Abstract
Intrathecal antibody production manifest as oligoclonal bands (OCBs) is a hallmark of multiple sclerosis (MS). Once present, OCBs can be detected in CSF throughout the lifetime of MS patients. To determine the specificity of the OCBs, we applied CSF IgG obtained from 2 consecutive lumbar punctures of 5 MS patients to screen phage-displayed random peptide libraries, and selected identical and related peptides that reacted with the paired CSF IgGs from each patient. Highly sensitive phage-mediated immuno-PCR revealed that the phage peptides bound specifically to IgG in MS CSF collected over time. IEF immunoblots also showed that these peptides were recognized by OCBs in MS CSF. We further demonstrated that the peptides represented linear epitopes, indicating that they represent natural epitopes of corresponding protein antigens. A database search combined with alanine scan mutagenesis of peptides that bound to CSF IgG from 3 MS patients revealed that they are derived from proteins including serine/threonine-protein kinase, protein ZIP2 and MHC class II. Identification of epitopes that are recognized by IgG in MS CSF over time provides a critical tool to investigate the specificity of OCBs, which may determine the cause of disease, leading to strategies for diagnostic and therapeutic intervention.
Keywords: Multiple sclerosis, Antigens, Peptides, Epitopes
1. Introduction
Multiple sclerosis (MS), the most common inflammatory demyelinating disease of the CNS, affects millions of individuals worldwide. The brain and CSF of more than 95% of MS patients contain increased amounts of IgG, manifested as oligoclonal bands (OCBs). OCBs are also found in chronic infectious diseases of the CNS such as neurosyphilis, tuberculous meningitis, cryptococcal and mumps meningitis, subacute sclerosing panencephalitis and progressive rubella panencephalitis. In each of those disorders, the oligoclonal IgG is directed against the agent that causes disease (reviewed in Gilden, 2005). Although the specificity of the oligoclonal IgG in MS is unknown, the antibody (Ab) response is not directed against myelin basic protein, proteolipid protein or myelin oligodendrocyte protein (Owens et al., 2009).
The presence of OCBs throughout the lifetime of MS patients (Hela-Felicitas and Reske, 2005) confirmed the earlier identification of persistent and clonally stable IgG in MS CSF (Walsh et al., 1986). Because elevated CSF IgG levels are seen more frequently in MS patients with aggressive disease than in patients with a benign course (Stendahl-Brodin and Link, 1980), and disease is less severe when OCBs are absent or present at only low levels (Avasarala et al., 2001; Joseph et al., 2009), the intrathecally synthesized Ab response is likely to be relevant to the pathogenesis of disease. In fact, identification of the specificity of OCBs in MS may reveal the cause of disease.
Several studies to identify possible targets of the Ab response in MS CSF by panning phage-displayed random peptide libraries found that peptide antigens (Ags) in MS are specific to the individual Ab response and that the peptides did not specifically bind oligoclonal IgG in MS CSF (Cortese et al., 1996, 1998, 2001; Archelos et al., 1998). Herein, we used phage-displayed random peptide libraries to identify peptide epitopes that were recognized by MS CSF IgG obtained longitudinally in 5 MS patients.
2. Materials and methods
2.1. Patients
With approval of the University of Colorado Institutional Review Board, CSF and sera from 5 MS and 2 non-MS CNS inflammatory disease control patients were collected at the University of Colorado Denver. CSFs were immediately centrifuged at 500×g for 10 min, and the supernatant was collected. Both CSF and sera were stored at −80 °C until use. The CSF of all patients contained OCBs. CSF from the 5 MS patients was collected at two intervals (Table 1).
Table 1.
Clinical characteristics of patients.
| Patient | CSF ID | Age/sex | Serum IgG (mg/mL) | CSF IgG (μg/mL) | CSF cellsa | IgG Index | Diagnosisb | OCB | Time intervals | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | MS02-24 | 40/F | 16.00 | 161 | 29 | 1.40 | SPMS | + | None | |
| P1 | MS05-09 | 42/F | 14.60 | 92 | 6 | 1.48 | SPMS | + | 3 years | None |
| P2 | MS04-03 | 44/M | 11.70 | 92 | 4 | 0.93 | RRMS | + | None | |
| P2 | MS07-04 | 47/M | 12.30 | 90 | 8 | 0.82 | RRMS | + | 3 years | None |
| P3 | MS05-04 | 57/F | 10.50 | 68 | 6 | 1.15 | RPMS | + | None | |
| P3 | MS07-12 | 59/F | 8.55 | 33 | 1 | 0.64 | RPMS | + | 2 years | None |
| P4 | MS05-06 | 63/M | 10.30 | 28 | 1 | 0.60 | PPMS | + | None | |
| P4 | ON06-03 | 64/M | 9.98 | 31 | 2 | 0.55 | PPMS | + | 1 year | None |
| P5 | ON05-02 | 31/F | 9.84 | 77 | 12 | 1.82 | RRMS | + | None | |
| P5 | ON06-02 | 32/F | 8.09 | 76 | 11 | 2.05 | RRMS | + | 1 year | None |
| P6 | IC07-1 | 42/M | 12.90 | 45 | 17 | 0.45 | Neurosyphilis | − | N/A | None |
| P7 | IC07-2 | 50/M | 7.66 | 79 | 7 | 0.72 | Chronic progressive meningoencephalitis | + | N/A | None |
RRMS: relapsing remitting multiple sclerosis.
RPMS: relapsing progressive multiple sclerosis.
PPMS: primary progressive multiple sclerosis.
Number of cells per cubic millimeter.
SPMS: secondary progressive multiple sclerosis.
2.2. Identification of phage peptides reactive to MS CSF IgG
The Ph.D.-7™ and Ph.D.-™ C7C Phage Display Peptide Library (New England BioLabs, Beverly, MA) kits were used for affinity selection of specific peptides by MS CSF IgG. The CSF panning with phage-displayed random peptide libraries was performed simultaneously for all patients. The panning procedure as well as characterization of positive phage peptides were as described (Yu et al., 2011). A streamlined protocol was used to determine phage peptide specificity after affinity selection (Yu et al., 2011). Briefly, individual phage plaques were amplified in U96 DeepWell plates and used to determine reactivity to panning MS CSF IgG by 96-well ELISA (Yu et al., 2006). Positive clones were confirmed by duplicate phage ELISA with a pre-immune human IgG control. DNA from positive phage clones were purified and sequenced.
