Abstract
Background:
To develop the Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: ACCP Evidence-Based Clinical Practice Guidelines (AT9), the American College of Chest Physicians (ACCP) assembled a panel of clinical experts, information scientists, decision scientists, and systematic review and guideline methodologists.
Methods:
Clinical areas were designated as articles, and a methodologist without important intellectual or financial conflicts of interest led a panel for each article. Only panel members without significant conflicts of interest participated in making recommendations. Panelists specified the population, intervention and alternative, and outcomes for each clinical question and defined criteria for eligible studies. Panelists and an independent evidence-based practice center executed systematic searches for relevant studies and evaluated the evidence, and where resources and evidence permitted, they created standardized tables that present the quality of the evidence and key results in a transparent fashion.
Results:
One or more recommendations relate to each specific clinical question, and each recommendation is clearly linked to the underlying body of evidence. Judgments regarding the quality of evidence and strength of recommendations were based on approaches developed by the Grades of Recommendations, Assessment, Development, and Evaluation Working Group. Panel members constructed scenarios describing relevant health states and rated the disutility associated with these states based on an additional systematic review of evidence regarding patient values and preferences for antithrombotic therapy. These ratings guided value and preference decisions underlying the recommendations. Each topic panel identified questions in which resource allocation issues were particularly important and, for these issues, experts in economic analysis provided additional searches and guidance.
Conclusions:
AT9 methodology reflects the current science of evidence-based clinical practice guideline development, with reliance on high-quality systematic reviews, a standardized process for quality assessment of individual studies and the body of evidence, an explicit process for translating the evidence into recommendations, disclosure of financial as well as intellectual conflicts of interest followed by management of disclosed conflicts, and extensive peer review.
This article describes the methodology used for the Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (AT9). This methodology incorporates current evidence-based approaches to the appraisal and synthesis of evidence and to the formulation of clinical practice recommendations. The process thus ensures explicit, transparent, evidence-based clinical practice guidelines.
The objective of AT9 is to optimize patient-important health outcomes and the processes of care for patients who have experienced or are at risk for thrombotic events. The targeted users of these guidelines are health-care providers in both primary and specialty care who assist patients in making treatment choices that optimize benefits, minimize harms and burdens, and are consistent with patient values and preferences.
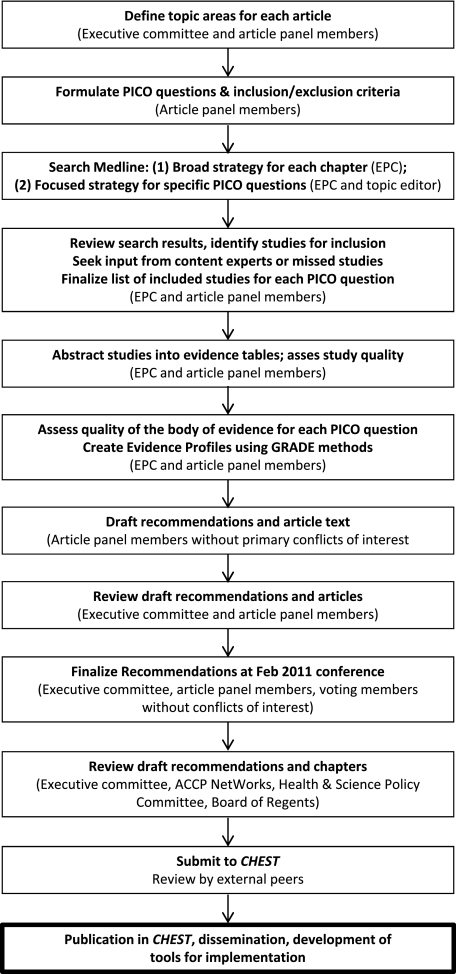
Figure 1 summarizes the process for the development of the AT9 recommendations. The primary responsibility for AT9 rests with the American College of Chest Physicians (ACCP) AT9 Executive Committee. This committee includes two methodologist-clinicians from the previous iteration, Antithrombotic and Thrombolytic Therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) (AT8), published in 20081 (Dr Guyatt, Panel Chair, and Dr Schünemann, Vice Chair of Methodology); a third methodologist-clinician (Dr Akl); a leading thrombosis expert (Dr Crowther, Vice Chair for Thrombosis); and two liaisons with the ACCP Health and Science Policy (HSP) Committee who had also served on the previous guideline Executive Committee (Dr Gutterman, Vice Chair and HSP Liaison, and Dr Lewis, Project Manager).
Figure 1.
The steps in the process of development of the Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. The Executive Committee, comprising systematic review and guideline methodologists, clinical experts, and ACCP Health and Science Policy Committee liaisons, coordinated the process. Information within parentheses indicates who performed each step in the process. ACCP = American College of Chest Physicians; EPC = Evidence-Based Practice Center; GRADE = Grades of Recommendations, Assessment, Development, and Evaluation; PICO = population, intervention, comparator, and outcome.
Articles within AT9 are defined by broad populations (eg, pediatric, obstetric) or clinical conditions (eg, prevention of VTE in medical patients or stroke prophylaxis in atrial fibrillation). In addition, AT9 includes three articles addressing the basic science of oral and parenteral anticoagulants and platelet-active drugs and an article addressing new antithrombotic and thrombolytic drugs.
1.0 Composition and Selection of Topic Panel Members
The ACCP AT9 Executive Committee selected panel members for each article. A topic editor and a deputy editor led each of the AT9 panels issuing recommendations. The topic editor was the person primarily responsible for each article and was required to be a methodologist without serious financial or intellectual conflict of interest for any of the article’s recommendations. In all but one case, the topic editor also was a clinician. The Executive Committee chose these individuals on the basis of their previous experience with guideline development and, in particular, their familiarity with methods developed by the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) Working Group.2 These topic editors and all panel members were approved by the ACCP HSP Committee after review of their conflict of interest disclosures (see section 7.0 “Disclosing and Managing Conflicts of Interest”).
Criteria for selection of the remainder of the panel members, including the deputy editor-thrombosis expert, were an established record in the relevant clinical or research area, international and gender representation, and an absence of financial conflicts of interest that were judged unacceptable. Some of the panelists had prior experience on ACCP guidelines in this area and represented the thrombosis community, but there was substantial turnover from the previous edition. After an international request for applications broadcast through multiple medical societies, the Executive Committee nominated individual topic editors and deputy editors and collaborated with them to identify and nominate other topic panel members.
The ACCP HSP Committee reviewed all nominees and approved all panel members after review of their curricula vitae and conflict of interest disclosures. Of 150 nominees, 137 were approved, 18 were approved with management of conflicts of interest (ie, regular disclosures and review of ongoing conflicts as the process progressed), and 13 were disapproved as a result of the magnitude of financial conflicts of interest. Articles associated with recommendations included from seven to 14 panel members. We did not include patients or representatives of specific stakeholder groups on topic panels.
Each topic panel also included a frontline physician working in the relevant area who was neither an expert in thrombosis nor a methodologist or clinical investigator. These individuals were chosen in consultation with the topic editors and the ACCP HSP Committee. These clinicians were charged with the following: (1) proposing important real-world clinical questions on the prevention, diagnosis, and treatment of thrombosis that were not addressed in AT8 and (2) reviewing the draft manuscripts and recommendations to assess the usability of the guidelines and the feasibility of implementation of AT9 recommendations.
To address issues of economic efficiency we included six health economist-physicians on the AT9 topic panels charged with making recommendations. These resource consultants were selected and approved through identical procedures to those for topic editors and panel members. We describe their roles more fully later in this discussion.
2.0 Ensuring Consistency Across Articles
We used a number of strategies to ensure consistency across articles, and one of us (M. C.) participated extensively in the formulation of clinical questions for each article. To ensure consistency of judgments regarding bleeding, one of us (S. S.) was responsible for standardizing the approach to bleeding outcomes and participated in multiple topic panels (described in more detail later in this article). Additionally, to ensure consistency in the trade-offs between thrombotic and bleeding events, all articles used the same ratings of values and preferences (also described in more detail later). Because some of the same evidence summaries were relevant to several articles, five individuals were chosen to participate in each of the articles addressing coronary artery disease, stroke, and peripheral arterial disease.
