Abstract
Background and Purpose
Carbon monoxide (CO) is a gaseous second messenger produced when heme oxygenase (HO) enzymes catabolize heme. We have demonstrated that CO can be therapeutic in ischemia-reperfusion brain injury; however, it is unclear whether CO can also offer protection in permanent ischemic stroke or what mechanism(s) underlies the effect. HO1 neuroprotection was shown to be regulated by Nrf2; therefore, we investigated whether CO might partially exert neuroprotection by modulating the Nrf2 pathway.
Methods
To evaluate potential protective effects of CO, we exposed male wildtype and Nrf2 knockout mice to 250 ppm CO or control air for 18 hours immediately after permanent middle cerebral artery occlusion. Infarct volume and neurological deficits were assessed on day 7. Molecular mechanisms of Nrf2 pathway activation by CO were also investigated.
Results
Mice exposed to CO after permanent ischemia had 29.6±12.6% less brain damage than did controls at 7 days, though amelioration in neurological deficits did not reach significance. Additionally, 18-hour CO treatment led to Nrf2 dissociation from Keap1, nuclear translocation, increased binding activity of Nrf2 to HO1 antioxidant response elements, and elevated HO1 expression 6–48 hours after CO exposure. The CO neuroprotection was completely abolished in Nrf2 knockout mice.
Conclusions
Low-concentration CO represent a neuroprotective agent for combination treatment of ischemic stroke, and its beneficial effect would be at least partially mediated by activation of the Nrf2 pathway.
Keywords: carboxyhemoglobin, heme oxygenase, mouse, neuroprotection, stroke
Low or near-physiological doses of carbon monoxide (CO) have been shown to protect cells through potential anti-inflammatory, anti-proliferative, or anti-apoptotic effects; however, the exact cellular pathway(s) is still under investigation.1,2 CO is a soluble gas that is generated in cells almost exclusively through the degradation of heme by heme oxygenase (HO) enzymes. HO1 is known to be induced by the transcriptional factor Nrf2 which is considered to be a multi-organ protector and mediate neuroprotection by binding to antioxidant response elements (AREs) of antioxidant genes to increase their transcription.3 Normally, Nrf2 is bound to Keap1, largely localized in the cytoplasm. This quenching interaction maintains low basal expression of Nrf2-regulated genes. However, when cells are subjected to oxidative or xenobiotic stress, Nrf2 dissociates from Keap1, traverses to the nucleus, and activates the expression of phase II enzymes such as HO1, which is known to have some of AREs.3 Exposure to CO produces protective effects similar to those of HO1 in many models, although it remains unclear in some cases whether the doses used represent physiological or supra-physiologic levels.1 We have demonstrated that low level of exogenous CO (250 ppm for 18 h) can be neuroprotective against transient cerebral ischemia.4 Here, our goal was to determine whether CO is also protective in permanent focal cerebral ischemia, which is considered by some to have higher clinical relevance than transient cerebral ischemia. Furthermore, we investigated whether CO acts as a neuroprotective messenger in vivo via Nrf2 pathway activation.
Materials and Methods
Animals
First all experiments were carried out in accordance with the National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee. All random-assigned mice were 8–10-week-old wildtype (WT) and Nrf2 knockout (Nrf2-/-) C57BL/6 mice.
CO exposure
Mice were placed into a Plexiglas chamber at room temperature for exposure to air-control, or 250 ppm CO as monitored by the Single Gas Analyzer, CO91 (Universal Enterprises) and at a 1 L/min flow. After exposure, mice were removed from the chamber to their original cages.
Permanent middle cerebral artery occlusion (pMCAO)
Mice were first anesthetized with halothane (3% induction, 1.2–1.5% maintenance), and then a short vertical skin incision was made between the right eye and ear, a 2.0 mm burr hole was drilled and the main trunk of the distal MCA was occluded by bipolar coagulation. Interruption of blood flow at the occlusion site was confirmed by severance and the blood flow reduction was monitored by laser-Doppler flowmetry. Infarct volume was determined 7 days after pMCAO by image analysis of five 2-mm thick coronal sections stained with 1% 2,3,5-triphenyltetrazolium chloride and calculated as a percentage of the contralateral cortex with correction for swelling.
