Abstract
Hyperactivity of hypothalamic-pituitary mediated hormone responses, such as to stimulation with a serotonin 1A (5-HT1A) receptor agonist, are a feature of depression which are normalized with clinical improvement during drug therapy. We previously reported that SSRIs induce desensitization of 5-HT1A receptor signaling in the paraventricular nucleus of the hypothalamus (PVN) while estradiol benzoate (EB) produces a more rapid, partial desensitization. In the current study, time-course and dose-response experiments demonstrated that two once daily doses of EB is the minimum needed to induce the desensitization response as indicated by 5-HT1A receptor-stimulated release of oxytocin and that 10 μg/kg/day EB produces the maximal response, a partial desensitization of approximately 40%. The effects of two once daily injections of 10 μg/kg/day EB on Gαz and RGSZ1 proteins were examined as components of the 5-HT1A receptor signaling system, which mediates the release of oxytocin and adrenocorticotropin hormone. RGSZ1 appears to be a major target for EB-mediated responses in the 5-HT1A receptor signaling system. A 55 kD membrane-associate RGSZ1 protein was greatly increased in the PVN and rest of the hypothalamus and moderately increased in the dorsal hippocampus and amygdala after EB treatment as well as after an acute dose of a 5-HT1A receptor agonist. These results suggest that EB is a candidate for adjuvant therapy with SSRIs to hasten the therapeutic response and that RGSZ1 is a major target of EB therapy which could be explored as a target for novel therapeutic approaches for the treatment of depression.
Keywords: 5-HT1A, neuroendocrine, oxytocin, regulators of G protein signaling proteins, estrogen, desensitization
1. INTRODUCTION
Recent clinical and preclinical evidence highlights the role of reproductive hormones in mood disorders in women. Indeed, women are more likely than men to experience depression by nearly 2 to 1 (Silva et al., 2010). In women, depression is associated with periods of hormone fluctuations such as during premenstrual and post-partum periods. Furthermore, women have increased risk for depression during transition periods in their reproductive cycle such peri-menopausal and early menopausal periods (Bromberger et al., 2011; Cohen et al., 2006; Soares, 2010).
One of the major shortcomings of current therapies for depression is the extensive delay in the clinical response; it takes weeks rather than hours or days for a therapeutic response as with most medications. It is paramount to develop approaches that shorten the delay in the clinical response. Estradiol is an effective treatment for depression in peri-menopausal women (Rasgon et al., 2002; Schmidt et al., 2000; Soares et al., 2001). Furthermore, estrogen may improve the clinical response to selective serotonin reuptake inhibitor (SSRI) therapy (Rasgon et al., 2002; Schneider et al., 2001; Schneider et al., 1997). In a 6 week clinical trial, fluoxetine significantly improved depressive symptoms in older women taking estrogen replacement therapy (ERT) compared to women without ERT (Schneider et al., 1997). In contrast, a 12 week trial of sertraline in older women with and without ERT demonstrated only a small increase in response to depression with ERT (Schneider et al., 2001). In a separate trial examining the effects of sertraline and ERT in depressed post-menopausal women, ERT did not alter the improvement in depressive symptoms at the end of the 12 week trial but did accelerate the anti-depressive responses at 2, 3 and 4 weeks of treatment (Rasgon et al., 2007). Similar to the findings in the study by Rasgon and colleagues (Rasgon et al., 2007), we and others have found an accelerated response to SSRIs with estradiol treatment in a pre-clinical studies. Estradiol alone produces, and in combination with fluoxetine facilitates antidepressant-like effects in rats as measured in a forced swim test (Estrada-Camarena et al., 2006a; Estrada-Camarena et al., 2006b). Furthermore, these affects are dependent on serotonin 1A (5-HT1A) receptors as a 5-HT1A receptor antagonist blocked the antidepressant-like effects.
