of SOC1, FUL and AP1, but also for up-regulation of FT, TWIN SISTER OF FT (TSF) and FD, and down-regulation of FLOWERING LOCUS C (FLC). These observations suggest that MED12/CRP is a novel flowering regulator with multiple regulatory target steps both upstream and downstream of the key flowering regulators including FT florigen. Our work, taken together with recent studies of other Mediator subunit genes, supports an emerging view that the Mediator plays multiple roles in the regulation of flowering.
Keywords: Arabidopsis, Flowering, FLOWERING LOCUS C, FLOWERING LOCUS T, Mediator, MED12
The nucleotide sequences reported in this paper have been submitted to DDBJ under accession numbers: MED12/CCT/CRP locus (AB690343) and MED12/CCT/CRP cDNA (AB690341 and AB690342).
Introduction
The plant life cycle is divided into distinct developmental phases by the morphological and functional features of the organs formed at the flank of the shoot apical meristem (SAM) (Araki 2001, Poethig 2003, Bäurle and Dean 2006). The proper timing of the transition from leaf-forming vegetative phase to flower-forming reproductive phase, called flowering, is especially important for reproductive success. Studies of Arabidopsis (Arabidopsis thaliana) have shown that regulation of flowering is orchestrated by inputs of multiple environmental and endogenous signals such as daylength, light quality, temperature and hormones, and that flowering time genes construct several parallel and interactive genetic pathways (Simpson and Dean 2002, Boss et al. 2004, Fornara et al. 2010). This integrative regulatory network has been proposed to create robustness as well as plasticity to increase fitness under genetic and environmental perturbation (Wilczek et al. 2009).
Several genes represent key nodes in the regulatory network. FLOWERING LOCUS T (FT) encodes a small 20 kDa protein, which belongs to the phosphatidylethanolamine-binding protein (PEBP)/Raf kinase inhibitor protein (RKIP) family, and plays a pivotal role in promoting flowering, integrating signals from converging pathways (Kardailsky et al. 1999, Kobayashi et al. 1999). Expression of FT is strictly controlled by transcription factors and chromatin-associated proteins in the leaf vasculature (Takada and Goto 2003, Kobayashi and Weigel 2007, Adrian et al. 2010, Imaizumi, 2010). A B-box zinc-finger protein CONSTANS (CO) plays a central role in FT regulation in the photoperiod pathway (Kardailsky et al. 1999, Kobayashi et al. 1999, Samach et al. 2000). The transcriptional and post-translational controls of CO are important for monitoring the seasonal daylength changes (Suárez-López et al. 2001, Yanovsky and Kay 2002, Valverde et al. 2004, Kobayashi and Weigel 2007). As the florigen, FT protein is translated in the leaf, moves via phloem to the SAM, and activates several positive floral regulators to initiate the formation of flowers (Corbesier et al. 2007, Jaeger and Wigge 2007, Mathieu et al. 2007, Notaguchi et al. 2008). FT forms a protein complex involving a bZIP (basic region/leucine zipper) transcription factor FD, and directly activates APETALA1 (AP1) expression (Abe et al. 2005, Wigge et al. 2005), and either directly or indirectly activates FRUITFULL (FUL) expression (Teper-Bamnolker and Samach 2005). FT also promotes expression of LEAFY (LFY) via up-regulation of SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1) (Searle et al. 2006, Liu et al. 2008). A MADS box transcription factor, FLOWERING LOCUS C (FLC), is a potent repressor of FT, TWIN SISTER OF FT (TSF), FD and SOC1, and thereby negatively regulates both florigen production and response (Searle et al. 2006). FLC expression is regulated by histone modification in response to vernalization and autonomous pathways (Bäurle and Dean 2006, Schmitz and Amasino 2007). Although knowledge of the regulation of key genes has been accumulated, there still remains much to learn about how they are organized into an integrative regulatory network.
Mediator is an evolutionarily conserved multiprotein complex in eukaryotes, whose biochemistry and functional roles have been extensively studied in yeast and animals. Mediator plays numerous roles in controlling the function of RNA polymerase II pre-initiation complex, and the structural plasticity of the multisubunit complex enables Mediator to regulate virtually all protein-coding genes (Malik and Roeder 2005). Genetic and molecular studies collectively suggest that the Mediator complex is essential for the proper development of the organism. Mediator complex consists of a ‘core module’ and ‘CDK8 module’. The CDK8 module contains cyclin-dependent kinase 8 (CDK8), cyclin C (CycC), Mediator complex subunit (MED) 12 (MED12) and MED13. Of the four subunits, MED12 and MED13 have been shown to be important for proper control of developmental programs in animals such as zebrafish, Drosophila and Caenorhabditis elegans (Treisman 2001, Janody et al. 2003, Hong et al. 2005, Yoda et al. 2005, Rau et al. 2006, Henteges 2011). Recently, plant Mediator subunits have been identified (Bäckström et al. 2007, Bourbon 2008), and the physiological roles of some subunits have been revealed (Autran et al. 2002, Kidd et al. 2009, Gillmor et al. 2010, Ito et al. 2011, Kidd et al. 2011, Kim et al. 2011). For example, a subunit of the core module, MED25, which was originally described as PHYTOCHROME AND FLOWERING TIME 1 (PFT1), was shown to act in jasmonate signaling and in the light quality pathway of flowering acting downstream of phytochrome B (phyB) to regulate expression of FT (Cerdán and Chory 2003, Wollenberg et al. 2008, Elfving et al. 2011).
In this study, we identified and investigated the CRYPTIC PRECOCIOUS (CRP) gene for the Arabidopsis counterpart of MED12. We isolated a novel dominant mutant, crp-1D, as a genetic modifier of the precocious-flowering phenotype in 35S::FT. Genetic and molecular analyses of both dominant crp-1D and recessive crp loss-of-function alleles suggest that MED12/CRP is a novel flowering regulator with multiple regulatory target steps both upstream and downstream of the key flowering regulators including FT florigen. Furthermore, our present work and previous works (Gillmor et al. 2010, Ito et al. 2011) strongly suggest that MED12/CENTER CITY (CCT)/CRP and MED13/GRAND CENTRAL (GCT)/MACCHI-BOU 2 (MAB2) proteins act closely together in the same pathways to regulate various aspects of plant development from embryogenesis to flowering and floral morphogenesis.
Results
cryptic precocious-1D, a dominant enhancer mutation of 35S::FT
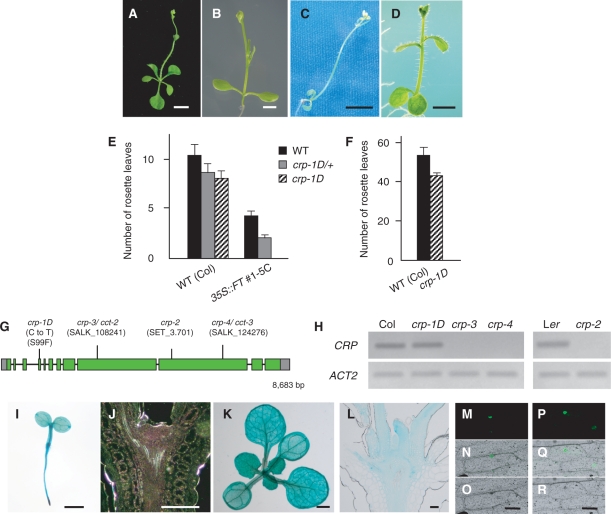
To understand the regulatory mechanisms acting downstream of the floral integrator FT, we carried out ethylmethane sulfonate (EMS) mutagenesis of a weak line of 35S::FT and screened for mutations that affect its precocious-flowering phenotype. We isolated one dominant mutation, cryptic precocious-1D (crp-1D), which strongly enhanced the precocious-flowering phenotype to skip the vegetative phase (Fig. 1A–C, E, Supplementary Table S1). The phenotype of crp-1D; 35S::FT plants was very similar to that of soc1-101D; 35S::FT plants (Fig. 1D), implying that crp-1D mutation causes up-regulation of genes acting downstream of FT, such as SOC1.
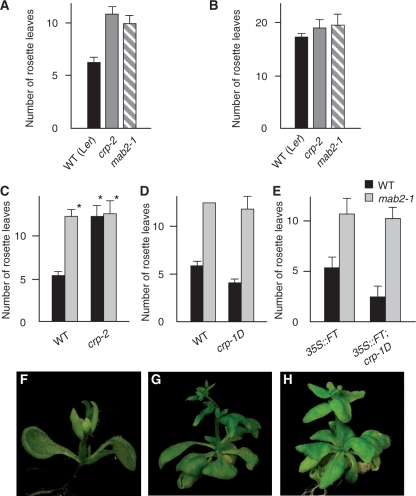
Fig. 1.
Flowering phenotype of the crp-1D mutant and gene structure and expression patterns of CRP. (A–D) Effect of crp-1D and soc1-101D on the early-flowering phenotype of 35S::FT. 35S::FT #1-5C (A), 35S::FT #1-5C/−; crp-1D/+ (B), 35S::FT #1-5C; crp-1D (C) and 35S::FT #1-5C; soc1-101D plants (D). ‘Transgene symbol/−’ and ‘mutation symbol/+’ indicate the hemizygote and heterozygote, respectively. (E) Flowering time of 35S::FT #1-5C/−; crp-1D/+ plants under long-day conditions. There is a statistically significant difference (Student’s t-test, P < 0.005) with CRP+ (solid bars). (F) Flowering time of the crp-1D mutant under short-day conditions. There is a statistically significant difference (Student’s t-test, P < 0.005) between the two genotypes. (G) CRP gene structure and positions of mutations and T-DNA or Ds insertions in crp alleles. Boxes and lines indicate exons and introns of CRP, respectively; gray and green boxes represent untranslated regions and coding regions, respectively. (H) Semi-quantitative RT–PCR analysis of CRP expression in the wild type and crp mutants. Plants were harvested on day 10 (Col background; left panel) or 7 (Ler background; right panel). (I–L) Spatial expression patterns of CRP. Whole-mount preparation and longitudinal section through the SAM of 3-day-old (I, J) and 10-day-old (K, L) CRP::GUS seedlings. Plants were grown under long-day conditions and subjected to GUS staining for 12–48 h. (M–R) Subcellular localizaton of CRP and crp-1D proteins. Onion epidermal cells were bombarded with GFP fusion constructs, 35S::CRP-GFP (M–O) and 35S::crp-1D-GFP (P–R). Dark field images (M, P), bright field images (N, Q) and merged images (O, R). Scale bars: 5 mm in A and C, 2 mm in B and D, 1 mm in I and K, and 0.1 mm in J, L, O and R. In E and F, the numbers of rosette leaves are the average of at least 11 plants. Error bars indicate the SD. Additional data and statistics of the data are summarized in Supplementary Tables S1 and S2.