In AT9, prevention of VTE is addressed in three articles as opposed to a single article as was done in AT8. The prevention topic editors and deputy editors and those of the stroke article (which includes thromboprophylaxis recommendations) participated in multiple conference calls to develop and harmonize the approach to prevention and to ensure consistency among final recommendations.3 Topic editors consulted with one another when issues overlapped. For example, the decision regarding the use of a vitamin K antagonist, aspirin, and clopidogrel simultaneously in patients with atrial fibrillation, valvular disease, and intravascular stents is relevant for the atrial fibrillation, coronary, and peripheral arterial disease articles. Topic panels deferred to the Evidence-Based Management of Anticoagulant Therapy AT9 topic panel for recommendations related to the dosing and monitoring of anticoagulation therapies.
The AT9 Executive Committee met at least once a month and regularly issued statements of clarification of methods to topic editors and deputy editors (eg, use of fixed- or random-effects models for meta-analysis), conflict of interest, preparation of tables, and issues of style and presentation. All these statements were communicated directly to the topic editors and deputy editors and made available in a central repository accessible to all AT9 panelists. The chair of the Executive Committee (G. H. G.) was available for resolving any challenging issues related to the aforementioned topics. Between September 2009 and September 2010, two members of the Executive Committee (E. A. A. and S. Z. L.) held regular (every 3 months), separate conference calls with each topic editor and deputy editor during which they addressed questions and concerns. Finally, the chair of the Executive Committee reviewed every article to ensure consistency of evidence presentation, evaluation, and writing style.
In terms of writing style, we used consistent language to describe effects that did not reach statistical significance. The approach was as follows:
For adverse outcomes (such as thrombosis and bleeding), “A is associated with a trend toward reduced thrombosis” if the lower boundary of the CI around a relative effect is ≤ 0.7 and the upper boundary of the CI is ≤ 1.1 or if the lower boundary of the CI is ≤ 0.8 and the upper boundary of the CI is ≤ 1.05. If the point estimate is > 1.0, the language used was, “A is associated with a trend toward increased bleeding” if the lower boundary of the CI around a relative effect is > 0.9 and the upper boundary of the CI is > 1.3 or if the lower boundary of the CI is > 0.95 and the upper boundary of the CI is > 1.2.
“A appears to have little or no effect on thrombosis” if the above conditions are not met, and the boundaries of the CI lie between 0.80 and 1.2.
For all other results that fail to exclude a relative risk of 1.0, the language was, “Results failed to demonstrate or exclude a beneficial effect or detrimental effect of A on thrombosis.” Alternative wording with regard to an association is “failed to establish or refute.”
3.0 Evidence Review
3.1 Defining the Clinical Questions—Population, Intervention, Comparator, and Outcome
The thrombosis expert on the Executive Committee (M. C.) along with the deputy editors took primary responsibility for defining the scope of the clinical questions that each article would address. For each question, the topic editor and deputy editor defined the relevant population, alternative management strategies (intervention and comparator), and the outcomes (ie, population, intervention, comparator, and outcome [PICO] format). Each clinical question provided the framework for formulating study inclusion and exclusion criteria and guided the search for relevant evidence (systematic reviews and original studies). Panels typically restricted included studies to randomized controlled trials (RCTs) for intervention questions but included observational studies when there was a paucity of RCT data addressing an intervention and for questions of risk assessment. Readers can find these PICO questions in the first table of each article. One or more recommendations could be formulated for each clinical question. The next subsections (3.2-3.5) deal with the approach to selection of outcomes.
3.2 Patient-Important and Surrogate Outcomes
The outcomes for each clinical question were chosen by the topic editors and their panel members and were generally consistent across articles. Outcomes were restricted to those of importance to patients.4 Panels considered the burden of anticoagulation therapy as a patient-important outcome when its consideration could tip the balance of benefits and harms. If we found no data for an outcome considered at the outset as patient-important, we nevertheless included uncertainty about the effects of the intervention on that outcome when weighing its benefits and harms.
In the absence of data on patient-important outcomes, surrogates could contribute to the estimation of the effect of an intervention on the outcomes that are important. Examples of surrogate outcomes include asymptomatic venous thrombosis detected by venographic or ultrasound surveillance and the percentage of time that an international normalized ratio was in therapeutic range (used as a surrogate for bleeding and thrombosis in the assessment of the effectiveness of centralized anticoagulation services).
The issue of asymptomatic thrombosis detected by venographic or ultrasound surveillance presented particular challenges to the articles addressing VTE prevention in orthopedic and nonorthopedic surgery populations, an article addressing nonsurgical prophylaxis, and an article addressing stroke prevention. We were explicit in considering the trade-offs between VTE and bleeding events. An article by Guyatt et al3 in this supplement addresses these issues in some detail.
3.3 Mortality
Options considered in summarizing mortal events were (1) all-cause mortality and (2) mortality related to antithrombotic therapy (ie, deaths from pulmonary emboli and deaths from bleeding). Advantages of the former include its being the most patient-important outcome and the difficulty of ascertaining cause of death. Difficulties ascertaining cause of death may be particularly problematic when adjudication is unblinded and therefore open to bias.
The disadvantage of all-cause mortality is that the signal from antithrombotic therapy-related deaths may be lost in noise from deaths due to other causes. The decision about which mortal outcome to use (all-cause mortality or antithrombotic therapy-related morality) was left to the authors of individual articles. Availability of data sometimes forced the choice of less satisfactory mortal outcomes (eg, if death related to pulmonary embolus but not death related to bleeding was reported). When mortality was one of the selected outcomes, we avoided double-counting by documenting nonfatal events for the remaining outcomes (eg, nonfatal thrombosis, nonfatal major bleeding) rather than all such events (fatal and nonfatal).
3.4 Composite End Points
Many of the primary studies we reviewed, particularly in the cardiovascular area, presented evidence in the form of composite end points.5 Particularly when the patient importance of the component end points and the magnitude of effect of the intervention on the components differ, composite end points can be misleading.6,7 Therefore, we present results and base inferences on the effect of interventions on individual outcomes.
3.5 Bleeding
In view of the wide variation in how bleeding was assessed and reported in the included primary studies across chapters, one individual (S. S.) was responsible for standardizing the approach to bleeding outcomes. He worked closely with the Executive Committee and the topic editors and deputy editors to ensure the uniform application of the approaches that were developed.
We began by specifying the bleeding outcomes that we believe patients consider important. We did not consider minor bleeding as incurring a burden that was important in comparison with symptomatic thromboembolic events.
We reported fatal hemorrhage as well as fatal stroke or pulmonary embolism in treatment-related or all-cause mortality. Likewise, hemorrhagic stroke and ischemic stroke were reported as “stroke.” We specified in footnotes the events attributable to each component.
We avoided any double-counting of events; for example, a fatal hemorrhagic stroke would only be reported under mortality. We also attempted to report more specific and homogeneous bleeding outcomes than “major bleeding,” which includes events with a wide range of patient importance and which investigators have defined in different ways. For example, in Falck-Ytter et al8 in this supplement, bleeding outcomes of primary interest were (1) bleeding requiring reoperation and (2) other major bleeding. Because some authors, particularly in older studies, failed to define subsets of major bleeding, we were not always able to achieve the desired specificity.
3.6 Identifying the Evidence
To identify the relevant evidence, a team of methodologists and medical librarians at the Oregon Health & Science University Evidence-based Practice Center conducted literature searches of Medline, the Cochrane Library, and the Database of Abstracts of Reviews of Effects. For each article, the team conducted a search for systematic reviews and another for original studies encompassing the main populations and interventions for that article. These searches included studies indexed from week 1, January 2005, forward because AT8 searches were carried out up to that date (search strategies are available on request). Many articles supplemented these searches with more-focused searches addressing specific clinical questions. When clinical questions had not been covered in AT8, searches commenced at a date relevant to each intervention.
Titles and abstracts retrieved from bibliographic database searches generally were screened in duplicate, and full-text articles were retrieved for further review. Consensus on whether individual studies fulfilled inclusion criteria was achieved for each study between two reviewers. If consensus could not be achieved, the topic editor and other topic panelists were brought into the discussion. Deputy editors reviewed lists of included studies from the database searches in order to identify any potentially missed studies. Additional studies identified were then retrieved for further evaluation.
Topic panels also searched the same bibliographic databases for systematic reviews addressing each PICO question. The quality of reviews was assessed using principles embodied in prior instruments addressing methodologic quality of systematic reviews,9,10 and wherever possible, current high-quality systematic reviews were used as the source of summary estimates. Reviews were also used to identify additional studies to complement the database searches.