Behavioral tests
One experimenter held the mouse while another placed an adhesive tape (3×4 mm2) onto the plantar surface of each forepaw, and the contact time and removal time were recorded. The contact time represented the time taken for the animal to begin shaking or bringing the forepaw to its mouth. The removal time represented the time taken, once the tape had been sensed, for the animal to remove the tape. The mice were given a maximum of 2 min.5 Mice were tested before surgery and 7 days after surgery. The 28-point scoring neurological system includes body symmetry, gait, climbing, circling behavior, front limb symmetry, compulsory circling, and whisker response. Each test is graded from 0 to 4, establishing a maximum deficit score of 28. For the gross locomotor test, mice were evaluated with the Home Cage Video Tracking System (MedAssociates) for 30 min at 7 days after CO or air exposure.
Electrophoresis mobility shift assay (EMSA) for Nrf2
Nuclear protein was extracted by the sucrose gradient centrifugation.6 Five micrograms of nuclear protein were incubated with 32P-labeled, double-stranded oligonucleotide probes containing ARE sequences of the HO1 promoter (5’-TTTTATGCTGTGTCATGGTTT-3’ and 3’-AAAATACGACACAGTACCAAA-5’); cold probes lacking 32P-label; and mutant probes containing mutant ARE sequences (5’-TTTTATGCGTAGATCTGGTT-3 ’ and 3 ’-AAAATACGCATCTAGACCAA-5’). Positive control nuclear protein was obtained from mouse neuronal cultures that were exposed to sulforaphane (an Nrf2 activator; 0.5 μM).
Immunological analysis
The cytosolic protein was prepared as described previously.6 For immunoprecipitation, 15 μL of antibody (anti-Nrf2 or anti-Keap1, Santa Cruz) were added to 300 μg of protein. The mixture was then incubated with glutathione sepharose 4B beads (GE Healthcare), and supernatant proteins were separated on gel, transferred to nitrocellulose membranes, and incubated with primary antibodies [anti-Keap1 or -Nrf2 (1:1000, Santa Cruz), anti-HO1 or -HO2 (1:1000, Stressgen Biotechnologies), anti-β-actin, (1:3000, Sigma), anti-histone (1:800, U.S. Biological), and anti-MnSOD (1:5000, from T. Dawson, Johns Hopkins University)]. For immunohistochemistry, the frozen sections were blocked in normal goat serum, incubated with Nrf2 antibody and then with biotinylated secondary antibody (Vector). Immunoreactions were developed with the avidin-peroxidase-labeled biotin complex and visualized by the DAB peroxidase substrate (Vector).
Statistical analysis
Data were analyzed with Sigmastat 3.0 software. All values are presented as mean±SD. For comparisons between two groups, a two-tailed unpaired Student’s t-test was used. For comparisons among multiple groups, one-way ANOVA with a Student-Newman-Keuls procedure was used. Statistical significance was set at P<0.05.
Results
Effect of post-pMCAO CO exposure on infarct size and neurobehavioral function
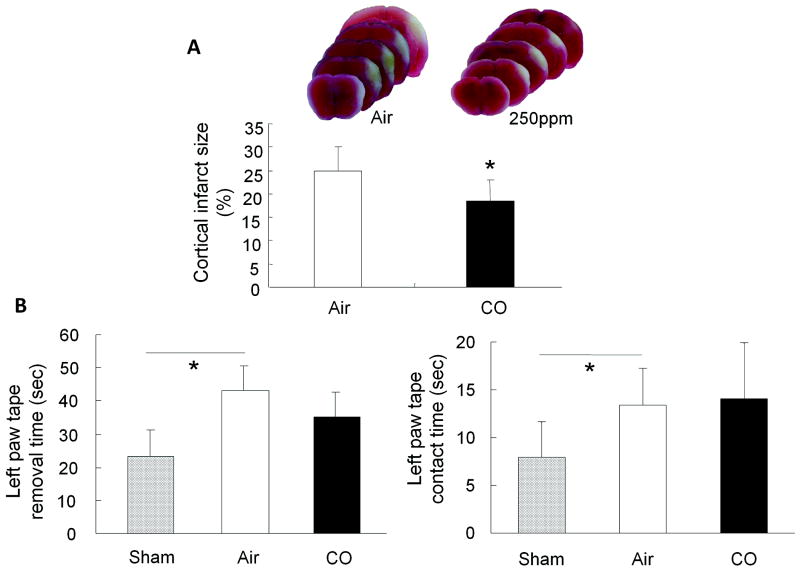
Eighteen hours of CO inhalation initiated immediately after ischemia significantly reduced infarct size from 25.2±4.8% (air) to 16.4±3.7% (CO) (Figure 1A). Behavioral performances between air- and CO-treated mice did not reach significance when assessed with neurological deficit score and locomotor test (data not shown). However, in the tape removal test, mice subjected to right-side pMCAO and exposed to air required significantly longer to contact (13.4±3.9 seconds) and remove (43.3±7.4 seconds) the tape from the left forepaw than did sham-operated mice (contact time: 7.9±3.7 seconds; removal time: 23.2±7.9 seconds). The mice exposed to CO had a trend toward shorter time to remove the tape from the left paw compared to animals exposed to air, but the contact time was unchanged (Figure 1B).