2.3. Western blotting
Unless specified, all antibody incubations were at room temperature. NuPAGE Bis-Tris Mini Gels (Invitrogen, Carlsbad, CA) were used for phage SDS-PAGE analysis with 1× MOPS SDS running buffer. Phage peptides (1×1010/well) in TBS were denatured and reduced by incubation with 1× protein sample buffer containing β-mercaptoethanol (Pierce Biotechnology, Rockford, IL) at 95 °C for 10 min. Triplicate gels were electrophoresed for 50 min at a constant 200 V and electro-blotted onto a PVDF membrane (Bio-Rad, Hercules, CA) for 60 min at a constant 15 V using Trans-Blot® Semi-Dry Cell (Bio-Rad). After blocking for 1 h with 1× casein/TBS (Vector Labs, Burlingame, CA) containing 0.1% Tween 20, filters were incubated for 1 h with corresponding consecutive MS CSF1 and CSF2 (1 μg/ml IgG) in 1× casein/TBS/0.1% Tween 20. CSF IgG binding was detected with HRP-conjugated goat anti-human IgG (H+L) (Sigma, St. Louis, MO) at a dilution of 1:5000, followed by incubation with SuperSignal® West Femto Maximum Sensitivity chemiluminescent substrate (Pierce Biotechnology) as recommended by the manufacturer. For detection of phage pIII protein, duplicate membranes were incubated with a 1:25,000 dilution of mouse anti-M13 pIII mAb (New England BioLabs, Ipswich, MA), followed by a 1:25,000 dilution of HRP-conjugated goat anti-mouse IgG (Vector Labs.) as secondary Ab and with SuperSignal® West Pico substrate for chemiluminescent detection (Pierce Biotechnology).
2.4. Dose–response phage-mediated immuno-PCR (IPCR)
Phage-mediated IPCR was performed as described (Yu et al., 2007). Reacti-Bind™ wells of protein A-coated clear strip plates (Thermo Scientific, Rockford, IL) were coated with 50 μl of CSF or serum (1 μg/ml IgG) and with pre-immune human IgG (Alpha Diagnostic) in TBS (50 mM Tris–HCl, 150 mM NaCl) at room temperature for 2 h, washed with TBS containing 0.05% Tween 20 (TBST) and blocked in 3% nonfat dry milk/0.05% TBST at room temperature for 1 h. Serial 10-fold phage dilutions in duplicate were added to MS CSF/serum IgG-coated wells and incubated at room temperature for 2 h. Bound phage were lysed in 50 μl of double-deionized water by heating the plates at 95 °C for 15 min to release single-stranded phage DNA as the template for real-time PCR in an Applied Biosystems 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA). For standard SYBR® Green PCR, each reaction (20 μl) consisted of 1× power SYBR® Green master mix (Applied Biosystems), 750 nM of each M13 phage primer and 4 μl of phage template. Thermal cycle conditions were 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 45 s. Fast real-time PCR was conducted using 1× Fast SYBR® Green master mix, with thermal cycling at 95 °C for 20 s, followed by 40 cycles at 95 °C for 3 s and 60 °C for 30 s. A control reaction without template was included in each run.
2.5. Isoelectric focusing (IEF) immunoblotting
CSF (200–500 μl) was centrifuged on an Amicon Ultra 0.5-ml 30 K cellulose centrifugal filter unit at 14,000×g for 30 min at room temperature before IEF using the SPIFE® IgG IEF kit (Helena Laboratories, Beaumont, TX) and a SPIFE 3000 electrophoresis analyzer. Wicks were soaked in an anode (0.3 M acetic acid) or cathode (1 M NaOH) solution and applied to the edge of a SPIFE® IgG IEF gel. Five microliters of MS CSF/sera [3–5 μg IgG for phage probe and 100 ng IgG for alkaline phosphatase (AP)-conjugated anti-human IgG probe] were loaded into wells of an SPIFE IEF gel. After electrophoresis at 700 V for 1 h at 15 °C, samples were transferred to PVDF membranes for 45 min, followed by blocking in Helena blocking agent (1 g bovine milk protein/50 ml 1× TBS) for 1 h at room temperature. Membranes were incubated with the respective phage peptide at concentrations ranging from 5.0×1010 to 1.5×1011 pfu/ml in 1:10 Helena blocking agent/TBST (blocking buffer) at room temperature for 2 h. After washing with 0.05% Tween-TBS, membranes were incubated with mouse anti-M13 mAb at a 1:500 dilution in blocking buffer, followed by incubation with 1:500 dilution of AP conjugated anti-mouse IgG at room temperature for 1 h. Membranes were developed with NBT/BCIP substrate. For control blots, membranes were incubated for 1 h with 1:1000 dilutions of AP-anti-human IgG (H+L) in blocking buffer, followed by NBT/BCIP detection.
2.6. Alanine scan mutagenesis and peptide SPOT analysis
Peptide alanine scan mutagenesis and candidate peptide SPOT membranes were prepared by Peptide Array Facility (Kinexus Bioinformatics Corp., Canada). Cellulose-bound peptides membranes were blocked with 3% milk containing 5% sucrose and incubated with MS CSF (1 μg/ml) overnight at 4 °C, followed by incubation with HRP-conjugated anti-human IgG (1:5000) at room temperature for 1 h and SuperSignal® West Pico detection.
2.7. DNA sequencing and database searches
Single-stranded phage DNA was purified and sequenced to deduce the amino acid sequence of the peptide. Consensus peptides were identified by sequence alignment using ClustalW (http://www.ebi.ac.uk/clustalw/). Peptide abundance was calculated based on the number of identical sequences of the total phage clones sequenced. To identify candidate proteins, the most abundant peptides panned by CSF from each patient were searched in BLAST (http://www.ncbi.nlm.nih.gov/) using the Swiss Prot protein sequence database. Peptides of candidate proteins were extracted based on critical residues identified in alanine scan mutagenesis.
3. Results
3.1. Selection of identical and similar high-affinity phage peptides by panning of sequential CSF IgG in 5 MS patients
We studied peptide antigen (Ag) specificity by panning phage-displayed random peptide libraries (Ph.D.-7™ and Ph.D.-™ C7C) with 2 consecutive CSF samples collected at intervals of 1- to 3-years from each of 5 MS patients (Table 1). After 3 to 5 rounds of affinity selection with each of the 2 CSF IgG samples, analysis using a recently developed streamlined protocol to determine phage peptide specificity by phage ELISA (Yu et al., 2011) showed that CSF from 4 MS patients (P1–P4) selected positive phage peptides from the linear peptide library, while the CSF of patient 5 (P5) selected positive peptides from the circular phage library. Multiple specific phage peptides were enriched and selected by each pair of CSF IgG samples (Table 2). Importantly, in all 5 patients, identical and similar peptides were identified by paired CSF IgG samples from every patient. Furthermore, in 4 patients, the most abundant peptides were those recognized by IgG from consecutive CSFs, with the abundance of common peptides among the paired CSFs ranging from 20 to 100%. In 3 MS patients (P1–P3), all peptides identified by the paired CSFs shared conserved amino acid residues (Table 2). Each selected phage was named according to its panning CSF and the location of the well from which the phage was initially identified. For example, CSF02-24-A6 indicates a phage peptide was panned by CSF IgG 02-24 (patient 1) from well A6.
Table 2.