4.0 Assessing Studies and Summarizing Evidence
4.1 Evaluating Risk of Bias in Individual Studies
We developed and applied uniform criteria for evaluating the risk of bias associated with individual RCTs based on the criteria recommended by the Cochrane Collaboration11 (Table 1). Although all authors assessed risk of bias for individual studies, because of resource limitations, we summarized the results of the risk of bias (eg, Table 112) for only a minority of the recommendations. Readers can find these assessments in the online data supplements. For most recommendations for which we did not develop such tables, we developed Evidence Profiles (see Table 213‐15) that typically provide information on the risk of bias in footnotes.
Table 1.
—[Section 4.1] Methodologic Quality of Randomized Trials: Fondaparinux vs No Fondaparinux for the Treatment of Superficial Vein Thrombosis
| Author, Year | Design | Randomization Concealed | Blinding | Analysis | Stopping Early for Benefit |
| Decousus et al12; CALISTO Study Group, 2010 | RCT: randomization sequence generated “using a computer-generated randomization list” | DY: “Through a central telephone system” | Patients: PY | ITT: DY for efficacy outcomes (as-treated analysis for safety outcomes) | No |
| Caregivers: PY | |||||
| Data collectors: PY | |||||
| Adjudicators: DY | Data for primary efficacy assessment available for 98.7% of randomized patients | ||||
| Data analysts: PN |
CALISTO = Comparison of ARIXTRA in Lower Limb Superficial Thrombophlebitis with placebo; DY = definitely yes; ITT = intent to treat; PN = probably no; PY = probably yes; RCT = randomized controlled trial.
Table 2.
—[Section 4.1] Evidence Profile: Question: Should LMWH Rather Than VKA be Used for Long-term Treatment of VTE?a,13-15
| Quality Assessment |
Summary of Findings |
||||||||||
| Participants (Studies), Median Follow-up | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Overall Quality of Evidence | Study Event Rates (%) |
Relative Effect, RR (95% CI) | Anticipated Absolute Effectsb |
||
| With VKA | With LMWH | Risk With VKA | Risk Difference With LMWH (95% CI) | ||||||||
| Overall mortality (critical outcome) | |||||||||||
| 2,496 (7 RCTs), 6 mo |
No serious risk of bias Selective outcome reporting not seriousc |
No serious inconsistency |
No serious indirectness |
Serious imprecision CI includes important benefit and harm |
Undetected |
Moderate due to imprecision |
202/1,231 (16.4) |
204/1,265 (16.1) |
0.96 (0.81-1.13) |
164 deaths per 1,000d |
7 fewer deaths per 1,000 (from 31 fewer to 21 more) |
| Recurrent symptomatic VTE (critical outcome): DVT and PE | |||||||||||
| 2,727 (8 RCTs), 6 mo |
Serious risk of bias |
No serious inconsistency |
No serious indirectness |
No serious imprecision |
Undetected |
Moderate due to risk of bias |
105/1,349 (7.8) |
67/1,378 (4.9) |
0.62 (0.46-0.84) |
No cancer |
|
| No studies were blinded | 30 VTEs per 1,000d |
11 fewer VTE per 1,000 (from 5 fewer to 16 fewer) |
|||||||||
| Nonmetastatic cancer | |||||||||||
| 80 VTEs per 1,000d |
30 fewer VTE per 1,000 (from 13 fewer to 43 fewer) |
||||||||||
| Metastatic cancer | |||||||||||
| 200 VTEs per 1,000d |
76 fewer VTE per 1,000 (from 32 fewer to 108 fewer) |
||||||||||
| Major bleeding (critical outcome) | |||||||||||
| 2,737 (8 RCTs), 6 mo |
No serious risk of bias Lack of blinding not seriouse |
No serious inconsistency |
No serious indirectness |
Serious imprecision CI includes important benefit and harm |
Undetected |
Moderate due to imprecision |
53/1,351 (3.9) |
45/1,386 (3.2) |
0.81 (0.55-1.2) |
No cancer or nonmetastatic cancer |
|
| 20 bleeds per 1,000f |
4 fewer bleeds per 1,000 (from 9 fewer to 4 more) |
||||||||||
| Metastatic cancer | |||||||||||
| 80 bleeds per 1,000f |
15 fewer bleeds per 1,000 (from 36 fewer to 16 more) |
||||||||||
| PTS (important outcome): self-reported leg symptoms and signs | |||||||||||
| 100 (1 RCT), 3 mo | Serious risk of bias Patients and investigators not blinded | No serious inconsistency | Serious indirectness Predictive value from 3 mo to long term uncertain | No serious imprecision | Undetected | Low due to risk of bias and indirectness | 31/44 (70.5) | 34/56 (60.7) | 0.85 (0.77- 0.94) | 200 PTS per 1,000g | 30 fewer per 1,000 (from 12 fewer to 46 fewer) |
LMWH = low-molecular-weight heparin; PTS = postthrombotic syndrome; RCT = randomized control trial; RR = risk ratio; VKA = vitamin K agonist. (Kearon C, unpublished data).h
Limited to LMWH regimens that used ≥ 50% of the acute treatment dose during the extended phase of treatment.
Time frame is 6 mo for all outcomes except PTS, which is 2 y.
One study did not report deaths: borderline decision.
Control event rates from cohort study by Prandoni et al,13 adjusted to 6-mo time frame.
Outcome less subjective: borderline decision.
Control event rates from cohort studies by Prandoni et al13 and Beth et al14 adjusted to 6-mo time frame.
Control event rate comes from observational studies in review by Kahn et al15 adjusted to 2-y time frame. All patients wore pressure stockings.
Meta-analysis is based on RCTs as referenced in the article text. The control event rate for mortality comes from this meta-analysis.
We also developed specific criteria for assessing the risk of bias of observational studies (cohort studies with concurrent controls, cohort studies with historical controls, case-control studies, or case series). Again, these were based on the evidence-based domains recommended by the Cochrane Collaboration for observational studies (eg, Table 316‐18).
Table 3.
—[Section 4.1] Methodologic Quality of Observational Studies: Cohort Studies of the Treatment of Heparin-Induced Thrombocytopenia
| Author/Year | Intervention/Control | Study Design | Intervention/Control Setting Similar | Intervention/Control Time Frame Similar | Adjustment | Effectively Blinded Assessment of Outcomes | Loss to Follow-up | Comments |
| Lubenow et al16/2005 |
Lepirudin: bolus 0.4 mg/kga; 0.15 mg/kg | Cohort with historical controls |
Very |
Not close |
None |
No |
None |
Cases and controls both required positive testing for HIT antibodies. |
| Control: varied (danaparoid, n = 24; phenoprocoumon, n = 21; other, n = 30) | ||||||||
| Lewis et al17/2003 |
Argatroban: 2 μg/kg per minb (no bolus) | Cohort with historical controls |
Somewhat |
Not close |
None |
No |
None |
HIT antibody data for cases were not provided. |
| Control: varied (typically heparin discontinuation and oral anticoagulation) | ||||||||
| Lewis et al18/2001 | Argatroban: 2 μg/kg per minb (no bolus) | Cohort with historical controls | Very | Not close | None | No | None | Sixty-five percent of cases and controls tested positive for HIT antibodies (remainder tested negative or were not tested). |
| Control: varied (typically discontinuation of heparin and oral anticoagulation) |
APTT = activated partial thromboplastin time; HIT = heparin-induced thrombocytopenia.
APTT adjusted to 1.5 to 2.5 times baseline APTT (or the mean laboratory normal range if the baseline APTT was unavailable).
APTT adjusted to 1.5 to 3.0 times baseline APTT.
Studies without internal comparisons were termed “cohort studies without internal controls” if they met the following criteria:
A protocol existed before the date of commencement of data collection.
A definition of inclusion and exclusion criteria was available.
The study reported the number of excluded patients.
The study conducted a standardized follow-up, including description of all of the following: schedule of follow-up, investigation of suspected outcomes, and criteria used to define outcomes.
The study reported all losses to follow-up.
We labeled studies that did not meet these criteria as “case series.” We did not make a distinction between prospective and retrospective studies because although prospective studies may on average be of higher quality, individual prospective studies may have a significant risk of bias and specific retrospective studies may not. For questions related to risk assessment, we evaluated the risk of bias of individual studies using the following criteria: valid outcome assessment, including blinding when appropriate; adjustment for between-group differences; and minimal loss to follow-up.