In contrast to the strong enhancement of the 35S::FT phenotype, crp-1D single mutation in the wild-type background had a moderate effect, leading to a slightly early-flowering phenotype in both inductive long-day and non-inductive short-day conditions (Fig. 1E, F, Supplementary Table S2). crp-1D also showed a dominant effect in the wild-type background (Fig. 1E, Supplementary Table S1).
Identification of the CRP gene
To obtain knowledge of the molecular nature of the CRYPTIC PRECOCIOUS (CRP) gene, map-based cloning was performed using crp-1D. The CRP locus was mapped to a 33.5 kb interval between F5l10 and F6N23 at the top of chromosome 4 where six genes are predicted (Supplementary Fig. S1). The only non-synonymous substitution found in the coding region of the six genes in the crp-1D mutant was a C-to-T substitution in At4g00450. No obvious differences in expression levels of the other genes were observed, indicating that nucleotide substitutions in the intergenic regions causing elevated or ectopic expression would be unlikely to be responsible for the crp-1D phenotype. Thus, we concluded that At4g00450 is the CRP gene. CRP was recently identified independently as the CENTER CITY (CCT) gene, which is required for proper embryogenesis (Gillmor et al. 2010). By cDNA cloning, we found that the actual coding region of CRP is comprised of 12 exons spanning 8.7 kb (Fig. 1G, DDBJ accession No. AB690343) and is different from the publicly available annotated unit adopted for CCT (encoding a protein of 2,144 amino acids; Gillmor et al. 2010). The CRP gene encodes a protein of 2,235 amino acids, which corresponds to the Arabidopsis counterpart of MED12 protein (Gillmor et al. 2010), a subunit of the transcriptional Mediator complex (Bäckström et al. 2007) (Supplementary Fig. S2). crp-1D mutation caused an amino acid substitution from serine (S) to phenylalanine (F) at position 99 (Fig. 1G, Supplementary Fig. S3). crp-1D mutation had no effect on the mRNA level (Fig. 1H).
Spatial expression patterns of CRP were examined using transgenic plants expressing the β-glucuronidase (GUS) reporter gene under the control of a 2.0 kb genomic sequence upstream of the initiation codon (CRP::GUS). Ubiquitous expression was observed in seedlings and young plants, with higher levels in vascular tissue and the shoot apex (Fig. 1I–L). In mature plants, strong expression was observed in young floral buds and developing floral organs as described below. To examine the subcellular localization of CRP protein, green fluorescent proteins (GFPs) fused to full-length wild-type CRP (CRP–GFP) and S99F mutant CRP (crp-1D–GFP) were expressed in onion epidermal cells. Both CRP–GFP and crp-1D–GFP fusion proteins were localized in the nuclear region in a similar manner (Fig. 1M–R).
Effects of crp loss-of-function mutations on flowering time
To investigate whether the CRP gene has any role in the flowering process, we obtained a Ds-transposon insertion line in the Landsberg erecta (Ler) background (crp-2) and two T-DNA insertion lines in the Columbia (Col) background (crp-3 and crp-4) (Fig. 1G). Reverse transcription–PCR (RT–PCR) analysis revealed no accumulation of the CRP full-length transcript in crp-2, crp-3 or crp-4 plants (Fig. 1H). As homozygous crp loss-of-function plants were sterile, we analyzed the phenotypes in homozygous mutants segregating from self-fertilized heterozygous crp plants.
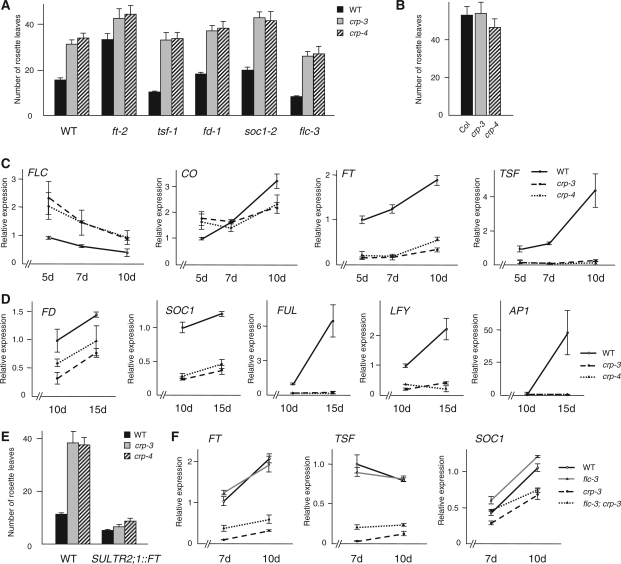
crp loss-of-function mutants flowered much later than the wild type in long-day conditions (Fig. 2A, Supplementary Table S2) (see also Gillmor et al. 2010), whereas no significant difference from the wild type was observed in short-day conditions (Fig. 2B, Supplementary Table S2). This phenotype is comparable with that of loss-of-function mutants of genes in the photoperiod pathway, such as ft, co and pft1/med25 (Koornneef et al. 1991, Cerdán and Chory 2003), suggesting that CRP is likely to be a promoter of flowering acting in the photoperiod pathway.
Fig. 2.
Effect of crp loss-of-function mutations on the flowering phenotype. (A) Flowering time of crp loss-of-function mutants and flowering-time mutants with wild-type CRP+ or crp loss-of-function alleles under long-day conditions. (B) Flowering time of crp loss-of-function mutants under short-day conditions. (C) CO, FLC, FT and TSF expression in wild-type Col, crp-3 and crp-4 plants. Aerial parts of seedlings grown under long-day conditions were harvested on days 5, 7 and 10 for qRT–PCR analysis. (D) FD, SOC1, FUL, LFY and AP1 expression in wild-type Col, crp-3 and crp-4 plants. Shoot apical regions of seedlings grown under long-day conditions were harvested on days 10 and 15 for qRT–PCR analysis. (E) Flowering time of crp loss-of-function mutants with or without the SULTR2;1::FT transgene. There is a statistically significant difference (Student’s t-test, P < 0.005) between CRP+ and crp in the SULTR2;1::FT background. (F) FT, TSF and SOC1 expression in wild-type Col, flc-3, crp-3 and flc-3; crp-3 plants. Plants were grown under long-day conditions and aerial parts were harvested on days 7 and 10 for qRT–PCR analysis. In A, B and E, the numbers of rosette leaves are the average of at least seven plants. Error bars indicate the SD. Additional data and statistics of the data are summarized in Supplementary Tables S2–S4. Error bars in C, D and F indicate the SEM (n = 9).
To explore further the molecular basis of the late-flowering phenotype in crp, we first examined expression levels of genes which are regulated by FT as well as genes with important roles in the FT-dependent flowering pathway, in crp loss-of-function mutants and in wild-type plants. While the mRNA level of CO, an upstream promoter of FT, remained unchanged, the mRNA level of FLC, a strong repressor of FT and TSF, was increased in crp-3 and crp-4 plants at every time point examined (Fig. 2C). Consistent with this, FT and TSF mRNA levels were much lower in crp loss-of-function mutants than in wild-type plants (Fig. 2C). The robust expression of the NRT1.7 gene in crp mutants, which has been reported as a marker gene of phloem companion cells of mature leaf minor veins (Fan et al. 2009), confirmed that the reduction of FT and TSF expression is likely to be due to the direct effect of crp mutation rather than the secondary effect of disturbed vascular patterning in crp (Supplementary Fig. S4A; details are described later). In the shoot apical region, expression of FD, encoding an FT partner bZIP transcription factor, and SOC1 and FUL, downstream targets of FT, were significantly reduced in crp mutants (Fig. 2D). This is again consistent with the increased level of FLC which is a negative regulator of FD and SOC1 expression in the shoot apex (Searle et al. 2006). That the reduced expression of FD, SOC1 and FUL was not the consequence of possible abnormal shoot apical morphology in the crp mutant or unequal collections of shoot apical samples among genotypes was confirmed by monitoring the expression level of the SHOOTMERISTEMLESS (STM) gene (Long et al. 1996) (Supplementary Fig. S4B). In accordance with the reduced expression of flowering promoters, LFY and AP1 expression was not induced in crp mutants even at day 15 when these floral meristem identity genes are up-regulated in wild-type plants (Fig. 2D).
Because CRP seems to act both upstream and downstream of the FT-dependent flowering pathway, we next analyzed the genetic interaction between CRP and FT. Since the 35S::FT transgene was silenced in F2 progeny from the cross between 35S::FT and crp T-DNA insertion alleles (data not shown), we instead used a SULTR2;1::FT transgenic line in which the FT gene is specifically expressed in the phloem (Abe et al. 2005) to examine whether the artificial expression of FT can suppress the late-flowering phenotype of crp loss-of-function mutants. Introduction of SULTR2;1::FT partially, but not completely, suppressed the late-flowering phenotype of crp-3 and crp-4 (Fig. 2E, Supplementary Table S3). This result is consistent with CRP controlling not only the expression of FT, but also the expression of downstream genes of FT in an, in part, FT-dependent manner. This was further supported by the observation that crp; ft-2 double mutants flowered later than the ft-2 single mutant (Fig. 2A, Supplementary Table S4). A tsf loss-of-function mutant (tsf-1) had no effect on the flowering time of crp, whereas both fd and soc1 mutations showed an additive effect on the late-flowering phenotype of the crp single mutant (Fig. 2A, Supplementary Table S4).