Identical and similar phage peptides were selected by sequential CSF IgG.
| Phage ID | Peptide | Abundance a | |
|---|---|---|---|
| Patient 1 (MS 02-24) | MS-1 CSF #1-A6b | S F G T F L W | 80% (4/5) |
| MS-1 CSF #1-B1 | E F G T F L W c | 20% (1/5) | |
| MS-1 CSF #2-C7 | E F G T F L W | 36% (4/11) | |
| MS-1 CSF #2-C9 | K F G T A L W | 27% (3/11) | |
| MS-1 CSF #2-C5 | Q F G T F L W | 27% (3/11) | |
| MS-1 CSF #2-D7 | S F G T A L W | 9% (1/11) | |
|
| |||
| Patient 2 (MS 04-3) | MS-2 CSF #1-B1 | V L N W H P F | 100% (8/8) |
| MS-2 CSF #2-C9 | V L N W H P F | 50% (1/2) | |
| MS-2 CSF #2-C6 | M F N W H P F | 50% (1/2) | |
|
| |||
| Patient 3 (MS 05-4) | MS-3 CSF #1-A1 | W G L D N P P | 33% (2/6) |
| MS-3 CSF #1-A2 | A P P H L P P | 17% (1/6) | |
| MS-3 CSF #1-E7 | A P S H P P P | 17% (1/6) | |
| MS-3 CSF #1-A6 | A P P H Q M P | 17% (1/6) | |
| MS-3 CSF #1-A12 | T Y M V P A P | 17% (1/6) | |
| MS-3 CSF #2-D9 | W G L D N P P | 56% (5/9) | |
| MS-3 CSF #2-F10 | A P A H Q I P | 11% (1/9) | |
| MS-3 CSF #2-B11 | A P A H H P P | 11% (1/9) | |
| MS-3 CSF #2-B10 | A P P H V M P | 11% (1/9) | |
| MS-3 CSF #2-C11 | G P V N M N L | 11% (1/9) | |
|
| |||
| Patient 4 (MS 05-6) | MS-4 CSF #1-E12 | F H L P W M Q | 100% (1/1) |
| MS-4 CSF#2-H4 | W Y Y K P P A | 60% (3/6) | |
| MS-4 CSF #2-D10 | K W P I I D T | 20% (1/5) | |
| MS-4 CSF #2-G9 | F H L P W M Q | 20% (1/5) | |
|
| |||
| Patient 5 (ON 05-2) | MS-5 CSF #1-E9 | c P V E L Q L Y c | 17% (1/6) |
| MS-5 CSF #1-A9 | c S Q S V AA S c | 17% (1/6) | |
| MS-5 CSF #1-E8 | c G R Q A Q Y Y c | 17% (1/6) | |
| MS-5 CSF #1-C10 | c P V G P W T N c | 17% (1/6) | |
| MS-5 CSF #1-C9 | c T V Q P Q F H c | 17% (1/6) | |
| MS-5 CSF #1-F9 | c M Q A H Q T K c | 17% (1/6) | |
| MS-5 CSF #2-F12 | c P L E L S V Y c | 60% (3/5) | |
| MS-5 CSF #2-H11 | c S H R P T T Q c | 20% (1/5) | |
| MS-5 CSF #2-C12 | c S T E G R H N c | 20% (1/5) | |
Positive phage were amplified, purified, and phage DNA was sequenced. Peptides are listed in order of their relative frequencies of the total clones sequenced.
Each phage peptide was named by panning CSF IgG (patient # and CSF #) followed by the location of the well from which the phage was initially identified. For example, peptide MS-1 CSF #1-A6 indicates that the phage was selected by CSF IgG of patient 1 and CSF 1 from well A6.
The identical/similar peptides were highlighted.
Circular peptides.
3.2. Specificity of phage peptides for the persistent CSF IgG
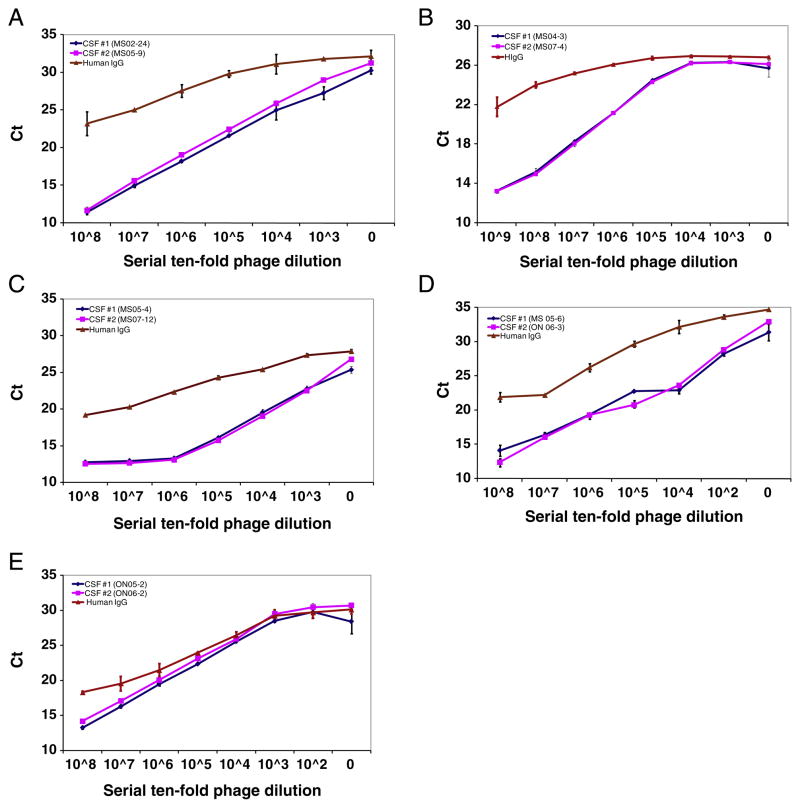
To determine whether the phage peptides selected by one CSF shared binding specificity for IgG of the second CSF from the same patient, we used highly sensitive phage-mediated IPCR (Yu et al., 2007). Serial 10-fold dilutions of phage were added to pairs of CSF IgG-coated wells of Reacti-Bind™ protein A plates, and bound phage were detected by real-time PCR. In all 5 MS patients, phage peptides selected by one CSF bound specifically to the paired CSF IgG with equal affinity (equal Ct) in a dose-dependent manner (Fig. 1). The negative control was pre-immune human IgG prepared from pooled sera of healthy humans (Alpha Diagnostic). These findings revealed that IgG obtained from paired MS CSF IgG at intervals of 1 to 3 years bound to the same peptides.
Fig. 1.
Dose-dependent phage-IPCR demonstrates peptide binding specificity to persistent IgG in the longitudinal CSF. Longitudinally obtained MS CSF and control pre-immune human IgG (50 μl at IgG concentration of 1 μl/ml) were coated in duplicate onto wells of protein A-plates before addition of the corresponding phage peptides (at serial 10-fold dilutions starting with 107 pfu) to each well. Bound phage were lysed and the DNA was amplified by real-time PCR. Peptide binding specificity was determined based on Ct values. Compared to control human IgG, phage peptides bound specifically to the paired CSF IgG with equal affinity in a dose-dependent manner. Experiments were repeated at least once. Error bars represent SD. A–E, Phage peptide binding specificity in patients 1–5, respectively. Data represent at least three independent experiments.
