Abstract
Background:
This guideline focuses on long-term administration of antithrombotic drugs designed for primary and secondary prevention of cardiovascular disease, including two new antiplatelet therapies.
Methods:
The methods of this guideline follow those described in Methodology for the Development of Antithrombotic Therapy and Prevention of Thrombosis Guidelines: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines in this supplement.
Results:
We present 23 recommendations for pertinent clinical questions. For primary prevention of cardiovascular disease, we suggest low-dose aspirin (75-100 mg/d) in patients aged > 50 years over no aspirin therapy (Grade 2B). For patients with established coronary artery disease, defined as patients 1-year post-acute coronary syndrome, with prior revascularization, coronary stenoses > 50% by coronary angiogram, and/or evidence for cardiac ischemia on diagnostic testing, we recommend long-term low-dose aspirin or clopidogrel (75 mg/d) (Grade 1A). For patients with acute coronary syndromes who undergo percutaneous coronary intervention (PCI) with stent placement, we recommend for the first year dual antiplatelet therapy with low-dose aspirin in combination with ticagrelor 90 mg bid, clopidogrel 75 mg/d, or prasugrel 10 mg/d over single antiplatelet therapy (Grade 1B). For patients undergoing elective PCI with stent placement, we recommend aspirin (75-325 mg/d) and clopidogrel for a minimum duration of 1 month (bare-metal stents) or 3 to 6 months (drug-eluting stents) (Grade 1A). We suggest continuing low-dose aspirin plus clopidogrel for 12 months for all stents (Grade 2C). Thereafter, we recommend single antiplatelet therapy over continuation of dual antiplatelet therapy (Grade 1B).
Conclusions:
Recommendations continue to favor single antiplatelet therapy for patients with established coronary artery disease. For patients with acute coronary syndromes or undergoing elective PCI with stent placement, dual antiplatelet therapy for up to 1 year is warranted.
Summary of Recommendations
Note on Shaded Text: Throughout this guideline, shading is used within the summary of recommendations sections to indicate recommendations that are newly added or have been changed since the publication of Antithrombotic and Thrombolytic Therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Recommendations that remain unchanged are not shaded.
2.1. For persons aged 50 years or older without symptomatic cardiovascular disease, we suggest low-dose aspirin 75 to 100 mg daily over no aspirin therapy (Grade 2B).
Remarks: Aspirin slightly reduces total mortality regardless of cardiovascular risk profile if taken over 10 years. In people at moderate to high risk of cardiovascular events, the reduction in myocardial infarction (MI) is closely balanced with an increase in major bleeds. Whatever their risk status, people who are averse to taking medication over a prolonged time period for very small benefits will be disinclined to use aspirin for primary prophylaxis. Individuals who value preventing an MI substantially higher than avoiding a GI bleed will be, if they are in the moderate or high cardiovascular risk group, more likely to choose aspirin.
3.1.1-3.1.5. For patients with established coronary artery disease (CAD), defined as patients 1-year post-acute coronary syndrome (ACS), with prior revascularization, coronary stenoses > 50% by coronary angiogram, and/or evidence for cardiac ischemia on diagnostic testing, (including patients after the first year post-ACS and/or with prior coronary artery bypass graft [CABG] surgery):
We recommend long-term single antiplatelet therapy with aspirin 75 to 100 mg daily or clopidogrel 75 mg daily over no antiplatelet therapy (Grade 1A).
We suggest single over dual antiplatelet therapy with aspirin plus clopidogrel (Grade 2B).
3.2.1-3.2.5. For patients in the first year after an ACS who have not undergone percutaneous coronary intervention (PCI):
We recommend dual antiplatelet therapy (ticagrelor 90 mg twice daily plus low-dose aspirin 75-100 mg daily or clopidogrel 75 mg daily plus low-dose aspirin 75-100 mg daily) over single antiplatelet therapy (Grade 1B).
We suggest ticagrelor 90 mg twice daily plus low-dose aspirin over clopidogrel 75 mg daily plus low-dose aspirin (Grade 2B).
For patients in the first year after an ACS who have undergone PCI with stent placement:
We recommend dual antiplatelet therapy (ticagrelor 90 mg twice daily plus low-dose aspirin 75-100 mg daily, clopidogrel 75 mg daily plus low-dose aspirin, or prasugrel 10 mg daily plus low-dose aspirin over single antiplatelet therapy) (Grade 1B).
Remarks: Evidence suggests that prasugrel results in no benefit or net harm in patients with a body weight of < 60 kg, age > 75 years, or with a previous stroke/transient ischemic attack.
We suggest ticagrelor 90 mg twice daily plus low-dose aspirin over clopidogrel 75 mg daily plus low-dose aspirin (Grade 2B).
For patients with ACS who undergo PCI with stent placement, we refer to sections 4.3.1 to 4.3.5 for recommendations concerning minimum and prolonged duration of treatment.
3.2.6-3.2.7. For patients with anterior MI and left ventricular (LV) thrombus, or at high risk for LV thrombus (ejection fraction < 40%, anteroapical wall motion abnormality), who do not undergo stenting:
We recommend warfarin (international normalized ratio [INR] 2.0-3.0) plus low-dose aspirin 75 to 100 mg daily over single antiplatelet therapy or dual antiplatelet therapy for the first 3 months (Grade 1B). Thereafter, we recommend discontinuation of warfarin and continuation of dual antiplatelet therapy for up to 12 months as per the ACS recommendations (see recommendations 3.2.1-3.2.5). After 12 months, single antiplatelet therapy is recommended as per the established CAD recommendations (see recommendations 3.1.1-3.1.5).
For patients with anterior MI and LV thrombus, or at high risk for LV thrombus (ejection fraction < 40%, anteroapical wall motion abnormality), who undergo bare-metal stent (BMS) placement:
We suggest triple therapy (warfarin [INR 2.0-3.0], low-dose aspirin, clopidogrel 75 mg daily) for 1 month over dual antiplatelet therapy (Grade 2C).
We suggest warfarin (INR 2.0-3.0) and single antiplatelet therapy for the second and third month post-BMS over alternative regimens and alternative time frames for warfarin use (Grade 2C). Thereafter, we recommend discontinuation of warfarin and use of dual antiplatelet therapy for up to 12 months as per the ACS recommendations (see recommendations 3.2.1-3.2.5). After 12 months, antiplatelet therapy is recommended as per the established CAD recommendations (see recommendations 3.1.1-3.1.5).
For patients with anterior MI and LV thrombus, or at high risk for LV thrombus (ejection fraction < 40%, anteroapical wall motion abnormality) who undergo drug-eluting stent (DES) placement:
We suggest triple therapy (warfarin INR 2.0-3.0, low-dose aspirin, clopidogrel 75 mg daily) for 3 to 6 months over alternative regimens and alternative durations of warfarin therapy (Grade 2C). Thereafter, we recommend discontinuation of warfarin and continuation of dual antiplatelet therapy for up to 12 months as per the ACS recommendations (see recommendations 3.2.1-3.2.5). After 12 months, antiplatelet therapy is recommended as per the established CAD recommendations (see recommendations 3.1.1-3.1.5).
4.1.1-4.3.5. For patients who have undergone elective PCI with placement of BMS:
For the first month, we recommend dual antiplatelet therapy with aspirin 75 to 325 mg daily and clopidogrel 75 mg daily over single antiplatelet therapy (Grade 1A).
For the subsequent 11 months, we suggest dual antiplatelet therapy with combination of low-dose aspirin 75 to 100 mg daily and clopidogrel 75 mg daily over single antiplatelet therapy (Grade 2C).
After 12 months, we recommend single antiplatelet therapy over continuation of dual antiplatelet therapy (Grade 1B).
For patients who have undergone elective PCI with placement of DES:
For the first 3 to 6 months, we recommend dual antiplatelet therapy with aspirin 75 to 325 mg daily and clopidogrel 75 mg daily over single antiplatelet therapy (Grade 1A).
Remarks: Absolute minimum duration will vary based on stent type (in general, 3 months for -limus stents and 6 months for -taxel stents).
After 3 to 6 months, we suggest continuation of dual antiplatelet therapy with low-dose aspirin 75 to 100 mg and clopidogrel (75 mg daily) until 12 months over single antiplatelet therapy (Grade 2C).
After 12 months, we recommend single antiplatelet therapy over continuation of dual antiplatelet therapy (Grade 1B). Single antiplatelet therapy thereafter is recommended as per the established CAD recommendations (see recommendations 3.1.1-3.1.5).
For patients who have undergone elective BMS or DES stent placement:
We recommend using low-dose aspirin 75 to 100 mg daily and clopidogrel 75 mg daily alone rather than cilostazol in addition to these drugs (Grade 1B).
We suggest aspirin 75 to 100 mg daily or clopidogrel 75 mg daily as part of dual antiplatelet therapy rather than the use of either drug with cilostazol (Grade 1B).
We suggest cilostazol 100 mg twice daily as substitute for either low-dose aspirin 75 to 100 mg daily or clopidogrel 75 mg daily as part of a dual antiplatelet regimen in patients with an allergy or intolerance of either drug class (Grade 2C).
For patients with CAD undergoing elective PCI but no stent placement:
We suggest for the first month dual antiplatelet therapy with aspirin 75 to 325 mg daily and clopidogrel 75 mg daily over single antiplatelet therapy (Grade 2C). Single antiplatelet therapy thereafter is recommended as per the established CAD recommendations (see recommendations 3.1.1-3.1.5).
5.1-5.3. For patients with systolic LV dysfunction without established CAD and no LV thrombus, we suggest not to use antiplatelet therapy or warfarin (Grade 2C).
Remarks: Patients who place a high value on an uncertain reduction in stroke and a low value on avoiding an increased risk of GI bleeding are likely to choose to use warfarin.
For patients with systolic LV dysfunction without established CAD with identified acute LV thrombus (eg, Takotsubo cardiomyopathy), we suggest moderate-intensity warfarin (INR 2.0-3.0) for at least 3 months (Grade 2C).
For patients with systolic LV dysfunction and established CAD, recommendations are as per the established CAD recommendations (see recommendations 3.1.1-3.1.5).
This article is devoted to long-term administration of antithrombotic drugs designed for primary and secondary prevention of cardiovascular disease. It does not address initial management of acute coronary syndromes (ACS) or periprocedural use of antithrombotic therapies.
We consider the desirable and undesirable consequences of antithrombotic treatment in the following populations and patient groups: (1) persons without established coronary artery disease (CAD); (2) patients with established CAD (established CAD is defined throughout as patients 1-year post ACS, with prior revascularization, coronary stenoses > 50% by coronary angiogram, and/or evidence for cardiac ischemia on diagnostic testing); including those post-ACS and post-coronary artery bypass graft (CABG) surgery; (3) patients with recent or remote percutaneous coronary intervention (PCI) with or without stents (bare-metal stents [BMS] or drug-eluting stents [DES]); and (4) patients with systolic left ventricular (LV) dysfunction (ischemic and nonischemic).
1.0 Methods
Table 1 describes the clinical questions (ie, population, intervention, comparator, and outcome) for each of the recommendations that follow. We define only patient characteristics relevant to our questions. For example, because whether ACS occurs with or without ST-segment elevation is not relevant to long-term secondary prevention, we provide a single set of recommendations for all patients following ACS. We have selected the same patient-important outcomes across all recommendations (eg, total mortality, nonfatal myocardial infarction [MI], nonfatal stroke, major extracranial bleed). We consider burden of treatment an important outcome for patients taking warfarin.
Table 1.
—Question Definition and Eligibility Criteria for Antithrombotic Treatments in Primary and Secondary Prevention of Cardiovascular Disease
| Section | Informal Question | PICO Question |
|||
| Population | Interventions | Comparator | Outcome(s) | ||
| 2.0 Primary prevention of cardiovascular disease | |||||
|
| |||||
| 2.1 | Choice of antithrombotic therapy | Persons without symptomatic cardiovascular disease | Aspirin | Placebo | Total mortality |
| Nonfatal MI | |||||
| Nonfatal stroke | |||||
| Major extracranial bleed | |||||
|
| |||||
| 3.0 Secondary prevention of cardiovascular disease (includes patients with prior CABG) | |||||
|
| |||||
| 3.1.1 | Choice of long-term antithrombotic therapy in patients with established CHD | Patients with established CHD | Aspirin | Placebo | Total mortality |
|
|
|
||||
| 3.1.2 | Clopidogrel | Aspirin | Nonfatal MI | ||
|
|
|
||||
| 3.1.3 | Clopidogrel + aspirin | Aspirin | Nonfatal stroke | ||
|
|
|
||||
| 3.1.4 | VKA moderate intensity + aspirin | Aspirin | Major extracranial bleed | ||
|
|
|
||||
| 3.1.5 | Dose of aspirin | Aspirin 75-100 mg | Aspirin > 100 mg | Burden of treatment (for VKA) | |
|
| |||||
| 3.2.1 | Choice of antithrombotic therapy the first year following ACS | Patients with recent ACS | Aspirin | Placebo | |
|
|
|
||||
| 3.2.2 | Clopidogrel | Aspirin | |||
|
|
|
||||
| 3.2.3 | Aspirin + clopidogrel | Aspirin | |||
|
|
|
||||
| 3.2.4 | Ticagrelor + aspirin | Clopidogrel + aspirin | |||
|
|
|
||||
| 3.2.5 | ACS + undergoing PCI | Prasugrel + aspirin | Clopidogrel + aspirin | ||
|
|
|
||||
| 3.2.6 | Patients with acute anterior STEMI and apical wall motion abnormality (± stent) | Aspirin + VKA | Aspirin ± clopidogrel | ||
|
|
|
||||
| 3.2.7 | Aspirin + clopidogrel + VKA | Aspirin + clopidogrel | |||
|
| |||||
| 4.0 Antithrombotic therapy following elective PCI | |||||
|
| |||||
| 4.1.1 | Choice of antithrombotic therapy following elective PCI | Patients undergoing elective PCI without stent placement | Aspirin + clopidogrel | Aspirin alone | Total mortality |
| Nonfatal MI | |||||
|
|
|
||||
| 4.1.2 | Patients undergoing elective PCI with stent placement | Thienopyridine + aspirin | VKA + aspirin | Stroke | |
|
|
|
||||
| 4.1.3 | Cilostazol + clopidogrel + aspirin | Clopidogrel + aspirin | Major extracranial bleeds | ||
|
|
|
||||
| 4.1.4 | Cilostazol + aspirin | Clopidogrel + aspirin | |||
|
| |||||
| 4.2 | Dose of aspirin following PCI | Patients undergoing PCI | ≤ 100 mg Aspirin | > 100 mg Aspirin | |
|
| |||||
| 4.3.1 | Duration of DAT (clopidogrel plus aspirin) following PCI with placement of BMS | Patients undergoing PCI with BMS | Minimum duration DAT 1 mo | No DAT | |
|
|
|
||||
| 4.3.2 | Extended duration DAT 6-12 mo | DAT 1 mo | |||
|
| |||||
| 4.3.3 | Duration of DAT following PCI with placement of DES | Patients undergoing PCI with DES | Minimum duration DAT 3-6 mo | No DAT | |
|
|
|
||||
| 4.3.4 | Extended duration DAT 1 y | DAT 3-6 mo | |||
|
|
|
||||
| 4.3.5 | Extended duration DAT > 1 y | DAT 1 y | |||
|
| |||||
| 5.0 Antithrombotic therapy in patients with systolic LV dysfunction | |||||
|
| |||||
| 5.1 | Choice of antithrombotic therapy in patients with nonischemic systolic LV dysfunction and no LV thrombus | Patients with nonischemic systolic LV dysfunction (without AF) and no LV thrombus | VKA | No VKA | Total mortality |
|
| |||||
| Aspirin | No aspirin | Nonfatal MI | |||
| Nonfatal stroke | |||||
| Major extracranial bleed | |||||
|
| |||||
| 5.2 | Choice of antithrombotic therapy in patients with non ischemic systolic LV dysfunction and LV thrombus | Patients with nonischemic systolic LV dysfunction (without AF) and LV thrombus | VKA | No warfarin | Burden of treatment (for VKA) |
|
| |||||
| 5.3 | Choice of antithrombotic therapy in patients with ischemic LV dysfunction | Patients with ischemic systolic LV dysfunction | Aspirin | Placebo | |
|
| |||||
| Clopidogrel | Aspirin | ||||
|
| |||||
| Clopidogrel + aspirin | Aspirin | ||||
|
| |||||
| VKA moderate intensity + aspirin | Aspirin | ||||
ACS = acute coronary syndrome; AF = atrial fibrillation; BMS = bare-metal stent; CABG = coronary artery bypass graft surgery; CHD = coronary heart disease; DAT = dual antiplatelet therapy; DES = drugeluting stent; LV = left ventricular; MI = myocardial infarction; PCI = percutaneous coronary intervention; PICO = population, intervention, comparator, outcome; STEMI = ST-segment elevation myocardial infarction; VKA = vitamin K antagonist.
Stent thrombosis frequently is reported in trials evaluating antiplatelet agents in patients undergoing PCI with stent placement. We have not included stent thrombosis as an important outcome because stent thrombosis derives its patient importance from consequent MI and deaths. Additional reporting of stent thrombosis along with MI and deaths would result in double counting of events and a distorted balance of benefits and harms.
Nonfatal hemorrhagic strokes and ischemic strokes are included together as nonfatal strokes. Although the former is a complication and prevention of the latter is a beneficial effect of antithrombotic therapy, their impact on patient morbidity is similar.