To investigate whether the elevated expression level of FLC is responsible for the late-flowering phenotype of crp loss-of-function mutants, crp; flc-3 double mutants were compared with crp single mutants. The crp; flc-3 plants flowered earlier than crp single mutants under long-day conditions (Fig. 2A, Supplementary Table S4), suggesting that the increased expression of FLC does partially explain the flowering delay of crp mutants. This was further confirmed by the observation that the reduction of FT and TSF in the crp single mutant was partially restored in crp; flc-3 double mutant plants (Fig. 2F, Supplementary Fig. S5).
Effect of overexpression of CRP on flowering time
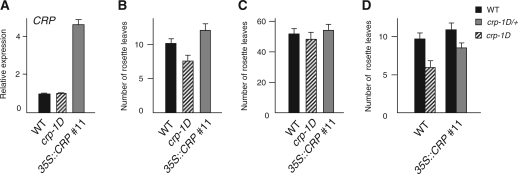
To understand further the function of CRP, we generated transgenic plants overexpressing CRP under the control of the 35S promoter (35S::CRP), and obtained 15 independent lines. Interestingly, the majority of 35S::CRP transgenic plants showed a flowering time phenotype either indistinguishable from that of wild-type plants or similar to that of crp loss-of-function mutants in both long-day and short-day conditions. In the latter case, the extent of delay in long days was not as severe as that of crp loss-of-function mutants (Fig. 3A–C, Supplementary Table S5). A similar observation has been reported for Drosophila kohtalo (kto), which is the Drosophila counterpart of CRP encoding MED12 (Janody et al. 2003). It is likely that the excessive amount of MED12 protein (CRP in Arabidopsis and kto in Drosophila) interferes with the proper formation of protein complex(es) that contain MED12. Consistently, expression levels of FT and TSF were reduced in the 35S::CRP plants as in the case of crp loss-of-function mutants (Supplementary Fig. S6).
Fig. 3.
CRP expression and flowering phenotype of CRP-overexpressing plants. (A) CRP expression levels in wild-type Col and CRP-overexpressing plants. Aerial parts of seedlings grown under long-day conditions were harvested on day 12 for qRT–PCR analysis. (B and C) Flowering time of wild-type Col and CRP-overexpressing plants under long-day (B) and short-day (C) conditions. There is a statistically significant difference (Student’s t-test, P < 0.005) between the wild type and 35S::CRP in B. (D) Flowering time of crp-1D plants with the 35S::CRP transgene under long-day conditions. There is a statistically significant difference (Student’s t-test, P < 0.005) between CRP+ (solid bar) and crp-1D in the 35S::CRP background. Error bars in A indicate the SE. In B–D, the numbers of rosette leaves are the average of at least 10 plants. Error bars indicate the SD. Additional data and statistics of the data are summarized in Supplementary Tables S5 and S6.
Based on the phenotypes of crp loss-of-function mutants and 35S::CRP transgenic plants, it is suggested that crp-1D acts as neither a dominant-negative mutation nor a gain-of-function mutation which increases protein stability as reported for other genes (El-Assal et al. 2001, Reed 2001, Dieterle et al. 2005). By taking advantage of 35S::CRP transgenic plants, we further addressed the question of whether the mutant crp-1D protein functions in the same molecular context as that of the wild-type CRP protein. If this is indeed the case, then increased amounts of wild-type CRP protein should attenuate the crp-1D phenotype. In contrast, there should be little effect if crp-1D protein functions in a different molecular context. As shown in Fig. 3D, the crp-1D phenotype was significantly attenuated by the introduction of the 35S::CRP transgene (see also Supplementary Table S6). Therefore, it is likely that crp-1D acts in the same molecular context as that which involves wild-type CRP, rather than acting as a ‘neomorphic’ allele whose activity is unrelated to normal CRP function. As described below, this was further supported by the observation that expression of the crp-1D phenotype required functional MED13/MAB2, another subunit functioning together with MED12/CRP (see Genetic interaction of MED12/CRP and MED13/MAB2 in the flowering process and auxin response).
Genetic interaction between the dominant crp-1D mutation and flowering-related genes
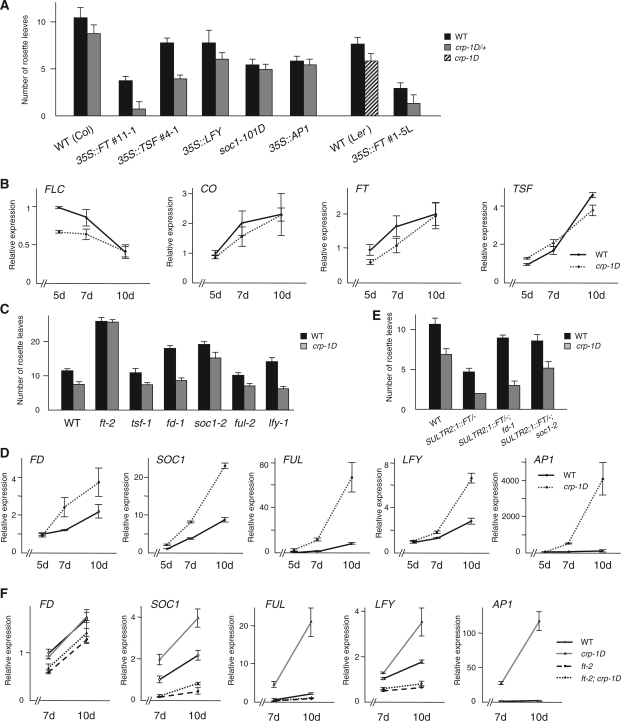
Since crp-1D is likely to act in the same molecular context as the wild-type CRP, it will shed some light on the normal CRP function. With this in mind, genetic and regulatory interactions between crp-1D and flowering-related genes were investigated. We first examined the effect of crp-1D mutation on the precocious-flowering phenotype of 35S::TSF. As expected from redundancy between FT and TSF, F1 progeny of crosses between crp-1D and 35S::TSF showed a much earlier flowering phenotype than 35S::TSF (Fig. 4A, Supplementary Table S1). Previously, it was reported that FT, TSF, SOC1 and LFY have both overlapping and independent functions such that soc1-101D and 35S::LFY, respectively, had additive effects on early-flowering phenotypes of 35S::FT or 35S::TSF (Kardailsky et al. 1999, Kobayashi et al. 1999, Moon et al. 2005, Yamaguchi et al. 2005). This raises the possibility that crp-1D may enhance the precocious-flowering phenotype of FT- or TSF-overexpressing plants via up-regulation of SOC1 or LFY. To test the interaction between crp-1D and these genes, we generated plants which have a crp-1D mutation with soc1-101D or 35S::LFY. F1 progeny of a cross between crp-1D and 35S::LFY flowered slightly earlier than 35S::LFY (Fig. 4A, Supplementary Table S1). In contrast, crp-1D did not enhance the early-flowering phenotype of soc1-101D (Fig. 4A, Supplementary Table S1). In addition, no enhancement by crp-1D was observed for the early-flowering phenotype of 35S::AP1 (Fig. 4A, Supplementary Table S1). These results suggest that crp-1D may affect step(s) upstream of SOC1 and AP1.
Fig. 4.
Effect of crp-1D mutation on the flowering phenotype. (A) Flowering time of plants overexpressing flowering regulator genes with or without the dominant crp-1D mutation. There is a statistically significant difference (Student’s t-test, P < 0.005) with CRP+ (solid bars). (B) CO, FLC, FT and TSF expression levels in wild-type Col and crp-1D plants. Aerial parts of seedlings grown under long-day conditions were harvested on days 5, 7 and 10 for qRT–PCR analysis. (C) Flowering time of double mutant plants with the crp-1D mutation and loss-of-function mutation of flowering regulator genes. (D) FD, SOC1, FUL, LFY and AP1 expression levels in wild-type Col and crp-1D plants. Shoot apical regions of seedlings grown under long-day conditions were harvested on days 5, 7 and 10 for qRT–PCR analysis. (E) Flowering time of SULTR2;1::FT/−; crp-1D/+ plants with an fd-1 or soc1-2 mutation. (F) FD, SOC1, FUL, LFY and AP1 expression levels in wild-type Col, crp-1D, ft-2 and ft-2; crp-1D plants. Shoot apical region of seedlings grown under long-day conditions were harvested on days 7 and 10 for qRT–PCR analysis. In A, C and E, the numbers of rosette leaves are the average of at least eight plants. Error bars indicate the SD. Additional data and statistics of the data are summarized in Supplementary Tables S1, S7 and S8. Error bars in B, D and F indicate the SEM (n = 9).
We next compared the temporal regulation of FLC, CO, FT and TSF in crp-1D and wild-type plants. With the possible exception of FLC in young seedlings (5 d), no significant differences were observed (Fig. 4B). This suggests that crp-1D may have an effect on step(s) downstream of FT and/or TSF. To test for genetic interaction between crp-1D and FT and TSF, we generated crp-1D; ft-2 and crp-1D; tsf-1 double mutants. The early-flowering phenotype of crp-1D was suppressed by ft-2, but was not affected by tsf-1 in long-day conditions (Fig. 4C, Supplementary Table S7), suggesting that crp-1D affects flowering mainly in the FT-dependent pathway.
