Abstract
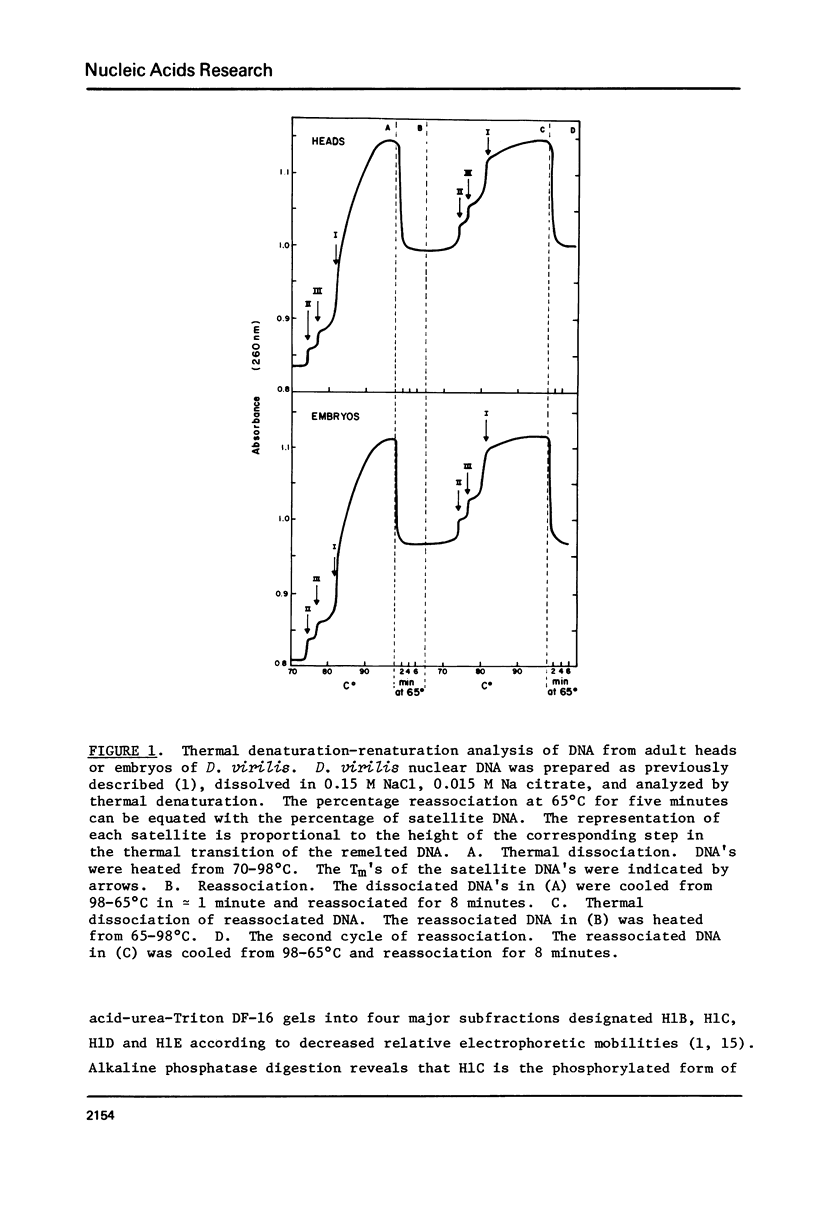
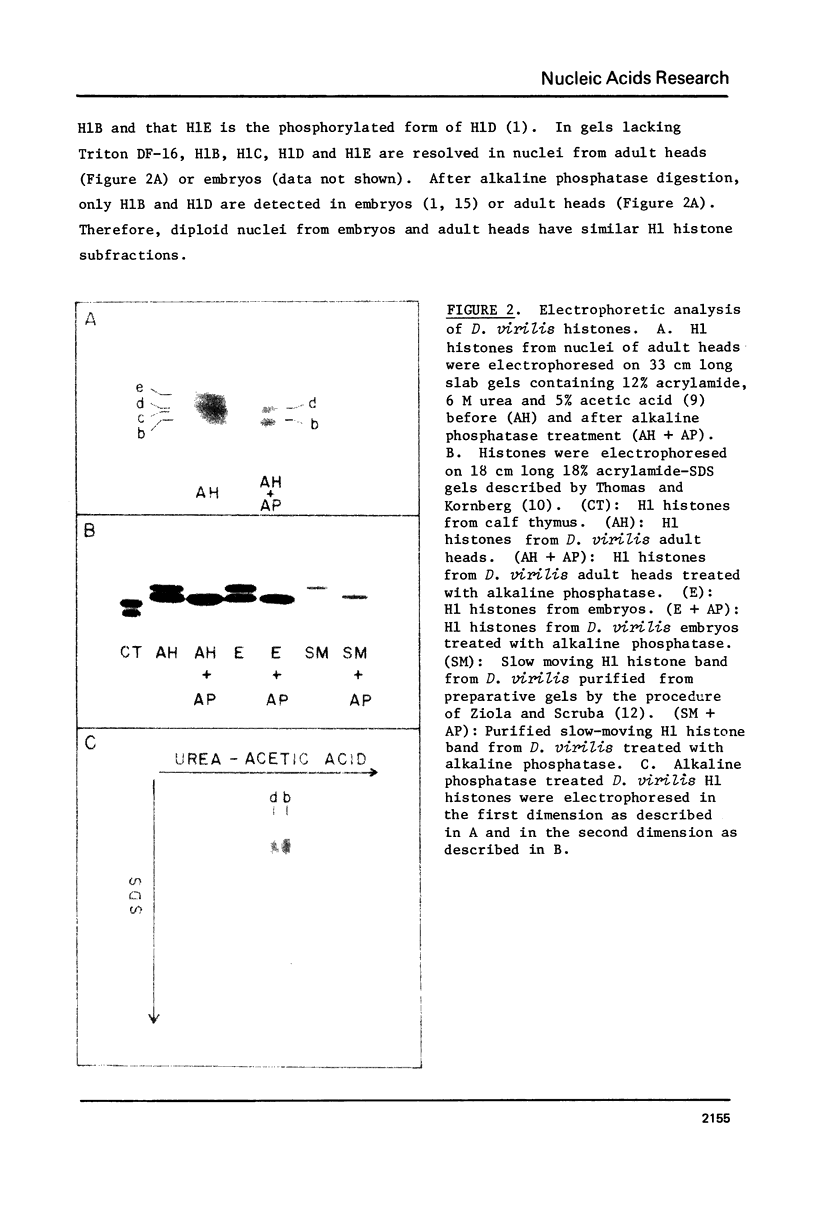
In embryonic nuclei of Drosophila virilis, 45% of the DNA is satellite, and congruent to 50% of the H1 histone is phosphorylated. In polytene salivary gland nuclei, less than 1% of the DNA is satellite, and less than 10tion. The phosphorylated H1's migrate 4% slower than the unphosphorylated H1's on SDS-acrylamide gels. The mobility difference may arise because the phosphorylated and unphosphorylated H1's have different conformations in SDS. This putative conformational difference could be essential to the compaction of satellite DNA into heterochromatin.
Full text
PDF



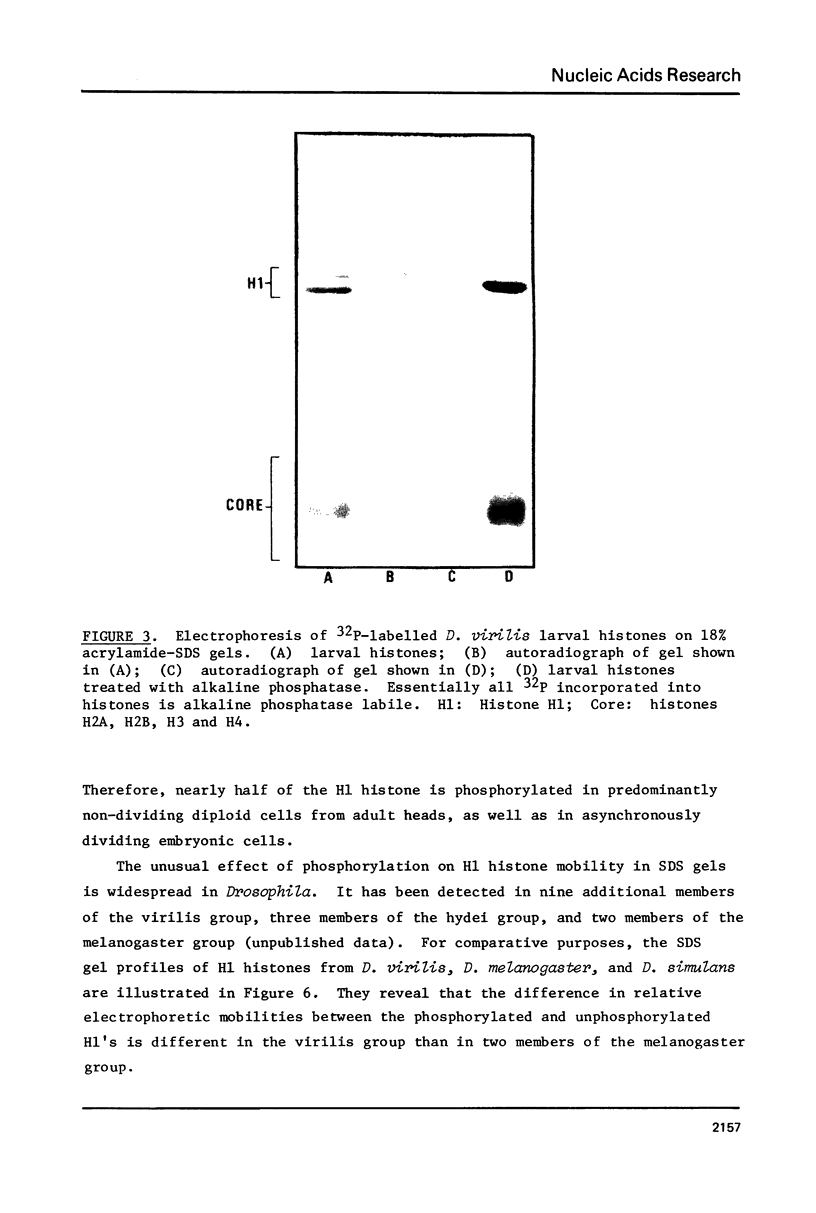
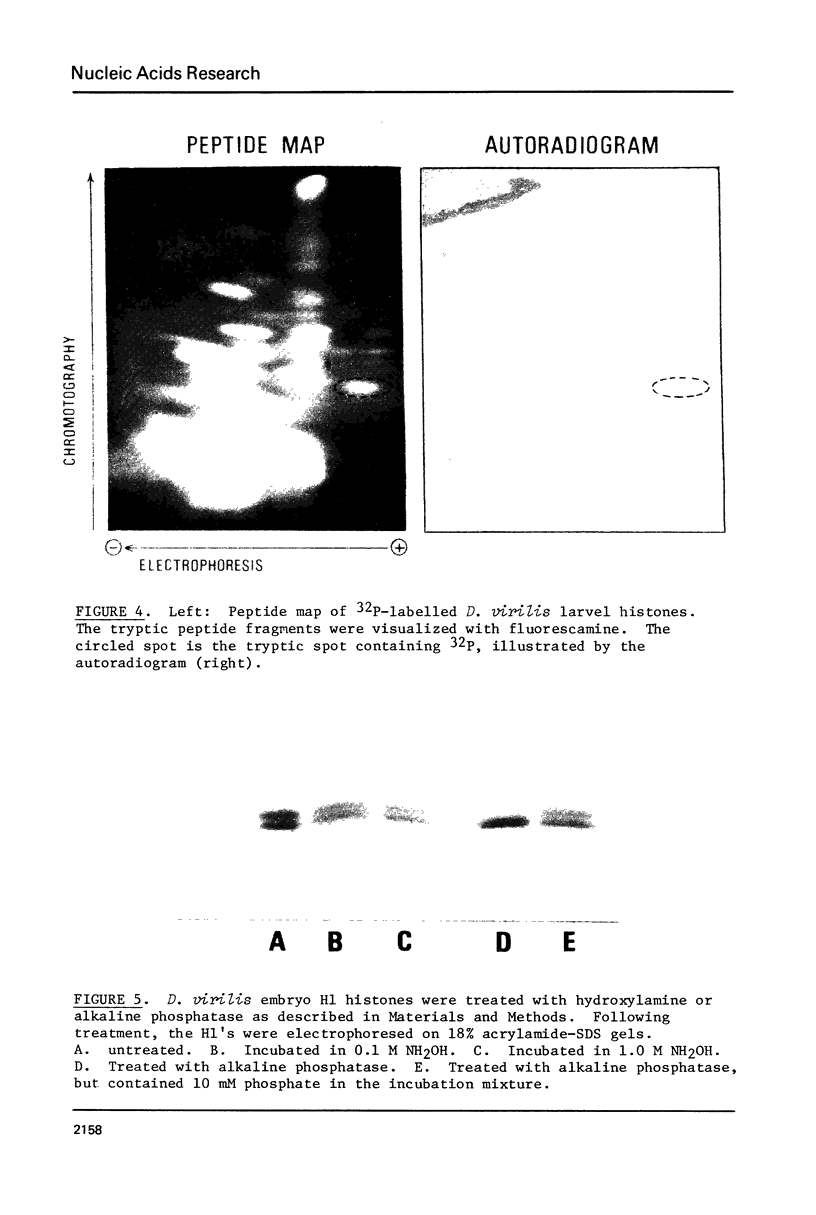

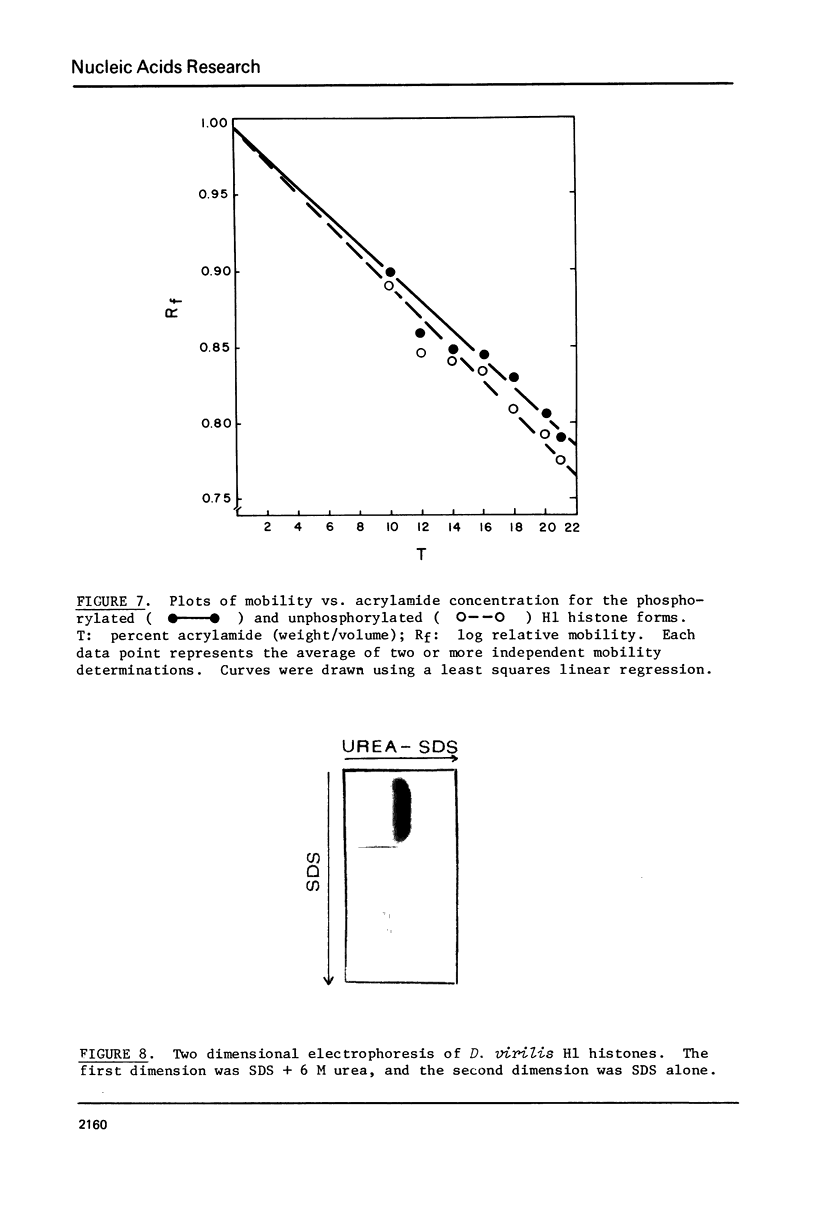




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balhorn R., Chalkley R., Granner D. Lysine-rich histone phosphorylation. A positive correlation with cell replication. Biochemistry. 1972 Mar 14;11(6):1094–1098. doi: 10.1021/bi00756a023. [DOI] [PubMed] [Google Scholar]