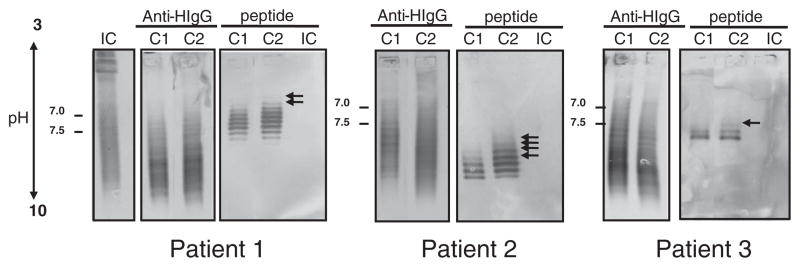
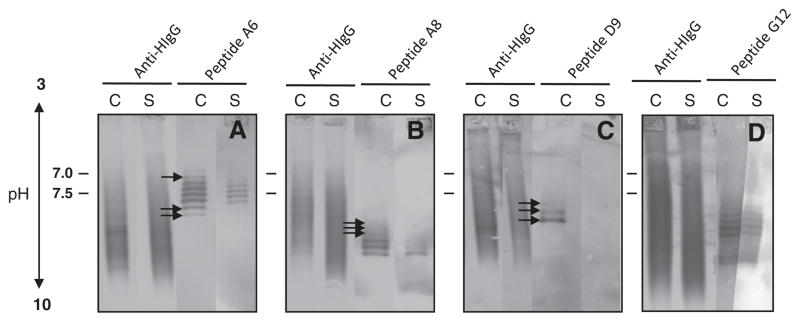
To further confirm the stability of the Ag-specific intrathecal IgG response over time, we used IEF immunoblotting to evaluate phage peptide reactivity to IgG in the paired CSFs. Paired MS CSFs were separated on SPIFE IEF agarose gels, and the blots were probed with phage peptides followed by colorimetric detection. The phage peptides were recognized by multiple IgG bands in paired CSF of 3 MS patients, but not by IgG from the CSF of a control patient with chronic progressive meningoencephalitis (Fig. 2). An irrelevant phage peptide also was not recognized by MS CSF IgG (data not shown). Furthermore, in all 3 MS patients, the peptides bound with greater intensity to the second CSF than to the first CSF (arrows), as revealed by bands in the lower pH region indicative of additional high-affinity IgG. Importantly, the pattern of bands of CSF IgG that bound to phage peptides was similar to that seen after binding of anti-human IgG (Fig. 2). Peptides selected by CSF IgGs from patients 4 and 5 were not recognized in the IEF blots, possibly due to either low affinity of IgG binding, or a conformation nature of the epitopes (data not shown).
Fig. 2.
IEF immunoblots reveal phage peptide recognition of persistent antibodies in MS CSF. Paired MS CSF (C1 and C2) and an inflammatory control patient with chronic progressive meningoencephalitis (IC) CSF (3–5 μg total IgG) were resolved on agarose IEF gels and transferred to nitrocellulose membranes. Blots were probed with corresponding phage peptides (1010 pfu/ml) and incubated with mouse anti-pIII antibody followed by AP-anti-mouse IgG. Phage peptides recognized multiple IgG bands in the paired MS CSF and revealed additional bands in the second CSF, but not in IC CSF. Positive controls were CSF (100 ng of total IgG) probed with AP-anti-human IgG. Experiments were repeated three times. A, patient 1 (02-24); B, patient 2 (04-3); C, patient 3 (05-4). Data represent at least three independent experiments.
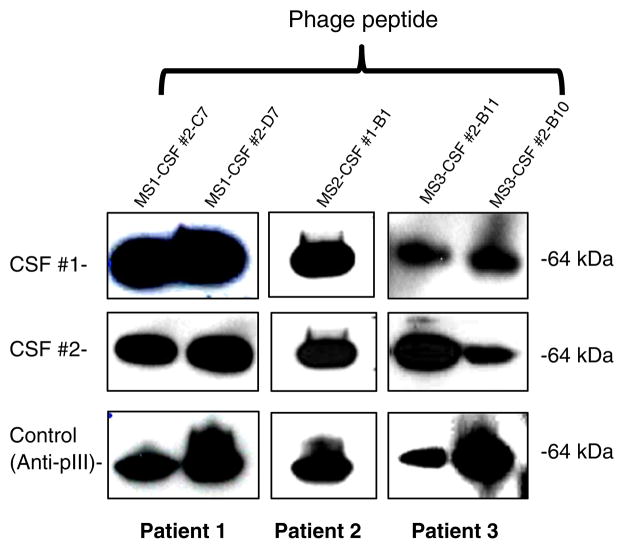
3.3. Paired MS CSF IgGs recognize linear phage peptides in Western blots
To determine the conformational nature of phage peptides, phage peptides identified by CSF IgG from 4 MS patients were examined by Western blotting after separation by 4–12% SDS-PAGE (circular peptides selected by CSF from MS patient 5 were not included). Phage peptides were recognized by 3 pairs of MS CSF (patients 1–3), shown as a 64-kDa band that resulted from fusion of the peptide to the pIII minor coat protein of M13 phage (Fig. 3, top two panels). The results confirmed that the peptides reacted with IgG from 2 consecutive MS CSFs, and that the epitopes were linear. The positive control anti-pIII Ab incubated with duplicate membranes revealed a 64-kDa band, which represents the minor coat protein for all 5 phage peptides (Fig. 3, lower panel). Peptides selected by CSF IgGs from patient 4 were not recognized in IEF blots, suggesting that the epitopes may be conformational.
Fig. 3.
Persistent peptide antigens in MS CSF represent linear epitopes. Phage peptides selected by MS CSF IgG (two peptides from patients 1 and 3, and one from patient 2) were separated on gradient 4–12% SDS-polyacrylamide gels, blotted to PVDF membranes and probed with corresponding CSF obtained at two time intervals (CSF #1 and CSF #2), followed by HRP-conjugated anti-human IgG detection. Duplicate blots were probed with anti-pIII antibody to detect phage minor coat protein as positive controls. In each patient, phage peptides were recognized by the paired CSF, indicating that they represent linear epitopes. The anti-pIII antibody blots showed a 64-kDa band of phage minor coat protein with all phage peptides. Data represent at least three independent experiments.