The results so far described prompted us to examine the relationship between crp-1D and genes acting at the shoot apex in steps downstream of FT, such as FD, SOC1 and FUL. The expression level of FD was not significantly affected in crp-1D (Fig. 4D), and fd-1 had little effect on the early-flowering phenotype of crp-1D (Fig. 4C, Supplementary Table S7). In addition, fd-1 only very weakly suppressed the early-flowering phenotype of crp-1D/+; SULTR2;1::FT/– (Fig. 4E, Supplementary Table S8). These results suggest that crp-1D is likely to regulate flowering independently of FD. In contrast, the expression level of SOC1 and FUL, both of which are regulatory targets of FT, was elevated in crp-1D at every time point tested (Fig. 4D). Consistently, the precocious-flowering phenotype of crp-1D plants was strongly suppressed by soc1-2 (Fig. 4C, Supplementary Table S7). In addition, soc1-2 suppressed the early-flowering phenotype of SULTR2;1::FT/–; crp-1D/+ plants (Fig. 4E, Supplementary Table S8). ful-2, which has little effect on flowering time in the wild-type background, did not delay flowering in crp-1D (Fig. 4C, Supplementary Table S7). These results suggest that crp-1D acts in the same pathway in which endogenous and overexpressed FT regulates SOC1 and FUL expression. This was further supported by the observation that up-regulation of SOC1 and FUL in crp-1D was strongly suppressed by introduction of ft-2, while the expression level of FD was little affected by genotypes (Fig. 4F). As expected from elevated SOC1 expression, levels of LFY and AP1 expression were increased in crp-1D compared with the wild type in an FT-dependent manner (Fig. 4D, F). Since lfy loss-of-function mutants (lfy-1) had no effect on the precocious-flowering phenotype of crp-1D (Fig. 4C, Supplementary Table S7), crp-1D may affect LFY (and AP1, as well) expression indirectly.
Genetic interaction between MED12/CRP and MED13/MAB2 in the flowering process and auxin response
It was reported that MED12 and MED13 act as a pair in the CDK8 Mediator kinase module in yeast and Drosophila (Samuelsen et al. 2003, Kornberg 2005, Bäckström et al. 2007, Loncle et al. 2007). Moreover, mutations in Drosophila MED12 and MED13 cause identical phenotypes across multiple traits, again supporting the idea that these two components act closely together in the same module of the Mediator (Treisman 2001, Janody et al. 2003). To test whether this is also true for Arabidopsis MED12/CRP and MED13/MACCHI-BOU2 (MAB2), we compared the flowering phenotype of mab2 loss-of-function mutant (mab2-1) with that of crp-2 in the Ler background. Similar to the crp-2 mutant, mab2-1 flowered later than the wild type in long-day conditions and no difference in flowering time was observed in short-day conditions (Fig. 5A, B, Supplementary Table S2). We further examined the genetic interaction between CRP and MAB2. In Drosophila, the phenotype of med12/kohtalo; med13/skuld double mutants was indistinguishable from that of either single mutant (Janody et al. 2003, Loncle et al. 2007). Similarly, the crp-2; mab2-1 double mutant and each parental single mutant showed the same phenotype in seedling morphology and flowering time in long-day conditions (Fig. 5C, Supplementary Table S9) (Ito et al. 2011). These observations suggest that MED12/CRP and MED13/MAB2 act closely together in the same pathway in Arabidopsis development. We further examined genetic interaction between crp-1D and mab2. mab2-1 completely suppressed the early-flowering phenotype of crp-1D and the precocious-flowering phenotype of 35S::FT/-; crp-1D/+ (Fig. 5D, E, F–H, Supplementary Table S10), indicating that crp-1D requires functional MED13/MAB2 for its effect on flowering. These results confirmed that MED13/MAB2 is required for crp-1D-mediated floral induction as well.
Fig. 5.
Effect of the mab2 loss-of-function mutation on the flowering time of crp mutants. (A and B) Flowering time of crp-2 and mab2-1 mutants under long-day (A) and short-day (B) conditions. (C) Flowering time of the crp-2 mutant with a wild-type MAB2+ or mab2 loss-of-function allele. There is no statistically significant difference (Student’s t-test, P > 0.5) among genotypes marked with an asterisk. (D) Flowering time of the crp-1D mutant with a wild-type MAB2+ or mab2 loss-of-function allele. (E) Flowering time of 35S::FT #1-5L/−; crp-1D L/+ plants with a wild-type MAB2+ or mab2 loss-of-function allele. (F–H) Effect of mab2-1 on the flowering phenotype of crp-1D plants. 35S::FT #1-5L/−; crp-1D L/+ (15 d old) (F), 35S::FT/−; crp-1D/+; mab2-1 (20 d old) (G) and crp-1D L; mab2-1 (30 d old) (H). Plants were grown under long-day conditions. In A, C and E, the numbers of rosette leaves are the average of at least 10, six and five plants, respectively. B and D include genotypes with two or three plants. Error bars in A–E indicate the SD, except for mab2-1 (n = 2) in D (left gray bar). Additional data and statistics of the data are summarized in Supplementary Tables S2, S9 and S10.
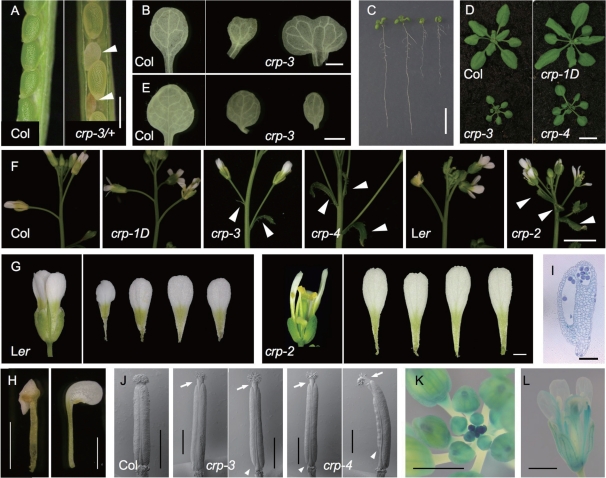
In addition to a late-flowering phenotype, crp loss-of-function mutants showed pleiotropic phenotypes. These include occasional embryo lethality, such that a certain fraction of seeds on the selfed crp/+ heterozygote were aborted (Fig. 6A), abnormal shape of cotyledons (Fig. 6B), delay in early growth (Fig. 6C), dwarfism (Fig. 6D), abnormal leaf shape and vascular patterning (Fig. 6E), ectopic bract-like organs subtending the first few flowers as reported for mab2 (Ito et al. 2011) (Fig. 6F), abnormal development of floral organs including chimerism (Fig. 6G, H), and sterility due to poor production of pollen grains and anther dehiscence (Fig. 6I). In accordance with the floral phenotype, strong expression was observed in young floral buds and developing floral organs (Fig. 6K, L). Interestingly, crp-1D showed the opposite effect in some of the seedling phenotypes (Fig. 6A, B). These phenotypes were in agreement with a recent report that center city-1 (cct-1), another loss-of-function allele of MED12, affected the timing of a variety of morphogenetic events, including embryogenesis, specification of peripheral–abaxial identity and vascular patterning (Gillmor et al. 2010), supporting the generally accepted concept that MED12 is involved in diverse developmental aspects through gene regulation.
Fig. 6.
Pleiotropic phenotype of crp mutants. (A) Opened siliques of wild-type Col (left) and crp-3/+ (right) plants. Arrowheads indicate aborted seeds. (B) Cotyledons from 10-day-old wild-type Col (left) and crp-3 (right) seedlings. (C) Eight-day-old seedlings of wild-type Col, crp-1D, crp-3 and crp-4 (from left to right). (D) Twenty-two-day-old wild-type Col (top left), crp-1D (top right), crp-3 (bottom left) and crp-4 (bottom right) plants. (E) First leaves from 10-day-old wild-type (left) and two crp-3 (right) seedlings. (F) First and second flowers with bract-like organs on the primary inflorescence shoot of wild-type Col, crp-1D, crp-3, crp-4, wild-type Ler and crp-2 (from left to right). Arrowheads indicate bract-like organs. (G) Flower and petals of wild-type Ler (left) and crp-2 (right). (H) Partial stamen-to-petal transformation observed in crp-3. (I) Longitudinal section through the anther of crp-2. (J) Scanning electron micrographs of pistils from wild-type Col (left), two crp-3 (middle) and two crp-4 (right) flowers. Arrows and arrowheads indicate elongated styles and basal internodes, respectively, of crp mutants. (K and L) Expression of CRP::GUS in inflorescence (K) and flower (L). Scale bars: 0.5 mm in A, 1 mm in B, E, G, H, J, K and L, 1 cm in C and D, 5 mm in F, 0.1 mm in I.
Interestingly, gynoecium morphology of crp loss-of-function mutants (Fig. 6J) resembles that of auxin-related mutants such as ettin (ett), namely shorter valves, longer style and elongated basal internode (Liu and Meyerowitz 1995, Nemhauser et al. 2000, Franks et al. 2002). A similar gynoecium phenotype was also observed in mab2 mutants. It was recently reported that double mutant combinations of mab2 and pinoid (pid), which abolishes directional flow of auxin in developing embryo (Robert and Offringa 2008), caused severe defects in organogenesis such that cotyledons and bilateral symmetry are completely missing (Ito et al. 2011). These observations prompted us to generate pid; crp double mutants. Similar to the pid-2; mab2-1 double mutant, seedlings of pid-2; crp-2, pid-3; crp-3 and pid-3; crp-4 double mutants completely lacked cotyledons (Fig. 7). These results suggest that MED12/CRP has a similar role in auxin-dependent organogenesis to that of MED13/MAB2, supporting the notion that these two act closely together possibly within the same module of the Mediator complex.
Fig. 7.
Phenotype of crp; pid seedlings. Five-day-old seedlings of wild-type Ler (A), pid-2 (B), crp-2 (C), pid-2; crp-2 (D), wild-type Col (E), pid-3 (F), crp-1D (G), pid-3; crp-1D (H), crp-3 (I), pid-3; crp-3 (J), crp-4 (K) and pid-3; crp-4 (L). Plants were grown under long-day conditions. Scale bars: 1 mm.