- Banker G. A., Cotman C. W. Measurement of free electrophoretic mobility and retardation coefficient of protein-sodium dodecyl sulfate complexes by gel electrophoresis. A method to validate molecular weight estimates. J Biol Chem. 1972 Sep 25;247(18):5856–5861. [PubMed] [Google Scholar]
- Blankstein L. A., Stollar B. D., Franklin S. G., Zweidler A., Levy S. B. Biochemical and immunological characterization of two distinct variants of histone H2A in Friend leukemia. Biochemistry. 1977 Oct 18;16(21):4557–4562. doi: 10.1021/bi00640a003. [DOI] [PubMed] [Google Scholar]
- Blumenfeld M., Forrest H. S. Differential under-replication of satellite DNAs during Drosophila development. Nat New Biol. 1972 Oct 11;239(93):170–172. doi: 10.1038/newbio239170a0. [DOI] [PubMed] [Google Scholar]
- Blumenfeld M., Orf J. W., Sina B. J., Kreber R. A., Callahan M. A., Mullins J. I., Snyder L. A. Correlation between phosphorylated H1 histones and satellite DNAs in Drosophila virilis. Proc Natl Acad Sci U S A. 1978 Feb;75(2):866–870. doi: 10.1073/pnas.75.2.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld M., Orf J. W., Sina B. J., Kreber R. A., Callahan M. A., Snyder L. A. Satellite DNA, H1 histone, and heterochromatin in Drosophila virilis. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):273–276. doi: 10.1101/sqb.1978.042.01.029. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R. Control of cell division by very lysine rich histone (F1) phosphorylation. Nature. 1974 Feb 1;247(5439):257–261. doi: 10.1038/247257a0. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R., Sarner N. Phosphorylation of very-lysine-rich histone in Physarum polycephalum. Correlation with chromosome condensation. Eur J Biochem. 1973 Feb 15;33(1):131–139. doi: 10.1111/j.1432-1033.1973.tb02664.x. [DOI] [PubMed] [Google Scholar]
- Cohen L. H., Gotchel B. V. Histones of polytene and nonpolytene nuclei of Drosophila melanogaster. J Biol Chem. 1971 Mar 25;246(6):1841–1848. [PubMed] [Google Scholar]