Estimation of Baseline Risks and Absolute Effects of Treatment
In order to estimate absolute benefits and harms associated with a given therapy, we performed the several steps. We first generated relative effect estimates (relative risks) from the highest-quality published meta-analysis of randomized controlled trials (RCTs) comparing therapies for a specific indication. If no such meta-analyses were available, we conducted our own meta-analyses of relevant RCTs or used relative risk estimates from single RCTs in the absence of other relevant RCTs.
Ideally, in order to approximate the benefit of a given therapy in the real world, population-based observational studies would inform estimates of baseline risk. Unfortunately, for most of our clinical questions, we were unable to identify observational studies of sufficient quality that reported all relevant outcomes. In such cases, we estimated control group risk from the control arm of either a relevant meta-analysis or a relevant RCT and adjusted them to our specified time frame. Individual sections present detailed explanations of our choices.
There are limited data to guide us with respect to the relative impact of outcomes on patient quality of life (see MacLean et al1 in this supplement). As described in the methodology article by Guyatt et al2 in these guidelines, we have used ratings from guideline panelists striving to infer a patient’s valuation of the outcomes of interest. The ratings suggest that major extracranial bleeding (which is usually readily treated and with few long-lasting consequences) carries only slightly less weight than a nonfatal MI (which also often has minimal long-term consequences) but substantially less weight than a stroke (which is often associated with long-term disability). Our decisions are based on a disutility of stroke of three times the disutility, or negative weight, of a major extracranial bleed.
Trade-offs between desirable and undesirable consequences of alternative management strategies sometimes represent close-call situations. For example, in the comparison of clopidogrel and aspirin vs aspirin alone in established CAD, available evidence from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management and Avoidance (CHARISMA) trial cannot rule out a benefit of dual antiplatelet therapy over aspirin alone, with a nonsignificant trend for benefit in cardiovascular outcomes such as vascular mortality, MI, and stroke.3 There is, however, suggested harm in terms of increased major bleeding events, with imprecise estimates of borderline statistical significance. In making recommendations in such situations, we have taken a primum non nocere approach, placing the burden of proof with those who would claim a benefit of treatment. In other words, when there is uncertain benefit and an appreciable probability of important harm (such as the aforementioned situation), we recommend against such treatments.
We identified the relevant evidence for our clinical questions with the assistance of a team of methodologists and medical librarians as outlined in the methodology article in this supplement.2 Systematic literature searches for systematic reviews and original studies were performed until the date of January 15, 2010. After that date, we scanned the literature regularly, although this was not performed as systematic literature searches.
2.0 Primary Prevention of Cardiovascular Disease
In this section, we address the effects of aspirin in primary prevention of cardiovascular disease. In addition, we consider recent meta-analyses demonstrating a reduction in cancer mortality and total mortality with long-term use of aspirin.4‐6 We do not include other antiplatelet therapies (eg, clopidogrel alone or in combination with aspirin) or oral anticoagulation (eg, warfarin) because they are not likely used in primary prevention. Whether aspirin should be prescribed in patients already receiving warfarin for atrial fibrillation (or other conditions) to enhance primary and secondary prevention of cardiovascular disease remains controversial. This topic is addressed in You et al.7
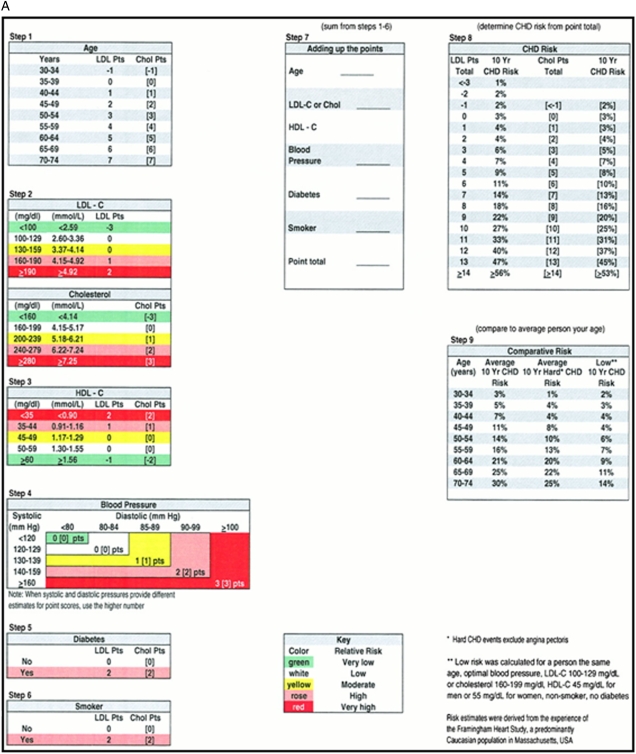
Users of this guideline require a tool to estimate risk of a cardiovascular event in the individual patient. Figure 1 shows the Framingham risk score that predicts the 10-year risk of developing a cardiovascular event (composite end point of MI and coronary death) as low (< 10%), moderate (10%-20%), and high (> 20%) risk.8
Figure 1.
[Section 2.0] Framingham risk score for cardiovascular events. A, Calculator for men. B, Calculator for women. Determine the number of points a patient receives for each risk factor (steps 1 through 6) and add them together (step 7). Using the point total in step 8 (using appropriate column - LDL or cholesterol depending on which was used in step 2), find the corresponding 10-year CHD risk. (Reprinted with permission from Wilson et al.101) CHD = coronary heart disease; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol.
We present absolute risk estimates for people at low, moderate, and high cardiovascular risk in a 10-year time frame based on the widely used Framingham risk score (Table 2). In order to derive our baseline control group risk estimates, we assumed patients with low, moderate, and high risk to have a 5%, 15%, and 25% risk of experiencing combined nonfatal and fatal MI, respectively.
Table 2.
—[Section 2.1] Aspirin (75-100 mg) Compared With No Aspirin in the Primary Prevention of Cardiovascular Diseasea,9
| Outcomes | Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects Over 10
y |
|
| Risk Without Aspirin | Risk Difference With Aspirin (95% CI) | ||||
| Total mortalitya | 100,076 (9), 3.8-10 y | Moderate due to imprecisionb | RR 0.94 (0.88 to 1.00) | 60-y-old manc | |
|
| |||||
| 100 deaths per 1,000c | 6 fewer deaths per 1,000 (from 12 fewer to 0 fewer) | ||||
|
| |||||
| MI nonfatal events | 95,000 (6), 3.8-10 y | High | RR 0.77 (0.69-0.86) | Low-cardiovascular-risk populationd | |
|
| |||||
| 27 MI per 1,000e | 6 fewer MI per 1,000 (from 8 fewer to 4 fewer) | ||||
|
| |||||
| Moderate-cardiovascular risk populationd | |||||
|
| |||||
| 83 MI per 1,000e | 19 fewer MI per 1,000 (from 26 fewer to 12 fewer) | ||||
|
| |||||
| High-cardiovascular-risk populationd | |||||
|
| |||||
| 136 per 1,000e | 31 fewer per 1,000 (from 42 fewer to 19 fewer) | ||||
|
| |||||
| Stroke includes nonfatal ischemic and hemorrhagic strokesf | 95,000 (6), 3.8-10 y | Moderate due to imprecisionb | RR 0.95 (0.85-1.06) | Low-cardiovascular-risk populationd | |
|
| |||||
| 23 strokes per 1,000e | No significant difference; 1 fewer stroke per 1,000 (from 3 fewer to 1 more) | ||||
|
| |||||
| Moderate-cardiovascular-risk populationd | |||||
|
| |||||
| 65 strokes per 1,000e | No significant difference; 3 fewer strokes per 1,000 (from 10 fewer to 4 more) | ||||
|
| |||||
| High-cardiovascular-risk populationd | |||||
|
| |||||
| 108 strokes per 1,000e | No significant difference; 5 fewer strokes per 1,000 (from 16 fewer to 8 more) | ||||
|
| |||||
| Major extracranial bleed | 95,000 (6), 3.8-10 y | High | RR 1.54 (1.30-1.82) | Low-cardiovascular-risk populationg | |
|
| |||||
| 8 bleeds per 1,000e | 4 more bleeds per 1,000 (from 2 more to 7 more) | ||||
|
| |||||
| Moderate-cardiovascular risk populationg | |||||
|
| |||||
| 24 bleeds per 1,000e | 16 more bleeds per 1,000 (from 7 more to 20 more) | ||||
|
| |||||
| High-cardiovascular-risk populationg | |||||
|
| |||||
| 40 bleeds per 1,000e | 22 more bleeds per 1,000 (from 12 more to 33 more) | ||||
GRADE = Grades of Recommendations, Assessment, Development, and Evaluation; RR = risk ratio. See Table 1 legend for expansion of other abbreviation.
This systematic review reports total mortality and includes the most recent trials but does not report specific causes of mortality. Other meta-analyses that use individual patient data report relative risk estimates for vascular mortality (RR, 0.97; 95% CI, 0.87-1.09), cancer mortality (RR, 0.66; 95% CI, 0.50-0.87), and fatal intracranial bleeds (RR, 1.73; 95% CI, 0.96-3.13). The risk of a fatal bleed (including extracranial and intracranial) was low (0.3% with aspirin and 0.2% with control).
The 95% CI for the absolute effect includes no benefit of aspirin. We did not rate down for risk of bias, but this was a borderline decision. Three of the trials did not blind patients, caregivers, or outcome adjudicators. Sensitivity analyses in meta-analysis by Raju et al4 did not show evidence of risk of bias.
Control group risk estimate for 10-y mortality apply to a 60-y-old man and came from population-based data from Statistics Norway. Mortality increases with age (eg, 50-y-old man; 50 deaths per 1,000 in 10 y) and is lower in women than in men (eg, 3% in women aged 50 y vs 5% in men aged 50 y).
Risk groups correspond to low risk (5%), medium risk (15%), high risk (25%) according to the Framingham score (or other risk tool) to estimate 10-y risk.
Control group risk estimates in low-, moderate-, and high-cardiovascular-risk groups are based on the Framingham score. As explained in the text, we have used data from an individual patient data meta-analysis to provide estimated risks for patient-important outcomes not covered by the Framingham risk score. We have also adjusted for 20% overestimation associated with Framingham risk score.
Of the strokes in the trials, 89 of 682 (13%) without aspirin were hemorrhagic and 116 of 655 (18%) with aspirin were hemorrhagic.
In the individual patient data meta-analysis risk for future major bleeding correlated with risk for future cardiovascular events. Therefore, we make the assumption that a patient at low, medium, or high risk of future cardiovascular events (determined by Framingham score) will be at low, medium, or high risk for future major bleeding events, respectively.
We believe that it is important to provide estimates separately for outcomes that patients value differently, as is the case for nonfatal MI, fatal MI, and stroke. The Framingham risk score does not allow separate calculation of nonfatal and fatal MI, and it does not include stroke or major extracranial bleeding. Therefore, to estimate the probability of each of these critical outcomes, we used the observed ratio of nonfatal MI to fatal MI to nonfatal stroke to major extracranial bleeding events in an individual participant data meta-analysis assessing benefits and harms of aspirin in primary prevention of cardiovascular disease.9 For example, a patient with a 5% (low) risk of fatal and nonfatal MI over 10 years based on the Framingham score would have a 3.3% risk of nonfatal MI, a 1.7% risk of a fatal MI, a 2.6% risk of nonfatal stroke, and a 1% risk of a major nonfatal extracranial bleed. Similar calculations were made to derive control group risk estimates for moderate- and high-risk strata.9
We made one additional modification to estimates from the Framingham risk score. The Framingham risk score overestimates 10-year coronary heart disease risk by 32% in men and 10% in women and is of little value in people aged > 85 years.10,11 We have adjusted our control group risk estimates accordingly, assuming 20% overestimation across sexes. For example, whereas Framingham estimates that 33 of 1,000 people at low cardiovascular risk will have a nonfatal MI without aspirin, our best estimate is that 27 of 1,000 people will have a nonfatal MI. Similar adjustments have been performed for vascular and bleeding outcomes because the Framingham risk estimate for nonfatal MI serves as the basis for the other risk estimates through our use of ratios from the individual participant data meta-analysis described later in this article.9
2.1 Aspirin
Table 2 (Table S1) summarizes results from an individual participant data meta-analysis that provides the best evidence regarding the benefits and harms of aspirin in primary prevention of cardiovascular disease.9 The meta-analysis includes 95,000 individuals (660,000 person-years, 3,554 vascular events) from six large trials (British Doctor Study, US Physicians’ Health Study, Thrombosis Prevention Trial, Hypertension Optimal Treatment Trial, Primary Prevention Project, and Women’s Health Study) that compared long-term aspirin use vs control.12‐17 Doses of aspirin varied between 75 mg and 300 mg without an apparent difference in benefit or harm. For total mortality, we used the relative-effect estimate derived from a high-quality systematic review and meta-analysis that included the most recent trials omitted from the individual participant data meta-analysis.4
Based on these analyses, aspirin use in patients at low risk would be associated with six fewer MIs and four more major bleeding events per 1,000 treated, with little or no effect on nonfatal stroke over a 10-year period (Table 2, Table S1). Aspirin would be associated with six fewer total deaths, but the 95% CI includes zero fewer deaths. For moderate- to high-risk patients, aspirin again would reduce nonfatal MI (19 fewer/1,000 treated and 31 fewer/1,000 treated, respectively) and increase major bleeding (16 more/1,000 treated and 22 more/1,000 treated, respectively), with a similar impact on total mortality (six fewer total deaths) as in the low-risk group. Our baseline risk estimate of 10-year mortality is derived from population-based data in Norway (www.ssb.no) and applies to a 60-year-old man. The overall quality of evidence is rated as moderate given the imprecision in the relative effect estimates for total mortality.
Patients averse to taking therapy for an extended duration for the potential of a very small decrease in total mortality may be disinclined to use long-term aspirin therapy for primary cardiovascular prevention. Patients (and physicians) may be interested in the effects on cause-specific mortality when considering aspirin prophylaxis. The individual participant data meta-analysis by Baigent et al9 reported a relative risk estimate for vascular mortality of 0.97 (95% CI, 0.87-1.09) associated with aspirin over a 10-year period. In another individual patient data meta-analysis, aspirin was associated with a reduction in cancer mortality (risk ratio [RR], 0.66; 95% CI, 0.50-0.87), which translated to ∼20 fewer cancer deaths (30 fewer to eight fewer) per 1,000 treated for 10 years.5 The impressive relative and anticipated absolute effect of aspirin therapy on cancer mortality contrast with the more-modest relative and absolute effect of aspirin on total mortality (three fewer deaths per 1,000). The difference in absolute effect is likely partly explained by the high 10-year risk of cancer mortality derived from the trials included in the individual participant data meta-analysis (60 per 1,000) compared with the low 10-year risk of total mortality derived from population-based data in a 50-year-old man (10 per 1,000). Apparently, patients enrolled in trials of aspirin aimed at reducing vascular risk were a population at high risk for cancer deaths.
We do not make specific recommendations for the use of aspirin based on patient characteristics, such as older age, sex, and diabetes mellitus. Other guidelines that do modify recommendations according to the presence or absence of such characteristics largely ignore any differences in bleeding risks and base their recommendations on evidence from what we believe are subgroup analyses of questionable validity.18‐22 Sophisticated risk calculators used in decision aids for specific populations may enhance individual decision-making, and when well done, we encourage their use.
Concerning diabetes, we (in contrast to some others) interpret current evidence as suggesting that the relative benefit of aspirin is similar in patients with and without diabetes. In two systematic reviews that include recent trials of patients with diabetes, CIs for the diabetes subgroup overlap with our estimates of relative effects from the combined population.23,24 Furthermore, analyses from the individual participant data meta-analysis provide no support for a difference in relative effect of aspirin in those with or without diabetes.9
Recommendation
2.1. For persons aged 50 years or older without symptomatic cardiovascular disease, we suggest low-dose aspirin 75 to 100 mg daily over no aspirin therapy (Grade 2B).
Remarks: Aspirin slightly reduces total mortality regardless of cardiovascular risk profile if taken over 10 years. In people at moderate to high risk of cardiovascular events, the reduction in MI is closely balanced with an increase in major bleeds. Whatever their risk status, people who are averse to taking medication over a prolonged time period for very small benefits will be disinclined to use aspirin for primary prophylaxis. Individuals who value preventing an MI substantially higher than avoiding a GI bleed will be, if they are in the moderate or high cardiovascular risk group, more likely to choose aspirin.
3.0 Secondary Prevention of Cardiovascular Disease
The evidence supporting the use of specific antithrombotic therapies sometimes differs between patients who have recently experienced an ACS and those with stable CAD. For purposes of these guidelines, and based on available data, recommendations for therapy following ACS will apply to the postdischarge period and extend to 1 year. Thereafter, patients will be considered to have established CAD. This definition is by necessity somewhat arbitrary, and we acknowledge that the higher-risk period following ACS may end before 1 year.
Most studies evaluating antithrombotic therapy immediately following CABG surgery have focused on a surrogate outcome, bypass graft patency, as the primary outcome. However, in making our recommendations, we focus exclusively on the relevant patient-important outcomes: nonfatal MI, nonfatal stroke, major extracranial bleeding, and death. Although substudies of large RCTs of antiplatelet therapy in patients with either CAD or recent ACS have examined clinical end points in patients with a history of remote CABG, these analyses do not suggest any significant differences in the associated relative benefit or harm compared with the overall study population.3,25‐27 In addition, loss of bypass graft patency derives its patient importance from consequent MI and deaths. Additional reporting of graft patency along with MI and death would result in double counting of events and a distorted balance of benefits and harms.