Discussion
In this study, we identified a dominant mutation, crp-1D, as a genetic modifier of the precocious-flowering phenotype of 35S::FT. Map-based cloning revealed that crp-1D is a missense mutation of the Arabidopsis gene for the MED12 subunit of the Mediator. Genetic and molecular analysis of both dominant crp-1D and recessive crp loss-of-function alleles showed that MED12/CRP is a novel flowering regulator which suppresses FLC expression, promotes FT and TSF expression and up-regulates SOC1 and FUL mainly in an FT-dependent manner under long-day conditions (Supplementary Fig. S7). Although protein interaction has not yet been successfully demonstrated for MED12/CRP and MED13/MAB2, our present work and previous work (Gillmor et al. 2010, Ito et al. 2011) strongly suggest that these two proteins act closely together in the same pathways to regulate various aspects of plant development from embryogenesis to floral morphogenesis, some of which are mediated through modulation of the auxin response (Ito et al. 2011). Among them, floral transition represents a developmental process largely independent of auxin signaling and a unique common target process shared by MED12/CRP and MED13/MAB2 and core subunits of the Mediator, such as MED8 and MED25/PFT1.
CRP encodes the MED12 subunit of the Mediator
We have shown that the CRP gene encodes a protein of 2,235 amino acids, which corresponds to the Arabidopsis counterpart of the MED12 subunit of the Mediator (Supplementary Fig. S2). The gene for MED12 was recently identified independently as the CCT gene required for proper embryogenesis (Gillmor et al. 2010). By cDNA analysis, we found that the actual coding region of MED12/CCT/CRP differs from the publicly available annotated unit (Fig. 1G). MED12/CCT/CRP is ubiquitously expressed in seedlings and young plants, with higher levels of expression in vascular tissues and the shoot apex.
A dominant crp-1D mutation caused an S-to-F amino acid substitution at position 99. This serine residue is not located in either the conserved MED12 domain or signature sequence motifs (SSMs) of MED12 defined by Bourbon (2008), and is not conserved even among plant MED12 proteins (Supplementary Fig. S3). This makes interpretation of the crp-1D mutation difficult. However, that crp-1D is likely to act in the same molecular context as that involving wild-type MED12/CRP is suggested by its requirement for MED13/MAB2 to exert its phenotypic effect and attenuation of the crp-1D phenotype by an increased level of wild-type CRP (Figs. 3, 5). One interesting feature of crp-1D is its limited effect on development (mainly restricted to the floral transition) as opposed to pleiotropic effects throughout the entire life cycle observed in cct and crp loss-of-function mutants (Figs. 5–7) (Gillmor et al. 2010). Even during flowering, crp-1D affects only a subset of genes whose expression is affected by crp loss-of-function mutations (Figs. 2, 4). These findings are reminiscent of a recently reported dominant allele of CURLY LEAF (CLF), the Arabidopsis ortholog of Enhancer of Zeste (Doyle and Amasino 2009). clf loss-of-function mutations cause a severe pleiotropic phenotype and ectopic expression of various genes including AGAMOUS (AG). In contrast, the effect of a dominant clf-59 mutation (P704S) is limited to flowering, and ectopic AG expression was not observed. crp-1D and clf-59 may be useful tools to understand further the biochemical function of the respective proteins.
MED12/CRP is a novel flowering regulator with multiple target steps
Genetic and molecular analysis of both dominant crp-1D and loss-of-function crp alleles suggested that MED12/CRP acts as a flowering regulator with multiple target steps. That the Mediator plays multiple roles in the regulation of flowering is an emerging scheme, as clearly demonstrated by recent studies of MED25/PFT1 (Kidd et al. 2009, Elfving et al. 2011, Iñigo et al. 2011). Our present work with MED12/CRP gives further supports to this idea (Supplementary Fig. S7).
First, MED12/CRP is a negative regulator of FLC expression (T-bar #1 in Supplementary Fig. S7). An increased FLC mRNA level in crp loss-of-function mutants (Fig. 2C) is in part responsible for the late-flowering phenotype (Fig. 2A). It also, at least in part, explains reduced expression levels of downstream target genes of FLC, namely FT and TSF in the vasculature, and FD and SOC1 in the shoot apex (Searle et al. 2006) (Fig. 2C, D). Genetic analysis suggests a similar role for MED13/MAB2 as well (Fig. 5). It is interesting to note that genome-wide association analysis of recombinant inbred lines revealed a promising association at the CRP locus with the FLC mRNA level, although its close linkage (<100 kb) with FRIGIDA, whose allelic variation is a major determinant of the FLC expression level among natural accessions, made it difficult to be conclusive (Atwell et al. 2010). Involvement of the Mediator in regulation of FLC expression has recently been shown for several core subunits, such as MED8 and MED25/PFT1 (Kidd et al. 2009, Elfving et al. 2011). Similar to crp loss-of-function mutants, med8 and med25/pft1 mutants have increased FLC and reduced FT expression levels and showed the late-flowering phenotype in long-day conditions. Taken together, these and our results suggest that the Mediator plays an important role in suppression of FLC. In zebrafish and humans, it was shown that the SOX9 transcription factor physically or functionally interacts with MED12 and MED25 (Zhou et al. 2002, Rau et al. 2006, Nakamura et al. 2011). Therefore, it is likely that MED12/CRP, and possibly MED13/MAB2 as well, act with MED25/PFT1 in the same Mediator complex in regulation of FLC.
Secondly, MED12/CRP also positively regulates FT and TSF independently of FLC (arrow #2 in Supplementary Fig. S7). Reduced expression levels of FT and TSF were observed in crp loss-of-function mutants. The increased FLC level mentioned above is not the sole cause, since only partial recovery of expression of these two genes was observed in crp; flc double mutants (Fig. 2F). There should be another pathway independent of FLC. This is not through the transcriptional regulation of CO, because neither dominant crp-1D nor loss-of-function crp mutants showed a significant change in CO expression (Figs. 2C, 4B). MED12/CRP, and possibly also MED13/MAB2, may act either downstream of CO expression or independently of the CO function. Interestingly, it was shown that MED25/PFT1 regulates FT (but not TSF) in both CO-dependent (through up-regulation of CO expression) and CO-independent pathways (Iñigo et al. 2011). MED12/CRP may participate in the latter pathway. Alternatively, since MED12/CRP regulates both FT and TSF, it may represent a unique pathway.
Thirdly, MED12/CRP acts downstream of FT in regulation of SOC1 and FUL (arrow #3 in Supplementary Fig. S7). SOC1 and FUL expression in the shoot apical region was severely reduced in crp-3 and crp-4 mutants and increased in the crp-1D mutant (Figs. 2D, 4D). Reduction of the SOC1 level in crp loss-of-function mutants is, again, only partly explained by increased FLC, which is a negative regulator of SOC1 (Fig. 2F). In contrast, increased SOC1 and FUL levels in crp-1D clearly required functional FT (Fig. 4F), suggesting that MED12/CRP acts with or downstream of FT. That MED12/CRP acts in part downstream of FT is further supported by partial suppression of the precocious-flowering phenotype of FT-overexpressing (SULTR2;1::FT) plants by crp loss-of-function mutations (Fig. 2E). It has been shown that FT is an upstream activator of SOC1 (Yamaguchi et al. 2005, Yoo et al. 2005, Searle et al. 2006) and FUL (Abe et al. 2005, Teper-Bamnolker and Samach 2005, Wigge et al. 2005) expression. MED12/CRP, and possibly also MED13/MAB2, may act with FT and transcription factors such as FD in transcriptional regulation of FUL and SOC1. Again, it is interesting to note that it was recently suggested that MED25/PFT1 is also involved in regulation of SOC1 expression (Iñigo et al. 2011). MED12/CRP, and possibly also MED13/MAB2, and MED25/PFT1 may act in the same pathway for SOC1 activation.
Recent studies in animals showed that the CDK8 module (comprising CDK8, CycC, MED12 and MED13) is stable and biochemically active apart from the Mediator (Knuesel et al. 2009). CDK8 phosphorylates histone H3S10, and the kinase activity of the CDK8 module is dependent on MED12 in humans (Knuesel et al. 2009). MED12 can repress transcription through interaction with histone H3K9 methyltransferase in HeLa cells (Ding et al. 2008). MED12 acts as a chromatin regulator and a ‘hub’ protein that modifies expression of a large number of genes from multiple signaling pathways in C. elegans (Moghal and Sternberg 2003, Lehner et al. 2006). These observations may support the possibility that MED12/CCT/CRP could be involved in the epigenetic control of genes such as FLC or FT in the context of the CDK8 module outside of the Mediator. However, our observations discussed above are slightly in favor of the alternative view that it functions in the context of the Mediator with core subunits.
MED12/CCT/CRP and MED13/GCT/MAB2 in plant development
In addition to the control of flowering, MED12/CRP and MED13/MAB2 are involved in various developmental processes. It was shown that MED12/CCT and MED13/GCT are absolutely required for KANADI expression during early embryogenesis and are important for the proper temporal regulation of genes involved in radial pattern formation during embryogenesis (Gillmor et al. 2010). This is likely to be achieved in part through modulation of the auxin response, as clearly demonstrated for MED13/MAB2 (Ito et al. 2010). That MED12/CRP closely shares the role in the auxin response with MED13/MAB2 was supported by a similar genetic interaction of crp and mab2 mutations with pid (Fig. 7). The fact that the gynoecium phenotype of the crp loss-of-function mutants is reminiscent of that of auxin-related mutants such as ett (Fig. 6J) lends additional support for the role of MED12/CRP in the auxin response. Taken together, MED12/CCT/CRP and MED13/GCT/MAB2 function closely together to regulate the auxin response. Dwarfism (Fig. 6D), inflorescence defects including ectopic bract formation (Fig. 6F), and various floral defects (Fig. 6G–I) observed in crp loss-of-function mutants, some of which are shared by mab2 (Ito et al. 2010), imply that MED12/CCT/CRP, and possibly also MED13/GCT/MAB2, are involved in other aspects of development. Some are probably through modulation of the auxin response. In contrast to the role in regulation of flowering, which is shared by core subunits of the Mediator, such as MED8 and MED25/PFT1, the roles of MED12/CCT/CRP and MED13/GCT/MAB2 in other aspects of development, such as embryogenesis, seem to be unique to these two subunits of the Mediator.