4.2 Evaluating Quality of Bodies of Evidence
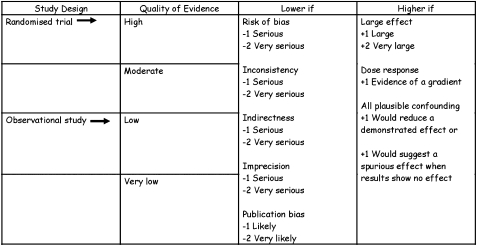
We assessed evidence across studies on an outcome-by-outcome basis using criteria suggested by the GRADE Working Group.19 We defined quality of evidence as our confidence in the estimate of the effect to support a recommendation.19 RCTs start as high-quality evidence and observational studies as low-quality evidence (Fig 2). Additional factors that affect this rating of quality include the risk of bias (as detailed earlier in this article); precision, consistency, and directness of results; likelihood of publication bias; and presence of very large effects.19 The ACCP adaptation of the GRADE system (Table 4) differs only in that the quality of a body of evidence can be high (A), moderate (B), or low (C) (Fig 2); GRADE also provides a category for very-low-quality evidence.
Figure 2.
GRADE approach to rating quality of evidence. See Figure 1 legend for expansion of abbreviation.
Table 4.
—Strength of the Recommendations Grading System
| Grade of Recommendation | Benefit vs Risk and Burdens | Methodologic Strength of Supporting Evidence | Implications |
| Strong recommendation, high-quality evidence (1A) | Benefits clearly outweigh risk and burdens or vice versa. | Consistent evidence from randomized controlled trials without important limitations or exceptionally strong evidence from observational studies. | Recommendation can apply to most patients in most circumstances. Further research is very unlikely to change our confidence in the estimate of effect. |
| Strong recommendation, moderate-quality evidence (1B) | Benefits clearly outweigh risk and burdens or vice versa. | Evidence from randomized controlled trials with important limitations (inconsistent results, methodologic flaws, indirect or imprecise) or very strong evidence from observational studies. | Recommendation can apply to most patients in most circumstances. Higher-quality research may well have an important impact on our confidence in the estimate of effect and may change the estimate. |
| Strong recommendation, low- or very-low-quality evidence (1C) | Benefits clearly outweigh risk and burdens or vice versa. | Evidence for at least one critical outcome from observational studies, case series, or randomized controlled trials, with serious flaws or indirect evidence. | Recommendation can apply to most patients in many circumstances. Higher-quality research is likely to have an important impact on our confidence in the estimate of effect and may well change the estimate. |
| Weak recommendation, high-quality evidence (2A) | Benefits closely balanced with risks and burden. | Consistent evidence from randomized controlled trials without important limitations or exceptionally strong evidence from observational studies. | The best action may differ depending on circumstances or patient or societal values. Further research is very unlikely to change our confidence in the estimate of effect. |
| Weak recommendation, moderate-quality evidence (2B) | Benefits closely balanced with risks and burden. | Evidence from randomized controlled trials with important limitations (inconsistent results, methodologic flaws, indirect or imprecise) or very strong evidence from observational studies. | Best action may differ depending on circumstances or patient or societal values. Higher-quality research may well have an important impact on our confidence in the estimate of effect and may change the estimate. |
| Weak recommendation, low- or very-low-quality evidence (2C) | Uncertainty in the estimates of benefits, risks, and burden; benefits, risk, and burden may be closely balanced. | Evidence for at least one critical outcome from observational studies, case series, or randomized controlled trials, with serious flaws or indirect evidence. | Other alternatives may be equally reasonable. Higher-quality research is likely to have an important impact on our confidence in the estimate of effect and may well change the estimate. |
Often, we found that the quality of the evidence differed across outcomes. For example, in assessing the quality of evidence for thienopyridines vs warfarin in patients undergoing percutaneous coronary interventions, we determined the evidence to be of moderate quality for mortality, nonfatal myocardial infarction, and revascularization but of low quality for major bleeding.
We then made a rating of the quality of the entire body of evidence bearing on the effect of alternative management strategies for each clinical question. In other words, we assessed the quality across outcomes, including both benefits and harms. Quality for each recommendation was the lowest quality rating of the outcomes judged as critical (as opposed to important, but not critical).19
4.3 Estimating Relative and Absolute Effects
Most patient-important outcomes in this guideline are binary or yes-no outcomes (death, stroke, VTE, myocardial infarction, bleeding). In general, relative effects are similar across subgroups of patients, including those with varying baseline risk.20,21 The evidence summaries (Evidence Profiles and Summary of Findings tables described later in this article), therefore, include a presentation of relative effects (where possible as relative risks because they are easier to understand than ORs) of intervention vs control management strategies.
Trading off desirable and undesirable consequences (eg, thrombosis vs bleeding) requires, however, estimates of absolute effect. For example, in patients with atrial fibrillation, warfarin results in a 66% relative risk reduction in nonfatal stroke. This comes at a cost of inconvenience, lifestyle restrictions, and risk of bleeding. For patients with a CHADS (congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke) score of ≥ 3, the 66% relative risk reduction translates into an absolute reduction of 6.3% (63 in 1,000) per year. Virtually all patients will consider this worthwhile. On the other hand, for patients with a CHADS score of 0, the 66% reduction translates into an absolute risk reduction of only 0.5% (5 in 1,000) per year. Many patients may consider this reduction not worth the undesirable consequences of warfarin use.
We calculated absolute effects by applying relative risks to estimates of control group risk. For instance, if control group risk of thrombosis is 4% and relative risk with an intervention is 50%, then the absolute difference between intervention and control is 4% of 50% or 2%, and the number needed to treat to prevent an episode of thrombosis is 100/2 or 50. In many cases, the Summary of Findings tables present effects as events prevented (or caused) per 1,000 patients. In this hypothetical example, the effect would be 20 events per 1,000 patients.
Whenever valid prognostic data were available from observational studies, they were used to estimate control group risks. When credible results from observational and prognostic studies were not available, risk estimates from control groups of RCTs were used.
4.4 Considering Subgroup-Specific Relative and Absolute Effects
Whenever we identified credible evidence that the relative effects vary across distinguishable subgroups of patients (ie, interaction between the intervention and a patient characteristic), we considered the respective relative effects separately. We then calculated the associated absolute effects.
Even when the relative effect is the same, the absolute magnitude of treatment effects may differ in patients with varying levels of risk. For instance, although the relative risk reduction of warfarin vs aspirin in stroke prevention for patients with atrial fibrillation is likely close to 50% across risk groups, this translates into an absolute risk reduction of < 1% per year in the lowest-risk groups and ∼ 5% per year in the highest-risk groups.
We included control group risks and absolute effect estimates for different groups in the summaries of effect when (and only when) two conditions were present. First, we required validated prognostic models or, at the very least, credible strategies for clinicians to easily identify higher- and lower-risk patients. Second, we identified varying risk groups only when recommendations differed in strength or direction between groups. Both conditions were met, for instance, in the atrial fibrillation recommendations in which strong recommendations in favor of anticoagulation were restricted to the higher-risk patients.
4.5 Conducting Meta-analyses
When pooled estimates of effects were not available from existing high-quality systematic reviews, we performed meta-analyses if the data were sufficiently homogeneous. When pooling two studies, we used a fixed-effects model. When three or more studies were available for generating a pooled estimate, we used a random-effects model as the primary analysis and a fixed-effects model as a secondary analysis. If there were discrepancies between the two, we considered the following reasons: If there was substantial heterogeneity leading to wider CIs with the random-effects model, we considered that model more trustworthy, and if the discrepancy was due to a single large dominant study with a result substantially different from smaller studies, we considered the fixed-effects model more trustworthy. We also assessed statistical heterogeneity using both a χ2 test and I2 as well as assessed possible explanations of heterogeneity considering a priori-generated hypotheses.22
4.6 Summary Tables
When resources permitted, we used a standardized approach for summarizing the evidence and methodology of individual studies (examples in Tables 1, 2, 5). These summaries appear in the online data supplements. Wherever possible, we report nonfatal events (eg, nonfatal stroke) so that there is no overlap with the number of fatal events reported.
Table 5.
