Abstract
Background:
This article addresses the treatment of VTE disease.
Methods:
We generated strong (Grade 1) and weak (Grade 2) recommendations based on high-quality (Grade A), moderate-quality (Grade B), and low-quality (Grade C) evidence.
Results:
For acute DVT or pulmonary embolism (PE), we recommend initial parenteral anticoagulant therapy (Grade 1B) or anticoagulation with rivaroxaban. We suggest low-molecular-weight heparin (LMWH) or fondaparinux over IV unfractionated heparin (Grade 2C) or subcutaneous unfractionated heparin (Grade 2B). We suggest thrombolytic therapy for PE with hypotension (Grade 2C). For proximal DVT or PE, we recommend treatment of 3 months over shorter periods (Grade 1B). For a first proximal DVT or PE that is provoked by surgery or by a nonsurgical transient risk factor, we recommend 3 months of therapy (Grade 1B; Grade 2B if provoked by a nonsurgical risk factor and low or moderate bleeding risk); that is unprovoked, we suggest extended therapy if bleeding risk is low or moderate (Grade 2B) and recommend 3 months of therapy if bleeding risk is high (Grade 1B); and that is associated with active cancer, we recommend extended therapy (Grade 1B; Grade 2B if high bleeding risk) and suggest LMWH over vitamin K antagonists (Grade 2B). We suggest vitamin K antagonists or LMWH over dabigatran or rivaroxaban (Grade 2B). We suggest compression stockings to prevent the postthrombotic syndrome (Grade 2B). For extensive superficial vein thrombosis, we suggest prophylactic-dose fondaparinux or LMWH over no anticoagulation (Grade 2B), and suggest fondaparinux over LMWH (Grade 2C).
Conclusion:
Strong recommendations apply to most patients, whereas weak recommendations are sensitive to differences among patients, including their preferences.
Summary of Recommendations
Note on Shaded Text: Throughout this guideline, shading is used within the summary of recommendations sections to indicate recommendations that are newly added or have been changed since the publication of Antithrombotic and Thrombolytic Therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Recommendations that remain unchanged are not shaded.
2.1. In patients with acute DVT of the leg treated with vitamin K antagonist (VKA) therapy, we recommend initial treatment with parenteral anticoagulation (low-molecular-weight heparin [LMWH], fondaparinux, IV unfractionated heparin [UFH], or subcutaneous [SC] UFH) over no such initial treatment (Grade 1B).
2.2.1. In patients with a high clinical suspicion of acute VTE, we suggest treatment with parenteral anticoagulants compared with no treatment while awaiting the results of diagnostic tests (Grade 2C).
2.2.2. In patients with an intermediate clinical suspicion of acute VTE, we suggest treatment with parenteral anticoagulants compared with no treatment if the results of diagnostic tests are expected to be delayed for more than 4 h (Grade 2C).
2.2.3. In patients with a low clinical suspicion of acute VTE, we suggest not treating with parenteral anticoagulants while awaiting the results of diagnostic tests, provided test results are expected within 24 h (Grade 2C).
2.3.1. In patients with acute isolated distal DVT of the leg and without severe symptoms or risk factors for extension, we suggest serial imaging of the deep veins for 2 weeks over initial anticoagulation (Grade 2C).
2.3.2. In patients with acute isolated distal DVT of the leg and severe symptoms or risk factors for extension (see text), we suggest initial anticoagulation over serial imaging of the deep veins (Grade 2C).
Remarks: Patients at high risk for bleeding are more likely to benefit from serial imaging. Patients who place a high value on avoiding the inconvenience of repeat imaging and a low value on the inconvenience of treatment and on the potential for bleeding are likely to choose initial anticoagulation over serial imaging.
2.3.3. In patients with acute isolated distal DVT of the leg who are managed with initial anticoagulation, we recommend using the same approach as for patients with acute proximal DVT (Grade 1B).
2.3.4. In patients with acute isolated distal DVT of the leg who are managed with serial imaging, we recommend no anticoagulation if the thrombus does not extend (Grade 1B); we suggest anticoagulation if the thrombus extends but remains confined to the distal veins (Grade 2C); we recommend anticoagulation if the thrombus extends into the proximal veins (Grade 1B).
2.4. In patients with acute DVT of the leg, we recommend early initiation of VKA (eg, same day as parenteral therapy is started) over delayed initiation, and continuation of parenteral anticoagulation for a minimum of 5 days and until the international normalized ratio (INR) is 2.0 or above for at least 24 h (Grade 1B).
2.5.1. In patients with acute DVT of the leg, we suggest LMWH or fondaparinux over IV UFH (Grade 2C) and over SC UFH (Grade 2B for LMWH; Grade 2C for fondaparinux).
Remarks: Local considerations such as cost, availability, and familiarity of use dictate the choice between fondaparinux and LMWH.
LMWH and fondaparinux are retained in patients with renal impairment, whereas this is not a concern with UFH.
2.5.2. In patients with acute DVT of the leg treated with LMWH, we suggest once- over twice-daily administration (Grade 2C).
Remarks: This recommendation only applies when the approved once-daily regimen uses the same daily dose as the twice-daily regimen (ie, the once-daily injection contains double the dose of each twice-daily injection). It also places value on avoiding an extra injection per day.
2.7. In patients with acute DVT of the leg and whose home circumstances are adequate, we recommend initial treatment at home over treatment in hospital (Grade 1B).
Remarks: The recommendation is conditional on the adequacy of home circumstances: well-maintained living conditions, strong support from family or friends, phone access, and ability to quickly return to the hospital if there is deterioration. It is also conditional on the patient feeling well enough to be treated at home (eg, does not have severe leg symptoms or comorbidity).
2.9. In patients with acute proximal DVT of the leg, we suggest anticoagulant therapy alone over catheter-directed thrombolysis (CDT) (Grade 2C).
Remarks: Patients who are most likely to benefit from CDT (see text), who attach a high value to prevention of postthrombotic syndrome (PTS), and a lower value to the initial complexity, cost, and risk of bleeding with CDT, are likely to choose CDT over anticoagulation alone.
2.10. In patients with acute proximal DVT of the leg, we suggest anticoagulant therapy alone over systemic thrombolysis (Grade 2C).
Remarks: Patients who are most likely to benefit from systemic thrombolytic therapy (see text), who do not have access to CDT, and who attach a high value to prevention of PTS, and a lower value to the initial complexity, cost, and risk of bleeding with systemic thrombolytic therapy, are likely to choose systemic thrombolytic therapy over anticoagulation alone.
2.11. In patients with acute proximal DVT of the leg, we suggest anticoagulant therapy alone over operative venous thrombectomy (Grade 2C).
2.12. In patients with acute DVT of the leg who undergo thrombosis removal, we recommend the same intensity and duration of anticoagulant therapy as in comparable patients who do not undergo thrombosis removal (Grade 1B).
2.13.1. In patients with acute DVT of the leg, we recommend against the use of an inferior vena cava (IVC) filter in addition to anticoagulants (Grade 1B).
2.13.2. In patients with acute proximal DVT of the leg and contraindication to anticoagulation, we recommend the use of an IVC filter (Grade 1B).
2.13.3. In patients with acute proximal DVT of the leg and an IVC filter inserted as an alternative to anticoagulation, we suggest a conventional course of anticoagulant therapy if their risk of bleeding resolves (Grade 2B).
Remarks: We do not consider that a permanent IVC filter, of itself, is an indication for extended anticoagulation.
2.14. In patients with acute DVT of the leg, we suggest early ambulation over initial bed rest (Grade 2C).
Remarks: If edema and pain are severe, ambulation may need to be deferred. As per section 4.1, we suggest the use of compression therapy in these patients.
3.0. In patients with acute VTE who are treated with anticoagulant therapy, we recommend long-term therapy (see section 3.1 for recommended duration of therapy) over stopping anticoagulant therapy after about 1 week of initial therapy (Grade 1B).
3.1.1. In patients with a proximal DVT of the leg provoked by surgery, we recommend treatment with anticoagulation for 3 months over (i) treatment of a shorter period (Grade 1B), (ii) treatment of a longer time-limited period (eg, 6 or 12 months) (Grade 1B), or (iii) extended therapy (Grade 1B regardless of bleeding risk).
3.1.2. In patients with a proximal DVT of the leg provoked by a nonsurgical transient risk factor, we recommend treatment with anticoagulation for 3 months over (i) treatment of a shorter period (Grade 1B), (ii) treatment of a longer time-limited period (eg, 6 or 12 months) (Grade 1B), and (iii) extended therapy if there is a high bleeding risk (Grade 1B). We suggest treatment with anticoagulation for 3 months over extended therapy if there is a low or moderate bleeding risk (Grade 2B).
3.1.3. In patients with an isolated distal DVT of the leg provoked by surgery or by a nonsurgical transient risk factor (see remark), we suggest treatment with anticoagulation for 3 months over treatment of a shorter period (Grade 2C) and recommend treatment with anticoagulation for 3 months over treatment of a longer time-limited period (eg, 6 or 12 months) (Grade 1B) or extended therapy (Grade 1B regardless of bleeding risk).
3.1.4. In patients with an unprovoked DVT of the leg (isolated distal [see remark] or proximal), we recommend treatment with anticoagulation for at least 3 months over treatment of a shorter duration (Grade 1B). After 3 months of treatment, patients with unprovoked DVT of the leg should be evaluated for the risk-benefit ratio of extended therapy.
3.1.4.1. In patients with a first VTE that is an unprovoked proximal DVT of the leg and who have a low or moderate bleeding risk, we suggest extended anticoagulant therapy over 3 months of therapy (Grade 2B).
3.1.4.2. In patients with a first VTE that is an unprovoked proximal DVT of the leg and who have a high bleeding risk, we recommend 3 months of anticoagulant therapy over extended therapy (Grade 1B).
3.1.4.3. In patients with a first VTE that is an unprovoked isolated distal DVT of the leg (see remark), we suggest 3 months of anticoagulant therapy over extended therapy in those with a low or moderate bleeding risk (Grade 2B) and recommend 3 months of anticoagulant treatment in those with a high bleeding risk (Grade 1B).
3.1.4.4. In patients with a second unprovoked VTE, we recommend extended anticoagulant therapy over 3 months of therapy in those who have a low bleeding risk (Grade 1B), and we suggest extended anticoagulant therapy in those with a moderate bleeding risk (Grade 2B).
3.1.4.5. In patients with a second unprovoked VTE who have a high bleeding risk, we suggest 3 months of anticoagulant therapy over extended therapy (Grade 2B).
3.1.5. In patients with DVT of the leg and active cancer, if the risk of bleeding is not high, we recommend extended anticoagulant therapy over 3 months of therapy (Grade 1B), and if there is a high bleeding risk, we suggest extended anticoagulant therapy (Grade 2B).
Remarks (3.1.3, 3.1.4, 3.1.4.3): Duration of treatment of patients with isolated distal DVT refers to patients in whom a decision has been made to treat with anticoagulant therapy; however, it is anticipated that not all patients who are diagnosed with isolated distal DVT will be prescribed anticoagulants (see section 2.3).
In all patients who receive extended anticoagulant therapy, the continuing use of treatment should be reassessed at periodic intervals (eg, annually).
3.2. In patients with DVT of the leg who are treated with VKA, we recommend a therapeutic INR range of 2.0 to 3.0 (target INR of 2.5) over a lower (INR < 2) or higher (INR 3.0-5.0) range for all treatment durations (Grade 1B).
3.3.1. In patients with DVT of the leg and no cancer, we suggest VKA therapy over LMWH for long-term therapy (Grade 2C). For patients with DVT and no cancer who are not treated with VKA therapy, we suggest LMWH over dabigatran or rivaroxaban for long-term therapy (Grade 2C).
3.3.2. In patients with DVT of the leg and cancer, we suggest LMWH over VKA therapy (Grade 2B). In patients with DVT and cancer who are not treated with LMWH, we suggest VKA over dabigatran or rivaroxaban for long-term therapy (Grade 2B).
Remarks (3.3.1-3.3.2): Choice of treatment in patients with and without cancer is sensitive to the individual patient’s tolerance for daily injections, need for laboratory monitoring, and treatment costs.
LMWH, rivaroxaban, and dabigatran are retained in patients with renal impairment, whereas this is not a concern with VKA.
Treatment of VTE with dabigatran or rivaroxaban, in addition to being less burdensome to patients, may prove to be associated with better clinical outcomes than VKA and LMWH therapy. When these guidelines were being prepared (October 2011), postmarketing studies of safety were not available. Given the paucity of currently available data and that new data are rapidly emerging, we give a weak recommendation in favor of VKA and LMWH therapy over dabigatran and rivaroxaban, and we have not made any recommendations in favor of one of the new agents over the other.
3.4. In patients with DVT of the leg who receive extended therapy, we suggest treatment with the same anticoagulant chosen for the first 3 months (Grade 2C).
3.5. In patients who are incidentally found to have asymptomatic DVT of the leg, we suggest the same initial and long-term anticoagulation as for comparable patients with symptomatic DVT (Grade 2B).
4.1. In patients with acute symptomatic DVT of the leg, we suggest the use of compression stockings (Grade 2B).
Remarks: Compression stockings should be worn for 2 years, and we suggest beyond that if patients have developed PTS and find the stockings helpful.
Patients who place a low value on preventing PTS or a high value on avoiding the inconvenience and discomfort of stockings are likely to decline stockings.
4.2.1. In patients with PTS of the leg, we suggest a trial of compression stockings (Grade 2C).
4.2.2. In patients with severe PTS of the leg that is not adequately relieved by compression stockings, we suggest a trial of an intermittent compression device (Grade 2B).
4.3. In patients with PTS of the leg, we suggest that venoactive medications (eg, rutosides, defibrotide, and hidrosmin) not be used (Grade 2C).
Remarks: Patients who value the possibility of response over the risk of side effects may choose to undertake a therapeutic trial.
5.1. In patients with acute PE, we recommend initial treatment with parenteral anticoagulation (LMWH, fondaparinux, IV UFH, or SC UFH) over no such initial treatment (Grade 1B).
5.2.1. In patients with a high clinical suspicion of acute PE, we suggest treatment with parenteral anticoagulants compared with no treatment while awaiting the results of diagnostic tests (Grade 2C).
5.2.2. In patients with an intermediate clinical suspicion of acute PE, we suggest treatment with parenteral anticoagulants compared with no treatment if the results of diagnostic tests are expected to be delayed for more than 4 h (Grade 2C).
5.2.3. In patients with a low clinical suspicion of acute PE, we suggest not treating with parenteral anticoagulants while awaiting the results of diagnostic tests, provided test results are expected within 24 h (Grade 2C).
5.3. In patients with acute PE, we recommend early initiation of VKA (eg, same day as parenteral therapy is started) over delayed initiation, and continuation of parenteral anticoagulation for a minimum of 5 days and until the INR is 2.0 or above for at least 24 h (Grade 1B).
5.4.1. In patients with acute PE, we suggest LMWH or fondaparinux over IV UFH (Grade 2C for LMWH; Grade 2B for fondaparinux) and over SC UFH (Grade 2B for LMWH; Grade 2C for fondaparinux).
Remarks: Local considerations such as cost, availability, and familiarity of use dictate the choice between fondaparinux and LMWH.
LMWH and fondaparinux are retained in patients with renal impairment, whereas this is not a concern with UFH.
In patients with PE where there is concern about the adequacy of SC absorption or in patients in whom thrombolytic therapy is being considered or planned, initial treatment with IV UFH is preferred to use of SC therapies.
5.4.2. In patients with acute PE treated with LMWH, we suggest once- over twice-daily administration (Grade 2C).
Remarks: This recommendation only applies when the approved once-daily regimen uses the same daily dose as the twice-daily regimen (ie, the once-daily injection contains double the dose of each twice-daily injection). It also places value on avoiding an extra injection per day.
5.5. In patients with low-risk PE and whose home circumstances are adequate, we suggest early discharge over standard discharge (eg, after first 5 days of treatment) (Grade 2B).
Remarks: Patients who prefer the security of the hospital to the convenience and comfort of home are likely to choose hospitalization over home treatment.
5.6.1.1. In patients with acute PE associated with hypotension (eg, systolic BP < 90 mm Hg) who do not have a high bleeding risk, we suggest systemically administered thrombolytic therapy over no such therapy (Grade 2C).
5.6.1.2. In most patients with acute PE not associated with hypotension, we recommend against systemically administered thrombolytic therapy (Grade 1C).
5.6.1.3. In selected patients with acute PE not associated with hypotension and with a low bleeding risk whose initial clinical presentation, or clinical course after starting anticoagulant therapy, suggests a high risk of developing hypotension, we suggest administration of thrombolytic therapy (Grade 2C).
5.6.2.1. In patients with acute PE, when a thrombolytic agent is used, we suggest short infusion times (eg, a 2-h infusion) over prolonged infusion times (eg, a 24-h infusion) (Grade 2C).
5.6.2.2. In patients with acute PE when a thrombolytic agent is used, we suggest administration through a peripheral vein over a pulmonary artery catheter (Grade 2C).
5.7. In patients with acute PE associated with hypotension and who have (i) contraindications to thrombolysis, (ii) failed thrombolysis, or (iii) shock that is likely to cause death before systemic thrombolysis can take effect (eg, within hours), if appropriate expertise and resources are available, we suggest catheter-assisted thrombus removal over no such intervention (Grade 2C).
5.8. In patients with acute PE associated with hypotension, we suggest surgical pulmonary embolectomy over no such intervention if they have (i) contraindications to thrombolysis, (ii) failed thrombolysis or catheter-assisted embolectomy, or (iii) shock that is likely to cause death before thrombolysis can take effect (eg, within hours), provided surgical expertise and resources are available (Grade 2C).
5.9.1. In patients with acute PE who are treated with anticoagulants, we recommend against the use of an IVC filter (Grade 1B).
5.9.2. In patients with acute PE and contraindication to anticoagulation, we recommend the use of an IVC filter (Grade 1B).
5.9.3. In patients with acute PE and an IVC filter inserted as an alternative to anticoagulation, we suggest a conventional course of anticoagulant therapy if their risk of bleeding resolves (Grade 2B).
Remarks: We do not consider that a permanent IVC filter, of itself, is an indication for extended anticoagulation.
6.1. In patients with PE provoked by surgery, we recommend treatment with anticoagulation for 3 months over (i) treatment of a shorter period (Grade 1B), (ii) treatment of a longer time-limited period (eg, 6 or 12 months) (Grade 1B), or (iii) extended therapy (Grade 1B regardless of bleeding risk).
6.2. In patients with PE provoked by a nonsurgical transient risk factor, we recommend treatment with anticoagulation for 3 months over (i) treatment of a shorter period (Grade 1B), (ii) treatment of a longer time-limited period (eg, 6 or 12 months) (Grade 1B), and (iii) extended therapy if there is a high bleeding risk (Grade 1B). We suggest treatment with anticoagulation for 3 months over extended therapy if there is a low or moderate bleeding risk (Grade 2B).
6.3. In patients with an unprovoked PE, we recommend treatment with anticoagulation for at least 3 months over treatment of a shorter duration (Grade 1B). After 3 months of treatment, patients with unprovoked PE should be evaluated for the risk-benefit ratio of extended therapy.
6.3.1. In patients with a first VTE that is an unprovoked PE and who have a low or moderate bleeding risk, we suggest extended anticoagulant therapy over 3 months of therapy (Grade 2B).
6.3.2. In patients with a first VTE that is an unprovoked PE and who have a high bleeding risk, we recommend 3 months of anticoagulant therapy over extended therapy (Grade 1B).
6.3.3. In patients with a second unprovoked VTE, we recommend extended anticoagulant therapy over 3 months of therapy in those who have a low bleeding risk (Grade 1B), and we suggest extended anticoagulant therapy in those with a moderate bleeding risk (Grade 2B).
6.3.4. In patients with a second unprovoked VTE who have a high bleeding risk, we suggest 3 months of therapy over extended therapy (Grade 2B).
6.4. In patients with PE and active cancer, if there is a low or moderate bleeding risk, we recommend extended anticoagulant therapy over 3 months of therapy (Grade 1B), and if there is a high bleeding risk, we suggest extended anticoagulant therapy (Grade 2B).
Remarks: In all patients who receive extended anticoagulant therapy, the continuing use of treatment should be reassessed at periodic intervals (eg, annually).
6.5. In patients with PE who are treated with VKA, we recommend a therapeutic INR range of 2.0 to 3.0 (target INR of 2.5) over a lower (INR < 2) or higher (INR 3.0-5.0) range for all treatment durations (Grade 1B).
6.6. In patients with PE and no cancer, we suggest VKA therapy over LMWH for long-term therapy (Grade 2C). For patients with PE and no cancer who are not treated with VKA therapy, we suggest LMWH over dabigatran or rivaroxaban for long-term therapy (Grade 2C).
6.7. In patients with PE and cancer, we suggest LMWH over VKA therapy (Grade 2B). In patients with PE and cancer who are not treated with LMWH, we suggest VKA over dabigatran or rivaroxaban for long-term therapy (Grade 2C).
Remarks (6.6-6.7): Choice of treatment in patients with and without cancer is sensitive to the individual patient’s tolerance for daily injections, need for laboratory monitoring, and treatment costs.
Treatment of VTE with dabigatran or rivaroxaban, in addition to being less burdensome to patients, may prove to be associated with better clinical outcomes than VKA and LMWH therapy. When these guidelines were being prepared (October 2011), postmarketing studies of safety were not available. Given the paucity of currently available data and that new data are rapidly emerging, we give a weak recommendation in favor of VKA and LMWH therapy over dabigatran and rivaroxaban, and we have not made any recommendation in favor of one of the new agents over the other.
6.8. In patients with PE who receive extended therapy, we suggest treatment with the same anticoagulant chosen for the first 3 months (Grade 2C).
6.9. In patients who are incidentally found to have asymptomatic PE, we suggest the same initial and long-term anticoagulation as for comparable patients with symptomatic PE (Grade 2B).
7.1.1. In patients with chronic thromboembolic pulmonary hypertension (CTPH), we recommend extended anticoagulation over stopping therapy (Grade 1B).
7.1.2. In selected patients with CTPH, such as those with central disease under the care of an experienced thromboendarterectomy team, we suggest pulmonary thromboendarterectomy over no pulmonary thromboendarterectomy (Grade 2C).
8.1.1. In patients with superficial vein thrombosis (SVT) of the lower limb of at least 5 cm in length, we suggest the use of a prophylactic dose of fondaparinux or LMWH for 45 days over no anticoagulation (Grade 2B).
Remarks: Patients who place a high value on avoiding the inconvenience or cost of anticoagulation and a low value on avoiding infrequent symptomatic VTE are likely to decline anticoagulation.
8.1.2. In patients with SVT who are treated with anticoagulation, we suggest fondaparinux 2.5 mg daily over a prophylactic dose of LMWH (Grade 2C).
9.1.1. In patients with acute upper-extremity DVT (UEDVT) that involves the axillary or more proximal veins, we recommend acute treatment with parenteral anticoagulation (LMWH, fondaparinux, IV UFH, or SC UFH) over no such acute treatment (Grade 1B).
9.1.2. In patients with acute UEDVT that involves the axillary or more proximal veins, we suggest LMWH or fondaparinux over IV UFH (Grade 2C) and over SC UFH (Grade 2B).
9.2.1. In patients with acute UEDVT that involves the axillary or more proximal veins, we suggest anticoagulant therapy alone over thrombolysis (Grade 2C).
Remarks: Patients who (i) are most likely to benefit from thrombolysis (see text); (ii) have access to CDT; (iii) attach a high value to prevention of PTS; and (iv) attach a lower value to the initial complexity, cost, and risk of bleeding with thrombolytic therapy are likely to choose thrombolytic therapy over anticoagulation alone.
9.2.2. In patients with UEDVT who undergo thrombolysis, we recommend the same intensity and duration of anticoagulant therapy as in similar patients who do not undergo thrombolysis (Grade 1B).
9.3.1. In most patients with UEDVT that is associated with a central venous catheter, we suggest that the catheter not be removed if it is functional and there is an ongoing need for the catheter (Grade 2C).
9.3.2. In patients with UEDVT that involves the axillary or more proximal veins, we suggest a minimum duration of anticoagulation of 3 months over a shorter period (Grade 2B).
Remarks: This recommendation also applies if the UEDVT was associated with a central venous catheter that was removed shortly after diagnosis.
9.3.3. In patients who have UEDVT that is associated with a central venous catheter that is removed, we recommend 3 months of anticoagulation over a longer duration of therapy in patients with no cancer (Grade 1B), and we suggest this in patients with cancer (Grade 2C).
9.3.4. In patients who have UEDVT that is associated with a central venous catheter that is not removed, we recommend that anticoagulation is continued as long as the central venous catheter remains over stopping after 3 months of treatment in patients with cancer (Grade 1C), and we suggest this in patients with no cancer (Grade 2C).
9.3.5. In patients who have UEDVT that is not associated with a central venous catheter or with cancer, we recommend 3 months of anticoagulation over a longer duration of therapy (Grade 1B).
9.4. In patients with acute symptomatic UEDVT, we suggest against the use of compression sleeves or venoactive medications (Grade 2C).
9.5.1. In patients who have PTS of the arm, we suggest a trial of compression bandages or sleeves to reduce symptoms (Grade 2C).
9.5.2. In patients with PTS of the arm, we suggest against treatment with venoactive medications (Grade 2C).
10.1. In patients with symptomatic splanchnic vein thrombosis (portal, mesenteric, and/or splenic vein thromboses), we recommend anticoagulation over no anticoagulation (Grade 1B).
10.2. In patients with incidentally detected splanchnic vein thrombosis (portal, mesenteric, and/or splenic vein thromboses), we suggest no anticoagulation over anticoagulation (Grade 2C).
11.1. In patients with symptomatic hepatic vein thrombosis, we suggest anticoagulation over no anticoagulation (Grade 2C).
11.2. In patients with incidentally detected hepatic vein thrombosis, we suggest no anticoagulation over anticoagulation (Grade 2C).
This article provides recommendations for the use of antithrombotic agents as well as the use of devices or surgical techniques in the treatment of patients with DVT and pulmonary embolism (PE), which are collectively referred to as VTE. We also provide recommendations for patients with (1) postthrombotic syndrome (PTS), (2) chronic thromboembolic pulmonary hypertension (CTPH), (3) incidentally diagnosed (asymptomatic) DVT or PE, (4) acute upper-extremity DVT (UEDVT), (5) superficial vein thrombosis (SVT), (6) splanchnic vein thrombosis, and (7) hepatic vein thrombosis.
Table 1 describes the populations, interventions, comparators, and outcomes (ie, PICO elements) for the questions addressed in this article and the design of the studies used to address them. Refer to Garcia et al,1 Ageno et al,2 and Holbrook et al3 in these guidelines for recommendations on the management of parenteral anticoagulation (dosing and monitoring) and oral anticoagulation (dosing and monitoring). Refer to Bates et al4 and Monagle et al5 in these guidelines for recommendations for pregnancy and neonates and children. The current article builds on previous versions of these guidelines and, most recently, the eighth edition.6
Table 1.
—Structured Clinical Questions
| Issue (Informal Question) | Structured PICO Question |
Methodology | |||
| Population | Intervention | Comparators | Outcome | ||
| Patient with acute DVT of the leg
(2.0-3.0) | |||||
| Initial anticoagulant (2.1) |
Patients with acute DVT of the leg |
Anticoagulation |
No anticoagulation |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs |
| Whether to treat while awaiting the results of the diagnostic
work-up (2.2.1-2.2.3) |
Patients with suspected acute DVT of the leg awaiting the results of
diagnostic tests |
Anticoagulation |
No anticoagulation |
PE, major bleeding, and mortality |
RCTs and cohort studies |
| Whether to treat isolated distal thrombosis (2.3.1-2.3.4) |
Patient with acute isolated distal DVT of the leg |
Anticoagulation |
No anticoagulation |
DVT extension, PE, major bleeding, mortality, QOL, and PTS |
RCTs and cohort studies |
| Timing of initiation of VKA relative to the initiation of parenteral
anticoagulation (2.4) |
Patients with acute DVT of the leg |
Early initiation of VKA |
Delayed initiation of VKA |
DVT extension, PE, major bleeding, mortality, QOL, and PTS |
RCTs and cohort studies |
| Duration of initial anticoagulation (2.4) |
Patients with acute DVT of the leg |
Longer duration |
Shorter duration |
DVT extension, PE, major bleeding, mortality, QOL, and PTS |
RCTs and cohort studies |
| Choice and route of initial anticoagulant (2.5.1, 2.5.2,
2.6) |
Patients with acute DVT of the leg |
UFH IV or SQ |
LMWH, fondaparinux, rivaroxaban |
DVT extension, PE, major bleeding, mortality, QOL, and PTS |
RCTs |
| Setting of initial anticoagulation (2.7) |
Patients with acute DVT of the leg |
In-hospital treatment |
At-home treatment |
DVT extension, PE, major bleeding, mortality, QOL, and PTS |
RCTs |
| Role of thrombolytic and mechanical interventions
(2.9-2.12) |
Patients with acute proximal DVT of the leg |
Catheter directed thrombolysis |
No active thrombus removal or another method of thrombus
removal |
Recurrent DVT and PE, major bleeding, mortality, QOL,
PTS, shorter ICU and hospital stays, and acute
complications |
RCTs and cohort studies |
| Systemic thrombolytic therapy | |||||
| Operative venous thrombectomy | |||||
| Role of IVC filters in addition to anticoagulation
(2.13.1) |
Patients with acute DVT of the leg started on
anticoagulation |
IVC filter |
No IVC filter |
Recurrent DVT and PE, major bleeding, mortality, QOL, PTS, and
complications of procedure |
RCTs |
| Role of IVC filters when anticoagulation is contraindicated
(2.13.2) |
Patients with acute DVT of the leg and a contraindication to
anticoagulation |
IVC filter |
No IVC filter |
Recurrent DVT and PE, major bleeding, mortality, QOL, PTS, and
complications of procedure |
RCTs and cohort studies |
| Role of anticoagulation in patients who initially received an IVC
filter when contraindication to anticoagulation resolves
(2.13.3) |
Patients with acute DVT of the leg who initially received an IVC
filter, now contraindication to anticoagulation resolved |
Anticoagulation in addition to IVC filter |
No anticoagulation in addition to IVC filter |
Recurrent DVT and PE, major bleeding, mortality, QOL, PTS, and
complications of procedure |
RCTs and cohort studies |
| Role of early ambulation (2.14) |
Patients with acute DVT of the leg started on anticoagulant
treatment |
Early ambulation |
Initial bed rest |
Recurrent DVT and PE, major bleeding, mortality, QOL, PTS, and
complications of procedure |
RCTs and cohort studies |
| Long-term anticoagulation therapy (3.0) |
Patients with acute VTE of the leg |
Long-term anticoagulation therapy |
No long-term anticoagulation therapy |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs |
| Duration of long-term anticoagulation (3.1.1- 3.1.5) |
Patients with an acute DVT of the leg |
Longer duration |
Shorter duration |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs |
| Intensity of VKA (3.2) |
Patients with acute DVT of the leg |
INR 2-3 |
Higher or lower INR ranges |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs |
| Choice of long-term anticoagulant (3.3.1, 3.3.2, 3.4) |
Patients with acute DVT of the leg. |
LMWH, dabigatran, rivaroxaban |
VKA |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs |
| Whether to treat an incidentally diagnosed asymptomatic acute DVT of
the leg (3.5) |
Patients with incidentally diagnosed asymptomatic DVT of the
leg |
Anticoagulation |
No anticoagulation |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs |
| Patients with PTS of the leg | |||||
| Role of compression stocking in preventing PTS (4.1) |
Patients with acute DVT of the leg started on anticoagulant
treatment |
Compression stockings |
No compression stockings |
QOL, PTS, and recurrent DVT |
RCTs |
| Role of compression stocking in PTS (4.2.1) |
Patients with PTS of the leg |
Compression stockings |
No compression stockings |
QOL, symptomatic relief, ulceration |
RCTs and cohort studies |
| Role of intermittent pneumatic compression in PTS (4.2.2) |
Patients with PTS of the leg |
Intermittent pneumatic compression |
No intermittent pneumatic compression |
QOL, symptomatic relief, ulceration |
RCTs and cohort studies |
| Role of venoactive medications in PTS (4.3) |
Patients with PTS of the leg |
Venoactive medications |
No venoactive medications |
QOL, PTS, and recurrent DVT |
RCTs and cohort studies |
| Patient with acute PE | |||||
| Initial anticoagulant (5.1) |
Patients with acute PE |
Anticoagulation |
No initial anticoagulation |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs |
| Whether to treat while awaiting the results of the diagnostic
work-up (5.2.1-5.2.3) |
Patients with suspected acute PE awaiting the results of the
diagnostic tests |
Anticoagulation |
No anticoagulation |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs and cohort studies |
| Timing of initiation of VKA relative to the initiation of parenteral
anticoagulation (5.3) |
Patients with acute PE |
Early initiation of VKA |
Delayed initiation of VKA |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs and cohort studies |
| Duration of initial anticoagulation (5.3) |
Patients with acute PE |
Longer duration |
Shorter duration |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs and cohort studies |
| Choice and route of initial anticoagulant (5.4.1, 5.4.2) |
Patients with acute DVT of the leg |
UFH IV or SQ |
LMWH, fondaparinux, and rivaroxaban |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs |
| Setting of initial anticoagulation (5.5) |
Patients with acute PE |
In-hospital treatment |
At-home treatment |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs |
| Thrombolytic therapy in patients with acute PE (5.6.1.1, 5.6.1.2,
5.6.1.3) |
Patients with acute PE |
Thrombolytic therapy |
No thrombolytic therapy |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs |
| Infusion time for thrombolytic therapy (5.6.2.1) |
Patients with acute PE requiring thrombolytic therapy |
Longer infusion time |
Shorter infusion time |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs and cohort studies |
| Venous access for thrombolytic therapy (5.6.2.2) |
Patients with acute PE requiring thrombolytic therapy |
Peripheral vein |
Pulmonary catheter |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs and cohort studies |
| Role of catheter-assisted thrombus removal (5.7) |
Patients with acute PE |
Use of catheter-assisted thrombus removal |
No use of catheter-assisted thrombus removal |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs and cohort studies |
| Role of surgical pulmonary embolectomy (5.8) |
Patients with acute PE |
Surgical pulmonary embolectomy |
No surgical pulmonary embolectomy |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs and cohort studies |
| Role of IVC filter in addition to anticoagulation in patients with
acute PE (5.9.1) |
Patients with acute PE started on anticoagulation |
IVC filter |
No IVC filter |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs and cohort studies |
| Role of IVC filters when anticoagulation is contraindicated
(5.9.2) |
Patients with acute PE and a contraindication to
anticoagulation |
IVC filter |
No IVC filter |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs and cohort studies |
| Role of anticoagulation in patients who initially received an IVC
filter when contraindication to anticoagulation resolves
(5.9.3) |
Patients with acute PE who initially received an IVC filter, now
contraindication to anticoagulation resolved |
Anticoagulation in addition to IVC filter |
No anticoagulation in addition to IVC filter |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs and cohort studies |
| Duration of long-term anticoagulation in patients with acute PE
(6.1-6.4) |
Patients with acute PE |
Longer duration |
Shorter duration |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs and cohort studies |
| Intensity of VKA (6.5) |
Patients with acute PE |
INR 2-3 |
Higher or lower INR range |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs and cohort studies |
| Choice of long-term anticoagulant (6.6, 6.7, 6.8) |
Patients with acute PE |
LMWH, dabigatran, rivaroxaban |
VKA |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs |
| Whether to treat an incidentally diagnosed asymptomatic acute PE
(6.9) |
Patients with incidentally diagnosed asymptomatic PE |
Anticoagulation |
No anticoagulation |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs and cohorts |
| Patient with CTPH | |||||
| Role of oral anticoagulation in CTPH (7.1.1) |
Patients with CTPH |
Oral anticoagulation |
No oral anticoagulation |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs and cohort studies |
| Role of pulmonary thromboendarterectomy in CTPH (7.1.2) |
Patients with CTPH |
Pulmonary thromboendarterectomy |
No pulmonary thromboendarterectomy |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs and cohort studies |
| Patient with SVT | |||||
| Role of anticoagulation in SVT (8.1.1, 8.1.2) |
Patients with SVT |
Anticoagulation |
No anticoagulation or other anticoagulant |
DVT and PE, major bleeding, mortality, QOL, symptomatic relief, and
PTS |
RCTs and cohort studies |
| Patient with acute UEDVT | |||||
| Acute anticoagulation (9.1.1, 9.1.2) |
Patients with UEDVT |
Parenteral anticoagulation |
No anticoagulation |
Recurrent DVT and PE, major bleeding, mortality, QOL, and
PTS |
RCTs and cohort studies |
| Role of thrombolytic therapy (9.2.1, 9.2.2) |
Patients with UEDVT |
Systemic thrombolytic therapy |
No systemic thrombolytic therapy |
Recurrent DVT and PE, major bleeding, mortality, QOL, PTS, shorter
ICU and hospital stays, and acute complications |
RCTs and cohort studies |
| Whether indwelling central venous catheter should be removed
(9.3.1) |
Patients with UEDVT and indwelling central venous catheter |
Removal of indwelling central venous catheter |
No removal of indwelling central venous catheter |
Recurrent DVT and PE, major bleeding, mortality, QOL, PTS, shorter
ICU and hospital stays, and acute complications |
RCTs and cohort studies |
| Duration of long-term anticoagulation (9.3.2-9.3.5) |
Patients with UEDVT and indwelling central venous catheter |
Longer duration |
Shorter duration |
Recurrent DVT and PE, major bleeding, mortality, QOL, PTS, shorter
ICU and hospital stays, and acute complications |
RCTs and cohort studies |
| Prevention of PTS of the arm (9.4) |
Patients with UEDVT |
Compression sleeves or venoactive medications |
No compression sleeves or venoactive medications |
QOL, PTS, and recurrent DVT |
RCTs and cohort studies |
| Treatment of PTS of the arm (9.5.1, 9.5.2) |
Patients with PTS of the arm |
Compression sleeves or venoactive medications |
No compression sleeves or venoactive medications |
QOL, symptomatic relief, and ulceration |
RCTs and cohort studies |
| Patient with thrombosis in unusual
sites | |||||
| Role of anticoagulation in splanchnic vein thrombosis (10.1,
10.2) |
Patients with splanchnic vein thrombosis |
Anticoagulation |
No anticoagulation |
Mortality, bowel ischemia, major bleeding, QOL, and symptomatic
relief |
RCTs and cohort studies |
| Role of anticoagulation in hepatic vein thrombosis (11.1, 11.2) | Patients with hepatic vein thrombosis | Anticoagulation | No anticoagulation | Mortality, liver failure, PE, major bleeding, QOL, and symptomatic relief | RCTs and cohort studies |
CTPH = chronic thromboembolic pulmonary hypertension; INR = international normalized ratio; IVC = inferior vena cava; LMWH = low-molecular-weight heparin, PE = pulmonary embolism; PICO = population, intervention, comparator, outcome; PTS = postthrombotic syndrome, QOL = quality of life; RCT = randomized controlled trial; SVT = superficial vein thrombosis; UEDVT = upper-extremity DVT; UFH = unfractionated heparin, VKA = vitamin K antagonist.
1.0 Methods
1.1 Presentation as DVT or PE
In addressing DVT, we first review studies that included (1) only patients who presented with symptomatic DVT or (2) patients who presented with DVT or PE (ie, meeting the broader criterion of VTE). For the PE components, we review studies (and subgroups within studies) that required patients to have presented with symptomatic PE (who may also have had symptoms of DVT). For this reason and because more patients with VTE present with symptoms of DVT alone than with symptoms of PE (including those who also have symptoms of DVT), the DVT section deals with a larger body of evidence than the PE section.
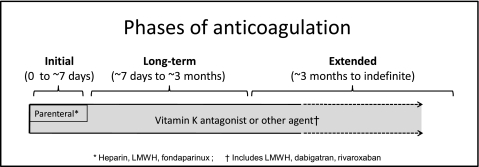
In the evaluation of anticoagulant therapy, there are a number of justifications for inclusion of patients who present with DVT and PE in the same study, and for extrapolating evidence obtained in patients with one presentation of VTE (eg, DVT) to the other presentation (eg, PE). First, a majority of patients with symptomatic DVT also have PE (symptomatic or asymptomatic), and a majority of those with symptomatic PE also have DVT (symptomatic or asymptomatic).7,8 Second, clinical trials of anticoagulant therapy have yielded similar estimates for efficacy and safety in patients with DVT alone, in those with both DVT and PE, and in those with only PE. Third, the risk of recurrence appears to be similar after PE and after proximal DVT.7,9 Consequently, the results of all studies of VTE have been considered when formulating recommendations for short- and long-term anticoagulation of proximal DVT and PE (Fig 1), and these recommendations are essentially the same for proximal DVT or PE.
Figure 1.
Phases of anticoagulation. LMWH = low-molecular-weight heparin.
There are, however, some important differences between patients who present with PE and those who present with DVT that justify separate consideration of some aspects of the treatment of PE. First, the risk of early death (within 1 month) from VTE due to either the initial acute episode or recurrent VTE is much greater after presenting with PE than after DVT9; this difference may justify more aggressive initial treatment of PE (eg, thrombolytic therapy, insertion of an inferior vena cava (IVC) filter, more intensive anticoagulant therapy) compared with DVT. Second, recurrent episodes of VTE are about three times as likely to be PE after an initial PE than after an initial DVT (ie, about 60% after a PE vs 20% after a DVT)7,9,10; this difference may justify more aggressive, or more prolonged, long-term therapy. Third, the long-term sequelae of PE are cardiorespiratory impairment, especially due to pulmonary hypertension, rather than PTS of the legs or arms. These differences are most important for recommendations about the use of thrombus removal procedures (eg, thrombolytic therapy) in patients who present with DVT and PE.
1.2 Outcomes Assessed
The outcomes important to patients we considered for most recommendations are recurrent VTE, major bleeding, and all-cause mortality. These outcomes are categorized in two different ways in the evidence profiles. Whenever data were available, fatal episodes of recurrent VTE and bleeding were included in the mortality outcome, and nonfatal episodes of recurrent VTE and bleeding were reported separately in their own categories to avoid reporting an outcome more than once in an evidence profile. However, many original reports and published meta-analyses did not report fatal and nonfatal events separately. In this situation, we have reported the outcome categories of mortality, recurrent VTE, and major bleeding, with fatal episodes of VTE and bleeding included in both mortality and two specific outcomes (ie, fatal episodes of VTE and bleeding are included in two outcomes of the evidence profile).
With both ways of reporting outcomes, we tried to specifically identify deaths from recurrent VTEs and major bleeds. As part of the assessment of the benefits and harms of a therapy, we generally assume that ∼5% of recurrent episodes of VTE are fatal11,12 and that ∼10% of major bleeds are fatal,12‐14 and if we deviated from these estimates, we noted the reasons for so doing. We did not consider surrogate outcomes (eg, vein patency) when there were adequate data addressing the corresponding outcome of importance to patients (eg, PTS).
When developing evidence profiles, we tried to obtain the baseline risk of outcomes (eg, risk of recurrent VTE or major bleeding) from observational studies because these estimates are most likely to reflect real-life incidence. In many cases, however, we used data from randomized trials because observational data were lacking or were of low quality. Methodologic issues specific to duration of anticoagulation are addressed in the section 3.1 under the subsection on general consideration in weighing the benefits and risks of different durations of anticoagulant therapy.
1.3 Patient Values and Preferences
In developing our recommendations, we took into account average patient values for each outcome and preferences for different types of antithrombotic therapy. As described in MacLean et al15 and Guyatt et al16 in these guidelines, these values and preferences for the most part were obtained from ratings that all panelists for these guidelines provided in response to standardized descriptions of different outcomes and treatments, supplemented with the findings of a systematic review of the literature on this topic.15 However, we also took into account that values and preferences vary markedly among individual patients and that often there is appreciable uncertainty about the average patient values we used.
On average, we assumed that patients attach equal value (or dislike [disutility]) to nonfatal thromboembolic and major bleeding events. Concern that the panelist rating exercise that attached a similar disutility to vitamin K antagonist (VKA) therapy (frequent blood testing and telephone or clinic visits, attention to changes in other medications) and long-term low-molecular-weight-heparin (LMWH) therapy (daily subcutaneous [SC] injection, injection site bruising or nodules) may have been misguided led us to request a review of this issue at the final meeting of all panelists. Our judgment that, on average, patients would prefer VKA therapy to long-term LMWH therapy was confirmed at that meeting.
1.4 Influence of Bleeding Risk and Cost
Usually, we did not assess how an individual patient’s risk of bleeding would influence each recommendation because (1) we considered that most recommendations would be unlikely to change based on differences in risk of bleeding (eg, anticoagulation vs no anticoagulation for acute VTE, comparison of anticoagulant regimens), (2) there are few data assessing outcomes in patients with different risks of bleeding, and (3) there is a lack of well-validated tools for stratifying risk of bleeding in patients with VTE. However, for a small number of the recommendations in which the risk of bleeding is very influential (eg, use of extended-duration anticoagulation), we stratified recommendations based on this risk (Table 2). Unless otherwise stated, the cost (eg, to the patient, a third-party payer, or society) associated with different treatments did not influence our recommendations. In most situations of uncertain benefit of a treatment, particularly if it was potentially harmful, we took the position of primum non nocere (first do no harm) and made a weak recommendation against the treatment.
Table 2.
—[Section 2.3, 3] Risk Factors for Bleeding With Anticoagulant Therapy and Estimated Risk of Major Bleeding in Low-, Moderate-, and High-Risk Categories
| Risk Factorsa | |||
| Age > 65 y17-25 | |||
| Age > 75 y17-21,23,25-34 | |||
| Previous
bleeding18,24,25,30,33-36 | |||
| Cancer20,24,30,37 | |||
| Metastatic cancer36.38, | |||
| Renal failure18,24,25,28,30,33 | |||
| Liver failure19,21,27,28 | |||
| Thrombocytopenia27,36 | |||
| Previous stroke18,25,27,39 | |||
| Diabetes18,19,28,32,34 | |||
| Anemia18,21,27,30,34 | |||
| Antiplatelet
therapy19,27,28,34,40 | |||
| Poor anticoagulant
control22,28,35 | |||
| Comorbidity and reduced functional
capacity24,28,36 | |||
| Recent surgery21,41,b | |||
| Frequent falls27 | |||
| Alcohol abuse24,25,27,34 | |||
| Estimated Absolute Risk of Major
Bleeding, % | |||
| Categorization of Risk of Bleedingc |
Low Riskd (0 Risk Factors) |
Moderate Riskd (1 Risk Factor) |
High Riskd (≥ 2 Risk
Factors) |
| Anticoagulation 0-3 moe | |||
| Baseline risk (%) |
0.6 |
1.2 |
4.8 |
| Increased risk (%) |
1.0 |
2.0 |
8.0 |
| Total risk (%) |
1.6e |
3.2 |
12.8f |
| Anticoagulation after first 3 mog | |||
| Baseline risk (%/y) |
0.3h |
0.6 |
≥ 2.5 |
| Increased risk (%/y) |
0.5 |
1.0 |
≥ 4.0 |
| Total risk (%/y) | 0.8i | 1.6i | ≥ 6.5 |
See Table 1 legend for expansion of abbreviations.
The increase in bleeding associated with a risk factor will vary with (1) severity of the risk factor (eg, location and extent of metastatic disease, platelet count), (2) temporal relationships (eg, interval from surgery or a previous bleeding episode),29 and (3) how effectively a previous cause of bleeding was corrected (eg, upper-GI bleeding).