MED12 and MED13 belong to the CDK8 module of Mediator which contains CDK8 and CycC subunits. There has been no report of a mutant phenotype and functional analysis of CycC orthologs in plants (Wang et al. 2004). In Arabidopsis, HUA ENHANCER3 (HEN3) encodes the CDK8 subunit, and is expressed in proliferating tissues, such as the SAM, young leaves and floral buds (Wang and Chen 2004, Wang et al. 2004). However, hen3 mutants do not show pleiotropic developmental defects such as those observed in cct/crp and gct/mab2 mutants. HEN3 protein interacts with LEUNIG (LUG) and SEUSS (SEU), which repress AG and are involved in auxin signaling during floral organ development (Navarro et al. 2004, Pfluger and Zambryski 2004, Gonzalez et al. 2007). Interestingly, crp and gct/mab2 mutants showed floral defects reminiscent of lug and seu mutants (Liu and Meyerowitz 1995, Franks et al. 2002, Gillmor et al. 2010) (Fig. 6G–J). It was reported that gct; kan double and hen3; hua1; hua2 triple mutants showed enhanced loss of floral organ identity, although gct/mab2 or hen3 single mutants did not show major defects in floral organ specification (Wang and Chen 2004, Gillmor et al. 2010). These observations suggest that floral organ development may represent a shared developmental program regulated by the CDK8 module proteins of the Mediator possibly through modulation of the auxin response.
Does Mediator contribute to feed-forward loops in flowering regulation?
Flowering of monocarpic plants such as A. thaliana is an irreversible phase transition. It has been suggested that feed-forward loops constructed from key regulators are important to minimize external noises to achieve robustness of developmental systems (Mangan and Alon 2003, Dekel et al. 2005, Pastore et al. 2011). As summarized in a simplified model (Supplementary Fig. S7), MED12/CRP, and possibly also MED13/MAB2, are involved in multiple steps in the regulation of production of florigen (FT and TSF proteins) and in multiple downstream steps of florigen action. Similarly, it has been shown that a core subunit of the Mediator, MED25/PFT1, regulates multiple steps both upstream and downstream of FT florigen. These observations and the possible involvement of genetic variation at the CRP locus in natural variation of the FLC expression level among various accessions (Atwell et al. 2010) prompt us to propose a hypothesis that the Mediator may make an important contribution to flowering as a key factor to construct a cohesive feed-forward regulatory motif. Further studies to examine the role of other core subunits of the Mediator in flowering and identification of transcription factors involved will be important steps to test this hypothesis.
Materials and Methods
Plant materials and growth conditions
Col-0 and Ler were used as wild types. T-DNA insertion alleles of CRP in the Col background (SALK_108241, crp-3; and SALK_124276; crp-4) were obtained from the Arabidopsis Biological Resource Center (ABRC). crp-3 and crp-4 were backcrossed with wild-type Col-0 three times prior to analysis. crp-3 and crp-4 correspond to cct-2 and cct-3, respectively (Gillmor et al. 2010). A Ds-transposon insertion allele in the Ler background (SET_3.701; crp-2) was kindly provided by Dr. V. Sundaresan (Parinov et al. 1999), and was backcrossed with wild-type Ler three times prior to analysis. crp-1D was isolated as an enhancer mutation of the 35S::FT #1-5C in the Col background (Kobayashi et al. 1999). Mutagenesis was carried out by soaking seeds in 0.1% EMS for 16 h. The resulting M1 population (5,000 seeds) was sown and self-fertilized, and the M2 population was screened for enhancers of the early-flowering phenotype in constant light conditions (24°C). crp-1D L was obtained by five backcrosses with wild-type Ler. CRP::GUS and 35S::CRP (see below for the transgene construction) were generated in this work. The ft-2 allele from the Ler accession was introgressed into Col by five backcrosses to generate ft-2 (Col). Previously published plant materials used in this work are as follows: 35S::FT #11-1 (a strong line), 35S::FT #1-5C (a weak line), SULTR2;1::FT #1-a, 35S::TSF #4-1 (a weak line), 35S::LFY (DW151.2.5C), 35S::AP1, soc1-101D, flc-3, tsf-1, fd-1 (Col). soc1-2, ful-2, lfy-1, pid-3 are in the Col background; mab2-1 and pid-2 are in the Ler background. Further information is given in Supplementary Table S11.
For analysis of the flowering time phenotype, plants were grown on soil or half-strength Murashige and Skoog (MS; Wako) medium supplemented with 0.5% sucrose and 0.4% Gellan Gum (Wako) at 22°C under long-day (16 h light/8 h dark) conditions with white fluorescent light (~80 μmol m−2 s−1) or short-day (8 h light/16 h dark) conditions with white fluorescent light (~100 μmol m−2 s−1). Flowering time was measured by counting rosette and cauline leaves. Bars in the figures show the number of rosette leaves. For expression analysis, plants were grown on half-strength MS medium supplemented with 0.5% sucrose and 0.4% Gellan Gum. Seeds were stratified by keeping them at 4°C for 2–4 d and then transferred to 22°C long-day conditions, which was defined as day 0. Plants were harvested at Zeitgeber time (ZT) 15 on each day.
RT–PCR analysis
RNA was extracted using TRIzol reagent (Invitrogen) and was treated with RNase-free DNase I (Invitrogen) according to the manufacturer’s instructions. Total RNA (0.5 μg) was reverse-transcribed in a 20 μl reaction mixture using SuperScript III (Invitrogen). After the reaction, 10 μl of the mixture was diluted with 240 μl of water, and 5 μl aliquots were analyzed. Quantitative RT–PCRs (qRT–PCRs) were performed using SYBR Premix Ex Taq II (TAKARA). Primers used in this study are listed in Supplementary Table S12. qRT–PCR results normalized to ACT2, NRT1.7 or STM show the average of nine different reactions (biological×technical triplicate), except for the data shown in Fig. 3A (only technical triplicate). Relative expression was obtained as the ratio to the level in wild-type Col harvested on the first day of the experiment. Semi-quantitative RT–PCR was performed using the primers listed in Supplementary Table S13. PCR products were electrophoresed on an agarose gel and visualized by ethidium bromide staining.
Plasmid construction and transgenic plants
To construct CRP::GUS, the GUS coding sequence from pBI101 was inserted downstream of the 2.0 kb CRP promoter fragment (2.0 kb sequence upstream of the presumptive initiation ATG codon) amplified by PCR from Col using the primers listed in Supplementary Table S14. To clone CRP and crp-1D cDNA, the coding region of CRP from Col and crp-1D, respectively, was amplified by PCR using the primers listed in Supplementary Table S14 and recombined into the SalI and XhoI sites of the pENTR1A vector (Invitrogen). To construct CRP-GFP and crp-1D-GFP, the coding region of sGFP was fused, in-frame, to the C-terminus of CRP or crp-1D in the pENTR vector as described above and, after sequencing, this translational fusion construct was cloned into the binary vector pGWB2 (Nakagawa et al. 2007) using LR Recombination Reactions (Invitrogen). To construct 35S::CRP, the CRP coding sequence in the pENTR1A vector was transferred to the binary vector pGWB2 (Nakagawa et al. 2007) using LR Recombination Reactions (Invitrogen). To generate stable transgenic plants, the constructs were introduced into Agrobacterium tumefaciens strain pMP90, and Col plants were transformed by the floral dip procedure (Clough and Bent 1998).
GUS staining, histological analysis and microscopy
CRP::GUS line #13.4 was chosen for analysis. Samples were collected at ZT15 from plants grown in long-day conditions. For GUS staining, tissues were incubated at 4°C for 15 min in 90% acetone, rinsed with phosphate-buffered saline (PBS), infiltrated with staining solution (0.5 mg ml−1 X-Gluc, 100 mM sodium phosphate buffer, pH 7.0, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 0.1% Triton X-100) under vacuum for 5 min and incubated at 37°C for about 12–48 h in the dark. After staining, for whole-mount observation, samples were cleared as described previously (Aida et al. 1997). For sectioning, samples were dehydrated through an ethanol series, embedded in Technovit 7100 (Heraeus Kulzer) and sectioned at a thickness of 4 μm with a microtome.
For visualization of seedling vasculature, plants were fixed and rehydrated as described previously (Aida et al. 1997). Gynoecium morphology was observed using a scanning electron microscope (TM3000 Miniscope, Hitachi).
Particle bombardment and GFP fluorescence detection
35S::CRP-GFP and 35S::crp-1D-GFP were used as bioluminescent reporter constructs. These plasmid vectors were introduced into onion cells using the particle bombardment PDS-1000/He Biolistic Particle Delivery System (Bio-Rad). A 1 μg aliquot of plasmids was mixed with 8 μl of a pre-washed 1 μm diameter gold particle suspension (60 mg ml−1), 3.3 μl of CaCl2 (2.5 M) and 3.3 μl of spermidine (0.1 M). After incubation, the particles were washed with 80% ethanol and resuspended in 10 μl of 100% ethanol. The DNA-coated particles were fired into the onion cells using a 1,100 p.s.i. rupture disk. Onion cells were incubated in the dark at 23°C for 24–40 h after particle delivery and the epidermal cells were observed with a confocal laser scanning microscope (FV1000, Olympus), excitation wavelength 488 nm by argon laser, emission wavelength 500–600 nm.
Supplementary data
Supplementary data are available at PCP online.
Funding
This work was supported by the Ministry of Education, Culture, Sport, Science and Technology of Japan [18370018 and 19060012 to T.A.]; the Japan Science and Technology Agency [the CREST program (to T.A.); the Mitsubishi Foundation [to T.A.].
Supplementary Material
Acknowledgments
We thank Dr. V. Sundaresan and the Arabidopsis Biological Resource Center for providing seeds of the Ds-insertion allele and T-DNA insertion alleles, respectively, Drs. Y. Daimon and Y. Tomita for excellent technical assistance and helpful discussions, and Dr. J. Ito and members of the Araki lab for discussion and comments. Thanks are also due to Dr. T. Oyama for the use of the particle bombardment facility.
