SUMMARY
Dendrites and axons show precise targeting and spacing patterns for proper reception and transmission of information in the nervous system. Self-avoidance promotes complete territory coverage and non-overlapping spacing between processes from the same cell [1, 2]. Neurons that lack Drosophila Down syndrome cell adhesion molecule 1 (Dscam1) show aberrant overlap, fasciculation, and accumulation of dendrites and axons, demonstrating a role in self-recognition and repulsion leading to self-avoidance [3–11]. Fasciculation and accumulation of processes suggested that Dscam1 might promote process spacing by counterbalancing developmental signals that otherwise promote self-association [9, 12]. Here we show that Dscam1 functions to counter sensory neuron dendritic targeting signals provided by secreted Netrin-B and Frazzled, a netrin receptor. Loss of Dscam1 function resulted in aberrant dendrite accumulation at a Netrin-B expressing target, whereas concomitant loss of Frazzled prevented accumulation and caused severe deficits in dendritic territory coverage. Netrin misexpression was sufficient to induce ectopic dendritic targeting in a Frazzled-dependent manner, whereas Dscam1 was required to prevent ectopic accumulation, consistent with separable roles for these receptors. Our results suggest that Dscam1-mediated self-avoidance counter extrinsic signals that are required for normal dendritic patterning, but whose action would otherwise favor neurite accumulation. Counterbalancing roles for Dscam1 may be deployed in diverse contexts during neural circuit formation.
Results and Discussion
Elimination of dendritic self-avoidance reveals focal dendrite targets for da sensory neurons
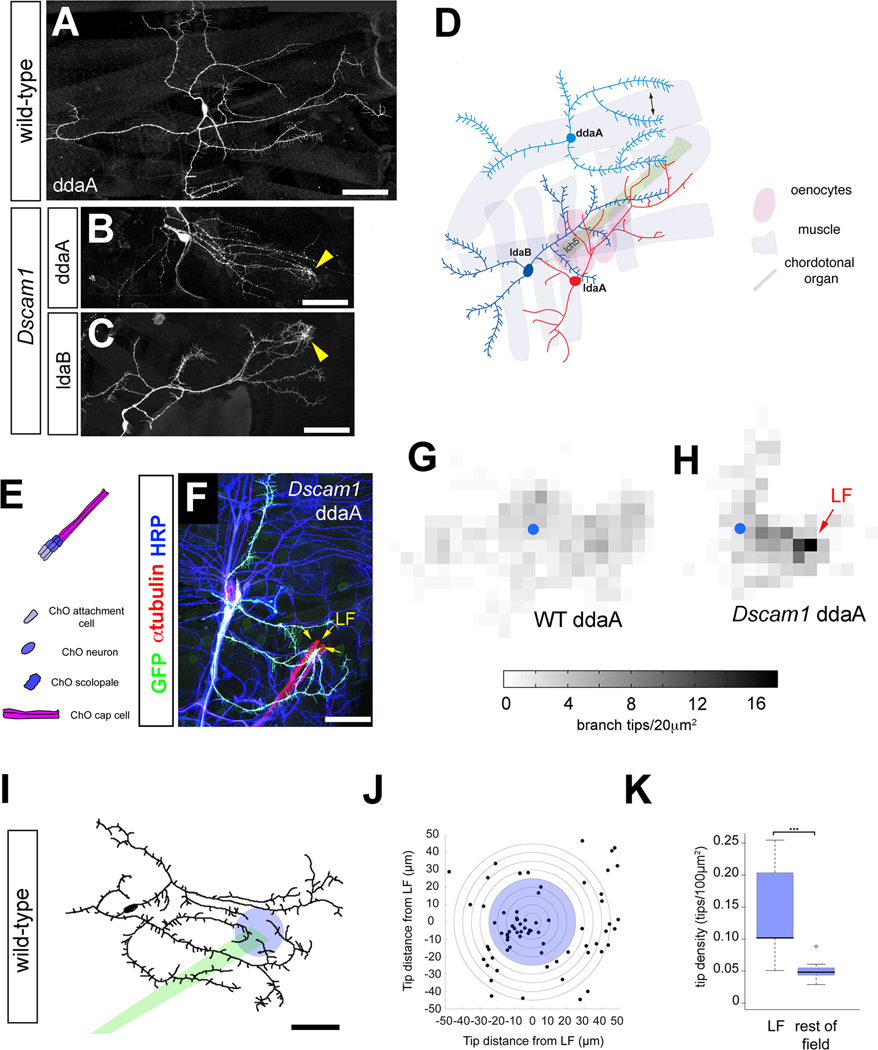
Dendritic territory formation involves both specific dendritic targeting and the spreading of branches across their target region. These processes are likely to be closely coordinated in time but under at least partially distinct molecular control by attractive and repulsive cues. To examine how these steps in territory formation are coordinated in individual arbors, we assessed the consequences of loss of Dscam1 on dendritic targeting of dendritic arborization (da) neurons. Sister dendrites of wild-type neurons rarely cross each other and thus spread evenly over their field (Figure 1A)[13], while dendrites of Dscam1 mutant sensory neurons aberrantly cross, fasciculate, and accumulate (Figures 1B and 1C) [9–11]. Sites of accumulation could correspond to preferred targets of sister dendrites that are revealed absent the spacing constraints of self-avoidance [9]. To characterize the targets that may be responsible for dendrite accumulation we focused on class III da neurons (ddaA and ldaB) that project dendrites near a peripheral sensory organ, the lateral chordotonal organ (LCHO) (Figure 1D) [9, 13]. Labeling of Dscam1 clones, together with anti-HRP, anti-βPS-integrin, or anti-α-tubulin to label surrounding cells, revealed accumulations of dendrites at a distal region of CHO cap cells, a type of support cell that links CHO sensory neurons to the body wall (Figures 1E–1F and S1A) [14]. For simplicity, we refer to this targeted zone of cap cells as the lateral focus (LF). Branch density was higher at LF than the rest of the field in Dscam1 clones (Figures 1G and 1H). Importantly, CHOs are positioned differently in thoracic and abdominal body segments, nevertheless dendrites of Dscam1 mutant sensory neurons accumulated at CHO cap cells regardless of their precise position (Figure S1B), strongly suggesting that it is this specific cell type, rather than other nearby cells, that directs targeting. Accumulations of dendrites in Dscam1 mutant clones represented excessive targeting rather than an abnormal ectopic targeting event, since single dendrites of wild-type ddaA neurons also targeted precisely to LF at each larval instar (Figures 1I–1K and S1C–S1F). These data together suggest that Dscam1 prevents excessive targeting of dendritic branches at peripheral cells that normally provide targeting instuctions to sensory dendrites.