Important for parenteral anticoagulation (eg, first 10 d) but less important for long-term or extended anticoagulation.
Although there is evidence that risk of bleeding increases with the prevalence of risk factors,20,21,25,27,30,33,34,36,42,43 this categorization scheme has not been validated. Furthermore, a single risk factor, when severe, will result in a high risk of bleeding (eg, major surgery within the past 2 d, severe thrombocytopenia).
Compared with low-risk patients, moderate-risk patients are assumed to have a twofold risk and high-risk patients an eightfold risk of major bleeding.18,20,21,27,28,30,36,44
The 1.6% corresponds to the average of major bleeding with initial UFH or LMWH therapy followed by VKA therapy (Table S6 Evidence Profile: LMWH vs IV UFH for initial anticoagulation of acute VTE). We estimated baseline risk by assuming a 2.6 relative risk of major bleeding with anticoagulation (footnote g in this table).
Consistent with frequency of major bleeding observed by Hull et al41 in high-risk patients.
We estimate that anticoagulation is associated with a 2.6-fold increase in major bleeding based on comparison of extended anticoagulation with no extended anticoagulation (Table S27 Evidence Profile: extended anticoagulation vs no extended anticoagulation for different groups of patients with VTE and without cancer). The relative risk of major bleeding during the first 3 mo of therapy may be greater that during extended VKA therapy because (1) the intensity of anticoagulation with initial parenteral therapy may be greater than with VKA therapy; (2) anticoagulant control will be less stable during the first 3 mo; and (3) predispositions to anticoagulant-induced bleeding may be uncovered during the first 3 mo of therapy.22,30,35 However, studies of patients with acute coronary syndromes do not suggest a ≥ 2.6 relative risk of major bleeding with parenteral anticoagulation (eg, UFH or LMWH) compared with control.45,46
Our estimated baseline risk of major bleeding for low-risk patients (and adjusted up for moderate- and high-risk groups as per footnote d in this table).
2.0 Treatment of Acute DVT
2.1 Initial Anticoagulation of Acute DVT of the Leg
The first and only randomized trial that compared anticoagulant therapy with no anticoagulant therapy in patients with symptomatic DVT or PE was published in 1960 by Barritt and Jordan.50 Trial results suggested that 1.5 days of heparin and 14 days of VKA therapy markedly reduced recurrent PE (0/16 vs 10/19) and appeared to reduce mortality (1/16 vs 5/19) in patients with acute PE. In the early 1990s, a single randomized trial established the need for an initial course of heparin in addition to VKA as compared with starting treatment with VKA therapy alone51 (Table 3, Table S1). (Tables that contain an “S” before the number denote supplementary tables not contained in the body of the article and available instead in an online data supplement. See the “Acknowledgments” for more information.) The need for an initial course of heparin is also supported by the observation that there are high rates of recurrent VTE during 3 months of follow-up in patients with acute VTE treated with suboptimal heparin therapy.1,3,52,53 We discuss whether isolated distal (calf) DVT should be sought and if isolated distal DVT is diagnosed, whether and how it should be treated in section 2.3.
Table 3.
—[Section 2.1] Summary of Findings: Parenteral Anticoagulation vs No Parenteral Anticoagulation in Acute VTEa,51
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated absolute
effects |
|
| Risk With No Parenteral Anticoagulation | Risk Difference With Parenteral Anticoagulation (95% CI) | ||||
| Mortality |
120 (1 study), 6 mo |
Moderateb,c due to imprecision |
RR 0.5 (0.05-5.37) |
33 per 1,000 |
16 fewer per 1,000 (from 31 fewer to 144 more) |
| VTE symptomatic extension or recurrence |
120 (1 study), 6 mo |
Moderateb,d due to imprecision |
RR 0.33 (0.11-0.98) |
200 per 1,000 |
134 fewer per 1,000 (from 4 fewer to 178 fewer) |
| Major bleeding | 120 (1 study), 6 mo | Moderateb,c due to imprecision | RR 0.67 (0.12-3.85) | 50 per 1,000 | 16 fewer per 1,000 (from 44 fewer to 142 more) |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. GRADE = Grades of Recommendations, Assessment, Development, and Evaluation; RR = risk ratio.
Both groups treated with acenocoumarol
Study described as double blinded; outcome adjudicators blinded. None of the study participants were lost to follow-up. Intention-to-treat analysis. Study was stopped early for benefit.
CI includes values suggesting no effect as well as values suggesting either appreciable benefit or appreciable harm.
Low number of events caused by the early stoppage of the trial.
Recommendation
2.1. In patients with acute DVT of the leg treated with VKA therapy, we recommend initial treatment with parenteral anticoagulation (LMWH, fondaparinux, IV unfractionated heparin [UFH], or SC UFH) over no such initial treatment (Grade 1B).
2.2 Whether to Treat With Parenteral Anticoagulation While Awaiting the Results of Diagnostic Work-up for VTE
We identified no trial addressing this question. The decision regarding treatment while awaiting test results requires balancing (1) minimizing thrombotic complications in patients with VTE and (2) avoiding bleeding in those without VTE. Our recommendations are based on two principles. First, the higher the clinical suspicion for VTE (use of validated prediction models for probability of having DVT54 or PE55,56 can usefully inform this assessment,57 the shorter the acceptable interval without treatment until results of diagnostic testing become available. Second, the higher the risk of bleeding, the longer the acceptable interval without treatment until results are available.
Our recommendations assume that patients do not have major risk factors for bleeding, such as recent surgery. The recommendations also take into account that starting anticoagulant therapy in patients who ultimately have DVT excluded is costly and is a burden to patients and the health-care system. Poor cardiopulmonary reserve may also encourage the use of anticoagulant therapy while awaiting diagnostic testing. If clinicians choose to administer anticoagulant therapy and diagnostic testing will be completed within 12 h, we suggest using a 12-h over a 24-h dose of LMWH. VKA therapy usually should not be started before VTE has been confirmed.
Recommendations
2.2.1. In patients with a high clinical suspicion of acute VTE, we suggest treatment with parenteral anticoagulants compared with no treatment while awaiting the results of diagnostic tests (Grade 2C).
2.2.2. In patients with an intermediate clinical suspicion of acute VTE, we suggest treatment with parenteral anticoagulants compared with no treatment if the results of diagnostic tests are expected to be delayed for more than 4 h (Grade 2C).
2.2.3. In patients with a low clinical suspicion of acute VTE, we suggest not treating with parenteral anticoagulants while awaiting the results of diagnostic tests, provided test results are expected within 24 h (Grade 2C).
2.3 Whether and How to Prescribe Anticoagulants to Patients With Isolated Distal DVT
Whether to Look for Isolated Distal DVT and When to Prescribe Anticoagulants if Distal DVT Is Found:
Whether patients with isolated distal DVT (DVT of the calf [peroneal, posterior tibial, anterior tibial veins] without involvement of the popliteal or more proximal veins) are identified depends on how suspected DVT is investigated.57 If all patients with suspected DVT have ultrasound examination of the calf veins (whole-leg ultrasound), isolated distal DVT accounts for about one-half of all DVT diagnosed.58 If a diagnostic approach is used that does not include ultrasound examination of the calf veins or that only performs ultrasound examination of the calf veins in selected patients, isolated distal DVT is rarely diagnosed.59
The primary goal of diagnostic testing for DVT is to identify patients who will benefit from anticoagulant therapy. This does not mean that all symptomatic DVT need to be identified. Isolated distal DVT do not need to be sought and treated provided that (1) there is strong evidence that the patient does not have a distal DVT that will extend into the proximal veins (ie, the patient is unlikely to have a distal DVT, and if a distal DVT is present, it is unlikely to extend); (2) if this criterion is not satisfied, a follow-up proximal ultrasound is done after 1 week to detect distal DVT that has extended into the proximal veins, in which case anticoagulant therapy is started; and (3) the patient does not have severe symptoms that would require anticoagulant therapy if the symptoms were due to a distal DVT.
Diagnostic approaches to suspected DVT that do not examine the calf veins (eg, use of a combination of clinical assessment, D-dimer testing, single and serial proximal vein ultrasound examination to manage patients) or only examine the calf veins in selected patients (eg, those who cannot have DVT excluded using the previously noted tests) have been proven safe and are presented in Bates et al57 in these guidelines. If the calf veins are imaged (usually with ultrasound) and isolated distal DVT is diagnosed, there are two management options: (1) treat patients with anticoagulant therapy or (2) do not treat patients with anticoagulant therapy unless extension of the DVT is detected on a follow-up ultrasound examination (eg, after 1 and 2 weeks or sooner if there is concern [there is no widely accepted protocol for surveillance ultrasound testing]).60 Natural history studies suggest that when left untreated, ∼15% of symptomatic distal DVT will extend into the proximal veins and that if extension does not occur within 2 weeks, it is unlikely to occur subsequently.7,60‐62 The risk of extension of isolated distal DVT will vary among patients (see later discussion).
As noted in Bates et al,57 these guidelines favor diagnostic approaches to suspected DVT other than routine whole-leg ultrasound. If isolated distal DVT is diagnosed, depending on the severity of patient symptoms (the more severe the symptoms, the stronger the indication for anticoagulation) and the risk for thrombus extension (the greater the risk, the stronger the indication for anticoagulation), we suggest either (1) anticoagulation or (2) withholding of anticoagulation while performing surveillance ultrasound examinations to detect thrombus extension. We consider the following to be risk factors for extension: positive D-dimer, thrombosis that is extensive or close to the proximal veins (eg, > 5 cm in length, involves multiple veins, > 7 mm in maximum diameter), no reversible provoking factor for DVT, active cancer, history of VTE, and inpatient status.7,60,63,64 Thrombosis that is confined to the muscular veins has a lower risk of extension than true isolated distal DVT.63,65 We anticipate that isolated distal DVT detected using a selective approach to whole-leg ultrasound often will satisfy criteria for initial anticoagulation, whereas distal DVT detected by routine whole-leg ultrasound often will not. A high risk for bleeding (Table 2) favors ultrasound surveillance over initial anticoagulation, and the decision to use surveillance or initial anticoagulation is expected to be sensitive to patient preferences. The evidence supporting recommendations to prescribe anticoagulants for isolated calf DVT is low quality because it is not based on direct comparisons of the two management strategies, and the ability to predict extension of distal DVT is limited.
How to Treat With Anticoagulants:
A single controlled trial of 51 patients with symptomatic isolated distal DVT, all of whom were initially treated with heparin, found that 3 months of VKA therapy prevented DVT extension and recurrent VTE (29% vs 0%, P < .01).66 The evidence in support of parenteral anticoagulation and VKA therapy for isolated distal DVT, which includes indirect evidence from patients with acute proximal DVT and PE that is presented elsewhere in this article, is of moderate quality (there is high-quality evidence that anticoagulation is effective, but uncertainty that benefits outweigh risks). There have not been evaluations of alternatives to full-dose anticoagulation of symptomatic isolated distal DVT, and it is possible that less-aggressive anticoagulant strategies may be adequate. Duration of anticoagulation for isolated distal DVT is discussed in section 3.1.
Recommendations
2.3.1. In patients with acute isolated distal DVT of the leg and without severe symptoms or risk factors for extension (see text), we suggest serial imaging of the deep veins for 2 weeks over initial anticoagulation (Grade 2C).
2.3.2. In patients with acute isolated distal DVT of the leg and severe symptoms or risk factors for extension (see text), we suggest initial anticoagulation over serial imaging of the deep veins (Grade 2C).
Remarks: Patients at high risk for bleeding are more likely to benefit from serial imaging. Patients who place a high value on avoiding the inconvenience of repeat imaging and a low value on the inconvenience of treatment and on the potential for bleeding are likely to choose initial anticoagulation over serial imaging.
Recommendations
2.3.3. In patients with acute isolated distal DVT of the leg who are managed with initial anticoagulation, we recommend using the same approach as for patients with acute proximal DVT (Grade 1B).
2.3.4. In patients with acute isolated distal DVT of the leg who are managed with serial imaging, we recommend no anticoagulation if the thrombus does not extend (Grade 1B); we suggest anticoagulation if the thrombus extends but remains confined to the distal veins (Grade 2C); we recommend anticoagulation if the thrombus extends into the proximal veins (Grade 1B).
2.4 Timing of Initiation of VKA and Associated Duration of Parenteral Anticoagulant Therapy
Until ∼20 years ago, initiation of VKA therapy was delayed until patients had received about 5 days of heparin therapy, which resulted in patients remaining in the hospital until they had received ∼10 days of heparin. Three randomized trials41,67,68 provided moderate-quality evidence that early initiation of VKA, with shortening of heparin therapy to ∼5 days, is as effective as delayed initiation of VKA with about a 10-day course of heparin (Table 4, Table S2). Shortening the duration of initial heparin therapy from about 10 to 5 days is expected to have the added advantage of reducing the risk of heparin-induced thrombocytopenia.69 If the international normalized ratio (INR) exceeds the therapeutic range (ie, INR > 3.0) prematurely, it is acceptable to stop parenteral therapy before the patient has received 5 days of treatment.
Table 4.
—[Recommendation 2.4] Summary of Findings: Early Warfarin (and Shorter Duration Heparin) vs Delayed Warfarin (and Longer Duration Heparin) for Acute VTEa-d,41,67,68
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With Delayed Warfarin Initiation (and Longer Duration Heparin) | Risk Difference With Early Warfarin Initiation (and Shorter Duration Heparin) (95% CI) | ||||
| Mortality |
688 (3 studies), 3 moe |
Moderatef,g due to imprecision |
RR 0.9 (0.41-1.95) |
24 per 1,000h |
2 fewer per 1,000 (from 14 fewer to 23 more) |
| Recurrent VTE |
688 (3 studies), 3 moe |
Moderatef,g due to imprecision |
RR 0.83 (0.4-1.74) |
47 per 1,000h |
8 fewer per 1,000 (from 28 fewer to 35 more) |
| Major bleeding | 688 (3 studies), 3 moi | Highf,j,k | RR 1.48 (0.68-3.23) | 16 per 1,000h | 14 more per 1,000 (from 9 fewer to 66 more) |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. See Table 1 and 3 legends for expansion of abbreviations.
Most patients had proximal DVT, some had isolated distal DVT, most DVT were symptomatic (asymptomatic DVT included in Hull et al41), and few had PE (only included in Gallus et al67).
The early initiation of VKA was associated with a fewer number of days of heparin therapy (4.1 vs 9.5 in Gallus et al67; 5 vs 10 in Hull et al41) and a fewer number of days of hospital stay (9.1 vs 13.0 in Gallus et al; 11.7 vs 14.7 in Hull et al; 11.9 vs 16.0 in Leroyer et al68).
VKA therapy started within 1 day of starting heparin therapy (UFH in two studies and LMWH in one study).
VKA therapy delayed for 4 to 10 d.
Outcome assessment was at hospital discharge in the study by Gallus et al67 (although there was also extended follow-up) and 3 mo in the studies by Hull et al41 and Leroyer et al.68
Patients and investigators were not blinded in two studies (Gallus et al67 and Leroyer et al68) and were blinded in one study (Hull et al41). Concealment was not clearly described but was probable in the three studies. Primary outcome appears to have been assessed after a shorter duration of follow-up in the shorter treatment arm of one study because of earlier discharge from hospital, and 20% of subjects in this study were excluded from the final analysis postrandomization (Gallus et al).
The 95% CI on relative effect includes both clinically important benefit and clinically important harm.
Event rate corresponds to the median event rate in the included studies.
Bleeding was assessed early (in hospital or in the first 10 d) in two studies (Gallus et al67 and Hull et al41) and at 3 mo in one study (Leroyer et al68).
It is unclear whether bleeding was assessed at 10 d in all subjects or just while heparin was being administered, which could yield a biased estimate in favor of short-duration therapy in one study (Hull et al41).
Because the shorter duration of heparin therapy is very unlikely to increase bleeding, the wide 95% CIs around the relative effect of shorter therapy on risk of bleeding is not a major concern.
Recommendation
2.4. In patients with acute DVT of the leg, we recommend early initiation of VKA (eg, same day as parenteral therapy is started) over delayed initiation, and continuation of parenteral anticoagulation for a minimum of 5 days and until the INR is 2.0 or above for at least 24 h (Grade 1B).
2.5 Choice of Initial Anticoagulant Regimen in Patients With Proximal DVT
Initial anticoagulant regimens vary according to the drug, the route of administration, and whether dose is adjusted in response to laboratory tests of coagulation. Six options are available for the initial treatment of DVT: (1) SC LMWH without monitoring, (2) IV UFH with monitoring, (3) SC UFH given based on weight initially, with monitoring, (4) SC UFH given based on weight initially, without monitoring, (5) SC fondaparinux given without monitoring, and (6) rivaroxaban given orally. We considered the SC UFH options as a single category because results were similar in studies that used SC UFH with and without laboratory monitoring (Table 5, Tables S3-S5). Rivaroxaban is used in the acute treatment of VTE without initial parenteral therapy; studies of its use for the acute treatment of VTE are reviewed under long-term treatment of DVT (section 3.1) and PE (section 6) of this article. Recommendations for dosing and monitoring of IV UFH, SC UFH, and SC LMWH are addressed in Garcia et al1 and Holbrook et al3 in these guidelines. Because LMWH, fondaparinux, and rivaroxaban have substantial renal excretion, these agents should be avoided (eg, use UFH instead) or should be used with coagulation monitoring (test selection is specific to each agent and requires expert interpretation) in patients with marked renal impairment (eg, estimated creatinine clearance < 30 mL/min [in a 70-year-old weighing 70 kg, a creatinine clearance of 30 mL/min corresponds to a serum creatinine of about 200 μmol/L (2.3 mg/dL) in a man and 175 μmol/L (2.0 mg/dL) in a woman] http://www.nephron.com/cgi-bin/CGSIdefault.cgi).
Table 5.
—[Section 2.5.1] Summary of Findings: LMWH vs SC UFH for Initial Anticoagulation of Acute VTE70,71,76,77
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With SC UFH | Risk Difference With LMWH (95% CI) | ||||
| All-cause mortality |
1,566 (3 studies), 3 mo |
Moderatea,b due to imprecision |
RR 1.1 (0.68-1.76) |
33 per 1,000c |
3 more per 1,000 (from 11 fewer to 25 more) |
| Recurrent VTE |
1,563 (3 studies), 3 mo |
Moderatea,b due to imprecision |
RR 0.87 (0.52-1.45) |
42 per 1,000c |
5 fewer per 1,000 (from 20 fewer to 19 more) |
| Major bleeding | 1,634 (4 studies), 3 mo | Moderatea,b due to imprecision | RR 1.27 (0.56-2.9) | 16 per 1,000c | 4 more per 1,000 (from 7 fewer to 30 more) |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. SC = subcutaneous. See Table 1 and 3 legends for expansion of abbreviations.
In the two largest trials (Prandoni et al,70 Kearon et al;71 87% of patients), allocation was concealed, outcome adjudicators and data analysts were concealed, analysis was intention to treat, and there were no losses to follow-up.
Precision judged from the perspective of whether SC heparin is noninferior to LMWH. The total number of events and the total number of participants were relatively low.
Event rate corresponds to the median event rate in the included studies.
LMWH Compared With IV UFH for the Initial Treatment of DVT:
A number of meta-analyses72‐75 have summarized the trials addressing this question. The evidence suggests that LMWH is associated with decreased mortality, lower recurrence of VTE, and decreased incidence of major bleeding compared with IV UFH (Table 6, Table S6). However, the quality of supporting evidence is low due to a high risk of bias in the primary studies, and evidence of publication bias in favor of LMWH. LMWH has the advantage over IV UFH that it is much easier to administer (which makes outpatient treatment feasible) and that it has a lower potential for heparin-induced thrombocytopenia,69 but the disadvantage is that it accumulates in patients with renal failure.
Table 6.
—[Section 2.5.1] Summary of Findings: LMWH vs IV UFH for Initial Anticoagulation of Acute VTE74
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With IV UFH | Risk Difference With LMWH (95% CI) | ||||
| All-cause mortality |
7,908 (17 studies), 3 mo |
Lowa,b due
to risk of bias, publication bias |
RR 0.79 (0.66-0.95) |
46 per 1,000c |
10 fewer per 1,000 (from 2 fewer to 16 fewer) |
| Recurrent VTE |
7,976 (17 studies), 3 mo |
Lowa,b due
to risk of bias, publication bias |
RR 0.72 (0.58-0.89) |
55 per 1,000c |
15 fewer per ,1000 (from 6 fewer to 23 fewer) |
| Major bleeding | 6,910 (20 studies), 3 mo | Lowa,b,d due to risk of bias, publication bias | RR 0.67 (0.45-1) | 15 per 1,000c | 5 fewer per 1,000 (from 8 fewer to 0 more) |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. See Table 1 and 3 legends for expansion of abbreviations.
Of the 20 trials, allocation was concealed in nine and was unclear whether concealed in the remaining 11. In 18 trials, outcome assessors were blinded. Seven trials did not have any postrandomization exclusions or losses to follow-up. Ten trials reported the number of participants lost to follow-up, which ranged from 1.0% to 12.7%. One trial did not report the drop-outs.
Inverted funnel plot very suggestive of publication bias. Many of the included studies are of small size, and all were funded by industry.
Event rate corresponds to the median event rate in the included studies.
CI includes values suggesting significant benefit and no effect.
SC UFH Compared With LMWH for the Initial Treatment of DVT:
Four randomized trials have compared SC UFH with SC LMWH (Table 6, Tables S3-S5).70,71,76,77 This evidence suggests that SC UFH is associated with a similar frequency of mortality, recurrent VTE, and major bleeding as LMWH. However, the quality of the evidence is moderate because of imprecision. LMWH has the disadvantage of a higher cost but is more convenient to use (LMWH can be administered once daily [see later discussion]), is more widely available for use in outpatients, has a lower potential for heparin-induced thrombocytopenia,69 and there is much more experience with its use than with SC UFH.
Fondaparinux Compared With LMWH for the Initial Treatment of DVT:
The Matisse-DVT trial78 compared fondaparinux with LMWH for short-term treatment of DVT (Table 7, Table S7). This study suggests that fondaparinux is associated with a similar frequency of mortality, recurrent VTE, and major bleeding as LMWH. However, the quality of the evidence from this study was moderate because of imprecision. Evidence that fondaparinux is effective for the treatment of PE79 (section 5.4) supports the equivalence of fondaparinux to LMWH for the treatment of acute VTE.
Table 7.
—[Section 2.5.1] Summary of Findings: Fondaparinux vs LMWH for Initial Anticoagulation of Acute DVTa-c,78
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With LMWH | Risk Difference With Fondaparinux (95% CI) | ||||
| Mortality |
2,205 (1 study), 3 mo |
Moderated,e due to imprecision |
RR 1.25 (0.8-1.97) |
30 per 1,000 |
7 more per 1,000 (from 6 fewer to 29 more) |
| Recurrent VTE |
2,205 (1 study), 3 mo |
Moderated,e due to imprecision |
RR 0.96 (0.64-1.45) |
41 per 1,000f |
2 fewer per 1,000 (from 15 fewer to 18 more) |
| Major bleeding | 2,205 (1 study), 3 mo | Moderated,e due to imprecision | RR 0.93 (0.43-2.03) | 12 per 1,000g | 1 fewer per 1,000 (from 7 fewer to 12 more) |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. See Table 1 and 3 legends for expansion of abbreviations.
All patients had acute symptomatic DVT.
Fondaparinux 7.5 mg (5.0 mg in patients weighing < 50 kg and 10.0 mg in patients weighing > 100 kg) SC once daily for at least 5 d and until VKAs induced an INR > 2.0.
Enoxaparin 1 mg/kg of body weight SC bid for at least 5 d and until VKAs induced an INR > 2.0.
Allocation was concealed. Patients, providers, data collectors, and outcome adjudicators were blinded. Analysis excluded 0.6% of randomized patients. Not stopped early for benefit.
CI includes values suggesting no effect and values suggesting either benefit or harm; relatively low number of events.
Five fatal VTE in fondaparinux group and five fatal VTE in LMWH group.
Twelve patients in the fondaparinux group and 13 in the LMWH group had a major bleeding event during the initial period (7 d). Of these, two in the fondaparinux group and none in the LMWH group were fatal.
Fondaparinux Compared With IV UFH for the Initial Treatment of DVT:
In the absence of direct evidence in patients with DVT, indirect evidence in patients with acute PE (section 5.4) suggests that fondaparinux is equivalent to IV UFH.79 As noted previously, we judge that fondaparinux and LMWH are equivalent; fondaparinux also shares the advantages that LMWH has over IV UFH and the disadvantage that it is renally excreted (section 2.5). The quality of the evidence regarding the comparison of fondaparinux and UFH is moderate as, although there is some indirectness, it is minor.
Fondaparinux Compared With SC UFH for the Initial Treatment of DVT:
There is no direct evidence for this comparison in any patient population. Our recommendation is based on our assessment that fondaparinux and LMWH are equivalent and that fondaparinux shares the advantages that LMWH has over SC UFH (section 2.5). This recommendation does not take into account difference in purchase cost between SC UFH and fondaparinux and is based on low-quality evidence.
Once- vs Twice-Daily Administration of LMWH for Initial Treatment of DVT:
Two meta-analyses80,81 summarized six studies comparing once-daily and twice-daily administrations of the same LMWH.82‐87 Table 8 and Table S8 summarize the findings of five of these studies83‐87 that had unconfounded comparisons. This evidence suggests that LMWH once daily and twice daily are associated with similar mortality, recurrent VTE, and major bleeding. However, the quality of the evidence is low because of imprecision and inconsistency. The sixth study that used a lower total daily dose of LMWH with once-daily compared with twice-daily administration (enoxaparin 1.5 mg/kg once daily vs 1.0 mg/kg bid; enoxaparin 2 mg/kg once daily is not used) suggested that outcomes might be inferior with this once-daily regimen.85
Table 8.
—[Section 2.5.2] Summary of Findings: LMWH Once vs Twice Daily for Initial Anticoagulation of Acute VTEa,b,81
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With Twice Daily | Risk Difference With LMWH Once Daily (95% CI) | ||||
| Mortality |
1,261 (3 studies), 3 mo |
Lowc-e due
to inconsistency and imprecision |
RR 1.05 (0.57-1.94) |
31 per 1,000 |
2 more per 1,000 (from 13 fewer to 29 more) |
| VTE recurrence |
1,261 (3 studies), 3 mo |
Lowc,e,f due
to inconsistency and imprecision |
RR 0.86 (0.52-1.42) |
49 per 1,000 |
7 fewer per 1,000 (from 24 fewer to 21 more) |
| Major bleeding | 1,522 (5 studies), 10 d | Moderatec,e due to imprecision | RR 1.13 (0.48-2.66) | 12 per 1,000 | 2 more per 1,000 (from 6 fewer to 20 more) |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. See Table 1 and 3 legends for expansion of abbreviations.
Of the five included studies, one included patients with PE and DVT and four included only patients with DVT. All studies addressed the initial management of VTE.
The five included studies used four brands of LMWH (enoxaparin, tinzaparin, dalteparin, and nadroparin). In Merli et al,85 enoxaparin 1 mg/kg bid was compared with 1.5 mg/kg once daily. Holmström et al84 adjusted the dose to anti-Xa levels, which resulted in different daily doses after a number of days. In the remaining studies, the dose of the once-daily administration was double the dose of the twice-daily administration (equal total daily dose).
All included studies concealed allocation. Two studies had a double-blind design, and two others were single blind. One study did not mention blinding. Intention to treat likely used in all studies. Participants were lost to follow-up in only two studies (0.3% and 2.2%).
I2 = 37%; point effect estimate in favor of twice-daily dose in Merli et al85 and in favor of once-daily dose in Charbonnier et al.83
Imprecision judged relative to no difference.
I2 = 65%; point effect estimate in favor of twice-daily dose in Merli et al85 and in favor of once-daily dose in Charbonnier.83
Recommendations
2.5.1. In patients with acute DVT of the leg, we suggest LMWH or fondaparinux over IV UFH (Grade 2C) and over SC UFH (Grade 2B for LMWH; Grade 2C for fondaparinux).
Remarks: Local considerations such as cost, availability, and familiarity of use dictate the choice between fondaparinux and LMWH. LMWH and fondaparinux are retained in patients with renal impairment, whereas this is not a concern with UFH.
2.5.2. In patients with acute DVT of the leg treated with LMWH, we suggest once- over twice-daily administration (Grade 2C).
Remarks: This recommendation only applies when the approved once-daily regimen uses the same daily dose as the twice-daily regimen (ie, the once-daily injection contains double the dose of each twice-daily injection). It also places value on avoiding an extra injection per day.
2.6 Initial Treatment With Rivaroxaban vs Parenteral Therapy
One trial directly compared short- and long-term rivaroxaban (without initial parenteral anticoagulation) with parenteral anticoagulation (LMWH) and VKA in patients with acute DVT.88 The findings of this study and associated recommendations are presented in section 3.3.
2.7 At-Home vs In-Hospital Initial Treatment of DVT
One trial of 201 patients directly compared outpatient and inpatient administration of the same initial anticoagulant regimen (three LMWH preparations were used); there were few recurrent VTE and major bleeds in each group.89 A number of trials90‐94 have compared LMWH administered at home (without hospital admission or after early discharge) in a substantial proportion of patients, with IV UFH administered in the hospital (Table 9, Table S9). This evidence suggests that home treatment is not associated with an increase in mortality, recurrent VTE, or major bleeding and may be associated with improved outcomes. However, the quality of the evidence is moderate because of indirectness (patients were not explicitly randomized to home therapy in most studies) and imprecision.
Table 9.
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence(GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With Hospital Treatment | Risk Difference With Home Treatment (95% CI) | ||||
| Mortality |
1,708 (6 studies), 3 mo |
Lowc-f due to
indirectness and imprecision |
RR 0.72 (0.45-1.15) |
46 per 1,000 |
13 fewer per 1,000 (from 25 fewer to 7 more) |
| Recurrent VTE |
1,708 (6 studies), 3 mo |
Moderatec-e due to indirectness |
RR 0.61 (0.42-0.9) |
74 per 1,000 |
29 fewer per 1,000 (from 7 fewer to 43 fewer) |
| Major bleeding |
1,708 (6 studies), 3 mo |
Moderatec-e,g due to indirectness |
RR 0.67 (0.33-1.36) |
21 per 1,000 |
7 fewer per 1,000 (from 14 fewer to 8 more) |
| QOL | 0 (3 studiesh), 3 mo | Lowi-k due to indirectness and imprecision | Not estimable | … | h |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. See Table 1 and 3 legends for expansion of abbreviations.
Studies included in the systematic review should have recruited patients whose home circumstances were adequate.
All studies included patients with lower-extremity DVT and excluded patients with suspected or confirmed PE. Studies also excluded patients who were pregnant.
Four studies had partial hospital treatment of many in the home arm: Koopman et al92 (mean hospital stay, 2.7 in home arm vs 8.1 d in hospital arm), Levine et al93 (2.1 vs 6.5 d), Boccalon et al89 (1 vs 9.6 d), and Ramacciotti et al94 (3 vs 7 d). In Daskalopoulos et al,91 there was no hospital stay at all in the home group. Chong et al90 did not report duration of hospital stay.
Only one study (Boccalon et al89) used LMWH in both treatment arms. Remaining studies used UFH in the inpatient arm and LMWH in the outpatient arm.
Out of six studies, allocation was clearly concealed in three (unclear in remaining three), outcome adjudicators were blinded in the two largest studies (unclear in remaining four), loss to follow-up was significant in only one small study, intention-to-treat analysis was conducted in four (unclear in remaining two), and no study was stopped early for benefit. Overall, the judgment was that these limitations would not warrant downgrading of quality; it has already been downgraded by at least one level based on other factors.
The CI includes values suggesting benefit and harm.
Judged as precise based on the narrow CI around absolute effect.
Bäckman et al95 reported evaluation of health-related QOL using the EQ-5D. They found no differences in mean QOL scores or in the proportion of patients showing improvement in self-rated health state. Koopman et al92 evaluated health-related QOL using the Medical Outcome Study Short Form-20 and an adapted version of the Rotterdam Symptom Checklist. The changes over time were similar in both groups, except that the patients receiving LMWH had better scores for physical activity (P = .002) and social functioning (P = .001) at the end of the initial treatment. The authors did not report enough data to assess precision and clinical significance of results. O’Brien et al96 assessed changes in QOL using the Medical Outcome Study Short Form-36 in 300 patients participating in Levine et al.93 They found that the change in scores from baseline to day 7 was not significantly different between the treatment groups for seven of the eight domains. The one exception was the domain of social functioning, where a greater improvement was observed for the outpatient group.
Potential inconsistency as Bäckman et al95 showed no effect, whereas Koopman et al92 and O’Brien et al96 showed potential benefit.
Two of the three studies had partial hospital treatment of many in the home arm: Koopman et al92 (mean hospital stay, 2.7 in home arm vs 8.1 d in hospital arm) and Levine et al93 (2.1 vs 6.5 d).
Not able to evaluate, but imprecision is possible. Taken together with the potential inconsistency, we downgraded the quality of evidence by one level.
Health economic evaluations that have assessed initial treatment of DVT at home, although they have weaknesses (eg, industry funded, not derived from trials in which LMWH was used both in the hospital and at home, short time horizon (ie, ≤ 3 months), and limited use of sensitivity analyses), all conclude that home treatment is cost-saving (about US $500-$2,500 per patient).95‐101
Recommendation
2.7. In patients with acute DVT of the leg and whose home circumstances are adequate, we recommend initial treatment at home over treatment in hospital (Grade 1B).
Remarks: The recommendation is conditional on the adequacy of the following home circumstances: well-maintained living conditions, strong support from family or friends, phone access, and ability to quickly return to hospital if there is deterioration. It is also conditional on the patient feeling well enough to be treated at home (eg, does not have severe leg symptoms or comorbidity).
2.8 Treatment Strategies of Thrombus Removal for Acute DVT
Treatments that actively remove thrombus in patients with acute DVT have the potential to reduce acute symptoms and the risk of developing PTS. Patients with DVT that involves the iliac and common femoral veins are at highest risk for PTS and, therefore, are the subset with the greatest potential to benefit from thrombus removal strategies.102 Thrombus removal strategies are indicated in patients with the very rare complication of impending venous gangrene despite optimal anticoagulant therapy; such patients are not the focus of the following sections. A recent trial that randomized 183 patients with proximal DVT to percutaneous endovascular intervention or to anticoagulant therapy alone reported reduced acute symptoms, hospital stay, recurrent VTE, and PTS at 6 months in the thrombus removal group.103 This trial, which had a high potential for bias (randomization not described, no blinding), is not considered further because it was not possible to determine outcomes in patients treated with mechanical thrombectomy alone and in those treated with thrombolytic therapy.
2.9 Catheter-Directed Thrombolysis for Acute DVT
The rationale for catheter-directed thrombolysis (CDT) is that compared with systemic thrombolysis, it will achieve lysis of thrombus more rapidly and with lower doses of thrombolytic therapy, thereby reducing serious bleeding. The addition of mechanical thrombus fragmentation (collectively referred to as pharmacomechanical thrombolysis) with or without aspiration can further reduce the dose of thrombolytic therapy and shorten the procedure.104
One randomized trial of CDT has been completed,105 and a second has reported short-term outcomes (but not the development of PTS).106,107 Table 10 and Table S10 present the combined findings from these studies (see also Tables S11 and S12). This evidence suggests that CDT may reduce PTS and improve quality of life without being associated with an unacceptable increase in bleeding. However, the quality of evidence is low for mortality, recurrent VTE, and major bleeding because of very serious imprecision, and is low for PTS because of indirectness (ie, use of surrogate outcome [PTS has yet to be measured directly during follow-up]).
Table 10.
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With No CDT | Risk Difference With CDT (95% CI) | ||||
| Mortality |
153 (2 studies), 3 mo |
Lowc,d due to
imprecision |
RR 0.14 (0.01-2.71) |
39 per 1,000e |
34 fewer per 1,000 (from 39 fewer to 67 more) |
| Nonfatal recurrent VTE |
153 (1 study), 3 mo |
Lowc,d due to
imprecision |
RR 0.35 (0-8.09) |
48 per 1,000f |
31 fewer per 1,000 (from 48 fewer to 340 more) |
| Nonfatal major bleeding |
153 (2 studies), 7 d |
Lowc,d due to
imprecision |
RR 2.00 (0.19-19.46) |
29 per 1,0006,7 |
29 more per 1,000 (from 23 fewer to 535 more) |
| PTS (complete lysis on venography [Elsharawy et
al73]; patency on ultrasound and air plethysmography
[Enden et al74]) |
138 (2 studies), 2 y |
Moderatec,g due to indirectness |
RR 0.46 (0-0.79) |
588 per 1,000h |
318 fewer per 1,000 (from 123 fewer to 588 fewer)i |
| QOL (SF-12, HUI Mark version 2/3 questionnaires) | 98 (1 studyj), 16 mo | Lowk,l | See footnotem | ||
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. HUI = Health Utilities Index; SF-12 = Medical Outcomes Survey Short Form-12; VETO = Venous Thrombosis Outcomes. See Table 1 and 3 legends for expansion of other abbreviations.
In selected patients with extensive acute proximal DVT (eg, iliofemoral DVT, symptoms for < 14 d, good functional status, life expectancy ≥ 1 y) who have a low risk of bleeding.
All patients prescribed anticoagulants per protocol, but the intervention group receives CDT in addition to anticoagulation.
Allocation was concealed in Enden et al106 but unclear in Elsharawy et al.105 Outcome assessor blinded in both studies. Follow-up rates were 87% in Enden et al and 100% in Elsharawy et al. Neither of the studies was stopped early for benefit.
CI includes values suggesting both benefit and harm.
Three control patients died of cancer.
Baseline risks for nonfatal recurrent VTE and for major bleeding derived from Douketis et al.108
Surrogate outcome: absence of patency at 6 mo in Enden et al106 study; absence of complete lysis at 6 mo in Elsharawy et al105 study.
This estimate is based on the findings of the VETO study.102 This probably underestimates PTS baseline risk given that overall, 52% of patients reported the current use of compression stockings during study follow-up.
Severe PTS: assuming the same RR of 0.46 and a baseline risk of 13.8%,102 the absolute reduction is 75 fewer severe PTS per 1,000 (from 29 fewer to 138 fewer) over 2 y.
Camerota et al.109
Participation rate was 65%.
Recall was used to measure QOL prior to the thrombotic event; we did not consider these measurements.
At the initial follow-up (mean, 16 mo), patients treated with CDT reported a trend toward a higher mental summary scale (P = .087) and improved HUI (P = .078). They reported better overall role physical functioning (P = .046), less stigma (P = .033), less health distress (P = .022), and fewer overall symptoms (P = .006) compared with patients who were treated with anticoagulation alone.
In addition to the two randomized trials,105,106 findings of observational studies suggest that CDT improves venous patency and preserves venous valve function (Tables S11 and S12). Use of CDT, however, requires substantial resources and expertise. Patients who are most likely to benefit from CDT have iliofemoral DVT, symptoms for < 14 days, good functional status, life expectancy of ≥ 1 year, and a low risk of bleeding (Table 11). Because the balance of risks and benefits with CDT is uncertain, anticoagulant therapy alone is an acceptable alternative to CDT in all patients with acute DVT who do not have impending venous gangrene.
Table 11.
—[Section 2.9, 2.10, 5.6, 9.2] Risk Factors for Bleeding With and Contraindications to Use of Thrombolytic Therapy (Both Systemic and Locally Administered)
| Major contraindicationsa |
| Structural intracranial disease |
| Previous intracranial hemorrhage |
| Ischemic stroke within 3 mo |
| Active bleeding |
| Recent brain or spinal surgery |
| Recent head trauma with fracture or brain injury |
| Bleeding diathesis |
| Relative contraindicationsb |
| Systolic BP > 180 mm Hg |
| Diastolic BP > 110 mm Hg |
| Recent bleeding (nonintracranial) |
| Recent surgery |
| Recent invasive procedure |
| Ischemic stroke more that 3 mo previously |
| Anticoagulation (eg, VKA therapy) |
| Traumatic cardiopulmonary resuscitation |
| Pericarditis or pericardial fluid |
| Diabetic retinopathy |
| Pregnancy |
| Age > 75 y |
| Low body weight (eg, < 60 kg) |
| Female sex |
| Black race |
Among 32,000 Medicare patients (≥ 65 y) with myocardial infarction who were treated with thrombolytic therapy, the following factors were independently associated with intracranial hemorrhage: age ≥ 75 y (OR, 1.6), black race (OR, 1.6), female sex (OR, 1.4), previous stroke (OR, 1.5), systolic BP ≥ 160 mm Hg (OR, 1.8), women weighing ≤ 65 kg or men weighing ≤ 80 kg (OR, 1.5), and INR > 4 (OR, 2.2).110 The rate of intracranial hemorrhage increased from 0.7% with none or one of these risk factors to 4.1% with five or more of these risk factors. Among 32,000 patients with myocardial infarction who were treated with thrombolytic therapy in five clinical trials, the following factors were independently associated with moderate or severe bleeding: older age (OR, 1.04 per year), black race (OR, 1.4), female sex (OR, 1.5), hypertension (OR, 1.2), and lower weight (OR, 0.99/kg).111 We estimated that systemic thrombolytic therapy is associated with a relative risk of major bleeding of 3.5 within 35 d (RR, ∼7 for intracranial bleeding); about three-fourths of the excess of major bleeds with thrombolytic therapy occur in the first 24 h.112 See Table 1 legend for expansion of abbreviations.
The presence of major contraindications usually precludes use of thrombolytic therapy and, consequently, these factors have not been well studied as risk factors for bleeding associated with thrombolytic therapy. The factors listed in this table are consistent with other recommendations for the use of thrombolytic therapy in patients with PE.72,259,260,454 104,111,113,114
Risk factors for bleeding during anticoagulant therapy noted in Table 10 that are not included in this table are also likely to be relative contraindications to thrombolytic therapy. The increase in bleeding associated with a risk factor will vary with (1) severity of the risk factor (eg, extent of trauma or recent surgery) and (2) temporal relationships (eg, interval from surgery or a previous bleeding episode believed to decrease markedly after ∼2 wk). Risk factors for bleeding at critical sites (eg, intracranial, intraocular) or noncompressible sites are stronger contraindications for thrombolytic therapy.
There is no single standardized approach to performing CDT or pharmacomechanical thrombolysis. If these interventions are performed, the technique used will vary with local resources and expertise. If CDT has been successful but there are residual lesions in the common femoral or more proximal veins, balloon angioplasty and stenting often are used to relieve obstruction. There are inadequate data to assess the benefit or risk of inserting an IVC filter in patients who have CDT performed (recommended by manufacturer with some endovascular devices and techniques, whereas not with others). Percutaneous mechanical venous thrombectomy without concomitant thrombolysis has not been evaluated in randomized trials, and its use is discouraged because small retrospective studies suggest that it often fails to remove much of the thrombus115,116 and is associated with a high risk of PE.117,118
Recommendation
2.9. In patients with acute proximal DVT of the leg, we suggest anticoagulant therapy alone over CDT (Grade 2C).
Remarks: Patients who are most likely to benefit from CDT (see text) and attach a high value to prevention of PTS and a lower value to the initial complexity, cost, and risk of bleeding with CDT are likely to choose CDT over anticoagulation alone.
2.10 Systemic Thrombolytic Therapy for Acute DVT
Many trials of systemic thrombolysis for the treatment of DVT assessed early lysis, often reported bleeding, but rarely reported recurrent VTE or development of PTS (Table S13 and S14).119‐137 A meta-analysis138 summarized the findings of trials that assessed mortality, recurrent VTE, major bleeding, and PTS (Table 12, Table S15). This evidence suggests that systemic thrombolysis has the potential to reduce PTS at the expense of an increase in major bleeding. However, the overall quality of this evidence is low because of imprecision and risk of bias.
Table 12.
—[Section 2.10] Summary of Findings: Systemic Lysis vs No Systemic Lysis for Extensive Acute DVT of the Leg138
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With No Systemic Lysis | Risk Difference With Systemic Lysis (95% CI) | ||||
| Mortality |
688 (5 studies), 3 moa |
Lowb-e due to
imprecision |
RR 0.86 (0.27-2.68) |
21 per 1,000 |
3 fewer per 1,000 (from 16 fewer to 36 more) |
| Nonfatal recurrent VTE |
687 (3 studies), 3 mof |
Lowd,e,g due to
imprecision |
RR 1.28 (0.25-6.68) |
48 per 1,000h |
13 more per 1,000 (from 36 fewer to 273 more) |
| Nonfatal major bleeding |
688 (10 studies), 3 mof |
Moderatec,d,i due to imprecision |
RR 1.84 (0.94-3.59) |
29 per 1,000h,j |
24 more per 1,000 (from 2 fewer to 75 more) |
| PTS |
678 (2 studies), 2 yk |
Lowd,e,l,m due to
risk of bias and imprecision |
RR 0.71 (0.49-1.04) |
588 per 1,000n |
171 fewer per 1,000 (from 300 fewer to 24 more)o |
| QOL not measured | … | … | … | … | … |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. See Table 1, 3, and 10 legends for expansion of abbreviations.
Range of follow-up in included studies, 1 to 72 mo.
Allocation was concealed in three of five studies. Follow-up inadequate in one of five studies (Common et al139). Excluding this study from the analysis does not change the effect estimate. All studies had blinded outcome assessors. None of the studies used a placebo control.
The population of one study (Schulman et al131) comprised patients with calf vein thrombosis.
Interventions varied across studies with regard to agent (eg, tissue plasminogen activator, streptokinase, urokinase), dose, use of the pedal vein administration, duration of treatment, and concomitant drugs (eg, steroids). However, we did not downgrade for indirectness given that there was no standard regimen and all analyses showed no heterogeneity in results.
CI included both no effect and a potentially significant effect.
Range of follow-up in included studies, 1 to 30 d.
Allocation was concealed in two of three studies. Follow-up adequate in all studies. All studies had blinded outcome assessors. None of the studies used a placebo control.
Baseline risks for nonfatal recurrent VTE and for major bleeding derived from Douketis et al.108
Allocation was concealed in seven of 10 studies. Follow-up inadequate in one of 10 studies (Common et al139). Excluding this study from the analysis does not affect the effect estimate. All studies had blinded outcome assessors. Two studies used placebo (Turpie et al135 and Verhaeghe et al136).
Only 4% of all major bleeding events were intracranial bleeds.
Range of follow-up in included studies, 1 to 6 y.
Allocation was concealed in two of two studies. Follow-up adequate in both studies. Both studies had blinded outcome assessors. Neither study used placebo control.
No use of a standardized validated tool reported.
This estimate is based on the findings of the VETO study.102 This probably underestimates PTS baseline risk, given that overall, 52% of patients reported the current use of compression stockings during study follow-up.
Severe PTS: Assuming the same RR of 0.71 and a baseline risk of 13.8%,102 the absolute reduction is 40 fewer severe PTS per 1,000 (from 70 fewer to 6 more) over 2 y.
There have been no direct comparisons of different thrombolytic agents; however, prolonged infusions of streptokinase that were used predominantly in the earlier studies appear to be associated with higher bleeding rates than other regimens. No randomized trial has compared systemic thrombolysis with CDT, but a single-center, retrospective study127 suggested that systemic thrombolysis achieves less lysis (31% vs 50%) and less preservation of valve function (13% vs 44%) (Tables S13 and S14), and the higher doses of thrombolytic agent used with systemic thrombolysis are expected to be associated with a higher risk of nonprocedure-related bleeding.