Accordingly, our recommendations for antithrombotic therapy in patients following elective CABG or CABG following ACS mirror those for patients with chronic CAD or recent ACS, respectively. For recommendations regarding continuation and discontinuation of antithrombotic therapy and timing of reinitiation relative to CABG, see Douketis et al28 in this supplement.
3.1 Choice of Long-term Antithrombotic Therapy in Patients With Established CAD
Control group risk estimates for nonfatal MI and stroke in patients not taking aspirin and in patients taking aspirin come from a meta-analysis of 16 RCTs adjusted to a 5-year time frame.9 Because this meta-analysis does not provide data on total mortality or nonfatal major extracranial bleeds, we derived baseline risk estimates from the aspirin arm in the CHARISMA trial (total mortality) and Clopidogrel Versus Aspirin in Patients at Risk of Ischaemic Events (CAPRIE) trial (major extracranial bleeds).3,29 To estimate control group risks for total mortality and major bleeds in patients not taking aspirin, we used estimates from the aspirin arm in these trials as the starting point and then applied the relative risks for total mortality and major bleeds to get to the control group risk estimate without aspirin.3,29 We used data regarding relative effects from the clopidogrel arm of the CAPRIE study, applied to baseline risks as previously mentioned, to generate control group risk estimates of vascular events and bleeding in patients taking clopidogrel alone.29
3.1.1 Aspirin:
Table 3 (Table S2) summarizes the quality of evidence and main findings from a meta-analysis of individual participant data from 16 randomized trials with 17,000 patients with established vascular disease (six trials of previous MI and 10 trials of previous transient ischemic attack [TIA] or stroke).9 In this population at high risk for a serious vascular event (8.2% yearly risk), aspirin significantly reduced total mortality, nonfatal MI, and nonfatal stroke at the cost of increased nonfatal extracranial bleeding events. The number of vascular events and total deaths prevented is far greater than the number of bleeding events that result from aspirin.
Table 3.
—[Sections 3.1.1-3.1.5, 3.2.1] Aspirin vs No Aspirin in Patients With Established CAD9
| Outcomes | Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects Over
5 y |
|
| Risk Without Aspirin | Risk Difference With Aspirin (95% CI) | ||||
| Total mortality |
17,000 (16 RCTs), 27 mo |
Moderate due to imprecisiona |
RR 0.90 (0.82-0.99) |
133 per 1,000b |
13 fewer per 1,000 (from 24 fewer to 1
fewer) |
| MI nonfatal events |
17,000 (16 RCTs), 27 mo |
High |
RR 0.69 (0.60-0.80) |
117 per 1,000b |
37 fewer per 1,000 (from 47 fewer to 23
fewer) |
| Stroke includes nonfatal ischemic and hemorrhagic
strokesc |
17,000 (16 RCTs), 27 mo |
High |
RR 0.81 (0.71-0.92) |
135 per 1,000b |
26 fewer per 1,000 (from 39 fewer to 11
fewer) |
| Major extracranial bleed | 17,000 (16 RCTs), 27 mo | Moderate due to indirectnessd | RR 2.69 (1.25-5.76) | 15 per 1,000e | 25 more per 1,000 (from 4 more to 71 more) |
CAD = coronary artery disease; CAPRIE = Clopidogrel vs Aspirin in Patients at Risk of Ischemic Events; CHARISMA = Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance; RCT = randomized controlled trial. See Table 1 and 2 legends for expansion of other abbreviations.
Rated down for imprecision because the 95% CI suggests possible benefit and no effect on total mortality.
Control group risk estimates (without aspirin) for MI and stroke come from observed yearly event rates in 16 RCTs reported in the meta-analysis, adjusted to a 5-y time frame. The control group rate estimate for total mortality without aspirin is derived from the event rate in the aspirin arm of the CHARISMA trial, using the RR of 0.90 to get the control group rate estimate without aspirin.
Of the strokes in the meta-analysis, 0.8% with aspirin were intracranial hemorrhages, and 0.4% of strokes without aspirin were intracranial hemorrhages.
Rated down for indirectness because bleeding events were only reported in a subset of trials with stroke and transient ischemic attack populations.
To estimate control group risks for major bleeds, we have used major bleed event rates from the aspirin arm in the CAPRIE trial adjusted to a 5-y time frame as the starting point (to ensure consistency across evidence profiles). We then used the RR of 2.69 for the comparison of aspirin to no aspirin observed in the meta-analysis to derive the control group rate estimate without aspirin.
The beneficial effects of aspirin are likely to also apply to patients with stable angina pectoris without prior MI. A well-performed systematic review and meta-analysis of antiplatelet therapy for prevention of vascular events in high-risk patients found that antiplatelet agents exerted similar effects on vascular events in patients with a history of MI (12 trials) and in patients with a history of stable angina and CAD (seven trials).30
3.1.2 Clopidogrel vs Aspirin:
The CAPRIE trial is the only randomized trial directly comparing clopidogrel and aspirin in the secondary prevention of cardiovascular events, and we consider this trial to be the most credible source of evidence.29 More than 19,000 patients with atherosclerotic vascular disease manifested as a recent stroke, recent MI, or symptomatic peripheral arterial disease received clopidogrel or aspirin. After a mean follow-up of 1.9 years, clopidogrel was associated with a possible reduction in nonfatal MI and nonfatal extracranial bleeding and little or no effect on total mortality. Table 4 (Table S3) summarizes the quality of evidence and main findings of the CAPRIE trial with anticipated absolute effects in a 5-year time frame for patients with established CAD. The results indicate no effect of clopidogrel on total mortality compared with aspirin. These results are consistent with a meta-analysis of 10 studies examining the effects of thienopyridine derivatives (eg, clopidogrel, ticlopidine) vs aspirin in patients at high vascular risk.31
Table 4.
—[Sections 3.1.1-3.1.5] Clopidogrel vs Aspirin for Patients With Established CAD29
| Outcomes | Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects Over
5 y |
|
| Risk With Aspirin | Risk Difference With Clopidogrel (95% CI) | ||||
| Total mortalitya |
19,185 (1 RCT), 1.9 y |
Moderate due to imprecisionb |
RR 0.98 (0.87-1.10) |
120 per 1,000c |
No significant difference; 2 fewer per 1,000 (from 16 fewer
to 12 more) |
| MI nonfatal events |
19,185 (1 RCT), 1.9 y |
Moderate due to imprecisionb |
RR 0.85 (0.72-1.00) |
80 per 1,000c |
12 fewer per 1,000 (from 22 fewer to 0 more) |
| Stroke includes nonfatal ischemic and hemorrhagic
strokesd |
19,185 (1 RCT), 1.9 y |
Moderate due to imprecisionb |
RR 0.94 (0.83-1.06) |
110 per 1,000c |
No significant difference; 7 fewer per 1,000 (from 19 fewer
to 7 more) |
| Major extracranial bleede | 19,185 (1 RCT), 1.9 y | Moderate due to imprecisionb | RR 0.88 (0.7-1.12) | 40 per 1,000f | No significant difference; 5 fewer per 1,000 (from 12 fewer to 5 more) |
Of the deaths in CAPRIE, 27 of 571 (4.7%) with aspirin were fatal bleeding events, and 23 of 560 (4.1%) with clopidogrel were fatal bleeding events.
Rated down for imprecision due to wide CIs for absolute effects, suggesting possible harm with clopidogrel for mortality, stroke, and bleeding and possible no effect for MI. Not rated down for inconsistency, although subgroup analysis of the composite end point reported a relative risk reduction of 7.3% for patients with stroke and 23.8% for patients with peripheral arterial disease and a relative risk increase of 3.7% for patients with MI (test for interaction P = .043). Based on criteria for credibility, we did not believe the results from the subgroup analysis; therefore, we did not rate down for inconsistency.
Control group risk estimates for total mortality come from the aspirin arm of the CHARISMA trial. Estimates for MI and stroke come from observed events in the aspirin arm of a meta-analysis of 16 RCTs in secondary prevention (Baigent et al9), adjusted to a 5-y time frame.
Of the strokes in CAPRIE, 24 of 486 (4.9%) with aspirin were hemorrhagic and 14 of 528 (2.6%) with clopidogrel were hemorrhagic.
Of the major extracranial bleeds in CAPRIE, 68 of 149 (45.6%) with aspirin were GI and 47 of 132 (35.6%) with clopidogrel were GI (P = .05).
Control group risk estimates come from observed major bleeding events in the CAPRIE trial, adjusted to a 5-y time frame, and not from the 16 studies included in the meta-analysis because these studies did not report major bleeds consistently.
Resource considerations—
Four studies that met criteria for review examined the cost-effectiveness of clopidogrel vs aspirin for secondary prevention of cardiovascular disease (Table S4). These studies considered multiple patient populations. Three studies32‐34 were based on the CAPRIE trial29 (patients with ischemic stroke in the prior 6 months, MI in the prior 35 days, or peripheral arterial disease). The fourth study was based on patients with prior TIA or nondisabling ischemic stroke.35 The latter study was included because patients with prior TIA or stroke are at higher risk for coronary heart disease. Coronary heart disease was considered as an outcome in all these studies. All these studies demonstrated that clopidogrel was cost-effective compared with aspirin, with incremental cost-effectiveness ratios similar after adjustment for the cost year. These results are limited in that they neglect any possible incremental benefit of aspirin over clopidogrel after > 5 years of use on cancer incidence (see section 2.1).
3.1.3 Dual Antiplatelet Therapy With Clopidogrel and Aspirin vs Single Antiplatelet Therapy:
A Cochrane systematic review evaluated short- and long-term dual antiplatelet therapy in patients with established CAD.36 Only one large-scale RCT, the CHARISMA trial, has evaluated the long-term efficacy of clopidogrel and aspirin vs aspirin alone.3 This trial followed 15,603 patients with established vascular disease or multiple risk factors for a mean period of 28 months. Table 5 (Table S5) summarizes the quality of the evidence and findings from this trial. Results of the study failed to demonstrate or exclude an effect of dual antiplatelet therapy relative to aspirin on total mortality or nonfatal MI. Dual antiplatelet therapy was associated with a possible reduction in nonfatal stroke and a possible increase in nonfatal extracranial bleeding. The quality of evidence is rated moderate because of imprecise effect estimates for all outcomes. Although this study included patients with other vascular diseases, we considered its findings directly applicable to patients with established CAD. We did not deem subgroup analyses suggesting different effects of dual antiplatelet therapy in symptomatic vs asymptomatic patients to be credible based on criteria by Sun et al.37
Table 5.
—[Secions 3.1.1-3.1.5] Aspirin Plus Clopidogrel vs Aspirin in the Secondary Prevention of Cardiovascular Events3
| Outcomes | Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects Over
5 y |
|
| Risk With Aspirin | Risk Difference With Aspirin + Clopidogrel (95% CI) | ||||
| Total mortalitya |
15,603 (1 RCT), 28 mo |
Moderate due to imprecisionb |
RR 0.99 (0.86-1.14) |
120 per 1,000c |
No significant difference; 1 fewer per 1,000 (from 17 fewer
to 17 more) |
| MI nonfatal events |
15,603 (1 RCT), 28 mo |
Moderate due to imprecisionb |
RR 0.94 (0.75-1.18) |
80 per 1,000c |
No significant difference; 5 fewer per 1,000 (from 20 fewer
to 14 more) |
| Stroke includes nonfatal ischemic and hemorrhagic
strokesd |
15,603 (1 RCT), 28 mo |
Moderate due to imprecisionb |
RR 0.81 (0.64-1.02) |
110 per 1,000c |
No significant difference; 21 fewer per 1,000 (from 40 fewer
to 2 more) |
| Major extracranial bleede | 15,603 (1 RCT), 28 mo | Moderate due to imprecisionb | RR 1.25 (0.97-1.61) | 40 per 1,000f | No significant difference; 10 more per 1,000 (from 1 fewer to 24 more) |
Of the deaths in the CHARISMA trial, 17 of 571 (3%) with aspirin were fatal bleeding events, and 26 of 574 (4.5%) with clopidogrel and aspirin were fatal bleeding events.
Rated down for imprecision because of wide CIs for absolute effects, suggesting important benefit, no benefit, or important harm with clopidogrel for all outcomes. Not rated down for inconsistency, although subgroup analysis found no significant effect of clopidogrel on vascular mortality in patients with established cardiovascular disease in contrast with increased mortality in asymptomatic patients. We judged claim of subgroup effect to be not credible (high number of subgroup hypotheses tested, unclear whether appropriate test for interaction used).
Control group risk estimates for total mortality come from the aspirin arm of the CHARISMA trial. Estimates for MI and stroke come from observed events in a meta-analysis of 16 RCTs in secondary prevention (Baigent et al9), adjusted to a 5-y time frame.
Of the strokes in CHARISMA, 27 of 189 (14%) with aspirin were intracranial hemorrhages, and 26 of 150 (17%) with clopidogrel were intracranial hemorrhages.
We excluded fatal bleeding and intracranial hemorrhage to avoid the double counting of events in the CHARISMA trial. Proportion of severe GI bleeds in CHARISMA was 0.65% (not reported separately for each treatment arm).
Control group risk estimates come from observed major bleeding events in the CAPRIE trial, adjusted to a 5-y time frame, and not from the 16 studies included in the meta-analysis or from CHARISMA because these studies did not report major bleeds consistently.
There are no studies comparing aspirin and clopidogrel to clopidogrel for secondary prevention in patients with CAD. The Management of Atherothrombosis With Clopidogrel in High-Risk Patients With Recent TIA or Ischemic Stroke (MATCH) study evaluated the efficacy and safety of clopidogrel plus aspirin compared with clopidogrel alone for 18 months in 7,599 patients with recent stroke or TIA and one other risk factor.38 Dual antiplatelet therapy was associated with a possible reduction in nonfatal stroke and a significant increase in major extracranial bleeding. Results failed to demonstrate or exclude an effect of dual antiplatelet therapy on vascular mortality or nonfatal MI (Table S6). We rated the overall quality of evidence from this trial as moderate given imprecision of point estimates for outcomes of MI, stroke, and total mortality. We did not rate down for indirectness because we considered the relative effects generated from this trial of patients with cerebrovascular disease to be directly applicable to patients with established CAD.
3.1.4 Moderate-Intensity Warfarin (International Normalized Ratio 2.0-3.0) Plus Aspirin vs Aspirin Alone:
Prior studies evaluating low-dose warfarin (international normalized ratio [INR] < 2.0) plus aspirin have not shown it to be more effective than aspirin alone in patients with CAD.39‐41 High-intensity warfarin (INR 2.8-4.2) without aspirin has proven to be more effective than aspirin alone in two prior randomized controlled clinical trials but was associated with increased bleeding risk.42,43 As a result, low-intensity warfarin plus aspirin or high-intensity warfarin are seldom used and will not be discussed further.
Rothberg et al44 performed a systematic review and meta-analysis of 10 randomized trials involving 5,938 patients with recent ACS who were randomized to moderate-to-high-intensity warfarin plus low-dose aspirin vs aspirin alone. We have performed our own meta-analysis of these studies (Table S7). In brief, the meta-analysis provides evidence of a substantial reduction in MI and nonfatal stroke with moderate-intensity warfarin plus aspirin at the costs of increased major extracranial bleeds.
These studies were completed in the pre-stent era, the majority started therapy immediately after ACS and had < 1-year follow-up, and we identified heterogeneity for the prevention of vascular events among patients with CAD, peripheral arterial disease, and nonembolic stroke. It is difficult to apply this evidence to patients with chronic CAD or ACS in the current era; therefore, we do not make recommendations for warfarin in these patient populations.
3.1.5 Aspirin Doses in Established CAD:
The best evidence of the effects of different aspirin doses on vascular and bleeding events comes from subgroup analyses in the Antithrombotic Trialists’ Collaboration30 meta-analysis of antiplatelet therapy, which included direct and indirect comparisons of different daily doses of aspirin (500-1,500 mg vs 160-325 mg vs 75-150 mg vs < 75 mg) on vascular events. In the direct comparisons of high- vs low-dose aspirin, there were no significant differences (ie, lower doses of aspirin were just as effective as higher doses). However, the small number of studies with aspirin < 75 mg left uncertainty about whether such low doses are as effective as daily doses of ≥ 75 mg. The indirect comparisons of higher daily doses of aspirin vs no aspirin provide no evidence to support that high doses of aspirin (eg, > 160 mg/d) are more effective than 75 to 160 mg. A subsequent systematic review of aspirin doses for the prevention of cardiovascular events in 2007 identified eight prospective trials that included nearly 10,000 patients taking aspirin 30 to 1,300 mg/d.45 A significant benefit of higher doses of aspirin was not identified in any of these studies, and in most, the lowest event rates were seen among patients randomized to the lower-dose group.