Glossary
Abbreviations
- AP1
APETALA1
- bZIP
basic region/leucine zipper
- CCT
CENTER CITY
- CDK
cyclin-dependent kinase
- CycC
cyclin C
- CO
CONSTANS
- CRP
CRYPTIC PRECOCIOUS
- EMS
ethylmethane sulfonate
- FLC
FLOWERING LOCUS C
- FT
FLOWERING LOCUS T
- FUL
FRUITFULL
- GCT
GRAND CENTRAL
- GFP
green fluorescent protein
- GUS
β-glucuronidase
- HEN
HUA ENHANCER
- LFY
LEAFY
- MAB
MACCHI-BOU
- MED
Mediator complex subunit
- MS
Murashige and Skoog
- NRT1.7
NITRATE TRANSPORTER 1.7
- PFT
PHYTOCHROME AND FLOWERING TIME
- PID
PINOID
- qRT–PCR
quantitative reverse transcription–PCR
- RT–PCR
reverse transcription–PCR
- 35S
Cauliflower mosaic virus 35S RNA promoter
- SAM
shoot apical meristem
- SOC1
SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1
- STM
SHOOTMERISTEMLESS
- SULTR2;1
SULFATE TRANSPORTER2
- 1 promoter; TSF
TWIN SISTER OF FT
- ZT
Zeitgeber time.
References
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, et al. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science. 2005;309:1052–1056. doi: 10.1126/science.1115983. [DOI] [PubMed] [Google Scholar]
- Adrian J, Farrona S, Reimer JJ, Albani MC, Coupland G, Turck F. cis-Regulatory elements and chromatin state coordinately control temporal and spatial expression of FLOWERING LOCUS T in Arabidopsis. Plant Cell. 2010;22:1425–1440. doi: 10.1105/tpc.110.074682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M. Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. Plant Cell. 1997;9:841–857. doi: 10.1105/tpc.9.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T. Transition from vegetative to reproductive phase. Curr. Opin. Plant Biol. 2001;4:63–68. doi: 10.1016/s1369-5266(00)00137-0. [DOI] [PubMed] [Google Scholar]
- Atwell S, Huang YS, Vilhjálmsson BJ, Willems G, Horton M, Li Y, et al. Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature. 2010;465:627–631. doi: 10.1038/nature08800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autran D, Jonak C, Belcram K, Beemster GT, Kronenberger J, Grandjean O, et al. Cell numbers and leaf development in Arabidopsis: a functional analysis of the STRUWWELPETER gene. EMBO J. 2002;21:6036–6049. doi: 10.1093/emboj/cdf614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckström S, Elfving N, Nilsson R, Wingsle G, Björklund S. Purification of a plant mediator from Arabidopsis thaliana identifies PFT1 as the Med25 subunit. Mol. Cell. 2007;26:717–729. doi: 10.1016/j.molcel.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Bäurle I, Dean C. The timing of developmental transitions in plants. Cell. 2006;125:655–664. doi: 10.1016/j.cell.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Boss PK, Bastow RM, Mylne JS, Dean C. Multiple pathways in the decision to flower: enabling, promoting, and resetting. Plant Cell. 2004;16:S18–S31. doi: 10.1105/tpc.015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbon HM. Comparative genomics supports a deep evolutionary origin for the large, four-module transcriptional mediator complex. Nucleic Acids Res. 2008;36:3993–4008. doi: 10.1093/nar/gkn349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdán PD, Chory J. Regulation of flowering time by light quality. Nature. 2003;423:881–885. doi: 10.1038/nature01636. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316:1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- Dekel E, Mangan S, Alon U. Environmental selection of the feed-forward loop circuit in gene-regulation networks. Phys. Biol. 2005;2:81–88. doi: 10.1088/1478-3975/2/2/001. [DOI] [PubMed] [Google Scholar]
- Dieterle M, Bauer D, Büche C, Krenz M, Schäfer E, Kretsch T. A new type of mutation in phytochrome A causes enhanced light sensitivity and alters the degradation and subcellular partitioning of the photoreceptor. Plant J. 2005;41:146–161. doi: 10.1111/j.1365-313X.2004.02286.x. [DOI] [PubMed] [Google Scholar]
- Ding N, Zhou H, Esteve PO, Chin HG, Kim S, Xu X, et al. Mediator links epigenetic silencing of neuronal gene expression with X-linked mental retardation. Mol. Cell. 2008;31:347–359. doi: 10.1016/j.molcel.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle MR, Amasino RM. A single amino acid change in the enhancer of zeste ortholog CURLY LEAF results in vernalization-independent, rapid flowering in Arabidopsis. Plant Physiol. 2009;151:1688–1697. doi: 10.1104/pp.109.145581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Assal SE, Alonso-Blanco C, Peeters AJ, Raz V, Koornneef M. A QTL for flowering time in Arabidopsis reveals a novel allele of CRY2. Nat. Genet. 2001;29:435–440. doi: 10.1038/ng767. [DOI] [PubMed] [Google Scholar]
- Elfving N, Davoine C, Benlloch R, Blomberg J, Brännström K, Müller D, et al. The Arabidopsis thaliana Med25 mediator subunit integrates environmental cues to control plant development. Proc. Natl Acad. Sci. USA. 2011;108:8245–8250. doi: 10.1073/pnas.1002981108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan SC, Lin CS, Hsu PK, Lin SH, Tsay YF. The Arabidopsis nitrate transporter NRT1.7, expressed in phloem, is responsible for source-to-sink remobilization of nitrate. Plant Cell. 2009;21:2750–2761. doi: 10.1105/tpc.109.067603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornara F, de Montaigu A, Coupland G. SnapShot: control of flowering in Arabidopsis. Cell. 2010;141:550. doi: 10.1016/j.cell.2010.04.024. e1-2. [DOI] [PubMed] [Google Scholar]
- Franks RG, Wang C, Levin JZ, Liu Z. SEUSS, a member of a novel family of plant regulatory proteins, represses floral homeotic gene expression with LEUNIG. Development. 2002;129:253–263. doi: 10.1242/dev.129.1.253. [DOI] [PubMed] [Google Scholar]
- Gillmor CS, Park MY, Smith MR, Pepitone R, Kerstetter RA, Poethig RS. The MED12–MED13 module of Mediator regulates the timing of embryo patterning in Arabidopsis. Development. 2010;137:113–122. doi: 10.1242/dev.043174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez D, Bowen AJ, Carroll TS, Conlan RS. The transcription corepressor LEUNIG interacts with the histone deacetylase HDA19 and mediator components MED14 (SWP) and CDK8 (HEN3) to repress transcription. Mol. Cell. Biol. 2007;27:5306–5315. doi: 10.1128/MCB.01912-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henteges KE. Mediator complex proteins are required for diverse developmental processes. Semin. Cell Dev. Biol. 2011;22:769–775. doi: 10.1016/j.semcdb.2011.07.025. [DOI] [PubMed] [Google Scholar]
- Hong SK, Haldin CE, Lawson ND, Weinstein BM, Dawid IB, Hukriede NA. The zebrafish kohtalo/trap230 gene is required for the development of the brain, neural crest, and pronephric kidney. Proc. Natl Acad. Sci. USA. 2005;102:18473–18478. doi: 10.1073/pnas.0509457102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T. Arabidopsis circadian clock and photoperiodism: time to think about location. Curr. Opin. Plant Biol. 2010;13:83–89. doi: 10.1016/j.pbi.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñigo S, Alvarez MJ, Strasser B, Califano A, Cerdán PD. PFT1, the MED25 subunit of the plant Mediator complex, promotes flowering through CONSTANS dependent and independent mechanisms in Arabidopsis. Plant J. 2011 doi: 10.1111/j.1365-313X.2011.04815.x. (in press) [DOI] [PubMed] [Google Scholar]
- Ito J, Sono T, Tasaka M, Furutani M. MACCHI-BOU 2 is required for early embryo patterning and cotyledon organogenesis in Arabidopsis. Plant Cell Physiol. 2011;52:539–552. doi: 10.1093/pcp/pcr013. [DOI] [PubMed] [Google Scholar]
- Jaeger KE, Wigge PA. FT protein acts as a long-range signal in Arabidopsis. Curr. Biol. 2007;17:1050–1054. doi: 10.1016/j.cub.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Janody F, Martirosyan Z, Benlali A, Treisman JE. Two subunits of the Drosophila mediator complex act together to control cell affinity. Development. 2003;130:3691–3701. doi: 10.1242/dev.00607. [DOI] [PubMed] [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, et al. Activation tagging of the floral inducer FT. Science. 1999;286:1962–1965. doi: 10.1126/science.286.5446.1962. [DOI] [PubMed] [Google Scholar]
- Kidd BN, Cahill DM, Manners JM, Schenk PM, Kazan K. Diverse roles of the Mediator complex in plants. Semin. Cell Dev. Biol. 2011;22:741–748. doi: 10.1016/j.semcdb.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Kidd BN, Edgar CI, Kumar KK, Aitken EA, Schenk PM, Manners JM, et al. The mediator complex subunit PFT1 is a key regulator of jasmonate-dependent defense in Arabidopsis. Plant Cell. 2009;21:2237–2252. doi: 10.1105/tpc.109.066910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Zheng B, Yu Y, Won SY, Mo B, Chen X. The role of Mediator in small and long noncoding RNA production in Arabidopsis thaliana. EMBO J. 2011;30:814–822. doi: 10.1038/emboj.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel MT, Meyer KD, Bernecky C, Taatjes DJ. The human CDK8 subcomplex is a molecular switch that controls Mediator coactivator function. Genes Dev. 2009;23:439–451. doi: 10.1101/gad.1767009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. A pair of related genes with antagonistic roles in mediating flowering signals. Science. 1999;286:1960–1962. doi: 10.1126/science.286.5446.1960. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Weigel D. Move on up, it’s time for change—mobile signals controlling photoperiod-dependent flowering. Genes Dev. 2007;21:2371–2384. doi: 10.1101/gad.1589007. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Hanhart CJ, van der Veen JJ. A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol. Gen. Genet. 1991;229:57–66. doi: 10.1007/BF00264213. [DOI] [PubMed] [Google Scholar]