We believe that systemic thrombolysis should be considered only in patients who meet all of the following criteria: iliofemoral DVT, symptoms for < 14 days, good functional status, life expectancy of ≥ 1 year, and low risk of bleeding (Table 11). Based on low-quality evidence of greater effectiveness and less bleeding, if resources and expertise are available to perform CDT, we consider it the preferable approach. Because the balance of risks and benefits with systemic thrombolysis is uncertain, and particularly because of concerns about major bleeding, anticoagulant therapy alone is an acceptable alternative to systemic thrombolysis in all patients with acute DVT who do not have impending venous gangrene.
Recommendation
2.10. In patients with acute proximal DVT of the leg, we suggest anticoagulant therapy alone over systemic thrombolysis (Grade 2C).
Remarks: Patients who are most likely to benefit from systemic thrombolytic therapy (see text), who do not have access to CDT, and who attach a high value to prevention of PTS and a lower value to the initial complexity, cost, and risk of bleeding with systemic thrombolytic therapy are likely to choose systemic thrombolytic therapy over anticoagulation alone.
2.11 Operative Venous Thrombectomy for Acute DVT
Operative venous thrombectomy, with contemporary operative techniques140 and more effective anticoagulant regimens, appears to achieve improved outcomes compared with earlier reports.141,142 A single small randomized trial with extended follow-up compared iliofemoral venous thrombectomy with a temporary arteriovenous fistula plus anticoagulation with anticoagulation alone.143‐145 Results at 6 months, 5 years, and 10 years suggested improved iliac vein patency, less leg swelling, and fewer leg ulcers with thrombectomy (Table 13, Tables S16-S18).143‐145 Evidence from this trial is of low quality because of imprecision and risk of bias.
Table 13.
—[Section 2.11] Summary of Findings: Surgical Thrombectomy vs No Surgical Thrombectomy for Extensive Acute DVT of the Lega,144
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With No Surgical Thrombectomy | Risk Difference With Surgical Thrombectomy (95% CI) | ||||
| Mortality not reported | … | … | … | … | … |
|
| |||||
| Nonfatal recurrent VTE | 51 (1 study), 3 mo | Lowb,c due to risk of bias and imprecision | RR 0.37 (0.02-8.75) | 48 per 1,000d,e | 30 fewer per 1,000 (from 47 fewer to 372 more) |
|
| |||||
| Nonfatal major bleeding | 51 (1 study), 3 mo | Lowb,c due to risk of bias and imprecision | Not estimable (no events) | … | - |
|
| |||||
| PTS | 51 (1 study), 2 y | Lowf,g due to risk of bias and imprecision | RR 0.63 (0.44-0.9)h | 588 per 1,000i | 218 fewer per 1,000 (from 59 fewer to 329 fewer)j |
|
| |||||
| QOL not measured | … | … | … | … | … |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. See Table 1, 3 and 10 legends for expansion of abbreviations.
The study included patients with DVT with symptoms of leg swelling not exceeding 7 d and a proximal extension of the thrombus above the inguinal ligament but not into the vena cava.
Not clear whether allocation was concealed. No blinding reported. Not clear whether analysis was intention to treat. Follow-up rate was 88% at 6 mo. Study not stopped early for benefit.
CI includes values suggesting either harm or benefit.
Baseline risks for nonfatal recurrent VTE derived from Douketis et al.108
One event was a symptomatic PE.
In addition to other study limitations, this outcome was assessed by those who did the surgery and anticoagulation. No standardized tool was used. One surgical patient had an amputation secondary to venous gangrene and was not counted in the PTS assessment.
Few number of events. This warrants rating down the quality of evidence by a second level when considered along with study limitations.
The RR is based on the 6-mo data.
This estimate is based on the findings of the VETO study.102 This probably underestimates PTS baseline risk, given that overall, 52% of patients reported the current use of compression stockings during study follow-up.
Severe PTS: assuming the same RR of 0.63 and a baseline risk of 13.8% over 2 y,102 the absolute reduction is 51 fewer severe PTS per 1,000 (from 14 fewer to 77 fewer) over 2 y.
We believe that operative venous thrombectomy should be considered only if all of the following criteria are met: iliofemoral DVT, symptoms for < 7 days (criterion used in the single randomized trial), good functional status, life expectancy of ≥ 1 year, and both resources and expertise are available. Based on low-quality evidence of greater effectiveness and less bleeding, we consider CDT preferable to the operative venous thrombectomy approach.
Recommendation
2.11. In patients with acute proximal DVT of the leg, we suggest anticoagulant therapy alone over operative venous thrombectomy (Grade 2C).
2.12 Anticoagulation in Patients Who Have Had Any Method of Thrombus Removal Performed
There are no randomized trials or observational studies that have compared different anticoagulant regimens or durations of therapy in patients with acute proximal DVT of the leg who have had any method of thrombus removal (including systemic thrombolysis). Mechanical components of these procedures are associated with a high early risk of early recurrent thrombosis, and thrombus removal is not known to alter the long-term risk of recurrent VTE. We used evidence from patients with DVT who did not have thrombus removal to guide anticoagulant decisions in those who had thrombus removal. This evidence is rated down to moderate quality because of its indirectness in this patient population.
Recommendation
2.12. In patients with acute DVT of the leg who undergo thrombosis removal, we recommend the same intensity and duration of anticoagulant therapy as in similar patients who do not undergo thrombosis removal (Grade 1B).
2.13 Vena Caval Filters for the Initial Treatment of DVT
No randomized trial or prospective observational study has evaluated IVC filters as sole therapy (ie, without concurrent anticoagulation) in patients with DVT. A single, large, randomized controlled trial evaluated permanent IVC filter insertion as an adjunct to anticoagulant therapy in patients with acute DVT who were considered to be at high risk for PE (Table 14, Table S19). The findings at 2 years146 and 8 years149 of follow-up, suggest that IVC filters increase the risk of recurrent DVT, reduce the risk of PE, do not alter the combined frequency of DVT and PE (ie, recurrent VTE), do not increase the risk of PTS, and do not alter mortality.
Table 14.
—[Section 2.13] Summary of Findings: Vena Cava Filter vs No Vena Cava Filter for Acute Proximal DVT of the Leg Treated With Anticoagulationa,b,146,443
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With No vena cava Filters | Risk Difference With Vena cava Filters (95% CI) | ||||
| Mortality |
400 (1 study), 8 y |
Moderatec,d due to imprecision |
RR 0.95 (0.78-1.16)e |
515 per 1,000 |
26 fewer per 1,000 (from 113 fewer to 82 more) |
| Symptomatic PE |
304 (1 study), 8 y |
Moderatec,f due to imprecision |
RR 0.41 (0.2-0.86)g |
151 per 1,000 |
89 fewer per 1,000 (from 21 fewer to 121 fewer) |
| Recurrent DVT |
310 (1 study), 8 y |
Moderatec,f due to imprecision |
RR 1.3 (0.93-1.82)h |
273 per 1,000 |
82 more per 1,000 (from 19 fewer to 224 more) |
| Major bleeding |
337 (1 study), 8 y |
Moderatec,d due to imprecision |
RR 0.83 (0.52-1.34)i |
185 per 1,000 |
31 fewer per 1,000 (from 89 fewer to 63 more) |
| PTS |
308 (1 study), 8 y |
Lowd,j due to
risk of bias and imprecision |
RR 0.87 (0.66-1.13) |
699 per 1,000 |
91 fewer per 1,000 (from 238 fewer to 91 more) |
| Complications | 379 (1 study), 2 y | Moderatef due to imprecision | … | … | k |
| QOL not reported | … | … | … | … | … |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. PREPIC = Prevention du Risque d’Embolie Pulmonaire par Interruption Cave. See Table 1 and 3 legends for expansion of other abbreviations.
Anticoagulation consisted of LMWH or UFH initially (according to a 2 × 2 factorial design) followed by oral anticoagulation for at least 3 mo.
Four types of permanent vena cava filters were used: Vena Tech LGM (B. Braun Melsugen AG), titanium Greenfield (Boston Scientific Corporation), Cardial (C.R. Bard, Inc), and Bird’s Nest (Cook Group Incorporated).
Allocation was concealed. Data collectors and outcome adjudicators were blinded. Intention-to-treat analysis. Data missing for 4% at 2 y and 1% at 8 y. Enrollment was stopped at 400 instead of targeted 800 because of slow recruitment.
CI includes both negligible effect and appreciable benefit or appreciable harm.
RR, 1.0 (95% CI, 0.29-3.4) at 12 d; RR, 1.08 (95% CI, 0.73-1.58) at 2 y.
Small number of events.
RR. 0.23 (95% CI, 0.05-1.05) at 12 d (both symptomatic and asymptomatic PE). RR, 0.54 (0.21-1.41) at 2 y (symptomatic PE).
RR, 1.78 (95% CI, 1.09-2.94) at 2 y.
RR, 1.5 (95% CI, 0.54-4.14) at 12 d. RR, 0.74 (95% CI, 0.41-1.36) at 2 y.
No standardized validated tool used to measure PTS.
No complications directly related to the filter or its insertion reported in the PREPIC trial.146 Mismetti et al147 (prospective study) reported an incidence of 3.2% (excluding filter tilting and puncture site hematoma) among 220 patients receiving a retrievable vena cava filter for secondary prevention of VTE, whereas Athanasoulis et al148 (retrospective study) reported an incidence of 0.3% for major complications among 1,731 patients receiving vena cava filters predominantly for secondary prevention of VTE.
In assessing the role of an IVC filter in patients who cannot receive anticoagulant therapy (eg, actively bleeding), we assume that the relative risk of outcomes will be the same as in patients who received anticoagulant therapy in the Prevention du Risque d’Embolie Pulmonaire par Interruption Cave (PREPIC) study. However, their absolute rate of symptomatic PE and recurrent DVT will be higher compared with the PREPIC participants who were prescribed anticoagulants. A comprehensive review of mostly retrospective case series of IVC filter insertions (6,500 patients in 89 reports) suggested that venous thrombosis at the site of filter insertion occurs in ∼10% of patients and that filters can be placed above the renal veins and in the superior vena cava if necessary.150 A prospective observational study also suggested that symptomatic VTE and asymptomatic filter thrombosis are common,151 and a systematic review suggested that the prevalence of PTS may be increased152 in patients with permanent IVC filters. A small single-center randomized trial suggested a higher complication rate with the Trapease compared with the Greenfield permanent filter.153
If an IVC filter is indicated in a patient with acute DVT or PE because anticoagulant therapy is temporarily contraindicated (eg, active bleeding), there is the option of inserting a retrievable filter and removing it when it is safe to start anticoagulant therapy. However, most retrievable filters are not removed; retrievable filters that are not removed may have a higher long-term complication rate than permanent filters, and there currently is no good evidence that retrievable IVC filters improve patient outcomes.104,147,154,155
Insertion of an IVC filter does not eliminate the risk of PE and increases the risk of DVT (Table 14, Table S19). Consequently, we suggest that patients who have an IVC filter inserted should receive a conventional course of anticoagulation (eg, parenteral and long-term anticoagulation) if the contraindication to anticoagulation resolves. Such patients should be treated for the same length of time as if the same patient had not had an IVC filter inserted (see section 3.1). The duration of anticoagulation, therefore, will vary according to whether the DVT was provoked by a temporary risk factor, was unprovoked, or was associated with cancer, and may be influenced by the patient’s ongoing risk of bleeding and preferences.
Our recommendation to treat patients with an IVC filter with anticoagulants when contraindications to anticoagulation resolve is weaker than for anticoagulation of most patients with VTE because the risks of bleeding may remain elevated, and the patient’s risk of recurrence is expected to be lower if the acute episode of thrombosis occurred remotely. The evidence for IVC filter use in patients with acute proximal DVT who cannot be treated with anticoagulation is moderate because of serious imprecision and indirectness (ie, extrapolated from the PREPIC study in which patients were routinely treated with anticoagulants; this indirectness, however, is minor).
Recommendations
2.13.1. In patients with acute DVT of the leg, we recommend against the use of an IVC filter in addition to anticoagulants (Grade 1B).
2.13.2. In patients with acute proximal DVT of the leg and contraindication to anticoagulation, we recommend the use of an IVC filter (Grade 1B).
2.13.3. In patients with acute proximal DVT of the leg and an IVC filter inserted as an alternative to anticoagulation, we suggest a conventional course of anticoagulant therapy if their risk of bleeding resolves (Grade 2B).
Remarks: We do not consider that a permanent IVC filter, of itself, is an indication for extended anticoagulation.
2.14 Early Ambulation of Patients With Acute DVT
Treatment of acute DVT with bed rest and anticoagulation (originally IV UFH) has given way to early mobilization with anticoagulation (often administered SC). Two meta-analyses156,157 summarized evidence from four relevant trials (Table 15, Tables S20-S22). This evidence is of low quality because of risk of bias and imprecision. We suggest early ambulation (eg, without a period of bed rest) when feasible because of its potential to decrease PTS and improve quality of life.
Table 15.
—[Section 2.14] Summary of Findings: Early Ambulation vs Delayed Ambulation for Acute DVT of the Lega,b,273,309,310,314,315
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With Delayed Ambulation | Risk Difference With Early Ambulation (95% CI) | ||||
| Mortality |
385 (4 studies), 3 moc |
Lowd,e due to
risk of bias, imprecision |
RR 1.3 (0.23-7.55) |
11 per 1,000 |
3 more per 1,000 (from 8 fewer to 70 more) |
| PE (symptomatic or asymptomatic) |
385 (4 studies), 4-12 d |
Lowd-g due to
risk of bias, imprecision |
RR 1.16 (0.66-2.05) |
118 per 1,000 |
19 more per 1,000 (from 40 fewer to 124 more) |
| QOL questionnaire in chronic limb venous insufficiency
(CIVIQ) |
53 (1 study), 2 y |
Lowh,i due to
risk of bias, indirectness |
|
|
See footnotej |
| PTS Villata-Prandoni scores (value, > 5) | 37 (1 study), 2 y | Lowe,h due to risk of bias, imprecision | RR 0.66 (0.42-1.03) | 400 per 1,000 | 136 fewer per 1,000 (from 232 fewer to 12 more) |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. CIVIQ = ChronIc Venous Insufficiency Questionnaire. See Table 1 and 3 legends for expansion of other abbreviations.
Two of four eligible studies excluded patients with symptomatic PE; in the third study, 24% of participants had symptomatic PE at baseline. It was not clear whether the fourth study excluded patients with symptomatic PE.
In two of four eligible trials, all patients received early compression therapy (bandages or stockings). In the two other trials, only patients randomized to early ambulation received early compression therapy.
Three studies reporting acute-phase mortality reported no deaths.
Concealment of allocation was reported in one of four studies; blinding of outcome assessors was reported in two of four studies; intention-to-treat analysis reported in two of four studies. Follow-up was 97% to 100%. In two of four trials, only patients randomized to early ambulation received early compression therapy (bandages or stockings). In the other two trials, all patients received early compression therapy.
CI includes both values of clinically significant benefit and values of clinically significant harms.
PE assessed as both symptomatic and asymptomatic PE.
Funnel plot reported as not asymmetrical by Aissaoui et al.156
Concealment of allocation was not reported; outcome assessors were not blinded for this outcome. Seventy percent follow-up rate; compression stockings used on patients with early mobilization but not in patients with delayed mobilization.
No explanation was provided.
Psychologic and overall somatic QOL did not differ significantly between the treatment groups, whereas DVT-related items, especially those reflecting the ease of locomotion, showed significantly greater improvement with compression than with bed rest (P < .001 for bandages, P < .05 for stockings).
Recommendation
2.14. In patients with acute DVT of the leg, we suggest early ambulation over initial bed rest (Grade 2C).
Remarks: If edema and pain are severe, ambulation may need to be deferred. As per section 4.1, we recommend the use of compression therapy in these patients.
3.0 Long-term Anticoagulation of Acute DVT of the Leg
In this review, the term long-term treatment refers to treatments (eg, VKA therapy, LMWH, dabigatran) that are continued after initial therapy (eg, parenteral anticoagulation, thrombolytic therapy) (Fig 1). In addition, we consider treatment with rivaroxaban, which is used without initial parenteral therapy. Long-term therapy has two goals: (1) to complete treatment of the acute episode of VTE and (2) to prevent new episodes of VTE that are not directly related to the acute event. During the early phase of long-term treatment (ie, first 3 months), treatment of the acute episode of VTE predominates. During the late phase of long-term treatment (ie, after the first 3 months), prevention of new episodes of VTE predominates. We use the term extended anticoagulation to refer to anticoagulation that is continued beyond 3 months without a scheduled stop date. However, regular (eg, yearly) reassessments are needed to assess whether a patient’s risk of bleeding increased or the patient’s preferences changed.
Three lines of evidence from randomized trials support the need for long-term anticoagulant treatment of DVT (ie, after 5-10 days of initial heparin therapy): (1) a randomized controlled trial of long-term anticoagulant therapy in 51 patients with symptomatic calf-vein thrombosis that documented a 25% rate of symptomatic extension of thrombosis within 3 months in the control group,66 (2) a randomized trial comparing long-term SC low-dose UFH (5,000 units bid) with VKA therapy in patients with proximal DVT that found that low-dose UFH was ineffective and resulted in a high rate of recurrent VTE (47% within 3 months),158 and (3) randomized trials in which reduced durations of treatment of 4 or 6 weeks resulted in important increases in recurrent VTE compared with conventional durations of treatment of 3 or 6 months (section 3.1).159‐161 This evidence is of moderate quality because of imprecision and indirectness.
Recommendation
3.0. In patients with acute VTE who are treated with anticoagulant therapy, we recommend long-term therapy (see section 3.1 for recommended duration of therapy) over stopping anticoagulant therapy after 1 week of initial therapy (Grade 1B).
3.1 Duration of Long-term Anticoagulant Therapy
Weighing the Benefits and Risks of Different Durations of Anticoagulant Therapy: General Considerations:
Anticoagulant therapy for VTE should be continued until (1) the reduction of recurrent VTE no longer clearly outweighs the increase in bleeding or (2) it is patient preference (which may be influenced by financial burden) to stop treatment, even if the reduction in VTE would outweigh the increase in bleeding.
Increase in Risk of Recurrent VTE After Stopping Therapy—
Current evidence suggests that the risk of recurrence after stopping therapy is largely determined by two factors: (1) whether the acute episode of VTE has been effectively treated (duration of therapy) and (2) the patient’s intrinsic risk of having a new episode of VTE (individual risk of recurrence).
Duration of therapy: The primary goal of trials that compare different time-limited durations of anticoagulation is to identify the shortest duration of therapy that results in a posttreatment risk of recurrence that is as low as can be achieved. The findings of these trials generally are not sensitive to differences in individual patient risk of bleeding.
Individual risk of recurrence:
Primary factors for estimating risk of recurrence: Presence of a reversible provoking risk factor,47,159,160,162‐168,169,170 unprovoked VTE,47,159,160,162‐168,169,170 and presence of active cancer9,47,165,166 are the most important factors that influence risk of recurrent VTE after stopping VKA. Among patients with VTE provoked by a reversible factor, the risk of recurrence is much lower if the provoking factor was recent surgery compared with a nonsurgical trigger (eg, estrogen therapy, pregnancy, leg injury, flight of > 8 h).162,171 In patients with proximal DVT and PE, the estimated cumulative risk of recurrent VTE after stopping anticoagulant therapy of each of these categories is as follows: VTE provoked by surgery, 1% after 1 year and 3% after 5 years; VTE provoked by a nonsurgical reversible risk factor, 5% after 1 year and 15% after 5 years; and unprovoked VTE, 10% recurrence after 1 year and 30% after 5 years.
There are sparse data addressing the risk of recurrent VTE after stopping therapy in patients with cancer because treatment is rarely stopped in these patients because of a high risk for recurrence.38,47,165,166,172 A reasonable estimate for this risk, expressed as an annualized rate, may be 15%. However, the risk of recurrence is expected to vary according to whether the cancer is metastatic, being treated with chemotherapy, or rapidly progressing.38,165 The high mortality in patients with VTE and cancer (40% at 6 months in one large study173) precludes estimating the cumulative risk of recurrence after long-term follow-up. We categorize patients with VTE according to these primary individual risk factors for recurrence when we make recommendations for duration of anticoagulant therapy.
Secondary factors for estimating risk of recurrence: Additional factors that influence the risk of recurrence strongly enough to modify some recommendations about duration of therapy include (1) whether DVT was confined to the distal veins (isolated distal [or calf] DVT), which is estimated to be associated with about one-half of the risk of recurrence of proximal DVT and PE,10,164,167,169,170 and (2) whether the VTE was a second or subsequent episode of VTE, which is estimated to be associated with about a 50% higher risk of recurrence compared with a first VTE.9,173,175
Additional factors for estimating risk of recurrence: Other factors predict risk of recurrence, but not strongly or consistently enough to influence recommendations on duration of therapy once the primary and secondary factors noted previously have been considered. These factors, which have mostly been evaluated in patients with unprovoked VTE, include negative D-dimer testing 1 month after withdrawal of VKA (risk ratio [RR], ∼0.4),48,176‐181 antiphospholipid antibody (RR, ∼2),182‐185 hereditary thrombophilia (RR, ∼1.5),162,163,174,177,180‐182,185‐190 male vs female sex (RR, ∼1.6),191,192 Asian ethnicity (RR, ∼0.8),193 and residual thrombosis in the proximal veins (RR, ∼1.5).149,182,185,194‐198 Combinations of factors have the potential to be more important predictions of recurrence risk than single factors (eg, low risk of recurrence in women with unprovoked proximal DVT or PE who have a negative D-dimer test before185 or 1 month after180,199 stopping anticoagulant therapy). PTS may be a risk factor for recurrent VTE,183,185,200 and recurrent ipsilateral DVT is a risk factor for development of PTS.201,202 Both associations may contribute to a decision to use extended therapy in a patient with established PTS.
Increase in Risk of Bleeding While Remaining on Anticoagulant Therapy—
Although the decision to treat patients with different time-limited durations of anticoagulant therapy generally are insensitive to an individual’s risk of bleeding, the decision to use extended anticoagulation, particularly in patients with an unprovoked proximal DVT or PE, is sensitive to risk of bleeding. There is no validated prediction tool to stratify the risk of major bleeding during extended anticoagulant therapy specifically in patients with VTE, but this risk appears to increase with the prevalence of the factors noted in Table 2. This table also provides our estimate of the absolute risk of bleeding without anticoagulation (baseline risk), the increase with anticoagulation, and the sum of these two risks (ie, risk of bleeding on therapy).
Comparisons of Time-Limited Durations of Therapy:
Randomized trials have compared either a short (eg, 4 or 6 weeks) with an intermediate (eg, 3 or 6 months) duration of therapy, or two intermediate durations of therapy, (eg, 3 months vs 6 or 12 months). VKA therapy targeted to an INR of 2.5 was the anticoagulant regimen in all comparisons.
Short vs Intermediate Durations of Therapy—
Five trials have evaluated shortening the duration of oral anticoagulant therapy from 3 or 6 months to 4 or 6 weeks in patients with mostly first episodes of VTE (Table 16, Tables S23-S25).159,160,167,169 This evidence, which is high quality, indicates that with the shorter duration of therapy, the absolute decrease in bleeding was small compared with the absolute increase in recurrent VTE. Patients with isolated distal DVT provoked by a major transient risk factor have a very low risk of recurrence after anticoagulant therapy is stopped (∼1% per year170). It is uncertain whether this risk is lowered by treating for 3 months compared with 4 or 6 weeks (hazard ratio [HR] for 4 or 6 weeks vs ≥ 3 months at 2 years after stopping therapy, 0.36; 95% CI, 0.09-1.54170). For this reason, we make a weaker recommendation for 3 months compared with a shorter duration of therapy in patients with isolated distal DVT that was provoked by a reversible risk factor. The evidence supporting this weaker recommendation is rated down to low quality because of serious imprecision and because it is a post hoc observation.
Table 16.
—[Section 3.1.1-3.1.4] Summary of Findings: Four or Six Weeks vs Three or Six Months as Minimum Duration of Anticoagulation for VTEa,b,16,159,167,169,195
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With Control | Risk Difference With 4 or 6 wk vs 3 or 6 mo Months of Anticoagulation (95% CI) | ||||
| Recurrent VTE |
2,185 (5 studiesc), 1-2 yd |
Highe-g |
RR 1.83 (1.39-2.42) |
64 per 1,000 |
53 more per 1,000 (from 25 more to 91 more) |
| Major bleeding |
2,185 (5 studies), 1-2 y |
Highf |
RR 0.54 (0.22-1.32) |
12 per 1,000 |
5 fewer per 1,000 (from 9 fewer to 4 more) |
| Mortality | 2,098 (5 studies), 1-2 y | Highe,f,h | RR 0.97 (0.68-1.38) | 55 per 1,000 | 2 fewer per 1,000 (from 18 fewer to 21 more) |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. See Table 1 and 3 legends for expansion of abbreviations.
Populations varied among studies: first provoked isolated distal DVT, proximal DVT, or PE provoked in Kearon et al195; first isolated distal DVT in Pinede et al167; first isolated distal DVT, proximal DVT, or PE in Schulman et al169; proximal DVT (21% had cancer) in Levine et al159; DVT or PE (29% not objectively confirmed) in British Thoracic Society.160
Short vs longer duration of anticoagulation was 6 wk vs 6 mo for Schulman et al169, 6 wk vs 3 mo for Pinede et al,167 and 4 wk vs 3 mo for the other three studies.
Timing of randomization relative to the start of treatment varied across studies: Pinede et al,167 Schulman et al,169 and British Thoracic Society160 randomized at diagnosis, and Kearon et al195 and Levine et al159 randomized to stop or to continue treatment for 2 mo more after the initial 4 wk of treatment.
Follow-up was for ∼1 y in all studied except for Schulman et al169 in which it was 2 y.
Generally, study design was strong. No study stopped early for benefit; two stopped early because of slow recruitment (Kearon et al,195 Pinede et al167). In one study (British Thoracic Society160), 44 randomized patients were excluded centrally as they did not satisfy eligibility criteria. Patients and caregivers were blinded in two studies (Kearon et al, Levine et al159). Adjudicators of outcomes were blinded in all but one study (British Thoracic Society). All studies appear to have used effective randomization concealment, intention-to-treat analysis, and a low unexplained drop-out frequency.
No heterogeneity with I2 = 0%.
No imprecision for overall estimates. However, for the subgroup of patients with isolated distal DVT, who are known to have a very low risk of recurrence, there is imprecision and the possibility that the shorter duration of anticoagulation is adequate and not associated with a clinically important higher risk of recurrence.
Differences in mortality are expected to be mediated by differences in recurrent VTE and bleeding.
Different Intermediate Durations of Therapy (6 or 12 months vs 3 months)—
We considered trials that randomized patients with VTE to 3 months vs to 6 or 12 months of treatment to determine, when using a time-limited duration of therapy, whether there was any benefit to treating for > 3 months. Five reports, which included six randomized comparisons, contributed to this analysis (Table 17, Tables S24-S26).167,194,203‐205 These studies found that 6 or 12 months of therapy did not convincingly lower risk of recurrence but increased major bleeding about 2.5-fold. In a meta-analysis of individual patient data from randomized trials, during the 2 years after stopping anticoagulant therapy, treatment of 3 months compared with ≥ 6 months was associated with an HR of 1.19 (95% CI, 0.86-1.65) in all patients and an HR of 1.39 (95% CI, 0.96-2.01) in patients with unprovoked DVT or PE.170 Therefore, although anticoagulants are very effective at preventing recurrence while patients are receiving therapy, when anticoagulants are stopped, there is a similar risk of recurrence whether patients have been treated for 3 months or longer.170 This evidence is of moderate quality because of serious imprecision.
Table 17.
—[Section 3.1.1-3.1.4] Summary of Findings: Six or Twelve Months vs Three Months as Minimum Duration of Anticoagulation for VTEa,b,167,203,204
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With 3 mo | Risk Difference With 6 or 12 mo (95% CI) | ||||
| Recurrent VTE |
2,061 (6 studies), 1-3 y |
Moderatec-e due to imprecision |
RR 0.89 (0.69-1.14) |
115 per 1,000 |
13 fewer per 1,000 (from 36 fewer to 16 more) |
| Major bleeding |
2,061 (6 studies), 1-3 y |
Highf |
RR 2.49 (1.2-5.16) |
9 per 1,000 |
13 more per 1,000 (from 2 more to 37 more) |
| Mortalityg | 1,331 (5 studies), 1-3 y | Moderated due to imprecision | RR 1.3 (0.81-2.08) | 44 per 1,000 | 13 more per 1,000 (from 8 fewer to 47 more) |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. See Table 1 and 3 legends for expansion of other abbreviations.
Study populations varied across studies: Pinede et al167 enrolled provoked and unprovoked proximal DVT and PE; Campbell et al203 enrolled provoked and unprovoked isolated distal DVT, proximal DVT, and PE; Agnelli et al194 had separate randomizations for provoked PE (3 vs 6 mo) and unprovoked (3 vs 12 mo); and Agnelli et al204 enrolled unprovoked proximal DVT.
Timing of randomization relative to the start of treatment and length of treatment in the non-3 mo group varied across studies: Pinede et al167 and Campbell et al203 randomized at diagnosis; and Agnelli et al194,204 randomized after the initial 3 mo of treatment to stop or continued treatment. The longer duration of treatment was 6 mo in Agnelli et al194 (provoked PE) and 12 mo in Agnelli et al204 and Agnelli et al194 (unprovoked PE).
Generally, study design was strong. No study stopped early for benefit; two stopped early because of slow recruitment (Campbell et al,203 Pinede et al167) and one because of lack of benefit (Agnelli et al204). In one study (Campbell et al), 20% of VTE outcomes were not objectively confirmed. Patients and caregivers were not blinded in any study. Adjudicators of outcomes were blinded in all but one study (Campbell et al). All studies used effective randomization concealment, intention-to-treat analysis, and a low unexplained drop-out frequency.
CIs include both values suggesting no effect and values suggesting either benefit or harm.
Low number of events and a total number of participants < 2,000.
One study may have confined the assessment of bleeding to when subjects were receiving anticoagulant therapy, which could have inflated the increase in bleeding associated with the longer duration of therapy (Campbell et al203).
Differences in mortality are expected to be mediated by differences in recurrent VTE and bleeding.
As an alternative to comparing two time-limited durations of anticoagulant therapy, the AESOPUS (Ultrasound Findings to Adjust the Duration of Anticoagulation) trial compared a predefined duration of therapy with a flexible duration of therapy that depended on whether there was residual thrombosis during follow-up in patients with a first proximal DVT206 (Tables S24 and S25). Its findings suggest that the latter approach may be helpful for tailoring the duration of therapy.
Extended vs Time-Limited Anticoagulant Therapy:
Five trials compared extended anticoagulation with VKA therapy (target INR 2.0-2.85,161 2.0-3.0,48,182,207 and 1.5-2.0174) with stopping VKA therapy at 3 or 6 months in patients who were judged to have a high risk of recurrence (Table 18, Table S24, S25, and S27). The results indicate that randomization to indefinite treatment with conventional-intensity VKA (target INR 2.5) reduces recurrent VTE by about 90% (RR for the four studies, 0.12; 95% CI, 0.05-0.2548,161,182,207), and randomization to low-intensity therapy (target INR 1.75) reduces VTE by 64% (95% CI for HR, 23%-81%),174 with about one-half of recurrent VTE in the active treatment groups in these studies occurring in patients who had prematurely stopped VKA therapy. Extended anticoagulant therapy was associated with about a 2.6-fold increase in major bleeding. The quality of evidence for the reduction in recurrent VTE with extended therapy is high but is rated down to moderate for bleeding and mortality because of imprecision.
Table 18.
—[Section 3.1.1-3.1.4] Summary of Findings: Extended Anticoagulation vs No Extended Anticoagulation for Different Groups of Patients with VTE and Without Cancera,b,48,161,182,207
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With No Extended Duration Oral Anticoagulation | Risk Difference With Extended Duration Oral Anticoagulation (95% CI) | ||||
| Mortality | 1,184 (4 studies), 10-36 mo | Moderatec-e due to imprecision | RR 0.57 (0.31-1.03) | 63 per 1,000 | 27 fewer per 1,000 (from 44 fewer to 2 more) |
|
| |||||
| Recurrent VTE at 1 y | 1,184 (4 studies), 10-36 mo | High | RR 0.12 (0.09-0.38) | First VTE provoked by surgeryf-j | |
|
| |||||
| 10 per 1,000 | 10 fewer per 1,000 (from 6 fewer to 9 fewer) | ||||
|
| |||||
| First proximal DVT or PE provoked nonsurgical/first unprovoked distal DVTf-j | |||||
|
| |||||
| 50 per 1,000 | 44 fewer per 1,000 (from 31 fewer to 45 fewer) | ||||
|
| |||||
| First unprovoked VTEf-j | |||||
|
| |||||
| 100 per 1,000 | 88 fewer per 1,000 (from 62 fewer to 91 fewer) | ||||
|
| |||||
| Second unprovoked VTEf-j | |||||
|
| |||||
| 150 per 1,000 | 132 fewer per 1,000 (from 93 fewer to 137 fewer) | ||||
|
| |||||
| Major bleeding at 1 y | 1,184 (4 studies), 10-36 mo | Moderate due to imprecision | RR 2.63 (1.02-6.76) | Lowk,l (see Table 2) | |
|
| |||||
| 3 per 1,000 | 5 more per 1,000 (from 0 more to 15 more) | ||||
|
| |||||
| Moderatek,l (see Table 2) | |||||
|
| |||||
| 6 per 1,000 | 10 more per 1,000 (from 1 more to 29 more) | ||||
|
| |||||
| Highk,l (see Table 2) | |||||
|
| |||||
| 25 per 1,000 | 40 more per 1,000 (from 3 more to 122 more) | ||||
|
| |||||
| Recurrent VTE at 5 y | 1,184 (4 studies), 10-36 mo | High | RR 0.12 (0.09-0.38) | First VTE provoked by surgeryf-j | |
|
| |||||
| 30 per 1,000 | 26 fewer per 1,000 (from 19 fewer to 27 fewer) | ||||
|
| |||||
| First proximal DVT or PE provoked nonsurgical/first unprovoked distal DVTf-j | |||||
|
| |||||
| 150 per 1,000 | 132 fewer per 1,000 (from 93 fewer to 137 fewer) | ||||
|
| |||||
| First unprovoked VTEf-j | |||||
|
| |||||
| 300 per 1,000 | 264 fewer per 1,000 (from 186 fewer to 273 fewer) | ||||
|
| |||||
| Second unprovoked VTEf-j | |||||
|
| |||||
| 450 per 1,000 | 396 fewer per 1,000 (from 279 fewer to 409 fewer) | ||||
|
| |||||
| Major bleeding at 5 y | 1,184 (4 studies), 10-36 mo | Moderate due to imprecision | RR 2.63 (1.02-6.76) | Lowk,l (see Table 2) | |
|
| |||||
| 15 per 1,000 | 24 more per 1,000 (from 2 more to 73 more) | ||||
|
| |||||
| Moderatek,l (see Table 2) | |||||
|
| |||||
| 30 per 1,000 | 48 more per 1,000 (from 4 more to 146 more) | ||||
|
| |||||
| Highk,l (see Table 2) | |||||
|
| |||||
| 125 per 1,000 | 199 more per 1,000 (from 17 more to 609 more) | ||||
|
| |||||
| Burden of anticoagulation not reported | … | … | … | Warfarin: daily medication, dietary and activity restrictions, frequent blood testing/monitoring, increased hospital/ clinic visitsm | … |
|
| |||||
| PTS not reported | … | … | … | n | … |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. PREVENT = Prevention of Recurrent Venous Thromboembolism. See Table 1 and3 legends for expansion of other abbreviations.
Studies vary in follow-up duration (10 mo to 3 y) and in duration of time-limited VKA (3-6 mo).
We excluded PREVENT trial because target INR was 1.75 (low intensity), which has been shown in an RCT44 to be less effective than a target of 2.5.
I2 = 0%.
CI includes both values suggesting no effect and values suggesting either appreciable harms or appreciable benefit.
Small number of events. Decision to rate down also takes into account that two studies were stopped early for benefit.
Annual risk of VTE recurrence after discontinuing oral anticoagulation therapy in patients with first VTE provoked by surgery: 1% (Iorio et al171); we assumed a 0.5% yearly risk thereafter (3% over 5 y).
Annual risk in patients with first VTE provoked by nonsurgical factor: ∼5% the first year (Iorio et al171); we assumed a 2.5% yearly thereafter (15% over 5 y).
Annual risk in patients with first episode of unprovoked VTE: 9.3% over 1 y in Rodger et al185; 11.0% over 1 y, 19.6% over 3 y, and 29.1% over 5 y in Prandoni et al.208 We assumed a risk of 10% the first year after discontinuation and 5% yearly thereafter (30% over 5 y).
Annual risk in patients with second episode of unprovoked VTE: we assumed an RR of 1.5 compared with a first episode of unprovoked VTE: 15% the first year after discontinuation, 7.5% yearly thereafter (45% over 5 y).
Case fatality rate of recurrent VTE after discontinuing oral anticoagulation therapy: 3.6% (Carrier et al12).
Annual risk of major bleeding is based on three risk levels: low, intermediate, and high. The corresponding 0.3%, 0.6%, and 1.2% risks are estimates based on control arms of included studies (see Table 2).
Case fatality rate of major bleeding during initial oral anticoagulation therapy: 11.3% (Carrier et al12) (no data available for after discontinuing oral anticoagulation therapy).
Burden of anticoagulation: endured by all patients who continue extended-duration anticoagulation (100%) and applies to patients who stop anticoagulation (no extended duration anticoagulation) who subsequently experienced a recurrent VTE (5%/10%/15% at 1 y; 15%/30%/45% at 5 y).
PTS: baseline risk over 2 y of 58.8% for PTS and 13.8% for severe PTS (Kahn et al102). There was a threefold (Prandoni et al202) to 10-fold (van Dongen et al209) increase in PTS with recurrent VTE in the ipsilateral leg.
Weighing the Benefits and Risks of Extended VKA Therapy—
The decision to extend anticoagulation therapy beyond 3 months is sensitive to both baseline risks of recurrent VTE and major bleeding. We did not identify a validated prediction tool for either outcome that takes into account all relevant risk factors. As an alternative, for patients without cancer, we chose to stratify our recommendations according to four primary risk groups for recurrent VTE (section 3.1) and three risk groups for major bleeding (section 3.1) (Table 2). This approach resulted in a total of 12 combinations of risk profiles. Table 19 shows the estimated total (and fatal) number of recurrent episodes of VTE prevented and the number of major bleeds caused by 5 years of extended therapy for each of the 12 combinations. In the absence of robust trial data for mortality for recurrent VTE and bleeding, we assumed that 3.6% of recurrent VTE and 11.3% of major bleeds will be fatal.12
Table 19.
—[Section 3.1.1-3.1.4] Estimated Absolute Difference in Recurrent VTE and Major Bleeding Events (Including Fatal Events) With 5 Years of vs No Extended Anticoagulation
| Outcomes After 5 y of Treatment | Risk of Bleeding |
|||
| Low | Intermediate | Higha | ||
| First VTE provoked by surgery |
Recurrent VTE reduction per 1,000 |
↓ 26 (19-27) (1 fatal)b |
↓ 26 (19-27) (1 fatal)b |
↓ 26 (19-27) (1 fatal)b |
| Major bleeding increase per 1,000 |
↑ 24 (2-73) (3 fatal)b |
↑ 49 (1-173) (5 fatal)b |
↑ 98 (1-346) (11 fatal)b |
|
| First VTE provoked by a nonsurgical
factor/first unprovoked distal DVT |
Recurrent VTE reduction per 1,000 |
↓ 132 (93-137) (5 fatal)c |
↓ 132 (93-137) (5 fatal)c |
↓ 132 (93-137) (5 fatal)b |
| Major bleeding increase per 1,000 |
↑ 24 (2-73) (3 fatal)c |
↑ 49 (1-173) (5 fatal)c |
↑ 98 (1-346) (11 fatal)b |
|
| First unprovoked proximal DVT or
PE |
Recurrent VTE reduction per 1,000 |
↓ 264 (186-273) (10 fatal)d |
↓ 264 (186-273) (10 fatal)d |
↓ 264(186-273) (10 fatal)b |
| Major bleeding increase per 1,000 |
↑ 24 (2-73) (3 fatal)d |
↑ 49 (1-173) (5 fatal)d |
↑ 98 (1-346) (11 fatal)b |
|
| second unprovoked VTE | Recurrent VTE reduction per 1,000 |
↓ 396 (279-409) (14 fatal)e |
↓ 396 (279-409) (14 fatal)d |
↓ 396 (279-409) (14 fatal)c |
| Major bleeding increase per 1,000 | ↑ 24 (2-73) (3 fatal)e | ↑ 49 (1-173) (5 fatal)d | ↑ 98 (1-346) (11 fatal)c | |
Recommendations:
Risk of dying in patients with a recurrent VTE or a major bleed:
• Case fatality rate of recurrent VTE after discontinuing oral anticoagulation therapy: 3.6% (Carrier et al12).
• Case fatality rate of major bleeding during initial oral anticoagulation therapy: 11.3% (Carrier et al12) (no data available for after discontinuing oral anticoagulation therapy).
Annual risks of recurrent VTE after discontinuation of anticoagulation:
• First VTE provoked by surgery: 1% (Iorio et al171); we assumed a 0.5% yearly risk thereafter (3% over 5 y).
• First episode of VTE provoked by nonsurgical factor: ∼5% the first year (Iorio et al171); we assumed a 2.5% yearly thereafter (15% over 5 y)
• First episode of unprovoked VTE: 9.3% over 1 y (Rodger et al185); 11.0% over 1 y, 19.6% over 3 y, 29.1% over 5 y (Prandoni et al208). We assumed a risk of 10% the first year after discontinuation and 5% yearly thereafter (30% over 5 y).
• Second episode of unprovoked VTE: we assumed that this inflicts 1.5 the risk of recurrent VTE relative to first episode of unprovoked VTE: 15% the first year after discontinuation, 7.5% yearly thereafter (45% over 5 y).
Relative risk reduction with extended anticoagulant therapy:
• 82% based on Table 18
Annual risks of major bleeding in patients not on anticoagulant therapy:
• Low risk, 0.3%/y; intermediate risk 0.6%/y; high risk, 2.4%/y (Table 2).
Relative risk of major bleeding with extended anticoagulant therapy:
• 2.6 based on Table 18.
Criteria used to decide on direction and strengths of recommendations:
• Criterion for a strong recommendation against whenever the estimated number of fatal bleeding events exceeded the estimated number of fatal recurrent VTE prevented.
• Criterion to go from a strong recommendation against to weak recommendation against: difference between the lower boundary of increased major bleeding and upper boundary of reduction in recurrent VTE < 2% (risk over 5 y averaged per year).
• Criterion to go from a weak recommendation against to a weak recommendation in favor of: difference between point estimate of reduction of recurrent VTE and point estimate for increase in major bleeding is > 2% (risk over 5 y averaged per year) (2% to account for the burden and cost of VKA).
• Criterion to go from a weak recommendation for to strong recommendation for: difference between the lower boundary of reduction in VTE and upper boundary of increased major bleeding > 4% (risk over 5 y averaged per year).
Another way of interpreting the direction and strength of recommendation based on the number of deaths (related to either bleeding or recurrent VTE) is as follows:
• A strong recommendation against: extended anticoagulation is estimated to be associated with an increase in deaths.
• A weak recommendation against: extended anticoagulation is estimated to be associated with from no effect on deaths to only a very small reduction in deaths (0-4/1,000 prevented over 5 y or < 0.5%/patient-y).
• A weak recommendation for: extended anticoagulation is estimated to be associated with a small reduction in deaths (5 to 9/1,000 prevented over 5 y or 0.5%-0.9%/patient-y).
• A strong recommendation for: extended anticoagulation is estimated to be associated with a large reduction in deaths (> 10/1,000 prevented over 5 y or > 1%/patient-y).
With an eightfold risk of bleeding in the high-risk group compared with the low-risk group, a strong recommendation against extended anticoagulation for a second unprovoked VTE is justified. The high-risk group, however, includes patients who have a risk of bleeding that is less than this estimate (eg, patients aged > 75 y without additional risk factors for bleeding [Table 2]) and, therefore, may benefit from extended anticoagulant therapy. For this reason, we provide a weak rather than a strong recommendation against extended anticoagulation for patients with a second unprovoked VTE in the high-bleeding-risk group.
Strong against
Weak against
Weak in favor
Strong in favor
We make (1) a strong recommendation for extended therapy when it is associated with a reduction in VTE that is substantially more frequent than the increase in major bleeding and with a mortality advantage, (2) a weak recommendation for extended therapy when it is associated with a reduction in VTE that is more frequent than the increase in major bleeding but the magnitude of this difference and the suggested mortality advantage are more modest, (3) a weak recommendation against extended therapy when extended therapy is associated with a reduction in VTE that is less frequent than the increase in major bleeding and no mortality advantage exists, and (4) a strong recommendation against extended therapy when extended therapy is associated with a reduction in VTE that is less frequent than the increase in major bleeding and a mortality disadvantage may exist. We assume that on average, extended anticoagulation with VKA therapy is a modest burden to patients.15 However, this differs markedly among patients; some do not find anticoagulant therapy a burden and have an enhanced feeling of well-being because they feel protected from recurrence, whereas others find it a major burden that greatly erodes their sense of well-being.210
The presence of additional risk factors for VTE recurrence (section 3.1), the patient’s relative value for the different outcome of interest (recurrence of VTE, major bleeding, PTS), and the patient’s perceived burden of anticoagulant therapy may influence decisions about the use of extended anticoagulant therapy in patient groups for which we provide a weak recommendation but are unlikely to influence this decision in patients groups for which we provide a strong recommendation.16 Similarly, the costs of therapy and how those costs are paid (eg, patient, third party) are more likely to influence treatment decisions when there is a weak recommendation (Grade 2) in favor of extended therapy.
Patients With VTE and Cancer: As previously noted (section 3.1), because they have a high risk of recurrence, patients with active cancer (eg, treated within the past 6 months, persistent or progressive) should benefit from extended anticoagulant therapy unless they have a very high risk of bleeding. The quality of the evidence supporting this recommendation is moderate because of indirectness (the relative effects of anticoagulation are based, in part, on evidence from patients without cancer). Presence of factors associated with a lower risk of recurrence that may support stopping anticoagulant therapy, particularly if the risk of bleeding was high, include the following: (1) VTE was associated with a superimposed reversible risk factor (eg, recent surgery, chemotherapy), (2) the cancer has responded to treatment, (3) the cancer has not metastasized, and (4) the VTE was an isolated distal DVT.
Follow-up of Patients on Extended Therapy: Patients who are treated with extended anticoagulant therapy should be reviewed regularly (eg, annually) to ensure that (1) they have not developed contraindications to extended therapy, (2) their preferences have not changed (eg, anticoagulation has become an excessive burden), (3) they can benefit from improved ways of selecting a patient for extended therapy if these have become available, and (4) they are being treated with the anticoagulant regimen that best suits them.
LMWH for Extended Therapy: We identified no direct evidence for LMWH compared with (1) VKA, (2) other anticoagulant strategies, or (3) control for the extended phase of anticoagulation in patients who were treated with a standardized initial long-term anticoagulant regimen. Based on indirect evidence from comparisons of LMWH with VKA therapy during the initial 3 or 6 months of long-term therapy, we judged LMWH to be at least as effective in terms of recurrent VTE and as safe in terms of major bleeding (Table 20, Table S28). The potential for drug-induced osteoporosis, however, may be greater with extended therapy LMWH than with VKA therapy.1
Table 20.