With respect to bleeding, a number of studies have suggested a potential relationship between increased aspirin doses and bleeding. A systematic review assessing bleeding rates associated with different doses of aspirin included > 190,000 patients enrolled in 31 RCTs.46 Aspirin > 200 mg was associated with an ∼30% increase in major bleeding compared with doses < 200 mg (P = .05). There was an increase in nonmajor bleeding in patients receiving 100 to 200 mg of aspirin per day compared with patients taking < 100 mg/d. The Antiplatelet Trialists’ Collaboration30 found no difference in the proportional increase in the risk of a major extracranial bleed between differing aspirin doses (< 75, 75-150, and 160-325 mg) compared with placebo but did not comment on doses > 325 mg. Taken together, the findings provide moderate-quality evidence (rated down for risk of bias because of indirect comparisons of different aspirin doses) to support the use of aspirin 75 to 100 mg/d for patients with established CAD.
Recommendations
3.1.1-3.1.5. For patients with established CAD (including patients after the first year post-ACS and/or with prior CABG surgery):
We recommend long-term single antiplatelet therapy with aspirin 75 to 100 mg daily or clopidogrel 75 mg daily over no antiplatelet therapy (Grade 1A).
We suggest single over dual antiplatelet therapy with aspirin plus clopidogrel (Grade 2B).
3.2 Choice of Antithrombotic Therapy Following ACS
For the purposes of these guidelines, we include patients with ST-segment elevation MI, non-ST-segment elevation MI, and unstable angina in the ACS population. This reflects our judgment that the relative efficacy and safety of specific therapies in the year following presentation does not differ substantially between these diagnostic entities. In addition, many studies evaluating antithrombotic therapy following ACS have included patients undergoing early PCI, stent placement, or both. Therefore, we use evidence from the total study cohorts, and for the most part, our recommendations apply to patients with ACS regardless of whether they undergo PCI. One exception is prasugrel, which has been studied primarily in patients with ACS with planned PCI; recommendations for this agent are restricted to this specific population. Recommendations for patients undergoing elective PCI/stenting (without ACS) are presented in a subsequent section.
Estimation of Baseline Risk—
There have been numerous studies of antithrombotic therapy following ACS. Depending on study population, date, and use of concomitant interventions, baseline risks vary widely. Ideally, we would identify a single population receiving different antithrombotic strategies in order to derive baseline risks. Because this is not possible, we use control group risks from Clopidogrel in Unstable Angina To Prevent Recurrent Events (CURE) for comparisons where aspirin constitutes the control group (as it did in CURE) and the Platelet Inhibition and Patient Outcomes (PLATO) study for comparisons where aspirin and clopidogrel constitute the control group.47,48 We selected CURE and PLATO because they were designed as large, simple trials; use accepted definitions for both vascular and bleeding events; and include a large proportion of patients who underwent cardiac catheterization/PCI, which reflects current practice in high-income countries.
3.2.1 Aspirin vs Placebo:
Table 3 summarizes the evidence from a meta-analysis with individual participant data from 16 RCTs with 17,000 patients with established vascular disease treated with aspirin vs placebo (including six trials of patients with previous MI).9 We deem this meta-analysis directly applicable to patients with recent ACS.
3.2.2 Clopidogrel vs Aspirin:
We again base our recommendation on evidence from the CAPRIE study, a randomized comparison of clopidogrel vs aspirin in the secondary prevention of cardiovascular events.29 Table 6 (Table S8) summarizes the evidence from the CAPRIE trial as it applies to an ACS population.
Table 6.
—[Sections 3.2.1-3.2.5] Clopidogrel vs Aspirin for Patients With Recent ACS29
| Outcomes | Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects Over
1 y |
|
| Risk With Aspirin | Difference With Clopidogrel (95% CI) | ||||
| Vascular mortalitya |
19,185 (1 RCT), 1.9 y |
Moderate due to imprecisionb |
RR 0.92 (0.80-1.07) |
60 per 1,000c |
No significant difference; 5 fewer per 1,000 (from 12 fewer
to 4 more) |
| MI nonfatal events |
19,185 (1 RCT), 1.9 y |
Moderate due to imprecisionb |
RR 0.85 (0.72-1.00) |
70 per 1,000c |
10 fewer per 1,000 (from 20 fewer to 0 more) |
| Stroke includes nonfatal ischemic and hemorrhagic
strokesd |
19,185 (1 RCT), 1.9 y |
High |
RR 0.94 (0.83-1.06) |
20 per 1,000c |
No significant difference; 1 fewer per 1,000 (from 3 fewer
to 1 more) |
| Major extracranial bleede | 19,185 (1 RCT), 1.9 y | Highf | RR 0.88 (0.7-1.12) | 30 per 1,000c | No significant difference; 3 fewer per 1,000 (from 9 fewer to 3 more) |
Of the deaths in CAPRIE 27 of 405 (6.7%) with aspirin were fatal bleeding events, and 23 of 372 (6.2%) with clopidogrel were fatal bleeding events.
Rated down for imprecision for MI because of a wide CI, including important benefit and no benefit with clopidogrel.
Control group risk estimates for death, MI, stroke, and bleeding come from the CURE trial (adjusted to 1-y time frame).
Of the strokes in CAPRIE, 24 of 486 (4.9%) with aspirin were hemorrhagic, and 14 of 528 (2.6%) with clopidogrel were hemorrhagic.
Of the major extracranial bleeds in CAPRIE, 68 of 149 (45.6%) with aspirin were GI, and 47 of 132 (35.6%) with clopidogrel were GI.
Our decision not to rate down for imprecision is due to the low control group risk for strokes and major bleeds that result in no important harm of clopidogrel (as judged by the upper limit of the 95% CI for the absolute effect).
3.2.3 Aspirin and Clopidogrel vs Aspirin:
During the past decade, the use of clopidogrel in addition to aspirin during the first 9 to 12 months after an ACS has become standard clinical practice. As recognized in a Cochrane systematic review,36 the CURE trial is the only study that has addressed the effects of clopidogrel in addition to aspirin in patients with ACS without ST-segment elevation.47 Table 7 (Table S9) presents the quality of the evidence and main findings of this trial that randomized 12,562 patients with a recent ACS to clopidogrel and aspirin or aspirin alone for 3 to 12 months, included 2,658 patients who underwent PCI following ACS, and provided moderate-quality evidence that dual antiplatelet therapy reduces MI and increases major bleeding events. Results failed to demonstrate or exclude an effect of dual antiplatelet therapy vs aspirin alone on vascular mortality or nonfatal stroke.
Table 7.
—[Sections 3.2.1-3.2.5] Aspirin Plus Clopidogrel vs Aspirin in Patients With a Recent ACS47
| Outcomes | Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects Over
1 y |
|
| Risk With Aspirin | Risk Difference With Clopidogrel + Aspirin (95% CI) | ||||
| Vascular mortalitya |
12,562 (1 RCT), 9 mo |
Moderate due to imprecisionb |
RR 0.93 (0.79-1.08) |
60 per 1,000c |
No significant difference; 4 fewer per 1,000 (from 13 fewer
to 5 more) |
| MI nonfatal events |
12,562 (1 RCT), 9 mo |
High |
RR 0.77 (0.67-0.89) |
70 per 1,000c |
16 fewer per 1000 (from 23 fewer to 8 fewer) |
| Stroke includes nonfatal ischemic and hemorrhagic
strokesd |
12,562 (1 RCT), 9 mo |
Moderate due to imprecisionb |
RR 0.86 (0.63-1.18) |
20 per 1,000c |
No significant difference; 3 fewer per 1,000 (from 7 fewer
to 4 more) |
| Major extracranial bleede | 12,562 (1 RCT), 9 mo | Moderate due to imprecisionb | RR 1.38 (1.13-1.67) | 30 per 1,000c | 11 more per 1,000 (from 4 more to 20 more) |
CURE = Clopidogrel in Unstable Angina to Prevent Recurrent Events. See Table 1-3 legends for expansion of other abbreviations.
Of the total deaths in the CURE trial, 15 of 390 (3.8%) with aspirin were fatal bleeding events, and 11 of 359 (3.1%) with clopidogrel were fatal bleeding events.
Rated down for imprecision because wide CIs included benefit and harm.
Control group risk estimates come from event rates in the aspirin arm of the CURE trial (adjusted to 1-y time frame).
Of the strokes in CURE, five of 87 (5.7%) with aspirin were hemorrhagic, and seven of 75 (9.3%) with clopidogrel were hemorrhagic.
Major bleed was defined as a substantially disabling bleed, intraocular bleed leading to the loss of vision, or bleeding necessitating the transfusion of at least 2 units of blood. Of the major extracranial bleeds in CURE, 47 of 169 (27.8%) with aspirin were GI, and 83 of 231 (35.9%) with clopidogrel were GI.
Resource Considerations—
Six studies33,49‐53 examined the cost-effectiveness of combined antiplatelet therapy with clopidogrel plus aspirin vs aspirin alone in patients after a recent ACS. These studies are consistent in demonstrating the cost-effectiveness of combined antiplatelet therapy with clopidogrel plus aspirin compared with aspirin alone after ACS. Schleinitz et al53 examined the effect of varying treatment duration and found that longer treatment duration was increasingly expensive, with incremental cost-effectiveness ratios (in 2010 US dollars) of $38,252/quality-adjusted life year (QALY) for 2 years, $74,204/QALY for 3 years, and $883,665/QALY for 5 years of treatment. Not only does cost-effectiveness decrease after 1 year but also the estimates represent extrapolation from the available data (patients were followed for only 1 year). Furthermore, evidence from a comparison of aspirin and clopidogrel vs aspirin raise serious questions about the extrapolation.3 Overall, the benefits of combined antiplatelet therapy with clopidogrel plus aspirin come at acceptable cost for the first year after ACS.
3.2.4 Ticagrelor and Aspirin vs Clopidogrel and Aspirin:
Ticagrelor is an oral, reversible, direct-acting inhibitor of the adenosine diphosphate receptor P2Y12 that has more-rapid onset and more-pronounced platelet inhibition than clopidogrel.54,55 Table 8 (Table S10) summarizes the quality of evidence and key findings from the PLATO trial that evaluated the effects of ticagrelor vs clopidogrel in patients with a recent ACS.56 In this study, 18,624 patients were randomized to receive, in addition to aspirin 75 mg/d, ticagrelor (180-mg loading dose, 90 mg bid thereafter) or clopidogrel (300-to 600-mg loading dose, 75 mg thereafter) for 6 to 12 months. At 12-month follow-up, ticagrelor significantly reduced vascular mortality and MI. Results failed to demonstrate or exclude an effect on nonfatal stroke. The rate of death from any cause was also reduced with ticagrelor (4.5% vs 5.9% with clopidogrel, P < .001). However, ticagrelor was associated with a higher rate of major bleeding not related to CABG (2.8% vs 2.2%, P = .03). The quality of evidence from this study was deemed moderate because of imprecision in nonfatal stroke and major extracranial bleeding.
Table 8.
—[Sections 3.2.1-3.2.5] Ticagrelor Plus Aspirin vs Clopidogrel Plus Aspirin in Patients With a Recent ACS56
| Outcomes | Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects Over
1 y |
|
| Risk With Clopidogrel and Aspirin | Risk Difference With Ticagrelor and Aspirin (95% CI) | ||||
| Vascular mortalitya |
18,624 (1 RCT), 6-12 mo |
High |
RR 0.79 (0.69-0.91) |
50 per 1,000b |
10 fewer per 1,000 (from 15 fewer to 4 fewer) |
| MI nonfatal events |
18,624 (1 RCT), 6-12 mo |
High |
RR 0.84 (075-0.95) |
70 per 1,000b |
11 fewer per 1,000 (from 17 fewer to 3 fewer) |
| Stroke includes nonfatal ischemic and hemorrhagic
strokesc |
18,624 (1 RCT), 6-12 mo |
Moderate due to imprecisiond |
RR 1.17 (0.91-1.52) |
13 per 1,000b |
No significant difference; 2 more per 1,000 (from 1 fewer to
7 more) |
| Major extracranial bleed | 18,624 (1 RCT), 6-12 mo | Moderate due to imprecisiond | RR 1.25 (1.01-1.53) | 22 per 1,000b | 6 more per 1,000 (from 0 more to 11 more) |
PLATO = Platelet Inhibition and Patient Outcomes. See Table 1-3 legends for expansion of other abbreviations.
Of the total deaths in the PLATO study, 20 of 399 (5.0%) with ticagrelor were fatal bleeding events, and 23 of 506 (4.5%) with clopidogrel were fatal bleeding events.
One-year control group risk estimates come from event rates in the clopidogrel arm of PLATO adjusted to a 1-y time frame.
Of the total strokes in PLATO, 23 of 125 (18.4%) with ticagrelor were hemorrhagic, and 13 of 106 (12.3%) with clopidogrel were hemorrhagic.
Rated down for imprecision due to wide CIs including harm with ticagrelor for stroke and bleeds.
A separate publication reports results from the subset of patients who underwent PCI.48 PCI was performed during the index hospitalization in 61% of patients, of whom 60% received intracoronary stents. The effects of ticagrelor compared with clopidogrel on vascular mortality, MI, stroke, and major bleeds appear to be similar in this subset of patients compared with the overall population.
Although the original study design was not intended to stratify observed outcomes by geographical region, patients enrolled in North America reportedly had a higher incidence of adverse cardiovascular outcomes (whereas net benefit was observed in other areas), which initially delayed US approval of ticagrelor pending further data review. After further post hoc analysis, the only baseline covariate identified as possibly contributing to geographic variation was use of higher doses of aspirin in the United States. To date, these data have not been published. The US Food and Drug Administration approved ticagrelor for patients with ACS in July 2010 but recommend against this agent in patients taking > 100 mg of aspirin per day.
3.2.5 Prasugrel and Aspirin vs Clopidogrel and Aspirin:
Prasugrel is a novel thienopyridine that achieves more-rapid and more-consistent platelet inhibition than standard-dose clopidogrel. Table 9 (Table S11) summarizes the quality of evidence and key findings from the TRITON-TIMI (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis in Myocardial Infarction) 38, the only published randomized trial to evaluate prasugrel vs clopidogrel in patients with recent ACS who undergo PCI.57 In this trial, 13,608 patients with moderate- to high-risk ACS and a scheduled PCI were randomized to receive, in addition to aspirin 75 mg/d, prasugrel (60-mg loading dose followed by 10 mg/d) or clopidogrel (300-mg loading dose followed by 75 mg/d) for 6 to 15 months. Ninety-nine percent of patients had PCI at the time of randomization, and 94% received intracoronary stents. Prasugrel significantly reduced MI but increased major bleeding, including life-threatening and fatal bleeds. Prasugrel was associated with a possible reduction in vascular mortality. Results failed to demonstrate or exclude an effect on nonfatal stroke. The quality of evidence is rated down because of imprecision in vascular mortality, nonfatal stroke, and major extracranial bleeding.
Table 9.
—[Sections 3.2.1-3.2.5] Prasugrel Plus Aspirin vs Clopidogrel Plus Aspirin in Patients With a Recent ACS and PCI57
| Outcomes | Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects Over
1 y |
|
| Risk With Clopidogrel and Aspirin | Risk Difference With Prasugrel and Aspirin (95% CI) | ||||
| Vascular mortalitya |
13,608 (1 RCT), 14.5 mo |
Low due to inconsistencyb and imprecisionc |
RR 0.89 (0.70-1.12) |
50 per 1,000d |
No significant difference; 5 fewer per 1,000 (from 15 fewer
to 6 more) |
| MI nonfatal events |
13,608 (1 RCT), 14.5 mo |
Moderate due to inconsistencyc |
RR 0.76 (0.67-0.85) |
70 per 1,000d |
17 fewer per 1,000 (from 23 fewer to 10 fewer) |
| Stroke includes nonfatal ischemic and hemorrhagic
strokese |
13,608 (1 RCT), 14.5 mo |
Low due to inconsistencyb and imprecisionc |
RR 1.02 (0.71-1.45) |
13 per 1,000d |
No significant difference; 0 more per 1,000 (from 4 fewer to
6 more) |
| Major extracranial bleed | 13,608 (1 RCT), 14.5 mo | Low due to inconsistencyb and imprecisionc | RR 1.32 (1.03-1.68) | 22 per 1,000d | 7 more per 1,000 (from 0 more to 15 more) |
Fatal bleeds were 0.4% with prasugrel and 0.1% with clopidogrel.
Rated down for inconsistency for all outcomes due to credible subgroup analyses showing net harm for composite end point in certain subgroups.
Rated down for imprecision due to wide CIs suggesting important benefit or harm with prasugrel.
Control group risk estimates come from the event rates in the clopidogrel arm of the PLATO study, adjusted to a 1-y time frame.
Hemorrhagic strokes constituted 0.3% of all strokes in both groups.
Post hoc exploratory subgroup analyses spurred by these observations suggested that patients with a history of stroke or TIA before enrollment had net harm from prasugrel treatment, whereas elderly (aged > 75 years) patients and patients with a body weight < 60 kg had no net benefit from prasugrel (composite outcome of all-cause mortality, MI, stroke, and non-CABG-related TIMI major bleeding) (tests for interaction P = .06 for both). We judged the claimed subgroup effects to be of moderate credibility. The Food and Drug Administration labeling includes a boxed warning that the drug should not be used in patients with a history of TIA or stroke or urgent need for surgery, including CABG. The manufacturer recommends a decreased maintenance dose of 5 mg/d for patients weighing < 60 kg, although this particular recommendation is based on pharmacokinetic/pharmacodynamic modeling rather than on clinical data. Experts have expressed concern about the increased bleeding risks with intensified platelet inhibition.58
Recommendations
3.2.1-3.2.5. For patients in the first year after an ACS who have not undergone PCI:
We recommend dual antiplatelet therapy (ticagrelor 90 mg twice daily plus low-dose aspirin 75-100 mg daily or clopidogrel 75 mg daily plus low-dose aspirin 75-100 mg daily) over single antiplatelet therapy (Grade 1B).