Figure 1.
Effects of 250 ppm CO on infarct size and neurological deficit after permanent ischemia. (A) Seven days after pMCAO, infarct size was significantly larger in the air-exposed group than in the CO-exposed group. (B) Mice subjected to pMCAO required significantly more time to contact and remove the tape on left forepaws than did the sham-operated group. No significant difference was observed between the air-exposed group and CO-exposed group in the contact time (right). However, mice exposed to CO had a trend toward improved ability to remove the tape compared with air-exposed mice (left). n = 9 per group; *P<0.05.
CO exposure increases dissociation of Keap1-Nrf2 and Nrf2 nuclear translocation
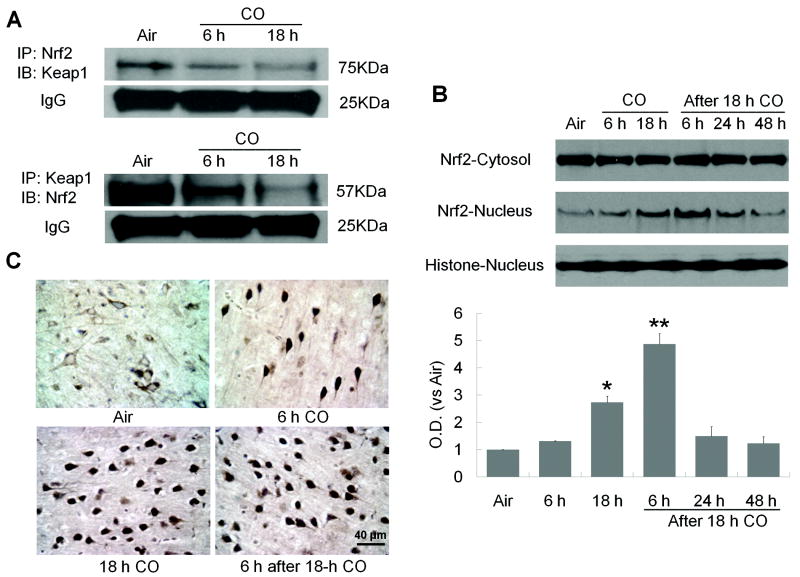
First, cytosolic fractions were immunoprecipitated with anti-Nrf2 antibody and immunoblotted with anti-Keap1 antibody. A 75-kDa protein, corresponding to Keap1 that had bound to Nrf2, was observed in the brains of mice exposed to air, but its expression was significantly lower after 18-hour CO exposure (Figure 2A). Next, the lysates were precipitated with anti-Keap1 antibody and immunoblotted with anti-Nrf2 antibody. The 57-KDa protein corresponding to Nrf2 that had bound to Keap1 was highly detected in the brains of mice exposed to air, but its expression decreased with increasing time of CO exposure (Figure 2A). These results support the concept that CO promotes dissociation of Nrf2 from Keap1, the first step in Nrf2 nuclear translocation.
Figure 2.