—[Section 3.3] Summary of Findings: LMWH vs VKA for Long-term Treatment of VTEa-c,173,211,213,223,224,226-228
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With VKA | Risk Difference With LMWH (95% CI) | ||||
| Death | 2,496 (7 studies), 6 mo | Moderated,e due to imprecision | RR 0.96 (0.81-1.13) | 164 per 1,000 | 7 fewer per 1,000 (from 31 fewer to 21 more) |
|
| |||||
| Recurrent VTE | 2,727 (8 studies), 6 mo | Moderatef due to risk of bias | RR 0.62 (0.46-0.84) | No cancerg | |
|
| |||||
| 30 per 1,000 | 11 fewer per 1,000 (from 5 fewer to 16 fewer) | ||||
|
| |||||
| Nonmetastatic cancerg | |||||
|
| |||||
| 80 per 1,000 | 30 fewer per 1,000 (from 13 fewer to 43 fewer) | ||||
|
| |||||
| Metastatic cancerg | |||||
|
| |||||
| 200 per 1,000 | 76 fewer per 1,000 (from 32 fewer to 108 fewer) | ||||
|
| |||||
| Major bleeding | 2,737 (8 studies), 6 mo | Moderateh,i due to imprecision | RR 0.81 (0.55-1.2) | No cancer or nonmetastatic cancerj | |
|
| |||||
| 20 per 1,000 | 4 fewer per 1,000 (from 9 fewer to 4 more) | ||||
|
| |||||
| Metastatic cancerj | |||||
|
| |||||
| 80 per 1,000 | 15 fewer per 1,000 (from 36 fewer to 16 more) | ||||
|
| |||||
| Burden of anticoagulation | … | Highk | … | Warfarin: daily medication, dietary restrictions, frequent blood testing/monitoring, increased hospital/clinic visits | LMWH: daily injection, no dietary restrictions, no frequent blood testing/monitoring |
|
| |||||
| PTS (self-reported leg symptoms and signs) | 100 (1 study), 2 y | Lowl,m due to risk of bias, indirectness | RR 0.85 (0.77-0.94) | 200 per 1,000n | 30 fewer per 1,000 (from 12 fewer to 46 fewer) |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. See Table 1 and 3 legends for expansion of abbreviations.
Two of these studies enrolled only patients without cancer, three enrolled only patients with cancer, and three enrolled both patients with and without cancer (separate data provided for patients with cancer and without cancer in one study).
Limited to LMWH regimens that used ≥ 50% of the acute treatment dose during the extended phase of treatment.
The initial parenteral anticoagulation was similar in both arms for all except one study (Hull et al211) in which patients randomized to LMWH initially received the same LWMH, whereas patients randomized to VKA received initially UFH.
One study did not report deaths, which is unusual and could reflect selective reporting of outcomes.
CI includes both no effect and harm with LMWH.
None of the studies were blinded, whereas the diagnosis of recurrent VTE has a subjective component and there could be a lower threshold for diagnosis of recurrent VTE in VKA-treated patients because switching the treatment of such patients to LMWH is widely practiced. At the same time, there is reluctance to diagnose recurrent VTE in patients who are already taking LMWH because there is no attractive alternative treatment option.
Risk of recurrent VTE: low corresponds to patients without cancer (3% estimate taken from recent large RCTs of acute treatment), intermediate corresponds to patients with local or recently resected cancer (based on average rate across the six studies in this analysis and appears to be consistent with Prandoni et al38 [particularly if low risk is increased to 4%]), and high corresponds to patients with locally advanced or distant metastatic cancer (Prandoni et al).
No study was blinded; diagnosis of major bleeding has a subjective component.
The 95% CIs for the RR for major bleeding includes a potentially clinically important increase or decrease with LMWH and may vary with the dose of LMWH used during the extended phase of therapy.
Risk of bleeding: low corresponds to patients without risk factor for bleeding (ie, age > 75 y, cancer, metastatic disease; chronic renal or hepatic failure; platelet count < 80,0000; requirement for antiplatelet therapy; history of bleeding without a reversible cause) (Table 2). Based on Prandoni et al38 and Beyth et al212 and adjusted to a 6-mo time frame.
Hull et al213 reported no significant difference in QOL but suggested greater satisfaction with LMWH over VKA (questionnaire did not directly assess the burden of injections).
Patients and investigators not blinded. Self-reported leg symptoms and signs after 3 mo of treatment.
The association between leg symptoms and signs at 3 mo and long-term PTS is uncertain.
Baseline risk assumes that patients all wear pressure stockings. Control event rate comes from observational studies in a review by Kahn et al,214 adjusted to a 2-y time frame.
Rivaroxaban for Extended Therapy: Use of rivaroxaban compared with initial parenteral therapy followed by VKA therapy for the short- and long-term treatment of DVT is reviewed in section 3.3 (Table 21, Table S29). In the current section, we consider rivaroxaban compared with no anticoagulation in patients with proximal DVT or PE who have completed 6 or 12 months of anticoagulant therapy, which has been evaluated in a single study (Table 22, Table S30).127 This study found that rivaroxaban markedly reduced recurrent VTE at the expense of a modest absolute increase in major bleeding. The evidence from this one study is of moderate quality because of serious imprecision.
Table 21.
—[Section 3.3] Summary of Findings: Rivaroxaban vs LMWH and VKA Therapy for Acute and Long-term Treatment of VTEa-c,88
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With LMWH and VKA Therapy | Risk Difference With Rivaroxaban (95% CI) | ||||
| Mortality |
3,449 (1 study), 6-12 mod |
Moderatee,f due to imprecision |
HR 0.67 (0.44-1.02) |
29 per 1,000 |
9 fewer per 1,000 (from 16 fewer to 1 more) |
| Recurrent VTE |
3,449 (1 study), 6-12 mod |
Moderatee,g due to imprecision |
HR 0.68 (0.44-1.04) |
30 per 1,000h |
9 fewer per 1,000 (from 17 fewer to 1 more) |
| Major bleeding |
3,429 (1 study), 6-12 mod |
Moderatee,g due to imprecision |
HR 0.68 (0.34-1.38)i |
11 per 1,000j |
4 fewer per 1,000 (from 7 fewer to 4 more) |
| Burden of anticoagulation not reported | … | … | … | Warfarin: daily medication, dietary restrictions, frequent blood testing/monitoring, increased hospital/clinic visits | Rivaroxaban: daily medication, no dietary restrictions, no frequent blood testing/monitoring |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. HR = hazard ratio. See Table 1 and 3 legends for expansion of other abbreviations.
Included patients had acute, symptomatic, and objectively verified proximal DVT of the legs (unprovoked, 62%; cancer, 6%; previous, VTE 19%).
Rivaroxaban 15 mg bid for 3 wk and then 20 mg/d for a total of 3 (12%), 6 (63%), or 12 (25%) mo.
Enoxaparin 1 mg/kg bid for ∼8 d and then VKA therapy targeted to an INR of 2.5 for 3, 6, or 12 mo.
Follow-up was prespecified to be 3 (12%), 6 (63%), or 12 (25%) mo.
Allocation was concealed. Patients, providers, and data collectors were not blinded, but outcome adjudicators were blinded. Intention-to-treat analysis; 1.0% were loss to follow-up. Not stopped early for benefit.
CI includes values suggesting benefit or no effect; relatively low number of events.
CI includes values suggesting benefit and harm.
One definite or possible fatal VTE in the rivaroxaban group and one in the LMWH/VKA group.
Calculated from reported data.
Bleeds contributing to death: one in the rivaroxaban group and five in the warfarin group.
Table 22.
—[Section 3.3] Summary of Findings: Rivaroxaban vs Placebo for Extended Anticoagulation of VTEa,b,88
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With Placebo | Risk Difference With Rivaroxaban (95% CI) | ||||
| Mortality | 1,196 (1 study), 6 or 12 moc | Moderated,e due to imprecision | RR 0.49 (0.04-5.4)f | 3 per 1,000 | 2 fewer per 1,000 (from 3 fewer to 15 more) |
|
| |||||
| Recurrent VTE | 1,196 (1 study), 6 or 12 moc | Highd | HR 0.18 (0.09-0.39) | 71 per 1,000g | 58 fewer per 1,000 (from 43 fewer to 64 fewer) |
|
| |||||
| Major bleeding | 1,188 (1 study), 6 or 12 mo | Moderated,h due to imprecision | RR 4.9 (0.58-42)f | i | 7 more per 1,000 (from 3 more to 16 more) |
|
| |||||
| Burden of anticoagulation not reported | … | Rivaroxaban: daily medication | |||
|
| |||||
| PTS not reported | … | … | … | … | j |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. See Table 1, 3, and 21 legends for expansion of abbreviations.
Included patients had acute, symptomatic, and objectively verified proximal DVT of the legs or PE (unprovoked, 73%; cancer, 5%; previous VTE, 19%).
Rivaroxaban 20 mg/d for 6 or 12 mo after initial long-term therapy.
Follow-up was prespecified to be 6 (60%) or 12 (40%) mo.
Allocation was concealed. Patients, providers, data collectors, and outcome adjudicators were blinded. Intention-to-treat analysis; 0.2% were loss to follow-up. Not stopped early for benefit.
CI includes values suggesting benefit or no effect; relatively low number of events.
Calculated from reported data with addition of one event to each event rate because event rate was 0 in the control group.
One definite or possible fatal VTE in the rivaroxaban group and one in the LMWH/VKA group.
CI includes values suggesting benefit and harm.
Bleeds contributing to death: none in the rivaroxaban group and none in the warfarin group.
PTS: baseline risk over 2 y of 58.8% for PTS and 13.8% for severe PTS (Kahn et al102). There is threefold (Prandoni et al202) to 10-fold (van Dongen et al209) increase in PTS with recurrent VTE in the ipsilateral leg.
Dabigatran for Extended Therapy: There are no completed studies that have compared dabigatran with no anticoagulation for extended treatment of VTE.
Choice of Anticoagulant Regimen for Extended Therapy: This question is addressed in section 3.3.
Recommendations
3.1.1. In patients with a proximal DVT of the leg provoked by surgery, we recommend treatment with anticoagulation for 3 months over: (i) treatment of a shorter period (Grade 1B), (ii) treatment of a longer time-limited period (eg, 6 or 12 months) (Grade 1B), or (iii) extended therapy (Grade 1B regardless of risk of bleeding).
3.1.2. In patients with a proximal DVT of the leg provoked by a nonsurgical transient risk factor, we recommend treatment with anticoagulation for 3 months over (i) treatment of a shorter period (Grade 1B), (ii) treatment of a longer time-limited period (eg, 6 or 12 months) (Grade 1B), and (iii) extended therapy if there is a high bleeding risk (Table 2) (Grade 1B). We suggest treatment with anticoagulation for 3 months over extended therapy if there is a low or moderate bleeding risk (Table 2) (Grade 2B).
3.1.3. In patients with an isolated distal DVT of the leg provoked by surgery or by a nonsurgical transient risk factor (see remark), we suggest treatment with anticoagulation for 3 months over treatment of a shorter period (Grade 2C) and recommend treatment with anticoagulation for 3 months over treatment of a longer time-limited period (eg, 6 or 12 months) (Grade 1B) or extended therapy (Grade 1B regardless of bleeding risk).
3.1.4. In patients with an unprovoked DVT of the leg (isolated distal [see remark] or proximal), we recommend treatment with anticoagulation for at least 3 months over treatment of a shorter duration (Grade 1B). After 3 months of treatment, patients with unprovoked DVT of the leg should be evaluated for the risk-benefit ratio of extended therapy.
3.1.4.1. In patients with a first VTE that is an unprovoked proximal DVT of the leg and who have a low or moderate bleeding risk (Table 2), we suggest extended anticoagulant therapy over 3 months of therapy (Grade 2B).
3.1.4.2. In patients with a first VTE that is an unprovoked proximal DVT of the leg and who have a high bleeding risk (Table 2), we recommend 3 months of anticoagulant therapy over extended therapy (Grade 1B).
3.1.4.3. In patients with a first VTE that is an unprovoked isolated distal DVT of the leg (see remark), we suggest 3 months of anticoagulant therapy over extended therapy in those with a low bleeding risk (Table 2) (Grade 2B), and recommend 3 months of anticoagulant treatment in those with a moderate or high bleeding risk (Table 2) (Grade 1B).
3.1.4.4. In patients with a second unprovoked VTE, we recommend extended anticoagulant therapy over 3 months of therapy in those who have a low bleeding risk (Table 2) (Grade 1B), and we suggest extended anticoagulant therapy in those with a moderate bleeding risk (Table 2) (Grade 2B).
3.1.4.5. In patients with a second unprovoked VTE who have a high bleeding risk (Table 2), we suggest 3 months of anticoagulant therapy over extended therapy (Grade 2B).
3.1.5. In patients with DVT of the leg and active cancer, if the risk of bleeding is not high (Table 2), we recommend extended anticoagulant therapy over 3 months of therapy (Grade 1B), and if there is a high bleeding risk (Table 2), we suggest extended anticoagulant therapy (Grade 2B).
Remarks (3.1.3, 3.1.4, 3.1.4.3): Duration of treatment of patients with isolated distal DVT refers to patients in whom a decision has been made to treat with anticoagulant therapy; however, it is anticipated that not all patients who are given a diagnosis of isolated distal DVT will be prescribed anticoagulants (see section 2.3). In all patients who receive extended anticoagulant therapy, the continuing use of treatment should be reassessed at periodic intervals (eg, annually).
3.2 Intensity of Anticoagulant Effect
Ageno et al2 and Holbrook et al3 in these guidelines present evidence for the optimal intensity of VKA therapy (ie, target INR) during the long-term (eg, first 3 months of treatment) and extended phases of treatment of VTE.
Recommendation
3.2. In patients with DVT of the leg who are treated with VKA, we recommend a therapeutic INR range of 2.0 to 3.0 (target INR of 2.5) over a lower (INR < 2) or higher (INR 3.0-5.0) range for all treatment durations (Grade 1B).
3.3 Choice of Anticoagulant Regimen for Long-term Therapy
VKA therapy has been the standard method of anticoagulant therapy for VTE. Adjusted-dose SC UFH is an effective alternative to VKA therapy, but it has never been popular because it requires initial laboratory monitoring and twice-daily injection and is associated with osteoporosis.215,216 SC LMWH has advantages over SC UFH in that it does not require laboratory monitoring, is less likely to cause osteoporosis,216 and can be given once a day. For these reasons, LMWH has been used for the long-term treatment of VTE. The synthetic pentasaccharide, fondaparinux, has not been evaluated or widely used for long-term treatment of VTE. Idraparinux, a long-acting pentasaccharide, is effective for the long-term treatment of VTE,217 but it is not being marketed because of concerns about bleeding given its prolonged duration of action (once weekly injection) and lack of reversibility. The direct antithrombin dabigatran and the direct factor Xa inhibitors apixaban and rivaroxaban have been evaluated for treatment of VTE and are now available in many countries. In this section, we compare VKA therapy (target INR 2.5), LMWH, dabigatran, and rivaroxaban for the long-term treatment of VTE (ie, first 3 months and extended therapy).
LMWH vs VKA Therapy for the Long-term Treatment of DVT:
Two meta-analyses compared LMWH in widely differing doses with VKAs.218,219 In an analysis by Iorio and colleagues,218 which included seven studies168,221‐225 and a total of 1,379 patients, among study differences of mean daily dose of LMWH, little effect on the efficacy of LMWH compared with VKA therapy was found, but the dose of LMWH appeared to influence the risk of major bleeding (OR, ∼0.2 with ∼4,000 International Units/d to ∼0.7 with 12,000 International Units/d, relative to the VKA groups; P = .03 for dose-dependent interaction). Because prophylactic doses of LMWH are rarely used as an alternative to VKA therapy in patients with VTE, we restricted our analysis to eight studies that used ≥ 50% of the full therapeutic dose of LMWH for long-term treatment of VTE (Table 20, Table S28).173,211,213,223,224,226‐228 This evidence suggests that compared with VKA therapy, LMWH is associated with a reduction of recurrent VTE and a similar frequency of major bleeding and mortality. The quality of this evidence is moderate because of potential for bias in the assessment of recurrent VTE (nonblinded outcome assessment) and serious imprecision for major bleeding and mortality.
Cancer vs No Cancer—
There are differences between patients with and without cancer with VTE that may influence response to anticoagulant therapies. These include about a 10-fold higher risk of dying and a threefold higher risk of recurrent VTE and major bleeding during the first 3 or 6 months of treatment38; different mechanisms of thrombosis that may be associated with a poor response to VKA therapy229; use of cancer chemotherapy that is associated with thrombocytopenia, vomiting, and anorexia and may have other interactions with VKA therapy; and the need for invasive therapeutic interventions (eg, drainage procedures) that require reversal of anticoagulation. Many of these factors make LMWH more attractive and VKA therapy less attractive in patients with VTE and cancer and suggest that cancer may alter the response of VTE to LMWH vs VKA therapy (ie, presence of an interaction).
Among the eight studies included in Table 20 and Table S28, separate data are provided for 1,114 patients with cancer and 660 patients without cancer. Subgroup analyses suggest the possibility that the response to LMWH vs VKA therapy may differ between patients with cancer and without cancer (recurrent VTE: RR, 0.52 with cancer [95% CI, 0.36-0.76] vs 0.99 without cancer [95% CI, 0.46-2.13]); major bleeding: RR, 0.92 with cancer [95% CI, 0.59-1.44] vs 0.43 without cancer [95% CI, 0.16-1.17]); mortality: RR, 0.93 with cancer [95% CI, 0.79-1.09] vs 1.85 without cancer [95% CI, 0.59-5.77]). However, none of these differences is statistically significant, making it less likely that there is a true difference in response to LMWH vs VKA in the two patient populations. For this reason, we have applied the same relative effects for LMWH vs VKA in patients with and without cancer. The baseline risks of events, however, are clearly different in the two populations.
Patient Preferences—
As discussed in the Methods section, the ultimate judgment of the entire Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (AT9) panel is that most patients prefer VKA therapy over LMWH therapy. The higher purchase cost of LMWH compared with VKA therapy is an additional barrier to the long-term use of LMWH.
Quality of Evidence and Strength of Recommendations—
Evidence for the comparison of long-term LMWH vs VKA therapy in patients without cancer is of low quality. The subgroup effect discussed previously was not sufficiently convincing to allow us to generate an effect estimate specifically for patients without cancer, but still reduced our confidence that the overall effect estimate applies to the noncancer subgroup that contributed a minority of data. Considerations favoring use of VKA over LMWH in patients without cancer include (1) the evidence of benefit with LMWH is of low quality, (2) the estimated absolute reductions in recurrent VTE events with LMWH compared with VKA therapy is small, (3) the high cost of LMWH, and (4) our assessment that LMWH is a greater burden to patients than VKA therapy. Considerations favoring use of LMWH over warfarin in patients with cancer include (1) a large absolute reduction in recurrent VTE with LMWH over VKA therapy and (2) that LMWH is better suited to the care of patients with cancer than is VKA therapy. Among patients with VTE and cancer, the advantages of LMWH over VKA therapy are expected to be greatest in patients (1) with metastatic disease, (2) being treated with aggressive chemotherapy, (3) presenting with extensive VTE, (4) with liver dysfunction, (5) with poor or unstable nutritional status, and (6) who wish to avoid laboratory monitoring of coagulation.
Dabigatran vs VKA Therapy for the Long-term Treatment of DVT:
One completed study has directly compared dabigatran and VKA for the first 6 months of treatment of VTE (Table 23, Table S31).343 Like patients treated with VKA therapy, patients treated with dabigatran initially received parenteral therapy (usually IV UFH or LMWH). This study suggests that treatment with dabigatran or VKA therapy is associated with a similar frequency of recurrent VTE, major bleeding, and death. This evidence is of moderate quality because of serious imprecision for each outcome and lack of long-term safety data for dabigatran in this patient population. Because the study included few patients with cancer, we were unable to assess whether its findings apply equally to patients with and without cancer. In the absence of evidence of such an interaction, however, we have not further rated down the quality of evidence for patients with VTE and cancer.
Table 23.
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With Warfarin | Risk Difference With Dabigatran (95% CI) | ||||
| Mortality |
2,539 (1 study), 6 mo |
Moderated,e due to imprecision |
HR 0.98 (0.53-1.79) |
17 per 1,000 |
0 fewer per 1,000 (from 8 fewer to 13 more) |
| Recurrent VTE |
2,539 (1 study), 6 mo |
Moderated,e due to imprecision |
HR 1.01 (0.65-1.84) |
19 per 1,000f |
0 more per 1,000 (from 7 fewer to 16 more) |
| Major bleeding |
2,539 (1 study), 6 mo |
Moderated,e due to imprecision |
HR 0.82 (0.45-1.48) |
19 per 1,000g |
3 fewer per 1,000 (from 10 fewer to 9 more) |
| Burden of anticoagulation not reported | … | … | … | Warfarin: daily medication, dietary restrictions, frequent blood testing/monitoring, increased hospital/clinic visits | Dabigatran: daily medication, no dietary restrictions, no frequent blood testing/monitoring |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. See Table 1, 3, and 21 legends for expansion of abbreviations.
Included patients had acute, symptomatic, and objectively verified proximal DVT of the legs or PE.
Dabigatran 150 mg bid taken orally for 6 mo after an initial treatment with LMWH or IV UFH.
Warfarin adjusted to achieve an INR of 2.0 to 3.0 for 6 mo after an initial treatment with LMWH or IV UFH.
Allocation was concealed. Patients, providers, data collectors, and outcome adjudicators were blinded. Modified intention-to-treat analysis; 1.1% loss to follow-up. Not stopped early for benefit.
CI includes values suggesting no effect and values suggesting either benefit or harm; relatively low number of events.
One fatal VTE in dabigatran group and three fatal VTE in warfarin group.
One fatal major bleeding in dabigatran group and one fatal major bleeding in warfarin group.
Rivaroxaban vs VKA Therapy for the Long-term Treatment of DVT:
A single study has directly compared rivaroxaban (without initial parenteral anticoagulation) with parenteral anticoagulation and VKA in patients with acute DVT (Table 21, Table S29).88 Results suggested that treatment with rivaroxaban and VKA therapy are associated with a similar frequency of recurrent VTE, major bleeding, and death. This evidence is of moderate quality because of serious imprecision for each outcome. Because the study included few patients with cancer, we were unable to assess whether its findings apply equally to patients with and without cancer. In the absence of evidence of such an interaction, however, we have not further rated down the quality of evidence for patients with VTE and cancer.
Comparisons Among LMWH, Dabigatran, and Rivaroxaban for the Long-term Treatment of DVT:
There are no direct comparisons of these three agents for the long-term treatment of VTE. Recommendations about the use of one of these agents over the other are based on indirect comparisons, and the evidence is low quality.
Recommendations
3.3.1. In patients with DVT of the leg and no cancer, we suggest VKA therapy over LMWH for long-term therapy (Grade 2C). For patients with DVT and no cancer who are not treated with VKA therapy, we suggest LMWH over dabigatran or rivaroxaban for long-term therapy (Grade 2C).
3.3.2. In patients with DVT of the leg and cancer, we suggest LMWH over VKA therapy (Grade 2B). In patients with DVT and cancer who are not treated with LMWH, we suggest VKA over dabigatran or rivaroxaban for long-term therapy (Grade 2B).
Remarks (3.3.1-3.3.2): Choice of treatment in patients with and without cancer is sensitive to individual patient’s tolerance for daily injections, need for laboratory monitoring, and treatment costs. LMWH, rivaroxaban, and dabigatran are retained in patients with renal impairment, whereas this is not a concern with VKA. Treatment of VTE with dabigatran or rivaroxaban, in addition to being less burdensome to patients, may prove to be associated with better clinical outcomes than VKA and LMWH therapy. When these guidelines were being prepared (October 2011), postmarketing studies of safety were not available. Given the paucity of currently available data and that new data are rapidly emerging, we give a weak recommendation in favor of VKA and LMWH therapy over dabigatran and rivaroxaban, and we have not made any recommendations in favor of one of the new agents over the other.
3.4 Choice of Anticoagulant Regimen for Extended Therapy
Other than a comparison of low-intensity (target INR 1.75) with conventional-intensity VKA therapy44 (Table S24 and S25), there are no completed studies that have compared different anticoagulant agents or regimens for extended therapy after a standardized initial period (eg, ≥ 3 months) of anticoagulation in either patients without or with cancer. Because a decision about using extended therapy occurs after an initial period of anticoagulation (eg, 3 months) and because the relative efficacy and safety of anticoagulant regimens are expected to be similar during the early and extended phases of therapy, we anticipate that most patients will continue to use their initial anticoagulant regimen for extended therapy. Possible reasons for switching from LMWH to VKA therapy include the following: cancer becomes less active, chemotherapy is completed, patient tires of SC injections, development of renal impairment causes concern about accumulation of LMWH (also applies to rivaroxaban and dabigatran), and LMWH costs become prohibitive. Reasons for switching from VKA therapy to LMWH could include difficulty with INR control and need for repeated invasive procedures. New anticoagulant therapies may expand indications for extended anticoagulant therapy because they are less burdensome than VKA or LMWH therapy and because they may be associated with improved clinical outcomes (ie, more effective or safer).
Recommendation
3.4. In patients with DVT of the leg who choose extended therapy, we suggest treatment with the same anticoagulant chosen for the first 3 months (Grade 2C).
3.5 Treatment of Asymptomatic DVT of the Leg
Rather than screen postoperative patients for the presence of asymptomatic DVT, clinicians should prescribe primary prophylaxis for VTE to surgical patients.230,231 If imaging studies performed for other reasons (eg, CT scanning for staging of cancer) incidentally detect asymptomatic proximal DVT, the high frequency of false-positive results in patients without a prior suspicion of DVT dictates caution in assuming that a DVT is truly present. Reasons for a high rate of false-positive results include (1) the imaging technique may not have been optimal for the diagnosis of DVT, (2) incidentally diagnosed DVT often is seen in the pelvis where DVT is harder to image (eg, unable to be assessed with compression ultrasound), and (3) the prevalence of DVT in asymptomatic patients is much lower than in symptomatic patients. Consequently, when there is evidence of incidental DVT, additional diagnostic testing (eg, ultrasound) to confirm the presence of DVT may be necessary. Because many cases of asymptomatic VTE are detected as PE, see also section 6.9 of this article for recommendations on the management of this condition.
No randomized trials have evaluated anticoagulant therapy in patients with incidental VTE; therefore, evidence is of moderate quality because of indirectness. Moreover, benefits of anticoagulant therapy may be less than in symptomatic patients because asymptomatic DVT may be chronic or less extensive and because the prevalence of false-positive results will be higher than in patients who were suspected of having DVT.
Factors that justify a more-aggressive approach to anticoagulation in patients with incidentally diagnosed DVT include certainty of diagnosis, extensive thrombosis that appears to be acute (eg, not present on a previous imaging study), progression of thrombosis on a follow-up imaging study, ongoing risk factors for VTE (eg, cancer), and a low risk of bleeding. A less-aggressive approach to anticoagulation could include (1) withholding of anticoagulation with surveillance to detect DVT extension or (2) limiting anticoagulant therapy to 3 months in patients with continuing risk factors for VTE (eg, cancer). Many patients have left the hospital by the time incidental DVT is reported. If it would be difficult for patients to return the same day, it is often reasonable to defer further assessment and anticoagulant therapy until the next day.
Recommendation
3.5. In patients who are incidentally found to have asymptomatic DVT of the leg, we suggest the same initial and long-term anticoagulation as for comparable patients with symptomatic DVT (Grade 2B).
4.0 PTS of the Leg
PTS is a cluster of leg symptoms and signs attributable to previous DVT. PTS occurs in about one-third of patients after acute DVT and up to two-thirds who have had an iliofemoral DVT.102,232 The initial treatment of acute DVT, particularly with the use of thrombus removal strategies, may influence the risk of developing PTS (section 2.8). The most prominent symptoms are chronic dependent swelling and pain, discomfort on walking, and skin discoloration. The severity of symptoms may vary over time, and the most severe manifestation is a venous ulcer of the lower leg. In this section, we address prevention of PTS first and then its treatment. Unlike the last edition of these guidelines,6 we do not address treatment of leg ulcers associated with venous insufficiency.
4.1 Compression Stockings and Bandages to Prevent PTS
Five randomized trials evaluated compression stockings used at various times after diagnosis of acute DVT for the prevention of PTS (Tables S32 and S33).201,202,233‐235 Two of these trials163,164 randomized patients soon after diagnosis of a first episode of symptomatic proximal DVT to prolonged use of stockings (30-40 mm Hg pressure at the ankles) or no stockings and otherwise treated patients the same way (Table 24, Table S34). These trials suggest that compression stockings started within 2 weeks of DVT and continued for 2 years reduce PTS by about 50% and do not alter the frequency of recurrent VTE. Patients with proximal DVT and a previous DVT in the same leg and who have marked symptoms are expected to gain the most benefit from compression stockings.102,201,202 The evidence is of moderate quality because the assessment of PTS, which includes a large subjective component, was not blinded, and the estimate for recurrent VTE was imprecise.
Table 24.
—[Section 4.1] Summary of Findings: Elastic Compression Stockings vs No Elastic Compression Stockings to Prevent PTS of the Lega,b,451
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With No Elastic Compression Stockings | Risk Difference With Elastic Compression Stockings (95% CI) | ||||
| PTS |
421 (2 studies), 2 y |
Moderatec due to risk of bias |
RR 0.46 (0.34-0.63)d |
479 per 1,000e,f |
259 fewer per 1,000 (from 177 fewer to 316 fewer)g |
| Recurrent VTE |
374 (2 studies), 5 y |
Moderateh,i due to imprecision |
RR 1.01 (0.61-1.67)d |
210 per 1,000j |
2 more per 1,000 (from 82 fewer to 141 more) |
| QOL not reported | … | … | k | … | |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. See Table 1,3 and 10 legends for expansion of abbreviations.
Prandoni et al202 excluded patients with recurrent ipsilateral DVT, preexisting leg ulcers, or signs of chronic venous insufficiency, bilateral thrombosis, a short life expectancy or a contraindication for use of stockings (eg, advanced-stage peripheral arterial insufficiency). Brandjes et al201 excluded patients with short life expectancy, paralysis of the leg, bilateral thrombosis, leg ulcers, or extensive varicosis.
Brandjes201 used graded elastic compression stockings (40 mm Hg of pressure at the ankle, 36 mm Hg at the lower calf, and 21 mm Hg at the upper calf); stockings were applied 2 to 3 wk after the first episode of proximal DVT. Prandoni et al202 used flat-knitted stockings (30 to 40 mm Hg of pressure at the ankle); stockings were started at hospital discharge, an average of 1 wk after admission. In both studies, stockings were used for 2 y.
Patients were not blinded to the treatment assignment, and outcomes were partly based on subjective report of symptoms.
The effect estimate shown here results from a meta-analysis (Mantel-Haenszel fixed-effects model) of the two relevant trials. A fixed-effects model was chosen because of the small number of studies available.
This estimate is based on the findings of the VETO study.70 This probably underestimates PTS baseline risk given that overall, 52% of patients reported the current use of compression stockings during study follow-up.
In Prandoni et al,202 most events occurred during the first 6 mo. The cumulative incidence of the PTS in the control group was 40% after 6 mo, 47% after 1 y, and 49% after 2 y.
Severe PTS: assuming the same RR of 0.46 and a baseline risk of 8.1% over 2 y, the absolute reduction is 44 fewer severe PTS per 1,000 (from 30 fewer to 53 fewer) over 2 y.
We did not rate down the quality of evidence for recurrent VTE for the lack of blinding because this a more objective outcome than PTS.
CI includes both negligible effect and appreciable benefit or appreciable harm.
This estimate is the mean of two estimates derived from two studies: 12.4% probable/definite VTE (Heit et al165) and 29.1% confirmed VTE (Prandoni et al208).
This is an important outcome that should be considered in future studies.
Compression stockings applying an ankle pressure of 30 to 40 mm Hg and a lower pressure higher up the leg (ie, graduated pressure) should be started as soon as feasible after starting anticoagulant therapy. Bandages may be used to provide initial compressive therapy because it may not be possible to fit compression stockings immediately and if stockings can be worn, a rapid decrease in leg swelling will require them to be refitted. The findings from a randomized trial of 69 patients suggest that immediate compression bandaging improves acute symptoms but does not reduce PTS at 1 year (RR, 0.9; 95% CI, 0.4-1.8).236 Patients or their caregivers need to be able to apply and remove stockings for their use to be feasible. Alternative approaches to the use of stockings, such as routinely wearing stockings after acute DVT but stopping them if there are no symptoms of PTS after 6 months235,237 or only wearing stockings if there is persistent leg swelling, have not been adequately evaluated.
Recommendation
4.1. In patients with acute symptomatic DVT of the leg, we suggest the use of compression stockings (Grade 2B).
Remarks: Compression stockings should be worn for 2 years, and we suggest beyond that if patients have developed PTS and find the stockings helpful. Patients who place a low value on preventing PTS or a high value on avoiding the inconvenience and discomfort of stockings are likely to decline stockings.
4.2 Physical Treatment of PTS
Treatment of PTS with compression stockings has only been evaluated in two small trials233,238 (Table 25, Tables S35 and S37) (all patients received rutosides in one study238). These studies did not find compression stockings to be of benefit. However, the quality of the evidence is low because of imprecision and risk of bias. There is anecdotal evidence that compression therapy is of benefit in many patients with PTS, and the potential benefit of a trial of compression stockings in individual patients is likely to outweigh its harm and cost. We suggest below-knee stockings in most patients, but thigh-length stockings may be preferable in those with marked thigh swelling.
Table 25.
—[Section 4.2.1] Summary of Findings: Compression Stockings vs No Compression Stockings for Patients With PTSa-c,233,238
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With No Compression Stockings | Risk Difference With Compression Stockings (95% CI) | ||||
| Symptomatic relief treatment successg | 115 (2 studies), 12 to 26 mo | Lowd-f due to risk of bias and imprecision | RR 0.96 (0.70-1.31) | 579 per 1,000 | 23 fewer per 1,000 (from 174 fewer to 179 more) |
|
| |||||
| QOL not reported | … | Not estimable | |||
|
| |||||
| Recurrent VTE not reported | … | Not estimable | |||
|
| |||||
| Ulcerationh not reported | … | Not estimableh | |||
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. CLOTS1 = Clots in Legs or Stocking After Stroke. See Table 1 and 3 legends for expansion of other abbreviations.
Ginsberg et al233 included patients with PTS 1 y after chronic, typical proximal DVT. Frulla et al238 included patients with clinical symptoms and signs suggestive of PTS.
Ginsberg et al233: Graduated compression stockings (30-40 mm Hg, calf or thigh length, depending on symptoms). Patients were encouraged to wear stockings as much as possible during waking hours. Frulla et al238: below-knee graded elastic compression stockings (ECS) (30-40 mm Hg at the ankle). Patients in both study arms received hydroxyethylrutosides (HR) (we considered the ECS 1 HR vs HR comparison).
Ginsberg et al233: placebo stockings (calf or thigh length, depending on symptoms).
Ginsberg et al233: Adequacy of sequence generation and allocation concealment were unclear; patients and outcome assessors were adequately blinded; unclear whether analysis followed the intention-to-treat principle; unclear whether follow-up was complete. Frulla et al238: outcome assessors were blinded; follow-up was complete; intention-to-treat principle was adhered to, but sequence generation and allocation concealment were unclear, and patients were not blinded.
Very small number of patients
Publication bias was not detected but not ruled out given that we identified only one small study partially supported by industry (provision of graduated compression stockings).
Ginsberg et al233 reported treatment failure (defined a priori based on any of five clinical criteria, including symptoms and ulcer development). Treatment success refers to the absence of treatment failure. Frulla et al238 used the Villalta scale.
Indirect evidence from the CLOTS1 trial suggests that compression stockings is associated with an RR of 4 for skin complications.
Two small crossover randomized trials have evaluated the treatment of severe PTS with intermittent compression devices239,240 (Table 26, Table S36). Both studies suggested benefit from intermittent compression therapy. The quality of the evidence, however, is moderate because of imprecision. The goal of intermittent compression therapy is to reduce PTS symptoms rather than to alter the natural history of its development. These devices can be used with or without compression stockings, depending on patient preference. Leg swelling and associated symptoms (eg, heaviness, tightness) are more likely to respond to compression stockings or intermittent compression devices than are other symptoms.
Table 26.
—[Section 4.2.2] Summary of Findings: Intermittent Compression Device vs No Intermittent Compression Device for Patients With Severe PTSa-c,233,240
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With No Intermittent Compression Device | Risk Difference With Intermittent Compression Device (95% CI) | ||||
| Symptomatic relief: symptom score includes scoring of pain, swelling, and limitation of activity on a scale of 10-70 | 82 (2 studiesd), 8 wk | Moderatee-i due to imprecision | The mean symptomatic relief in the control groups was 0 | The mean symptomatic relief in the intervention groups was 0.41 SDs higher (0.02 lower to 0.85 higher) | |
|
| |||||
| QOL: VEINES-QOL scale of 0-100. | 0 (1 studyd,j), 8 wk | Moderateg-i,k,l due to imprecision | The mean QOL in the control groups was 50.2 | The mean QOL in the intervention groups was 2.3 higher (1.04 lower to 5.64 higher) | |
|
| |||||
| Recurrent VTEm not reported | … | Not estimablem | |||
|
| |||||
| Ulcerationn not reported | … | Not estimablen | |||
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. VEINES = Venous Insufficiency Epidemiological and Economic Study. See Table 1, 3, and 25 legends for expansion of other abbreviations.
Patients with previous DVT with symptoms of severe PTS.
Intervention group: Ginsberg et al239: Extremity pump used bid for 20 min each session; 50 mm Hg (therapeutic pressure) for 1 mo. O’Donnell et al240: Venowave lower-limb venous return assist device to wear for most of the day for 8 wk.
Control group: Ginsberg et al239: Extremity pump used bid for 20 min each session; 15 mm Hg (placebo pressure) for 1 mo. O’Donnell et al240: Venowave lower-limb venous return assist device with no connection between motor and planar sheet for 8 wk.
Crossover RCTs.
In both studies, sequence generation was adequate; patients were blinded, analysis adhered to intention-to-treat principle, and there were no missing outcome data. In Ginsberg et al239 (but not O’Donnell et al240), outcome assessors were not blinded, and it was not clear whether allocation was concealed.
I2 = 0%.
Some concerns with indirectness, given relatively short follow-up (8 wk).
Very small number of patients. CI includes both values suggesting no effect and values suggesting a beneficial effect.
Publication bias not detected but not ruled out given that we identified only two small studies, with one (Ginsberg et al239) partially supported by industry (provision of devices).
O’Donnell et al et al.240
Sequence generation was adequate; patients were blinded, analysis adhered to intention-to-treat principle, and there were no missing outcome data. However, outcome assessors were not blinded, and it was not clear whether allocation was concealed.
Publication bias was not detected but not ruled out given that we identified only a small study.
O’Donnell et al240 indicated no cases of recurrent VTE by the end of this study but judged the follow-up period to be short.
O’Donnell et al240 indicated that one patient in the control group developed a venous ulceration. Three other participants developed nonserious skin-related side effects. Indirect evidence from the CLOTS1 suggests that compression stockings are associated with an RR of 4 for skin complications. Common side effects attributed to Venowave were heat sensation, skin irritation, and increased sweating.
Recommendations
4.2.1. In patients with PTS of the leg, we suggest a trial of compression stockings (Grade 2C).
4.2.2. In patients with severe PTS of the leg that is not adequately relieved by compression stockings, we suggest a trial of an intermittent compression device (Grade 2B).
4.3 Pharmacologic Treatment of PTS
Hydroxyrutosides, a class of flavonoid drug produced from plant glycosides, may reduce capillary permeability, reduce inflammation, improve lymphatic function, and promote ulcer healing in patients with chronic venous insufficiency.6,241,242 Two studies compared treatment of PTS (without ulceration) with rutosides vs control238,243 (all patients wore compression stockings in one study238), and one study compared rutosides with hidrosmina244(Table S37). The two controlled studies suggest that rutosides do not reduce most symptoms of PTS, although they may reduce ankle swelling (Table 27). This evidence is of low quality because of inconsistency and imprecision. Furthermore, rutosides may be associated with important side effects.
Table 27.
—[Section 4.3] Summary of Findings: Venoactive Medication vs No Venoactive Medication for Patients With PTSa,b,202,238
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With No Venoactive Medication | Risk Difference With Venoactive Medication (95% CI) | ||||
| Symptomatic relief: PTS score (Villalta scale) < 5 or decreased by 30% at 12 mo compared with baseline in Frulla et al238; improved tiredness of the leg at 8 wk in de Jongste et al243,c | 163 (2 studies) | Lowd-g due to inconsistency, imprecision | RR 1.14 (0.85-1.52) | 476 per 1,000 | 67 more per 1,000 (from 71 fewer to 247 more) |
|
| |||||
| QOL not reported | … | Not estimable | |||
|
| |||||
| Recurrent VTE not reported | … | Not estimable | |||
|
| |||||
| Ulceration not reported | … | Not estimable | |||
|
| |||||
| Side effects | 203 (2 studies) | Moderated,f-h due to imprecision | RR 2.04 (0.76-5.51) | 61 per 1,000 | 63 more per 1,000 (from 15 fewer to 275 more) |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. See Table 1 and 3 legends for expansion of abbreviations.
Patients with PTS and history of DVT in PTS leg.
Included studies that assessed rutosides.
Investigators assessed other symptoms (pain, heaviness, swelling feeling, restless legs, and cramps) but did not report a composite score. The symptom we chose to report showed the most benefit; the effect estimates for the other symptoms ranged from 0.8 to 1.4, and none were statistically significant.
In both studies, sequence generation and allocation concealment were unclear. Both studies blinded outcome assessors and had complete follow-up. Although de Jongste et al243 blinded patients, the authors did not adhere to the intention-to-treat principle and did not use a validated scale to measure symptomatic relief. Although Frulla et al238 adhered to the intention-to-treat principle, the author did not blind patients.
I2 = 77%.
Small number of patients. CI included both values suggesting harms and values suggesting benefits.
Publication bias was not detected but not ruled out given that we identified only two small studies, and it unclear whether they were funded by industry.
I2 = 7%.
Recommendation
4.3. In patients with PTS of the leg, we suggest that venoactive medications (eg, rutosides, defibrotide, and hidrosmin) not be used (Grade 2C).
Remarks: Patients who value the possibility of response over the risk of side effects may choose to undertake a therapeutic trial
5.0 Initial Treatment of Acute PE
As we noted in Methods (section 1.1), recommendations for management of patients with PE, particularly those addressing anticoagulant therapy and IVC filter insertion, are based on studies that enrolled patients with only DVT , patients with both DVT and PE, and patients with only symptoms of PE. The following sections emphasize studies that enrolled only patients with symptoms of PE (who could also have symptoms of DVT), emphasize differences in the management of patients who present with PE compared with DVT, and make recommendations for the management of patients with PE. We do not repeat evidence that was presented in the corresponding section that addresses treatment of DVT; instead, the reader is directed to those sections of the article and to the related tables. We do not comment in the text on the quality of the evidence that underlies treatment recommendations for PE unless the quality of this evidence differs from that for patients who present with DVT.
5.1 Initial Anticoagulation for Acute PE
See section 2.1, Table 3, and Table S1.
Recommendation
5.1. In patients with acute PE, we recommend initial treatment with parenteral anticoagulation (LMWH, fondaparinux, IV UFH, or SC UFH) over no such initial treatment (Grade 1B).
5.2 Whether to Treat With Parenteral Anticoagulation While Awaiting the Results of Diagnostic Work-up for PE
See section 2.2. For the purpose of implementing this recommendation, validated prediction rules help with estimation of clinical probability of having PE.55,56
Recommendations
5.2.1. In patients with a high clinical suspicion of acute PE, we suggest treatment with parenteral anticoagulants compared with no treatment while awaiting the results of diagnostic tests (Grade 2C).
5.2.2. In patients with an intermediate clinical suspicion of acute PE, we suggest treatment with parenteral anticoagulants compared with no treatment if the results of diagnostic tests are expected to be delayed for more than 4 h (Grade 2C).
5.2.3. In patients with a low clinical suspicion of acute PE, we suggest not treating with parenteral anticoagulants while awaiting the results of diagnostic tests provided that test results are expected within 24 h (Grade 2C).
5.3 Timing of Initiation of VKA and Associated Duration of Parenteral Anticoagulant Therapy
See section 2.4, Table 4, and Table S2.
Recommendation
5.3. In patients with acute PE, we recommend early initiation of VKA (eg, same day as parenteral therapy is started) over delayed initiation, and continuation of parenteral anticoagulation for a minimum of 5 days and until the INR is 2.0 or above for at least 24 h (Grade 1B).
5.4 Choice of Initial Parenteral Anticoagulant Regimen in Patients With PE
See section 2.5.
LMWH Compared With IV UFH for the Initial Treatment of PE:
See section 2.5 and Table 6. Consistent with findings in patients with DVT, LMWH has been found to be as effective and safe as IV UFH in studies that included both patients with PE and DVT or only in patients with PE (Table 6). A meta-analysis of 12 studies85,146,245‐254 that included a total of 1,951 patients with either submassive symptomatic PE or asymptomatic PE in conjunction with symptomatic DVT failed to demonstrate or exclude a beneficial or detrimental effect of LMWH on recurrent VTE (OR, 0.63; 95% CI, 0.33-1.18), major bleeding (OR, 0.67; 95% CI, 0.36-1.27), and all-cause mortality (OR, 1.20; 95% CI, 0.59-2.45).255
SC UFH Compared With SC LMWH for the Initial Treatment of PE:
Fondaparinux Compared With IV UFH for the Initial Treatment of PE:
The Matisse-PE trial compared fondaparinux with IV UFH for acute treatment of PE79 (Table 28, Table S38). This study suggested that fondaparinux is associated with a similar frequency of mortality, recurrent VTE, and major bleeding as LMWH. The quality of this evidence is moderate because of imprecision. In making recommendations, we also considered evidence that fondaparinux is equivalent to LMWH for the treatment of DVT (see section 2.5, Table 7, and Table S38) and that fondaparinux shares the advantages that LMWH has over IV UFH.
Table 28.
—[Section 5.4] Summary of Findings: Fondaparinux vs IV UFH for Initial Anticoagulation of Acute PEa-c,79
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With UFH | Risk Difference With Fondaparinux (95% CI) | ||||
| Mortality |
2,213 (1 study), 3 mo |
Moderated,e due to imprecision |
RR 1.20 (0.82-1.74) |
43 per 1,000 |
9 more per 1,000 (from 8 fewer to 32 more) |
| Recurrent VTE |
2,213 (1 study), 3 mo |
Moderated,e due to imprecision |
RR 0.75 (0.51-1.12) |
50 per 1,000f |
13 fewer per 1,000 (from 25 fewer to 6 more) |
| Major bleeding | 2,213 (1 study), 3 mo | Moderated,e due to imprecision | RR 0.85 (0.49-1.49) | 23 per 1,000g | 4 fewer per 1,000 (from 12 fewer to 11 more) |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. See Table 1 and 3 legends for expansion of abbreviations.
All patients had acute, symptomatic, hemodynamically stable PE.
Fondaparinux (5.0, 7.5, or 10.0 mg in patients weighing < 50, 50 to 100, or > 100 kg, respectively) SC once daily given for at least 5 days and until the use of VKAs resulted in an INR > 2.0.