We suggest ticagrelor 90 mg twice daily plus low-dose aspirin over clopidogrel 75 mg daily plus low-dose aspirin (Grade 2B).
For patients in the first year after an ACS who have undergone PCI with stent placement:
We recommend dual antiplatelet therapy (ticagrelor 90 mg twice daily plus low-dose aspirin 75-100 mg daily, clopidogrel 75 mg daily plus low-dose aspirin, or prasugrel 10 mg daily plus low-dose aspirin over single antiplatelet therapy) (Grade 1B).
Remarks: Evidence suggests that prasugrel results in no benefit or net harm in patients with a body weight of less than 60 kg, age above 75 years, or with a previous stroke/TIA.
We suggest ticagrelor 90 mg twice daily plus low-dose aspirin over clopidogrel 75 mg daily plus low-dose aspirin (Grade 2B).
For patients with ACS who undergo PCI with stent placement, we refer to sections 4.3.1 to 4.3.5 for recommendations concerning minimum and prolonged duration of treatment.
3.2.6 Antithrombotic Therapy in Patients With Acute Anterior MI and LV Thrombus (or at Risk for LV Thrombus):
Patients with large anterior MI have a high risk of developing LV thrombus and subsequent systemic embolization (eg, stroke, peripheral embolization). Observational studies prior to the advent of thrombolysis and PCI suggested rates of LV thrombus formation as high as 20% to 50%.59‐62 More recent studies suggest LV thrombus rates of ∼15% in patients with anterior MI63,64 and up to 27% with anterior ST-segment elevation MI and LV ejection fraction < 40%.65
Embolization rates in patients with anterior MI who develop LV thrombus and who are not treated with warfarin therapy are more difficult to estimate. In a natural history study of 198 consecutive patients with MI conducted from 1985 to 1987,62 LV thrombus occurred in 38 of 124 (31%) of patients with anterior MI. Deterioration in LV function, discharge ejection fraction ≤ 35%, or apical aneurysm/dyskinesis predicted development of LV thrombus by logistic regression analysis. Six of 35 patients (17%) with LV thrombus on predischarge echocardiogram experienced systemic embolization. Unfortunately, presence or absence of warfarin treatment was not included as a variable in regression analyses.
Vaitkus et al66 performed a meta-analysis of 11 observational studies of the effects of anticoagulation on the incidence of LV thrombosis and systemic embolization in patients with Q-wave (transmural) anterior MI. Anticoagulation with vitamin K antagonists decreased the risk of LV thrombus formation (adjusted OR, 0.32; 95% CI, 0.20-0.52) (four studies, 307 patients) and embolization (adjusted OR, 0.14; 95% CI, 0.04-0.52) (seven studies, 270 patients). Systemic embolization was ∼11% in patients with LV thrombus vs 2% in those without LV thrombus (adjusted OR, 5.45; 95% CI, 3.02-9.83).
Given these data as well as prior studies suggesting that warfarin plus aspirin is more effective in patients with established CAD than aspirin alone (Table S7), the benefits of adding warfarin to aspirin in patients with large anterior MI (ejection fraction < 40%, anteroapical wall motion abnormality) who are not undergoing stent placement, particularly those with LV thrombus, likely outweighs the bleeding risk.
3.2.7 Anterior MI, LV Thrombus, and Stent Placement:
Extrapolating these data to the current era in which most patients with a large anterior MI will undergo PCI and stent placement is difficult. Although aspirin and clopidogrel are superior to warfarin for the prevention of acute stent thrombosis, their relative effect on the prevention of systemic embolization in patients with LV thrombus is largely unknown. Physicians must attempt to weigh the potential benefits and risks of adding warfarin to dual antiplatelet therapy in these patients.
Table 10 (Table S12) summarizes the evidence and anticipated absolute effects of triple therapy vs dual antiplatelet therapy in patients with large anterior MI at risk for or with LV thrombus who undergo PCI with stent placement. In the absence of direct comparisons, we used indirect evidence to address this question. For nonstroke outcomes (death, MI, and major bleeds), we make the assumption that the relative impact of triple therapy (aspirin, clopidogrel, and warfarin) vs dual therapy (aspirin plus clopidogrel) is similar to that of warfarin plus aspirin vs aspirin alone. We use data from studies included in the meta-analysis by Rothberg et al44 that compared warfarin plus aspirin to aspirin alone following ACS to derive relative risk estimates for the outcomes of mortality, nonfatal MI, and major bleeding (Table S7).
Table 10.
—[Sections 3.2.6-3.2.7] Triple Therapy (Warfarin, Aspirin, Clopidogrel) vs Dual Antiplatelet Therapy in Patients With Acute Large Anterior MI at Risk for or With LV Thrombus Who Undergo PCI With Stent Placement44
| Outcomes | Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects Over
3 mo |
|
| Risk With Clopidogrel and Aspirin | Risk Difference With Warfarin + Clopidogrel and Aspirin (95% CI) | ||||
| Total mortality | 10,883 (10 RCT), 3-60 mo | Low due to indirectnessa and imprecisionb | RR 1.00 (0.82-1.22) | 25 per 1,000c | No significant difference; 0 fewer per 1,000 (from 4 fewer to 6 more) |
|
| |||||
| MI nonfatal events | 10,883 (10 RCTs), 3-60 mo | Low due to serious indirectnessa | RR 0.69 (0.54-0.88) | 35 per 1,000c | 11 fewer per 1,000 (from 16 fewer to 4 fewer) |
|
| |||||
| Stroke includes nonfatal ischemic and hemorrhagic strokesd | 6,709 (1 RCT), 1.3 y | Low due to indirectnessd and imprecisionb | RR 0.56 (0.39-0.82) | Anteroapical MI without LV thrombus | |
|
| |||||
| 15 per 1,000e | 7 fewer per 1,000 (from 9 fewer to 3 fewer) | ||||
|
| |||||
| Anteroapical MI with LV thrombus | |||||
|
| |||||
| 100 per 1,000e | 44 fewer per 1,000 (from 18 fewer to 61 fewer) | ||||
|
| |||||
| Major extracranial bleed | 10,883 (10 RCTs), 3-60 mo | Low due to indirectnessa | RR 2.37 (1.62-3.47) | 11 per 1,000c | 15 more per 1,000 (from 7 more to 27 more) |
|
| |||||
| Burden of treatmentf | Not applicable | High | Warfarin > aspirin | Warfarin: daily medication, dietary and activity restrictions, frequent blood testing/monitoring, increased hospital/clinic visits | |
|
| |||||
| Aspirin: daily medication only | |||||
ACTIVE-W = Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events. See Table 1-3, and 8 legends for expansion of other abbreviations.
Relative risk for warfarin, aspirin, and clopidogrel vs dual antiplatelet therapy was derived from a meta-analysis of studies comparing warfarin plus aspirin to aspirin alone in patients following ACS.
Rated down for imprecision for total mortality due to wide CIs suggesting important harm and benefit with warfarin plus aspirin. For stroke, we rated down for imprecise baseline risk estimates.
Control group risk estimates (aspirin + clopidogrel) come from PLATO trial, adjusted to a 3-mo time frame assuming that one-half of the total events at 1 y occurred in the first 3 mo (as was the case in the PLATO trial).
We assumed that the relative risk for the outcome of nonfatal stroke (ischemic and hemorrhagic) would be the same as observed in the ACTIVE-W study, which compared warfarin to dual antiplatelet therapy (aspirin + clopidogrel). We calculated the RR and 95% CI after extracting the number of nonfatal strokes (ischemic and hemorrhagic) in each group from the published report because it did not directly report RR in the article.
Control group risk estimates for nonfatal stroke is based on an ∼1.5% rate/3 mo (see text) with clopidogrel and aspirin following anterior MI and 10% rate/3 mo in patients with anterior MI and LV thrombus. There is considerable imprecision in these estimates.
There are studies evaluating quality of life in patients during warfarin treatment (with disparate findings), but these are limited by small sample size, lack of comparator, and other design issues.
We also assumed that the relative effects of triple therapy vs dual therapy on nonfatal stroke would be similar to that of warfarin alone vs aspirin plus clopidogrel. We used data from the Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events (ACTIVE-W) study to derive the relative risk estimate for nonfatal stroke.67 This assumption may underestimate the potential benefit of triple therapy relative to dual antiplatelet therapy on vascular outcomes.
In patients with large anterior MI but no thrombus, LV thrombus is estimated to develop in ∼15%.62,66 Given the estimated 10% associated risk of embolic stroke, there is 1.5% risk of stroke at 3 months without warfarin therapy. As shown in Table 10 (Table S12), we estimated that patients with large anterior MI but no initial thrombus who receive warfarin in addition to dual antiplatelet therapy will have seven fewer nonfatal strokes and 15 more extracranial nonfatal bleeds per 1,000 treated. For patients with large anterior MI and demonstrated LV thrombus, the addition of warfarin to antiplatelet therapy would be expected to result in 44 fewer nonfatal strokes and 15 more nonfatal extracranial bleeds. The addition of warfarin to dual antiplatelet therapy following MI may result in an absolute decrease of 11 MIs per 1,000 patients treated.
Given the increased risk of major bleeding, the duration of triple therapy, if chosen, should be minimized. Although the formation of LV thrombus was observed in most patients in the first few weeks, additional clots developed up to 3 months after anterior MI. For patients at risk for LV thrombus (but no thrombus identified on initial echocardiogram) in whom warfarin therapy is withheld, repeat echocardiogram in 1 to 2 weeks to rule out subsequent development of thrombus may be advisable.
As is discussed subsequently, we suggest that the minimal duration of dual antiplatelet therapy should be 1 month following BMS and 3 to 6 months following DES. These time periods were considered in developing our recommendations for this section.
Recommendations
3.2.6-3.2.7. For patients with anterior MI and LV thrombus or at high risk for LV thrombus (ejection fraction < 40%, anteroapical wall motion abnormality) who do not undergo stenting:
We recommend warfarin (INR 2.0-3.0) plus low-dose aspirin 75 to 100 mg daily over single antiplatelet therapy or dual antiplatelet therapy for the first 3 months (Grade 1B). Thereafter, we recommend discontinuation of warfarin and continuation of dual antiplatelet therapy for up to 12 months as per the ACS recommendations (see recommendations 3.2.1-3.2.5). After 12 months, single antiplatelet therapy is recommended as per the established CAD recommendations (see recommendations 3.1.1-3.1.5).
For patients with anterior MI and LV thrombus, or at high risk for LV thrombus (ejection fraction < 40%, anteroapical wall motion abnormality), who undergo BMS placement:
We suggest triple therapy (warfarin [INR 2.0-3.0], low-dose aspirin, clopidogrel 75 mg daily) for 1 month over dual antiplatelet therapy (Grade 2C).
We suggest warfarin (INR 2.0-3.0) and single antiplatelet therapy for the second and third month post-BMS over alternative regimens and alternative time frames for warfarin use (Grade 2C). Thereafter, we recommend discontinuation of warfarin and use of dual antiplatelet therapy for up to 12 months as per the ACS recommendations (see recommendations 3.2.1-3.2.5). After 12 months, antiplatelet therapy is recommended as per the established CAD recommendations (see recommendations 3.1.1-3.1.5).
For patients with anterior MI and LV thrombus or at high risk for LV thrombus (ejection fraction < 40%, anteroapical wall motion abnormality) who undergo DES placement:
We suggest triple therapy (warfarin [INR 2.0-3.0], low-dose aspirin, clopidogrel 75 mg daily) for 3 to 6 months over alternative regimens and alternative durations of warfarin therapy (Grade 2C). Thereafter, we recommend discontinuation of warfarin and continuation of dual antiplatelet therapy for up to 12 months as per the ACS recommendations (see recommendations 3.2.1-3.2.5). After 12 months, antiplatelet therapy is recommended as per the established CAD recommendations (see recommendations 3.1.1-3.1.5).
4.0 Antithrombotic Therapy Following Elective PCI
Choice and duration of antiplatelet therapy following PCI depends on the setting (acute vs elective), whether a stent is placed, and the type of stent (DES vs BMS) placed. We have previously discussed evidence for antithrombotic therapy following PCI in patients with ACS. In this section, we discuss antithrombotic therapy following elective PCI. As in prior sections, we address the patient-important outcomes of death, nonfatal MI, nonfatal stroke (if reported), and major bleeding.
Estimation of Baseline Risk—
For the comparison of thienopyridines plus aspirin vs warfarin plus aspirin following elective PCI, we chose vascular and bleeding risks from the warfarin plus aspirin arm of a systematic review of four RCTs.68 For the comparisons involving cilostazol as part of dual or triple antiplatelet therapy vs aspirin plus clopidogrel, we chose baseline risks from the clopidogrel plus aspirin arm of a systematic review of 10 RCTs examining cilostazol following elective PCI.69 For the comparison of high- vs low-dose aspirin following PCI, we chose the low-dose aspirin arm of the CURRENT-OASIS 7 (Clopidogrel Optimal Loading Dose Usage To Reduce Recurrent Events/Optimal Antiplatelet Strategy for Interventions) study.70 For duration of dual antiplatelet therapy following placement of BMS (12 months vs 1 month), we chose baseline risks from the 1-month dual antiplatelet therapy arm from a meta-analysis we performed of relevant RCTs. For duration of dual antiplatelet therapy following placement of DES (> 1 vs < 1 year), we used the risk estimate from the < 1 year arm of the merged REAL LATE (Correlation of Clopidogrel Therapy Duration in Real-World Patients Treated with Drug-Eluting Stent Implantation and Late Coronary Arterial Thrombotic Events) and ZEST LATE (Evaluation of the Long-Term Safety after Zotarolimus-Eluting Stent, Sirolimus-Eluting Stent, or Paclitaxel-Eluting Stent Implantation for Coronary Lesions-Late Coronary Arterial Thrombotic Events) studies.71 These studies were merged while ongoing because of slow enrollment and similar study designs.
4.1.1 Antithrombotic Therapy Following Balloon Angioplasty Without Stent Placement:
All patients undergoing stent procedures undergo balloon angioplasty, but on rare occasions, balloon angioplasty is not followed by stent placement. In many respects, balloon angioplasty can be considered a controlled rupture of a coronary plaque. Short-term antithrombotic therapy following this iatrogenic plaque rupture is necessary to prevent initiation of subsequent thrombotic events that may lead to MI. In the prestent era, patients undergoing balloon angioplasty generally were treated with aspirin alone. Extrapolation of evidence from patients with ACS and undergoing stent placement suggests that dual antiplatelet therapy with low-dose aspirin plus clopidogrel may achieve additional reduction in thrombosis (see sections 3.2.3, 3.2.4, and 4.3.1).
4.1.2 Short-term Dual Antiplatelet Therapy (Thienopyridine and Aspirin) Following Elective PCI With Stenting:
Stent placement following balloon angioplasty was initially limited by high rates of acute or subacute stent thrombosis (6%-24%) secondary to the thrombogenicity of metal stent struts.72‐75 Concomitantly, a number of studies compared a new strategy, aspirin plus ticlopidine, to the previously most successful strategy of aspirin plus warfarin in patients undergoing stent placement. A Cochrane systematic review of four randomized trials including 2,436 patients found that a 30- to 42-day course of ticlopidine plus aspirin vs warfarin plus aspirin reduced the 30- to 42-day risk of nonfatal MI (RR, 0.50; 95% CI, 0.30-0.83; number needed to treat, 55) and revascularization (RR, 0.29; 95% CI, 0.16-0.56; number needed to treat, 33), with a possible reduction in major bleeding (RR, 0.36; 95% CI, 0.14-1.02).68 Table 11 (Table S13) summarizes the quality of evidence and main findings from the meta-analysis. Given the thrombocytopenia/neutropenia as well as rare cases of thrombotic thrombocytopenic purpura associated with ticlopidine, ticlopidine has been largely replaced by clopidogrel. In the current era of dual antiplatelet therapy, early stent thrombosis occurs rarely (< 2%).
Table 11.
—[Sections 4.1.1-4.3] Thienopyridine Plus Aspirin vs Warfarin Plus Aspirin in the First Month Following PCI68
| Outcomes | Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects Over
30 d |
|
| Risk With Warfarin and Aspirin | Risk Difference With Thienopyridine and Aspirin (95% CI) | ||||
| Total mortality |
2,436 (4 RCTs), 4-6 wk |
Moderate due to imprecisiona |
RR 0.73 (0.25-2.18) |
7 per 1,000b |
No significant difference; 2 fewer per 1,000 (from 5 fewer
to 8 more) |
| MI nonfatal events |
13,608 (1 RCT), 14.5 mo |
Moderate due to risk of biasc |
RR 0.50 (0.29-0.83) |
39 per 1,000b |
19 fewer per 1,000 (from 28 fewer to 7 fewer) |
| Stroke |
This critical outcome was not
reported in the meta-analysis |
||||
| Major extracranial bleedd | 2,436 (4 RCTs), 4-6 wk | Low due to inconsistency,e imprecision,a and risk of biasc | RR 0.38 (0.14-1.02) | 64 per 1,000b | No significant difference; 40 fewer per 1,000 (from 55 fewer to 1 more) |
Wide CIs including benefit and harm (total mortality) or no benefit (major bleeding events).