CO increases Nrf2 nuclear translocation. (A) Nrf2 dissociates from Keap1 after 6- or 18-hour CO exposure. Immunoblotting showed that each antibody precipitated significantly less of the other protein after CO exposure, indicating a reduction in interaction between Nrf2 and Keap1. IgG was a protein-loading control. (B) Nrf2 protein expression was assessed in nuclear and cytosolic fractions at the times shown. Nrf2 expression was unchanged in the cytosolic fraction, but it was significantly increased in the nuclear fraction after 18-hour CO exposure and remained elevated for at least 6 hours after CO termination. Histone was used as a nuclear marker and as a protein-loading control. (C) Immunohistochemistry shows Nrf2 nuclear localization in the cortex of CO-exposed mice. In the air-treated group, most Nrf2 was cytoplasmic in cells with neuronal morphology. Nuclear staining of Nrf2 increased with increasing CO exposure time and remained high through 6 hours after CO exposure. The percentage of cells with nuclear Nrf2 staining was assessed on six non-overlapping fields for each section (n = 6 mice per group); *P<0.05, **P<0.01.
To further test whether CO could induce Nrf2 nuclear translocation, we measured Nrf2 protein in cytosolic and nuclear brain extracts. CO exposure did not significantly alter cytosolic Nrf2 levels. However, nuclear Nrf2 accumulation was significantly enhanced at 0 and 6 hours after 18-hour CO exposure; the expression then decreased to basal levels over 24 hours (Figure 2B). We used immunohistochemistry to confirm localization of Nrf2 in the cortex. In the air-exposed group, we observed Nrf2 staining in the cytosol of cells, whereas Nrf2 immunostaining was predominantly nuclear at 0 and 6 hours after CO exposure (Figure 2C).
CO increases nuclear Nrf2 occupancy of ARE in the HO1 promoter
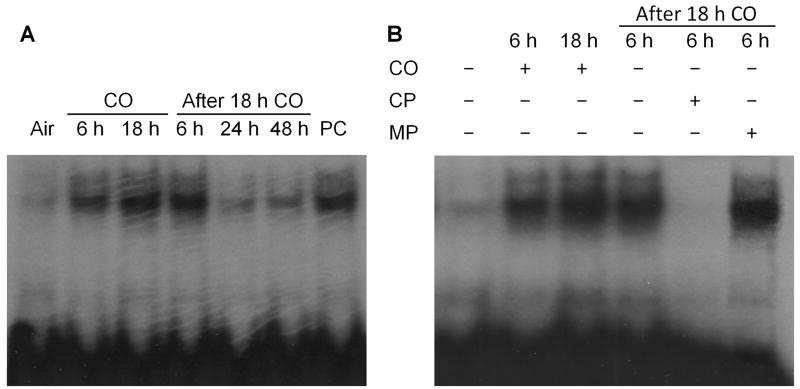
Binding to the ARE by Nrf2 represents an indispensable process for transcriptional activation of ARE-responsive genes. Therefore, we measured Nrf2-ARE binding activity with Nrf2 specific, double-stranded oligonucleotide probes. We found that Nrf2-ARE was significantly increased at 0 and 6 hours after 18-hour CO exposure but had returned to nearly baseline by 24 hours (Figure 3A). Cultured neurons treated with the Nrf2 activator sulforaphane served as a positive control. To confirm that up-regulation of HO1 expression was mediated by functional Nrf2, we used an excess concentration of cold probes to compete for radioactive complex formation, and dominant-negative mutant probes to demonstrate Nrf2-ARE interactional specificity. Competition from cold probes completely inhibited Nrf2 binding with radioactive probes. In contrast, mutant probes failed to suppress binding activity (Figure 3B).
Figure 3.
Effect of CO on Nrf2-ARE binding activity. (A) Nrf2-ARE binding activity was substantially increased from 0 to 6 hours after 18-hour CO exposure. As a positive control (PC), cultured mouse neurons were treated with the Nrf2 activator sulforaphane (0.5 μM) for 24 hours. (B) Competition with excess nonradioactive probes markedly suppressed Nrf2-ARE binding, but mutant probes were unable to disrupt this binding. CP, cold probe without 32P; MP, mutant probe with 32P.