UFH continuous IV infusion (ratio of the activated partial thromboplastin time to a control value of 1.5-2.5) given for at least 5 days and until the use of VKAs resulted in an INR > 2.0.
Allocation was concealed. Patients, providers, and data collectors were not blinded. Outcome adjudicators were blinded; 0.6% of randomized patients were lost to follow-up. Not stopped early for benefit.
CI includes values suggesting no effect and values suggesting either benefit or harm; relatively low number of events.
Sixteen fatal VTE in fondaparinux group and 15 fatal VTE in UFH group.
Fourteen patients in the fondaparinux group and 12 patients in the LMWH group had a major bleeding event during the initial period (6-7 d). Of these, one in the fondaparinux group and one in the UFH group were fatal.
Fondaparinux Compared With LMWH for the Initial Treatment of PE:
In the absence of direct evidence in patients with PE, indirect evidence in patients with acute DVT (see section 2.5, Table 7, and Table S7) suggests that fondaparinux is equivalent to LMWH.
Fondaparinux Compared With SC UFH for the Initial Treatment of PE:
There is no direct evidence for this comparison. In making recommendations, we considered that fondaparinux and LMWH are equivalent and that fondaparinux shares the advantages that LMWH has over IV UFH. We did not take into account the lower purchase cost of SC UFH compared with fondaparinux.
Once- vs Twice-Daily Administration of LMWH for Initial Treatment of PE:
See section 2.5, Table 8, and Table S8. Patients who presented with PE were included in only one of the five studies with an unconfounded comparison of once- and twice-daily LMWH85 and in one additional large study that compared once-daily LMWH therapy with IV UFH in patients who presented with PE.252
Recommendations
5.4.1. In patients with acute PE, we suggest LMWH or fondaparinux over IV UFH (Grade 2C for LMWH; Grade 2B for fondaparinux), and over SC UFH (Grade 2B for LMWH; Grade 2C for fondaparinux).
Remarks: Local considerations such as cost, availability, and familiarity of use dictate the choice between fondaparinux and LMWH. LMWH and fondaparinux are retained in patients with renal impairment, whereas this is not a concern with UFH. In patients with PE where there is concern about the adequacy of SC absorption or in patients in whom thrombolytic therapy is being considered or planned, initial treatment with IV UFH is preferred to use of SC therapies.
5.4.2. In patients with acute PE treated with LMWH, we suggest once- over twice-daily administration (Grade 2C).
Remarks: This recommendation only applies when the approved once-daily regimen uses the same daily dose as the twice-daily regimen (ie, the once-daily injection contains double the dose of each twice-daily injection). It also places value on avoiding an extra injection per day.
5.5 Early vs Standard Discharge of Patients With Acute PE
Consistent with our discussion of outpatient treatment of acute DVT (section 2.7), LMWH has made it feasible to treat acute PE at home either without admission to the hospital (ie, discharge from the emergency department) or with admission and early discharge. However, because acute PE is associated with much higher short-term mortality than acute DVT, the safety of treating PE at home is uncertain. Consequently, PE is treated at home much less often than DVT, and the proportion of outpatients with PE that clinical centers treat at home varies from almost none to about 50%.
Two studies randomized patients with acute PE and a low risk of complications to receive LMWH either (1) in the hospital for only 3 days vs entirely in the hospital256 or (2) entirely out of the hospital (discharged within 24 h) vs at least partly in hospital257 (Table 29, Table S39). This evidence suggests that treating appropriately selected patients with acute PE at home does not increase recurrent VTE, bleeding, or mortality.
Table 29.
—[Section 5.5] Summary of Findings: Early Discharge vs Standard Discharge in the Treatment of Acute PEa,b,256,257
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With Standard Discharge | Risk Difference With Early Discharge (95% CI) | ||||
| Mortality |
471 (2 studies), 3 mo |
Moderatec,d due to imprecision |
RR 0.58 (0.17-1.97) |
26 per 1,000 |
11 fewer per 1,000 (from 22 fewer to 26 more) |
| Nonfatal recurrent PE |
471 (2 studies), 3 mo |
Moderatec,d due to imprecision |
RR 1.23 (0.25-6.03) |
9 per 1,000 |
2 more per 1,000 (from 7 fewer to 44 more) |
| Major bleeding |
471 (2 studies), 3 mo |
Moderatec,d due to imprecision |
RR 2.74 (0.45-16.71) |
4 per 1,000 |
8 more per 1,000 (from 2 fewer to 69 more) |
| QOL not reported |
… |
… |
… |
… |
… |
| PTS not reported | … | … | … | … | … |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. See Table 1 and 3 legends for expansion of abbreviations.
The two RCTs included only patients with low risk: risk classes I or II on the Pulmonary Embolism Severity Index in Aujesky et al216; low risk on clinical prediction rule by Uresandi et al.258
Mean length of hospital stay: 3.4 (SD 1.1) vs 9.3 (SD 5.7) d in Otero et al256 and 0.5 (SD 1.0) vs 3.9 (SD 3.1) d in Aujesky et al257; low risk on clinical prediction rule by Uresandi et al258 in Otero et al.
Otero et al256: allocation concealed; no patients lost to follow-up; intention-to-treat analysis; no blinding of outcome assessors reported; study stopped early because the rate of short-term mortality was unexpectedly high in the early discharge group (2 [2.8%] vs 0 [0%]). Aujesky et al257: unclear whether allocation was concealed; three (1%) patients had missing outcome data; intention-to-treat analysis; blinding of outcome adjudicators; no early stoppage.
CI includes both values suggesting no effect and values suggesting appreciable harm or appreciable benefit.
There are a number of prediction rules for identifying patients with acute PE who have a low risk of serious complications and may be suitable for treatment at home.258‐263 Of these, the PE Severity Index (PESI) is best validated261,262,264‐266 and was used to select patients for home treatment in the larger of the previously noted clinical trials (Table 29, Table S39). Patients with acute PE who meet the following criteria appear to be suitable for treatment out of the hospital: (1) Clinically stable with good cardiopulmonary reserve (eg, PESI score of < 85257 or simplified PESI score of 0,262 including none of hypoxia, systolic BP < 100, recent bleeding, severe chest pain, platelet count < 70,000/mm3, PE while on anticoagulant therapy, and severe liver or renal disease)257, (2) good social support with ready access to medical care, and (3) expected to be compliant with follow-up. Patients also need to feel well enough to be treated at home (eg, absence of severe symptoms or comorbidity).
Consistent with the findings of these two trials, a systematic review of 11 observational studies (seven prospective, four retrospective; 928 patients)267 and four more recent observational studies (two retrospective with 584 patients268,269; two prospective with 449 patients270,271) reported a very low frequency of complications in low-risk patients with acute PE who were initially treated partially or entirely at home. About one-third to one-half of outpatients with acute PE appear to be in this low-risk group.272 The evidence from the randomized trials is of moderate quality (rated down for serious imprecision), with additional supportive findings from the observational studies.
Recommendation
5.5. In patients with low-risk PE and whose home circumstances are adequate, we suggest early discharge over standard discharge (eg, after the first 5 days of treatment) (Grade 2 B).
Remarks: Patients who prefer the security of the hospital to the convenience and comfort of home are likely to choose hospitalization over home treatment.
5.6 Systemic Thrombolytic Therapy for PE
5.6.1 Systemic Thrombolytic Therapy vs Anticoagulation Alone for PE:
Randomized trials have established that, at 24 h, thrombolytic therapy improves (1) pulmonary artery hemodynamic measurements (eg, mean pulmonary artery pressure improvement, 4.4 mm Hg; 95% CI, −4.6-4.2 mm Hg), (2) arteriovenous oxygen (difference of −0.3 [−0.4 to −0.2]), (3) pulmonary perfusion (50% early improvement in perfusion scan, OR, 3.8; 95% CI, 0.9-15.7), and (4) echocardiographic assessment (OR for improved right ventricular wall movement, 3.1; 95% CI, 1.5-6.3).273 Thrombolytic therapy, however, does not appear to reduce the extent of residual thrombosis. It is uncertain whether the benefits of more-rapid resolution of PE outweigh the risk of increased bleeding associated with thrombolytic therapy. In patients with PE, severity of presentation is expected to depend on the extent of embolism (ie, degree of pulmonary artery obstruction) and the presence and severity of chronic cardiopulmonary impairment.104,274,275 Patients with the most severe presentations who have the highest risk of dying from an acute PE have the most to gain from thrombolysis.
Prognosis in Patients With Acute PE—
Of patients who are diagnosed with PE and start treatment, ∼5% die of the initial PE or another PE within the next 7 days.9,104,276‐279 However, although the risk of dying of PE differs markedly among patients, no validated risk prediction tool is available. Risk of dying of PE is estimated to be ∼70% if cardiopulmonary arrest occurs (∼1% of patients at presentation), 30% if there is shock requiring inotropic support (∼5% of patients), and ∼2% in patients who are not hypotensive.104,276‐278,280,281 In the presence of normal systemic arterial pressure, prognosis can also differ, depending on (1) clinical evaluation,276 (2) cardiac biomarkers such as troponin or brain natriuretic peptide,279,282‐291 and (3) assessment of right ventricular size and function.279,280,283,285,290‐295
Clinical evaluation involves assessment of general appearance, BP, heart rate, respiratory rate, temperature, pulse oximetry, and signs of right ventricular dysfunction (eg, distended jugular veins, tricuspid regurgitation, accentuated P2).104 Clues on the ECG include right bundle branch block, S1Q3T3, and T-wave inversion in leads V1 through V4.296 Elevation of cardiac troponins indicates right ventricular microinfarction, and echocardiography may show right ventricular hypokinesis; both are risk factors for early mortality and are associated with a worse outcome when they occur together.238,241‐245,250 Right ventricular enlargement on the CT pulmonary angiogram, defined as a right ventricular diameter ≥ 90% than the left ventricular diameter may also be an independent risk factor for death and nonfatal complications.279,291,293,295,297
Risk of Bleeding With Thrombolytic Therapy—
We have not identified any validated risk prediction tool for bleeding with thrombolytic therapy in patients with PE. However, we assume that the assessment of bleeding risk with thrombolytic therapy is similar in patients with PE and with acute ST-segment elevation myocardial infarction.104,110‐113,298,299 Table 11 lists risk factors for bleeding with thrombolytic therapy, categorized as major and relative contraindications.
Trials Evaluating Thrombolytic Therapy in Patients With Acute PE—
The findings of 13 randomized trials that compared thrombolytic therapy to anticoagulant therapy alone in patients with acute PE are summarized in Table 30 and Tables S40 through S42.300‐313 A number of meta-analyses of these studies have been performed.104,273,315,315, This evidence suggests that thrombolysis may be associated with a reduction in mortality and recurrent PE and is associated with an increase in major bleeding, as has been established in patients with myocardial infarction.112 The quality of evidence regarding mortality and recurrent PE is low because of risk of bias, serious imprecision, and suspected publication bias. A previous meta-analysis that categorized studies as either including, or not including, patients with cardiopulmonary compromise, suggested that thrombolytic therapy reduced the composite outcome of death and recurrent PE in studies that included the sickest patients.315 However, we found that the data available from these studies are not sufficiently detailed to enable a subgroup analysis evaluating outcomes in patients with hemodynamic compromise or other markers of heightened risk of death (eg, right ventricular dysfunction).
Table 30.
—[Section 5.6.1] Summary of Findings: Systemic Thrombolytic Therapy vs Anticoagulation Alone in Patients With Acute PEa-d,273,309,310,314,315
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute
Effects |
|
| Risk With No Systemically Administered Thrombolytic Therapy | Risk Difference With Systemically Administered Thrombolytic Therapy (95% CI) | ||||
| Mortality | 847 (12 studies), 30 d | Lowe-h due to risk of bias and imprecision | RR 0.7 (0.37-1.31) | Lowi,j | |
|
| |||||
| 11 per 1,000 | 3 fewer per 1,000 (from 7 fewer to 3 more) | ||||
|
| |||||
| Highi,j | |||||
|
| |||||
| 89 per 1,000 | 27 fewer per 1,000 (from 56 fewer to 28 more) | ||||
|
| |||||
| Recurrent PE | 801 (9 studies), 30 d | Lowe-h due to risk of bias and imprecision | RR 0.7 (0.4-1.21) | 57 per 1,000 | 17 fewer per 1,000 (from 34 fewer to 12 more) |
|
| |||||
| Major bleeding | 847 (12 studies), 10 d | Moderatee,f,h,k due to risk of bias and imprecision | RR 1.63 (1-2.68)l | Lowm | |
|
| |||||
| 1 per 1,000 | 1 more per 1000 (from 0 more to 2 more) | ||||
|
| |||||
| Highm | |||||
|
| |||||
| 62 per 1,000 | 39 more per 1,000 (from 0 more to 104 more) | ||||
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. See Table 1 and 3 legends for expansion of abbreviations.
One study included exclusively patients with hemodynamic compromise (shock), six excluded them, whereas the rest either included a number of such patients or did not specify related eligibility criteria. Of studies not restricted to patients with hemodynamic compromise (n = 11), only three were clearly restricted to patients with right ventricular dysfunction; the rest either did not specify related eligibility criteria or included both patients with and without right ventricular dysfunction. As a result, it was not possible to perform reliable categorization of studies to conduct subgroup analyses based on the presence or absence of right ventricular dysfunction or hemodynamic compromise.
Studies included patients at low risk for bleeding.
Included studies that used different thrombolytic agents with varying doses and durations of administration; no statistical heterogeneity was noted.
Thrombolysis was in addition to anticoagulation (most of the studies used heparin followed by warfarin; three studies used warfarin only).
Report of methodologic quality was poor in most studies. Of the 12 eligible studies, allocation was concealed in five, three were single blind (outcome assessor), six were double blind, and three were not blinded. Most studies did not report on missing outcome data. None of the studies were stopped early for benefit. For the increase in bleeding with thrombolytic therapy, quality of evidence is increased from low to moderate because there is high quality evidence of this association in patients with myocardial infarction and the indirectness of this evidence to patients with PE is minor.
I2 = 0%.
CI includes values suggesting both benefit and no effect or harm; small number of events.
Inverted funnel plots suggestive of possible publication bias in favor of thrombolytics.
Recurrent PE stratification based on the simplified Pulmonary Embolism Severity Index validated in the RIETE (RegistroInformatizado de la Enfermedad Tromboembólica) cohort.262
Some studies suggested that the baseline risk of mortality in patients with hemodynamic instability is as high as 30%.274 In that case, the absolute number of deaths associated with thrombolytics would be 90 fewer per 1,000 (from 189 fewer to 93 more).
CI includes values suggesting both harm and no effect; small number of events.
Major bleeding risk stratification derived from the RIETE cohort.30 The median risk of bleeding over the first 10 d reported in the eligible trials was 3.1%. In that case, the absolute number of major bleeding events with thrombolysis would be 20 more per 1,000 (from 0 more to 52 more).
Indirect evidence from studies of thrombolysis for myocardial infarction and acute stroke provide more precise estimates of increase major bleeding with thrombolytics use.
Balancing Benefits and Harms of Thrombolytic Therapy—
In patients who present with PE and hypotension (eg, systolic pressure BP < 90 mm Hg or a documented drop in systolic BP > 40 mm Hg with evidence of poor perfusion), especially if they have a low risk of bleeding, even modest efficacy of thrombolytic therapy is likely to reduce deaths from PE more than it would increase fatal bleeds and nonfatal intracranial bleeds (Table 30, Tables S40-S42). The ultimate judgment of the entire AT9 panel was to issue a weak recommendation for patients with PE and hypotension given the uncertainty of the benefit. In most patients with PE, given the certain risks of bleeding and less-certain benefits, thrombolysis is likely to be harmful. Selected patients without hypotension may benefit from thrombolysis because their initial clinical presentation or clinical course after starting anticoagulant therapy suggest that they are at high risk of dying.
There is no explicit clinical prediction rule that identifies this subgroup of patients. We suggest that such patients are identified predominantly by clinical evidence of instability (eg, a decrease in systolic BP that still remains > 90 mm Hg, tachycardia, elevated jugular venous pressure, clinical evidence of poor tissue perfusion, hypoxemia) and failure to improve on anticoagulant therapy. As noted previously, laboratory (eg, troponin, brain natriuretic peptide), ECG, echocardiography, and CT evidence of right ventricular dysfunction or enlargement, can supplement the clinical evaluation of instability; however, they are not sufficiently predictive to serve as indications for thrombolytic therapy on their own,289 and we do not recommend that they are routinely measured.
Recommendations
5.6.1.1. In patients with acute PE associated with hypotension (eg, systolic BP < 90 mm Hg) who do not have a high risk of bleeding, we suggest systemically administered thrombolytic therapy over no such therapy (Grade 2C).
5.6.1.2. In most patients with acute PE not associated with hypotension, we recommend against systemically administered thrombolytic therapy (Grade 1C).
5.6.1.3. In selected patients with acute PE not associated with hypotension and with a low risk of bleeding whose initial clinical presentation or clinical course after starting anticoagulant therapy suggests a high risk of developing hypotension, we suggest administration of thrombolytic therapy (Grade 2C).
5.6.2 Systemic Thrombolytic Therapy Regimen for PE:
Twelve randomized trials (total of 938 patients) have compared the rate of thrombus resolution achieved with various IV thrombolytic regimens.316‐327 These regimens included urokinase given over 2 h319,326 or 12 h316,321,326; streptokinase given over 2 h, 12 h,320 or 24 h316; and recombinant tPA (rt-PA) given over 15 min317,322,325 or 2 h,317‐325,327 reteplase in two boluses 30 min apart,325 and desmoteplase in three different doses as a bolus323.
An additional study compared IV with pulmonary artery catheter administration of rt-PA (50 mg over 2 h).328 The results of studies that compared different approaches to thrombolysis in patients with PE (noted previously) suggest that (1) prolonged infusions of thrombolytic agents (eg, ≥ 12 h) are associated with higher rates of bleeding316,318; (2) 2-h infusions achieve more rapid clot lysis than 12- or 24-h infusions318,320,321; (3) when a high-concentration 2-h infusion of thrombolysis is administered, there is no clear difference in the efficacy or safety of rt-PA vs streptokinase327; (4) bolus rt-PA regimens (eg, ∼50 mg in ≤ 15 min) appears to be as effective and safe as a 2-h infusion of 100 mg of rt-PA313,317,322,325; and (5) infusion of rt-PA directly into a pulmonary artery as opposed to a peripheral vein does not accelerate thrombolysis but does cause more frequent bleeding at the catheter insertion site (there was no attempt to infuse rt-PA directly into or to mechanically disrupt the thrombus in this study from 1988).328 When a lytic agent is appropriate for PE, current evidence supports that thrombolytic therapy should be infused into a peripheral vein over ≤ 2 h. At a dose of 100 mg over 2 h, rt-PA is currently the most widely used and evaluated regimen. In patients with imminent or actual cardiac arrest, bolus infusion of thrombolytic therapy is indicated.
The quality of evidence for comparisons of systemic thrombolytic agents and regimens (eg, different doses or durations of infusion) is low based on very serious imprecision and risk of bias. In addition, there is substantial potential for publication bias. Based on this evidence, we provide only weak recommendations for all comparisons of thrombolytic agents and regimens in the short-term treatment of PE.
Recommendations
5.6.2.1. In patients with acute PE, when a thrombolytic agent is used, we suggest short infusion times (eg, a 2-h infusion) over prolonged infusion times (eg, a 24-h infusion) (Grade 2C).
5.6.2.2. In patients with acute PE, when a thrombolytic agent is used, we suggest administration through a peripheral vein over a pulmonary artery catheter (Grade 2C).
5.6.3 Initial Anticoagulant Therapy in Patients Treated With Thrombolytic Therapy:
Trials that evaluated thrombolysis for PE used IV UFH in conjunction with thrombolytic therapy (Table 30, Tables S40-S42), and no randomized trials have compared different regimens of IV UFH in this setting. IV UFH should be given in full therapeutic doses1,3 before thrombolytic therapy is administered, and it is acceptable to either continue or suspend the UFH infusion during administration of thrombolytic therapy (these two practices have never been compared). During a 2-h infusion of 100 mg of tPA, US regulatory bodies recommend suspension of IV UFH, whereas IV UFH is continued during the tPA infusion in many other countries. US authorities recommend checking the activated partial thromboplastin time immediately after completion of the tPA infusion and, provided that the activated antithrombin time is not > 80 s, restarting IV UFH without a bolus at the same infusion rate as before tPA was started.
5.7 Catheter-Based Thrombus Removal for the Initial Treatment of PE
Interventional catheterization techniques for massive PE include mechanical fragmentation of thrombus with a standard pulmonary artery catheter, clot pulverization with a rotating basket catheter, percutaneous rheolytic thrombectomy, or pigtail rotational catheter embolectomy.329‐336 Pharmacologic thrombolysis and mechanical interventions are usually combined unless bleeding risk is high. Catheter embolectomy does not result in extraction of intact pulmonary arterial thrombus; instead, clot fragments are suctioned through the catheter or displaced distally with modest angiographic improvement.
No randomized trials have evaluated interventional catheterization techniques for PE. Most observation studies are retrospective series of < 30 patients.334 Consequently, evidence for the use of interventional catheter techniques in patients with acute PE is of low quality, and our recommendations are weak. Catheter selection, catheter deployment, and adjunctive thrombolytic regimen should be based on local expertise and resources.
Recommendation
5.7. In patients with acute PE associated with hypotension and who have (i) contraindications to thrombolysis, (ii) failed thrombolysis, or (iii) shock that is likely to cause death before systemic thrombolysis can take effect (eg, within hours), if appropriate expertise and resources are available, we suggest catheter-assisted thrombus removal over no such intervention (Grade 2C).
5.8 Surgical Embolectomy for the Initial Treatment of PE
Emergency surgical embolectomy with cardiopulmonary bypass is another management strategy for acute PE associated with hypotension.337‐340 This operation is also suited for patients with acute PE who require surgical excision of a right atrial thrombus, paradoxical arterial embolism, or closure of a patent foramen ovale. Surgical embolectomy also can be performed to rescue patients in whom thrombolysis has been unsuccessful. The procedure is best performed on a warm, beating heart, without aortic cross-clamping, cardioplegia, or fibrillatory arrest.
No randomized trials or prospective observational studies have evaluated surgical embolectomy in patients with acute PE. Consequently, evidence related to surgical embolectomy in patients with acute PE is of low quality, and our recommendations are weak.
Recommendation
5.8. In patients with acute PE associated with hypotension, we suggest surgical pulmonary embolectomy over no such intervention if they have (i) contraindications to thrombolysis, (ii) failed thrombolysis or catheter-assisted embolectomy, or (iii) shock that is likely to cause death before thrombolysis can take effect (eg, within hours), provided surgical expertise and resources are available (Grade 2C).
5.9. Vena Caval Filters for the Initial Treatment of PE
As previously noted in section 2.13, IVC filters can be used instead of initial anticoagulant therapy in patients with acute PE if there is an unacceptable risk of bleeding or as an adjunct to anticoagulation. As in DVT, no randomized trials or prospective cohort studies have evaluated IVC filters as sole therapy for acute PE (ie, without concurrent anticoagulation). As described in section 2.13, the PREPIC study, which evaluated IVC filters as an adjunct to anticoagulation in 400 high-risk patients with proximal DVT, showed that filters reduced PE, increased DVT, and did not change overall frequency of VTE (DVT and PE combined) or mortality146,149 (Table 14; Table S19).
The PREPIC study included 145 (36%) patients with symptomatic PE and 52 (13%) patients with asymptomatic PE at enrollment. If a patient has an acute PE and a short-term contraindication to anticoagulation, provided there is no proximal DVT on ultrasound, it is reasonable not to insert an IVC filter immediately; serial ultrasound examinations can be performed to ensure that the patient remains free of proximal DVT while anticoagulation is withheld.
There is uncertainty about the risk and benefits of inserting IVC filters as an adjunct to anticoagulant and thrombolytic therapy in patients with PE and hypotension. Among patients with hemodynamic compromise in the International Cooperative Pulmonary Embolism Registry, insertion of an IVC filter was associated with a reduction of early recurrent PE and death.280 Consequently, our recommendation against insertion of an IVC filter in patients with acute PE who are treated with anticoagulants may not apply to this select subgroup of patients.
Recommendations
5.9.1. In patients with acute PE who are treated with anticoagulants, we recommend against the use of an IVC filter (Grade 1B).
5.9.2. In patients with acute PE and contraindication to anticoagulation, we recommend the use of an IVC filter (Grade 1B).
5.9.3. In patients with acute PE and an IVC filter inserted as an alternative to anticoagulation, we suggest a conventional course of anticoagulant therapy if their risk of bleeding resolves (Grade 2B).
Remarks: We do not consider that a permanent IVC filter of itself is an indication for extended anticoagulation.
6.0 Long-term Treatment of PE
In the following sections, we emphasize studies that were performed exclusively in patients with PE and patients with PE who were enrolled in other studies. For the reasons noted in section 1.1, we make the same recommendations for long-term treatment of PE as for DVT and rate the quality of the underlying evidence as the same (see corresponding sections for treatment of DVT).
VKA for the Long-term Treatment of PE:
There has been only one evaluation of duration of VKA therapy exclusively in patients with PE. After 3 months of initial treatment, patients with PE provoked by a temporary risk factor were randomized to stop or to receive 3 more months of therapy, and those with unprovoked PE were randomized to stop or to receive 6 more months of therapy (WODIT PE [Warfarin Optimal Duration Italian Trial in patients with Pulmonary Embolism]) (Table S24 and S25).194 Consistent with studies that included patients who presented with DVT, extended VKA therapy was effective while treatment was being received. However, extending the duration of treatment beyond 3 months did not lower the rates of recurrence that were observed when anticoagulants were subsequently stopped.
LMWH for the Long-term Treatment of PE:
Two small studies from the same investigator group have compared long-term LMWH (enoxaparin 1 mg/kg SC bid for ∼14 days followed by 1.5 mg/kg SC daily) with long-term VKA exclusively in patients who presented with PE.341,342 The two studies combined found a similar frequency of recurrent VTE (enoxaparin, 4/60; VKA, 1/40) and major bleeding (enoxaparin, 1/60; VKA, 2/40) with the two treatments.341 Of the 12 other studies that compared LMWH with VKA therapy for long-term treatment of VTE (see section 3.3), only two173,227 included patients with PE. In these two studies, all patients had cancer, and 295 had PE (36% of all enrolled patients; some PE may have been asymptomatic in one study227); subgroup analyses were not reported for the patients with PE.
Dabigatran for the Long-term Treatment of PE:
In the one completed study that compared dabigatran with VKA therapy after initial parenteral therapy (Table 23, Table S31), 786 (31%) patients had symptomatic PE at enrollment.343 Subgroup analysis did not suggest that patients with symptomatic PE have a different response to dabigatran vs VKA therapy in terms of either recurrent VTE or bleeding.
Rivaroxaban for the Long-term Treatment of PE:
In the Einstein Extension study that compared rivaroxaban with placebo after an initial period of long-term anticoagulation (Table 22, Table S30), 454 (38%) patients had symptomatic PE at enrollment.88 Subgroup analysis did not suggest that patients with symptomatic PE had a different response to rivaroxaban vs VKA therapy in terms of either recurrent VTE or bleeding.
Recommendations
6.1. In patients with PE provoked by surgery, we recommend treatment with anticoagulation for 3 months over (i) treatment of a shorter period (Grade 1B), (ii) treatment of a longer time-limited period (eg, 6 or 12 months) (Grade 1B), or (iii) extended therapy (Grade 1B regardless of bleeding risk).
6.2. In patients with PE provoked by a nonsurgical transient risk factor, we recommend treatment with anticoagulation for 3 months over (i) treatment of a shorter period (Grade 1B), (ii) treatment of a longer time-limited period (eg, 6 or 12 months) (Grade 1B), and (iii) extended therapy if there is a high bleeding risk (Table 2) (Grade 1B). We suggest treatment with anticoagulation for 3 months over extended therapy if there is a low or moderate bleeding risk (Table 2) (Grade 2B).
6.3. In patients with an unprovoked PE, we recommend treatment with anticoagulation for at least 3 months over treatment of a shorter duration (Grade 1B). After 3 months of treatment, patients with unprovoked PE should be evaluated for the risk-benefit ratio of extended therapy.
6.3.1. In patients with a first VTE that is an unprovoked PE and who have a low or moderate bleeding risk (Table 2), we suggest extended anticoagulant therapy over 3 months of therapy (Grade 2B).
6.3.2. In patients with a first VTE that is an unprovoked PE and who have a high bleeding risk, we recommend 3 months of anticoagulant therapy over extended therapy (Grade 1B).
6.3.3. In patients with a second unprovoked VTE, we recommend extended anticoagulant therapy over 3 months of therapy in those who have a low bleeding risk (Table 2) (Grade 1B), and we suggest extended anticoagulant therapy in those with a moderate bleeding risk (Table 2) (Grade 2B).
6.3.4. In patients with a second unprovoked VTE who have a high bleeding risk (Table 2), we suggest 3 months of therapy over extended therapy (Grade 2B).
6.4. In patients with PE and active cancer, if the risk of bleeding is not high (Table 2), we recommend extended anticoagulant therapy over 3 months of therapy (Grade 1B), and if there is a high bleeding risk (Table 2), we suggest extended anticoagulant therapy (Grade 2B).
Remarks: In all patients who receive extended anticoagulant therapy, the continuing use of treatment should be reassessed at periodic intervals (eg, annually).
6.5. In patients with PE who are treated with VKA, we recommend a therapeutic INR range of 2.0 to 3.0 (target INR of 2.5) over a lower (INR < 2) or higher (INR 3.0-5.0) range for all treatment durations (Grade 1B).
6.6. In patients with PE and no cancer, we suggest VKA therapy over LMWH for long-term therapy (Grade 2C). For patients with PE and no cancer who are not treated with VKA therapy, we suggest LMWH over dabigatran or rivaroxaban for long-term therapy (Grade 2C).
6.7. In patients with PE and cancer, we suggest LMWH over VKA therapy (Grade 2B). In patients with PE and cancer who are not treated with LMWH, we suggest VKA over dabigatran or rivaroxaban for long-term therapy (Grade 2C).
Remarks (6.6-6.7): Choice of treatment in patients with and without cancer is sensitive to individual patient tolerance for daily injections, need for laboratory monitoring, and treatment costs. Treatment of VTE with dabigatran or rivaroxaban, in addition to being less burdensome to patients, may prove to be associated with better clinical outcomes that VKA and LMWH therapy. When these guidelines were being prepared (October 2011), postmarketing studies of safety were not available. Given the paucity of currently available data and that new data are rapidly emerging, we give a weak recommendation in favor of VKA and LMWH therapy over dabigatran and rivaroxaban, and we have not made any recommendations in favor of one of the new agents over the other.
6.8. In patients with PE who receive extended therapy, we suggest treatment with the same anticoagulant chosen for the first 3 months (Grade 2C).
6.9 Treatment of Asymptomatic PE
Diagnosis of asymptomatic PE occurs in ∼1% of outpatients and ∼4% of inpatients who have contrast-enhanced CT scans, with a majority being in patients with known malignancy.40,344‐355 When PE is diagnosed unexpectedly in patients with cancer, in retrospect, the clinical history may reveal symptoms that were aggravated by the PE (eg, an increase in fatigue).345 About one-half of such incidental PE involve the lobar or more central pulmonary arteries, whereas the other one-half are more distal.40,353
When there is evidence of an asymptomatic PE, the first priority is to review the CT scans to determine whether the findings are convincing for acute PE. Other recent CT scans may be available for comparison, or the current scan may also reveal DVT in the central deep veins (eg, subclavian vein, IVC, iliac vein). If there is any uncertainty about the presence of an acute PE, additional diagnostic testing is required (eg, ultrasonography of the deep veins, dedicated CT pulmonary angiography, D-dimer).
Consistent with recommendations for the treatment of asymptomatic DVT (section 3.5) in patients in whom clinicians are convinced that an asymptomatic PE has occurred, based on moderate-quality evidence, we suggest the same initial and long-term anticoagulation as for similar patients with symptomatic PE. The indication for anticoagulation is most compelling when the presence of PE is unequivocal, PE involves the lobar and more central pulmonary arteries, PE is a new finding on CT, ultrasound reveals proximal DVT, there are ongoing risk factors for VTE such as active cancer, and the patient is not at high risk for bleeding (Table 2). Many patients have left the hospital by the time an incidental PE is reported. If PE is less extensive and it would be difficult for patients to return the same day, it often is reasonable to defer further assessment and anticoagulant therapy until the next day.
Recommendation
6.9. In patients who are incidentally found to have asymptomatic PE, we suggest the same initial and long-term anticoagulation as for similar patients with symptomatic PE (Grade 2B).
7.0 Chronic Thromboembolic Pulmonary Hypertension
Prospective studies suggest that CTPH occurs in ∼3% of patients who are treated for PE.104,354‐358 About one-third of patients have a history of VTE, whereas two-thirds have had single or recurrent episodes of PE that were not diagnosed and may have been asymptomatic.359 Patients with CTPH are likely to have a high risk of recurrent VTE because they have had previous VTE and have cardiopulmonary impairment. Recurrent VTE may be fatal more often in patients with severe cardiopulmonary impairment than in those without such impairment. After PE initiates CTPH, pulmonary vascular remodeling may cause severe pulmonary hypertension out of proportion with pulmonary vascular thrombosis.104,359,360
7.1 Pulmonary Thromboendarterectomy, Anticoagulant Therapy, and Vena Caval Filter for the Treatment of CTPH
Primary therapy for CTPH is pulmonary thromboendarterectomy, which, if successful, can reduce or cure pulmonary hypertension and associated symptoms.104,359‐367 The operation is lengthy and complex, requiring a median sternotomy, cardiopulmonary bypass, deep hypothermia with periods of circulatory arrest, and exploration of both pulmonary arteries. At the most experienced centers, mortality is ∼5%.104,360,363,365‐367 Management often includes insertion of a permanent IVC filter before or during pulmonary endarterectomy and indefinite anticoagulant therapy.359,361,363,365 Patients with CTPH who are not candidates for pulmonary endarterectomy because of comorbid disease or surgically inaccessible lesions may be candidates for vasodilator therapy, balloon pulmonary angioplasty, or lung transplantation and may benefit from referral to a center with expertise in pulmonary hypertension.359,363‐365,368‐370
There are no randomized trials of CTPH therapy and, overall, evidence is low quality. There is, however, high-quality indirect evidence that anticoagulant therapy is very effective at preventing recurrent VTE in other patient populations (see section 3.1; Table 18; and Tables S24, S25, and S27). Consequently, the evidence supporting long-term anticoagulation in patients with CTPH is of moderate quality (rated down for indirectness). Features that are expected to be associated with greater benefit with pulmonary thromboendarterectomy include younger age, central disease, progressive clinical deterioration, and access to an expert multidisciplinary thromboendarterectomy team.
Recommendations
7.1.1. In patients with CTPH, we recommend extended anticoagulation over stopping therapy (Grade 1B).
7.1.2. In selected patients with CTPH, such as those with central disease under the care of an experienced thromboendarterectomy team, we suggest pulmonary thromboendarterectomy over no pulmonary thromboendarterectomy (Grade 2C).
8.0 Superficial Vein Thrombosis
SVT has been less well studied than DVT but is estimated to occur more often.371,372 It usually affects the lower limbs; often involves a varicose vein; is associated with chronic venous insufficiency, malignancy, thrombophilia, pregnancy or estrogen therapy, obesity, sclerotherapy, long-distance travel, and a history of VTE; or may be unprovoked.371‐373 The long saphenous vein is involved in about two-thirds of lower-limb SVT.374
Although traditionally considered a benign disease, a number of studies indicate that the consequences of SVT may be more serious.371,372 A prospective study of 844 patients with acute SVT of ≥ 5 cm found that at initial presentation, ∼4% of patients had symptomatic PE, and routine ultrasound detected proximal DVT in 10% and distal DVT in an additional 13% of patients.374 In patients without VTE at presentation, despite 90% being treated with anticoagulant therapy (therapeutic doses in two-thirds, prophylactic doses in one-third, median duration of 11 days), 3.1% developed symptomatic VTE (0.5% PE, 1.2% proximal DVT, 1.4% distal DVT), 1.9% had recurrent SVT (different location), and 3.3% had an extension of SVT (same location) at 3 months. Male sex, history of VTE, cancer, and absence of varicose veins each was associated with about a doubling of the risk of VTE during follow-up.
Given the high prevalence of concomitant proximal DVT in patients with SVT and the need to treat such patients with higher doses of anticoagulant therapy (ie, therapeutic doses), patients with SVT above the knee should have ultrasonography to exclude proximal DVT. Ultrasound can also help with the diagnosis of SVT if the clinical presentation is uncertain. With greater appreciation of the seriousness of SVT, investigators have evaluated anticoagulant therapy, often in prophylactic or intermediate doses, as a way to reduce acute symptoms, extension, recurrence, and progression to VTE (Table 31, Tables S43-S45).375
Table 31.
| Outcomes | No. of Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects |
|
| Risk With No Fondaparinux | Risk Difference With Fondaparinux (95% CI) | ||||
| Mortality |
3,002 (1 study), 3 mo |
Moderated-g due to
imprecision |
RR 1.99 (0.18-21.87) |
4 per 1,000h |
4 more per 1,000 (from 3 fewer to 83 more) |
| VTE |
3,002 (1 study), 3 mo |
Highd |
RR 0.18 (0.06-0.53) |
33 per 1,000h |
27 fewer per 1,000 (from 16 fewer to 31 fewer) |
| SVT recurrence |
3,002 (1 study), 3 mo |
Highd |
RR 0.31 (0.14-0.68) |
19 per 1,000h |
13 fewer per 1,000 (from 6 fewer to 16 fewer) |
| Major bleeding |
2,987(1 study), 47 d |
Moderated,e,i due to
imprecision |
RR 0.99 (0.06-15.86)e |
1 per 1,000 |
0 fewer per 1,000 (from 1 fewer to 10 more) |
| QOL not measured | … | … | … | … | … |
The basis for the assumed risk (eg, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Working group grades of evidence are as follow: High quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very-low quality, we are very uncertain about the estimate. See Table 1 and 3 legends for expansion of abbreviations.
Patients with infusion-related SVT were excluded from CALISTO (Comparison of ARIXTRA in lower Limb Superficial Thrombophlebitis with Placebo).
Fondaparinux 2.5 mg for 45 d.
Patients in the two treatment groups benefited from close clinical monitoring with adequate diagnostic procedures in the event of new or persistent symptoms.
Allocation concealed. Outcome adjudicators, steering committee, patients, providers, and data collectors blinded. Follow-up rate was 98%. Intention-to-treat analysis for efficacy outcomes. Not stopped early for benefit.
CI includes values suggesting large benefit and values suggesting large harm.
We rated down by only one level because of the low event rate and large sample size.
Small number of events.
Baseline risk derived from a large prospective cohort study.374
The upper limit of the CI for absolute effect (10 more bleeds) is not low enough to suggest a clear balance of benefits vs harms.
8.1 Treatment of SVT
Most studies that have evaluated anticoagulant therapy for SVT have been small (eg, ≤ 100 patients per treatment group), with additional methodologic weaknesses376‐381 (Tables S44, S45). Although these studies suggest that prophylactic-dose LMWH, intermediate-dose UFH or LMWH, warfarin therapy, and oral nonsteroidal antiinflammatory agents are beneficial in patients with SVT, the supporting evidence is of low quality. The recently published Comparison of ARIXTRA™ in lower LImb Superficial Thrombophlebitis with placebo (CALISTO) study, which compared fondaparinux (2.5 mg/d for 45 days) with placebo in 3,000 patients with SVT (≥ 5 cm in length), has helped to clarify the role of anticoagulants for the treatment of SVT (Table 31, Table S43), and the natural history of this condition.382
CALISTO found that fondaparinux is very effective at reducing VTE, recurrent SVT, extension of SVT, and the need for venous surgery, and is associated with little bleeding. In the placebo group, thrombotic complications occurred more often if SVT involved the greater saphenous vein (92% of patients in the control group), extended to within 10 cm from the saphenofemoral junction (9% of patients), and involved veins above the knee (46% of patients) and if VTE (7% of patients) or SVT (12% of patients) had occurred previously.382 Age, sex, and presence of varicose veins were not convincingly associated with the frequency of thrombotic complications, and there were too few patients with cancer in CALISTO to assess that association.
The evidence is moderate quality. We have interpreted the findings of the CALISTO study as evidence for anticoagulation in general and assume that prophylactic doses of LMWH and fondaparinux have similar antithrombotic efficacy and safety. Because it is direct and more extensive, the evidence in support of fondaparinux is higher quality than the evidence in support of LMWH. Quality of the evidence for comparison of fondaparinux with LMWH is low because there is no direct comparison in patients with SVT. Factors that favor the use of anticoagulant therapy in patients with SVT (see Recommendation 8.1.1) include: extensive SVT; involvement above the knee, particularly if close to the saphenofemoral junction; severe symptoms; involvement of the greater saphenous vein; history of VTE or SVT; active cancer; and recent surgery. An economic evaluation found that treatment of SVT with fondaparinux was not cost-effective; it cost $500,000 per quality-adjusted life year gained compared with no treatment.383
Graduated compression stockings often are used in patients with SVT (eg, 83% of patients in the CALISTO study). Oral nonsteroidal antiinflammatory agents may be used to alleviate symptoms if patients are not treated with anticoagulants. Topical nonsteroidal antiinflammatory agents may reduce symptoms and can be used with anticoagulant therapy. Surgical therapy, with ligation of the saphenofemoral junction or stripping of thrombosed superficial veins appears to be associated with higher rates of VTE than treatment with anticoagulants.380,384,385 Anticoagulant therapy generally is not used to treat SVT that occurs in association with an IV infusion (ie, infusion thrombophlebitis).
Recommendations
8.1.1. In patients with SVT of the lower limb of at least 5 cm in length, we suggest the use of a prophylactic dose of fondaparinux or LMWH for 45 days over no anticoagulation (Grade 2B).
Remarks: Patients who place a high value on avoiding the inconvenience or cost of anticoagulation and a low value on avoiding infrequent symptomatic VTE are likely to decline anticoagulation.
8.1.2. In patients with SVT who are treated with anticoagulation, we suggest fondaparinux 2.5 mg daily over a prophylactic dose of LMWH (Grade 2C).
9.0 Acute Upper-Extremity DVT
About 5% to 10% of VTE involve the upper extremities.386‐390 UEDVT includes two etiologic groups: primary (unprovoked with or without thrombophilia, effort-related and thoracic outlet syndrome) and secondary (provoked by central venous catheters, pacemakers, or cancer). Secondary UEDVT accounts for ∼75% of cases.386,388,389,391‐394
UEDVT involves the subclavian, axillary, or brachial veins and may include extension to the brachiocephalic vein, superior vena cava, or the internal jugular vein. Clinical manifestations include acute and chronic arm pain, swelling, discoloration, and dilated collateral veins over the arm, neck, or chest. UEDVT may lead to complications, including symptomatic PE (∼5% of patients387‐389,393,395), recurrent UEDVT (∼8% at 5 years of follow-up389,390,395), and PTS of the arm (∼20% of patients).386,387,395,396 In the absence of a central venous catheter, the dominant arm is more often affected.387 Complications of UEDVT are expected to occur much more often and to be more severe when UEDVT involves the axillary or more-proximal veins than if thrombosis is confined to the brachial vein. In general, when we refer to UEDVT, we are referring to thrombosis that involves the axillary or more-proximal veins.
The most frequent risk factor for UEDVT is a central venous catheter.386,388,397,398 If UEDVT occurs in association with a central venous catheter and the catheter is no longer required, it should be removed. There are no data to guide whether catheter removal should be preceded by an initial period of anticoagulation, and we do not have a preference for immediate or deferred removal. If UEDVT occurs in association with a central venous catheter and there is a continuing need for the catheter, the catheter need not be removed. If the catheter is not functioning and cannot be made to function (even after a period of systemic anticoagulation), it should be removed.
As with treatment of leg DVT and PE, treatment of UEDVT may be divided into acute (eg, parenteral anticoagulants, thrombolytic therapy) and long-term phases (eg, anticoagulation, treatment of upper-extremity PTS). Because no randomized trials have evaluated treatment of UEDVT, recommendations are based on indirect evidence from studies performed in patients with leg DVT, observational studies (generally small), and understanding of the natural history of UEDVT. Therefore, quality of evidence is, at best, moderate.
9.1 Acute Anticoagulation for UEDVT
No randomized controlled studies have evaluated acute anticoagulation for initial treatment of UEDVT. Several small prospective cohort studies have reported low rates of recurrent DVT, PE, and major bleeding when UEDVT was treated similarly to leg DVT (Tables S46 and S47).395,399‐401 Anticoagulant therapy is used to treat UEDVT because (1) UEDVT causes acute symptoms, can cause PE (including fatal episodes), and is associated with PTS; (2) observational studies support its use; and (3) there is strong evidence for benefit in patients with leg DVT.
Uncertainty exists about the need to prescribe anticoagulants to patients with thrombosis confined to the brachial vein. Acceptable alternatives to full-dose anticoagulation in such patients include clinical or ultrasound surveillance to detect extension of UEDVT while withholding anticoagulation, or treatment with prophylactic-dose anticoagulation, or treatment with therapeutic doses of anticoagulation for < 3 months. We favor anticoagulation if isolated brachial vein thrombosis is symptomatic, associated with a central venous catheter that will remain in place, or associated with cancer in the absence of a central venous catheter. A high risk of bleeding argues against full-dose anticoagulation.
Recommendations
9.1.1. In patients with acute UEDVT that involves the axillary or more proximal veins, we recommend acute treatment with parenteral anticoagulation (LMWH, fondaparinux, IV UFH, or SC UFH) over no such acute treatment (Grade 1B).
9.1.2. In patients with acute UEDVT that involves the axillary or more proximal veins, we suggest LMWH or fondaparinux over IV UFH (Grade 2C) and over SC UFH (Grade 2B).
9.2 Thrombolytic Therapy for the Initial Treatment of UEDVT
No randomized controlled studies have evaluated thrombolytic therapy compared with anticoagulation alone in patients with UEDVT. A number of retrospective and small prospective observational studies have evaluated streptokinase, urokinase, or rt-PA administered with varying doses, methods of administration (IV, catheter directed), and infusion durations.402‐409 Three of these studies included nonrandomized control groups who received anticoagulation alone.402,407,408 In some studies, a few patients also had venous angioplasty405 or surgical decompression402,405,408 (Tables S48 and S49).
These studies suggest that thrombolysis can improve early and late venous patency but is associated with increased bleeding. However, it is not known whether thrombolytic therapy reduces PTS of the arm or recurrent VTE. PTS of the arm appears to be a less common complication of thrombosis than PTS of the leg.386‐388,393,395,410 We believe that thrombolysis should be considered only in patients who meet all of the following criteria: severe symptoms, thrombus involving most of the subclavian vein and the axillary vein, symptoms for < 14 days, good functional status, life expectancy of ≥ 1 year, and low risk for bleeding (Table 11). If thrombolysis is used, in order to reduce the dose of thrombolytic therapy and the associated risk of bleeding, we encourage catheter-based therapy over systemic thrombolysis. In addition, because the balance of risks and benefits with all forms of thrombolytic therapy is uncertain, anticoagulant therapy alone is acceptable initial therapy in all patients with UEDVT. There is no evidence to suggest that thrombolysis reduces the risk of recurrent VTE.