3.4. Phage peptides target the CSF intrathecal IgG
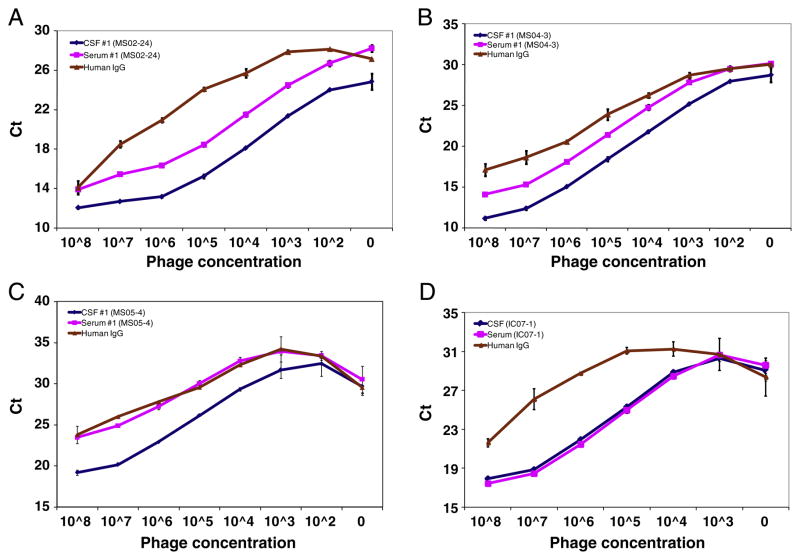
To determine whether CSF-selected peptides were specific for in-trathecally synthesized IgG in MS patients, we tested equal amounts of serum IgG (50 ng per well) from the same patients for peptide binding using highly sensitive dose-dependent phage-IPCR. Paired serum and CSF from all MS patients were coated onto wells of protein A plates, followed by addition of serial 10-fold dilutions of corresponding phage peptides to each well, and phage binding was assessed by real-time PCR. Phage peptides selected by CSF IgG of all MS patients bound ~10-fold (3- to 4-fold Ct difference) more to CSF IgG than to serum IgG of the same patient in a dose-dependent manner. Figs. 4A to C show a representative example of greater binding of phage peptides to CSF IgG than to serum IgG in 3 MS patients; furthermore, the same phage peptides did not bind to CSF or serum from a inflammatory CNS control patient with neurosyphilis (Fig. 4D). Overall, these results demonstrate that the phage peptides were preferentially targeted by intrathecally-synthesized IgG in MS CSF.
Fig. 4.
Targeting of intrathecally synthesized IgG by persistent phage peptides in dose-dependent phage-IPCR. Paired MS serum and CSF, as well as pre-immune human IgG control (50 μl at IgG concentration of 1 μl/ml), were coated in duplicate wells of protein A-plates before addition of the corresponding phage peptides (at serial 10-fold dilutions starting with 108 pfu) each well. Bound phage was determined by real-time PCR. Phage peptide bound at levels ~10-fold (3–4 Ct difference) higher to IgG in CSF than in serum in a dose-dependent manner. Preimmune human IgG served as negative control. Note that phage peptide selected by IC control IgG bound specifically to both serum and CSF IgG, indicating that it did not target intrathecal IgG. Experiments were repeated at least once. Error bars represent SD. A, patient 1 (02-24); B, patient 2 (04-3); C, patient 3; D, IC control patient (IC07-1, neurosyphilis). Data represent at least three independent experiments.
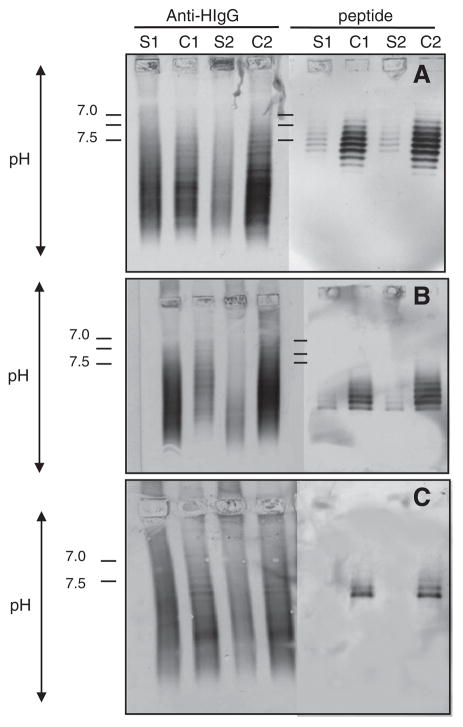
To further confirm that peptides bound to CSF IgG more than to serum IgG from the same MS patient, we evaluated phage binding by IEF immunoblotting. Paired MS CSF and serum from all 5 MS patients were separated on agarose IEF gels, transferred to PVDF membranes, and the blots were probed with corresponding phage peptides. In all 5 patients, the peptides were recognized by multiple high-intensity IgG bands in CSF, while fewer and less intense bands were seen in the paired serum. Figs. 5A to C show representative findings in patients 1–3. To demonstrate that IgG bands recognized by phage peptides represented oligoclonal bands in CSF, paired serum and CSF IEF blots were probed with anti-human IgG. The peptide reactive bands in the CSF corresponded to bands of oligoclonal IgG. The negative control peptide panned by CSF IgG from a patient with neurosyphilis was recognized by equal numbers of bands in both CSF and serum (Fig. 5D). To ensure that bands seen in CSF but not in serum were not due to lower amounts of serum IgG loaded onto the IEF gel, we applied 30 μg serum IgG from patient 1 to IEF gel, and no additional bands were seen when probed with phage peptides (data not shown). Moreover, in 2 of 3 MS patients, phage peptides were recognized by bands of IgG in serum and CSF of the same patient (Fig. 6).
Fig. 5.
Phage peptides recognize specific CSF oligoclonal IgG bands in IEF-immunoblots. Paired MS CSF and serum (3–5 μg total IgG) from three patients were resolved on agarose IEF gels and transferred to nitrocellulose membranes. Blots were probed with corresponding phage peptides (1010 pfu/ml) and incubated with mouse anti-pIII antibody followed by AP-anti-mouse antibody. Duplicate blots were probed with anti-human IgG as positive controls to reveal total oligoclonal bands. Peptides selected by MS IgG recognized multiple high-density IgG bands in the CSF, but weaker and reduced number of bands in the paired serum. Arrows indicate extra bands detected in the CSF. The peptide-specific OCBs correspond to some of the major bands in the OCB pattern of the MS patients. The control peptide panned by an IC CSF IgG recognized equal numbers of bands in both CSF and serum. Experiments were repeated three times. A, patient 1 (02-24); B, patient 2 (04-3); C, patient 3 (05-4); D, IC control patient. Data represent at least three independent experiments.
Fig. 6.
Phage peptides recognize persistent antibodies in corresponding serum. Consecutive pairs of MS sera and CSF (S1, C1, S2, C2) were examined by IEF-immunoblotting (see Fig. 5 legend). In two of three patients, phage peptides recognized persistent oligoclonal IgG bands in the paired sera, although all three CSF showed persistent IgG reactivity (see Fig. 2). Positive controls were sera and CSF probed with AP-anti-human IgG. Experiments were repeated twice. A, patient 1 (02-24); B, patient 2 (04-3); C, patient 3 (05-4). Data represent at least three independent experiments.