HO1 induction after CO exposure
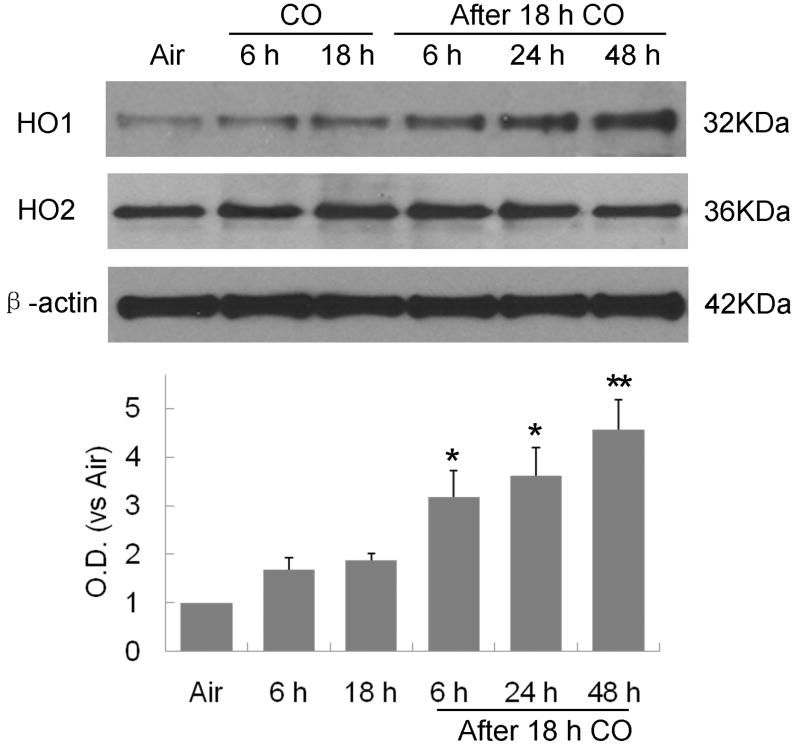
HO1 has a large number of AREs within its promoter and is highly dependent on Nrf2 action. Therefore, we investigated the expression of HO1 and HO2 after CO exposure. As shown in Figure 4, HO1 expression gradually increased from 6 to 48 hours after 18-hour CO exposure. In contrast, CO had no effect on HO2 expression.
Figure 4.
HO1, but not HO2, time-dependently increased from 6 to 48 hours after 18-hour CO exposure; *P<0.05, **P<0.01.
CO is not protective in Nrf2-/- mice
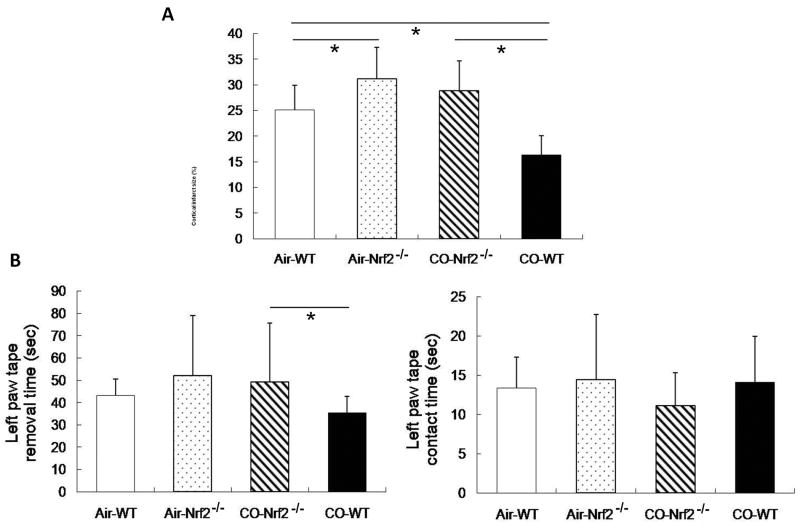
To further clarify the beneficial role of Nrf2 activation after CO treatment, we subjected Nrf2-/-and WT mice to pMCAO and exposure to 250 ppm CO or air for 18 hours and then measured infarct size and neurological deficits at 7 days. Nrf2-/- mice had significantly larger infarct size and prolonged left-paw tape removal time than did WT mice and did not show any improvements after CO exposure (Figure 5). Additionally, CO did not induce HO1 expression in Nrf2-/- mice after CO exposure (see http://stroke.ahajournals.org).
Figure 5.