Desensitization of 5-HT1A receptor signaling in both midbrain and forebrain regions (hypothalamus, amygdala, frontal cortex) may be an underlying mechanism for the therapeutic effects of estrogen and of SSRIs such as fluoxetine for mood disorders and hot flushes in peri- and post-menopausal women (Bosker et al., 2001; Gomez-Gil et al., 2010; Navines et al., 2007; Nikisch et al., 2005; Shen et al., 2002). However, unlike SSRIs, which require 7–14 days to induce desensitization of 5-HT1A receptor signaling and begin to induce a therapeutic response, estrogens can produce a partial desensitization of 5-HT1A receptor signaling within 2 days of administration in rodent models (Li et al., 1997; Li et al., 1996; Mize and Alper, 2000; Mize et al., 2001; Raap et al., 2000; Uphouse et al., 1994). In particular, two days of treatment with 17β-estradiol-3-benzoate (EB) results in partial desensitization of 5-HT1A receptor signaling in the rat paraventricular nucleus of the hypothalamus (PVN) (D’Souza et al., 2004; Raap et al., 2000). Stimulation of 5-HT1A receptors in the PVN by the 5-HT1A/7 agonist (+)8-hydroxy-2-dipropylaminotetralin ((+)8-OH-DPAT), stimulates the release of oxytocin and adrenocorticotropic (ACTH) hormones (Osei-Owusu et al., 2003). Furthermore, the (+)8-OH-DPAT stimulated release of oxytocin and ACTH is mediated by 5-HT1A receptors as demonstrated by inhibition with the selective 5-HT1A receptor antagonist WAY-100635 (Vicentic et al., 1998). Plasma oxytocin and ACTH levels thus serve as markers for 5-HT1A receptor function in the PVN (Lerer et al., 1999). In the PVN, 5-HT1A receptors couple to the Gαz subunit to mediate oxytocin and ACTH hormone release (Serres et al., 2000). Gαz signaling is modulated by regulator of G-protein signaling protein Z1 (RGSZ1), a GTPase-activating protein with approximately 20-fold selectivity for Gαz over other members of the Gαi/o family (Glick et al., 1998). RGSZ1 accelerates hydrolysis of Gαz signaling over 400-fold, quickly blunting further downstream signaling (Wang et al., 1998). Treatment with EB for two days increases the levels of a 30 kD RGSZ1 protein in the membrane of the PVN in a dose-dependent manner without affecting Gαz levels (Carrasco et al., 2004; Raap et al., 2000).
In the present study, we examined the dose-response and time course of the effects of EB treatment on desensitization of 5-HT1A receptor signaling in the PVN. We further explored possible mechanisms underlying the desensitization of 5-HT1A receptor signaling by examining the expression of Gαz and other RGSZ1 isoforms as well as their interaction following treatment with EB for 2 days. For comparison, we also examined the effects of acute stimulation with the 5-HT1A receptor agonist (+)8-OH-DPAT on RGSZ1, Gαz and their interaction. We examined expression of RGSZ1 and Gαz in several brain regions that express 5-HT1A receptors and are associated with depression including the hypothalamus, hippocampus and amygdala.
2. MATERIALS AND METHODS
2.1 Radioimmunoassays
Plasma oxytocin concentrations were determined by radioimmunoassay as previously described (Rossi et al., 2010). Briefly, 1 ml of cold acetone followed by 2.5 ml petroleum ether was used to extract oxytocin from 0.5 ml of plasma. The samples were dried in a Centrivap vacuum concentrator at 4°C after the top ether layer was removed. The dried residue was resuspended in 1 ml of cold assay buffer containing 0.05 M phosphate buffer pH 7.4, 0.125% bovine serum albumin and 1.0 mM EDTA. The plasma extract was used for the radioimmunoassay using radioactive 125I oxytocin (specific activity: 2200 Ci/mmol; PerkinElmer; Waltham, MA). Plasma ACTH concentrations were determined as previously described (Li et al., 1993).
2.2 Tissue preparation
The PVN, the rest of the hypothalamus, the dorsal hippocampus and amygdala brain regions were punched out of 300 μm brain slices sectioned on a cryostat. The PVN was collected from brain sections at the level of about −1.80 to ~ −2.7 mm from bregma. The rest of hypothalamus was collected at the level of −1.80 to ~ −3.3 mm from bregma, and the amygdala and dorsal hippocampus were collected from the sections at −2.4 to ~ −4.5 mm of bregma based on co-ordinates from a rat brain atlas (Paxinos and Watson, 2007). Tissue was stored at −80°C until use. The tissue was homogenized in 10 volumes (volume/tissue weight) in homogenate buffer (50mM Tris, pH 7.4 containing 150mM NaCl, 10% sucrose, 20mM N-ethymaleimide isopeptidase inhibitor, 1X protease inhibitor cocktail (Sigma; St. Louis, MO) and 1X phosphatase inhibitor cocktail I and II (Sigma; St. Louis, MO), using the Powergene 1000 homogenizer (Fisher-Scientific; Waltham, MA) with a 5mm probe at speed 5 for 10 seconds on ice. The homogenates were then centrifuged at 25,000 × g at 4°C for 60 minutes. The supernatant was collected as the cytosol fraction. The pellet was then sonicated at setting 3 for 5 seconds, at 4°C in 3 volumes of solubilization buffer containing 20mM Tris, pH 8, 1mM EDTA, 100mM NaCl, 1% sodium cholate, 20mM N-ethymaleimide, 1X protease inhibitor cocktail and 1X phosphatase inhibitor cocktails I and II. Vials were shaken horizontally at 4°C for 60 minutes, centrifuged at 25,000 × g at 4°C for 1 hour and the supernatant was collected as the membrane fraction. The cytosol and membrane fractions were aliquoted and stored at −80°C. The protein concentration in these brain tissue fractions was determined with the Pierce BCA protein assay using bovine serum albumin as a standard (Thermo Fisher Scientific, Rockford, IL).
