Abstract
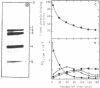
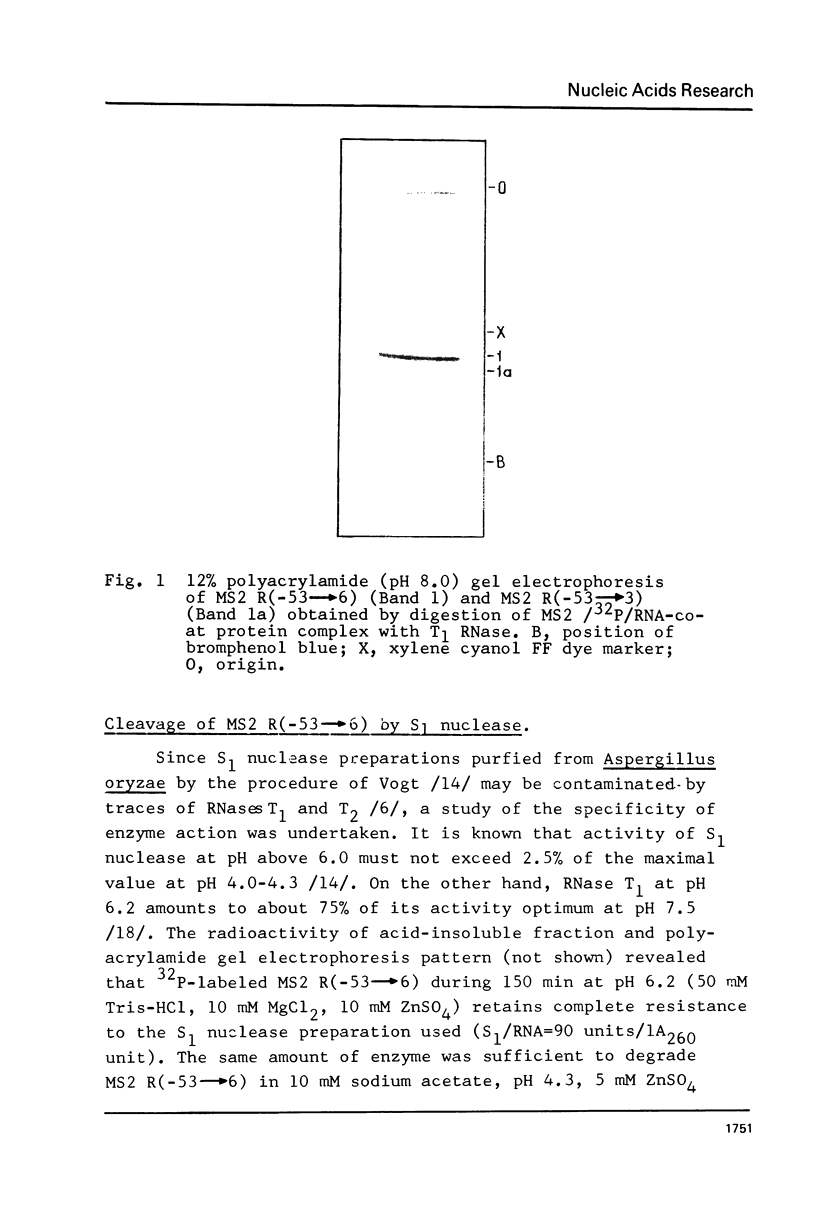
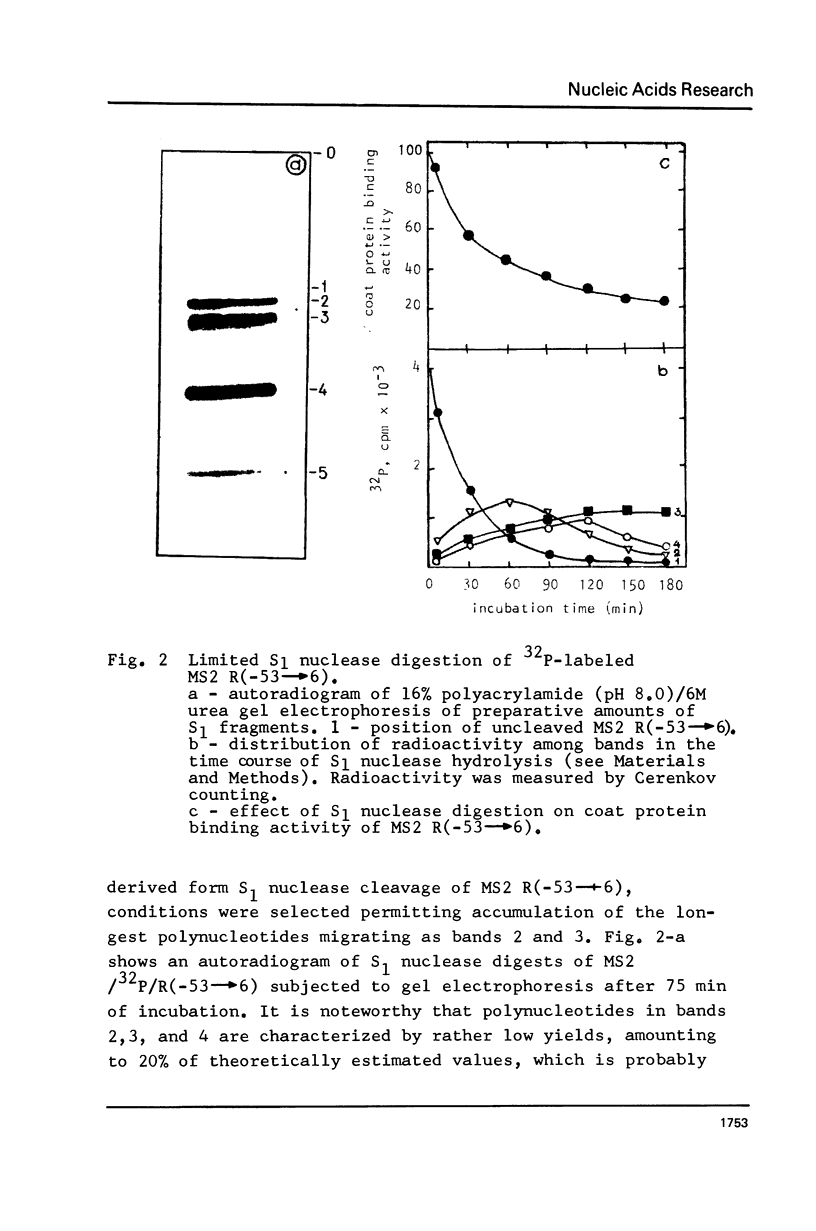
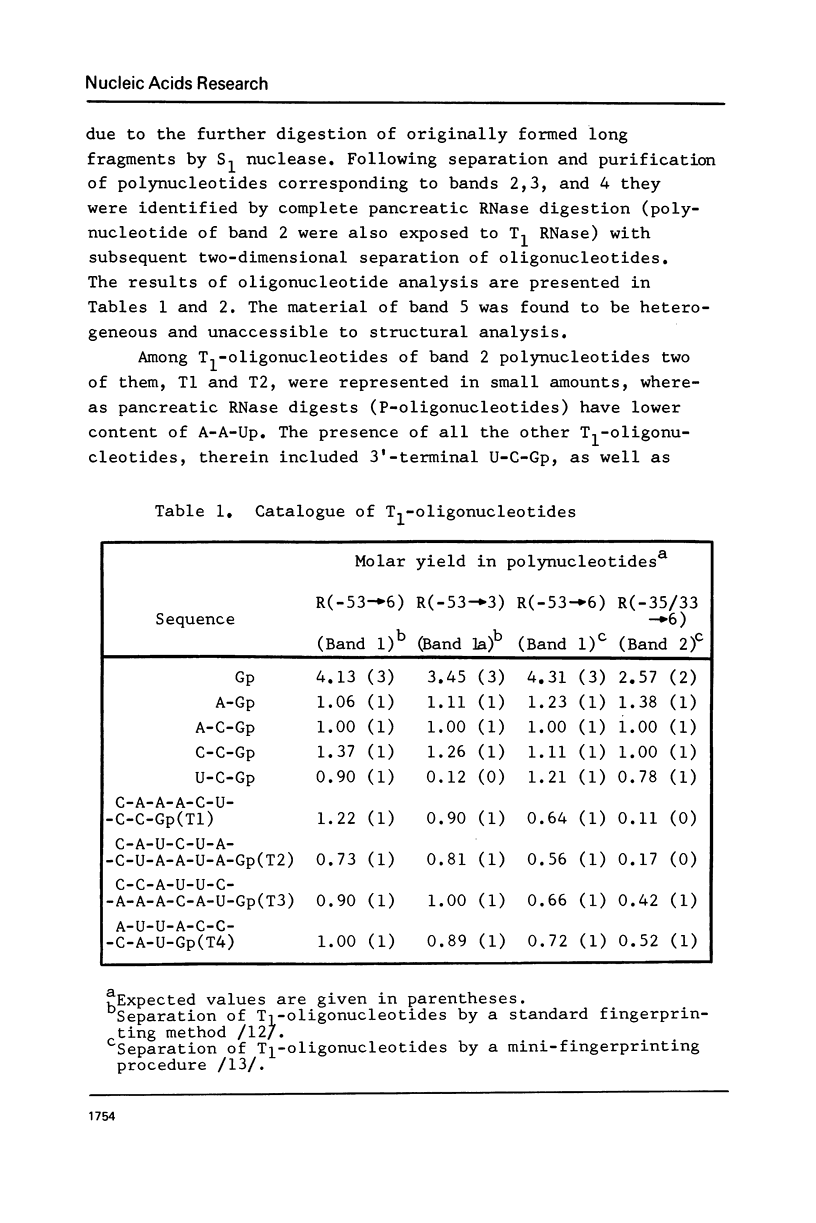
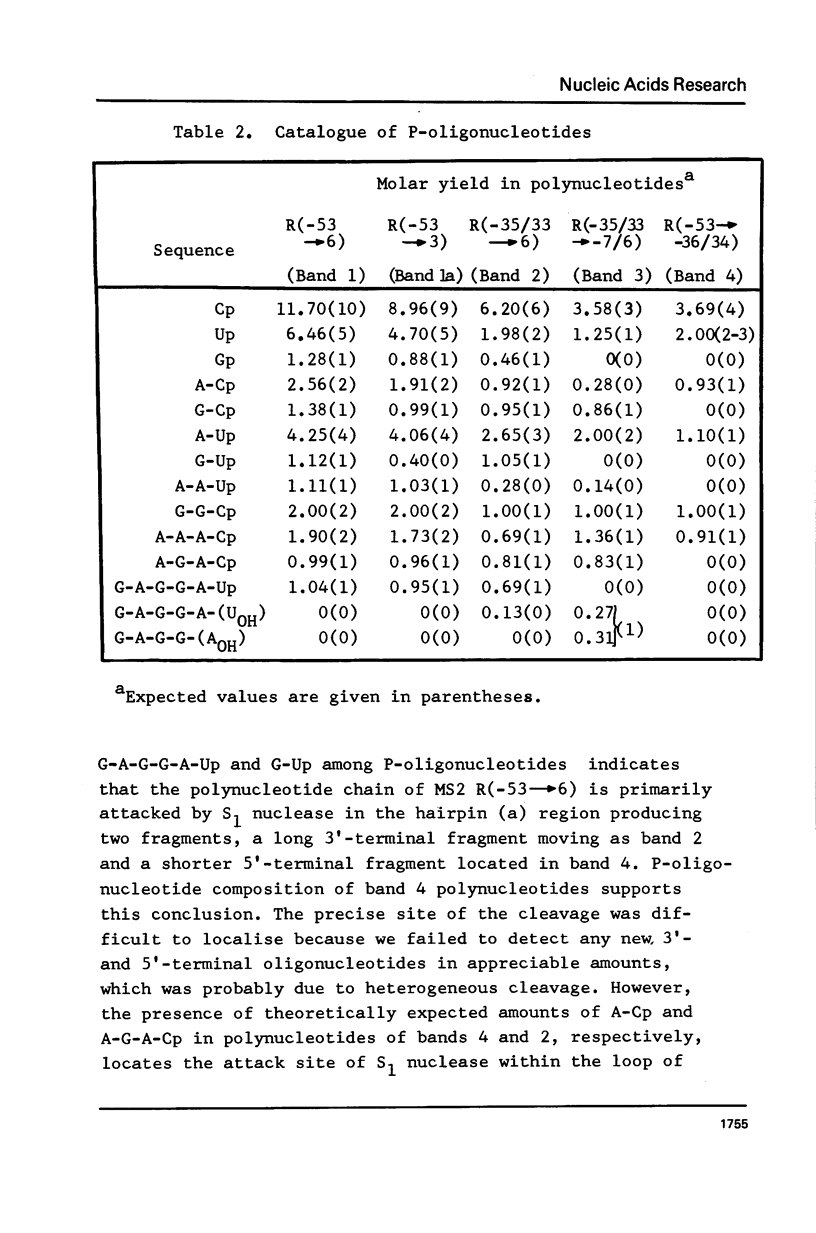
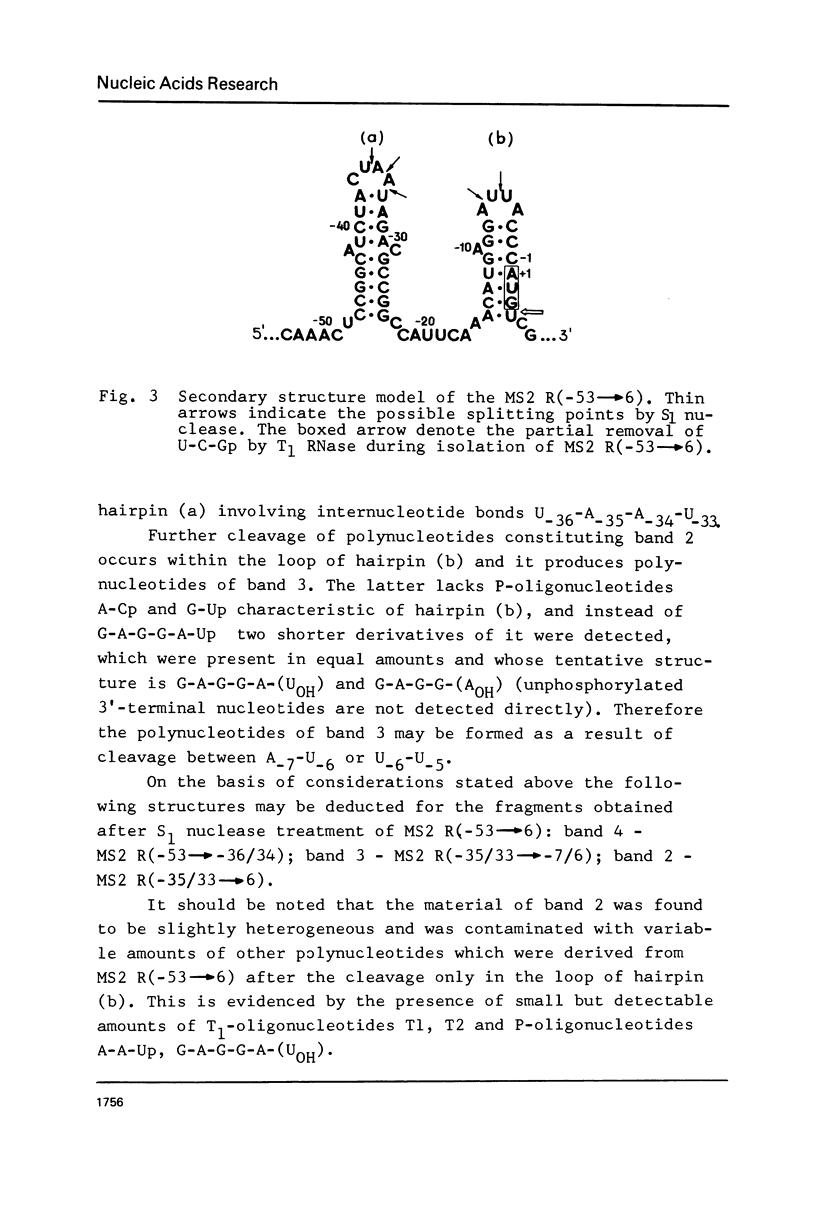
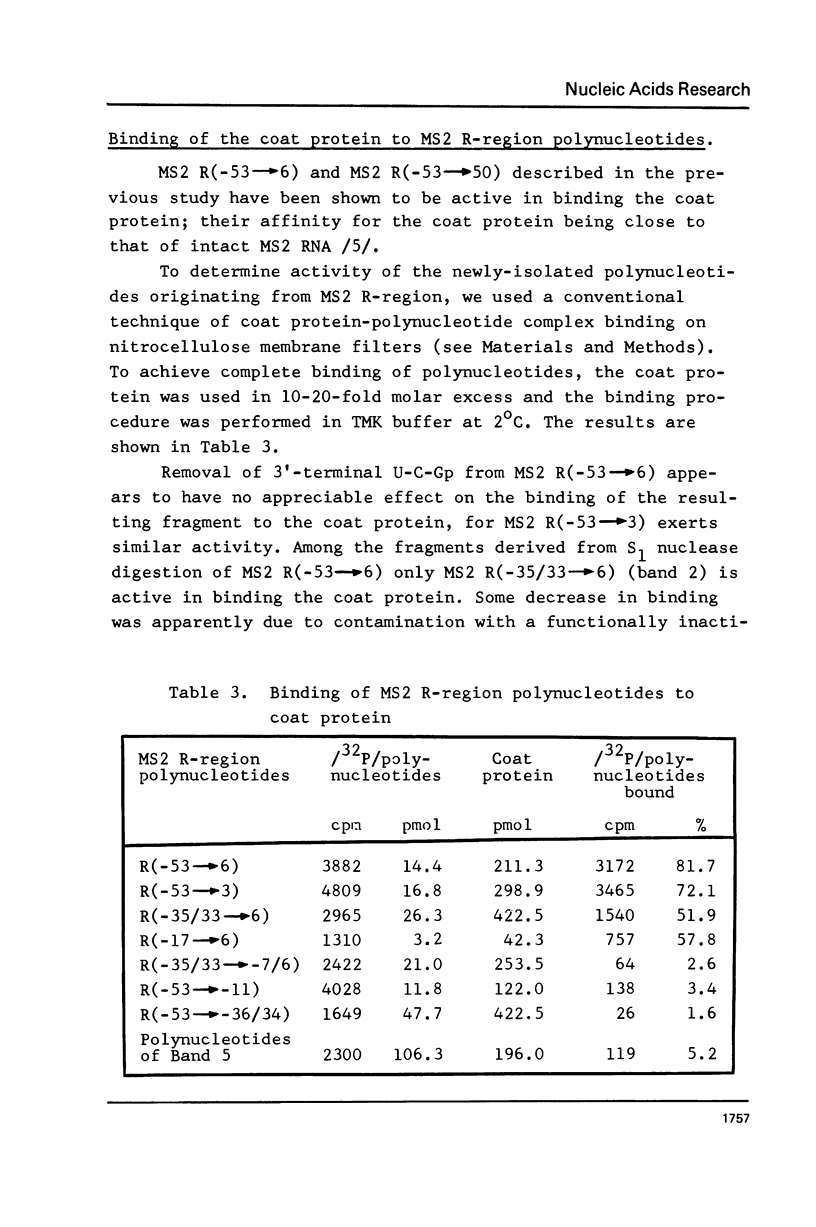
The functionally active fragments MS2 R(-53 leads to 6) and MS2 R(-53 leads to 3) comprising the regulatory region for the replicase cistron have been isolated from MS2 RNA-coat protein complex following T1 RNase digestion. In order to obtain shorter fragments, active in coat protein binding and initiation of translation, MS2 R(-53 leads to 6) was cleaved with S1 nuclease. The results indicate that S1 nuclease attacks the most susceptible loop