Figure 1. Targeting of Dscam1 mutant neurons to chordotonal organs.
(A) Wild-type MARCM clone of the class III neuron ddaA. Sister dendrites show robust self-avoidance.
(B) Dscam121 mutant MARCM clone of class III neuron ddaA shows branch overlap and dendrite accumulation (yellow arrowhead).
(C) Dscam123 mutant MARCM clone of class III neuron ldaB shows branch overlap and dendrite accumulation (yellow arrowhead).
(D) Schematic of da neurons and nearby non-neuronal cells, including muscle, oenocytes, and chordotonal organs. Epidermal cells upon which dendrites grow are not included in the schematic.
(E) The lateral chordotonal organ (LCHO) consists of a distal cap cell, a scolopale cell, neuron, and proximal attachment cell.
(F) Labeling of a Dscam121 mutant ddaA MARCM clone with anti-GFP, anti-α-tubulin, and anti-HRP reveals accumulation at the distal margin of LCHO in abdominal segments, termed the lateral focus (LF; yellow arrows).
(G) Density plot of all ddaA dendritic termini for wild-type (WT; n=8) MARCM clones. The average numbers of branch endings per cell that terminate within each 20µm2 square are plotted, with higher terminal density indicated by increasing grayscale. Blue circle indicates the position of the cell body.
(H) Density plot of ddaA dendritic termini for Dscam1 MARCM clones (n=4). The average numbers of branch endings per cell that terminate within each 20µm2 square are plotted as in (G). Blue circle indicates the position of the cell body. The accumulation of dendrite tips at LF in Dscam1 mutant clones is indicated by a red arrow.
(I) Tracing of a wild-type ddaA neuron labeled with 189Y-Gal4, UAS-mCD8::GFP. A green bar indicates the trajectory of the chordotonal organ, LCHO. A blue circle with a 25μm radius is centered at LF.
(J) Dendritic tips of ddaA in wild-type larvae are enriched within a 25μm radius of LF when branch tips of multiple cells (n=11) are plotted with respect to LF.
(K) The density of branch tips in a circle of radius 25μm is significantly greater than the rest of the dendritic field.
All scale bars = 50μm.
Datasets are presented in boxplots as median (thick line), quartiles Q1–Q3 (25%–75% quantiles; shaded box), and data in 1.5× quartile range (dashed bars). Data points outside these ranges are indicated as open circles.
* = p < 0.05; ** = p < 0.01; *** = p < 0.001 by Wilcoxon rank-sum test
See also Supplemental Figure S1
Targeting of sensory dendrites requires Frazzled
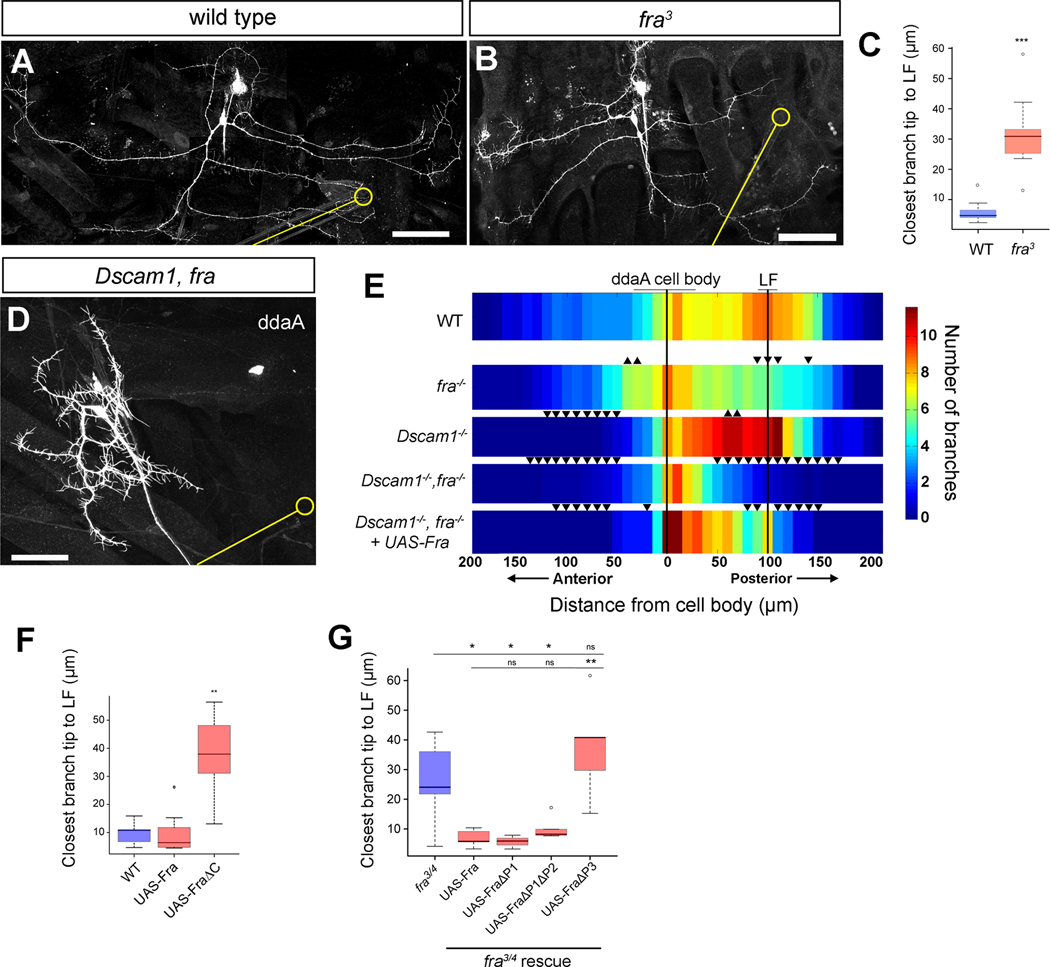
Dscam1 shows homophilic binding activity [15, 16], and in addition can serve as a receptor for the secreted netrins [17–19]. However, since dendrites showed robust targeting both in the presence and absence of Dscam1, we inferred that a signal for an alternate receptor is presented at LF. In a candidate screen of possible attractive receptors for LF targeting, we identified another netrin receptor, Frazzled/Deleted in Colorectal Cancer (Fra/DCC) [20–25]. Whereas wild-type ddaA clones showed robust targeting of a single branch to LF (Figures 2A and 2C), frazzled3 (fra3) mosaic analysis with a repressible cell marker (MARCM) clones showed significant disruptions in targeting (Figures 2B and 2C). Importantly, coincident loss of Frazzled suppressed the accumulation phenotypes seen in Dscam1−/− neurons (Dscam1−/− = 100% accumulated, n=8; Dscam1−/−, fra−/− = 0% accumulated, n=13; Figure 2D). These data are consistent with a cell-autonomous role for Frazzled in sensory dendrite targeting and suggest that Dscam1 prevents excessive Frazzled-dependent targeting by sister dendrites.
Figure 2. Frazzled is required for sensory neuron dendrite targeting.