Resection of the first rib has been advocated when UEDVT is believed to have been due to entrapment of the subclavian vein as it passes between the clavicle and the first rib.6,386,411‐421 Insertion of a filter in the superior vena cava has also been used in patients with acute UEDVT who cannot be given anticoagulants. Complications, however, may be more than with IVC filters.389,413,422 The evidence in support of these procedures is of low quality, and because there is the potential to cause harm, their use should be confined to exceptional circumstances in specialized centers.
Recommendations
9.2.1. In patients with acute UEDVT that involves the axillary or more proximal veins, we suggest anticoagulant therapy alone over thrombolysis (Grade 2C).
Remarks: Patients who (i) are most likely to benefit from thrombolysis (see text); (ii) have access to CDT; (iii) attach a high value to prevention of PTS; and (iv) attach a lower value to the initial complexity, cost, and risk of bleeding with thrombolytic therapy are likely to choose thrombolytic therapy over anticoagulation alone.
9.2.2. In patients with UEDVT who undergo thrombolysis, we recommend the same intensity and duration of anticoagulant therapy as in similar patients who do not undergo thrombolysis (Grade 1B).
9.3 Long-term Anticoagulation for UEDVT
No randomized studies have evaluated duration or intensity of long-term anticoagulation in patients with UEDVT. In prospective observational studies, patients with UEDVT generally were treated with VKA (target INR 2.5) for periods of 3 to 6 months.393,399‐401,423 Rates of recurrent VTE and PTS varied (Tables S50 and S51), but as previously noted, these rates generally were lower than those observed in patients with leg DVT.
The factors that influence long-term anticoagulation in patients with leg DVT (section 3.1) are relevant to long-term treatment of UEDVT. Some differences between UEDVT and leg DVT are worthy of emphasis, especially that UEDVT often is associated with a central venous catheter that may or may not be removed (section 9.0). The most important continuing risk factors for UEDVT are (1) the presence of a central venous catheter in the same arm and (2) active cancer in patients with UEDVT not associated with a central venous catheter.
Another important distinction between UEDVT and leg DVT relates to long-term anticoagulation in patients with unprovoked thrombosis. Because the risk of recurrent VTE is substantially lower in patients with UEDVT compared with in those with proximal leg DVT,387,388,393,395 we discourage extended anticoagulant therapy (ie, beyond 3 months) in patients with an unprovoked UEDVT.
No data are available for the long-term use of LMWH monotherapy or newer anticoagulants for the long-term treatment of UEDVT. We make the same recommendations for choice of initial, long-term, and extended anticoagulant regimens for UEDVT as for leg DVT (recommendations 3.1.1, 3.1.2, and 3.1.4) and note that the supporting evidence for these weak recommendations is further weakened in this population because of indirectness.
Recommendations
9.3.1. In most patients with UEDVT that is associated with a central venous catheter, we suggest that the catheter not be removed if it is functional and there is an ongoing need for the catheter (Grade 2C).
9.3.2. In patients with UEDVT that involves the axillary or more proximal veins, we suggest a minimum duration of anticoagulation of 3 months over a shorter period (Grade 2B).
Remarks: This recommendation also applies if the UEDVT is associated with a central venous catheter that was removed shortly after diagnosis.
9.3.3. In patients who have UEDVT that is associated with a central venous catheter that is removed, we recommend 3 months of anticoagulation over a longer duration of therapy in patients with no cancer (Grade 1B), and we suggest this in patients with cancer (Grade 2C).
9.3.4. In patients who have UEDVT that is associated with a central venous catheter that is not removed, we recommend that anticoagulation is continued as long as the central venous catheter remains over stopping after 3 months of treatment in patients with cancer (Grade 1C), and we suggest this in patients with no cancer (Grade 2C).
9.3.5. In patients who have UEDVT that is not associated with a central venous catheter or with cancer, we recommend 3 months of anticoagulation over a longer duration of therapy (Grade 1B).
9.4 Prevention of PTS of the Arm
PTS of the arm occurs in ∼20% of patients after treatment for UEDVT38,395,396 and can be a disabling condition that adversely affects quality of life, particularly if the dominant arm is involved.410,424 No randomized trials have evaluated compression bandages, compression sleeves, or venoactive drugs to prevent PTS after UEDVT. We have not considered indirect evidence from the legs for use of compression therapy to prevent PTS of the arms because (1) the pathophysiology of PTS is believed to be different in the arms than in the legs (less dependent venous hypertension), (2) arm sleeves are more difficult to fit than stockings, and (3) PTS occurs less often after UEDVT than after leg DVT.
Recommendation
9.4. In patients with acute symptomatic UEDVT, we suggest against the use of compression sleeves or venoactive medications (Grade 2C).
9.5 Treatment of PTS of the Arm
Symptoms of PTS of the arm include swelling, heaviness, and limb fatigue with exertion.395,410 No randomized trials have evaluated compression bandages, compression sleeves (as are used for lymphedema), or venoactive drugs to treat PTS after UEDVT. We considered anecdotal evidence that compression therapy benefits some patients with PTS of the arm and that the benefits of a trial of compression therapy will outweigh its harms and costs. There is no evidence that venoactive drugs are of benefit in PTS of the arm.
Recommendations
9.5.1. In patients who have PTS of the arm, we suggest a trial of compression bandages or sleeves to reduce symptoms (Grade 2C).
9.5.2. In patients with PTS of the arm, we suggest against treatment with venoactive medications (Grade 2C).
10.0 Splanchnic Vein Thrombosis
Thrombosis in the portal venous system, which includes the superior mesenteric, inferior mesenteric, splenic, and portal veins, is collectively termed splanchnic vein thrombosis. Depending on the location and extent of thrombosis, how rapidly thrombosis develops, speed and extent of thrombus recannulation, presence of collateral portal venous drainage, and adequacy of arterial inflow, splanchnic vein thrombosis may result in bowel or splenic infarction and chronic portal hypertension.425‐430 Acute and chronic splanchnic vein thrombosis may be symptomatic, but many episodes are detected incidentally in imaging studies performed for other indications, such as assessing response to surgical or medical therapy in patients with cancer.348,429 Limited understanding of the natural history of both symptomatic and incidentally detected splanchnic vein thrombosis in patients who are not treated with anticoagulants (ie, frequency of bowel infarction, development of portal hypertension, recurrence), a paucity of data from prospective cohort studies,428,431 and a lack of randomized trials of standardized anticoagulant therapy for splanchnic vein thrombosis result in uncertainty about the role of anticoagulation for this condition. Increased risk of bleeding associated with esophageal varices (secondary to portal hypertension), thrombocytopenia (secondary to hypersplenism), and the presence of cirrhosis and malignancy (which predispose to splanchnic vein thrombosis) add to this uncertainty.
A number of retrospective,425,429,430 and two prospective,428,431 studies suggested that bowel ischemia is uncommon in patients with symptomatic splanchnic vein thrombosis who are treated with anticoagulants (∼2%428), that recurrent venous thrombosis (both involving the splanchnic and nonsplanchnic veins) is common without anticoagulation or after stopping anticoagulation (∼5% per year425,429,430), and that anticoagulation is effective at preventing progression and recurrent thrombosis,425,428‐431 although it is associated with an increased (particularly GI), but usually acceptable, frequency of bleeding.428‐431 Efficacy of anticoagulant therapy in other forms of symptomatic venous thrombosis also provides indirect evidence for anticoagulation of patients with symptomatic splanchnic vein thrombosis and, supported by the previously noted observational studies, this evidence is of moderate quality. We are not aware of studies of treated or untreated asymptomatic splanchnic vein thrombosis.
Factors that may encourage anticoagulant therapy in patients with incidental splanchnic vein thrombosis include extensive thrombosis that appears to be acute (eg, not present on a previous imaging study, presence of an intraluminal filling defect, lack of cavernous transformation), progression of thrombosis on a follow-up imaging study, and ongoing cancer chemotherapy. Esophageal varices secondary to acute portal vein thrombosis are not necessarily a contraindication to anticoagulant therapy because such treatment may improve the portal hypertension. LMWH may be preferred over VKA if there is active malignancy, liver disease, or thrombocytopenia.431 The presence of a reversible provoking factor for splanchnic vein thrombosis, such as intraabdominal sepsis or recent surgery, supports stopping anticoagulant therapy after 3 months. Absence of a reversible risk factor (eg, “unprovoked” thrombosis or presence of a persistent risk factor, such as myeloproliferative disease) and a low risk of bleeding support extended anticoagulant therapy.
Recommendations
10.1. In patients with symptomatic splanchnic vein thrombosis (portal, mesenteric, and/or splenic vein thromboses), we recommend anticoagulation over no anticoagulation (Grade 1B).
10.2. In patients with incidentally detected splanchnic vein thrombosis (portal, mesenteric, and/or splenic vein thromboses), we suggest no anticoagulation over anticoagulation (Grade 2C).
11.0 Hepatic Vein Thrombosis
Hepatic vein thrombosis, particularly Budd-Chiari syndrome with occlusion of the main hepatic vein, can result in impairment of liver function and an associated coagulopathy.432,433 Because there is limited understanding of the natural history of this condition and a paucity of prospective studies that have evaluated anticoagulant therapy, the role of anticoagulant therapy is uncertain.432
In a prospective registry of 163 patients with Budd-Chiari syndrome of variable extent, of whom 86% were treated with anticoagulation, one-half of patients did not require invasive interventions (ie, transjugular intrahepatic portosystemic shunting in 34% of all patients, surgical portosystemic shunting in 2% of patients, liver transplantation in 12% of patients), and survival was 82% after 2 years.432 In an earlier retrospective study of 237 patients with Budd-Chiari syndrome performed when use of surgical portosystemic shunting was common (49% of patients), use of anticoagulant therapy (72% of patients) had no apparent effect on survival (RR, 1.05; 95% CI, 0.62-1.76).
Factors that encourage anticoagulant therapy in patients with incidental hepatic vein thrombosis include extensive thrombosis that appears to be acute (eg, not present on a previous imaging study, presence of an intraluminal filling defect), progression of thrombosis on a follow-up imaging study, and ongoing cancer chemotherapy. Coagulopathy due to liver dysfunction caused by hepatic vein thrombosis is not a contraindication to anticoagulant therapy because anticoagulants may improve hepatic function. LMWH usually will be preferred to VKA therapy when there is hepatic dysfunction and if there is active malignancy. Presence of a reversible provoking factor for hepatic vein thrombosis, such as oral contraceptive therapy, encourages a time-limited course of therapy. Absence of a reversible risk factor encourages the use of extended therapy. Treatment of hepatic vein obstruction is complex and best undertaken by a multidisciplinary team.
Recommendations
11.1. In patients with symptomatic hepatic vein thrombosis, we suggest anticoagulation over no anticoagulation (Grade 2C).
11.2. In patients with incidentally detected hepatic vein thrombosis, we suggest no anticoagulation over anticoagulation (Grade 2C).
12.0 Future Research
Several questions in the treatment of VTE need to be answered. Current evidence relating to these questions is of moderate or low quality. We list the questions roughly as they arise in this article rather than in order of importance. We do not present the rationale for each question because this is addressed in the corresponding sections of the article. We have confined ourselves to the primary question (eg, Should patients with proximal DVT be treated with anticoagulant therapy alone, or should they be treated with pharmacomechanical CDT?); however, once the primary question is answered, we anticipate that secondary questions will need to be addressed (eg, Which patients with proximal DVT should, or should not, be treated with CDT?). We are pleased to note that many of the these questions are being addressed in ongoing trials.
Should patients with an isolated distal DVT routinely be treated with anticoagulant therapy, or should they have serial testing to determine whether the DVT is extending and only be treated if extension is detected?
Should patients with proximal DVT be treated with anticoagulant therapy alone, or should they be treated with pharmacomechanical CDT?
Which patients with unprovoked proximal DVT or PE or cancer-associated VTE should stop anticoagulant therapy at 3 months, and which should remain on extended anticoagulant therapy?
Which patients with unprovoked VTE or cancer-associated VTE have an unacceptable risk of bleeding if they remain on extended anticoagulant therapy?
How should risk of recurrent VTE if anticoagulant therapy is stopped be balanced against risk of bleeding is anticoagulant therapy is continued?
What is the preferred anticoagulant regimen for the short- and long-term treatment of VTE in patients with and without cancer?
Should patients receiving an incidental diagnosis of asymptomatic VTE routinely be treated with anticoagulant therapy, or should they have serial testing to determine whether they have evolving DVT and only be treated if this is detected?
Should patients with symptomatic DVT routinely wear graduated compression stockings from the time of diagnosis, or should stockings be used selectively (eg, in selected patients, in patients whose symptoms do not rapidly resolve)?
Should patients with PE that causes right ventricular dysfunction be treated with anticoagulant therapy alone, or should they be treated with thrombolytic therapy?
If patients have catheter-associated UEDVT and the catheter is removed, should they be treated with anticoagulant therapy or can they be treated without anticoagulant therapy?
Can UEDVT be treated with less-intense or a shorter duration of anticoagulant therapy than leg DVT?
Data Supplement
Acknowledgments
Author contributions: As Topic Editor, Dr Akl oversaw the development of this article, including the data analysis and subsequent development of the recommendations contained herein.
Dr Kearon: served as Deputy Editor.
Dr Akl: served as Topic Editor.
Dr Comerota: served as a panelist.
Dr Prandoni: served as a panelist.
Dr Bounameaux: served as a panelist.
Dr Goldhaber: served as a panelist.
Dr Nelson: served as a frontline clinician.
Dr Wells: served as a panelist.
Dr Gould: served as a resource consultant.
Dr Dentali: served as a panelist.
Dr Crowther: served as a panelist.
Dr Kahn: served as a panelist.
Financial/nonfinancial disclosures: The authors of this guideline provided detailed conflict of interest information related to each individual recommendation made in this article. A grid of these disclosures is available online at http://chestjournal.chestpubs.org/content/141/2_suppl/e419S/suppl/DC1. In summary, the authors have reported to CHEST the following conflicts of interest: Dr Kearon is a consultant to Boehringer Ingelheim and has received peer-reviewed funding for studies in the treatment of VTE. Dr Bounameaux has received grant monies and honoraria for consultancy or lectures from Bayer Schering Pharma AG; Daiichi-Sankyo Co, Ltd; Sanofi-Aventis LLC; Pfizer Inc; Bristol-Myers Squibb; Servier; and Canonpharma Production Ltd. Dr Goldhaber has received funds from Eisai Co, Ltd; EKOS Corporation; Sanofi-Aventis LLC; and Johnson & Johnson. He serves as a consultant for Boehringer Ingelheim GmbH; Bristol-Myers Squibb; Daiichi Sankyo Co, Ltd; Eisai Co, Ltd; EKOS Corporation; Medscape; Merck & Co, Inc; Portola Pharmaceuticals, Inc; and Sanofi-Aventis LLC. Dr Wells has received peer-reviewed and investigator-initiated industry research funding for projects related to venous thrombosis treatment. He has received honoraria for industry-sponsored (Bayer, Boehringer-Ingelheim, Pfizer, BioMerieux, sanofi-aventis) talks pertaining to venous thrombosis and has attended advisory boards for Bayer, Boehringer-Ingelheim, Pfizer and Bristol-Myers-Squibb. Dr Crowther has served on various advisory boards, has assisted in the preparation of educational materials, has sat on data safety management boards, and his institution has received research funds from the following companies: Leo Pharma A/S, Pfizer Inc, Boerhinger Ingelheim GmbH, Bayer Healthcare Pharmaceuticals, Octapharm AG, CSL Behring, and Artisan Pharma. Personal total compensation for these activities over the past 3 years totals less than US $10,000. Dr Kahn has received peer-reviewed and investigator-initiated industry research funding for projects related to venous thrombosis and postthrombotic syndrome prevention and treatment. She has received honoraria for industry-sponsored talks pertaining to venous thrombosis. Dr Akl is a member of and prominent contributor to the GRADE Working Group. Dr Comerota discloses that he is a consultant to and speaker for Covidien, Inc. Drs Prandoni, Nelson, Gould, and Dentali have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors played no role in the development of these guidelines. Sponsoring organizations cannot recommend panelists or topics, nor are they allowed prepublication access to the manuscripts and recommendations. Guideline panel members, including the chair, and members of the Health & Science Policy Committee are blinded to the funding sources. Further details on the Conflict of Interest Policy are available online at http://chestnet.org.
Other contributions: We acknowledge the contributions of the writing groups of previous editions of this guideline and thank Gordon H. Guyatt, MD, FCCP, for his comments and suggestions and Aman Rajpal, MD, for his help with reviewing the proofs.
Endorsements: This guideline is endorsed by the American Association for Clinical Chemistry, the American College of Clinical Pharmacy, the American Society of Health-System Pharmacists, the American Society of Hematology, and the International Society of Thrombosis and Hematosis.
Additional information: The supplement Tables can be found in the Online Data Supplement at http://chestjournal.chestpubs.org/content/141/2_suppl/e419S/suppl/DC1.
Abbreviations
- CALISTO
Comparison of ARIXTRA in Lower Limb Superficial Thrombophlebitis With Placebo
- CDT
catheter-directed thrombolysis
- CTPH
chronic thromboembolic pulmonary hypertension
- HR
hazard ratio
- INR
international normalized ratio
- IVC
inferior vena cava
- LMWH
low-molecular-weight heparin
- PE
pulmonary embolism
- PESI
Pulmonary Embolism Severity Index
- PREPIC
Prevention du Risque d’Embolie Pulmonaire par Interruption Cave
- PTS
postthrombotic (phlebitic) syndrome
- RR
risk ratio
- rt-PA
recombinant tissue plasminogen activator
- SC
subcutaneous
- SVT
superficial vein thrombosis
- tPA
tissue plasminogen activator
- UEDVT
upper-extremity DVT
- UFH
unfractionated heparin
- VKA
vitamin K antagonist
Footnotes
Funding/Support: The Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines received support from the National Heart, Lung, and Blood Institute [R13 HL104758] and Bayer Schering Pharma AG. Support in the form of educational grants were also provided by Bristol-Myers Squibb; Pfizer, Inc; Canyon Pharmaceuticals; and sanofi-aventis US.
Disclaimer: American College of Chest Physician guidelines are intended for general information only, are not medical advice, and do not replace professional medical care and physician advice, which always should be sought for any medical condition. The complete disclaimer for this guideline can be accessed at http://chestjournal.chestpubs.org/content/141/2_suppl/1S.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2) suppl:e24S–e43S. doi: 10.1378/chest.11-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2) suppl:e44S–e88S. doi: 10.1378/chest.11-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2) suppl:e152S–e184S. doi: 10.1378/chest.11-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos A-M, Vandvik PO. VTE, thrombophilia, antithrombotic therapy, and pregnancy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2) suppl:e691S–e736S. doi: 10.1378/chest.11-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monagle P, Chan AKC, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2) suppl:e737S–e801S. doi: 10.1378/chest.11-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. American College of Chest Physicians Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th Edition) Chest. 2008;133(6) suppl:454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 7.Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107(23) suppl 1:I-22-I30. doi: 10.1161/01.CIR.0000078464.82671.78. [DOI] [PubMed] [Google Scholar]
- 8.Stein PD, Matta F, Musani MH, Diaczok B. Silent pulmonary embolism in patients with deep venous thrombosis: a systematic review. Am J Med. 2010;123(5):426–431. doi: 10.1016/j.amjmed.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 9.Murin S, Romano PS, White RH. Comparison of outcomes after hospitalization for deep venous thrombosis or pulmonary embolism. Thromb Haemost. 2002;88(3):407–414. [PubMed] [Google Scholar]
- 10.Baglin T, Douketis J, Tosetto A, et al. Does the clinical presentation and extent of venous thrombosis predict likelihood and type of recurrence? A patient-level meta-analysis. J Thromb Haemost. 2010;8(11):2436–2442. doi: 10.1111/j.1538-7836.2010.04022.x. [DOI] [PubMed] [Google Scholar]
- 11.Douketis JD, Kearon C, Bates S, Duku EK, Ginsberg JS. Risk of fatal pulmonary embolism in patients with treated venous thromboembolism. JAMA. 1998;279(6):458–462. doi: 10.1001/jama.279.6.458. [DOI] [PubMed] [Google Scholar]
- 12.Carrier M, Le Gal G, Wells PS, Rodger MA. Systematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med. 2010;152(9):578–589. doi: 10.7326/0003-4819-152-9-201005040-00008. [DOI] [PubMed] [Google Scholar]
- 13.Linkins L, O’Donnell M, Julian JA, Kearon C. Intracranial and fatal bleeding according to indication for long-term oral anticoagulant therapy. J Thromb Haemost. 2010;8(10):2201–2207. doi: 10.1111/j.1538-7836.2010.04016.x. [DOI] [PubMed] [Google Scholar]
- 14.Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med. 2003;139(11):893–900. doi: 10.7326/0003-4819-139-11-200312020-00007. [DOI] [PubMed] [Google Scholar]
- 15.MacLean S, Mulla S, Akl EA, et al. Patient values and preferences for decision making in antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2) suppl:e1S–e23S. doi: 10.1378/chest.11-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyatt GH, Norris SL, Schulman S, et al. Methodology for the development of antithrombotic therapy and prevention of thrombosis guidelines: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2) suppl:53S–70S. doi: 10.1378/chest.11-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Meer FJ, Rosendaal FR, Vandenbroucke JP, Briët E. Bleeding complications in oral anticoagulant therapy. An analysis of risk factors. Arch Intern Med. 1993;153(13):1557–1562. doi: 10.1001/archinte.153.13.1557. [DOI] [PubMed] [Google Scholar]
- 18.Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med. 1998;105(2):91–99. doi: 10.1016/s0002-9343(98)00198-3. [DOI] [PubMed] [Google Scholar]
- 19.Douketis JD, Arneklev K, Goldhaber SZ, Spandorfer J, Halperin F, Horrow J. Comparison of bleeding in patients with nonvalvular atrial fibrillation treated with ximelagatran or warfarin: assessment of incidence, case-fatality rate, time course and sites of bleeding, and risk factors for bleeding. Arch Intern Med. 2006;166(8):853–859. doi: 10.1001/archinte.166.8.853. [DOI] [PubMed] [Google Scholar]
- 20.Kuijer PMM, Hutten BA, Prins MH, Büller HR. Prediction of the risk of bleeding during anticoagulant treatment for venous thromboembolism. Arch Intern Med. 1999;159(5):457–460. doi: 10.1001/archinte.159.5.457. [DOI] [PubMed] [Google Scholar]
- 21.Landefeld CS, McGuire E, III, Rosenblatt MW. A bleeding risk index for estimating the probability of major bleeding in hospitalized patients starting anticoagulant therapy. Am J Med. 1990;89(5):569–578. doi: 10.1016/0002-9343(90)90174-c. [DOI] [PubMed] [Google Scholar]
- 22.Palareti G, Leali N, Coccheri S, et al. Italian Study on Complications of Oral Anticoagulant Therapy Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT) Lancet. 1996;348(9025):423–428. doi: 10.1016/s0140-6736(96)01109-9. [DOI] [PubMed] [Google Scholar]
- 23.Torn M, Bollen WL, van der Meer FJ, van der Wall EE, Rosendaal FR. Risks of oral anticoagulant therapy with increasing age. Arch Intern Med. 2005;165(13):1527–1532. doi: 10.1001/archinte.165.13.1527. [DOI] [PubMed] [Google Scholar]
- 24.White RH, Beyth RJ, Zhou H, Romano PS. Major bleeding after hospitalization for deep-venous thrombosis. Am J Med. 1999;107(5):414–424. doi: 10.1016/s0002-9343(99)00267-3. [DOI] [PubMed] [Google Scholar]
- 25.Olesen JB, Lip GY, Hansen PR, et al. Bleeding risk in ‘real world’ patients with atrial fibrillation: comparison of two established bleeding prediction schemes in a nationwide cohort. J Thromb Haemost. 2011;9(8):1460–1467. doi: 10.1111/j.1538-7836.2011.04378.x. [DOI] [PubMed] [Google Scholar]
- 26.Fihn SD, Callahan CM, Martin DC, McDonell MB, Henikoff JG, White RH. The National Consortium of Anticoagulation Clinics The risk for and severity of bleeding complications in elderly patients treated with warfarin. Ann Intern Med. 1996;124(11):970–979. doi: 10.7326/0003-4819-124-11-199606010-00004. [DOI] [PubMed] [Google Scholar]
- 27.Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J. 2006;151(3):713–719. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Lip GY, Frison L, Halperin JL, Lane DA. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol. 2011;57(2):173–180. doi: 10.1016/j.jacc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 29.Nieto JA, Bruscas MJ, Ruiz-Ribo D, et al. RIETE Investigators Acute venous thromboembolism in patients with recent major bleeding. The influence of the site of bleeding and the time elapsed on outcome. J Thromb Haemost. 2006;4(11):2367–2372. doi: 10.1111/j.1538-7836.2006.02188.x. [DOI] [PubMed] [Google Scholar]
- 30.Ruíz-Giménez N, Suárez C, González R, et al. RIETE Investigators Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost. 2008;100(1):26–31. doi: 10.1160/TH08-03-0193. [DOI] [PubMed] [Google Scholar]
- 31.van der Meer FJ, Rosendaal FR, Vandenbroucke JP, Briët E. Assessment of a bleeding risk index in two cohorts of patients treated with oral anticoagulants. Thromb Haemost. 1996;76(1):12–16. [PubMed] [Google Scholar]
- 32.Pengo V, Legnani C, Noventa F, Palareti G. ISCOAT Study Group.(Italian Study on Complications of Oral Anticoagulant Therapy) Oral anticoagulant therapy in patients with nonrheumatic atrial fibrillation and risk of bleeding. A Multicenter Inception Cohort Study. Thromb Haemost. 2001;85(3):418–422. [PubMed] [Google Scholar]
- 33.Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58(4):395–401. doi: 10.1016/j.jacc.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shireman TI, Mahnken JD, Howard PA, Kresowik TF, Hou Q, Ellerbeck EF. Development of a contemporary bleeding risk model for elderly warfarin recipients. Chest. 2006;130(5):1390–1396. doi: 10.1378/chest.130.5.1390. [DOI] [PubMed] [Google Scholar]
- 35.Fihn SD, McDonell M, Martin D, et al. Warfarin Optimized Outpatient Follow-up Study Group Risk factors for complications of chronic anticoagulation. A multicenter study. Ann Intern Med. 1993;118(7):511–520. doi: 10.7326/0003-4819-118-7-199304010-00005. [DOI] [PubMed] [Google Scholar]
- 36.Nieto JA, Solano R, Ruiz-Ribó MD, et al. Riete Investigators Fatal bleeding in patients receiving anticoagulant therapy for venous thromboembolism: findings from the RIETE registry. J Thromb Haemost. 2010;8(6):1216–1222. doi: 10.1111/j.1538-7836.2010.03852.x. [DOI] [PubMed] [Google Scholar]
- 37.Hutten BA, Prins MH, Gent M, Ginsberg J, Tijssen JG, Büller HR. Incidence of recurrent thromboembolic and bleeding complications among patients with venous thromboembolism in relation to both malignancy and achieved international normalized ratio: a retrospective analysis. J Clin Oncol. 2000;18(17):3078–3083. doi: 10.1200/JCO.2000.18.17.3078. [DOI] [PubMed] [Google Scholar]
- 38.Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100(10):3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 39.Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120(11):897–902. doi: 10.7326/0003-4819-120-11-199406010-00001. [DOI] [PubMed] [Google Scholar]
- 40.Dentali F, Ageno W, Becattini C, et al. Prevalence and clinical history of incidental, asymptomatic pulmonary embolism: a meta-analysis. Thromb Res. 2010;125(6):518–522. doi: 10.1016/j.thromres.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Hull RD, Raskob GE, Rosenbloom D, et al. Heparin for 5 days as compared with 10 days in the initial treatment of proximal venous thrombosis. N Engl J Med. 1990;322(18):1260–1264. doi: 10.1056/NEJM199005033221802. [DOI] [PubMed] [Google Scholar]
- 42.Dahri K, Loewen P. The risk of bleeding with warfarin: a systematic review and performance analysis of clinical prediction rules. Thromb Haemost. 2007;98(5):980–987. [PubMed] [Google Scholar]
- 43.Palareti G, Cosmi B. Bleeding with anticoagulation therapy-who is at risk, and how best to identify such patients. Thromb Haemost. 2009;102(2):268–278. doi: 10.1160/TH08-11-0730. [DOI] [PubMed] [Google Scholar]
- 44.Kearon C, Ginsberg JS, Kovacs MJ, et al. Extended Low-Intensity Anticoagulation for Thrombo-Embolism Investigators Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med. 2003;349(7):631–639. doi: 10.1056/NEJMoa035422. [DOI] [PubMed] [Google Scholar]
- 45.Collins R, MacMahon S, Flather M, et al. Clinical effects of anticoagulant therapy in suspected acute myocardial infarction: systematic overview of randomised trials. BMJ. 1996;313(7058):652–659. doi: 10.1136/bmj.313.7058.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yusuf S, Mehta SR, Xie C, et al. CREATE Trial Group Investigators Effects of reviparin, a low-molecular-weight heparin, on mortality, reinfarction, and strokes in patients with acute myocardial infarction presenting with ST-segment elevation. JAMA. 2005;293(4):427–435. doi: 10.1001/jama.293.4.427. [DOI] [PubMed] [Google Scholar]
- 47.Prandoni P, Lensing AWA, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125(1):1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 48.Palareti G, Cosmi B, Legnani C, et al. PROLONG Investigators D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. 2006;355(17):1780–1789. doi: 10.1056/NEJMoa054444. [DOI] [PubMed] [Google Scholar]
- 49.Wells PS, Forgie MA, Simms M, et al. The outpatient bleeding risk index: validation of a tool for predicting bleeding rates in patients treated for deep venous thrombosis and pulmonary embolism. Arch Intern Med. 2003;163(8):917–920. doi: 10.1001/archinte.163.8.917. [DOI] [PubMed] [Google Scholar]
- 50.Barritt DW, Jordan SC. Anticoagulant drugs in the treatment of pulmonary embolism. A controlled trial. Lancet. 1960;1(7138):1309–1312. doi: 10.1016/s0140-6736(60)92299-6. [DOI] [PubMed] [Google Scholar]
- 51.Brandjes DPM, Heijboer H, Büller HR, de Rijk M, Jagt H, ten Cate JW. Acenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal-vein thrombosis. N Engl J Med. 1992;327(21):1485–1489. doi: 10.1056/NEJM199211193272103. [DOI] [PubMed] [Google Scholar]
- 52.Hull RD, Raskob GE, Hirsh J, et al. Continuous intravenous heparin compared with intermittent subcutaneous heparin in the initial treatment of proximal-vein thrombosis. N Engl J Med. 1986;315(18):1109–1114. doi: 10.1056/NEJM198610303151801. [DOI] [PubMed] [Google Scholar]
- 53.Raschke RA, Reilly BM, Guidry JR, Fontana JR, Srinivas S. The weight-based heparin dosing nomogram compared with a “standard care” nomogram. A randomized controlled trial. Ann Intern Med. 1993;119(9):874–881. doi: 10.7326/0003-4819-119-9-199311010-00002. [DOI] [PubMed] [Google Scholar]
- 54.Wells PS, Hirsh J, Anderson DR, et al. A simple clinical model for the diagnosis of deep-vein thrombosis combined with impedance plethysmography: potential for an improvement in the diagnostic process. J Intern Med. 1998;243(1):15–23. doi: 10.1046/j.1365-2796.1998.00249.x. [DOI] [PubMed] [Google Scholar]
- 55.Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144(3):165–171. doi: 10.7326/0003-4819-144-3-200602070-00004. [DOI] [PubMed] [Google Scholar]
- 56.Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416–420. [PubMed] [Google Scholar]
- 57.Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2) suppl:e351S–e418S. doi: 10.1378/chest.11-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson SA, Stevens SM, Woller SC, et al. Risk of deep vein thrombosis following a single negative whole-leg compression ultrasound: a systematic review and meta-analysis. JAMA. 2010;303(5):438–445. doi: 10.1001/jama.2010.43. [DOI] [PubMed] [Google Scholar]
- 59.Bernardi E, Camporese G, Büller HR, et al. Erasmus Study Group Serial 2-point ultrasonography plus D-dimer vs whole-leg color-coded Doppler ultrasonography for diagnosing suspected symptomatic deep vein thrombosis: a randomized controlled trial. JAMA. 2008;300(14):1653–1659. doi: 10.1001/jama.300.14.1653. [DOI] [PubMed] [Google Scholar]
- 60.Masuda EM, Kistner RL. The case for managing calf vein thrombi with duplex surveillance and selective anticoagulation. Dis Mon. 2010;56(10):601–613. doi: 10.1016/j.disamonth.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Palareti G, Cosmi B, Lessiani G, et al. Evolution of untreated calf deep-vein thrombosis in high risk symptomatic outpatients: the blind, prospective CALTHRO study. Thromb Haemost. 2010;104(5):1063–1070. doi: 10.1160/TH10-06-0351. [DOI] [PubMed] [Google Scholar]
- 62.Righini M, Paris S, Le Gal G, Laroche JP, Perrier A, Bounameaux H. Clinical relevance of distal deep vein thrombosis. Review of literature data. Thromb Haemost. 2006;95(1):56–64. [PubMed] [Google Scholar]
- 63.Macdonald PS, Kahn SR, Miller N, Obrand D. Short-term natural history of isolated gastrocnemius and soleal vein thrombosis. J Vasc Surg. 2003;37(3):523–527. doi: 10.1067/mva.2003.149. [DOI] [PubMed] [Google Scholar]
- 64.Parisi R, Visonà A, Camporese G, et al. Isolated distal deep vein thrombosis: efficacy and safety of a protocol of treatment. Treatment of Isolated Calf Thrombosis (TICT) Study. Int Angiol. 2009;28(1):68–72. [PubMed] [Google Scholar]
- 65.Schwarz T, Buschmann L, Beyer J, Halbritter K, Rastan A, Schellong S. Therapy of isolated calf muscle vein thrombosis: a randomized, controlled study. J Vasc Surg. 2010;52(5):1246–1250. doi: 10.1016/j.jvs.2010.05.094. [DOI] [PubMed] [Google Scholar]
- 66.Lagerstedt CI, Olsson CG, Fagher BO, Oqvist BW, Albrechtsson U. Need for long-term anticoagulant treatment in symptomatic calf-vein thrombosis. Lancet. 1985;2(8454):515–518. doi: 10.1016/s0140-6736(85)90459-3. [DOI] [PubMed] [Google Scholar]
- 67.Gallus AS, Jackaman J, Tillett J, Mills W, Wycherley A. Safety and efficacy of warfarin started early after submassive venous thrombosis or pulmonary embolism. Lancet. 1986;2(8519):1293–1296. doi: 10.1016/s0140-6736(86)91431-5. [DOI] [PubMed] [Google Scholar]
- 68.Leroyer C, Bressollette L, Oger E, et al. The ANTENOX Study Group Early versus delayed introduction of oral vitamin K antagonists in combination with low-molecular-weight heparin in the treatment of deep vein thrombosis. a randomized clinical trial. Haemostasis. 1998;28(2):70–77. doi: 10.1159/000022415. [DOI] [PubMed] [Google Scholar]
- 69.Linkins L-A, Dans AL, Moores LK, et al. Treatment and prevention of heparin-induced thrombocytopenia: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2) suppl:e495S–e530S. doi: 10.1378/chest.11-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prandoni P, Carnovali M, Marchiori A. Galilei Investigators Subcutaneous adjusted-dose unfractionated heparin vs fixed-dose low-molecular-weight heparin in the initial treatment of venous thromboembolism. Arch Intern Med. 2004;164(10):1077–1083. doi: 10.1001/archinte.164.10.1077. [DOI] [PubMed] [Google Scholar]
- 71.Kearon C, Ginsberg JS, Julian JA, et al. Fixed-Dose Heparin (FIDO) Investigators Comparison of fixed-dose weight-adjusted unfractionated heparin and low-molecular-weight heparin for acute treatment of venous thromboembolism. JAMA. 2006;296(8):935–942. doi: 10.1001/jama.296.8.935. [DOI] [PubMed] [Google Scholar]
- 72.Dolovich LR, Ginsberg JS, Douketis JD, Holbrook AM, Cheah G. A meta-analysis comparing low-molecular-weight heparins with unfractionated heparin in the treatment of venous thromboembolism: examining some unanswered questions regarding location of treatment, product type, and dosing frequency. Arch Intern Med. 2000;160(2):181–188. doi: 10.1001/archinte.160.2.181. [DOI] [PubMed] [Google Scholar]
- 73.Gould MK, Dembitzer AD, Doyle RL, Hastie TJ, Garber AM. Low-molecular-weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis. A meta-analysis of randomized, controlled trials. Ann Intern Med. 1999;130(10):800–809. doi: 10.7326/0003-4819-130-10-199905180-00003. [DOI] [PubMed] [Google Scholar]
- 74.van Dongen CJJ, van den Belt AG, Prins MH. Lensing AW. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev. 2004;(4):CD001100. doi: 10.1002/14651858.CD001100.pub2. [DOI] [PubMed] [Google Scholar]
- 75.Erkens PM, Prins MH. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev. 2010;(9):CD001100. doi: 10.1002/14651858.CD001100.pub3. [DOI] [PubMed] [Google Scholar]
- 76.Faivre R, Neuhart Y, Kieffer Y, et al. [A new treatment of deep venous thrombosis: low molecular weight heparin fractions. Randomized study] Presse Med. 1988;17(5):197–200. [PubMed] [Google Scholar]
- 77.Lopaciuk S, Meissner AJ, Filipecki S, et al. Subcutaneous low molecular weight heparin versus subcutaneous unfractionated heparin in the treatment of deep vein thrombosis: a Polish multicenter trial. Thromb Haemost. 1992;68(1):14–18. [PubMed] [Google Scholar]
- 78.Büller HR, Davidson BL, Decousus H, et al. Matisse Investigators Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med. 2004;140(11):867–873. doi: 10.7326/0003-4819-140-11-200406010-00007. [DOI] [PubMed] [Google Scholar]
- 79.Büller HR, Davidson BL, Decousus H, et al. Matisse Investigators Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med. 2003;349(18):1695–1702. doi: 10.1056/NEJMoa035451. [DOI] [PubMed] [Google Scholar]
- 80.Couturaud F, Julian JA, Kearon C. Low molecular weight heparin administered once versus twice daily in patients with venous thromboembolism: a meta-analysis. Thromb Haemost. 2001;86(4):980–984. [PubMed] [Google Scholar]
- 81.van Dongen CJ, MacGillavry MR, Prins MH. Once versus twice daily LMWH for the initial treatment of venous thromboembolism. Cochrane Database Syst Rev. 2005;(3):CD003074. doi: 10.1002/14651858.CD003074.pub2. [DOI] [PubMed] [Google Scholar]
- 82.Breddin HK, Hach-Wunderle V, Nakov R, Kakkar VV. CORTES Investigators. Clivarin: Assessment of Regression of Thrombosis, Efficacy, and Safety Effects of a low-molecular-weight heparin on thrombus regression and recurrent thromboembolism in patients with deep-vein thrombosis. N Engl J Med. 2001;344(9):626–631. doi: 10.1056/NEJM200103013440902. [DOI] [PubMed] [Google Scholar]
- 83.Charbonnier BA, Fiessinger JN, Banga JD, Wenzel E, d’Azemar P, Sagnard L. Comparison of a once daily with a twice daily subcutaneous low molecular weight heparin regimen in the treatment of deep vein thrombosis. FRAXODI group. Thromb Haemost. 1998;79(5):897–901. [PubMed] [Google Scholar]
- 84.Holmoström M, Berglund MC, Granquist S, Bratt G, Törnebohm E, Lockner D. Fragmin once or twice daily subcutaneously in the treatment of deep venous thrombosis of the leg. Thromb Res. 1992;67(1):49–55. doi: 10.1016/0049-3848(92)90257-b. [DOI] [PubMed] [Google Scholar]
- 85.Merli G, Spiro TE, Olsson CG, et al. Enoxaparin Clinical Trial Group Subcutaneous enoxaparin once or twice daily compared with intravenous unfractionated heparin for treatment of venous thromboembolic disease. Ann Intern Med. 2001;134(3):191–202. doi: 10.7326/0003-4819-134-3-200102060-00009. [DOI] [PubMed] [Google Scholar]
- 86.Partsch H, Kechavarz B, Mostbeck A, Köhn H, Lipp C. Frequency of pulmonary embolism in patients who have iliofemoral deep vein thrombosis and are treated with once- or twice-daily low-molecular-weight heparin. J Vasc Surg. 1996;24(5):774–782. doi: 10.1016/s0741-5214(96)70012-5. [DOI] [PubMed] [Google Scholar]
- 87.Siegbahn A, Y-Hassan S, Boberg J, et al. Subcutaneous treatment of deep venous thrombosis with low molecular weight heparin. A dose finding study with LMWH-Novo. Thromb Res. 1989;55(6):767–778. doi: 10.1016/0049-3848(89)90307-1. [DOI] [PubMed] [Google Scholar]
- 88.Bauersachs R, Berkowitz SD, Brenner B, et al. EINSTEIN Investigators Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 89.Boccalon H, Elias A, Chalé JJ, Cadène A, Gabriel S. Clinical outcome and cost of hospital vs home treatment of proximal deep vein thrombosis with a low-molecular-weight heparin: the Vascular Midi-Pyrenees study. Arch Intern Med. 2000;160(12):1769–1773. doi: 10.1001/archinte.160.12.1769. [DOI] [PubMed] [Google Scholar]
- 90.Chong BH, Brighton TA, Baker RI, Thurlow P, Lee CH. ASTH DVT Study Group Once-daily enoxaparin in the outpatient setting versus unfractionated heparin in hospital for the treatment of symptomatic deep-vein thrombosis. J Thromb Thrombolysis. 2005;19(3):173–181. doi: 10.1007/s11239-005-1848-x. [DOI] [PubMed] [Google Scholar]
- 91.Daskalopoulos ME, Daskalopoulou SS, Tzortzis E, et al. Long-term treatment of deep venous thrombosis with a low molecular weight heparin (tinzaparin): a prospective randomized trial. Eur J Vasc Endovasc Surg. 2005;29(6):638–650. doi: 10.1016/j.ejvs.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 92.Koopman MMW, Prandoni P, Piovella F, et al. The Tasman Study Group Treatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low-molecular-weight heparin administered at home. N Engl J Med. 1996;334(11):682–687. doi: 10.1056/NEJM199603143341102. [DOI] [PubMed] [Google Scholar]
- 93.Levine M, Gent M, Hirsh J, et al. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med. 1996;334(11):677–681. doi: 10.1056/NEJM199603143341101. [DOI] [PubMed] [Google Scholar]
- 94.Ramacciotti E, Araújo GR, Lastoria S, et al. CLETRAT Investigators An open-label, comparative study of the efficacy and safety of once-daily dose of enoxaparin versus unfractionated heparin in the treatment of proximal lower limb deep-vein thrombosis. Thromb Res. 2004;114(3):149–153. doi: 10.1016/j.thromres.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 95.Bäckman K, Carlsson P, Kentson M, Hansen S, Engquist L, Hallert C. Deep venous thrombosis: a new task for primary health care. A randomised economic study of outpatient and inpatient treatment. Scand J Prim Health Care. 2004;22(1):44–49. doi: 10.1080/02813430310003543. [DOI] [PubMed] [Google Scholar]