Control group risk estimates come from the meta-analysis.
Lack of blinding in RCTs.
Bleeding definitions varied greatly across studies.
Heterogeneity was observed for major bleeding events (I = 72%).
4.1.3 Cilostazol Plus Clopidogrel Plus Aspirin vs Clopidogrel Plus Aspirin:
Cilostazol is a phosphodiesterase III inhibitor that has antiplatelet and antithrombotic effects and reduces intimal hyperplasia after endothelial injury, properties that have led to trials evaluating its efficacy for the prevention of restenosis after PCI. A systematic review by Tamhane and colleagues69 identified 10 RCTs (n = 2,809) comparing cilostazol + clopidogrel + aspirin vs clopidogrel and aspirin following stent placement. Treatment and follow-up ranged from 6 to 9 months. Table 12 (Table S14) summarizes the quality of evidence and main findings from the meta-analysis of triple therapy with cilostazol vs dual therapy. Results failed to demonstrate or exclude an effect of cilostazol on reinfarction, major bleeding, and mortality between the two groups. Triple therapy showed an increased risk of skin rash (OR, 3.67; 95% CI, 1.86-7.24) (three RCTs). Sensitivity analyses did not materially affect the results, and there was no evidence of publication bias or statistical heterogeneity.
Table 12.
—[Sections 4.1.1-4.3.5] Triple Therapy With Cilostazol vs Clopidogrel + Aspirin Following Elective PCI With Stenting69
| Outcomes | Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects Over
6-9 mo |
|
| Risk With Clopidogrel and Aspirin | Risk Difference With Cilostazol + Clopidogrel and Aspirin (95% CI) | ||||
| Total mortality |
2,809 (10 RCTs), 6-9 mo |
Moderate due to imprecisiona |
RR 0.73 (0.25-2.12) |
20 per 1,000b |
No significant difference; 5 fewer per 1,000 (from 15 fewer
to 22 more) |
| MI nonfatal events |
2,809 (10 RCTs), 6-9 mo |
Moderate due to imprecisiona |
RR 1.12 (0.57-2.24) |
50 per 1,000b |
No significant difference; 6 more per 1,000 (from 21 fewer
to 62 more) |
| Stroke |
This critical outcome was not
reported in the meta-analysis |
||||
| Major extracranial bleed | 2,809 (10 RCTs), 6-9 mo | Moderate due to imprecisiona | RR 0.87 (0.44-1.74) | 50 per 1,000b | No significant difference; 6 fewer per 1,000 (from 28 fewer to 37 more) |
The recently published randomized trial Influence of Cilostazol-Based Triple Antiplatelet Therapy on Ischemic Complications After Drug-Eluting Stent Implantation (CILON-T) confirms and extends these findings.76 In this open-label study, 960 patients undergoing DES implantation were randomized to either 6 months of aspirin plus clopidogrel vs aspirin, clopidogrel, and cilostazol 100 mg bid. At 6 months, there was no significant difference in the prespecified primary outcome (cardiac death, nonfatal MI, clinically driven target vessel revascularization, ischemic stroke) (9.2% vs 8.5%, P = .74), any of the individual components of the primary outcome, or TIMI major bleeding (0.2% vs 0.4%, P = .51).
4.1.4 Cilostazol as Part of Dual Antiplatelet Therapy:
A systematic review by Biondi-Zoccai and colleagues77 identified 23 randomized trials (5,428 patients; median follow-up, 6 months) comparing the effects of cilostazol to a range of control therapies (including thienopyridines) on stent thrombosis, revascularization, major adverse cardiac events, and bleeding. Table S15 summarizes the findings from the meta-analysis of 13 studies of 3,437 patients comparing cilostazol + aspirin vs thienopyridine + aspirin. We rate the quality of evidence as very low because of risk of bias, indirectness (lacking reporting of death, MI, and stroke), publication bias, and imprecision. Cilostazol was not associated with significant improvement in clinical outcomes but was associated with a reduction in repeat revascularization and binary angiographic restenosis. Again, we consider the latter outcomes to be of little relevance to patients.
4.2 Aspirin Dose Following PCI With Stent Placement
We do not address loading doses of aspirin or clopidogrel prior to PCI in this section, but we do review evidence for aspirin therapy dosing following PCI. There has been only one RCT comparing higher- vs lower-dose aspirin post-PCI. The Clopidogrel Optimal Loading Dose Usage to Reduce Recurrent Events/Optimal Antiplatelet Strategy for Interventions (CURRENT OASIS-7) trial randomized 25,086 patients with ACS referred for PCI in a two-by-two fashion to (1) clopidogrel 600 mg load followed by 150 mg for 6 days vs clopidogrel 300 mg load followed by 75 mg for 6 days and (2) aspirin 325 mg load followed by 300 to 325 mg/d for 29 days vs 75 mg/d for 29 days.70 The investigators published a separate article reporting on the prespecified analysis of a subset of 17,263 patients who actually underwent PCI.78 Table 13 (Table S16) summarizes the relevant evidence, data, and quality of evidence for aspirin from this analysis.
Table 13.
—[Sections 4.1.1-4.3.5] High-Dose Aspirin vs Low-Dose Aspirin for 30 Days Post PCI78
| Outcomes | Participants (studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI)a | Anticipated Absolute Effects Over 30
d |
|
| Risk With Aspirin 75-100 mg | Risk Difference With Aspirin 300-325 mg (95% CI) | ||||
| Total mortalityb |
17,236 (1 RCT), 30 d |
Moderate due to imprecisionc |
RR 0.87 (0.74-1.03) |
25 per 1,000d |
No significant difference; 3 fewer per 1,000 (from 7 fewer to 1
more) |
| MI nonfatal events |
17,236 (1 RCT), 30 d |
Moderate due to imprecisionc |
RR 0.97 (0.82-1.16) |
21 per 1,000d |
No significant difference; 1 fewer per 1,000 (from 4 fewer to 3
more) |
| Strokee |
17,236 (1 RCT), 30 d |
Moderate due to imprecisionc |
RR 1.19 (0.84-1.68) |
5 per 1,000d |
No significant difference; 1 more per 1,000 (from 1 fewer to 3
more) |
| Major extracranial bleedf | 17,236 (1 RCT), 30 d | Moderate due to imprecisionc | RR 1.09 (0.89-1.34) | 14 per 1,000d | No significant difference; 1 more per 1,000 (from 2 fewer to 5 more) |
CURRENT-OASIS 7 = Clopidogrel Optimal Loading Dose Usage to Reduce Recurrent Events/Optimal Antiplatelet Strategy for Interventions; TIMI = Thombolysis in Myocardial Infarction. See Table 1-3 legends for expansion of other abbreviations.
Study reports hazard ratios. We have converted to relative risks (RR) for consistency.
Of the total deaths in the CURRENT-OASIS 7 study, nine of 314 (2.9%) with low-dose aspirin and 10 of 273 (2.7%) with higher-dose aspirin were fatal bleeding events.
Wide CIs suggest benefit and harm with high-dose aspirin.
Control group risk estimates come from event rates in patients allocated to low-dose aspirin undergoing PCI in CURRENT-OASIS 7.
It is unclear from the article whether hemorrhagic and fatal strokes were included in total strokes.
TIMI criteria used. It is unclear from the article whether hemorrhagic and fatal bleeding were included in total major bleeding.
The American College of Cardiology/American Heart Association Guidelines79 recommend aspirin 162 to 325 mg for 1 month following PCI with BMS, 3 months for sirolimus stent, and 6 months for paclitaxel stent (to be followed by aspirin 75-162 mg thereafter). This recommendation is based on aspirin doses used in prior clinical studies evaluating stent type or adjunctive therapy with stent placement. In contrast, the European Society of Cardiology recommends low-dose aspirin following PCI.80 In a post hoc analysis of data from PCI-CURE, patients were stratified into three groups based on aspirin dose (≥ 200, 101-199, and ≤ 100 mg).81 All three groups had similar rates of the composite end point of cardiovascular death, MI, or stroke at long-term follow-up (8.6%, 7.4%, 7.1%, respectively). Major bleeding was significantly increased with high-dose aspirin compared with medium- or low-dose aspirin (3.9%, 1.5%, 1.9%, respectively).
4.3 Duration of Dual Antiplatelet Therapy Following PCI With Placement of BMS or DES
4.3.1.,4.3.3 Minimum Duration of Dual Antiplatelet Therapy Following Stent Placement:
Antithrombotic therapy following PCI with stent placement is necessary to prevent thrombosis due to exposure of blood to metal stent struts. This risk is decreased after healing of the lesion and endothelialization of the bare metal struts (in ∼4-6 weeks).82,83
In the past decade, there has been an increased use of DES. These have been shown to decrease the rate of angiographic restenosis and need for repeat revascularization, although the effect relative to BMS on more important outcomes remains less certain.84 The antiinflammatory/antiproliferative effects of drug-coated stents result in delayed healing characterized by poor endothelialization that increases the duration of stent thrombogenicity. As a result, extended dual antiplatelet therapy has been used: a minimum of 3 months for -limus stents and 6 months for -taxel stents. Initial comparative studies (DES vs BMS; sirolimus vs paclitaxel) used these or longer durations of dual antiplatelet therapy.85 Discontinuation of clopidogrel therapy before this minimum duration has been associated with stent thrombosis and clinically adverse outcomes.86‐88 In a prospective observational study of 2,229 consecutive patients undergoing DES implantation, 1.3% of patients had stent thrombosis at 9 months; case fatality was 45% (13/29) in these patients.86 Premature clopidogrel therapy discontinuation (< 3 months sirolimus, < 6 months paclitaxel) was the strongest predictor of stent thrombosis (hazard ratio, 89.8; 95% CI, 29.9-269.6). There are no RCTs evaluating shorter duration of dual antiplatelet therapy for these different stent subtypes.
4.3.2 Extended Duration of Dual Antiplatelet Therapy Following Elective PCI and BMS Placement:
As described previously, the risk of BMS thrombosis is decreased after 1 month of dual antiplatelet therapy. Potential benefit of extended dual antiplatelet therapy beyond 1 month might result from a decrease in later stent thrombosis events or a decrease in coronary vascular events occurring at other plaque sites. Table 14 (Table S17) summarizes the quality of evidence and main findings from our systematic review and meta-analysis of RCTs identified by a systematic literature search (updated January 2010) comparing 1 month of dual antiplatelet therapy vs 6 to 12 months in patients undergoing PCI with placement of BMS.89‐93 The quality of evidence is rated as low because of risk of bias, indirectness (populations varied from PCI in ACS [PCI-CURE] to elective PCI in stable angina), and large imprecision in effect estimates for all outcomes. The results suggest that dual antiplatelet therapy for 6 to 12 months significantly reduces MI (RR, 0.66) but does not confirm or exclude a significant effect on mortality, stroke, or major bleeds.
Table 14.
—[Sections 4.1.1-4.3.5] Six to Twelve Months vs One Month of Clopidogrel Plus Aspirin Following PCI With Placement of BMS89-92
| Outcomes | Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects Over
6-9 mo |
|
| Risk With 1 mo Clopidogrel + Aspirin | Risk Difference With 6-12 mo Clopidogrel + Aspirin (95% CI) | ||||
| Total mortalitya |
3,390 (3 RCT), 6-12 mo |
Low due to risk of biasb and imprecisionc |
RR 0.73 (0.48-1.13) |
28 per 1,000d |
No significant difference; 8 fewer per 1,000 (from 15 fewer
to 4 more) |
| MI nonfatal events |
4,852 (3 RCTs), 6-12 mo |
Moderate due to risk of biasa |
RR 0.66 (0.50-86) |
28 per 1,000d |
9 fewer per 1,000 (from 14 fewer to 4 fewer) |
| Stroked |
2,194 (2 RCTs), 6-12 mo |
Low due to risk of biasa and imprecisionc |
RR 0.46 (0.16-1.32) |
10 per 1,000d |
No significant difference; 5 fewer per 1,000 (from 8 fewer
to 3 more) |
| Major extracranial bleede | 5,052 (3 RCTs), 6-12 mo | Low due to risk of biasa and imprecisionc | RR 1.17 (0.86-1.60) | 50 per 1,000d | No significant difference; 8 more per 1,000 (from 7 fewer to 30 more) |
Fatal bleeding events not reported.
Bernardi et al92 and Pekdemir et al90 were not blinded, and there was no placebo control; Bernardi et al stopped early for benefit. The Akbulut et al93 design was unclear (no mention of randomization, but the Health Technology Assessment report referred to it as randomized). Mehta et al89 had variable follow-up.
CIs include important benefit and harm.
Control group risk estimates derived from rates in subjects treated with dual antiplatelet therapy for 1 mo in included trials.
Major bleeding not stratified by type of bleed; unclear whether major bleeding included any fatalities.
4.3.4 Extended Duration of Dual Antiplatelet Therapy Following Elective PCI and DES Placement:
Dual Antiplatelet Therapy for Up to One Year—
No randomized trials have evaluated the efficacy and safety of dual antiplatelet therapy in patients undergoing DES for up to 1 year (compared with the minimum of 3-6 months). A number of observational studies have suggested that patients with DES are at increased risk of late-stent thrombosis and poor outcomes after discontinuation of dual antiplatelet therapy at 6 months. A consecutive series of 746 unselected patients enrolled in the Basel Stent Kosteneffektivitäts Trial (BASKET) study (a randomized trial of DES vs BMS) received aspirin and clopidogrel for 6 months and were followed for another 1 year.94 The incidence of cardiac death and MI after discontinuation of clopidogrel was higher in patients undergoing DES than those undergoing BMS (4.9% vs 1.3%).
In another observational study, 4,666 consecutive patients undergoing PCI with either BMS (n = 3,165) or DES (n = 1,501) were followed up at 6, 12, and 24 months.95 In patients with DES who were event free at 6 months, clopidogrel use at 6 months was associated with lower rates of adjusted death (2% vs 5.3% without, P = .03) and death and MI (3.1% vs 7.2%, P = .02) at 24 months. There was a trend for decreased rates of nonfatal MI (2.6% vs 1.3%, P = .24). Bleeding outcomes were not reported in either study. Based on these and other observational studies, it has become standard practice to treat patients with DES with dual antiplatelet therapy for 12 months.
4.3.5 Dual Antiplatelet Therapy for More Than One Year:
Table 15 (Table S18) summarizes the quality of evidence and main findings from two merged RCTs (REAL LATE and ZEST LATE), examining the effects of prolonged dual antiplatelet therapy (clopidogrel 75 mg + aspirin 100-200 mg/d for a median of 19 months) vs 12 months in patients who had undergone implantation of DES.71 These studies were merged by their respective executive committees because of slower-than-expected enrollment and similar study designs. The indication for the initial PCI with DES placement was stable angina (37%), unstable angina (41%), or ACS (21%, equally distributed between non-ST-elevation ACS and ST-elevation ACS). Sirolimus-eluting stents were most commonly used (57%) followed by paclitaxel- (24%) and zotarolimus-eluting stents (19%).
Table 15.
—[Sections 4.1.1-4.3.5] Extended Duration of Clopidogrel Plus Aspirin Following PCI With Placement of DES71
| Outcomes | Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects Over
19 mo |
|
| Risk With 12 mo Clopidogrel + Aspirin | Risk Difference With 19 mo Clopidogrel + Aspirin (95% CI) | ||||
| Total mortality |
2,701 (2 RCTs), 19 mo |
Moderate due to imprecisiona |
RR 1.65 (0.80-3.36) |
6 per 1,000b |
No significant difference; 4 more per 1,000 (from 1 fewer to
14 more) |
| MI nonfatal events |
2,701 (2 RCTs), 19 mo |
Moderate due to imprecisiona |
RR 0.66 (0.16-1.32) |
3 per 1,000b |
No significant difference; 2 more per 1,000 (from 1 fewer to
13 more) |
| Stroke |
2,701 (2 RCTs), 19 mo |
Moderate due to imprecisiona |
RR 0.46 (0.16-1.32) |
2 per 1,000b |
No significant difference; 3 more per 1,000 (from 1 fewer to
16 more) |
| Major extracranial bleedc | 2,701 (2 RCTs), 19 mo | Moderate due to imprecisiona | RR 1.17 (0.86-1.60) | 1 per 1,000b | No significant difference; 2 more per 1,000 (from 1 fewer to 19 more) |
Rated down for imprecision due to wide CI for absolute effects, including substantial harm with extended duration of dual antiplatelet therapy. We did not rate down for risk of bias, although it was an open-label study because end points were adjudicated by blinded assessors.
Control group risk estimates come from subjects receiving dual antiplatelet therapy for 1 y in the merged trials.
Major bleeding defined by TIMI (Thrombolysis in Myocardial Infarction) criteria; no information was provided on the type of major bleeding event in either group. No fatal bleeding was reported.
As shown in Table 15 (Table S18), these data did not confirm or exclude benefit of an extended duration of dual antiplatelet therapy vs 12 months of dual antiplatelet therapy for any of the outcomes. The very-low baseline risk for all outcomes results in only moderately imprecise absolute effects, although the relative risk estimates are considerably more imprecise. The results suggest a trend favoring short-term over prolonged dual antiplatelet therapy for all outcomes. In summary, the available evidence suggests no benefit and possible harm of continuing dual antiplatelet therapy beyond 12 months.