regions of the two hairpin helices of MSZ R(-53) leads to 6). Among the three fragments which have been isolated, only MS2 R(-35/33 leads to 6) containing the intact hairpin (b) region with initiation codon AUG is active in the coat protein binding. Functional activity exerted by another polynucleotide MS R(-17 leads to 6) supports the assumption that specific binding with the coat protein is determined by the hairpin (b) region prior to the replicase cistron.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beard P., Morrow J. F., Berg P. Cleavage of circular, superhelical simian virus 40 DNA to a linear duplex by S1 nuclease. J Virol. 1973 Dec;12(6):1303–1313. doi: 10.1128/jvi.12.6.1303-1313.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi A., Spahr P. F. Nucleotide sequence at the binding site for coat protein on RNA of bacteriophage R17. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3033–3037. doi: 10.1073/pnas.69.10.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzin' V. M., Borisova G. P., Gribanov V. A., Rozental' G. F., Tsielens I. E. Reguliatornaia oblast' tsistrona replikazy RNK faga MS2. Vydelenie i kharakteristika spetsificheskikh fragmentov RNK. Dokl Akad Nauk SSSR. 1976;229(3):741–744. [PubMed] [Google Scholar]
- Berzin' V. M., Iansone I. V., Gribanov V. A., Tsimanis A. Iu, Gren E. Ia. Reguliatornyi uchastok tsistrona replikazy RNK faga MS2. Dostupnost' Spetsificheskogo fragmenta RNK faga MS2 deistviiu RNKazy T1 i khimicheskoi. Mol Biol (Mosk) 1978 Nov-Dec;12(6):1288–1298. [PubMed] [Google Scholar]