—[Section 4.1] Descriptive Table: Randomized Controlled Trials of Fondaparinux vs No Fondaparinux for the Treatment of Superficial Vein Thrombosis
| Author/Year | Patients | Intervention | Control | Outcomes | Results |
| Decousus et al12; CALISTO Study Group/2010 | Patients aged ≥ 18 years with acute symptomatic, objectively confirmed SVT of the legs | Fondaparinux, 2.5 mg subcutaneous once daily for 45 d Use of GCS encouraged for all patients | Placebo for 45 d | Primary efficacy outcome:
|
Primary efficacy outcome: 13/1,502 vs 88/1,500; RR, 0.15; 95% CI, 0.08-0.26 |
Exclusion criteria:
|
Secondary efficacy outcomes:
|
Composite of DVT and PE up to day 77: 4/1,502 vs 22/1,500; RR, 0.18; 95% CI, 0.06-0.53 | |||
Safety outcomes (up to day 47 or up to day 77):
|
All other efficacy outcomes: P <.05; major bleeding by day 47, one event per group; RR, 1 |
GCS = graduated compression stockings; NSAID = nonsteroidal antiinflammatory drug; PE = pulmonary embolism; RR = risk ratio; SVT = superficial vein thrombosis. See Table 1 for expansion of other abbreviation.
For a large number of recommendations, we summarized the quality of the body of evidence (Fig 2) and estimates of relative and absolute effect of alternative management strategies using the methods of the GRADE Working Group.23 Evidence Profiles summarize the quality of the body of evidence and when evidence comes from randomized trials, generally include a presentation of reviewer assessment of risk of bias, precision, consistency, directness, and publication bias associated with each outcome (eg, see Table 2). As specified in GRADE methodology,19 the overall quality of evidence represents the lowest quality of any critical outcome.
Evidence Profiles can be found in the online data supplement. The format for these tables was determined through a formal survey of panelists that evaluated the panelists’ preferences for alternative presentations and the impact of these presentations on their understanding of the evidence. The text in the printed version of the AT9 recommendations includes more succinct Summary of Findings tables (eg, see Table 624), which include the overall quality assessment as well as the relative and absolute effect sizes for each outcome. Use of an associated computer program facilitated the production of the Evidence Profiles and Summary of Findings tables.25
Table 6.
—[Section 4.6] Summary of Findings Table: Prasugrel + Aspirin vs Clopidogrel + Aspirin in Patients With a Recent ACS and PCI24
| Outcomes | No. of Participants (Studies), Mean Follow-up | Quality of the Evidence (GRADE) | Relative Effect, RR (95% CI) | Anticipated Absolute Effects (1-y Time Frame) |
|
| Risk With Clopidogrel | Risk Difference With Prasugrel (95% CI) | ||||
| Vascular mortality | 13,608 (1 study), 14.5 mo | Moderate due to imprecision | 0.89 (0.7-1.12) | 24 deaths per 1,000a | 3 fewer deaths per 1,000 (from 7 fewer to 3 more) |
| Nonfatal MI | 13,608 (1 study), 14.5 mo | High | 0.76 (0.67-0.85) | 95 MI per 1,000a | 22 fewer MI per 1,000 (from 33 fewer to 14 fewer) |
| Nonfatal stroke | 13,608 (1 study), 14.5 mo | Moderate due to imprecision | 1.02 (0.71-1.45) | 10 strokes per 1,000a | 0 fewer strokes per 1,000 (from 3 fewer to 5 more |
| Major extracranial bleed Non-CABG- related TIMI major bleeding | 13,457 (1 study); median, 14.5 mo | High | 1.32 (1.03-1.68) | 22 major bleeds per 1,000a | 6 more major bleeds per 1,000 (from 0 more to 12 more) |
The anticipated absolute effect is expressed as risk difference (and its 95% CI) and is based on the baseline risk in the comparison group and the relative effect of the intervention (and its 95% CI). High quality indicates further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; and very-low quality, we are very uncertain about the estimate. ACS = acute coronary syndromes; CABG = coronary artery bypass graft; GRADE = Grades of Recommendations, Assessment, Development, and Evaluation; MI = myocardial infarction; PCI = percutaneous coronary intervention; RR = risk ratio; TIMI = thrombolysis in myocardial infarction.
5.0 Values and Preferences
Making trade-offs between desirable and undesirable consequences of alternative management strategies—the fundamental process of making recommendations—requires making value and preference judgments. For antithrombotic therapy guidelines, this trade-off involves, in most instances, a reduction in thrombotic events compared with an increase in bleeding events. Ideally, the values and preferences applied to this decision would be the average values and preferences of the patient population. We know, however, that patient values for health outcomes vary substantially from patient to patient. Knowledge of the extent to which patient values and preferences vary is one factor in deciding on the strength of a recommendation. The greater the variability in values and preferences, the more likely a weak recommendation is appropriate.23
To inform these decisions, we conducted a systematic review of the literature bearing on patient values and preferences regarding antithrombotic therapy.26 The methodology of conducting such studies remains to be fully developed, and the area remains underinvestigated. Nevertheless, the results of the review provided guidance for the values and preferences that we adopted for these guidelines.26
As an additional strategy for achieving meaningful value and preference decisions by each topic panel and to facilitate consistency across articles, we conducted a values rating exercise. Topic editors and deputy editors constructed patient scenarios for key outcomes of thrombosis and bleeding relevant to their articles. Then informed by the systematic review of values and preferences, panelists used these scenarios to rate each outcome from 0 (death) to 100 (full health). The mean values of these ratings guided the trade-offs between thrombotic and bleeding events and, thus, the determination of strong vs weak recommendations. The scenarios and results of the rating exercise are available in the online data supplements.
The introductory section in each chapter includes a summary, quantitative wherever possible, of the key values and preferences underlying the recommendations. Where value and preference judgments were particularly relevant or controversial, explicit statements of values and preferences accompany individual recommendations.
The literature review26 revealed extensive heterogeneity of results across studies of patient values and preferences—variability that often is difficult to explain. Both the variability between studies and the considerable variability in values and preferences among patients within studies, mandated circumspection in making strong recommendations. Therefore, we restricted strong recommendations to situations in which the desirable consequences of an intervention substantially and convincingly outweighed the undesirable consequences (or the reverse) and to unusual situations in which there was reason to believe that values and preferences are relatively uniform.
6.0 Resource Use Issues
In addressing resource use (cost) issues in AT9, we followed previously developed principles.27 In particular, we restricted economic evaluation to recommendations in which it was plausible that resource use considerations might change the direction or strength of the recommendation and in which high-quality economic evaluations were available. When this was not the case, we did not consider resource use in the recommendations.
Six clinicians with the requisite expertise in decision and economic analyses participated in the guideline development process; each article had the benefit of one of these experts as a full committee member. In the following subsections, we present key points in the process of considering resource allocation issues in the recommendations.
6.1 Overview of the Process
Panelists, in consultation with resource use consultants, determined questions for which resource use might change the direction or strength of recommendations. For those questions, we sought high-quality economic analyses. If such analyses were available, we applied the evidence regarding resource use to the relevant recommendation. If net costs or marginal cost-effectiveness ratios were very high, panelists considered rating down the quality of evidence for an intervention from high to low or possibly changing the direction of the recommendation using guides described in section 6.4 “Criteria for Resource Allocation Issues to Affect Recommendations—Thresholds for Cost-Effectiveness.”
6.2 Identifying the Literature
The Oregon Health & Science University Evidence-based Practice Center conducted thorough literature searches for economic analyses relevant to the different AT9 articles. The resource use experts supplemented these by searches focused on the specific questions of interest for each article. The searches were conducted in Medline and the Cochrane Central Register of Clinical Trials. On the basis that data from studies appreciably more than a decade old would not reflect the current situation, searches were restricted to published studies from 1999 forward. Thus, bibliographic database searches encompassed publications from January 1999 forward: The end date varied across articles and ranged between November 2009 and March 2010 when the searches were executed.
6.3 Evaluating the Evidence
A standardized data extraction form was used to ensure uniform evaluation of the quality of relevant economic analyses. Quality assessment was based on published criteria27‐33 and included specification of perspective of analysis (eg, societal, health system), appropriateness of time horizon (preferably lifetime), use of high-quality evidence for probabilities and rates, use of high-quality sources for costs (eg, primary data, Medicare payments, claims data as proxies), use of appropriate methods for measurement of preferences, and performance of sensitivity analyses to explore uncertainty (both deterministic and probabilistic).