(A) A wild-type MARCM ddaA neuron showing targeting of branches to the chordotonal organ. The chordotonal organ and LF are indicated by a yellow line and yellow circle, respectively in this, and subsequent, panels.
(B) A fra3 MARCM clone of ddaA showing disrupted targeting to LF.
(C) Plot of the distance of the closest branch tip to LF in wild-type (WT, n=8) and fra3 (n=9) mutant clones.
(D) Dendrites of Dscam121, fra4 double mutant MARCM clone lack enrichment of dendritic termini at LF, and show extensive dendritic clumping near the cell body.
(E) Horizontal Sholl analysis showing mean branch density values at 10μm intervals for wild type (n=8), fra (n=8), Dscam1 (n=5), Dscam121, fra4 (n=14) and Dscam121, fra4 + UAS-Fra (n=6) MARCM clones of ddaA. Upwards-pointing arrowheads denote significant increase in branch density relative to control (p < .05), while downwards-pointing arrowheads denote significant decrease in branch density (p < .05) as compared to topmost control strip.
(F) Quantification of the single closest branch tip to LF in control (n=8), UAS-Fra (n=7), and UAS-FraΔC (n=6) FLP-out clones reveals no effect of UAS-Fra on targeting, but a disruption of targeting caused by UAS-FraΔC. The pan-da neuron driver 109(2)80-Gal4 was used in these experiments.
(G) Quantification of closest branch tip to LF in FLP-out rescue experiments in fra3/fra4 mutant (n=9) background. Introduction of UAS-Fra (n=5), UAS-FraΔP1 (n=3), UAS-FraΔP1ΔP2 (n=5) significantly rescues the targeting defect seen in fra3/fra4 mutant larvae, while introduction of UAS-FraΔP3 (n=5) does not. 109(2)80-Gal4 was used as a driver in these experiments.
Datasets are presented in boxplots as median (thick line), quartiles Q1–Q3 (25%–75% quantiles; shaded box), and data in 1.5× quartile range (dashed bars). Data points outside these ranges are indicated as open circles. In (E), * = p < .05 by Wilcoxon rank-sum test (no further delineations of significance are presented for clarity of presentation).
* = p < 0.05; ** = p < 0.01;*** = p < 0.001 by Wilcoxon rank-sum test.
All scale bars = 50μm
See also Supplemental Figure S2
We asked whether Dscam1 and Frazzled control larger-scale patterning of sensory neuron territories in addition to precise targeting. Modified Sholl analysis of the anterior-posterior arborization of ddaA indicated that wild-type arbors are polarized toward LF and have a relatively sparse anterior arborization (Figure 2E). We observed an anterior shift of ddaA territories (increased anterior and decreased posterior arborization) in fra mutant neurons (Figure 2E). By contrast, anterior coverage was reduced and arborization toward LF was increased in Dscam1 mutant neurons (Figure 2E). Loss of anterior coverage could reflect an early role for Dscam1 in segregation of anterior and posterior processes [5], or Dscam1-mediated responses to other, non-netrin, extrinsic cues. Thus, Frazzled and Dscam1 are both required for proper anterior-posterior positioning of ddaA sensory arbors, but with opposite polarities. Removing activity of both Dscam1 and Frazzled diminished both anterior and posterior regions of the arbor, and territory footprints were strongly reduced relative to wild-type (Figures 2D, 2E, and S2A). The simplest interpretation of these phenotypes is that Dscam1 and Frazzled have separable functions in dendritic targeting. It appears that Frazzled promotes directional spreading of dendrites, presumably to sources of guidance cues near LF, while Dscam1 controls territory positioning by promoting dendrite separation and counterbalancing Frazzled-mediated targeting activity through branch-branch repulsion.