3.5. MS CSF IgG recognizes candidate peptides derived from protein database searches
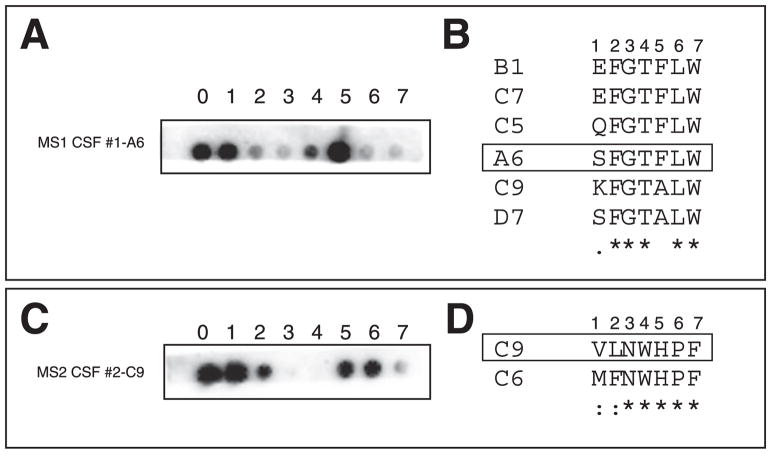
Alanine scan mutagenesis, which allows identification of key residues for antibody binding by virtue of an alanine substitution at each peptide residue position (Reineke, 1996), was used to identify critical amino acid residues of peptides that bound to IgG from CSF of 3 MS patients. A cellulose-bound peptide blot was incubated with MS CSF, followed by incubation with HRP-anti-human IgG and chemiluminescent detection. The critical amino acid residues were those with individual amino acids (SPOTS) in which little or no signal was revealed (Fig. 7A, C). As expected from peptide alignments, the critical residues for peptides MS1 CSF #1-A6 and MS2 CSF #1-C9 revealed by SPOT analysis were those shown to be conserved by analysis of sequence alignments (Fig. 7A–D).
Fig. 7.
Critical amino acid residues of peptides for MS IgG binding. Alanine scan muta-genesis was performed using SPOT synthesis technology and each mutated peptide was spotted on nitrocellulose membranes (A, peptide MS1 CSF #1-A6 of patient 1; C, peptide MS2 CSF #2-A8 of patient 2). Note peptide MS2 CSF #2-A8 has the same sequence as MS2 CSF #2-B1 in Table 2. Numbers above each blot indicate the locations of alanine replacement. “0” indicates the original peptide. Membranes were incubated with the respective MS CSF followed by HRP-anti-human IgG detection. The critical amino acid residues were spots with little or no signal. Sequence alignment results in B and D show the conserved (*), conservative (:), and semi-conservative (.) residues. The highlighted sequences were peptides used in mutagenesis studies in A and C.
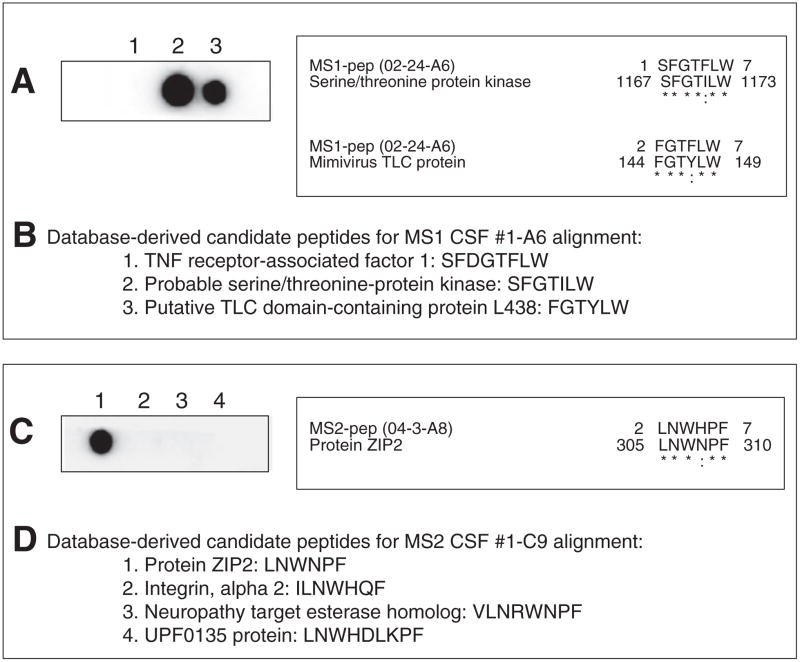
Based on results of alanine scan mutagenesis, candidate peptides were identified from database searches and synthesized using SPOT synthesis technology. Blots were probed with MS CSF. Peptides derived from two candidate proteins (serine/threonine-protein kinase and TLC domain-containing protein L438) were recognized by CSF from MS patient 1 (02-24); from the CSF of MS patient 2 (04-3), peptides from candidate protein ZIP2 were recognized (Fig. 8). The CSF of patient 3 recognized peptides derived from MHC class II (HLA-DQA2) and ATP-binding cassette, sub-family D (ALD) member 4 (data not shown).
Fig. 8.
Reactivity of MS CSF with candidate peptides derived from database searches. Based on results of alanine scan mutagenesis analysis, candidate peptides were identified from database searches and synthesized for phage peptides MS1 #1-A6 (A) and MS2 #1-A8 (C) using SPOT synthesis technology. Blots were probed with the respective MS CSF followed by horseradish peroxidase-anti-human IgG incubation and chemiluminescent detection. Peptides derived from likely candidate proteins serine/threonine-protein kinase and TLC domain-containing protein L438 were recognized by patient 1 CSF (02-24), while peptides derived from candidate protein ZIP2 were recognized by patient 2 CSF (04-3). Sequence alignments for the positive peptides are shown on the right (*=identical amino acid residues, : = conserved amino acid residues). B, D, Lists of candidate peptide sequences on the SPOT membranes.
Together, our data demonstrate the presence of stable peptide epitopes in MS CSF and the feasibility of using phage peptides to identify candidate protein antigens specific for MS CSF. IEF blots probed with phage peptides revealed higher-affinity specific OCBs in CSF than in the corresponding serum in 3 of 5 MS patients, providing qualitative evidence of antigen-specific intrathecal antibody synthesis.
4. Discussion
We used a phage-displayed random peptide library approach to investigate the specificity of the persistent IgG in the CSF of 5 MS patients. CSF IgG obtained from 2 consecutive lumbar punctures (1- to 3-yr intervals) from each patient affinity-selected phage peptides that were identical and/or similar in paired CSF samples from each MS patient. Highly sensitive phage-mediated IPCR showed that the phage peptides bound specifically to the persistent IgG in longitudinally obtained CSF. These peptide Ags were recognized preferentially by intrathecally synthesized IgG in CSF, revealing that they represent epitopes targeted by OCBs in MS patients. Linear peptides selected by 3 MS CSFs represented epitopes of proteins serine/threonine-protein kinase, TLC domain-containing protein, protein ZIP2 and MHC class II (HLA-DQA2) by alanine scan SPOT analysis and sequence alignment analysis.