- Berzin V., Borisova G. P., Cielens I., Gribanov V. A., Jansone I., Rosenthal G., Gren E. J. The regulatory region of MS2 phage RNA replicase cistron. Functional activity of individual MS2 RNA fragments. J Mol Biol. 1978 Feb 15;119(1):101–131. doi: 10.1016/0022-2836(78)90272-3. [DOI] [PubMed] [Google Scholar]
- Flashner M. S., Vournakis J. N. Specific hydrolysis of rabbit globin messenger RNA by S1 nuclease. Nucleic Acids Res. 1977 Jul;4(7):2307–2319. doi: 10.1093/nar/4.7.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralla J., Steitz J. A., Crothers D. M. Direct physical evidence for secondary structure in an isolated fragment of R17 bacteriophage mRNA. Nature. 1974 Mar 15;248(445):204–208. doi: 10.1038/248204a0. [DOI] [PubMed] [Google Scholar]
- Hilbers C. W., Shulman R. G., Yamane T., Steitz J. A. High resolution proton NMR study of an isolated fragment of R17 bacteriophage mRNA. Nature. 1974 Mar 15;248(445):225–226. doi: 10.1038/248225a0. [DOI] [PubMed] [Google Scholar]
- Rushizky G. W., Mozejko J. H. Optimization of conditions for cleavage of tRNA at the anticodon loop by S1 nucleases. Anal Biochem. 1977 Feb;77(2):562–566. doi: 10.1016/0003-2697(77)90274-3. [DOI] [PubMed] [Google Scholar]
- Rushizky G. W., Shaternikov V. A., Mozejko J. H., Sober H. A. S1 nuclease hydrolysis of single-stranded nucleic acids with partial double-stranded configuration. Biochemistry. 1975 Sep 23;14(19):4221–4226. doi: 10.1021/bi00690a011. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Specific recognition of the isolated R17 replicase initiator region by R17 coat protein. Nature. 1974 Mar 15;248(445):223–225. doi: 10.1038/248223a0. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Volckaert G., Jou W. M., Fiers W. Analysis of 32P-labeled bacteriophage MS2 RNA by a mini-fingerprinting procedure. Anal Biochem. 1976 May 7;72:433–446. doi: 10.1016/0003-2697(76)90551-0. [DOI] [PubMed] [Google Scholar]
- Warrington R. C. Ribonuclease T1 catalyzed degradation of transfer RNA: an unusual alteration induced by urea. Biochim Biophys Acta. 1974 Jun 14;353(1):63–68. doi: 10.1016/0005-2787(74)90097-5. [DOI] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]