Recommendations
4.1.1-4.3.5. For patients who have undergone elective PCI with placement of BMS:
For the first month, we recommend dual antiplatelet therapy with aspirin 75 to 325 mg daily and clopidogrel 75 mg daily over single antiplatelet therapy (Grade 1A).
For the subsequent 11 months, we suggest dual antiplatelet therapy with combination of low-dose aspirin 75 to 100 mg daily and clopidogrel 75 mg daily over single antiplatelet therapy (Grade 2C).
After 12 months, we recommend single antiplatelet therapy over continuation of dual antiplatelet therapy (Grade 1B).
For patients who have undergone elective PCI with placement of DES:
For the first 3 to 6 months, we recommend dual antiplatelet therapy with aspirin 75 to 325 mg daily and clopidogrel 75 mg daily over single antiplatelet therapy (Grade 1A).
Remarks: Absolute minimum duration will vary based on stent type (in general 3 months for -limus stents and 6 months for -taxel stents).
After 3 to 6 months, we suggest continuation of dual antiplatelet therapy with low-dose aspirin 75 to 100 mg and clopidogrel (75 mg daily) until 12 months over single antiplatelet therapy (Grade 2C).
After 12 months, we recommend single antiplatelet therapy over continuation of dual antiplatelet therapy (Grade 1B). Single antiplatelet therapy thereafter is recommended as per the established CAD recommendations (see recommendations 3.1.1-3.1.5).
For patients who have undergone elective BMS or DES stent placement:
We recommend use of low-dose aspirin 75 to 100 mg daily and clopidogrel 75 mg daily alone rather than cilostazol in addition to these drugs (Grade 1B).
We suggest aspirin 75 to 100 mg daily and clopidogrel 75 mg daily as part of dual antiplatelet therapy rather than the use of either drug with cilostazol (Grade 1B).
We suggest cilostazol 100 mg twice daily as substitute for either low-dose aspirin 75 to 100 mg daily or clopidogrel 75 mg daily as part of a dual antiplatelet regimen in patients with an allergy or intolerance of either drug class (Grade 2C).
For patients with CAD undergoing elective PCI but no stent placement:
We suggest for the first month, dual antiplatelet therapy with aspirin 75 to 325 mg daily and clopidogrel 75 mg daily over single antiplatelet therapy (Grade 2C). Single antiplatelet therapy thereafter is recommended as per the established CAD recommendations (see recommendations 3.1.1-3.1.5).
5.0 Antithrombotic Therapy in Patients With Systolic LV Dysfunction
Approximately 70% of patients with systolic LV dysfunction and heart failure have ischemic heart disease. The remaining 30% of patients with systolic heart failure are considered to have a nonischemic etiology (eg, hypertensive heart disease, valvular heart disease, idiopathic). Because the majority of these latter patients are free of concomitant CAD, risk for MI is lower than that of patients with ischemic systolic LV dysfunction.
Assessment of Baseline Risk
For the comparison of warfarin vs aspirin in patients with systolic LV dysfunction (ischemic and nonischemic), we used risks from the aspirin-only arm of a meta-analysis we performed of three RCTs pertinent to this question.
A prior Cochrane systematic review had identified only one pilot RCT.96 We performed an updated systematic literature search and performed a meta-analysis based on four randomized trials evaluating antithrombotic therapy in patients with symptomatic heart failure and ejection fraction < 35%.97‐100 In brief, results could not demonstrate or exclude a significant difference for patient-important outcomes between patients receiving warfarin or aspirin compared with those receiving no antithrombotic therapy. Table 16 presents evidence from our meta-analysis of data from the three studies comparing warfarin to aspirin (Table S19).97‐99 Warfarin was associated with a significant decrease in strokes. The data do not confirm or exclude a benefit of warfarin vs aspirin for the other end points. Quality of this evidence is low because of imprecision and risk of bias. Approximately 75% of patients were designated as having systolic LV dysfunction of an ischemic etiology. Unfortunately, there were insufficient data for us to examine possible differences in antithrombotic efficacy and safety in patients classified by type of heart failure (ischemic vs nonischemic).
Table 16.
—[Recommendations 5.1-5.3] Warfarin vs Aspirin in Patients With Systolic LV Dysfunction (Ischemic and Nonischemic)97-99
| Outcomes | Participants (Studies), Follow-up | Quality of the Evidence (GRADE) | Relative Effect (95% CI) | Anticipated Absolute Effects Over 5
y |
|
| Risk With Aspirin | Risk Difference With Warfarin (95% CI) | ||||
| Total mortality |
1,358 (3 RCT), 23-27 mo |
Low due to risk of biasa and imprecisionb |
RR 0.95 (0.76-1.19) |
193 per 1,000c |
No significant difference; 10 fewer per 1,000 (from 46 fewer to
36 more) |
| MId |
1,358 (3 RCT), 23-27 mo |
Low due to risk of biasa and imprecisionb |
RR 0.99 (0.35-2.84) |
33 per 1,000c |
No significant difference; 0 fewer per 1,000 (from 21 fewer to
60 more) |
| Strokee |
1,358 (3 RCT), 23-27 mo |
Low due to risk of biasa and imprecisionb |
RR 0.34 (0.13-0.97) |
24 per 1,000c |
16 fewer per 1000 (from 21 fewer to 1 fewer) |
| Major extracranial bleedf |
1,358 (3 RCT), 23-27 mo |
Low due to risk of biasa and imprecisionb |
RR 1.97 (0.89-4.3) |
30 per 1,000c |
No significant difference; 29 more per 1,000 (from 3 fewer to 99
more) |
| Burden of treatment | Not applicable | High | Warfarin > Aspirin | Warfarin: daily medication, dietary
and activity restrictions, frequent blood testing/monitoring,
increased hospital/clinic visits |
|
| Aspirin: daily medication only | |||||
Two of three trials were stopped early (one for benefit, one for slow enrollment); problems with blinding.
Wide CIs include benefit and harm.
Control group risk estimates derived from event rates from aspirin arm of the pooled studies.
Fatal and nonfatal MIs not reported separately in all studies.
Fatal and nonfatal strokes not reported separately in all studies, types of strokes (ischemic/hemorrhagic) not reported.
Definition of major hemorrhage varied.
Finally, there will be patients who develop acute dilated cardiomyopathy from noncardiac causes (eg, acute viral myocarditis, Takotsubo cardiomyopathy) who may develop acute LV thrombosis. We found no studies comparing anticoagulation strategies in such patients. Based on indirect evidence from studies of patients with anterior MI and LV thrombus (see section 3.6), we assume that systemic embolization rates from acute LV thrombus in patients with nonischemic cardiomyopathy are similarly high (∼10%).
Recommendations
5.1-5.3. For patients with systolic LV dysfunction without established CAD and no LV thrombus, we suggest not to use antiplatelet therapy or warfarin (Grade 2C).
Remarks: Patients who place a high value on an uncertain reduction in stroke and a low value on avoiding an increased risk of GI bleeding are likely to choose to use warfarin.
For patients with systolic LV dysfunction without established CAD with identified acute LV thrombus (eg, Takotsubo cardiomyopathy), we suggest moderate-intensity warfarin (INR 2.0-3.0) for at least 3 months (Grade 2C).
For patients with systolic LV dysfunction and established CAD, recommendations are as per the established CAD recommendations (see recommendations 3.1.1-3.1.5).
Data Supplement
Acknowledgments
Author contributions: As Topic Editor, Dr Vandvik oversaw the development of this article, including the data analysis and subsequent development of the recommendations contained herein.
Dr Vandvik: served as Topic Editor.
Dr Lincoff: served as a panelist.
Dr Gore: served as a panelist.
Dr Gutterman: served as a panelist.
Dr Sonnenberg: served as a resource consultant.
Dr Alonso-Coello: served as a panelist.
Dr Akl: served as a panelist.
Dr Lansberg: served as a panelist.
Dr Guyatt: served as a panelist.
Dr Spencer: served as Deputy Editor.
Financial/nonfinancial disclosures: The authors of this guideline provided detailed conflict of interest information related to each individual recommendation made in this article. A grid of these disclosures is available online at http://chestjournal.chestpubs.org/content/141/2_suppl/e637S/suppl/DC1. In summary, the authors have reported to CHEST the following conflicts of interest: Dr Lincoff is Director of the Cleveland Clinic Coordinating Center for Clinical Research (C5Research), which has research grants from Anthera Pharmaceuticals, Inc; AstraZeneca; Bristol-Myers Squibb; Eli Lilly and Company; Kai Pharmaceuticals, Inc; Pfizer, Inc; Hoffmann La-Roche Inc; Novartis AG; Sanofi-Aventis LLC; Merck/Schering-Plough; Scios, Inc; Takeda Pharmaceutical Company Limited, and Johnson & Johnson. He has received honoraria for consultations or advisory board activities from AstraZeneca; Avanir Pharmaceuticals; Baxter; Bristol-Myers Squibb; Ikaria, Inc; Hoffmann La-Roche Inc; and Merck/Schering-Plough. Dr Gutterman has had the following relationships that are entirely unrelated to the AT9 guidelines: ACCP President, GlaxoSmithKline plc grant to study vasodilation in adipose tissue, National Institutes of Health grant to study human coronary dilation, and GE Healthcare consultation on a study for ECG evaluation of chronic heart disease. Dr Guyatt is co-chair of the GRADE Working Group. Drs Vandvik, Alonso-Coello, and Akl are members of and prominent contributors to the GRADE Working Group. Drs Gore, Sonnenberg, Lansberg, and Spencer have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors played no role in the development of these guidelines. Sponsoring organizations cannot recommend panelists or topics, nor are they allowed prepublication access to the manuscripts and recommendations. Guideline panel members, including the chair, and members of the Health & Science Policy Committee are blinded to the funding sources. Further details on the Conflict of Interest Policy are available online at http://chestnet.org.
Other contributions: We thank Louis Kuritzky, MD, for providing his frontline primary-care clinician perspective on the content of this article, John You, MD, for methodologic contributions (triple therapy in patients with acute LV thrombus), and Colin Baigent, MD, for sharing his methodologic expertise on primary prevention of cardiovascular disease with aspirin
Endorsements: This guideline is endorsed by the American Association for Clinical Chemistry, the American College of Clinical Pharmacy, the American Society of Health-System Pharmacists, the American Society of Hematology, and the International Society of Thrombosis and Hematosis.
Additional information: The supplement Tables can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/141/2_suppl/e637S/suppl/DC1.
Abbreviations
- ACS
acute coronary syndrome
- BMS
bare-metal stent
- CAD
coronary artery disease
- CAGB
coronary artery bypass graft
- CAPRIE
Clopidogrel vs Aspirin in Patients at Risk of Ischaemic Events
- CHARISMA
Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance
- CURE
Clopidogrel in Unstable Angina to Prevent Recurrent Events
- DES
drug-eluting stent
- INR
international normalized ratio
- LV
left ventricular
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- PLATO
Platelet Inhibition and Patient Outcomes
- QALY
quality-adjusted life year
- RCT
randomized controlled trial
- RR
risk ratio
- TIA
transient ischemic attack
Footnotes
Funding/Support: The Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines received support from the National Heart, Lung, and Blood Institute [R13 HL104758] and Bayer Schering Pharma AG. Support in the form of educational grants was also provided by Bristol-Myers Squibb; Pfizer, Inc; Canyon Pharmaceuticals; and sanofi-aventis US.
Disclaimer: American College of Chest Physician guidelines are intended for general information only, are not medical advice, and do not replace professional medical care and physician advice, which always should be sought for any medical condition. The complete disclaimer for this guideline can be accessed at http://chestjournal.chestpubs.org/content/141/2_suppl/1S.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.MacLean S, Mulla S, Akl EA, et al. Patient values and preferences in decision making for antithrombotic therapy: a systematic review: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2) suppl:e1S–e23S. doi: 10.1378/chest.11-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guyatt GH, Norris SL, Schulman S, et al. Methodology for the development of antithrombotic therapy and prevention of thrombosis guidelines: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2) suppl:53S–70S. doi: 10.1378/chest.11-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt DL, Fox KA, Hacke W, et al. CHARISMA Investigators Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354(16):1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 4.Raju NC, Sobieraj-Teague M, Hirsh J, O’Donnell M, Eikelboom J. Effect of aspirin on mortality in the primary prevention of cardiovascular disease. Am J Med. 2011;24(7):621–629. doi: 10.1016/j.amjmed.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377(9759):31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 6.Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 7.You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2) suppl:e531S–e575S. doi: 10.1378/chest.11-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 9.Baigent C, Blackwell L, Collins R, et al. Antithrombotic Trialists’ (ATT) Collaboration Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins GS, Altman DG. An independent external validation and evaluation of QRISK cardiovascular risk prediction: a prospective open cohort study. BMJ. 2009;339:b2584. doi: 10.1136/bmj.b2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Ruijter W, Westendorp RG, Assendelft WJ, et al. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ. 2009;338:a3083. doi: 10.1136/bmj.a3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peto R, Gray R, Collins R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br Med J (Clin Res Ed) 1988;296(6618):313–316. doi: 10.1136/bmj.296.6618.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steering Committee of the Physicians’ Health Study Research Group Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med. 1989;321(3):129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 14.Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council’s General Practice Research Framework. Lancet. 1998;351(9098):233–241. [PubMed] [Google Scholar]
- 15.Hansson L, Zanchetti A, Carruthers SG, et al. HOT Study Group Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351(9118):1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 16.de Gaetano G. Collaborative Group of the Primary Prevention Project Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Lancet. 2001;357(9250):89–95. doi: 10.1016/s0140-6736(00)03539-x. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 18.Bulugahapitiya U, Siyambalapitiya S, Sithole J, Fernando DJ, Idris I. Age threshold for vascular prophylaxis by aspirin in patients without diabetes. Heart. 2008;94(11):1429–1432. doi: 10.1136/hrt.2008.150698. [DOI] [PubMed] [Google Scholar]
- 19.Buse JB, Ginsberg HN, Bakris GL, et al. American Heart Association American Diabetes Association Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care. 2007;30(1):162–172. doi: 10.2337/dc07-9917. [DOI] [PubMed] [Google Scholar]
- 20.Colwell JA. American Diabetes Association Aspirin therapy in diabetes. Diabetes Care. 2004;27(suppl 1):S72–S73. doi: 10.2337/diacare.27.2007.s72. [DOI] [PubMed] [Google Scholar]
- 21.Elwood P, Morgan G, Brown G, Pickering J. Aspirin for everyone older than 50? For. BMJ. 2005;330(7505):1440–1441. doi: 10.1136/bmj.330.7505.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rydén L, Standl E, Bartnik M, et al. Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) European Association for the Study of Diabetes (EASD) Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. Eur Heart J. 2007;28(1):88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 23.De Berardis G, Sacco M, Strippoli GF, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: meta-analysis of randomised controlled trials. BMJ. 2009;339:b4531. doi: 10.1136/bmj.b4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang C, Sun A, Zhang P, et al. Aspirin for primary prevention of cardiovascular events in patients with diabetes: A meta-analysis. Diabetes Res Clin Pract. 2010;87(2):211–218. doi: 10.1016/j.diabres.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 25.Saw J, Topol EJ, Steinhubl SR, Brennan D, Berger PB, Moliterno DJ. CREDO Investigators Comparison of long-term usefulness of clopidogrel therapy after the first percutaneous coronary intervention or coronary artery bypass grafting versus that after the second or repeat intervention. Am J Cardiol. 2004;94(5):623–625. doi: 10.1016/j.amjcard.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Fox KA, Mehta SR, Peters R, et al. Clopidogrel in Unstable angina to prevent Recurrent ischemic Events Trial Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation. 2004;110(10):1202–1208. doi: 10.1161/01.CIR.0000140675.85342.1B. [DOI] [PubMed] [Google Scholar]