When OCBs are absent or only present in few in number, MS tends to be less severe than when band numbers are high (Avasarala et al., 2001; Joseph et al., 2009). Furthermore, the number of plasma blasts in MS CSF correlates with the abundance of intrathecal Ig production and inflammatory parenchymal disease activity as measured by magnetic resonance imaging (Cepok et al., 2005). Combined with the concordance between the CSF Ig transcriptome and the CSF Ig proteome (Obermeier et al., 2008), the collective data suggest that the intrathecal antibody response is relevant to the pathogenesis of MS, and that a greater number of OCBs correlates with disease progression. Our findings of a temporal stability of intrathecal IgG specificity in CSF, with enhanced IgG binding intensity in CSF and a greater number of negatively charged epitope-specific IgGs in the second CSF from the same patient, suggest the generation of higher-affinity CSF IgG during disease progression. The peptide reactivity was with only a subset of OCBs, suggesting that only a portion of the OCB reactivity was identified, and that the other OCBs not reacting with a dominant peptide have other unidentified specificities for epitopes of the same antigens or additional antigens, and then that potentially additional epitopes or self-antigens are to be identified which may reveal the cause of disease.
The presence of increased numbers of negatively charged bands of IgG in the second CSF suggests that these IgG isoforms may be related to disease severity. Furthermore, only one dominant peptide group was selected by both CSF IgGs in each MS patient, confirming the presence of restricted clonal expression of IgG in the CSF of MS patients (Ritchie et al., 2004). Luxton et al. (1995) noted that the intrathecal polyspecific IgG in MS is usually low affinity, unlike the high-affinity IgG directed against a specific pathogen. Our consistent selection of high-affinity peptides demonstrated that they represent epitopes of specific autoantigens. Their relevance to disease pathogenesis requires further study. Conformational epitopes may not be identified using linear phage libraries. For future experiments, we will identify conformational epitopes using CLIPS technology (Chemical Linkage of Peptides onto Scaffolds) technology from the Mimotopes (www.mimotopes.com), as well as screening a custom constructed library with longer peptides (Noren and Noren, 2001).
Three non-mutually exclusive theories of persistent Ig production against pathogens or autoantigens have been proposed: (Archelos et al., 1998) continuous activation of memory B cells and generation of short-lived plasma blasts driven by antigen (Ochsenbein et al., 2000); (Avasarala et al., 2001) persistent Ig secretion by long-lived plasma cells in bone marrow and at sites of inflammation (Odendahl et. al, 2005); and (Bernasconi et al., 2002) bystander activation of memory B cells in the absence of Ag and generation of short-lived plasmablasts (Bernasconi et al., 2002). Our data suggest that persistent Ig production reflects continuous activation of memory B cells by specific Ag over time. The absence of reagents capable of detecting individual CSF Ab species has hampered the possibility of ascertaining whether Abs with the same specificity are also present in patients’ sera. Using peptides selected by CSF IgG to address this issue, we examined serum obtained from the same MS patients for reactivity with these peptides. Highly sensitive phage-IPCR analysis showed that the peptides bound to IgG in CSF at ~10-fold higher levels than in paired serum in a dose-dependent manner, revealing that the peptides were targeted by intrathecal IgG in the CSF. This is in contrast to a report (Cortese et al., 1996) that Abs recognizing the selected peptides are commonly found with equal frequency in sera and CSF of MS patients.
OCBs are defined by the presence of 2 or more IgG bands detected in CSF that are not present in the corresponding serum, reflecting a localized B-cell response that accompanies CNS inflammation (Link and Huang, 2006). Our IEF immunoblotting analysis of paired serum and CSF IgG to evaluate phage binding specificity to OCBs and to determine in which compartment OCBs are synthesized showed that while phage peptides recognized specific IgG bands in both MS sera and CSF, they were targeted by a stronger and more focused intrathecal immune response in CSF, providing qualitative evidence of Ag-specific intrathecal Ab synthesis. The OCB profiles highlighted in our IEF blots with specific peptides are consistent with typical OCB patterns found in MS (Giovannoni and Thompson in Raine et al. 2008). For example, the “greater than” pattern C (C++>S+) in which OCBs are detected in both CSF and serum but with additional bands in the CSF, was seen in patients 1 and 2 (Fig. 5A, B), while Pattern B (C+, S−), with OCBs present in the CSF but no apparent corresponding abnormality in serum, was seen in patient 3 (Fig. 5C). IEF blots from inflammatory control neurosyphilis IgGs revealed identical bands in CSF and serum (C+, S+) (Pattern D), indicative of local CNS synthesis, consistent with passive transfer from a systemic B cell response (Giovannoni and Thompson in Raine et al. 2008).
Peptide-specific IgGs in CSF and serum appear to represent a spectrum of IgG bands that differ in overall net charge (pH values), raising the possibility that the IgG isoforms result from post-translational modifications of clonally related IgG, such as deamidation, differential glycosylation or proteolysis (Walsh et al., 1986). The post-translationally modified CSF-restricted IgG is most likely the result of local intrathecal synthesis. The enrichment may also represent a consequence of a rare stochastic variation in IgG production. These peptides likely represent cognate Ags of the OCBs.
OCB-specific peptides provide a novel tool to identify intrathecally produced Abs. Alanine scan mutagenesis (Figs. 7 and 8) and database alignment identified candidate peptides representing potential protein Ags of MS. Alanine scan mutagenesis combined with database searches identified candidate peptides representing potential protein antigens of MS. The different protein candidates identified in each MS CSF may reflect the small sample size (3 MS CSFs) studied. These candidate peptides will be used to screen large numbers of MS CSFs to search for common antigens. In addition, phage libraries with be panned with large numbers of MS CSF IgGs to identify epitopes and antigens shared by MS patients. Furthermore, it is not surprising that different antigens were identified in MS since even in subacute sclerosing panencephalitis, a chronic encephalitis caused by measles virus, not all Abs produced by clonally expanded plasma cells in the patient’s brain and CSF are demonstrably measles-virus-specific (Burgoon et al., 2005). Thus, more extensive analyses with larger sets of peptides may identify common Ags in MS, some of which may be non-human.
Acknowledgments
We thank Andrew Shearer for technical assistance. The authors also thank Marina Hoffman for editorial assistance and Cathy Allen for manuscript preparation.
Abbreviations
- AP
alkaline phosphatase
- IC
inflammatory controls
- IEF
isoelectric focusing
- IPCR
immuno-PCR
- HIgG
human IgG
- MS
multiple sclerosis
- OCB
oligoclonal band
Footnotes
This work was supported by a research grant (RG) from the National Multiple Sclerosis Society (RG 3934A1/1).