We next performed misexpression experiments to identify the domains of Frazzled that are required for precise dendritic targeting. Overexpression of full-length Frazzled in otherwise wild-type neurons did not alter branch targeting at LF (Figures 2F and S2B). By contrast, misexpression of FraΔC, which lacks the cytoplasmic tail and acts as a dominant negative [26], strongly suppressed targeting (Figures 2F and S2B). Three conserved domains within the Frazzled cytoplasmic tail - P1, P2 and P3 - are differentially required for axon guidance downstream of Frazzled, but have not been examined for activity in dendrite targeting [24, 26, 27]. We assessed requirements in dendrites by testing the ability of Frazzled transgenes lacking different combinations of these domains to rescue fra mutant dendrite targeting phenotypes [26]. Transheterozygous fra3/fra4 mutant larvae showed deficits in targeting similar to those seen in fra MARCM clones (Figure 2G). Targeting was rescued by full-length UAS-Fra-myc, UAS-FraΔP1-myc, and UAS-FraΔP1ΔP2-myc (Figure 2G). By contrast, UAS-FraΔP3-myc did not significantly restore targeting in fra3/fra4 mutant larvae (p > 0.05, Figure 2G). We observed qualitatively similar levels of expression of the different transgenes (data not shown). Thus, sequences within the P3 domain of Frazzled, but not within P1 or P2, appear to be important for dendritic targeting.
Netrin-B is essential for Frazzled-dependent sensory dendrite targeting
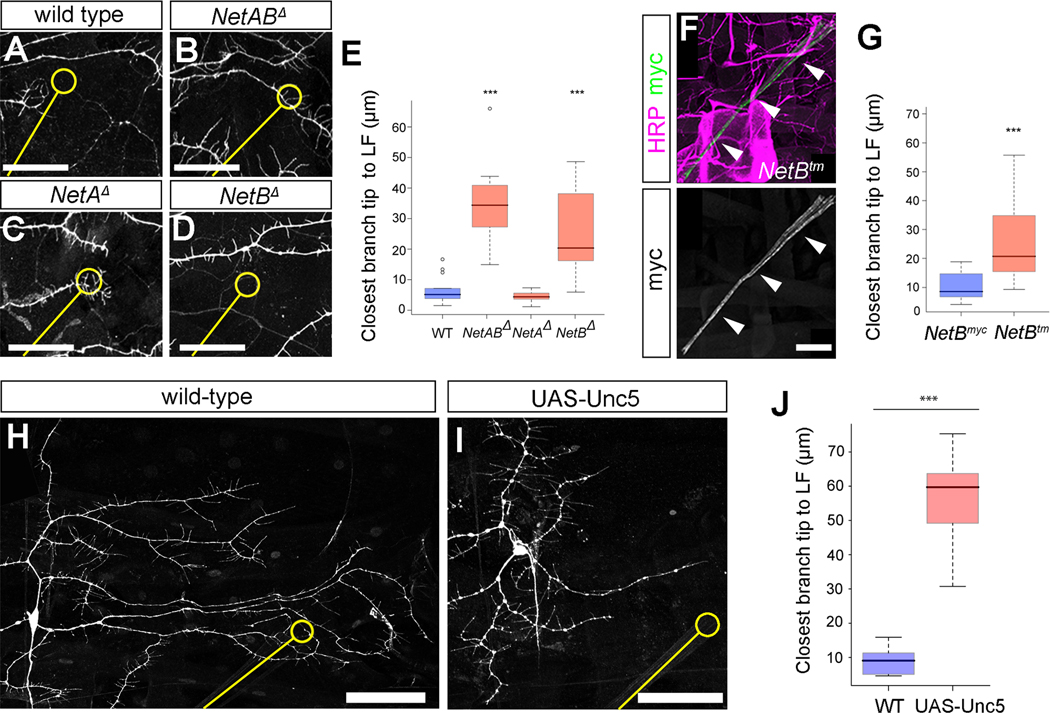
Two closely related Drosophila netrins are encoded by the Netrin-A (NetA) and Netrin-B (NetB) genes [28, 29]. We analyzed targeting in larvae lacking one or both netrins using 189Y-Gal4>UAS-mCD8::GFP to label class III neurons. Targeting to LF in 189Y-Gal4>UAS-mCD8::GFP animals was robust (Figures 3A and 3E), but was strongly disrupted in NetABΔ mutant animals (Figures 3B and 3E), implicating netrins in precise targeting. We examined dendrite targeting in mutant animals lacking either Netrin gene alone and found that NetB, but not NetA, is required for targeting (Figures 3C–3E). Dendrite targeting in NetABΔ mutant larvae was not significantly different from fra3/fra4 mutants (p > 0.05), suggesting that the activity of netrins during targeting occurs largely, or exclusively, through Frazzled.
Figure 3. Function of Netrins in peripheral dendritic targeting and expression of Netrin-B.
(A–D) Analysis of targeting of class III da neuron ddaA (marked by 189Y-Gal4, UAS-mCD8::GFP) in (A) WT (n=13), (B) NetABΔ (n=7), (C) NetAΔ (n=7) and (D) NetBΔ (n=13) third instar larvae. A–D show representative images of area surrounding LF. The location of the chordotonal organ, labeled in the HRP channel (not shown), is indicated by a yellow line, and the region corresponding to LF indicated by a yellow circle. Targeting is disrupted in NetABΔ and NetBΔ mutant larvae.
(E) The distances of the closest major branch tip to LF in WT, NetABΔ, NetAΔ, and NetBΔ animals.
(F) NetBtm levels are highly enriched in the chordotonal organ of third instar larvae (arrowheads).
(G) Quantification of closest tip to LF in ΔNetA,NetBmyc (n=10) and ΔNetA,NetBtm (n=17) larvae.
(H) Morphology and chordotonal organ targeting of single wild-type FLP-out clone of ddaA.
(I) Lack of chordotonal targeting of ddaA neuron as revealed by a single FLP-out clone expressing Unc5 under the control of 109(2)80-Gal4.
(J) Plot of the closest branch tip to LF in wild-type (WT, n=10) and UAS-Unc5-expressing (n=6) ddaA neurons.
All scale bars = 50μm.