6.4 Criteria for Resource Allocation Issues To Affect Recommendations—Thresholds for Cost-Effectiveness
The results of economic analyses may either increase the strength of an otherwise weak recommendation or weaken the strength of a strong recommendation. If cost-effectiveness studies bolstered an already strong recommendation, no change to the recommendation was necessary. We chose the following thresholds for cost-effectiveness considerations affecting recommendations:
- When the clinical evidence warrants a strong recommendation for A over B:
- Strong recommendation favoring A when high-quality evidence from economic evaluations shows that A costs < 3 times the gross domestic product (GDP) per capita (approximately US $150,000)34 per quality-adjusted life year (QALY) gained relative to B
- Weak recommendation favoring A when high-quality evidence from economic evaluations shows that A costs 3 to 5 times the GDP per capita (∼$150,000-$250,000) per QALY gained relative to B
- Weak recommendation favoring B when high-quality evidence from economic evaluations shows that A costs > 5 times the GDP per capita (∼$250,000) per QALY gained relative to B
- When the clinical evidence warrants a weak recommendation for A over B:
- Strong recommendation favoring A if A results in cost savings of > 10% to 20% of the GDP per capita (∼$5,000-$10,000) relative to B (Cost savings must represent all downstream costs and not just the actual cost of the intervention, and analysis must demonstrate a high level of confidence that there is a cost savings.)
- Continued weak recommendation favoring A when B is marginally more costly than A (< 10% the GDP per capita)
- Continued weak recommendation favoring A when A costs 0 to 5 times the GDP per capita per QALY gained relative to B
- Weak recommendation favoring B if A costs > 5 times the GDP per capita (∼$250,000) per QALY gained relative to B
6.5 Extension of Economic Analyses to Low- and Middle-Income Countries
Although certain interventions may be cost-effective in high-income countries (eg, < $20,000 per QALY gained), in poor countries, $20,000 gained per QALY may be prohibitive. The choice of a threshold will vary depending on who is making resource allocation decisions. To facilitate the use of already published cost-effectiveness analyses, the World Health Organization (WHO), through its WHO-CHOICE (Choosing Interventions that are Cost Effective) program35 has used criteria suggested by the Commission on Macroeconomics and Health.36 Interventions that cost < 1 times the average per-capita income for a given country or region per QALY gained are considered very cost-effective. Interventions that cost up to three times the average per-capita income per QALY gained are still considered cost-effective, whereas those that exceed this level are not considered to be cost-effective. To facilitate this process, WHO has developed tables of such threshold values for different regions and countries around the world.37 Thus, the thresholds discussed in the previous section have been defined in terms of GDP per capita. Although referencing thresholds for cost-effectiveness to average per-capita income in middle- and low-income countries can help to extend results of economic analyses performed in high-income countries, such analyses may be less relevant in low-income countries because of significantly different material and labor costs and, thus, may be difficult to extrapolate. Furthermore, the comparator strategies may not be feasible or customary in these locales.
7.0 Disclosing and Managing Conflicts of Interest
All panelists were required to disclose both financial conflicts of interest, such as receipt of funds for consulting with industry, and intellectual conflicts of interest, such as publication of original data bearing directly on a recommendation. Financial and intellectual conflicts of interest were classified as primary (more serious) or secondary (less serious).38 The operational definition of primary intellectual conflicts of interest included authorship of original studies and peer-reviewed grant funding (government, not-for-profit organizations) directly bearing on a recommendation. The operational definition of primary financial conflicts of interest included consultancies, advisory board membership, and the like from industry. Topic editors had no primary conflicts of interest, as noted. Some deputy editors, who were clinical experts in the topic of the article, had relevant primary conflicts of interest. The ACCP HSP Committee deemed some of these conflicts serious enough to require “management.” Management involved more frequent updates of disclosures than required of the approved panelists without any conflicts and recusal from activities relevant to that conflict.
Topic panel members, including the deputy editor, with primary conflicts related to a particular recommendation did not participate in the final deliberations that led to the decision regarding the direction or strength of a recommendation, nor did they vote on recommendations for which they were primarily conflicted. Panelists with primary conflicts could, however, participate in discussions and offer their opinions on interpretations of the evidence. Readers will find a record of panelist conflicts of interest on a recommendation-by-recommendation basis in the online data supplement.
8.0 Finalizing the Recommendations
8.1 Formulating Recommendations
Following approaches recommended by the GRADE Working Group,23 the topic editor, in some cases aided by a panelist without conflicts, formulated the draft recommendations. The formulation of recommendations considered the balance between the desirable and undesirable consequences of an intervention; the quality of evidence; the variability in patient values and preferences; and, on occasion, resource use issues. The recommendations were graded as strong when desirable effects were much greater than undesirable effects or vice versa. Strong recommendations were worded as “We recommend” and labeled 1. Recommendations were graded as weak when desirable effects were not clearly greater or less great than undesirable effects. Weak recommendations were worded as “We suggest” and labeled 2. The rating of the quality of the evidence—high, A; moderate, B; or low, C—is provided with the strength of each recommendation.
8.2 Finalizing Recommendations
After completing the steps described previously, the topic panel members without primary conflicts discussed draft recommendations (Fig 1). Initial discussions generally led to a consensus at the article level on the quality of evidence and the direction and strength of recommendations. At least two members of the Executive Committee reviewed in detail drafts of articles, including recommendations. Written critiques were prepared and returned to the authors for revision. Articles were then made available to the entire AT9 panel.
Recommendations on which topic panels had difficulty coming to a consensus were discussed at a final conference in February 2011 attended by the topic editors and deputy editors and at least one other panel member from each article. Prior to the conference, all AT9 panelists updated their conflict of interest disclosures. The ACCP invited a number of clinical organizations with interest in the guideline topic to attend the final conference as observers.
At this final conference, a representative of each article presented potentially controversial issues in their article’s recommendations. Following discussion, which included those present and those attending by videoconference, all panelists without primary conflicts of interest voted on each recommendation. The voting process used a GRADE grid and required that for a strong recommendation, ≥ 80% of those voting had to agree that a strong recommendation was appropriate.39
The AT9 Executive Committee members (G. H. G., M. C., E. A. A., and D. D. G.) harmonized the articles and resolved remaining disagreements among them through facilitated discussion with topic editors and deputy editors without primary conflicts. All major correspondence and decisions at the final conference were recorded in written and audio formats and are available on request to science@chestnet.org.
9.0 Review by ACCP and External Reviewers
The ACCP HSP Committee established a process for the thorough review of all ACCP evidence-based clinical practice guidelines. After final review by the AT9 Executive Committee, the guidelines underwent review by the Cardiovascular and Pulmonary Vascular NetWorks of the ACCP, the HSP Committee, and the ACCP Board of Regents. The latter two groups had the right of approval or disapproval but usually worked with the topic panelists and editors to make necessary revisions prior to final approval. Both the HSP Committee and the Board of Regents identified primary reviewers who read the full set of articles, and the remaining HSP Committee members were responsible for reviewing several articles each. The reviewers considered both content and methodology as well as whether there was balanced reporting and adherence to HSP Committee processes. All reviewers were vetted through the same conflict of interest disclosure and management process as described previously. Finally, the Editor in Chief of CHEST read and forwarded the manuscripts for independent, external peer review prior to acceptance for publication. No recommendations or assessments of the quality of the evidence could be changed without the express approval of the topic panel members, AT9 Executive Committee, HSP Committee, and ACCP Board of Regents.
10.0 Organization of Articles
In order to provide a transparent, explicit link among PICO questions, evidence, tables, and recommendations, the section numbering in each article corresponds to numbers in Table 1 in each article, which specifies the patients, interventions, and outcomes for each question. The section numbering also corresponds to the numbering of the recommendations themselves. Evidence Profiles and other tables include these corresponding numbers in brackets in the title, as is true for the online data supplement tables.
11.0 Revisions in the Process Since AT8
AT9 includes improvements from AT8 that reflect the evolution of the science of systematic reviews and clinical practice guidelines. In this supplement,40 some of these improvements include augmented provisions to decrease the likelihood of conflict of interest influence, more stringent application of GRADE criteria for evidence and recommendations (both facilitated by methodologists without primary conflicts taking the role of topic editor), and a systematic review of values and preferences to guide the recommendations.