References
- Archelos JJ, Trotter J, Previtali S, Weissbrich B, Toyka KV, Hartung HP. Isolation and characterization of an oligodendrocyte precursor-derived B-cell epitope in multiple sclerosis. Ann Neurol. 1998;43:15–24. doi: 10.1002/ana.410430107. [DOI] [PubMed] [Google Scholar]
- Avasarala JR, Cross AH, Trotter JL. Oligoclonal band number as a marker for prognosis in multiple sclerosis. Arch Neurol. 2001;58:2044–2045. doi: 10.1001/archneur.58.12.2044. [DOI] [PubMed] [Google Scholar]
- Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- Burgoon MP, Keays KM, Owens GP, Ritchie AM, Rai PR, Cool CD, Gilden DH. Laser-capture microdissection of plasma cells from subacute sclerosing panencephalitis brain reveals intrathecal disease-relevant antibodies. Proc Natl Acad Sci USA. 2005;102:7245–7250. doi: 10.1073/pnas.0502323102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepok S, Rosche B, Grummel V, Vogel F, Zhou D, Sayn J, Sommer N, Hartung HP, Hemmer B. Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain. 2005;128:1667–1676. doi: 10.1093/brain/awh486. [DOI] [PubMed] [Google Scholar]
- Cortese I, Tafi R, Grimaldi LM, Martino G, Nicosia A, Cortese R. Identification of peptides specific for cerebrospinal fluid antibodies in multiple sclerosis by using phage libraries. Proc Natl Acad Sci USA. 1996;93:11063–11067. doi: 10.1073/pnas.93.20.11063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese I, Capone S, Tafi R, Grimaldi LM, Nicosia A, Cortese R. Identification of peptides binding to IgG in the CSF of multiple sclerosis patients. Mult Scler. 1998;4:31–36. doi: 10.1177/135245859800400108. [DOI] [PubMed] [Google Scholar]
- Cortese I, Capone S, Luchetti S, Cortese R, Nicosia A. Cross-reactive phage-displayed mimotopes lead to the discovery of mimicry between HSV-1 and a brain-specific protein. J Neuroimmunol. 2001;113:119–128. doi: 10.1016/s0165-5728(00)00398-2. [DOI] [PubMed] [Google Scholar]
- Gilden DH. Infectious causes of multiple sclerosis. Lancet Neurol. 2005;4:195–202. doi: 10.1016/S1474-4422(05)01017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine CS, McFarland HF, Hohlfeld R Giovannoni and Thompson. Multiple Sclerosis, A Comprehensive Text. Saunders; Edinburgh(?): 2008. pp. 88–99. [Google Scholar]
- Hela-Felicitas P, Reske D. Expansion of antibody reactivity in the cerebrospinal fluid of multiple sclerosis patients — follow-up and clinical implications. Cerebrospinal Fluid Res. 2005;2:3. doi: 10.1186/1743-8454-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph FG, Hirst CL, Pickersgill TP, Ben-Shlomo Y, Robertson NP, Scolding NJ. CSF oligoclonal band status informs prognosis in multiple sclerosis: a case control study of 100 patients. J Neurol Neurosurg Psychiatry. 2009;80:292–296. doi: 10.1136/jnnp.2008.150896. [DOI] [PubMed] [Google Scholar]
- Link H, Huang YM. Oligoclonal bands in multiple sclerosis cerebrospinal fluid: an update on methodology and clinical usefulness. J Neuroimmunol. 2006;180:17–28. doi: 10.1016/j.jneuroim.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Luxton RW, Zeman A, Holzel H, Harvey P, Wilson J, Kocen R, Morgan-Hughes J, Miller DH, Compston A, Thompson EJ. Affinity of antigen-specific IgG distinguishes multiple sclerosis from encephalitis. J Neurol Sci. 1995;132:11–19. doi: 10.1016/0022-510x(95)00115-i. [DOI] [PubMed] [Google Scholar]
- Obermeier B, Mentele R, Malotka J, Kellermann J, Kumpfel T, Wekerle H, Lottspeich F, Hohlfeld R, Dornmair K. Matching of oligoclonal immunoglobulin transcriptomes and proteomes of cerebrospinal fluid in multiple sclerosis. Nat Med. 2008;14:688–693. doi: 10.1038/nm1714. [DOI] [PubMed] [Google Scholar]
- Ochsenbein AF, Pinschewer DD, Sierro S, Horvath E, Hengartner H, Zinkernagel RM. Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs. Proc Natl Acad Sci USA. 2000;97:13263–13268. doi: 10.1073/pnas.230417497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odendahl M, Mei H, Hoyer BF, Jacobi AM, Hansen A, Muehlinghaus G, Berek C, Hiepe F, Manz R, Radbruch A, Dorner T. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood. 2005;105:1614–1621. doi: 10.1182/blood-2004-07-2507. [DOI] [PubMed] [Google Scholar]
- Owens GP, Bennett JL, Lassmann H, O’Connor KC, Ritchie AM, Shearer A, Lam C, Yu X, Birlea M, Dupree C, Williamson RA, Hafler DA, Burgoon MP, Gilden D. Antibodies produced by clonally expanded plasma cells in multiple sclerosis cerebrospinal fluid. Ann Neurol. 2009;65:639–649. doi: 10.1002/ana.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke U, Sabat R, Kramer A, Stigler RD, Seifert M, Michel T, Volk HD, Schneider-Mergener J. Mapping protein–protein contact sites using cellulose-bound peptide scans. Mol Divers. 1996;1:141–148. doi: 10.1007/BF01544952. [DOI] [PubMed] [Google Scholar]
- Ritchie AM, Gilden DH, Williamson RA, Burgoon MP, Yu X, Helm K, Corboy JR, Owens GP. Comparative analysis of the CD19+ and CD138+ cell antibody repertoires in the cerebrospinal fluid of patients with multiple sclerosis. J Immunol. 2004;173:649–656. doi: 10.4049/jimmunol.173.1.649. [DOI] [PubMed] [Google Scholar]
- Stendahl-Brodin L, Link H. Relation between benign course of multiple sclerosis and low-grade humoral immune response in cerebrospinal fluid. J Neurol Neurosurg Psychiatry. 1980;43:102–105. doi: 10.1136/jnnp.43.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MJ, Tourtellotte WW, Shapshak P. Immunoglobulin heavy chain associated protein in multiple sclerosis cerebrospinal fluid. Mol Immunol. 1986;23:1117–1123. doi: 10.1016/0161-5890(86)90010-6. [DOI] [PubMed] [Google Scholar]
- Yu X, Owens GP, Gilden DH. Rapid and efficient identification of epitopes/mimotopes from random peptide libraries. J Immunol Methods. 2006;316:67–74. doi: 10.1016/j.jim.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Yu X, Burgoon MP, Shearer AJ, Gilden DH. Characterization of phage peptide interaction with antibody using phage-mediated immuno-PCR. J Immunol Methods. 2007;326:33–40. doi: 10.1016/j.jim.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Gilden D, Schambers L, Barmina O, Burgoon M, Bennett J, Owens G. Peptide reactivity between multiple sclerosis (MS) CSF IgG and recombinant antibodies generated from clonally expanded plasma cells in MS CSF. J Neuroimmunol. 2011;233:192–203. doi: 10.1016/j.jneuroim.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]