- Kornberg RD. Mediator and the mechanism of transcriptional activation. Trends Biochem. Sci. 2005;30:235–239. doi: 10.1016/j.tibs.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Lehner B, Crombie C, Tischler J, Fortunato A, Fraser AG. Systematic mapping of genetic interactions in Caenorhabditis elegans identifies common modifiers of diverse signaling pathways. Nat. Genet. 2006;38:896–903. doi: 10.1038/ng1844. [DOI] [PubMed] [Google Scholar]
- Liu C, Chen H, Er HL, Soo HM, Kumar PP, Han JH, et al. Direct interaction of AGL24 and SOC1 integrates flowering signals in Arabidopsis. Development. 2008;135:1481–1491. doi: 10.1242/dev.020255. [DOI] [PubMed] [Google Scholar]
- Liu Z, Meyerowitz EM. LEUNIG regulates AGAMOUS expression in Arabidopsis flowers. Development. 1995;121:975–991. doi: 10.1242/dev.121.4.975. [DOI] [PubMed] [Google Scholar]
- Loncle N, Boube M, Joulia L, Boschiero C, Werner M, Cribbs DL, et al. Distinct roles for Mediator cdk8 module subunits in Drosophila development. EMBO J. 2007;26:1045–1054. doi: 10.1038/sj.emboj.7601566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JA, Moan EI, Medford JI, Barton MK. A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature. 1996;379:66–69. doi: 10.1038/379066a0. [DOI] [PubMed] [Google Scholar]
- Malik S, Roeder RG. Dynamic regulation of pol II transcription by the mammalian Mediator complex. Trends Biochem. Sci. 2005;30:256–263. doi: 10.1016/j.tibs.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Mangan S, Alon U. Structure and function of the feed-forward loop network motif. Proc. Natl Acad. Sci. USA. 2003;100:11980–11985. doi: 10.1073/pnas.2133841100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu J, Warthmann N, Küttner F, Schmid M. Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Curr. Biol. 2007;17:1055–1060. doi: 10.1016/j.cub.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Moghal N, Sternberg PW. A component of the transcriptional mediator complex inhibits RAS-dependent vulval fate specification in C. elegans. Development. 2003;130:57–69. doi: 10.1242/dev.00189. [DOI] [PubMed] [Google Scholar]
- Moon J, Lee H, Kim M, Lee I. Analysis of flowering pathway integrators in Arabidopsis. Plant Cell Physiol. 2005;46:292–299. doi: 10.1093/pcp/pci024. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, et al. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 2007;104:34–41. doi: 10.1263/jbb.104.34. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Yamamoto K, He X, Otsuki B, Kim Y, Murao H, et al. Wwp2 is essential for palatogenesis mediated by the interaction between Sox9 and mediator subunit 25. Nat. Commun. 2011;2:251. doi: 10.1038/ncomms1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro C, Efremova N, Golz JF, Rubiera R, Kuckenberg M, Castillo R, et al. Molecular and genetic interactions between STYLOSA and GRAMINIFOLIA in the control of Antirrhinum vegetative and reproductive development. Development. 2004;131:3649–3659. doi: 10.1242/dev.01205. [DOI] [PubMed] [Google Scholar]
- Nemhauser JL, Feldman LJ, Zambryski PC. Auxin and ETTIN in Arabidopsis gynoecium morphogenesis. Development. 2000;127:3877–3888. doi: 10.1242/dev.127.18.3877. [DOI] [PubMed] [Google Scholar]
- Notaguchi M, Abe M, Kimura T, Daimon Y, Kobayashi T, Yamaguchi A, et al. Long-distance, graft-transmissible action of Arabidopsis FLOWERING LOCUS T protein to promote flowering. Plant Cell Physiol. 2008;49:1645–1658. doi: 10.1093/pcp/pcn154. [DOI] [PubMed] [Google Scholar]
- Parinov S, Sevugan M, Ye D, Yang WC, Kumaran M, Sundaresan V. Analysis of flanking sequences from dissociation insertion lines: a database for reverse genetics in Arabidopsis. Plant Cell. 1999;11:2263–2270. doi: 10.1105/tpc.11.12.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore JJ, Limpuangthip A, Yamaguchi N, Wu MF, Sang Y, Han SK, et al. LATE MERISTEM IDENTITY2 acts together with LEAFY to activate APETALA1. Development. 2011;138:3189–3198. doi: 10.1242/dev.063073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfluger J, Zambryski P. The role of SEUSS in auxin response and floral organ patterning. Development. 2004;131:4697–4707. doi: 10.1242/dev.01306. [DOI] [PubMed] [Google Scholar]
- Poethig RS. Phase change and the regulation of developmental timing in plants. Science. 2003;301:334–336. doi: 10.1126/science.1085328. [DOI] [PubMed] [Google Scholar]
- Rau MJ, Fischer S, Neumann CJ. Zebrafish Trap230/Med12 is required as a coactivator for Sox9-dependent neural crest, cartilage and ear development. Dev. Biol. 2006;296:83–93. doi: 10.1016/j.ydbio.2006.04.437. [DOI] [PubMed] [Google Scholar]
- Reed JW. Roles and activities of Aux/IAA proteins in Arabidopsis. Trends Plant Sci. 2001;6:420–425. doi: 10.1016/s1360-1385(01)02042-8. [DOI] [PubMed] [Google Scholar]
- Robert HS, Offringa R. Regulation of auxin transport polarity by AGC kinases. Curr. Opin. Plant Biol. 2008;11:495–502. doi: 10.1016/j.pbi.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, et al. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science. 2000;288:1613–1616. doi: 10.1126/science.288.5471.1613. [DOI] [PubMed] [Google Scholar]
- Samuelsen CO, Baraznenok V, Khorosjutina O, Spahr H, Kieselbach T, Holmberg S, et al. TRAP230/ARC240 and TRAP240/ARC250 Mediator subunits are functionally conserved through evolution. Proc. Natl Acad. Sci. USA. 2003;100:6422–6427. doi: 10.1073/pnas.1030497100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz RJ, Amasino RM. Vernalization: a model for investigating epigenetics and eukaryotic gene regulation in plants. Biochim. Biophys. Acta. 2007;1769:269–275. doi: 10.1016/j.bbaexp.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Searle I, He Y, Turck F, Vincent C, Fornara F, Kröber S, et al. The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Genes Dev. 2006;20:898–912. doi: 10.1101/gad.373506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GG, Dean C. Arabidopsis, the Rosetta stone of flowering time? Science. 2002;296:285–289. doi: 10.1126/science.296.5566.285. [DOI] [PubMed] [Google Scholar]
- Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature. 2001;410:1116–1120. doi: 10.1038/35074138. [DOI] [PubMed] [Google Scholar]
- Takada S, Goto K. TERMINAL FLOWER2, an Arabidopsis homolog of HETEROCHROMATIN PROTEIN1, counteracts the activation of FLOWERING LOCUS T by CONSTANS in the vascular tissues of leaves to regulate flowering time. Plant Cell. 2003;15:2856–2865. doi: 10.1105/tpc.016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teper-Bamnolker P, Samach A. The flowering integrator FT regulates SEPALLATA3 and FRUITFULL accumulation in Arabidopsis leaves. Plant Cell. 2005;17:2661–2675. doi: 10.1105/tpc.105.035766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman J. Drosophila homologues of the transcriptional coactivation complex subunits TRAP240 and TRAP230 are required for identical processes in eye-antennal disc development. Development. 2001;128:603–615. doi: 10.1242/dev.128.4.603. [DOI] [PubMed] [Google Scholar]
- Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science. 2004;303:1003–1006. doi: 10.1126/science.1091761. [DOI] [PubMed] [Google Scholar]
- Wang G, Kong H, Sun Y, Zhang X, Zhang W, Altman N, et al. Genome-wide analysis of the cyclin family in Arabidopsis and comparative phylogenetic analysis of plant cyclin-like proteins. Plant Physiol. 2004;135:1084–1099. doi: 10.1104/pp.104.040436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Chen X. HUA ENHANCER3 reveals a role for a cyclin-dependent protein kinase in the specification of floral organ identity in Arabidopsis. Development. 2004;131:3147–3156. doi: 10.1242/dev.01187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, et al. Integration of spatial and temporal information during floral induction in Arabidopsis. Science. 2005;309:1056–1059. doi: 10.1126/science.1114358. [DOI] [PubMed] [Google Scholar]
- Wilczek AM, Roe JL, Knapp MC, Cooper MD, Lopez-Gallego C, Martin LJ, et al. Effects of genetic perturbation on seasonal life history plasticity. Science. 2009;323:930–934. doi: 10.1126/science.1165826. [DOI] [PubMed] [Google Scholar]
- Wollenberg AC, Strasser B, Cerdán PD, Amasino RM. Acceleration of flowering during shade avoidance in Arabidopsis alters the balance between FLOWERING LOCUS C-mediated repression and photoperiodic induction of flowering. Plant Physiol. 2008;148:1681–1694. doi: 10.1104/pp.108.125468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Kobayashi Y, Goto K, Abe M, Araki T. TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant Cell Physiol. 2005;46:1175–1189. doi: 10.1093/pcp/pci151. [DOI] [PubMed] [Google Scholar]
- Yanovsky MJ, Kay SA. Molecular basis of seasonal time measurement in Arabidopsis. Nature. 2002;419:308–312. doi: 10.1038/nature00996. [DOI] [PubMed] [Google Scholar]
- Yoda A, Kouike H, Okano H, Sawa H. Components of the transcriptional Mediator complex are required for asymmetric cell division in C. elegans. Development. 2005;132:1885–1893. doi: 10.1242/dev.01776. [DOI] [PubMed] [Google Scholar]
- Yoo SK, Chung KS, Kim J, Lee JH, Hong SM, Yoo SJ, et al. CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiol. 2005;139:770–778. doi: 10.1104/pp.105.066928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Bonneaud N, Yuan CX, de Santa Barbara P, Boizet B, Schomber T, et al. SOX9 interacts with a component of the human thyroid hormone receptor-associated protein complex. Nucleic Acids Res. 2002;30:3245–52. doi: 10.1093/nar/gkf443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.