Datasets are presented in boxplots as median (thick line), quartiles Q1–Q3 (25%–75% quantiles; shaded box), and data in 1.5× quartile range (dashed bars). Data points outside these ranges are indicated as open circles.
* = p < 0.05; ** = p < 0.01; *** = p < 0.001 by Wilcoxon rank-sum test
See also Supplemental Figure S3
We next assessed NetB expression in animals carrying a modified NetB locus that generates a transmembrane tethered NetB protein tagged with c-myc (NetBtm) [20]. NetBtm reflects endogenous NetB distribution in the central nervous system, but because it is not secreted is more closely associated with membranes of cells in which it is produced [20]. NetBtm was enriched in scolopale cells of LCHO in late embryos (Figure S3A) and accumulated in LCHO cap cells in both abdominal and thoracic segments throughout all larval stages (Figure 3F and S3B–S3D). By contrast, using in situ hybridization we detected NetA at segment borders (Figure S3E; [29]) but not in LCHO. Notably, dendritic targeting to LF was disrupted in ΔNetA NetBtm larvae compared to ΔNetA NetBmyc controls (Figure 3G). Targeting was not significantly different in ΔNetA NetBtm animals compared to NetABΔ null animals (p > 0.05), together suggesting that secretion of NetB is required for targeting. These data suggest that LCHO cap cells provide the source of secreted NetB that underlies da neuron dendrite targeting.
Although NetA was dispensable for LF targeting, we cannot exclude a role for NetA in da neuron dendritic patterning outside of LF. Notably, we found that misexpression of Unc5, a repulsive netrin receptor [23], in all da neurons using 109(2)80-Gal4 caused defects in dendritic morphology, including disruption of LF targeting (Figures 3H–3J), and aberrant exclusion of dendrites from segment boundaries (Figures S3F and S3G). These results raise the possibility that other sources of Netrins, perhaps including NetA at segment borders, could also be important for da neuron dendrite patterning.
Ectopic expression of Netrin directs dendritic targeting and reshapes sensory neuron territories in a Frazzled-dependent manner
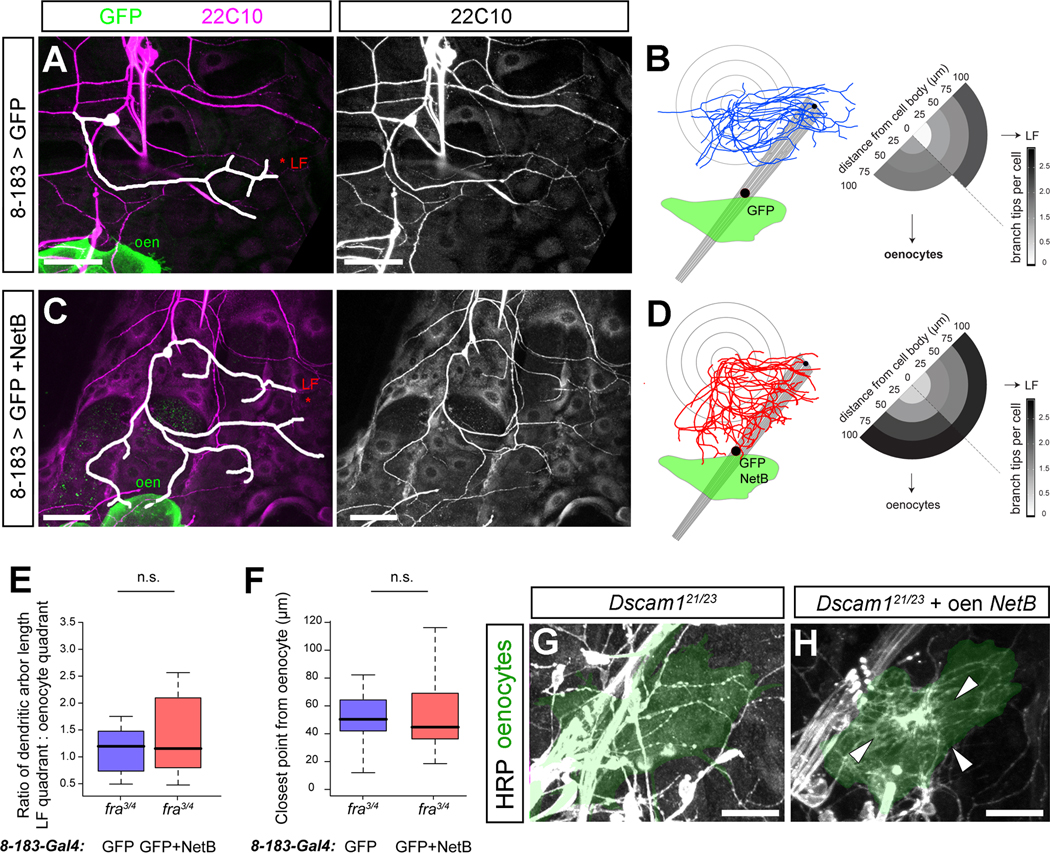
To test whether localized netrin expression is also sufficient for da neuron dendrite targeting, and to further test the respective roles of Dscam1 and Frazzled, we developed an ectopic targeting assay. We used 8-183-Gal4 to drive netrin expression in larval oenocytes, which are clusters of 5–6 large hepatocyte-like cells in the lateral body wall [30]. We assessed directional growth of the ddaA dendritic arbor by measuring the relative enrichment of dendrites in either the ‘LF’ quadrant posterior to the cell body or an adjacent ‘oenocyte’ quadrant directly ventral to the cell body (Figures 4A–4D). Wild-type ddaA dendrites were polarized toward LF and projected more branches to the LF quadrant than the oenocyte quadrant (Figures 4A and 4B). NetB misexpression in the oenocytes (8-183-Gal4>NetB) did not significantly alter LF quadrant arborization (Figures 4B, 4D, and S4A), however, in the oenocyte quadrant, overall branch number and proportion of arbor increased relative to control, while the median distance of the closest branch to the oenocytes decreased (Figures 4B, 4D, and S4A–S4B). Both NetA and NetB had qualitatively similar effects on dendritic targeting in this assay (Figure S4C), suggesting that the selective endogenous role for NetB arises due to differential expression rather than differential activity. Dendritic territories of ddaA failed to adjust toward ectopic sources of NetB in fra mutant larvae (Figures 4E and 4F). Furthermore, while we did not find evidence for dendrite accumulation at the oenocytes in Dscam1 mutant larvae (Figure 4G), or in density plots generated from mutant clones (Figure 1H), 8-183-Gal4 driving NetB induced dendritic accumulation at oenocytes in larvae mutant for Dscam1 (Figure 4H). Thus, localized expression of netrins in peripheral tissue was sufficient to direct the targeting of dendrites and reshape arbor territories, and dendrites from a single cell appeared able to respond to multiple such cues. Since opposite patterning defects arose when guidance activity was provided in the absence of Frazzled or Dscam1, our data support a role for Frazzled in attractive responses to netrin and a role for Dscam in self-repulsion and counterbalancing of Frazzled-dependent attraction in normal and ectopic targeting.