- 27.Patel JH, Stoner JA, Owora A, Mathew ST, Thadani U. Evidence for using clopidogrel alone or in addition to aspirin in post coronary artery bypass surgery patients. Am J Cardiol. 2009;103(12):1687–1693. doi: 10.1016/j.amjcard.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 28.Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2) suppl:e326S–e350S. doi: 10.1378/chest.11-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CAPRIE Steering Committee A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348(9038):1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 30.Antithrombotic Trialists’ Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sudlow CL, Mason G, Maurice JB, Wedderburn CJ, Hankey GJ. Thienopyridine derivatives versus aspirin for preventing stroke and other serious vascular events in high vascular risk patients. Cochrane Database Syst Rev. 2009;(4):CD001246. doi: 10.1002/14651858.CD001246.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schleinitz MD, Weiss JP, Owens DK. Clopidogrel versus aspirin for secondary prophylaxis of vascular events: a cost-effectiveness analysis. Am J Med. 2004;116(12):797–806. doi: 10.1016/j.amjmed.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 33.Karnon J, Bakhai A, Brennan A, et al. A cost-utility analysis of clopidogrel in patients with non-ST-segment-elevation acute coronary syndromes in the UK. Int J Cardiol. 2006;109(3):307–316. doi: 10.1016/j.ijcard.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 34.Durand-Zaleski I, Bertrand M. The value of clopidogrel versus aspirin in reducing atherothrombotic events: the CAPRIE study. Pharmacoeconomics. 2004;22(suppl 4):19–27. doi: 10.2165/00019053-200422004-00005. [DOI] [PubMed] [Google Scholar]
- 35.Sarasin FP, Gaspoz JM, Bounameaux H. Cost-effectiveness of new antiplatelet regimens used as secondary prevention of stroke or transient ischemic attack. Arch Intern Med. 2000;160(18):2773–2778. doi: 10.1001/archinte.160.18.2773. [DOI] [PubMed] [Google Scholar]
- 36.Keller TT, Squizzato A, Middeldorp S. Clopidogrel plus aspirin versus aspirin alone for preventing cardiovascular disease. Cochrane Database Syst Rev. 2007;(3):CD005158. doi: 10.1002/14651858.CD005158.pub2. [DOI] [PubMed] [Google Scholar]
- 37.Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ. 2010;340:c117. doi: 10.1136/bmj.c117. [DOI] [PubMed] [Google Scholar]
- 38.Diener HC, Bogousslavsky J, Brass LM, et al. MATCH investigators Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364(9431):331–337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 39.Coumadin Aspirin Reinfarction Study (CARS) Investigators Randomised double-blind trial of fixed low-dose warfarin with aspirin after myocardial infarction. Coumadin Aspirin Reinfarction Study (CARS) Lancet. 1997;350(9075):389–396. [PubMed] [Google Scholar]
- 40.The Post Coronary Artery Bypass Graft Trial Investigators The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. N Engl J Med. 1997;336(3):153–162. doi: 10.1056/NEJM199701163360301. [DOI] [PubMed] [Google Scholar]
- 41.Anand SS, Yusuf S, Pogue J, Weitz JI, Flather M. Long-term oral anticoagulant therapy in patients with unstable angina or suspected non-Q-wave myocardial infarction: organization to assess strategies for ischemic syndromes (OASIS) pilot study results. Circulation. 1998;98(11):1064–1070. doi: 10.1161/01.cir.98.11.1064. [DOI] [PubMed] [Google Scholar]
- 42.Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347(13):969–974. doi: 10.1056/NEJMoa020496. [DOI] [PubMed] [Google Scholar]
- 43.van Es RF, Jonker JJ, Verheugt FW, Deckers JW, Grobbee DE. Antithrombotics in the Secondary Prevention of Events in Coronary Thrombosis-2 (ASPECT-2) Research Group Aspirin and Coumadin after acute coronary syndromes (the ASPECT-2 study): a randomised controlled trial. Lancet. 2002;360(9327):109–113. doi: 10.1016/S0140-6736(02)09409-6. [DOI] [PubMed] [Google Scholar]
- 44.Rothberg MB, Celestin C, Fiore LD, Lawler E, Cook JR. Warfarin plus aspirin after myocardial infarction or the acute coronary syndrome: meta-analysis with estimates of risk and benefit. Ann Intern Med. 2005;143(4):241–250. doi: 10.7326/0003-4819-143-4-200508160-00005. [DOI] [PubMed] [Google Scholar]
- 45.Campbell CL, Smyth S, Montalescot G, Steinhubl SR. Aspirin dose for the prevention of cardiovascular disease: a systematic review. JAMA. 2007;297(18):2018–2024. doi: 10.1001/jama.297.18.2018. [DOI] [PubMed] [Google Scholar]
- 46.Serebruany VL, Steinhubl SR, Berger PB, et al. Analysis of risk of bleeding complications after different doses of aspirin in 192,036 patients enrolled in 31 randomized controlled trials. Am J Cardiol. 2005;95(10):1218–1222. doi: 10.1016/j.amjcard.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 47.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 48.Cannon CP, Harrington RA, James S, et al. PLATelet inhibition and patient Outcomes Investigators Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet. 2010;375(9711):283–293. doi: 10.1016/S0140-6736(09)62191-7. [DOI] [PubMed] [Google Scholar]
- 49.Lamy A, Jönsson B, Weintraub WS, et al. CURE Economic Group The cost-effectiveness of the use of clopidogrel in acute coronary syndromes in five countries based upon the CURE study. Eur J Cardiovasc Prev Rehabil. 2004;11(6):460–465. doi: 10.1097/01.hjr.0000152239.28456.36. [DOI] [PubMed] [Google Scholar]
- 50.Main C, Palmer S, Griffin S, et al. Clopidogrel used in combination with aspirin compared with aspirin alone in the treatment of non-ST-segment-elevation acute coronary syndromes: a systematic review and economic evaluation. Health Technol Assess. 2004;8(40):1–141. doi: 10.3310/hta8400. [DOI] [PubMed] [Google Scholar]
- 51.Lindgren P, Jönsson B, Yusuf S. Cost-effectiveness of clopidogrel in acute coronary syndromes in Sweden: a long-term model based on the CURE trial. J Intern Med. 2004;255(5):562–570. doi: 10.1111/j.1365-2796.2004.01324.x. [DOI] [PubMed] [Google Scholar]
- 52.Weintraub WS, Mahoney EM, Lamy A, et al. CURE Study Investigators Long-term cost-effectiveness of clopidogrel given for up to one year in patients with acute coronary syndromes without ST-segment elevation. J Am Coll Cardiol. 2005;45(6):838–845. doi: 10.1016/j.jacc.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 53.Schleinitz MD, Heidenreich PA. A cost-effectiveness analysis of combination antiplatelet therapy for high-risk acute coronary syndromes: clopidogrel plus aspirin versus aspirin alone. Ann Intern Med. 2005;142(4):251–259. doi: 10.7326/0003-4819-142-4-200502150-00007. [DOI] [PubMed] [Google Scholar]
- 54.Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J. 2006;27(9):1038–1047. doi: 10.1093/eurheartj/ehi754. [DOI] [PubMed] [Google Scholar]
- 55.Storey RF, Husted S, Harrington RA, et al. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol. 2007;50(19):1852–1856. doi: 10.1016/j.jacc.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 56.Wallentin L, Becker RC, Budaj A, et al. PLATO Investigators Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 57.Wiviott SD, Braunwald E, McCabe CH, et al. TRITON-TIMI 38 Investigators Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 58.Unger EF. Weighing benefits and risks—the FDA’s review of prasugrel. N Engl J Med. 2009;361(10):942–945. doi: 10.1056/NEJMp0907122. [DOI] [PubMed] [Google Scholar]
- 59.Asinger RW, Mikell FL, Elsperger J, Hodges M. Incidence of left-ventricular thrombosis after acute transmural myocardial infarction. Serial evaluation by two-dimensional echocardiography. N Engl J Med. 1981;305(6):297–302. doi: 10.1056/NEJM198108063050601. [DOI] [PubMed] [Google Scholar]
- 60.Weinreich DJ, Burke JF, Pauletto FJ. Left ventricular mural thrombi complicating acute myocardial infarction. Long-term follow-up with serial echocardiography. Ann Intern Med. 1984;100(6):789–794. doi: 10.7326/0003-4819-100-6-789. [DOI] [PubMed] [Google Scholar]
- 61.Lamas GA, Vaughan DE, Pfeffer MA. Left ventricular thrombus formation after first anterior wall acute myocardial infarction. Am J Cardiol. 1988;62(1):31–35. doi: 10.1016/0002-9149(88)91360-4. [DOI] [PubMed] [Google Scholar]
- 62.Keren A, Goldberg S, Gottlieb S, et al. Natural history of left ventricular thrombi: their appearance and resolution in the posthospitalization period of acute myocardial infarction. J Am Coll Cardiol. 1990;15(4):790–800. doi: 10.1016/0735-1097(90)90275-t. [DOI] [PubMed] [Google Scholar]
- 63.Osherov AB, Borovik-Raz M, Aronson D, et al. Incidence of early left ventricular thrombus after acute anterior wall myocardial infarction in the primary coronary intervention era. Am Heart J. 2009;157(6):1074–1080. doi: 10.1016/j.ahj.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 64.Solheim S, Seljeflot I, Lunde K, et al. Frequency of left ventricular thrombus in patients with anterior wall acute myocardial infarction treated with percutaneous coronary intervention and dual antiplatelet therapy. Am J Cardiol. 2010;106(9):1197–1200. doi: 10.1016/j.amjcard.2010.06.043. [DOI] [PubMed] [Google Scholar]
- 65.Schwalm JD, Ahmad M, Salehian O, Eikelboom JW, Natarajan MK. Warfarin after anterior myocardial infarction in current era of dual antiplatelet therapy: a randomized feasibility trial. J Thromb Thrombolysis. 2010;30(2):127–132. doi: 10.1007/s11239-010-0448-6. [DOI] [PubMed] [Google Scholar]
- 66.Vaitkus PT, Barnathan ES. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol. 1993;22(4):1004–1009. doi: 10.1016/0735-1097(93)90409-t. [DOI] [PubMed] [Google Scholar]
- 67.Healey JS, Hart RG, Pogue J, et al. Risks and benefits of oral anticoagulation compared with clopidogrel plus aspirin in patients with atrial fibrillation according to stroke risk: the Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events (ACTIVE-W) Stroke. 2008;39(5):1482–1486. doi: 10.1161/STROKEAHA.107.500199. [DOI] [PubMed] [Google Scholar]
- 68.Cosmi B, Rubboli A, Castelvetri C, Milandri M. Ticlopidine versus oral anticoagulation for coronary stenting. Cochrane Database Syst Rev. 2001;(4):CD002133. doi: 10.1002/14651858.CD002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tamhane U, Meier P, Chetcuti S, et al. Efficacy of cilostazol in reducing restenosis in patients undergoing contemporary stent based PCI: a meta-analysis of randomised controlled trials. EuroIntervention. 2009;5(3):384–393. doi: 10.4244/v5i3a60. [DOI] [PubMed] [Google Scholar]
- 70.Mehta SR, Bassand JP, Chrolavicius S, et al. CURRENT-OASIS 7 Investigators Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med. 2010;363(10):930–942. doi: 10.1056/NEJMoa0909475. [DOI] [PubMed] [Google Scholar]
- 71.Park SJ, Park DW, Kim YH, et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N Engl J Med. 2010;362(15):1374–1382. doi: 10.1056/NEJMoa1001266. [DOI] [PubMed] [Google Scholar]
- 72.Cook S, Windecker S. Early stent thrombosis: past, present, and future. Circulation. 2009;119(5):657–659. doi: 10.1161/CIRCULATIONAHA.108.842757. [DOI] [PubMed] [Google Scholar]
- 73.Foley JB, Brown RI, Penn IM. Thrombosis and restenosis after stenting in failed angioplasty: comparison with elective stenting. Am Heart J. 1994;128(1):12–20. doi: 10.1016/0002-8703(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 74.Roubin GS, Cannon AD, Agrawal SK, et al. Intracoronary stenting for acute and threatened closure complicating percutaneous transluminal coronary angioplasty. Circulation. 1992;85(3):916–927. doi: 10.1161/01.cir.85.3.916. [DOI] [PubMed] [Google Scholar]
- 75.Serruys PW, Strauss BH, Beatt KJ, et al. Angiographic follow-up after placement of a self-expanding coronary-artery stent. N Engl J Med. 1991;324(1):13–17. doi: 10.1056/NEJM199101033240103. [DOI] [PubMed] [Google Scholar]
- 76.Suh JW, Lee SP, Park KW, et al. Multicenter randomized trial evaluating the efficacy of cilostazol on ischemic vascular complications after drug-eluting stent implantation for coronary heart disease: results of the CILON-T (influence of CILostazol-based triple antiplatelet therapy ON ischemic complication after drug-eluting stenT implantation) trial. J Am Coll Cardiol. 2011;57(3):280–289. doi: 10.1016/j.jacc.2010.08.631. [DOI] [PubMed] [Google Scholar]
- 77.Biondi-Zoccai GG, Lotrionte M, Anselmino M, et al. Systematic review and meta-analysis of randomized clinical trials appraising the impact of cilostazol after percutaneous coronary intervention. Am Heart J. 2008;155(6):1081–1089. doi: 10.1016/j.ahj.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 78.Mehta SR, Tanguay JF, Eikelboom JW, et al. CURRENT-OASIS 7 trial investigators Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376(9748):1233–1243. doi: 10.1016/S0140-6736(10)61088-4. [DOI] [PubMed] [Google Scholar]
- 79.King SB, III, Smith SC, Jr, Hirshfeld JW, Jr, et al. ACC/AHA/SCAI 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol. 2008;51(2):172–209. doi: 10.1016/j.jacc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 80.Silber S, Albertsson P, Avilés FF, et al. Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology Guidelines for percutaneous coronary interventions. Eur Heart J. 2005;26(8):804–847. doi: 10.1093/eurheartj/ehi138. [DOI] [PubMed] [Google Scholar]
- 81.Jolly SS, Pogue J, Haladyn K, et al. Effects of aspirin dose on ischaemic events and bleeding after percutaneous coronary intervention: insights from the PCI-CURE study. Eur Heart J. 2009;30(8):900–907. doi: 10.1093/eurheartj/ehn417. [DOI] [PubMed] [Google Scholar]
- 82.Bergeron P, Rudondy P, Poyen V, Pinot JJ, Alessandri C, Martelet JP. Long-term peripheral stent evaluation using angioscopy. Int Angiol. 1991;10(3):182–186. [PubMed] [Google Scholar]
- 83.Ueda Y, Nanto S, Komamura K, Kodama K. Neointimal coverage of stents in human coronary arteries observed by angioscopy. J Am Coll Cardiol. 1994;23(2):341–346. doi: 10.1016/0735-1097(94)90417-0. [DOI] [PubMed] [Google Scholar]
- 84.Stettler C, Wandel S, Allemann S, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370(9591):937–948. doi: 10.1016/S0140-6736(07)61444-5. [DOI] [PubMed] [Google Scholar]
- 85.Schömig A, Dibra A, Windecker S, et al. A meta-analysis of 16 randomized trials of sirolimus-eluting stents versus paclitaxel-eluting stents in patients with coronary artery disease. J Am Coll Cardiol. 2007;50(14):1373–1380. doi: 10.1016/j.jacc.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 86.Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293(17):2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 87.Spertus JA, Kettelkamp R, Vance C, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation. 2006;113(24):2803–2809. doi: 10.1161/CIRCULATIONAHA.106.618066. [DOI] [PubMed] [Google Scholar]
- 88.Jeremias A, Sylvia B, Bridges J, et al. Stent thrombosis after successful sirolimus-eluting stent implantation. Circulation. 2004;109(16):1930–1932. doi: 10.1161/01.CIR.0000127105.99982.21. [DOI] [PubMed] [Google Scholar]
- 89.Mehta SR, Yusuf S, Peters RJ, et al. Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358(9281):527–533. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 90.Pekdemir H, Cin VG, Camsari A, et al. A comparison of 1-month and 6-month clopidogrel therapy on clinical and angiographic outcome after stent implantation. Heart Vessels. 2003;18(3):123–129. doi: 10.1007/s00380-003-0704-1. [DOI] [PubMed] [Google Scholar]
- 91.Steinhubl SR, Berger PB, Mann JT, III, et al. CREDO Investigators. Clopidogrel for the Reduction of Events During Observation Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288(19):2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 92.Bernardi V, Szarfer J, Summay G, et al. Long-term versus short-term clopidogrel therapy in patients undergoing coronary stenting (from the Randomized Argentine Clopidogrel Stent [RACS] trial) Am J Cardiol. 2007;99(3):349–352. doi: 10.1016/j.amjcard.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 93.Akbulut M, Ozbay Y, Karaca I, Ilkay E, Gundogdu O, Arslan N. The effect of long-term clopidogrel use on neointimal formation after percutaneous coronary intervention. Coron Artery Dis. 2004;15(6):347–352. doi: 10.1097/00019501-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 94.Pfisterer M, Brunner-La Rocca HP, Buser PT, et al. BASKET-LATE Investigators Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol. 2006;48(12):2584–2591. doi: 10.1016/j.jacc.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 95.Eisenstein EL, Anstrom KJ, Kong DF, et al. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA. 2007;297(2):159–168. doi: 10.1001/jama.297.2.joc60179. [DOI] [PubMed] [Google Scholar]
- 96.Lip GY, Gibbs CR. Antiplatelet agents versus control or anticoagulation for heart failure in sinus rhythm. Cochrane Database Syst Rev. 2010;(4):CD003333. doi: 10.1002/14651858.CD003333. [DOI] [PubMed] [Google Scholar]
- 97.Cleland JG, Findlay I, Jafri S, et al. The Warfarin/Aspirin Study in Heart failure (WASH): a randomized trial comparing antithrombotic strategies for patients with heart failure. Am Heart J. 2004;148(1):157–164. doi: 10.1016/j.ahj.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 98.Massie BM, Collins JF, Ammon SE, et al. WATCH Trial Investigators Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation. 2009;119(12):1616–1624. doi: 10.1161/CIRCULATIONAHA.108.801753. [DOI] [PubMed] [Google Scholar]
- 99.Cokkinos DV, Haralabopoulos GC, Kostis JB, Toutouzas PK. HELAS investigators Efficacy of antithrombotic therapy in chronic heart failure: the HELAS study. Eur J Heart Fail. 2006;8(4):428–432. doi: 10.1016/j.ejheart.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 100.Jafri SM, Mammen EF, Masura J, Goldstein S. Effects of warfarin on markers of hypercoagulability in patients with heart failure. Am Heart J. 1997;134(1):27–36. doi: 10.1016/s0002-8703(97)70103-0. [DOI] [PubMed] [Google Scholar]
- 101.Wilson PWF, D'Agostino RB, Levy D, Belanger AM, Silbershatz S, Kannel WB. Prediction of coronary artery disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.