- Endow S. A., Gall J. G. Differential replication of satellite DNA in polyploid tissues of Drosophila virilis. Chromosoma. 1975;50(2):175–179. doi: 10.1007/BF00283238. [DOI] [PubMed] [Google Scholar]
- Gorovsky M. A., Keevert J. B. Absence of histone F1 in a mitotically dividing, genetically inactive nucleus. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2672–2676. doi: 10.1073/pnas.72.7.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEPPEL L. A., HARKNESS D. R., HILMOE R. J. A study of the substrate specificity and other properties of the alkaline phosphatase of Escherichia coli. J Biol Chem. 1962 Mar;237:841–846. [PubMed] [Google Scholar]
- Hardison R., Chalkley R. Polyacrylamide gel electrophoretic fractionation of histones. Methods Cell Biol. 1978;17:235–251. doi: 10.1016/s0091-679x(08)61146-2. [DOI] [PubMed] [Google Scholar]
- Hohmann P., Tobey R. A., Gurley L. R. Phosphorylation of distinct regions of f1 histone. Relationship to the cell cycle. J Biol Chem. 1976 Jun 25;251(12):3685–3692. [PubMed] [Google Scholar]
- Peacock W. J., Lohe A. R., Gerlach W. L., Dunsmuir P., Dennis E. S., Appels R. Fine structure and evolution of DNA in heterochromatin. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1121–1135. doi: 10.1101/sqb.1978.042.01.113. [DOI] [PubMed] [Google Scholar]
- Postlethwait J. H., Schneiderman H. A. Developmental genetics of Drosophila imaginal discs. Annu Rev Genet. 1973;7:381–433. doi: 10.1146/annurev.ge.07.120173.002121. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Omachi A., Kawaichi M., Hayaishi O. Natural occurrence of poly(ADP-ribosyl) histones in rat liver. Proc Natl Acad Sci U S A. 1975 Jan;72(1):205–209. doi: 10.1073/pnas.72.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziola B. R., Scraba D. G. Recovery of SDS-proteins from polyacrylamide gels by electrophoresis into hydroxylapatite. Anal Biochem. 1976 May 7;72:366–371. doi: 10.1016/0003-2697(76)90543-1. [DOI] [PubMed] [Google Scholar]