- 96.O’Brien B, Levine M, Willan A, et al. Economic evaluation of outpatient treatment with low-molecular-weight heparin for proximal vein thrombosis. Arch Intern Med. 1999;159(19):2298–2304. doi: 10.1001/archinte.159.19.2298. [DOI] [PubMed] [Google Scholar]
- 97.Huse DM, Cummins G, Taylor DC, Russell MW. Outpatient treatment of venous thromboembolism with low-molecular-weight heparin: an economic evaluation. Am J Manag Care. 2002;8(1) suppl:S10–S16. [PubMed] [Google Scholar]
- 98.Spyropoulos AC, Hurley JS, Ciesla GN, de Lissovoy G. Management of acute proximal deep vein thrombosis: pharmacoeconomic evaluation of outpatient treatment with enoxaparin vs inpatient treatment with unfractionated heparin. Chest. 2002;122(1):108–114. doi: 10.1378/chest.122.1.108. [DOI] [PubMed] [Google Scholar]
- 99.Tillman DJ, Charland SL, Witt DM. Effectiveness and economic impact associated with a program for outpatient management of acute deep vein thrombosis in a group model health maintenance organization. Arch Intern Med. 2000;160(19):2926–2932. doi: 10.1001/archinte.160.19.2926. [DOI] [PubMed] [Google Scholar]
- 100.Rodger M, Bredeson C, Wells PS, Beck J, Kearns B, Huebsch LB. Cost-effectiveness of low-molecular-weight heparin and unfractionated heparin in treatment of deep vein thrombosis. CMAJ. 1998;159(8):931–938. [PMC free article] [PubMed] [Google Scholar]
- 101.van den Belt AG, Bossuyt PM, Prins MH, Gallus AS, Büller HR. TASMAN Study Group Replacing inpatient care by outpatient care in the treatment of deep venous thrombosis—an economic evaluation. Thromb Haemost. 1998;79(2):259–263. [PubMed] [Google Scholar]
- 102.Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149(10):698–707. doi: 10.7326/0003-4819-149-10-200811180-00004. [DOI] [PubMed] [Google Scholar]
- 103.Sharifi M, Mehdipour M, Bay C, Smith G, Sharifi J. Endovenous therapy for deep venous thrombosis: the TORPEDO trial. Catheter Cardiovasc Interv. 2010;76(3):316–325. doi: 10.1002/ccd.22638. [DOI] [PubMed] [Google Scholar]
- 104.Jaff MR, McMurtry MS, Archer SL, et al. American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriocsclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788–1830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 105.Elsharawy M, Elzayat E. Early results of thrombolysis vs anticoagulation in iliofemoral venous thrombosis. A randomised clinical trial. Eur J Vasc Endovasc Surg. 2002;24(3):209–214. doi: 10.1053/ejvs.2002.1665. [DOI] [PubMed] [Google Scholar]
- 106.Enden T, Kløw NE, Sandvik L, et al. CaVenT study group Catheter-directed thrombolysis vs. anticoagulant therapy alone in deep vein thrombosis: results of an open randomized, controlled trial reporting on short-term patency. J Thromb Haemost. 2009;7(8):1268–1275. doi: 10.1111/j.1538-7836.2009.03464.x. [DOI] [PubMed] [Google Scholar]
- 107.Enden T, Sandvik L, Kløw NE, et al. Catheter-directed venous thrombolysis in acute iliofemoral vein thrombosis—the CaVenT study: rationale and design of a multicenter, randomized, controlled, clinical trial ( NCT00251771) Am Heart J. 2007;154(5):808–814. doi: 10.1016/j.ahj.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 108.Douketis JD, Foster GA, Crowther MA, Prins MH, Ginsberg JS. Clinical risk factors and timing of recurrent venous thromboembolism during the initial 3 months of anticoagulant therapy. Arch Intern Med. 2000;160(22):3431–3436. doi: 10.1001/archinte.160.22.3431. [DOI] [PubMed] [Google Scholar]
- 109.Comerota AJ, Throm RC, Mathias SD, Haughton S, Mewissen M. Catheter-directed thrombolysis for iliofemoral deep venous thrombosis improves health-related quality of life. J Vasc Surg. 2000;32(1):130–137. doi: 10.1067/mva.2000.105664. [DOI] [PubMed] [Google Scholar]
- 110.Brass LM, Lichtman JH, Wang Y, Gurwitz JH, Radford MJ, Krumholz HM. Intracranial hemorrhage associated with thrombolytic therapy for elderly patients with acute myocardial infarction: results from the Cooperative Cardiovascular Project. Stroke. 2000;31(8):1802–1811. doi: 10.1161/01.str.31.8.1802. [DOI] [PubMed] [Google Scholar]
- 111.Mehta RH, Stebbins A, Lopes RD, et al. Race, Bleeding, and Outcomes in STEMI Patients Treated with Fibrinolytic Therapy. Am J Med. 2011;124(1):48–57. doi: 10.1016/j.amjmed.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 112.Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Lancet. 1994;343(8893):311–322. [PubMed] [Google Scholar]
- 113.Piazza G, Goldhaber SZ. Fibrinolysis for acute pulmonary embolism. Vasc Med. 2010;15(5):419–428. doi: 10.1177/1358863X10380304. [DOI] [PubMed] [Google Scholar]
- 114.Todd JL, Tapson VF. Thrombolytic therapy for acute pulmonary embolism: a critical appraisal. Chest. 2009;135(5):1321–1329. doi: 10.1378/chest.08-2125. [DOI] [PubMed] [Google Scholar]
- 115.Kasirajan K, Gray B, Ouriel K. Percutaneous AngioJet thrombectomy in the management of extensive deep venous thrombosis. J Vasc Interv Radiol. 2001;12(2):179–185. doi: 10.1016/s1051-0443(07)61823-5. [DOI] [PubMed] [Google Scholar]
- 116.Vedantham S, Vesely TM, Parti N, Darcy M, Hovsepian DM, Picus D. Lower extremity venous thrombolysis with adjunctive mechanical thrombectomy. J Vasc Interv Radiol. 2002;13(10):1001–1008. doi: 10.1016/s1051-0443(07)61864-8. [DOI] [PubMed] [Google Scholar]
- 117.Delomez M, Beregi JP, Willoteaux S, et al. Mechanical thrombectomy in patients with deep venous thrombosis. Cardiovasc Intervent Radiol. 2001;24(1):42–48. doi: 10.1007/s002700001658. [DOI] [PubMed] [Google Scholar]
- 118.Kinney TB, Valji K, Rose SC, et al. Pulmonary embolism from pulse-spray pharmacomechanical thrombolysis of clotted hemodialysis grafts: urokinase versus heparinized saline. J Vasc Interv Radiol. 2000;11(9):1143–1152. doi: 10.1016/s1051-0443(07)61355-4. [DOI] [PubMed] [Google Scholar]
- 119.Arnesen H, Heilo A, Jakobsen E, Ly B, Skaga E. A prospective study of streptokinase and heparin in the treatment of deep vein thrombosis. Acta Med Scand. 1978;203(6):457–463. doi: 10.1111/j.0954-6820.1978.tb14908.x. [DOI] [PubMed] [Google Scholar]
- 120.Arnesen H, Høiseth A, Ly B. Streptokinase of heparin in the treatment of deep vein thrombosis. Follow-up results of a prospective study. Acta Med Scand. 1982;211(1-2):65–68. [PubMed] [Google Scholar]
- 121.Browse NL, Thomas ML, Pim HP. Streptokinase and deep vein thrombosis. BMJ. 1968;3(5620):717–720. doi: 10.1136/bmj.3.5620.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Duckert F, Müller G, Nyman D, et al. Treatment of deep vein thrombosis with streptokinase. BMJ. 1975;1(5956):479–481. doi: 10.1136/bmj.1.5956.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Elliot MS, Immelman EJ, Jeffery P, et al. A comparative randomized trial of heparin versus streptokinase in the treatment of acute proximal venous thrombosis: an interim report of a prospective trial. Br J Surg. 1979;66(12):838–843. doi: 10.1002/bjs.1800661203. [DOI] [PubMed] [Google Scholar]
- 124.Goldhaber SZ, Meyerovitz MF, Green D, et al. Randomized controlled trial of tissue plasminogen activator in proximal deep venous thrombosis. Am J Med. 1990;88(3):235–240. doi: 10.1016/0002-9343(90)90148-7. [DOI] [PubMed] [Google Scholar]
- 125.Kakkar VV, Flanc C, Howe CT, O’Shea M, Flute PT. Treatment of deep vein thrombosis. A trial of heparin, streptokinase, and arvin. BMJ. 1969;1(5647):806–810. doi: 10.1136/bmj.1.5647.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kiil J, Carvalho A, Saksø P, Nielsen HO. Urokinase or heparin in the management of patients with deep vein thrombosis? Acta Chir Scand. 1981;147(7):529–532. [PubMed] [Google Scholar]
- 127.Laiho MK, Oinonen A, Sugano N, et al. Preservation of venous valve function after catheter-directed and systemic thrombolysis for deep venous thrombosis. Eur J Vasc Endovasc Surg. 2004;28(4):391–396. doi: 10.1016/j.ejvs.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 128.Marder VJ, Soulen RL, Atichartakarn V, et al. Quantitative venographic assessment of deep vein thrombosis in the evaluation of streptokinase and heparin therapy. J Lab Clin Med. 1977;89(5):1018–1029. [PubMed] [Google Scholar]
- 129.Porter JM, Seaman AJ, Common HH, Rösch J, Eidemiller LR, Calhoun AD. Comparison of heparin and streptokinase in the treatment of venous thrombosis. Am Surg. 1975;41(9):511–519. [PubMed] [Google Scholar]
- 130.Robertson BR, Nilsson IM, Nylander G. Value of streptokinase and heparin in treatment of acute deep venous thrombosis. A coded investigation. Acta Chir Scand. 1968;134(3):203–208. [PubMed] [Google Scholar]
- 131.Schulman S, Granqvist S, Juhlin-Dannfelt A, Lockner D. Long-term sequelae of calf vein thrombosis treated with heparin or low-dose streptokinase. Acta Med Scand. 1986;219(4):349–357. doi: 10.1111/j.0954-6820.1986.tb03323.x. [DOI] [PubMed] [Google Scholar]
- 132.Schweizer J, Elix H, Altmann E, Hellner G, Forkmann L. Comparative results of thrombolysis treatment with rt-PA and urokinase: a pilot study. Vasa. 1998;27(3):167–171. [PubMed] [Google Scholar]
- 133.Schweizer J, Kirch W, Koch R, et al. Short- and long-term results after thrombolytic treatment of deep venous thrombosis. J Am Coll Cardiol. 2000;36(4):1336–1343. doi: 10.1016/s0735-1097(00)00863-9. [DOI] [PubMed] [Google Scholar]
- 134.Tsapogas MJ, Peabody RA, Wu KT, Karmody AM, Devaraj KT, Eckert C. Controlled study of thrombolytic therapy in deep vein thrombosis. Surgery. 1973;74(6):973–984. [PubMed] [Google Scholar]
- 135.Turpie AG, Levine MN, Hirsh J, et al. Tissue plasminogen activator (rt-PA) vs heparin in deep vein thrombosis. Results of a randomized trial. Chest. 1990;97(4) suppl:172S–175S. [PubMed] [Google Scholar]
- 136.Verhaeghe R, Besse P, Bounameaux H, Marbet GA. Multicenter pilot study of the efficacy and safety of systemic rt-PA administration in the treatment of deep vein thrombosis of the lower extremities and/or pelvis. Thromb Res. 1989;55(1):5–11. doi: 10.1016/0049-3848(89)90451-9. [DOI] [PubMed] [Google Scholar]
- 137.Watz R, Savidge GF. Rapid thrombolysis and preservation of valvular venous function in high deep vein thrombosis. A comparative study between streptokinase and heparin therapy. Acta Med Scand. 1979;205(4):293–298. doi: 10.1111/j.0954-6820.1979.tb06050.x. [DOI] [PubMed] [Google Scholar]
- 138.Watson LI, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev. 2004;(4):CD002783. doi: 10.1002/14651858.CD002783.pub2. [DOI] [PubMed] [Google Scholar]
- 139.Common HH, Seaman AJ, Rösch J, Porter JM, Dotter CT. Deep vein thrombosis treated with streptokinase or heparin. Follow-up of a randomized study. Angiology. 1976;27(11):645–654. doi: 10.1177/000331977602701105. [DOI] [PubMed] [Google Scholar]
- 140.Comerota AJ, Gale SS. Technique of contemporary iliofemoral and infrainguinal venous thrombectomy. J Vasc Surg. 2006;43(1):185–191. doi: 10.1016/j.jvs.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 141.Karp RB, Wylie EJ. Recurrent thrombosis after iliofemoral venous thrombectomy. Surg Forum. 1966;17:147. [PubMed] [Google Scholar]
- 142.Lansing AM, Davis WM. Five-year follow-up study of iliofemoral venous thrombectomy. Ann Surg. 1968;168(4):620–628. doi: 10.1097/00000658-196810000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Plate G, Akesson H, Einarsson E, Ohlin P, Eklöf B. Long-term results of venous thrombectomy combined with a temporary arterio-venous fistula. Eur J Vasc Surg. 1990;4(5):483–489. doi: 10.1016/s0950-821x(05)80788-1. [DOI] [PubMed] [Google Scholar]
- 144.Plate G, Einarsson E, Ohlin P, Jensen R, Qvarfordt P, Eklöf B. Thrombectomy with temporary arteriovenous fistula: the treatment of choice in acute iliofemoral venous thrombosis. J Vasc Surg. 1984;1(6):867–876. doi: 10.1067/mva.1984.avs0010867. [DOI] [PubMed] [Google Scholar]
- 145.Plate G, Eklöf B, Norgren L, Ohlin P, Dahlström JA. Venous thrombectomy for iliofemoral vein thrombosis—10-year results of a prospective randomised study. Eur J Vasc Endovasc Surg. 1997;14(5):367–374. doi: 10.1016/s1078-5884(97)80286-9. [DOI] [PubMed] [Google Scholar]
- 146.Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338(7):409–415. doi: 10.1056/NEJM199802123380701. [DOI] [PubMed] [Google Scholar]
- 147.Mismetti P, Rivron-Guillot K, Quenet S, et al. A prospective long-term study of 220 patients with a retrievable vena cava filter for secondary prevention of venous thromboembolism. Chest. 2007;131(1):223–229. doi: 10.1378/chest.06-0631. [DOI] [PubMed] [Google Scholar]
- 148.Athanasoulis CA, Kaufman JA, Halpern EF, Waltman AC, Geller SC, Fan CM. Inferior vena caval filters: review of a 26-year single-center clinical experience. Radiology. 2000;216(1):54–66. doi: 10.1148/radiology.216.1.r00jl1254. [DOI] [PubMed] [Google Scholar]
- 149.Cosmi B, Legnani C, Cini M, Guazzaloca G, Palareti G. D-dimer levels in combination with residual venous obstruction and the risk of recurrence after anticoagulation withdrawal for a first idiopathic deep vein thrombosis. Thromb Haemost. 2005;94(5):969–974. doi: 10.1160/TH05-02-0095. [DOI] [PubMed] [Google Scholar]
- 150.Streiff MB. Vena caval filters: a comprehensive review. Blood. 2000;95(12):3669–3677. [PubMed] [Google Scholar]
- 151.Hajduk B, Tomkowski WZ, Malek G, Davidson BL. Vena cava filter occlusion and venous thromboembolism risk in persistently anticoagulated patients: a prospective, observational cohort study. Chest. 2010;137(4):877–882. doi: 10.1378/chest.09-1533. [DOI] [PubMed] [Google Scholar]
- 152.Fox MA, Kahn SR. Postthrombotic syndrome in relation to vena cava filter placement: a systematic review. J Vasc Interv Radiol. 2008;19(7):981–985. doi: 10.1016/j.jvir.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 153.Usoh F, Hingorani A, Ascher E, et al. Prospective randomized study comparing the clinical outcomes between inferior vena cava Greenfield and TrapEase filters. J Vasc Surg. 2010;52(2):394–399. doi: 10.1016/j.jvs.2010.02.280. [DOI] [PubMed] [Google Scholar]
- 154.Nicholson W, Nicholson WJ, Tolerico P, et al. Prevalence of fracture and fragment embolization of Bard retrievable vena cava filters and clinical implications including cardiac perforation and tamponade. Arch Intern Med. 2010;170(20):1827–1831. doi: 10.1001/archinternmed.2010.316. [DOI] [PubMed] [Google Scholar]
- 155.Dabbagh O, Nagam N, Chitima-Matsiga R, Bearelly S, Bearelly D. Retrievable inferior vena cava filters are not getting retrieved: where is the gap? Thromb Res. 2010;126(6):493–497. doi: 10.1016/j.thromres.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 156.Aissaoui N, Martins E, Mouly S, Weber S, Meune C. A meta-analysis of bed rest versus early ambulation in the management of pulmonary embolism, deep vein thrombosis, or both. Int J Cardiol. 2009;137(1):37–41. doi: 10.1016/j.ijcard.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 157.Kahn SR, Shrier I, Kearon C. Physical activity in patients with deep venous thrombosis: a systematic review. Thromb Res. 2008;122(6):763–773. doi: 10.1016/j.thromres.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 158.Hull R, Delmore T, Genton E, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med. 1979;301(16):855–858. doi: 10.1056/NEJM197910183011602. [DOI] [PubMed] [Google Scholar]
- 159.Levine MN, Hirsh J, Gent M, et al. Optimal duration of oral anticoagulant therapy: a randomized trial comparing four weeks with three months of warfarin in patients with proximal deep vein thrombosis. Thromb Haemost. 1995;74(2):606–611. [PubMed] [Google Scholar]
- 160.Research Committee of the British Thoracic Society Optimum duration of anticoagulation for deep-vein thrombosis and pulmonary embolism. Lancet. 1992;340(8824):873–876. [PubMed] [Google Scholar]
- 161.Schulman S, Granqvist S, Holmström M, et al. The Duration of Anticoagulation Trial Study Group The duration of oral anticoagulant therapy after a second episode of venous thromboembolism. N Engl J Med. 1997;336(6):393–398. doi: 10.1056/NEJM199702063360601. [DOI] [PubMed] [Google Scholar]
- 162.Baglin T, Luddington R, Brown K, Baglin C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet. 2003;362(9383):523–526. doi: 10.1016/S0140-6736(03)14111-6. [DOI] [PubMed] [Google Scholar]
- 163.Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352–2361. doi: 10.1001/jama.293.19.2352. [DOI] [PubMed] [Google Scholar]
- 164.Hansson PO, Sörbo J, Eriksson H. Recurrent venous thromboembolism after deep vein thrombosis: incidence and risk factors. Arch Intern Med. 2000;160(6):769–774. doi: 10.1001/archinte.160.6.769. [DOI] [PubMed] [Google Scholar]
- 165.Heit JA, Mohr DN, Silverstein MD, Petterson TM, O’Fallon WM, Melton LJ., III Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med. 2000;160(6):761–768. doi: 10.1001/archinte.160.6.761. [DOI] [PubMed] [Google Scholar]
- 166.Palareti G, Legnani C, Cosmi B, Guazzaloca G, Pancani C, Coccheri S. Risk of venous thromboembolism recurrence: high negative predictive value of D-dimer performed after oral anticoagulation is stopped. Thromb Haemost. 2002;87(1):7–12. [PubMed] [Google Scholar]
- 167.Pinede L, Ninet J, Duhaut P, et al. Investigators of the “Durée Optimale du Traitement AntiVitamines K” (DOTAVK) Study Comparison of 3 and 6 months of oral anticoagulant therapy after a first episode of proximal deep vein thrombosis or pulmonary embolism and comparison of 6 and 12 weeks of therapy after isolated calf deep vein thrombosis. Circulation. 2001;103(20):2453–2460. doi: 10.1161/01.cir.103.20.2453. [DOI] [PubMed] [Google Scholar]
- 168.Pini M, Aiello S, Manotti C, et al. Low molecular weight heparin versus warfarin in the prevention of recurrences after deep vein thrombosis. Thromb Haemost. 1994;72(2):191–197. [PubMed] [Google Scholar]
- 169.Schulman S, Rhedin AS, Lindmarker P, et al. Duration of Anticoagulation Trial Study Group A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. N Engl J Med. 1995;332(25):1661–1665. doi: 10.1056/NEJM199506223322501. [DOI] [PubMed] [Google Scholar]
- 170.Boutitie F, Pinede. L, Schulman S, et al. Influence of preceding duration of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping therapy: analysis of individual participants’ data from seven trials. BMJ. 2011;342:d3036. doi: 10.1136/bmj.d3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med. 2010;170(19):1710–1716. doi: 10.1001/archinternmed.2010.367. [DOI] [PubMed] [Google Scholar]
- 172.Palareti G, Legnani C, Lee A, et al. A comparison of the safety and efficacy of oral anticoagulation for the treatment of venous thromboembolic disease in patients with or without malignancy. Thromb Haemost. 2000;84(5):805–810. [PubMed] [Google Scholar]
- 173.Lee AY, Levine MN, Baker RI, et al. Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 174.Ridker PM, Goldhaber SZ, Danielson E, et al. PREVENT Investigators Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348(15):1425–1434. doi: 10.1056/NEJMoa035029. [DOI] [PubMed] [Google Scholar]
- 175.Schulman S, Wåhlander K, Lundström T, Clason SB, Eriksson H. THRIVE III Investigators Secondary prevention of venous thromboembolism with the oral direct thrombin inhibitor ximelagatran. N Engl J Med. 2003;349(18):1713–1721. doi: 10.1056/NEJMoa030104. [DOI] [PubMed] [Google Scholar]
- 176.Eichinger S, Minar E, Bialonczyk C, et al. D-dimer levels and risk of recurrent venous thromboembolism. JAMA. 2003;290(8):1071–1074. doi: 10.1001/jama.290.8.1071. [DOI] [PubMed] [Google Scholar]
- 177.Palareti G, Legnani C, Cosmi B, et al. Predictive value of D-dimer test for recurrent venous thromboembolism after anticoagulation withdrawal in subjects with a previous idiopathic event and in carriers of congenital thrombophilia. Circulation. 2003;108(3):313–318. doi: 10.1161/01.CIR.0000079162.69615.0F. [DOI] [PubMed] [Google Scholar]
- 178.Shrivastava S, Ridker PM, Glynn RJ, et al. D-dimer, factor VIII coagulant activity, low-intensity warfarin and the risk of recurrent venous thromboembolism. J Thromb Haemost. 2006;4(6):1208–1214. doi: 10.1111/j.1538-7836.2006.01935.x. [DOI] [PubMed] [Google Scholar]
- 179.Cosmi B, Legnani C, Tosetto A, et al. PROLONG Investigators (on behalf of Italian Federation of Anticoagulation Clinics) Usefulness of repeated D-dimer testing after stopping anticoagulation for a first episode of unprovoked venous thromboembolism: the PROLONG II prospective study. Blood. 2010;115(3):481–488. doi: 10.1182/blood-2009-08-237354. [DOI] [PubMed] [Google Scholar]
- 180.Douketis J, Tosetto A, Marcucci M, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med. 2010;153(8):523–531. doi: 10.7326/0003-4819-153-8-201010190-00009. [DOI] [PubMed] [Google Scholar]
- 181.Verhovsek M, Douketis JD, Yi Q, et al. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med. 2008;149(7):481–490. doi: 10.7326/0003-4819-149-7-200810070-00008. [DOI] [PubMed] [Google Scholar]
- 182.Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med. 1999;340(12):901–907. doi: 10.1056/NEJM199903253401201. [DOI] [PubMed] [Google Scholar]
- 183.Schulman S, Lindmarker P, Holmström M, et al. Post-thrombotic syndrome, recurrence, and death 10 years after the first episode of venous thromboembolism treated with warfarin for 6 weeks or 6 months. J Thromb Haemost. 2006;4(4):734–742. doi: 10.1111/j.1538-7836.2006.01795.x. [DOI] [PubMed] [Google Scholar]
- 184.Schulman S, Svenungsson E, Granqvist S. Duration of Anticoagulation Study Group Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Am J Med. 1998;104(4):332–338. doi: 10.1016/s0002-9343(98)00060-6. [DOI] [PubMed] [Google Scholar]
- 185.Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ. 2008;179(5):417–426. doi: 10.1503/cmaj.080493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Eichinger S, Pabinger I, Stümpflen A, et al. The risk of recurrent venous thromboembolism in patients with and without factor V Leiden. Thromb Haemost. 1997;77(4):624–628. [PubMed] [Google Scholar]
- 187.Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med. 2006;166(7):729–736. doi: 10.1001/archinte.166.7.729. [DOI] [PubMed] [Google Scholar]
- 188.Lindmarker P, Schulman S, Sten-Linder M, Wiman B, Egberg N, Johnsson H. The risk of recurrent venous thromboembolism in carriers and non-carriers of the G1691A allele in the coagulation factor V gene and the G20210A allele in the prothrombin gene. DURAC Trial Study Group. Duration of Anticoagulation. Thromb Haemost. 1999;81(5):684–689. [PubMed] [Google Scholar]
- 189.Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA. 2009;301(23):2472–2485. doi: 10.1001/jama.2009.853. [DOI] [PubMed] [Google Scholar]
- 190.Kearon C, Julian JA, Kovacs MJ, et al. ELATE Investigators Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood. 2008;112(12):4432–4436. doi: 10.1182/blood-2008-06-163279. [DOI] [PubMed] [Google Scholar]
- 191.McRae S, Tran H, Schulman S, Ginsberg J, Kearon C. Effect of patient’s sex on risk of recurrent venous thromboembolism: a meta-analysis. Lancet. 2006;368(9533):371–378. doi: 10.1016/S0140-6736(06)69110-1. [DOI] [PubMed] [Google Scholar]
- 192.Douketis J, Tosetto A, Marcucci M, et al. Risk of recurrence after venous thromboembolism in men and women: patient level meta-analysis. BMJ. 2011;342:d813. doi: 10.1136/bmj.d813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.White RH, Zhou H, Romano PS. Incidence of idiopathic deep venous thrombosis and secondary thromboembolism among ethnic groups in California. Ann Intern Med. 1998;128(9):737–740. doi: 10.7326/0003-4819-128-9-199805010-00006. [DOI] [PubMed] [Google Scholar]
- 194.Agnelli G, Prandoni P, Becattini C, et al. Warfarin Optimal Duration Italian Trial Investigators Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med. 2003;139(1):19–25. doi: 10.7326/0003-4819-139-1-200307010-00008. [DOI] [PubMed] [Google Scholar]
- 195.Kearon C, Ginsberg JS, Anderson DR, et al. SOFAST Investigators Comparison of 1 month with 3 months of anticoagulation for a first episode of venous thromboembolism associated with a transient risk factor. J Thromb Haemost. 2004;2(5):743–749. doi: 10.1046/j.1538-7836.2004.00698.x. [DOI] [PubMed] [Google Scholar]
- 196.Piovella F, Crippa L, Barone M, et al. Normalization rates of compression ultrasonography in patients with a first episode of deep vein thrombosis of the lower limbs: association with recurrence and new thrombosis. Haematologica. 2002;87(5):515–522. [PubMed] [Google Scholar]
- 197.Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med. 2002;137(12):955–960. doi: 10.7326/0003-4819-137-12-200212170-00008. [DOI] [PubMed] [Google Scholar]
- 198.Carrier M, Rodger MA, Wells PS, Righini M, LE Gal G. Residual vein obstruction to predict the risk of recurrent venous thromboembolism in patients with deep vein thrombosis: a systematic review and meta-analysis. J Thromb Haemost. 2011;9(6):1119–1125. doi: 10.1111/j.1538-7836.2011.04254.x. [DOI] [PubMed] [Google Scholar]
- 199.Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation. 2010;121(14):1630–1636. doi: 10.1161/CIRCULATIONAHA.109.925214. [DOI] [PubMed] [Google Scholar]
- 200.Stain M, Schönauer V, Minar E, et al. The post-thrombotic syndrome: risk factors and impact on the course of thrombotic disease. J Thromb Haemost. 2005;3(12):2671–2676. doi: 10.1111/j.1538-7836.2005.01648.x. [DOI] [PubMed] [Google Scholar]
- 201.Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet. 1997;349(9054):759–762. doi: 10.1016/S0140-6736(96)12215-7. [DOI] [PubMed] [Google Scholar]
- 202.Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141(4):249–256. doi: 10.7326/0003-4819-141-4-200408170-00004. [DOI] [PubMed] [Google Scholar]
- 203.Campbell IA, Bentley DP, Prescott RJ, Routledge PA, Shetty HG, Williamson IJ. Anticoagulation for three versus six months in patients with deep vein thrombosis or pulmonary embolism, or both: randomised trial. BMJ. 2007;334(7595):674–680. doi: 10.1136/bmj.39098.583356.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Agnelli G, Prandoni P, Santamaria MG, et al. Warfarin Optimal Duration Italian Trial Investigators Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. N Engl J Med. 2001;345(3):165–169. doi: 10.1056/NEJM200107193450302. [DOI] [PubMed] [Google Scholar]
- 205.Siragusa S, Malato A, Anastasio R, et al. Residual vein thrombosis to establish duration of anticoagulation after a first episode of deep vein thrombosis: the Duration of Anticoagulation based on Compression UltraSonography (DACUS) study. Blood. 2008;112(3):511–515. doi: 10.1182/blood-2008-01-131656. [DOI] [PubMed] [Google Scholar]
- 206.Prandoni P, Prins MH, Lensing AW, et al. AESOPUS Investigators Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med. 2009;150(9):577–585. doi: 10.7326/0003-4819-150-9-200905050-00003. [DOI] [PubMed] [Google Scholar]
- 207.Farraj RS. Anticoagulation period in idiopathic venous thromboembolism. How long is enough? Saudi Med J. 2004;25(7):848–851. [PubMed] [Google Scholar]
- 208.Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92(2):199–205. doi: 10.3324/haematol.10516. [DOI] [PubMed] [Google Scholar]
- 209.van Dongen CJ, Prandoni P, Frulla M, Marchiori A, Prins MH, Hutten BA. Relation between quality of anticoagulant treatment and the development of the postthrombotic syndrome. J Thromb Haemost. 2005;3(5):939–942. doi: 10.1111/j.1538-7836.2005.01333.x. [DOI] [PubMed] [Google Scholar]
- 210.Locadia M, Bossuyt PM, Stalmeier PF, et al. Treatment of venous thromboembolism with vitamin K antagonists: patients’ health state valuations and treatment preferences. Thromb Haemost. 2004;92(6):1336–1341. doi: 10.1160/TH04-02-0075. [DOI] [PubMed] [Google Scholar]
- 211.Hull RD, Pineo GF, Brant RF, et al. LITE Trial Investigators Self-managed long-term low-molecular-weight heparin therapy: the balance of benefits and harms. Am J Med. 2007;120(1):72–82. doi: 10.1016/j.amjmed.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 212.Beyth RJ, Cohen AM, Landefeld CS. Long-term outcomes of deep-vein thrombosis. Arch Intern Med. 1995;155(10):1031–1037. [PubMed] [Google Scholar]
- 213.Hull RD, Pineo GF, Brant R, et al. Home therapy of venous thrombosis with long-term LMWH versus usual care: patient satisfaction and post-thrombotic syndrome. Am J Med. 2009;122(8):762–769. doi: 10.1016/j.amjmed.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 214.Kahn SR, Ginsberg JS. Relationship between deep venous thrombosis and the postthrombotic syndrome. Arch Intern Med. 2004;164(1):17–26. doi: 10.1001/archinte.164.1.17. [DOI] [PubMed] [Google Scholar]
- 215.Hull R, Delmore T, Carter C, et al. Adjusted subcutaneous heparin versus warfarin sodium in the long-term treatment of venous thrombosis. N Engl J Med. 1982;306(4):189–194. doi: 10.1056/NEJM198201283060401. [DOI] [PubMed] [Google Scholar]
- 216.Monreal M, Lafoz E, Olive A, del Rio L, Vedia C. Comparison of subcutaneous unfractionated heparin with a low molecular weight heparin (Fragmin) in patients with venous thromboembolism and contraindications to coumarin. Thromb Haemost. 1994;71(1):7–11. [PubMed] [Google Scholar]
- 217.Buller HR, Cohen AT, Davidson B, et al. van Gogh Investigators. Idraparinux versus standard therapy for venous thromboembolic disease. N Engl J Med. 2007;357(11):1094–1104. doi: 10.1056/NEJMoa064247. [DOI] [PubMed] [Google Scholar]
- 218.Iorio A, Guercini F, Pini M. Low-molecular-weight heparin for the long-term treatment of symptomatic venous thromboembolism: meta-analysis of the randomized comparisons with oral anticoagulants. J Thromb Haemost. 2003;1(9):1906–1913. doi: 10.1046/j.1538-7836.2003.00364.x. [DOI] [PubMed] [Google Scholar]
- 219.van der Heijden JF, Hutten BA, Büller HR, et al. Prins MH Vitamin K antagonists or low-molecular-weight heparin for the long term treatment of symptomatic venous thromboembolism. Cochrane Database Syst Rev. 2002;(4):CD002001. doi: 10.1002/14651858.CD002001. [DOI] [PubMed] [Google Scholar]
- 220.Das SK, Cohen AT, Edmondson RA, Melissari E, Kakkar VV. Low-molecular-weight heparin versus warfarin for prevention of recurrent venous thromboembolism: a randomized trial. World J Surg. 1996;20(5):521–526.. doi: 10.1007/s002689900081. [DOI] [PubMed] [Google Scholar]
- 221.Gonzalez-Fajardo JA, Arreba E, Castrodeza J, et al. Venographic comparison of subcutaneous low-molecular weight heparin with oral anticoagulant therapy in the long-term treatment of deep venous thrombosis. J Vasc Surg. 1999;30(2):283–292. doi: 10.1016/s0741-5214(99)70139-4. [DOI] [PubMed] [Google Scholar]
- 222.Hull R, Pineo G, Mah A, et al. Long-term low molecular weight heparin treatment versus oral anticoagulant therapy for proximal deep vein thrombosis [abstract] Blood. 2000;96:449a. [Google Scholar]
- 223.Lopaciuk S, Bielska-Falda H, Noszczyk W, et al. Low molecular weight heparin versus acenocoumarol in the secondary prophylaxis of deep vein thrombosis. Thromb Haemost. 1999;81(1):26–31. [PubMed] [Google Scholar]
- 224.López-Beret P, Orgaz A, Fontcuberta J, et al. Low molecular weight heparin versus oral anticoagulants in the long-term treatment of deep venous thrombosis. J Vasc Surg. 2001;33(1):77–90. doi: 10.1067/mva.2001.109336. [DOI] [PubMed] [Google Scholar]
- 225.Veiga F, Escribá A, Maluenda MP, et al. Low molecular weight heparin (enoxaparin) versus oral anticoagulant therapy (acenocoumarol) in the long-term treatment of deep venous thrombosis in the elderly: a randomized trial. Thromb Haemost. 2000;84(4):559–564. [PubMed] [Google Scholar]
- 226.Deitcher SR, Kessler CM, Merli G, Rigas JR, Lyons RM, Fareed J. ONCENOX Investigators Secondary prevention of venous thromboembolic events in patients with active cancer: enoxaparin alone versus initial enoxaparin followed by warfarin for a 180-day period. Clin Appl Thromb Hemost. 2006;12(4):389–396. doi: 10.1177/1076029606293692. [DOI] [PubMed] [Google Scholar]
- 227.Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162(15):1729–1735. doi: 10.1001/archinte.162.15.1729. [DOI] [PubMed] [Google Scholar]
- 228.Romera A, Cairols MA, Vila-Coll R, et al. A randomised open-label trial comparing long-term sub-cutaneous low-molecular-weight heparin compared with oral-anticoagulant therapy in the treatment of deep venous thrombosis. Eur J Vasc Endovasc Surg. 2009;37(3):349–356. doi: 10.1016/j.ejvs.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 229.Varki A. Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110(6):1723–1729. doi: 10.1182/blood-2006-10-053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2) suppl:e278S–e325S. doi: 10.1378/chest.11-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Robinson KS, Anderson DR, Gross M, et al. Ultrasonographic screening before hospital discharge for deep venous thrombosis after arthroplasty: the post-arthroplasty screening study. A randomized, controlled trial. Ann Intern Med. 1997;127(6):439–445. doi: 10.7326/0003-4819-127-6-199709150-00004. [DOI] [PubMed] [Google Scholar]
- 232.Kahn SR, Ginsberg JS. The post-thrombotic syndrome: current knowledge, controversies, and directions for future research. Blood Rev. 2002;16(3):155–165. doi: 10.1016/s0268-960x(02)00008-5. [DOI] [PubMed] [Google Scholar]
- 233.Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3-part study. Arch Intern Med. 2001;161(17):2105–2109. doi: 10.1001/archinte.161.17.2105. [DOI] [PubMed] [Google Scholar]
- 234.Partsch H, Kaulich M, Mayer W. Immediate mobilisation in acute vein thrombosis reduces post-thrombotic syndrome. Int Angiol. 2004;23(3):206–212. [PubMed] [Google Scholar]
- 235.Aschwanden M, Jeanneret C, Koller MT, Thalhammer C, Bucher HC, Jaeger KA. Effect of prolonged treatment with compression stockings to prevent post-thrombotic sequelae: a randomized controlled trial. J Vasc Surg. 2008;47(5):1015–1021. doi: 10.1016/j.jvs.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 236.Roumen-Klappe EM, den Heijer M, van Rossum J, et al. Multilayer compression bandaging in the acute phase of deep-vein thrombosis has no effect on the development of the post-thrombotic syndrome. J Thromb Thrombolysis. 2009;27(4):400–405. doi: 10.1007/s11239-008-0229-7. [DOI] [PubMed] [Google Scholar]
- 237.Ten Cate-Hoek AJ, Ten Cate H, Tordoir J, Hamulyák K, Prins MH. Individually tailored duration of elastic compression therapy in relation to incidence of the postthrombotic syndrome. J Vasc Surg. 2010;52(1):132–138. doi: 10.1016/j.jvs.2010.01.089. [DOI] [PubMed] [Google Scholar]
- 238.Frulla M, Marchiori A, Sartor D, et al. Elastic stockings, hydroxyethylrutosides or both for the treatment of post-thrombotic syndrome. Thromb Haemost. 2005;93(1):183–185. [PubMed] [Google Scholar]
- 239.Ginsberg JS, Magier D, Mackinnon B, Gent M, Hirsh J. Intermittent compression units for severe post-phlebitic syndrome: a randomized crossover study. CMAJ. 1999;160(9):1303–1306. [PMC free article] [PubMed] [Google Scholar]
- 240.O’Donnell MJ, McRae S, Kahn SR, et al. Evaluation of a venous-return assist device to treat severe post-thrombotic syndrome (VENOPTS). A randomized controlled trial. Thromb Haemost. 2008;99(3):623–629. doi: 10.1160/TH07-09-0546. [DOI] [PubMed] [Google Scholar]
- 241.Lyseng-Williamson KA, Perry CM. Micronised purified flavonoid fraction: a review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs. 2003;63(1):71–100. doi: 10.2165/00003495-200363010-00005. [DOI] [PubMed] [Google Scholar]
- 242.Shoab SS, Porter J, Scurr JH, Coleridge-Smith PD. Endothelial activation response to oral micronised flavonoid therapy in patients with chronic venous disease—a prospective study. Eur J Vasc Endovasc Surg. 1999;17(4):313–318. doi: 10.1053/ejvs.1998.0751. [DOI] [PubMed] [Google Scholar]
- 243.de Jongste AB, Jonker JJ, Huisman MV, ten Cate JW, Azar AJ. A double blind three center clinical trial on the short-term efficacy of 0-(beta-hydroxyethyl)-rutosides in patients with post-thrombotic syndrome. Thromb Haemost. 1989;62(3):826–829. [PubMed] [Google Scholar]
- 244.Monreal M, Callejas JM, Martorell A, et al. A prospective study of the long-term efficacy of two different venoactive drugs in patients with post-thrombotic syndrome. Phlebology. 1994;9:37–40. [Google Scholar]
- 245.Campbell IA, Yeoh J, Medlicott S. Duration of hospital stay in patients with pulmonary venous thromboembolism: a randomized comparison of unfractionated heparinversus low molecular weight heparin [Abstract] Thorax. 1998;53:254. [Google Scholar]
- 246.Duroux P. A randomised trial of subcutaneous low molecular weight heparin (CY 216) compared with intravenous unfractionated heparin in the treatment of deep vein thrombosis. A collaborative European multicentre study. Thromb Haemost. 1991;65(3):251–256. [PubMed] [Google Scholar]
- 247.Hull RD, Raskob GE, Pineo GF, et al. Subcutaneous low-molecular-weight heparin compared with continuous intravenous heparin in the treatment of proximal-vein thrombosis. N Engl J Med. 1992;326(15):975–982. doi: 10.1056/NEJM199204093261502. [DOI] [PubMed] [Google Scholar]
- 248.Kirchmaier CM, Wolf H, Schäfer H, Ehlers B, Breddin HK. Certoparin-Study Group Efficacy of a low molecular weight heparin administered intravenously or subcutaneously in comparison with intravenous unfractionated heparin in the treatment of deep venous thrombosis. Int Angiol. 1998;17(3):135–145. [PubMed] [Google Scholar]
- 249.Kuijer PM, Gallus AS, Cade J, et al. Randomized comparison of LMWH versus standard heparin in the initial treatment of pulmonary embolism [Abstract] Thromb Haemost. 1995;73:974. [Google Scholar]
- 250.Meyer G, Brenot F, Pacouret G, et al. Subcutaneous low-molecular-weight heparin fragmin versus intravenous unfractionated heparin in the treatment of acute non massive pulmonary embolism: an open randomized pilot study. Thromb Haemost. 1995;74(6):1432–1435. [PubMed] [Google Scholar]
- 251.Pérez de Llano LA, Baloira Villar A, Veres Racamonde A, Veiga F, Golpe Gómez R, Pajuelo Fernández F. Multicenter, prospective study comparing enoxaparin with unfractionated heparin in the treatment of submassive pulmonary thromboembolism [in Spanish] Arch Bronconeumol. 2003;39(8):341–345. doi: 10.1016/s0300-2896(03)75401-5. [DOI] [PubMed] [Google Scholar]
- 252.Simonneau G, Sors H, Charbonnier B, et al. A comparison of low-molecular-weight heparin with unfractionated heparin for acute pulmonary embolism. The THESEE Study Group. Tinzaparine ou Heparine Standard: Evaluations dans l’Embolie Pulmonaire. N Engl J Med. 1997;337(10):663–669. doi: 10.1056/NEJM199709043371002. [DOI] [PubMed] [Google Scholar]
- 253.The Columbus Investigators Low-molecular-weight heparin in the treatment of patients with. N Engl J Med. 1997;337(10):657–662. doi: 10.1056/NEJM199709043371001. [DOI] [PubMed] [Google Scholar]
- 254.Théry C, Simonneau G, Meyer G, et al. Randomized trial of subcutaneous low-molecular-weight heparin CY 216 (Fraxiparine) compared with intravenous unfractionated heparin in the curative treatment of submassive pulmonary embolism. A dose-ranging study. Circulation. 1992;85(4):1380–1389. doi: 10.1161/01.cir.85.4.1380. [DOI] [PubMed] [Google Scholar]
- 255.Quinlan DJ, McQuillan A, Eikelboom JW. Low-molecular-weight heparin compared with intravenous unfractionated heparin for treatment of pulmonary embolism: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2004;140(3):175–183. doi: 10.7326/0003-4819-140-3-200402030-00008. [DOI] [PubMed] [Google Scholar]
- 256.Otero R, Uresandi F, Jiménez D, et al. Home treatment in pulmonary embolism. Thromb Res. 2010;126(1):e1–e5. doi: 10.1016/j.thromres.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 257.Aujesky D, Roy PM, Verschuren F, et al. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet. 2011;378(9785):41–48. doi: 10.1016/S0140-6736(11)60824-6. [DOI] [PubMed] [Google Scholar]
- 258.Uresandi F, Otero R, Cayuela A, et al. [A clinical prediction rule for identifying short-term risk of adverse events in patients with pulmonary thromboembolism] Arch Bronconeumol. 2007;43(11):617–622. doi: 10.1016/s1579-2129(07)60139-6. [DOI] [PubMed] [Google Scholar]
- 259.Wicki J, Perrier A, Perneger TV, Bounameaux H, Junod AF. Predicting adverse outcome in patients with acute pulmonary embolism: a risk score. Thromb Haemost. 2000;84(4):548–552. [PubMed] [Google Scholar]
- 260.Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005;172(8):1041–1046. doi: 10.1164/rccm.200506-862OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Jiménez D, Yusen RD, Otero R, et al. Prognostic models for selecting patients with acute pulmonary embolism for initial outpatient therapy. Chest. 2007;132(1):24–30. doi: 10.1378/chest.06-2921. [DOI] [PubMed] [Google Scholar]
- 262.Jiménez D, Aujesky D, Moores L, et al. RIETE Investigators Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010;170(15):1383–1389. doi: 10.1001/archinternmed.2010.199. [DOI] [PubMed] [Google Scholar]
- 263.Jakobsson C, Jiménez D, Gómez V, Zamarro C, Méan M, Aujesky D. Validation of a clinical algorithm to identify low-risk patients with pulmonary embolism. J Thromb Haemost. 2010;8(6):1242–1247. doi: 10.1111/j.1538-7836.2010.03836.x. [DOI] [PubMed] [Google Scholar]
- 264.Chan CM, Woods C, Shorr AF. The validation and reproducibility of the pulmonary embolism severity index. J Thromb Haemost. 2010;8(7):1509–1514. doi: 10.1111/j.1538-7836.2010.03888.x. [DOI] [PubMed] [Google Scholar]
- 265.Moores L, Aujesky D, Jiménez D, et al. Pulmonary Embolism Severity Index and troponin testing for the selection of low-risk patients with acute symptomatic pulmonary embolism. J Thromb Haemost. 2010;8(3):517–522. doi: 10.1111/j.1538-7836.2009.03725.x. [DOI] [PubMed] [Google Scholar]
- 266.Fraga M, Taffé P, Méan M, et al. The inter-rater reliability of the Pulmonary Embolism Severity Index. Thromb Haemost. 2010;104(6):1258–1262. doi: 10.1160/TH10-07-0426. [DOI] [PubMed] [Google Scholar]
- 267.Squizzato A, Galli M, Dentali F, Ageno W. Outpatient treatment and early discharge of symptomatic pulmonary embolism: a systematic review. Eur Respir J. 2009;33(5):1148–1155. doi: 10.1183/09031936.00133608. [DOI] [PubMed] [Google Scholar]
- 268.Erkens PM, Gandara E, Wells P, et al. Safety of outpatient treatment in acute pulmonary embolism. J Thromb Haemost. 2010;8(11):2412–2417. doi: 10.1111/j.1538-7836.2010.04041.x. [DOI] [PubMed] [Google Scholar]
- 269.Kovacs MJ, Hawel JD, Rekman JF, Lazo-Langner A. Ambulatory management of pulmonary embolism: a pragmatic evaluation. J Thromb Haemost. 2010;8(11):2406–2411. doi: 10.1111/j.1538-7836.2010.03981.x. [DOI] [PubMed] [Google Scholar]
- 270.Zondag W, Mos IC, Creemers-Schild D, et al. Hestia Study Investigators Outpatient treatment in patients with acute pulmonary embolism: the Hestia Study. J Thromb Haemost. 2011;9(8):1500–1507. doi: 10.1111/j.1538-7836.2011.04388.x. [DOI] [PubMed] [Google Scholar]
- 271.Agterof MJ, Schutgens RE, Snijder RJ, et al. Out of hospital treatment of acute pulmonary embolism in patients with a low NT-proBNP level. J Thromb Haemost. 2010;8(6):1235–1241. doi: 10.1111/j.1538-7836.2010.03831.x. [DOI] [PubMed] [Google Scholar]
- 272.Baglin T. Fifty per cent of patients with pulmonary embolism can be treated as outpatients. J Thromb Haemost. 2010;8(11):2404–2405. doi: 10.1111/j.1538-7836.2010.04055.x. [DOI] [PubMed] [Google Scholar]
- 273.Dong B, Jirong Y, Wang Q. Wu T Thrombolytic treatment for pulmonary embolism. Cochrane Database Syst Rev. 2006;(2):CD004437. doi: 10.1002/14651858.CD004437.pub2. [DOI] [PubMed] [Google Scholar]
- 274.Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002;121(3):877–905. doi: 10.1378/chest.121.3.877. [DOI] [PubMed] [Google Scholar]
- 275.McIntyre KM, Sasahara AA. Determinants of right ventricular function and hemodynamics after pulmonary embolism. Chest. 1974;65(5):534–543. doi: 10.1378/chest.65.5.534. [DOI] [PubMed] [Google Scholar]
- 276.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353(9162):1386–1389. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 277.Laporte S, Mismetti P, Décousus H, et al. RIETE Investigators Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) Registry. Circulation. 2008;117(13):1711–1716. doi: 10.1161/CIRCULATIONAHA.107.726232. [DOI] [PubMed] [Google Scholar]
- 278.Pollack CV, Schreiber D, Goldhaber SZ, et al. Clinical characteristics, management, and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department: initial report of EMPEROR (Multicenter Emergency Medicine Pulmonary Embolism in the Real World Registry) J Am Coll Cardiol. 2011;57(6):700–706. doi: 10.1016/j.jacc.2010.05.071. [DOI] [PubMed] [Google Scholar]