The beneficial effect of CO is lost in Nrf2 knockout (Nrf2-/-) mice. (A) Infarct size was significantly larger in Nrf2-/- than in WT mice regardless of air or CO exposure. CO exposure reduced the infarct size in WT but not in Nrf2-/- mice relative to their respective air-exposed controls; n = 8 per group. (B) Neurological deficits were assessed by the tape removal test. No significant differences were noted among the groups in the contact time (right). However, in the CO-exposed group, Nrf2-/- mice had prolonged left paw tape removal time (left) compared with WT mice. No differences existed in tape removal time between CO-exposed and air-exposed Nrf2-/- mice, indicating that CO provided no benefits in Nrf2-/- mice. n = 8 per group; *P<0.05.
Discussion
The major findings are that 250 ppm CO exposure: 1) significantly reduces infarct size but has no significant effect on behavioral deficits after permanent ischemia; 2) increases dissociation of Nrf2 from Keap1 and causes Nrf2 nuclear translocation in the brain; 3) increases the nuclear Nrf2 occupancy of ARE within the HO1 promoter; 4) induces time-dependent increases in HO1 expression; and 5) does not provide beneficial effects in Nrf2-/- mice. These data suggest that CO exposure after stroke can provide neurological protection by activating the Nrf2 pathway.
Our previous study showed that 250 ppm CO exposure for 18 h provided better neuroprotection than other concentrations and durations (125 or 500 ppm for 12 or 18 h).4 In addition, we found 250 ppm CO treatment for 18 h given immediately or 1 h delay after ischemia showed significant protection against brain injury, as evidenced by decreased infarct volume. However, delaying CO exposure for 3 h resulted in weaker protection (data not shown). Therefore, the therapeutic window for CO treatment in mice might be within 3 h after ischemia. We chose to give CO immediately after pMCAO.
We first accessed neurological deficit score and locomotor activity for behavioral evaluation. However, we did not find any significant difference between air- and CO-treated mice subjected to pMCAO on day 7 (data not shown). Nevertheless, according to Freret et al.,5, the tape removal test is more sensitive than other methods for detecting behavioral differences after pMCAO. We employed this test and found CO-exposed mice had a trend toward removing the tape from the left paw in less time than air-exposed mice. A few factors that may contribute to detect no significant effect on neurological deficits: 1) the injury in pMCAO is mainly confined to the cortex of the temporal lobe, but the primary somatosensory area is located in the postcentral gyrus of the parietal lobe; 2) the tape removal test may have intrinsic limitations; 3) the limitations of behavioral testing in mice may be partly due to the high level of spontaneous recovery; 4) CO may not be sufficiently robust per se to provide functional improvement with the give neurobehavioral tests. Taken together, we plan to consider more sensitive evaluation of neurological deficits to reveal a benefit of CO treatment in future studies.
High CO concentrations can cause hypoxemia by competitive binding to oxygen-binding sites of hemoglobin to form carboxyhemoglobin (COHb). In humans, symptoms of CO poisoning begin to appear at 20% COHb, while death occurs between 50 and 80% COHb.7 In contrast, CO exposures associated with cytoprotection in rodents resulted in attained COHb levels within the 15-20% range.8 Based on our study, the blood COHb levels reached only 16.5±1.5% at 18 h, and returned to baseline relatively quickly after exposure. Whether compensation could be possible; no changes in total hemoglobin levels we noted (data not shown). Therefore, oxygen carrying capacity of blood did not significantly changed during CO exposure although subtle changes cannot be ignored.
Our data revealed that activation of the Nrf2 pathway might partially contribute to CO neuroprotection. Nrf2 has been considered to be a key regulator in cell survival mechanisms, and its activation induces expression of phase II enzymes, which attenuate tissue injury caused by oxidative stress.3 Dulak and colleagues.9 reported that CO gas activates different kinases such as phosphatidylinositol 3-kinase, protein kinase C, c-Jun NH2-terminal kinase, p38 and extracellular-signal-regulated kinase, which lead to Nrf2 activation and downstream gene expression. Although a lot of work has been conducted in regard to the Nrf2 regulation its exact activation remains unclear. It has been shown that CO-induced guanylate cyclase activation with cGMP production involves in vasoregulation. However, it is not clear whether cGMP production is directly involved in Nrf2 activation by CO. Nrf2 gene function did not appear to be necessary for overall mouse development, growth, and fertility. No detectable development defects are found in Nrf2-/- mice.10 Existing data have demonstrated that treatment with chemical activators of Nrf2, such as sulforaphane11, can reduce cellular damage in wildtype mice but not in Nrf2-/-mice. In agreement with these findings, we found that exogenous CO induced Nrf2 activation and its neuroprotection was abolished in Nrf2-/- mice. Our group and others have shown that Nrf2 activation may confer protection to stroke12 or oxidative stress-related neurodegenerative insults.13 Previous and current data position the Nrf2 as a prime candidate for prevention of oxidative stress and subsequent neurotoxicity.