Figure 4. Ectopic expression of Netrin-B generates new dendritic targets.
(A) The dendritic arbor of ddaA in a wild-type larva, visualized by anti-Futsch/22C10 staining. The ventral and posterior portion of the arbor of ddaA is traced in white. mCD8::GFP (GFP) is expressed in oenocytes (oen) driven by 8-183-Gal4.
(B) A plot of multiple registered ddaA arbors (n=12) with concentric circles placed 25, 50, 75, and 100μm from the cell body. Dendritic intersections were segregated into two quadrants, one centered towards LF (LF quadrant) and one centered towards the oenocytes (oenocyte quadrant).
(C) Dendritic arbor morphology of ddaA upon expression of mCD8::GFP and NetB under the control of 8-183-Gal4.
(D) A plot of ddaA dendritic arbors (n=12) upon misexpression of mCD8::GFP and NetB in the oenocytes. Dendrites are plotted as in (B).
(E) Quantification of the ratio of total arbor length within the quadrant centered on LF and the quadrant centered on the oenocytes reveals no change when NetB is expressed under the control of 8-183-Gal4 in fra3/fra4 animals.
(F) Quantification of the closest point of each arbor to the oenocytes reveals no change when NetB is expressed under the control of 8-183-Gal4 in fra3/fra4 animals.
(G) Dendrites do not accumulate at oenocytes (shaded in green) in Dscam121/Dscam123 mutant larvae (n=3 animals examined).
(H) Dendrites accumulate at oenocytes (arrows) in Dscam121/Dscam123 mutant larvae when NetB is expressed under the control of 8-183-Gal4 (n=6 animals examined).
All scale bars = 50μm
Datasets are presented in boxplots as median (thick line), quartiles Q1–Q3 (25%–75% quantiles; shaded box), and data in 1.5× quartile range (dashed bars).
* = p < 0.05; ** = p < 0.01; *** = p < 0.001 by Wilcoxon rank-sum test.
See also Supplemental Figure S4
Conclusions
Dendritic targeting is an important component of neural circuit assembly in diverse systems [25, 31–35]. Our data suggest that targeting cues can generate a patterning problem for complex dendrites that must be overcome by self-avoidance signaling. Our findings together suggest a model in which repulsive self-avoidance counters netrin-based attractive signals to permit both precise targeting and full and even territory coverage by sister dendrites. Dendritic branches will be repelled only when their number at any given target becomes excessive. We suggest that control of dendritic targeting by a combination of attractive and self-repulsive cues provides a system for the regulation of branch positioning and spacing that can be implemented dynamically in growing dendrites, and without regard for the exact number and position of guidance cue sources. Indeed, our overexpression results suggest that one advantage of this mechanism is that counterbalancing can be executed in response to multiple, spatially distinct, targeting cues impinging on a single arbor. Conceivably, such action could greatly diversify the shapes taken by dendritic fields. It is worth noting that, in da neurons, counterbalancing is implemented in arbors that are largely restricted to two-dimensional growth. However, in a similar fashion, Dscam1 appears to prevent local accumulations of dendritic and axonal arbors in regions of the brain where arbors elaborate in three dimensions [5, 8]. These observations raise the possibility that repulsive self-avoidance functions as a counterbalancing cue more broadly, perhaps revealing a general role for Dscam1-based self-recognition and repulsion during the formation of neural circuits.
Recent work has shown that Dscams mediate self-avoidance in Drosophila and mammals. In Drosophila, discrimination between self and non-self dendrites depends on extensive alternative splicing of the Dscam1 ectodomain and selective binding between identical isoforms [15, 16, 36]. Other Drosophila Dscams, as well as vertebrate DSCAM, lack the same degree of molecular diversity but nevertheless appear to prevent inappropriate associations and fasciculation between like-type isoneuronal or heteroneuronal processes. Drosophila Dscam2 mediates axonal tiling and prevents inappropriate pairings of like-type postsynaptic elements in the visual system [37, 38]. Vertebrate DSCAM and the related DSCAML1 prevent neurite fasciculation and clumping of cell bodies in classes of amacrine cells and retinal ganglion cells [12, 39]. DSCAM and DSCAML1 have been proposed to function in mammalian neurons to mask as yet unknown cell-type specific adhesive cues that promote clumping and fasciculation of processes [12, 39]. An important future goal is to understand how these opposing cues are integrated by dendrites or axons. In sensory neurons, the integration of self-avoidance and targeting cues is apparently biased in favor of repulsion since the initiation of Dscam1-mediated self-avoidance appears non-permissive for Frazzled-dependent branch targeting.