- 279.Sanchez O, Trinquart L, Caille V, et al. Prognostic factors for pulmonary embolism: the prep study, a prospective multicenter cohort study. Am J Respir Crit Care Med. 2010;181(2):168–173. doi: 10.1164/rccm.200906-0970OC. [DOI] [PubMed] [Google Scholar]
- 280.Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation. 2006;113(4):577–582. doi: 10.1161/CIRCULATIONAHA.105.592592. [DOI] [PubMed] [Google Scholar]
- 281.Torbicki A, Perrier A, Konstantinides S, et al. ESC Committee for Practice Guidelines (CPG) Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Eur Heart J. 2008;29(18):2276–2315. doi: 10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]
- 282.Douketis JD, Leeuwenkamp O, Grobara P, et al. The incidence and prognostic significance of elevated cardiac troponins in patients with submassive pulmonary embolism. J Thromb Haemost. 2005;3(3):508–513. doi: 10.1111/j.1538-7836.2005.01189.x. [DOI] [PubMed] [Google Scholar]
- 283.Giannitsis E, Katus HA. Risk stratification in pulmonary embolism based on biomarkers and echocardiography. Circulation. 2005;112(11):1520–1521. doi: 10.1161/CIRCULATIONAHA.105.566182. [DOI] [PubMed] [Google Scholar]
- 284.Konstantinides S, Geibel A, Olschewski M, et al. Importance of cardiac troponins I and T in risk stratification of patients with acute pulmonary embolism. Circulation. 2002;106(10):1263–1268. doi: 10.1161/01.cir.0000028422.51668.a2. [DOI] [PubMed] [Google Scholar]
- 285.Scridon T, Scridon C, Skali H, Alvarez A, Goldhaber SZ, Solomon SD. Prognostic significance of troponin elevation and right ventricular enlargement in acute pulmonary embolism. Am J Cardiol. 2005;96(2):303–305. doi: 10.1016/j.amjcard.2005.03.062. [DOI] [PubMed] [Google Scholar]
- 286.Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation. 2007;116(4):427–433. doi: 10.1161/CIRCULATIONAHA.106.680421. [DOI] [PubMed] [Google Scholar]
- 287.Lankeit M, Friesen D, Aschoff J, et al. Highly sensitive troponin T assay in normotensive patients with acute pulmonary embolism. Eur Heart J. 2010;31(15):1836–1844. doi: 10.1093/eurheartj/ehq234. [DOI] [PubMed] [Google Scholar]
- 288.Lega JC, Lacasse Y, Lakhal L, Provencher S. Natriuretic peptides and troponins in pulmonary embolism: a meta-analysis. Thorax. 2009;64(10):869–875. doi: 10.1136/thx.2008.110965. [DOI] [PubMed] [Google Scholar]
- 289.Bova C, Pesavento R, Marchiori A, et al. TELESIO Study Group Risk stratification and outcomes in hemodynamically stable patients with acute pulmonary embolism: a prospective, multicentre, cohort study with three months of follow-up. J Thromb Haemost. 2009;7(6):938–944. doi: 10.1111/j.1538-7836.2009.03345.x. [DOI] [PubMed] [Google Scholar]
- 290.Stein PD, Matta F, Janjua M, Yaekoub AY, Jaweesh F, Alrifai A. Outcome in stable patients with acute pulmonary embolism who had right ventricular enlargement and/or elevated levels of troponin I. Am J Cardiol. 2010;106(4):558–563. doi: 10.1016/j.amjcard.2010.03.071. [DOI] [PubMed] [Google Scholar]
- 291.Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J. 2008;29(12):1569–1577. doi: 10.1093/eurheartj/ehn208. [DOI] [PubMed] [Google Scholar]
- 292.Goldhaber SZ. Echocardiography in the management of pulmonary embolism. Ann Intern Med. 2002;136(9):691–700. doi: 10.7326/0003-4819-136-9-200205070-00012. [DOI] [PubMed] [Google Scholar]
- 293.Schoepf UJ, Kucher N, Kipfmueller F, Quiroz R, Costello P, Goldhaber SZ. Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation. 2004;110(20):3276–3280. doi: 10.1161/01.CIR.0000147612.59751.4C. [DOI] [PubMed] [Google Scholar]
- 294.ten Wolde M, Söhne M, Quak E, Mac Gillavry MR, Büller HR. Prognostic value of echocardiographically assessed right ventricular dysfunction in patients with pulmonary embolism. Arch Intern Med. 2004;164(15):1685–1689. doi: 10.1001/archinte.164.15.1685. [DOI] [PubMed] [Google Scholar]
- 295.Frémont B, Pacouret G, Jacobi D, Puglisi R, Charbonnier B, de Labriolle A. Prognostic value of echocardiographic right/left ventricular end-diastolic diameter ratio in patients with acute pulmonary embolism: results from a monocenter registry of 1,416 patients. Chest. 2008;133(2):358–362. doi: 10.1378/chest.07-1231. [DOI] [PubMed] [Google Scholar]
- 296.Vanni S, Polidori G, Vergara R, et al. Prognostic value of ECG among patients with acute pulmonary embolism and normal blood pressure. Am J Med. 2009;122(3):257–264. doi: 10.1016/j.amjmed.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 297.Stein PD, Beemath A, Matta F, et al. Enlarged right ventricle without shock in acute pulmonary embolism: prognosis. Am J Med. 2008;121(1):34–42. doi: 10.1016/j.amjmed.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Fiumara K, Kucher N, Fanikos J, Goldhaber SZ. Predictors of major hemorrhage following fibrinolysis for acute pulmonary embolism. Am J Cardiol. 2006;97(1):127–129. doi: 10.1016/j.amjcard.2005.07.117. [DOI] [PubMed] [Google Scholar]
- 299.Kanter DS, Mikkola KM, Patel SR, Parker JA, Goldhaber SZ. Thrombolytic therapy for pulmonary embolism. Frequency of intracranial hemorrhage and associated risk factors. Chest. 1997;111(5):1241–1245. doi: 10.1378/chest.111.5.1241. [DOI] [PubMed] [Google Scholar]
- 300.The urokinase pulmonary embolism trial. A national cooperative study. Circulation. 1973;47(2 suppl):II1–II108. [PubMed] [Google Scholar]
- 301.Dalla-Volta S, Palla A, Santolicandro A, et al. PAIMS 2: alteplase combined with heparin versus heparin in the treatment of acute pulmonary embolism. Plasminogen activator Italian multicenter study 2. J Am Coll Cardiol. 1992;20(3):520–526. doi: 10.1016/0735-1097(92)90002-5. [DOI] [PubMed] [Google Scholar]
- 302.Dotter CT, Seamon AJ, Rosch J, et al. Streptokinase and heparin in the treatment of pulmonary embolism: a randomized comparison. Vasc Surg. 1979;13:42–52. [Google Scholar]
- 303.PIOPED Investigators Tissue plasminogen activator for the treatment of acute pulmonary embolism. A collaborative study by the PIOPED Investigators. Chest. 1990;97(3):528–533. doi: 10.1378/chest.97.3.528. [DOI] [PubMed] [Google Scholar]
- 304.Jerjes-Sanchez C, Ramírez-Rivera A. de Lourdes García M, et al Streprokinase and heparin versus heparin alone in massive pulmonary embolism: a randomized controlled trial. J Thromb Thrombolysis. 1995;2(3):227–229. doi: 10.1007/BF01062714. [DOI] [PubMed] [Google Scholar]
- 305.Ly B, Arnesen H, Eie H, Hol R. A controlled clinical trial of streptokinase and heparin in the treatment of major pulmonary embolism. Acta Med Scand. 1978;203(6):465–470. doi: 10.1111/j.0954-6820.1978.tb14909.x. [DOI] [PubMed] [Google Scholar]
- 306.Marini C, Di Ricco G, Rossi G, Rindi M, Palla R, Giuntini C. Fibrinolytic effects of urokinase and heparin in acute pulmonary embolism: a randomized clinical trial. Respiration. 1988;54(3):162–173. doi: 10.1159/000195517. [DOI] [PubMed] [Google Scholar]
- 307.Tibbutt DA, Davies JA, Anderson JA, et al. Comparison by controlled clinical trial of streptokinase and heparin in treatment of life-threatening pulmonay embolism. BMJ. 1974;1(5904):343–347. doi: 10.1136/bmj.1.5904.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Urokinase pulmonary embolism trial. Phase I results: a cooperative study. JAMA. 1970;214(12):2163–2172. [PubMed] [Google Scholar]
- 309.Becattini C, Agnelli G, Salvi A, et al. TIPES Study Group Bolus tenecteplase for right ventricle dysfunction in hemodynamically stable patients with pulmonary embolism. Thromb Res. 2010;125(3):e82–e86. doi: 10.1016/j.thromres.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 310.Fasullo S, Scalzo S, Maringhini G, et al. Six-month echocardiographic study in patients with submassive pulmonary embolism and right ventricle dysfunction: comparison of thrombolysis with heparin. Am J Med Sci. 2010;341(1):33–39. doi: 10.1097/MAJ.0b013e3181f1fc3e. [DOI] [PubMed] [Google Scholar]
- 311.Goldhaber SZ, Haire WD, Feldstein ML, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet. 1993;341(8844):507–511. doi: 10.1016/0140-6736(93)90274-k. [DOI] [PubMed] [Google Scholar]
- 312.Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W. Management Strategies and Prognosis of Pulmonary Embolism-3 Trial Investigators Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347(15):1143–1150. doi: 10.1056/NEJMoa021274. [DOI] [PubMed] [Google Scholar]
- 313.Levine M, Hirsh J, Weitz J, et al. A randomized trial of a single bolus dosage regimen of recombinant tissue plasminogen activator in patients with acute pulmonary embolism. Chest. 1990;98(6):1473–1479. doi: 10.1378/chest.98.6.1473. [DOI] [PubMed] [Google Scholar]
- 314.Agnelli G, Becattini C, Kirschstein T. Thrombolysis vs heparin in the treatment of pulmonary embolism: a clinical outcome-based meta-analysis. Arch Intern Med. 2002;162(22):2537–2541. doi: 10.1001/archinte.162.22.2537. [DOI] [PubMed] [Google Scholar]
- 315.Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation. 2004;110(6):744–749. doi: 10.1161/01.CIR.0000137826.09715.9C. [DOI] [PubMed] [Google Scholar]
- 316.Urokinase-streptokinase embolism trial. Phase 2 results. A cooperative study. JAMA. 1974;229(12):1606–1613. [PubMed] [Google Scholar]
- 317.Goldhaber SZ, Agnelli G, Levine MN. The Bolus Alteplase Pulmonary Embolism Group Reduced dose bolus alteplase vs conventional alteplase infusion for pulmonary embolism thrombolysis. An international multicenter randomized trial. Chest. 1994;106(3):718–724. doi: 10.1378/chest.106.3.718. [DOI] [PubMed] [Google Scholar]
- 318.Goldhaber SZ, Kessler CM, Heit J, et al. Randomised controlled trial of recombinant tissue plasminogen activator versus urokinase in the treatment of acute pulmonary embolism. Lancet. 1988;2(8606):293–298. doi: 10.1016/s0140-6736(88)92354-9. [DOI] [PubMed] [Google Scholar]
- 319.Goldhaber SZ, Kessler CM, Heit JA, et al. Recombinant tissue-type plasminogen activator versus a novel dosing regimen of urokinase in acute pulmonary embolism: a randomized controlled multicenter trial. J Am Coll Cardiol. 1992;20(1):24–30. doi: 10.1016/0735-1097(92)90132-7. [DOI] [PubMed] [Google Scholar]
- 320.Meneveau N, Schiele F, Vuillemenot A, et al. Streptokinase vs alteplase in massive pulmonary embolism. A randomized trial assessing right heart haemodynamics and pulmonary vascular obstruction. Eur Heart J. 1997;18(7):1141–1148. doi: 10.1093/oxfordjournals.eurheartj.a015410. [DOI] [PubMed] [Google Scholar]
- 321.Meyer G, Sors H, Charbonnier B, et al. The European Cooperative Study Group for Pulmonary Embolism Effects of intravenous urokinase versus alteplase on total pulmonary resistance in acute massive pulmonary embolism: a European multicenter double-blind trial. J Am Coll Cardiol. 1992;19(2):239–245. doi: 10.1016/0735-1097(92)90472-y. [DOI] [PubMed] [Google Scholar]
- 322.Sors H, Pacouret G, Azarian R, Meyer G, Charbonnier B, Simonneau G. Hemodynamic effects of bolus vs 2-h infusion of alteplase in acute massive pulmonary embolism. A randomized controlled multicenter trial. Chest. 1994;106(3):712–717. doi: 10.1378/chest.106.3.712. [DOI] [PubMed] [Google Scholar]
- 323.Tebbe U, Bramlage P, Graf A, et al. Desmoteplase in acute massive pulmonary thromboembolism. Thromb Haemost. 2009;101(3):557–562. [PubMed] [Google Scholar]
- 324.Tebbe U, Graf A, Kamke W, et al. Hemodynamic effects of double bolus reteplase versus alteplase infusion in massive pulmonary embolism. Am Heart J. 1999;138(1 Pt 1):39–44. doi: 10.1016/s0002-8703(99)70243-7. [DOI] [PubMed] [Google Scholar]
- 325.Wang C, Zhai Z, Yang Y, et al. China Venous Thromboembolism (VTE) Study Group Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest. 2010;137(2):254–262. doi: 10.1378/chest.09-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Wang C, Zhai Z, Yang Y, et al. China Venous Thromboembolism Study Group Efficacy and safety of 2-hour urokinase regime in acute pulmonary embolism: a randomized controlled trial. Respir Res. 2009;10:128. doi: 10.1186/1465-9921-10-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Meneveau N, Schiele F, Metz D, et al. Comparative efficacy of a two-hour regimen of streptokinase versus alteplase in acute massive pulmonary embolism: immediate clinical and hemodynamic outcome and one-year follow-up. J Am Coll Cardiol. 1998;31(5):1057–1063. doi: 10.1016/s0735-1097(98)00068-0. [DOI] [PubMed] [Google Scholar]
- 328.Verstraete M, Miller GAH, Bounameaux H, et al. Intravenous and intrapulmonary recombinant tissue-type plasminogen activator in the treatment of acute massive pulmonary embolism. Circulation. 1988;77(2):353–360. doi: 10.1161/01.cir.77.2.353. [DOI] [PubMed] [Google Scholar]
- 329.de Gregorio M, Gimeno M, Alfonso R, et al. [Mechanical fragmentation and intrapulmonary fibrinolysis in the treatment of massive pulmonary embolism hemodynamic repercussions] Arch Bronconeumol. 2001;37(2):58–64. doi: 10.1016/s0300-2896(01)75015-6. [DOI] [PubMed] [Google Scholar]
- 330.Fava M, Loyola S, Flores P, Huete I. Mechanical fragmentation and pharmacologic thrombolysis in massive pulmonary embolism. J Vasc Interv Radiol. 1997;8(2):261–266. doi: 10.1016/s1051-0443(97)70552-9. [DOI] [PubMed] [Google Scholar]
- 331.Schmitz-Rode T, Janssens U, Duda SH, Erley CM, Günther RW. Massive pulmonary embolism: percutaneous emergency treatment by pigtail rotation catheter. J Am Coll Cardiol. 2000;36(2):375–380. doi: 10.1016/s0735-1097(00)00734-8. [DOI] [PubMed] [Google Scholar]
- 332.Schmitz-Rode T, Janssens U, Schild HH, Basche S, Hanrath P, Günther RW. Fragmentation of massive pulmonary embolism using a pigtail rotation catheter. Chest. 1998;114(5):1427–1436. doi: 10.1378/chest.114.5.1427. [DOI] [PubMed] [Google Scholar]
- 333.Kucher N. Catheter embolectomy for acute pulmonary embolism. Chest. 2007;132(2):657–663. doi: 10.1378/chest.07-0665. [DOI] [PubMed] [Google Scholar]
- 334.Kuo WT, Gould MK, Louie JD, Rosenberg JK, Sze DY, Hofmann LV. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol. 2009;20(11):1431–1440. doi: 10.1016/j.jvir.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 335.Margheri M, Vittori G, Vecchio S, et al. Early and long-term clinical results of AngioJet rheolytic thrombectomy in patients with acute pulmonary embolism. Am J Cardiol. 2008;101(2):252–258. doi: 10.1016/j.amjcard.2007.07.087. [DOI] [PubMed] [Google Scholar]
- 336.Zhou WZ, Shi HB, Yang ZQ, et al. Value of percutanous catheter fragmentation in the management of massive pulmonary embolism. Chin Med J (Engl) 2009;122(15):1723–1727. [PubMed] [Google Scholar]
- 337.Leacche M, Unic D, Goldhaber SZ, et al. Modern surgical treatment of massive pulmonary embolism: results in 47 consecutive patients after rapid diagnosis and aggressive surgical approach. J Thorac Cardiovasc Surg. 2005;129(5):1018–1023. doi: 10.1016/j.jtcvs.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 338.Meneveau N, Séronde MF, Blonde MC, et al. Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest. 2006;129(4):1043–1050. doi: 10.1378/chest.129.4.1043. [DOI] [PubMed] [Google Scholar]
- 339.Sukhija R, Aronow WS, Lee J, et al. Association of right ventricular dysfunction with in-hospital mortality in patients with acute pulmonary embolism and reduction in mortality in patients with right ventricular dysfunction by pulmonary embolectomy. Am J Cardiol. 2005;95(5):695–696. doi: 10.1016/j.amjcard.2004.10.055. [DOI] [PubMed] [Google Scholar]
- 340.Fukuda I, Taniguchi S, Fukui K, Minakawa M, Daitoku K, Suzuki Y. Improved outcome of surgical pulmonary embolectomy by aggressive intervention for critically ill patients. Ann Thorac Surg. 2011;91(3):728–732. doi: 10.1016/j.athoracsur.2010.10.086. [DOI] [PubMed] [Google Scholar]
- 341.Beckman JA, Dunn K, Sasahara AA, Goldhaber SZ. Enoxaparin monotherapy without oral anticoagulation to treat acute symptomatic pulmonary embolism. Thromb Haemost. 2003;89(6):953–958. [PubMed] [Google Scholar]
- 342.Kucher N, Quiroz R, McKean S, Sasahara AA, Goldhaber SZ. Extended enoxaparin monotherapy for acute symptomatic pulmonary embolism. Vasc Med. 2005;10(4):251–256. doi: 10.1191/1358863x05vm634oa. [DOI] [PubMed] [Google Scholar]
- 343.Schulman S, Kearon C, Kakkar AK, et al. RE-COVER Study Group Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342–2352. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 344.Gosselin MV, Rubin GD, Leung AN, Huang J, Rizk NW. Unsuspected pulmonary embolism: prospective detection on routine helical CT scans. Radiology. 1998;208(1):209–215. doi: 10.1148/radiology.208.1.9646815. [DOI] [PubMed] [Google Scholar]
- 345.O’Connell CL, Boswell WD, Duddalwar V, et al. Unsuspected pulmonary emboli in cancer patients: clinical correlates and relevance. J Clin Oncol. 2006;24(30):4928–4932. doi: 10.1200/JCO.2006.06.5870. [DOI] [PubMed] [Google Scholar]
- 346.Sebastian AJ, Paddon AJ. Clinically unsuspected pulmonary embolism—an important secondary finding in oncology CT. Clin Radiol. 2006;61(1):81–85. doi: 10.1016/j.crad.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 347.Storto ML, Di Credico A, Guido F, Larici AR, Bonomo L. Incidental detection of pulmonary emboli on routine MDCT of the chest. AJR Am J Roentgenol. 2005;184(1):264–267. doi: 10.2214/ajr.184.1.01840264. [DOI] [PubMed] [Google Scholar]
- 348.Douma RA, Kok MG, Verberne LM, Kamphuisen PW, Büller HR. Incidental venous thromboembolism in cancer patients: prevalence and consequence. Thromb Res. 2010;125(6):e306–e309. doi: 10.1016/j.thromres.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 349.Farrell C, Jones M, Girvin F, Ritchie G, Murchison JT. Unsuspected pulmonary embolism identified using multidetector computed tomography in hospital outpatients. Clin Radiol. 2010;65(1):1–5. doi: 10.1016/j.crad.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 350.Hui GC, Legasto A, Wittram C. The prevalence of symptomatic and coincidental pulmonary embolism on computed tomography. J Comput Assist Tomogr. 2008;32(5):783–787. doi: 10.1097/RCT.0b013e31815a7aea. [DOI] [PubMed] [Google Scholar]
- 351.den Exter PL, Hooijer J, Dekkers OM, Huisman MV. Risk of recurrent venous thromboembolism and mortality in patients with cancer incidentally diagnosed with pulmonary embolism: a comparison with symptomatic patients. J Clin Oncol. 2011;29(17):2405–2409. doi: 10.1200/JCO.2010.34.0984. [DOI] [PubMed] [Google Scholar]
- 352.Dentali F, Ageno W, Pierfranceschi MG, et al. Prognostic relevance of an asymptomatic venous thromboembolism in patients with cancer. J Thromb Haemost. 2011;9(5):1081–1083. doi: 10.1111/j.1538-7836.2011.04259.x. [DOI] [PubMed] [Google Scholar]
- 353.O’Connell C, Razavi P, Ghalichi M, et al. Unsuspected pulmonary emboli adversely impact survival in patients with cancer undergoing routine staging multi-row detector computed tomography scanning. J Thromb Haemost. 2011;9(2):305–311. doi: 10.1111/j.1538-7836.2010.04114.x. [DOI] [PubMed] [Google Scholar]
- 354.Becattini C, Agnelli G, Pesavento R, et al. Incidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary embolism. Chest. 2006;130(1):172–175. doi: 10.1378/chest.130.1.172. [DOI] [PubMed] [Google Scholar]
- 355.Pengo V, Lensing AWA, Prins MH, et al. Thromboembolic Pulmonary Hypertension Study Group Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350(22):2257–2264. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 356.Ribeiro A, Lindmarker P, Johnsson H, Juhlin-Dannfelt A, Jorfeldt L. Pulmonary embolism: one-year follow-up with echocardiography Doppler and five-year survival analysis. Circulation. 1999;99(10):1325–1330. doi: 10.1161/01.cir.99.10.1325. [DOI] [PubMed] [Google Scholar]
- 357.Dentali F, Donadini M, Gianni M, et al. Incidence of chronic pulmonary hypertension in patients with previous pulmonary embolism. Thromb Res. 2009;124(3):256–258. doi: 10.1016/j.thromres.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 358.Kline JA, Steuerwald MT, Marchick MR, Hernandez-Nino J, Rose GA. Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest. 2009;136(5):1202–1210. doi: 10.1378/chest.08-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Hoeper MM, Mayer E, Simonneau G, Rubin LJ. Chronic thromboembolic pulmonary hypertension. Circulation. 2006;113(16):2011–2020. doi: 10.1161/CIRCULATIONAHA.105.602565. [DOI] [PubMed] [Google Scholar]
- 360.Jamieson SW, Kapelanski DP, Sakakibara N, et al. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg. 2003;76(5):1457–.. doi: 10.1016/s0003-4975(03)00828-2. [DOI] [PubMed] [Google Scholar]
- 361.Fedullo PF, Auger WR, Kerr KM, Rubin LJ. Chronic thromboembolic pulmonary hypertension. N Engl J Med. 2001;345(20):1465–1472. doi: 10.1056/NEJMra010902. [DOI] [PubMed] [Google Scholar]
- 362.Bernard J, Yi ES. Pulmonary thromboendarterectomy: a clinicopathologic study of 200 consecutive pulmonary thromboendarterectomy cases in one institution. Hum Pathol. 2007;38(6):871–877. doi: 10.1016/j.humpath.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 363.Thistlethwaite PA, Kaneko K, Madani MM, Jamieson SW. Technique and outcomes of pulmonary endarterectomy surgery. Ann Thorac Cardiovasc Surg. 2008;14(5):274–282. [PubMed] [Google Scholar]
- 364.Doyle RL, McCrory D, Channick RN, Simonneau G, Conte J. American College of Chest Physicians Surgical treatments/interventions for pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1) suppl:63S–71S. doi: 10.1378/chest.126.1_suppl.63S. [DOI] [PubMed] [Google Scholar]
- 365.Mehta S, Helmersen D, Provencher S, et al. for the Canadian Thoracic Society Pulmonary Vascular Disease - CTEPH CPG Development Committee and the Canadian Thoracic Society Canadian Respiratory Guidelines Committee Diagnostic evaluation and management of chronic thromboembolic pulmonary hypertension: A clinical practice guideline. Can Respir J. 2010;17(6):301–334. doi: 10.1155/2010/704258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141(3):702–710. doi: 10.1016/j.jtcvs.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 367.Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH). Results from an international prospective registry. Circulation. 124(18):1973–1981. doi: 10.1161/CIRCULATIONAHA.110.015008. [DOI] [PubMed] [Google Scholar]
- 368.Feinstein JA, Goldhaber SZ, Lock JE, Ferndandes SM, Landzberg MJ. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation. 2001;103(1):10–13. doi: 10.1161/01.cir.103.1.10. [DOI] [PubMed] [Google Scholar]
- 369.Bresser P, Pepke-Zaba J, Jaïs X, Humbert M, Hoeper MM. Medical therapies for chronic thromboembolic pulmonary hypertension: an evolving treatment paradigm. Proc Am Thorac Soc. 2006;3(7):594–600. doi: 10.1513/pats.200605-115LR. [DOI] [PubMed] [Google Scholar]
- 370.Becattini C, Manina G, Busti C, Gennarini S, Agnelli G. Bosentan for chronic thromboembolic pulmonary hypertension: findings from a systematic review and meta-analysis. Thromb Res. 2010;126(1):e51–e56. doi: 10.1016/j.thromres.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 371.Decousus H, Epinat M, Guillot K, Quenet S, Boissier C, Tardy B. Superficial vein thrombosis: risk factors, diagnosis, and treatment. Curr Opin Pulm Med. 2003;9(5):393–397. doi: 10.1097/00063198-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 372.Wichers IM, Di Nisio M, Büller HR, Middeldorp S. Treatment of superficial vein thrombosis to prevent deep vein thrombosis and pulmonary embolism: a systematic review. Haematologica. 2005;90(5):672–677. [PubMed] [Google Scholar]
- 373.Quenet S, Laporte S, Décousus H, Leizorovicz A, Epinat M, Mismetti P. STENOX Group Factors predictive of venous thrombotic complications in patients with isolated superficial vein thrombosis. J Vasc Surg. 2003;38(5):944–949. doi: 10.1016/s0741-5214(03)00607-4. [DOI] [PubMed] [Google Scholar]
- 374.Decousus H, Quéré I, Presles E, et al. POST (Prospective Observational Superficial Thrombophlebitis) Study Group Superficial venous thrombosis and venous thromboembolism: a large, prospective epidemiologic study. Ann Intern Med. 2010;152(4):218–224. doi: 10.7326/0003-4819-152-4-201002160-00006. [DOI] [PubMed] [Google Scholar]
- 375.Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev. 2007;(2):CD004982. doi: 10.1002/14651858.CD004982.pub3. [DOI] [PubMed] [Google Scholar]
- 376.Superficial Thrombophlebitis Treated By Enoxaparin Study Group A pilot randomized double-blind comparison of a low-molecular-weight heparin, a nonsteroidal anti-inflammatory agent, and placebo in the treatment of superficial vein thrombosis. Arch Intern Med. 2003;163(14):1657–1663. doi: 10.1001/archinte.163.14.1657. [DOI] [PubMed] [Google Scholar]
- 377.Titon JP, Auger D, Grange P, et al. [Therapeutic management of superficial venous thrombosis with calcium nadroparin. Dosage testing and comparison with a non-steroidal anti-inflammatory agent] Ann Cardiol Angeiol (Paris) 1994;43(3):160–166. [PubMed] [Google Scholar]
- 378.Prandoni P, Tormene D, Pesavento R. Vesalio Investigators Group High vs. low doses of low-molecular-weight heparin for the treatment of superficial vein thrombosis of the legs: a double-blind, randomized trial. J Thromb Haemost. 2005;3(6):1152–1157. doi: 10.1111/j.1538-7836.2005.01391.x. [DOI] [PubMed] [Google Scholar]
- 379.Marchiori A, Verlato F, Sabbion P, et al. High versus low doses of unfractionated heparin for the treatment of superficial thrombophlebitis of the leg. A prospective, controlled, randomized study. Haematologica. 2002;87(5):523–527. [PubMed] [Google Scholar]
- 380.Belcaro G, Nicolaides AN, Errichi BM, et al. Superficial thrombophlebitis of the legs: a randomized, controlled, follow-up study. Angiology. 1999;50(7):523–529. doi: 10.1177/000331979905000701. [DOI] [PubMed] [Google Scholar]
- 381.Andreozzi GM, Signorelli S, Di Pino L, et al. Tolerability and clinical efficacy of desmin in the treatment of superficial thrombovaricophlebitis. Angiology. 1996;47(9):887–894. doi: 10.1177/000331979604700907. [DOI] [PubMed] [Google Scholar]
- 382.Decousus H, Prandoni P, Mismetti P, et al. CALISTO Study Group Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med. 2010;363(13):1222–1232. doi: 10.1056/NEJMoa0912072. [DOI] [PubMed] [Google Scholar]
- 383.Blondon M, Righini M, Bounameaux H, et al. Fondaparinux for isolated superficial-vein thrombosis of the legs: a cost-effectiveness analysis. Chest. doi: 10.1378/chest.11-0625. In press. 10.1378/chest.11-0625. [DOI] [PubMed] [Google Scholar]
- 384.Lozano FS, Almazan A. Low-molecular-weight heparin versus saphenofemoral disconnection for the treatment of above-knee greater saphenous thrombophlebitis: a prospective study. Vasc Endovascular Surg. 2003;37(6):415–420. doi: 10.1177/153857440303700605. [DOI] [PubMed] [Google Scholar]
- 385.Sullivan V, Denk PM, Sonnad SS, Eagleton MJ, Wakefield TW. Ligation versus anticoagulation: treatment of above-knee superficial thrombophlebitis not involving the deep venous system. J Am Coll Surg. 2001;193(5):556–562. doi: 10.1016/s1072-7515(01)01043-2. [DOI] [PubMed] [Google Scholar]
- 386.Flinterman LE, Van Der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost. 2008;6(8):1262–1266. doi: 10.1111/j.1538-7836.2008.03017.x. [DOI] [PubMed] [Google Scholar]
- 387.Lechner D, Wiener C, Weltermann A, Eischer L, Eichinger S, Kyrle PA. Comparison between idiopathic deep vein thrombosis of the upper and lower extremity regarding risk factors and recurrence. J Thromb Haemost. 2008;6(8):1269–1274. doi: 10.1111/j.1538-7836.2008.02998.x. [DOI] [PubMed] [Google Scholar]
- 388.Spencer FA, Emery C, Lessard D, Goldberg RJ. Worcester Venous Thromboembolism Study Upper extremity deep vein thrombosis: a community-based perspective. Am J Med. 2007;120(8):678–684. doi: 10.1016/j.amjmed.2006.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Owens CA, Bui JT, Knuttinen MG, Gaba RC, Carrillo TC. Pulmonary embolism from upper extremity deep vein thrombosis and the role of superior vena cava filters: a review of the literature. J Vasc Interv Radiol. 2010;21(6):779–787. doi: 10.1016/j.jvir.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 390.Mai C, Hunt D. Upper-extremity deep venous thrombosis: a review. Am J Med. 2011;124(5):402–407. doi: 10.1016/j.amjmed.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 391.Becker DM, Philbrick JT, Walker FB., IV Axillary and subclavian venous thrombosis. Prognosis and treatment. Arch Intern Med. 1991;151(10):1934–1943. [PubMed] [Google Scholar]
- 392.Joffe HV, Kucher N, Tapson VF, Goldhaber SZ. Deep Vein Thrombosis (DVT) FREE Steering Committee Upper-extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110(12):1605–1611. doi: 10.1161/01.CIR.0000142289.94369.D7. [DOI] [PubMed] [Google Scholar]
- 393.Prandoni P, Polistena P, Bernardi E, et al. Upper-extremity deep vein thrombosis. Risk factors, diagnosis, and complications. Arch Intern Med. 1997;157(1):57–62. [PubMed] [Google Scholar]
- 394.Kucher N. Clinical practice. Deep-vein thrombosis of the upper extremities. N Engl J Med. 2011;364(9):861–869. doi: 10.1056/NEJMcp1008740. [DOI] [PubMed] [Google Scholar]
- 395.Prandoni P, Bernardi E, Marchiori A, et al. The long term clinical course of acute deep vein thrombosis of the arm: prospective cohort study. BMJ. 2004;329(7464):484–485. doi: 10.1136/bmj.38167.684444.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Elman EE, Kahn SR. The post-thrombotic syndrome after upper extremity deep venous thrombosis in adults: a systematic review. Thromb Res. 2006;117(6):609–614. doi: 10.1016/j.thromres.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 397.Baskin JL, Pui CH, Reiss U, et al. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet. 2009;374(9684):159–169. doi: 10.1016/S0140-6736(09)60220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Saber W, Moua T, Williams EC, et al. Risk factors for catheter-related thrombosis (CRT) in cancer patients: a patient-level data (IPD) meta-analysis of clinical trials and prospective studies. J Thromb Haemost. 2011;9(2):312–319. doi: 10.1111/j.1538-7836.2010.04126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Savage KJ, Wells PS, Schulz V, et al. Outpatient use of low molecular weight heparin (Dalteparin) for the treatment of deep vein thrombosis of the upper extremity. Thromb Haemost. 1999;82(3):1008–1010. [PubMed] [Google Scholar]
- 400.Karabay O, Yetkin U, Onol H. Upper extremity deep vein thrombosis: clinical and treatment characteristics. J Int Med Res. 2004;32(4):429–435. doi: 10.1177/147323000403200413. [DOI] [PubMed] [Google Scholar]
- 401.Kovacs MJ, Kahn SR, Rodger M, et al. A pilot study of central venous catheter survival in cancer patients using low-molecular-weight heparin (dalteparin) and warfarin without catheter removal for the treatment of upper extremity deep vein thrombosis (The Catheter Study) J Thromb Haemost. 2007;5(8):1650–1653. doi: 10.1111/j.1538-7836.2007.02613.x. [DOI] [PubMed] [Google Scholar]
- 402.AbuRahma AF, Short YS, White JF, III, Boland JP. Treatment alternatives for axillary-subclavian vein thrombosis: long-term follow-up. Cardiovasc Surg. 1996;4(6):783–787. doi: 10.1016/s0967-2109(96)00025-7. [DOI] [PubMed] [Google Scholar]
- 403.Horne MK, III, Mayo DJ, Cannon RO, III, Chen CC, Shawker TH, Chang R. Intraclot recombinant tissue plasminogen activator in the treatment of deep venous thrombosis of the lower and upper extremities. Am J Med. 2000;108(3):251–255. doi: 10.1016/s0002-9343(99)00407-6. [DOI] [PubMed] [Google Scholar]
- 404.Lee JT, Karwowski JK, Harris EJ, Haukoos JS, Olcott C., IV Long-term thrombotic recurrence after nonoperative management of Paget-Schroetter syndrome. J Vasc Surg. 2006;43(6):1236–1243. doi: 10.1016/j.jvs.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 405.Lokanathan R, Salvian AJ, Chen JC, Morris C, Taylor DC, Hsiang YN. Outcome after thrombolysis and selective thoracic outlet decompression for primary axillary vein thrombosis. J Vasc Surg. 2001;33(4):783–788. doi: 10.1067/mva.2001.112708. [DOI] [PubMed] [Google Scholar]
- 406.Pegis JD, Papon X, Pasco A, Regnard O, Abraham P, Enon B. [In situ thrombolysis in the treatment of venous thrombosis of effort in the arm [in French] J Mal Vasc. 1997;22(3):187–192. [PubMed] [Google Scholar]
- 407.Petrakis IE, Katsamouris A, Kafassis E, D’Anna M, Sciacca V., V Two Different Therapeutic Modalities in the Treatment of the Upper Extremity Deep Vein Thrombosis: Preliminary Investigation With 20 Case Reports. Int J Angiol. 2000;9(1):46–50. doi: 10.1007/BF01616331. [DOI] [PubMed] [Google Scholar]
- 408.Sabeti S, Schillinger M, Mlekusch W, Haumer M, Ahmadi R, Minar E. Treatment of subclavian-axillary vein thrombosis: long-term outcome of anticoagulation versus systemic thrombolysis. Thromb Res. 2002;108(5-6):279–285. doi: 10.1016/s0049-3848(03)00093-8. [DOI] [PubMed] [Google Scholar]
- 409.Schindler J, Bona RD, Chen HH, et al. Regional thrombolysis with urokinase for central venous catheter-related thrombosis in patients undergoing high-dose chemotherapy with autologous blood stem cell rescue. Clin Appl Thromb Hemost. 1999;5(1):25–29. doi: 10.1177/107602969900500106. [DOI] [PubMed] [Google Scholar]
- 410.Kahn SR, Elman EA, Bornais C, Blostein M, Wells PS. Post-thrombotic syndrome, functional disability and quality of life after upper extremity deep venous thrombosis in adults. Thromb Haemost. 2005;93(3):499–502. doi: 10.1160/TH04-10-0640. [DOI] [PubMed] [Google Scholar]
- 411.Machleder HI. Evaluation of a new treatment strategy for Paget-Schroetter syndrome: spontaneous thrombosis of the axillary-subclavian vein. J Vasc Surg. 1993;17(2):305–315. doi: 10.1016/0741-5214(93)90416-j. [DOI] [PubMed] [Google Scholar]
- 412.Schneider DB, Dimuzio PJ, Martin ND, et al. Combination treatment of venous thoracic outlet syndrome: open surgical decompression and intraoperative angioplasty. J Vasc Surg. 2004;40(4):599–603. doi: 10.1016/j.jvs.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 413.Spence LD, Gironta MG, Malde HM, Mickolick CT, Geisinger MA, Dolmatch BL. Acute upper extremity deep venous thrombosis: safety and effectiveness of superior vena caval filters. Radiology. 1999;210(1):53–58. doi: 10.1148/radiology.210.1.r99ja1353. [DOI] [PubMed] [Google Scholar]
- 414.Feugier P, Aleksic I, Salari R, Durand X, Chevalier JM. Long-term results of venous revascularization for Paget-Schroetter syndrome in athletes. Ann Vasc Surg. 2001;15(2):212–218. doi: 10.1007/s100160010043. [DOI] [PubMed] [Google Scholar]
- 415.Lee MC, Grassi CJ, Belkin M, Mannick JA, Whittemore AD, Donaldson MC. Early operative intervention after thrombolytic therapy for primary subclavian vein thrombosis: an effective treatment approach. J Vasc Surg. 1998;27(6):1101–1107. doi: 10.1016/s0741-5214(98)70012-6. [DOI] [PubMed] [Google Scholar]
- 416.Malcynski J, O’Donnell TF, Jr, Mackey WC, Millan VA. Long-term results of treatment for axillary subclavian vein thrombosis. Can J Surg. 1993;36(4):365–371. [PubMed] [Google Scholar]
- 417.Meier GH, Pollak JS, Rosenblatt M, Dickey KW, Gusberg RJ. Initial experience with venous stents in exertional axillary-subclavian vein thrombosis. J Vasc Surg. 1996;24(6):974–981. doi: 10.1016/s0741-5214(96)70043-5. [DOI] [PubMed] [Google Scholar]
- 418.Sanders RJ, Cooper MA. Surgical management of subclavian vein obstruction, including six cases of subclavian vein bypass. Surgery. 1995;118(5):856–863. doi: 10.1016/s0039-6060(05)80276-4. [DOI] [PubMed] [Google Scholar]
- 419.Sheeran SR, Hallisey MJ, Murphy TP, Faberman RS, Sherman S. Local thrombolytic therapy as part of a multidisciplinary approach to acute axillosubclavian vein thrombosis (Paget-Schroetter syndrome) J Vasc Interv Radiol. 1997;8(2):253–260. doi: 10.1016/s1051-0443(97)70551-7. [DOI] [PubMed] [Google Scholar]
- 420.Urschel HC, Jr, Patel AN. Paget-Schroetter syndrome therapy: failure of intravenous stents. Ann Thorac Surg. 2003;75(6):1693–1696. doi: 10.1016/s0003-4975(03)00116-4. [DOI] [PubMed] [Google Scholar]
- 421.Yilmaz EN, Vahl AC, van Heek NT, Vermeulen EGJ, Rauwerda JA. Long-term results of local thrombolysis followed by first rib resection: an encouraging clinical experience in treatment of subclavian vein thrombosis. Vasc Surg. 2000;34(1):17–23. [Google Scholar]
- 422.Usoh F, Hingorani A, Ascher E, et al. Long-term follow-up for superior vena cava filter placement. Ann Vasc Surg. 2009;23(3):350–354. doi: 10.1016/j.avsg.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 423.Martinelli I, Battaglioli T, Bucciarelli P, Passamonti SM, Mannucci PM. Risk factors and recurrence rate of primary deep vein thrombosis of the upper extremities. Circulation. 2004;110(5):566–570. doi: 10.1161/01.CIR.0000137123.55051.9B. [DOI] [PubMed] [Google Scholar]
- 424.Berzaczy D, Popovic M, Reiter M, et al. Quality of life in patients with idiopathic subclavian vein thrombosis. Thromb Res. 2010;125(1):25–28. doi: 10.1016/j.thromres.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 425.Dentali F, Ageno W, Witt D, et al. WARPED consortium Natural history of mesenteric venous thrombosis in patients treated with vitamin K antagonists: a multi-centre, retrospective cohort study. Thromb Haemost. 2009;102(3):501–504. doi: 10.1160/TH08-12-0842. [DOI] [PubMed] [Google Scholar]
- 426.Kumar S, Sarr MG, Kamath PS. Mesenteric venous thrombosis. N Engl J Med. 2001;345(23):1683–1688. doi: 10.1056/NEJMra010076. [DOI] [PubMed] [Google Scholar]
- 427.Martinelli I, Franchini M, Mannucci PM. How I treat rare venous thromboses. Blood. 2008;112(13):4818–4823. doi: 10.1182/blood-2008-07-165969. [DOI] [PubMed] [Google Scholar]
- 428.Plessier A, Darwish-Murad S, Hernandez-Guerra M, et al. European Network for Vascular Disorders of the Liver (EN-Vie) Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology. 2010;51(1):210–218. doi: 10.1002/hep.23259. [DOI] [PubMed] [Google Scholar]
- 429.Amitrano L, Guardascione MA, Scaglione M, et al. Prognostic factors in noncirrhotic patients with splanchnic vein thromboses. Am J Gastroenterol. 2007;102(11):2464–2470. doi: 10.1111/j.1572-0241.2007.01477.x. [DOI] [PubMed] [Google Scholar]
- 430.Condat B, Pessione F, Hillaire S, et al. Current outcome of portal vein thrombosis in adults: risk and benefit of anticoagulant therapy. Gastroenterology. 2001;120(2):490–497. doi: 10.1053/gast.2001.21209. [DOI] [PubMed] [Google Scholar]
- 431.Amitrano L, Guardascione MA, Menchise A, et al. Safety and efficacy of anticoagulation therapy with low molecular weight heparin for portal vein thrombosis in patients with liver cirrhosis. J Clin Gastroenterol. 2010;44(6):448–451. doi: 10.1097/MCG.0b013e3181b3ab44. [DOI] [PubMed] [Google Scholar]
- 432.Darwish Murad S, Plessier A, Hernandez-Guerra M, et al. EN-Vie (European Network for Vascular Disorders of the Liver) Etiology, management, and outcome of the Budd-Chiari syndrome. Ann Intern Med. 2009;151(3):167–175. doi: 10.7326/0003-4819-151-3-200908040-00004. [DOI] [PubMed] [Google Scholar]
- 433.Darwish Murad S, Valla DC, de Groen PC, et al. Determinants of survival and the effect of portosystemic shunting in patients with Budd-Chiari syndrome. Hepatology. 2004;39(2):500–508. doi: 10.1002/hep.20064. [DOI] [PubMed] [Google Scholar]
- 434.Mohiuddin SM, Hilleman DE, Destache CJ, Stoysich AM, Gannon JM, Sketch MH., Sr Efficacy and safety of early versus late initiation of warfarin during heparin therapy in acute thromboembolism. Am Heart J. 1992;123(3):729–732. doi: 10.1016/0002-8703(92)90513-u. [DOI] [PubMed] [Google Scholar]
- 435.van Dongen CJJ, van den Belt AG, Prins MH, Lensing AW. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev. 2004;(4):CD001100. doi: 10.1002/14651858.CD001100.pub2. [DOI] [PubMed] [Google Scholar]
- 436.Fiessinger JN, Lopez-Fernandez M, Gatterer E, et al. Once-daily subcutaneous dalteparin, a low molecular weight heparin, for the initial treatment of acute deep vein thrombosis. Thromb Haemost. 1996;76(2):195–199. [PubMed] [Google Scholar]
- 437.Harenberg J, Schmidt JA, Koppenhagen K, Tolle A, Huisman MV, Büller HR. EASTERN Investigators Fixed-dose, body weight-independent subcutaneous LMW heparin versus adjusted dose unfractionated intravenous heparin in the initial treatment of proximal venous thrombosis. Thromb Haemost. 2000;83(5):652–656. [PubMed] [Google Scholar]
- 438.Lindmarker P, Holmström M, Granqvist S, Johnsson H, Lockner D. Comparison of once-daily subcutaneous Fragmin with continuous intravenous unfractionated heparin in the treatment of deep vein thrombosis. Thromb Haemost. 1994;72(2):186–190. [PubMed] [Google Scholar]
- 439.Prandoni P, Lensing AWA, Büller HR, et al. Comparison of subcutaneous low-molecular-weight heparin with intravenous standard heparin in proximal deep-vein thrombosis. Lancet. 1992;339(8791):441–445. doi: 10.1016/0140-6736(92)91054-c. [DOI] [PubMed] [Google Scholar]
- 440.Riess H, Koppenhagen K, Tolle A, et al. TH-4 Study Group Fixed-dose, body weight-independent subcutaneous low molecular weight heparin Certoparin compared with adjusted-dose intravenous unfractionated heparin in patients with proximal deep venous thrombosis. Thromb Haemost. 2003;90(2):252–259. doi: 10.1160/TH02-09-0061. [DOI] [PubMed] [Google Scholar]
- 441.Simonneau G, Charbonnier B, Decousus H, et al. Subcutaneous low-molecular-weight heparin compared with continuous intravenous unfractionated heparin in the treatment of proximal deep vein thrombosis. Arch Intern Med. 1993;153(13):1541–1546. [PubMed] [Google Scholar]
- 442.Othieno R, Abu Affan M, Okpo E. Home versus in-patient treatment for deep vein thrombosis. Cochrane Database Syst Rev. 2007;(3):CD003076. doi: 10.1002/14651858.CD003076.pub2. [DOI] [PubMed] [Google Scholar]
- 443.PREPIC Study Group Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation. 2005;112(3):416–422. doi: 10.1161/CIRCULATIONAHA.104.512834. [DOI] [PubMed] [Google Scholar]
- 444.Aschwanden M, Labs KH, Engel H, et al. Acute deep vein thrombosis: early mobilization does not increase the frequency of pulmonary embolism. Thromb Haemost. 2001;85(1):42–46. [PubMed] [Google Scholar]
- 445.Blättler W, Partsch H. Leg compression and ambulation is better than bed rest for the treatment of acute deep venous thrombosis. Int Angiol. 2003;22(4):393–400. [PubMed] [Google Scholar]
- 446.Jünger M, Diehm C, Störiko H, et al. Mobilization versus immobilization in the treatment of acute proximal deep venous thrombosis: a prospective, randomized, open, multicentre trial. Curr Med Res Opin. 2006;22(3):593–602. doi: 10.1185/030079906X89838. [DOI] [PubMed] [Google Scholar]
- 447.Partsch H, Blättler W. Compression and walking versus bed rest in the treatment of proximal deep venous thrombosis with low molecular weight heparin. J Vasc Surg. 2000;32(5):861–869. doi: 10.1067/mva.2000.110352. [DOI] [PubMed] [Google Scholar]
- 448.Schellong SM, Schwarz T, Kropp J, Prescher Y, Beuthien-Baumann B, Daniel WG. Bed rest in deep vein thrombosis and the incidence of scintigraphic pulmonary embolism. Thromb Haemost. 1999;82(suppl 1):127–129. [PubMed] [Google Scholar]
- 449.Hull RD, Pineo GF, Brant RF, et al. LITE Trial Investigators Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119(12):1062–1072. doi: 10.1016/j.amjmed.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 450.Kakkar VV, Gebska M, Kadziola Z, Saba N, Carrasco P. Bemiparin Investigators Low-molecular-weight heparin in the acute and long-term treatment of deep vein thrombosis. Thromb Haemost. 2003;89(4):674–680. [PubMed] [Google Scholar]
- 451.Kolbach DN, Sandbrink MW, Neumann HA, Prins MH. Compression therapy for treating stage I and II (Widmer) post-thrombotic syndrome. Cochrane Database Syst Rev. 2003;(4):CD004177. doi: 10.1002/14651858.CD004177. [DOI] [PubMed] [Google Scholar]
- 452.Belcaro G, Laurora G, Cesarone MR, De Sanctis MT. Prophylaxis of recurrent deep venous thrombosis. A randomized, prospective study using indobufen and graduated elastic compression stockings. Angiology. 1993;44(9):695–699. doi: 10.1177/000331979304400904. [DOI] [PubMed] [Google Scholar]
- 453.Arpaia G, Cimminiello C, Mastrogiacomo O, de Gaudenzi E. Efficacy of elastic compression stockings used early or after resolution of the edema on recanalization after deep venous thrombosis: the COM.PRE Trial. Blood Coagul Fibrinolysis. 2007;18(2):131–137. doi: 10.1097/MBC.0b013e328011f2dd. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.