12.0 Limitations of Methods
Although encouraged to use Evidence Profiles and Summary of Findings tables for all recommendations, there were some for which the authors were unable to produce such tables. However, those recommendations used an evidence-based systematic review and assessment of relevant studies. Some recommendations would have benefited from meta-analyses that would have clarified aspects of the evidence. Although panelists were instructed in completing the value and preference rating exercise to estimate patient values and preferences rather than to use their own, we cannot be assured that they succeeded in all instances.41
13.0 Plans for Updating AT9
We plan to continue the tradition of the antithrombotic guidelines to update recommendations when important new studies are published that might change the current recommendations. In March 2011, the ACCP Board of Regents approved a proposal to revise the guideline development and updating process to a “living guidelines” process, whereby the evidence-based guidelines will be periodically assessed and updated as the literature warrants. From 1 year after the publication of this ninth edition onward, all clinical questions or sets of related questions will become their own units. This process will be discussed in greater depth in future publications.
In addition to the published guidelines, ACCP has historically provided clinical resources, including a quick reference to the recommendations, patient education materials, and slide sets for presentations. These resources will continue to be based on the guidelines, but they will be accessed online through the ACCP Web site. In addition, there will be related resources, tools, and links to make the content more easily useful and searchable.
14.0 Conclusion
For AT9, we used an explicit, transparent process that seeks to produce highly relevant and unbiased recommendations for clinical practice. This process involved the a priori specification of clinical questions in the PICO format along with study inclusion and exclusion criteria, an exhaustive search for relevant literature, an evaluation of the risk of bias of included studies, and a rigorous and standardized assessment of the quality of the body of evidence and its translation into recommendations using GRADE Working Group methodologies. We incorporated specification of values and preferences and resource considerations into recommendations where particularly relevant and when such data were available. Finally, we sought to minimize bias potentially introduced by intellectual and financial conflicts of interest by comprehensive disclosure requirements and aggressive management of relevant conflicts.
Acknowledgments
Author contributions: Authors contributed to the AT9 guideline process in the roles described in the article. As Topic Editor and Chair of the guideline, Dr Guyatt oversaw the development of this article.
Dr Guyatt: produced the first draft and was responsible for the final article.
Dr Norris: undertook a major revision of a late draft of the article.
Dr Schulman: took responsibility for sections relevant to their role in AT9 and reviewed and approved the final article.
Dr Hirsh: took responsibility for sections relevant to their role in AT9 and reviewed and approved the final article.
Dr Eckman: took responsibility for sections relevant to their role in AT9 and reviewed and approved the final article.
Dr Akl: took responsibility for sections relevant to their role in AT9 and reviewed and approved the final article.
Dr Crowther: took responsibility for sections relevant to their role in AT9 and reviewed and approved the final article.
Dr Vandvik: took responsibility for sections relevant to their role in AT9 and reviewed and approved the final article.
Dr Eikelbloom: took responsibility for sections relevant to their role in AT9 and reviewed and approved the final article.
Dr McDonagh: took responsibility for sections relevant to their role in AT9 and reviewed and approved the final article.
Dr Lewis: took responsibility for sections relevant to their role in AT9 and reviewed and approved the final article.
Dr Gutterman: took responsibility for sections relevant to their role in AT9 and reviewed and approved the final article.
Dr Cook: took responsibility for sections relevant to their role in AT9 and reviewed and approved the final article.
Dr Schünemann: took responsibility for sections relevant to their role in AT9 and reviewed and approved the final article.
Financial/nonfinancial disclosures: In summary, the authors have reported to CHEST the following conflicts of interest: Dr Eckman has received the following university grants: “Using Decision Analytic Modeling to Guide the ACCP Guideline Development Process for Antithrombotic Therapy in Atrial Fibrillation” (Foundation for Informed Medical Decision Making; October 2011-September 2013; $185,000); “Cost-Effectiveness of Screening for Chronic Hepatitis C Infection” (Merck/Schering-Plough; October 2011- September 2012; $58,000); “Greater Cincinnati BEACON Collaborative” (Office of the National Coordinator for Health Information Technology [90BC0016/01]; September 2010-March 2012; ∼15% effort); “Cincinnati Center for Clinical and Translational Science and Training (CTSA) ARRA Supplement for Development of Distance Learning Program in Medical Informatics” (National Institutes of Health [NIH]/National Center for Research Resources [NCRR] [UL1 RR026314-01S1]; August 2009-August 2011; ∼20% effort); “Cincinnati Center for Clinical and Translational Science and Training (CTSA)” (NIH/NCRR [1U54 RR 025216]; January 2009-February 2014; ∼15% effort); “A Patient Specific Decision Support Tool for Bariatric Surgery” (National Institute of Diabetes and Digestive and Kidney Diseases [K23 DK075599]; August 2007-June 2012; no financial support); National Heart, Lung, and Blood Institute (K23 HL085387; June 2008-March 2013; no financial support); and “Cost-Effectiveness of Screening for Chronic Hepatitis B Infection” (Gilead Sciences Inc; March 2008-August 2010; ∼$56,000). He has also served as consultant for Savient Pharmaceuticals (“Cost Effectiveness Analysis of Gout Medication”; 2010; ∼$300) and as editorial consultant for the ACCP (“Physicians’ Information and Education Resource [PIER]: Module on Pre-Operative Assessment for Bleeding Disorders”; 2006-present; ∼$250/y). Dr Crowther has served on various advisory boards, has assisted in the preparation of educational materials, and has sat on data safety and management boards. His institution has received research funds from the following companies: Leo Pharma A/S, Pfizer Inc, Boerhinger Ingelheim GmbH, Bayer Healthcare Pharmaceuticals, Octapharm AG, CSL Behring, and Artisan Pharma. Personal total compensation for these activities over the past 3 years totals less than US $10,000. Dr Eikelboom has received consulting fees and honoraria from AstraZeneca; Boehringer-Ingelheim GmbH; Bristol-Myers Squibb; Corgenix; Daiichi-Sankyo, Inc; Eisai Co, Inc; Eli Lilly and Company; GlaxoSmithKline plc; Haemonetics Corp; McNeil Consumer Healthcare; and Sanofi-Aventis LLC and grants and in-kind support from Accumetrics, Inc; AspirinWorks; Bayer Healthcare Pharmaceuticals; Boehringer Ingelheim GmbH; Bristol-Myers Squibb; Corgenix; Dade Behring Inc; GlaxoSmithKline plc; and Sanofi-Aventis LLC. Dr Gutterman has had the following relationships that are entirely unrelated to the AT9 guidelines: ACCP President, GlaxoSmithKline plc grant to study vasodilation in adipose tissue, National Institutes of Health grant to study human coronary dilation, and GE Healthcare consultation on a study for ECG evaluation of chronic heart disease. Drs Guyatt and Schünemann are co-chairs of the GRADE Working Group, and Drs Akl and Vandvik are members and prominent contributors to the Grade Working Group. Dr Lewis is a full-time employee of the ACCP. Drs Norris, Schulman, Hirsh, McDonagh, and Cook have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors played no role in the development of these guidelines. Sponsoring organizations cannot recommend panelists or topics, nor are they allowed prepublication access to the manuscripts and recommendations. Guideline panel members, including the chair, and members of the Health & Science Policy Committee are blinded to the funding sources. Further details on the Conflict of Interest Policy are available online at http://chestnet.org.
Endorsements: This guideline is endorsed by the American Association for Clinical Chemistry, the American College of Clinical Pharmacy, the American Society of Health-System Pharmacists, the American Society of Hematology, and the International Society of Thrombosis and Hematosis.
Abbreviations
- ACCP
American College of Chest Physicians
- AT8
Antithrombotic and Thrombolytic Therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition)
- AT9
Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines
- GDP
gross domestic product
- GRADE
Grades of Recommendation, Assessment, Development, and Evaluation
- HSP
Health and Science Policy
- PICO
population, intervention, comparator, and outcome
- QALY
quality-adjusted life year
- RCT
randomized controlled trial
- WHO
World Health Organization
Footnotes
Funding/Support: The Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines received support from the National Heart, Lung, and Blood Institute [R13 HL104758] and Bayer Schering Pharma AG. Support in the form of educational grants was also provided by Bristol-Myers Squibb; Pfizer, Inc; Canyon Pharmaceuticals; and sanofi-aventis US.