In da neuron dendrite targeting, roles for Dscam1 appear to be separable from those for Netrin and Frazzled, since several of our experiments support roles for Dscam1 in self-avoidance, and Netrin together with Frazzled in attractive guidance. In addition to Frazzled/DCC, Dscam1 in Drosophila and DSCAM in mammals have been shown to act as receptors for netrins during axon guidance [17–19]. In the Drosophila CNS, dendritic attraction by the action of netrins appears to depend largely, or entirely, on Frazzled [34], similar to our findings for da neurons. NetA and Frazzled also instruct the development of the v’ch1 chordotonal organ cap cell, which, in turn, influences dendrite orientation of associated chordotonal neurons [40].
Our results demonstrate how analysis of Dscam1 mutant phenotypes can uncover new sources of signals that affect neurite patterning. Evidence for targeting cues acting on complex dendrites may be difficult to detect because of the dispersed nature of many dendritic fields and constraints imposed by other cues that may exclude arborization, such as repulsive self-avoidance and tiling. Interfering with self-avoidance appears to provide one approach for identifying preferred targets of neurites, which may then be interrogated further to identify patterning cues for highly branched dendritic arbors. The precision of targeting shown by da neuron dendrites should prove useful for dissecting signaling mechanisms of targeting and self-avoidance.
Highlights.
The guidance cue Netrin-B and Frazzled control da sensory neuron dendritic targeting
Dscam1 prevents aberrant dendrite accumulation at Netrin-B expressing targets
NetB is sufficient for ectopic dendritic targeting in wild-type or Dscam1−/− neurons
Self-avoidance counterbalances uniform responses of sister dendrites to guidance cues
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to O. Hobert, L. Zipursky and members of the Grueber lab for comments on earlier versions of the manuscript and for discussions, M. Corty for contributing to Unc5 overexpression experiments and E. Pyronneau for help with early work on this project. We thank G. Bashaw, B. Dickson, U. Heberlein, F. Roegiers, R. Yang, L. Zipursky and the Bloomington Stock Center for providing fly stocks and reagents. B.J.M. was supported by an NIH Predoctoral Fellowship F31NS060341. This work is supported by NIH NS061908 from NINDS, the Searle Scholars Program, the Klingenstein Foundation, and the McKnight Endowment Fund (to W.B.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes four figures and Supplemental Experimental Procedures and can be found with this article online at XXXXXXXX
References
- 1.Grueber WB, Sagasti A. Self-avoidance and tiling: Mechanisms of dendrite and axon spacing. Cold Spring Harb Perspect Biol. 2010;2:a001750. doi: 10.1101/cshperspect.a001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cameron S, Rao Y. Molecular mechanisms of tiling and self-avoidance in neural development. Mol Brain. 2010;3:28. doi: 10.1186/1756-6606-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmucker D, Clemens JC, Shu H, Worby C, Xiao J, Muda M, Dixon J, Zipursky SL. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell. 2000;101:671–684. doi: 10.1016/s0092-8674(00)80878-8. [DOI] [PubMed] [Google Scholar]
- 4.Zhan X, Clemens JC, Neves G, Hattori D, Flanagan J, Hummel T, Vasconcelos M, Chess A, Zipursky SL. Analysis of Dscam diversity in regulating axon guidance in Drosophila mushroom bodies. Neuron. 2004;43:673–686. doi: 10.1016/j.neuron.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Zugates CT, Liang IH, Lee CH, Lee T. Drosophila Dscam is required for divergent segregation of sister branches and suppresses ectopic bifurcation of axons. Neuron. 2002;33:559–571. doi: 10.1016/s0896-6273(02)00570-6. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Ma X, Yang J, Zheng X, Zugates C, Lee C, Lee T. Transmembrane/juxtamembrane domain-dependent Dscam distribution and function during mushroom body neuronal morphogenesis. Neuron. 2004;43:663–672. doi: 10.1016/j.neuron.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Hattori D, Demir E, Kim HW, Viragh E, Zipursky SL, Dickson BJ. Dscam diversity is essential for neuronal wiring and self-recognition. Nature. 2007;449:223–227. doi: 10.1038/nature06099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu H, Hummel T, Clemens JC, Berdnik D, Zipursky SL, Luo L. Dendritic patterning by Dscam and synaptic partner matching in the Drosophila antennal lobe. Nat Neurosci. 2006;9:349–355. doi: 10.1038/nn1652. [DOI] [PubMed] [Google Scholar]
- 9.Matthews BJ, Kim ME, Flanagan JJ, Hattori D, Clemens JC, Zipursky SL, Grueber WB. Dendrite self-avoidance is controlled by Dscam. Cell. 2007;129:593–604. doi: 10.1016/j.cell.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Soba P, Zhu S, Emoto K, Younger SH, Yang JS-J, Yu H-H, Lee T, Jan LY, Jan Y-N. Drosophila sensory neurons require Dscam for dendritic self-avoidance and proper dendritic field organization. Neuron. 2007;54:403–416. doi: 10.1016/j.neuron.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes ME, Bortnick R, Tsubouchi A, Bäumer P, Kondo M, Uemura T, Schmucker D. Homophilic Dscam interactions control complex dendrite morphogenesis. Neuron. 2007;54:417–427. doi: 10.1016/j.neuron.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuerst PG, Bruce F, Tian M, Wei W, Elstrott J, Feller MB, Erskine L, Singer JH, Burgess RW. DSCAM and DSCAML1 Function in Self-Avoidance in Multiple Cell Types in the Developing Mouse Retina. Neuron. 2009;64:484–497. doi: 10.1016/j.neuron.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grueber WB, Jan LY, Jan Y-N. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- 14.McIver SB. Mechanoreception. In: GAaG, Kerkut LI, editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Oxford: Pergamon; 1985. pp. 71–132. [Google Scholar]