Nrf2 activation can coordinately upregulate expression of several antioxidative enzymes recognized to play important roles in combating oxidative stress, including HO1, SOD, glutathione S-transferase, as well as NADPH-regenerating enzymes. However, compared to other enzymes, HO1 promoter is known to have a large number of ARE sequences to which Nrf2 can bind to induce its expression in a preferential manner.3 In addition, HO1 is more likely to exert a central role in neuroprotection because it is inducible to degrade the free heme, and its metabolites CO or biliverdin/bilirubin can directly provide cytoprotection.4, 14 It has been shown that HO1 may provide protection against excitotoxicity15 and cerebral ischemia16. Therefore, we believe HO1 could be a significant player although other enzymes can also assist to the CO neuroprotection. We further plan to use HO1 knockout mice or enzymatic inhibitors to further determine its unique contribution in CO protection although we have found that Nrf2 activation caused by CO led to an increase in HO1 levels.
Recent findings have suggested the central role of reactive oxygen species (ROS) induced by low levels of CO in CO-initiated preconditioning and protection by induction of antioxidant enzymes and protective signaling pathways.2 Eighteen hours of CO exposure may create such an oxidative preconditioning to induce Nrf2 activation for modulation of cerebral ischemia. Meanwhile, the ROS formation in response to low concentrations of CO may have both positive (signaling) and negative (damaging) effects depending on the amount of ROS formed and the cell type under investigation. Therefore, we cannot rule out that 18 h-CO exposure might result in some factors that might contribute to ischemic injury although no overall detectable toxicity was reported at the levels tested.
Our data imply that controlled exposure to exogenous CO could be one means to harness the neuroprotective Nrf2 pathway for stroke treatment. The beneficial effects of CO are likely caused by a combination of factors. CO may also act as a vasodilator by modulating soluble guanylate cyclase17 or activating calcium-activated potassium channels18 to induce vasorelaxation. Activation of p38 MAPK signaling pathway modulated by CO has been implicated in anti-inflammatory19 and anti-apoptotic20 effects. In addition, CO has been reported to have early thrombolytic effects after ischemia.21 Taken together, further investigation of the various mechanisms by which CO might be protective in permanent cerebral ischemia is warranted.
Summary
CO could be a neuroprotective agent in combination treatment of permanent ischemia although it did not reach significance in attenuating the given behavioral tests. As optimal delivery of CO is refined, the protective effects of CO could be extended to the treatment of ischemic stroke.
Acknowledgments
We especially thank Dr. Raymond Koehler, Dr. Xiaoling Li, Claire Levine, and all members of the Doré lab for their assistance in this project. We also thank Thomas Kensler (Johns Hopkins University) and Masayuki Yamamoto (Tohoku University) for the Nrf2-/- mice.
Sources of Funding This study was supported in part by NS046400 and the GEMI Fund grants (SD).
Footnotes
Conflicts of Interest/Disclosures None.