Disclaimer: American College of Chest Physician guidelines are intended for general information only, are not medical advice, and do not replace professional medical care and physician advice, which always should be sought for any medical condition. The complete disclaimer for this guideline can be accessed at http://chestjournal.chestpubs.org/content/141/2_suppl/1S
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Hirsh J, Guyatt G, Albers GW, Harrington R, Schünemann HJ. American College of Chest Physicians Antithrombotic and thrombolytic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(suppl 6):110S–112S. doi: 10.1378/chest.08-0652. [DOI] [PubMed] [Google Scholar]
- 2.Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guyatt GH, Eikelboom JW, Gould MK, et al. Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2) suppl:e185S–e194S. doi: 10.1378/chest.11-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guyatt G, Montori V, Devereaux PJ, Schünemann H, Bhandari M. Patients at the center: in our practice, and in our use of language. ACP J Club. 2004;140(1):A11–A12. [PubMed] [Google Scholar]
- 5.Ferreira-González I, Busse JW, Heels-Ansdell D, et al. Problems with use of composite end points in cardiovascular trials: systematic review of randomised controlled trials. BMJ. 2007;334(7597):786. doi: 10.1136/bmj.39136.682083.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira-González I, Permanyer-Miralda G, Busse JW, et al. Methodologic discussions for using and interpreting composite endpoints are limited, but still identify major concerns. J Clin Epidemiol. 2007;60(7):651–662.. doi: 10.1016/j.jclinepi.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Montori VM, Permanyer-Miralda G, Ferreira-González I, et al. Validity of composite end points in clinical trials. BMJ. 2005;330(7491):594–596. doi: 10.1136/bmj.330.7491.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2) suppl:e278S–e325S. doi: 10.1378/chest.11-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013–1020. doi: 10.1016/j.jclinepi.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Oxman AD, Guyatt GH, Singer J, et al. Agreement among reviewers of review articles. J Clin Epidemiol. 1991;44(1):91–98. doi: 10.1016/0895-4356(91)90205-n. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Altman D. Assessing the risk of bias in included studies. In: Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.1. Chichester, England:: John Wiley & Sons; 2008. pp. 17–21. [Google Scholar]
- 12.Decousus H, Prandoni P, Mismetti P, et al. CALISTO Study Group. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med. 2010;363(13):1222, 1232. doi: 10.1056/NEJMoa0912072. [DOI] [PubMed] [Google Scholar]
- 13.Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 14.Beyth RJ, Cohen AM, Landefeld CS. Long-term outcomes of deep-vein thrombosis. Arch Intern Med. 1995;155:1031–1037. [PubMed] [Google Scholar]
- 15.Kahn SR, Ginsberg JS. Relationship between deep venous thrombosis and the postthrombotic syndrome. Arch Intern Med. 2004;164:17–26. doi: 10.1001/archinte.164.1.17. [DOI] [PubMed] [Google Scholar]
- 16.Lewis BE, Wallis DE, Berkowitz SD, et al. Argatroban anticoagulant therapy in patients with heparin-induced thrombocytopenia. Circulation. 2001;103:1838–1843. doi: 10.1161/01.cir.103.14.1838. [DOI] [PubMed] [Google Scholar]
- 17.Lewis BE, Wallis DE, Leya F, et al. Argatroban anticoagulation in patients with heparin-induced thrombocytopenia. Arch Intern Med. 2003;163:1849–1856. doi: 10.1001/archinte.163.15.1849. [DOI] [PubMed] [Google Scholar]
- 18.Lubenow N, Eichler P, Lietz T, et al. Lepirudin in patients with heparin-induced thrombocytopenia—results of the third prospective study (HAT-3) and a combined analysis of HAT-1, HAT-2, and HAT-3. J Thromb Haemost. 2005;3:2428–2436. doi: 10.1111/j.1538-7836.2005.01623.x. [DOI] [PubMed] [Google Scholar]
- 19.Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. GRADE Working Group What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336(7651):995–998. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med. 2002;21(11):1575–1600. doi: 10.1002/sim.1188. [DOI] [PubMed] [Google Scholar]
- 21.Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the ‘number needed to treat’? An empirical study of summary effect measures in meta-analyses. Int J Epidemiol. 2002;31(1):72–76. doi: 10.1093/ije/31.1.72. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyatt GH, Oxman AD, Kunz R, et al. GRADE Working Group Going from evidence to recommendations. BMJ. 2008;336(7652):1049–1051. doi: 10.1136/bmj.39493.646875.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 25.Brozek J, Oxman A. Schünemann HS. GRADEpro [Computer program] Version 3.2 for Windows 2008 http://www.cc-ims.net/gradepro. or http://mcmaster.flintbox.com/technology.asp?page=3993.
- 26.MacLean S, Mulla S, Akl EA, et al. Patient values and preferences in decision making for antithrombotic therapy: a systematic review: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2) suppl:e1S–e23S. doi: 10.1378/chest.11-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guyatt G, Baumann M, Pauker S, et al. Addressing resource allocation issues in recommendations from clinical practice guideline panels: suggestions from an American College of Chest Physicians task force. Chest. 2006;129(1):182–187. doi: 10.1378/chest.129.1.182. [DOI] [PubMed] [Google Scholar]
- 28.Detsky AS, Naglie IG. A clinician’s guide to cost-effectiveness analysis. Ann Intern Med. 1990;113(2):147–154. doi: 10.7326/0003-4819-113-2-147. [DOI] [PubMed] [Google Scholar]
- 29.Drummond MF, Richardson WS, O’Brien BJ, Levine M, Heyland D. Users’ guides to the medical literature. XIII. How to use an article on economic analysis of clinical practice. A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA. 1997;277(19):1552–1557. doi: 10.1001/jama.277.19.1552. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien BJ, Heyland D, Richardson WS, Levine M, Drummond MF. Users’ guides to the medical literature. XIII. How to use an article on economic analysis of clinical practice. B. What are the results and will they help me in caring for my patients? Evidence-Based Medicine Working Group. JAMA. 1997;277(22):1802–1806. doi: 10.1001/jama.277.22.1802. [DOI] [PubMed] [Google Scholar]
- 31.Eisenberg JM. Clinical economics. A guide to the economic analysis of clinical practices. JAMA. 1989;262(20):2879–2886. doi: 10.1001/jama.262.20.2879. [DOI] [PubMed] [Google Scholar]
- 32.Gold MR, Siegel JE, Russell LB, et al. Cost-Effectiveness in Health and Medicine. 1st ed. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 33.Guyatt GH, Oxman AD, Kunz R, et al. GRADE Working Group Incorporating considerations of resources use into grading recommendations. BMJ. 2008;336(7654):1170–1173. doi: 10.1136/bmj.39504.506319.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Economic Outlook Database-April 2011. International Monetary Fund World Health Organization Web site. http://www.who.int/macrohealth/documents/tough_choices/en/index.html. Accessed June 27, 2011.
- 35.Hutubessy R, Chisholm D, Edejer TT. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Eff Resour Alloc. 2003;1(1):8. doi: 10.1186/1478-7547-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.WHO Commission on Macroeconomics and Health . Macroeconomics and Health: Investing in Health for Economic Development. Report of the Commission on Macroeconomics and Health: Executive Summary. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 37.World Health Organization . Threshold Values for Intervention Cost-Effectiveness by Region. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 38.Guyatt G, Akl EA, Hirsh J, et al. The vexing problem of guidelines and conflict of interest: a potential solution. Ann Intern Med. 2010;152(11):738–741. doi: 10.7326/0003-4819-152-11-201006010-00254. [DOI] [PubMed] [Google Scholar]
- 39.Jaeschke R, Guyatt GH, Dellinger P, et al. GRADE Working Group Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ. 2008;337:a744. doi: 10.1136/bmj.a744. [DOI] [PubMed] [Google Scholar]
- 40.Guyatt GH, Akl EA, Crowther M, Schünemann HJ, Gutterman DD, Lewis SZ, et al. Introduction to the ninth edition: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2) suppl:48S–52S. doi: 10.1378/chest.11-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ubel PA, Angott AM, Zikmund-Fisher BJ. Physicians recommend different treatments for patients than they would choose for themselves. Arch Intern Med. 2011;171(7):630–634. doi: 10.1001/archinternmed.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]