- 15.Wojtowicz WM, Flanagan J, Millard SS, Zipursky SL, Clemens JC. Alternative splicing of Drosophila Dscam generates axon guidance receptors that exhibit isoform-specific homophilic binding. Cell. 2004;118:619–633. doi: 10.1016/j.cell.2004.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wojtowicz WM, Wu W, Andre I, Qian B, Baker D, Zipursky SL. A vast repertoire of Dscam binding specificities arises from modular interactions of variable Ig domains. Cell. 2007;130:1134–1145. doi: 10.1016/j.cell.2007.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews GL, Tanglao S, Farmer WT, Morin S, Brotman S, Berberoglu MA, Price H, Fernandez GC, Mastick GS, Charron F, et al. Dscam guides embryonic axons by Netrin-dependent and -independent functions. Development. 2008;135:3839–3848. doi: 10.1242/dev.023739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu G, Li W, Wang L, Kar A, Guan K-L, Rao Y, Wu JY. DSCAM functions as a netrin receptor in commissural axon pathfinding. Proc Natl Acad Sci USA. 2009;106:2951–2956. doi: 10.1073/pnas.0811083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ly A, Nikolaev A, Suresh G, Zheng Y, Tessier-Lavigne M, Stein E. DSCAM is a netrin receptor that collaborates with DCC in mediating turning responses to netrin-1. Cell. 2008;133:1241–1254. doi: 10.1016/j.cell.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brankatschk M, Dickson BJ. Netrins guide Drosophila commissural axons at short range. Nat Neurosci. 2006;9:188–194. doi: 10.1038/nn1625. [DOI] [PubMed] [Google Scholar]
- 21.Chan SS, Zheng H, Su MW, Wilk R, Killeen MT, Hedgecock EM, Culotti JG. UNC-40 a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. doi: 10.1016/s0092-8674(00)81337-9. [DOI] [PubMed] [Google Scholar]
- 22.Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- 23.Keleman K, Dickson BJ. Short- and long-range repulsion by the Drosophila Unc5 netrin receptor. Neuron. 2001;32:605–617. doi: 10.1016/s0896-6273(01)00505-0. [DOI] [PubMed] [Google Scholar]
- 24.Kolodziej PA, Timpe LC, Mitchell KJ, Fried SR, Goodman CS, Jan LY, Jan YN. frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell. 1996;87:197–204. doi: 10.1016/s0092-8674(00)81338-0. [DOI] [PubMed] [Google Scholar]
- 25.Suli A, Mortimer N, Shepherd I, Chien C-B. Netrin/DCC signaling controls contralateral dendrites of octavolateralis efferent neurons. J Neurosci. 2006;26:13328–13337. doi: 10.1523/JNEUROSCI.2858-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garbe DS, O'Donnell M, Bashaw GJ. Cytoplasmic domain requirements for Frazzled-mediated attractive axon turning at the Drosophila midline. Development. 2007;134:4325–4334. doi: 10.1242/dev.012872. [DOI] [PubMed] [Google Scholar]
- 27.Dorsten JN, VanBerkum MFA. Frazzled cytoplasmic P-motifs are differentially required for axon pathway formation in the Drosophila embryonic CNS. Int J Dev Neurosci. 2008;26:753–761. doi: 10.1016/j.ijdevneu.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Harris R, Sabatelli LM, Seeger MA. Guidance cues at the Drosophila CNS midline: identification and characterization of two Drosophila Netrin/UNC-6 homologs. Neuron. 1996;17:217–228. doi: 10.1016/s0896-6273(00)80154-3. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell K, Doyle J, Serafini T, Kennedy T, Tessier-Lavigne M, Goodman C, Dickson BJ. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron. 1996;17:203–215. doi: 10.1016/s0896-6273(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez E, Wiggins D, Fielding B, Gould AP. Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature. 2007;445:275–280. doi: 10.1038/nature05382. [DOI] [PubMed] [Google Scholar]
- 31.Brierley DJ, Blanc E, Reddy OV, Vijayraghavan K, Williams DW. Dendritic targeting in the leg neuropil of Drosophila: the role of midline signalling molecules in generating a myotopic map. PLoS Biol. 2009;7:e1000199. doi: 10.1371/journal.pbio.1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furrer M-P, Kim S, Wolf B, Chiba A. Robo and Frazzled/DCC mediate dendritic guidance at the CNS midline. Nat Neurosci. 2003;6:223–230. doi: 10.1038/nn1017. [DOI] [PubMed] [Google Scholar]
- 33.Komiyama T, Luo L. Intrinsic control of precise dendritic targeting by an ensemble of transcription factors. Curr Biol. 2007;17:278–285. doi: 10.1016/j.cub.2006.11.067. [DOI] [PubMed] [Google Scholar]
- 34.Mauss A, Tripodi M, Evers JF, Landgraf M. Midline signalling systems direct the formation of a neural map by dendritic targeting in the Drosophila motor system. PLoS Biol. 2009;7:e1000200. doi: 10.1371/journal.pbio.1000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature. 2000;404:567–573. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- 36.Sawaya MR, Wojtowicz WM, Andre I, Qian B, Wu W, Baker D, Eisenberg D, Zipursky SL. A double S shape provides the structural basis for the extraordinary binding specificity of Dscam isoforms. Cell. 2008;134:1007–1018. doi: 10.1016/j.cell.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Millard SS, Flanagan JJ, Pappu KS, Wu W, Zipursky SL. Dscam2 mediates axonal tiling in the Drosophila visual system. Nature. 2007;447:720–724. doi: 10.1038/nature05855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Millard SS, Lu Z, Zipursky SL, Meinertzhagen IA. Drosophila dscam proteins regulate postsynaptic specificity at multiple-contact synapses. Neuron. 2010;67:761–768. doi: 10.1016/j.neuron.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuerst PG, Koizumi A, Masland RH, Burgess RW. Neurite arborization and mosaic spacing in the mouse retina require DSCAM. Nature. 2008;451:470–474. doi: 10.1038/nature06514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mrkusich EM, Osman ZB, Bates KE, Marchingo JM, Duman-Scheel M, Whitington PM. Netrin-guided accessory cell morphogenesis dictates the dendrite orientation and migration of a Drosophila sensory neuron. Development. 2010;137:2227–2235. doi: 10.1242/dev.047795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.