References
- 1.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 2.Bilban M, Haschemi A, Wegiel B, Chin BY, Wagner O, Otterbein LE. Heme oxygenase and carbon monoxide initiate homeostatic signaling. J Mol Med. 2008;86:267–279. doi: 10.1007/s00109-007-0276-0. [DOI] [PubMed] [Google Scholar]
- 3.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the keap1-nrf2-are pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 4.Zeynalov E, Doré S. Low doses of carbon monoxide protect against experimental focal brain ischemia. Neurotox Res. 2009;15:133–137. doi: 10.1007/s12640-009-9014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freret T, Bouet V, Leconte C, Roussel S, Chazalviel L, Divoux D, Schumann-Bard P, Boulouard M. Behavioral deficits after distal focal cerebral ischemia in mice: Usefulness of adhesive removal test. Behav Neurosci. 2009;123:224–230. doi: 10.1037/a0014157. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Nemoto M, Xu Z, Yu SW, Shimoji M, Andrabi SA, Haince JF, Poirier GG, Dawson TM, Dawson VL, Koehler RC. Influence of duration of focal cerebral ischemia and neuronal nitric oxide synthase on translocation of apoptosis-inducing factor to the nucleus. Neuroscience. 2007;144:56–65. doi: 10.1016/j.neuroscience.2006.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Von Burg R. Carbon monoxide. J Appl Toxicol. 1999;19:379–386. doi: 10.1002/(sici)1099-1263(199909/10)19:5<379::aid-jat563>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Hoetzel A, Dolinay T, Vallbracht S, Zhang Y, Kim HP, Ifedigbo E, Alber S, Kaynar AM, Schmidt R, Ryter SW, Choi AM. Carbon monoxide protects against ventilator-induced lung injury via ppar-gamma and inhibition of egr-1. Am J Respir Crit Care Med. 2008;177:1223–1232. doi: 10.1164/rccm.200708-1265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dulak J, Loboda A, Jozkowicz A. Effect of heme oxygenase-1 on vascular function and disease. Curr Opin Lipidol. 2008;19:505–512. doi: 10.1097/MOL.0b013e32830d81e9. [DOI] [PubMed] [Google Scholar]
- 10.Chan K, Lu R, Chang JC, Kan YW. Nrf2, a member of the nfe2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci U S A. 1996;93:13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao J, Kobori N, Aronowski J, Dash PK. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci Lett. 2006;393:108–112. doi: 10.1016/j.neulet.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 12.Shah ZA, Li RC, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, Doré S. Role of reactive oxygen species in modulation of nrf2 following ischemic reperfusion injury. Neuroscience. 2007;147:53–59. doi: 10.1016/j.neuroscience.2007.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calkins MJ, Johnson DA, Townsend JA, Vargas MR, Dowell JA, Williamson TP, Kraft AD, Lee JM, Li J, Johnson JA. The nrf2/are pathway as a potential therapeutic target in neurodegenerative disease. Antioxid Redox Signal. 2009;11:497–508. doi: 10.1089/ars.2008.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doré S, Takahashi M, Ferris CD, Hester LD, Guastella D, Snyder SH. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci U S A. 1999;96:2445–2450. doi: 10.1073/pnas.96.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmad AS, Zhuang H, Doré S. Heme oxygenase-1 protects brain from acute excitotoxicity. Neuroscience. 2006;141:1703–1708. doi: 10.1016/j.neuroscience.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 16.Panahian N, Yoshiura M, Maines MD. Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J Neurochem. 1999;72:1187–1203. doi: 10.1111/j.1471-4159.1999.721187.x. [DOI] [PubMed] [Google Scholar]
- 17.Leffler CW, Parfenova H, Jaggar JH, Wang R. Carbon monoxide and hydrogen sulfide: Gaseous messengers in cerebrovascular circulation. J Appl Physiol. 2006;100:1065–1076. doi: 10.1152/japplphysiol.00793.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu L, Cao K, Lu Y, Wang R. Different mechanisms underlying the stimulation of k(ca) channels by nitric oxide and carbon monoxide. J Clin Invest. 2002;110:691–700. doi: 10.1172/JCI15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen- activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Shan P, Otterbein LE, Alam J, Flavell RA, Davis RJ, Choi AM, Lee PJ. Carbon monoxide inhibition of apoptosis during ischemia-reperfusion lung injury is dependent on the p38 mitogen-activated protein kinase pathway and involves caspase 3. J Biol Chem. 2003;278:1248–1258. doi: 10.1074/jbc.M208419200. [DOI] [PubMed] [Google Scholar]
- 21.Chen YH, Tsai HL, Chiang MT, Chau LY. Carbon monoxide-induced early thrombolysis contributes to heme oxygenase-1-mediated inhibition of neointimal growth after vascular injury in hypercholesterolemic mice. J Biomed Sci. 2006;13:721–730. doi: 10.1007/s11373-006-9093-7. [DOI] [PubMed] [Google Scholar]