2.3 Immunoblot Analysis
RGSZ1 and Gαz protein levels in the membrane and cytosol fractions of the PVN, the rest of the hypothalamus, amygdala and hippocampus were determined by immunoblot analysis. Briefly, after adding 4X sample buffer (350 mM Tris pH 6.8, 0.1% SDS, 45% glycerol, 0.001% bromophenol blue), samples were denatured at 95°C for 5 minutes then cooled on ice. Since the RGSZ1 antibody reacts with the reducing agents DTT and β-mercaptoethanol, the reducing agents were excluded from sample buffer. The samples (containing 10 μg of protein) were resolved on 12% SDS-PAGE gels (acrylamide: bis-acrylamide = 30: 0.2), then proteins were transferred to PVDF membranes. Non-specific binding was blocked by incubating the membrane in 5% milk in TBST (50 mM Tris, pH 7.4 containing 150 mM NaCl and 0.1% Tween 20). The blots were next incubated with rabbit-anti-RGSZ1 (1:2000) or rabbit-anti-Gαz (Santa Cruz; Santa Cruz, CA, 1:2000) in TBST containing 2% milk overnight at 4°C. After washing with TBST and incubation with goat-anti rabbit IgG (1:10,000, Jackson Lab), the bands were then detected using ECL substrate solution (GE Healthcare Biosciences, Piscataway, NJ) and captured using a BioRad ChemiDoc XRS+ molecular imager (BioRad, Hercules, CA). To normalize protein loading, the blots were then incubated with β-actin antibodies (1:20,000, MP Biomedicals, Solon, OH). The integrated optical density (IOD) of protein bands were analyzed densitometrically using ImageLab software (BioRad, Hercules, CA). All samples were run in triplicate. RGSZ1 and Gαz protein levels were normalized to β-actin protein levels and calculated as percent of the mean of the vehicle-treated control group in each blot.
2.4 Co-immunoprecipitation
To determine the interaction between Gαz protein and RGSZ1, we immunoprecipitated proteins binding to Gαz protein and then examined the proteins on immunoblots using an antibody against RGSZ1. The membrane and cytosol fractions of the hypothalamus (without the PVN) containing 200 μg of protein were incubated with 25 μl pre-washed protein G agarose beads (Invitrogen Carlsbad, CA) in total volume of 500 μl of IP buffer (50mM Tris, pH 7.4, 10mM EGTA, 100mM NaCl, 0.5% Triton X-100, 20mM N-ethymaleimide, 1X protease inhibitor cocktail and 1X phosphatase inhibitor cocktail I and II) with rotation at 4°C for one hour to absorb the endogenous IgG. After centrifugation at 3,000 × g at 4°C for 5 minutes, the supernatant was incubated with 4 μg Gαz primary antibody (Santa Cruz; Santa Cruz, CA) or 4 μg of rabbit IgG and rotated at 4°C overnight. A negative control containing antibody but no tissue was also included in some assays. 50 μl of prewashed protein G beads were added to each tube and rotated at 4°C for 2 hours. Protein G beads were pelleted by centrifugation at 1,000 × g, at 4°C for 3 minutes and then resuspended in 0.5 mL ice cold IP buffer. After washing 3 times, the Gáz bound protein complexes were eluted in 25 μl 2X sample buffer without β-mercaptoethanol by heating at 95°C for 5 minutes, then centrifuging at 3,000 rpm for 5 minutes. The supernatant was collected and stored at −80°C.
Immunoprecipitated samples and 10 μg of original sample (input) were resolved on 12% SDS-PAGE gels and transferred on PVDF membrane as described above. The membranes were then incubated with rabbit-antiRGSZ1 antibody (1:2000) overnight at 4°C. An HRP-conjugated secondary antibody that only recognizes intact IgG (Cleanblot, 1:1000, Thermo Scientific, Rockford, IL) was used to reduce background from the rabbit antibody (anti-Gαz) used for immunoprecipitation. Proteins were visualized by enhanced chemi-luminescence detection (SuperSignal West Dura, Thermo Scientific, Rockford, IL). Membranes were then incubated with Gαz primary antibody (Invitrogen; Carlsbad, CA, 1:2000) to be used as a protein loading control. Each blot contained at least 3 samples from each treatment group i.e., samples from EB, 8-OH-DPAT and vehicle-treated rats. Gαz-bound RGSZ1 protein levels for each sample were first normalized to the Gαz protein level as a loading control and then the samples in the EB and 8-OH-DPAT treatment groups were compared to the vehicle-treated samples on that blot. Each sample was run in triplicate and the mean of the normalized values for each sample was used for statistical comparisons.
2.5 Data Analysis
Hormone data were analyzed using 2- or 3-way ANOVA followed by Newman Keuls post-hoc tests. The protein levels were analyzed by comparing the vehicle and 8-OH-DPAT treated groups or the vehicle and EB treated groups using 1-way ANOVA via the Statview® statistical software package.
3. EXPERIMENTAL PROCEDURES
Female Sprague-Dawley rats (200–225g) were purchased from Harlan Laboratories (Indianapolis, IN). Animals were housed two per cage in a temperature-, humidity-, and light- controlled room (12 hr light/dark cycle). Food and water were available ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and as approved by the Loyola University School of Medicine Institutional Animal Care and Use Committee. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize available alternatives to in vivo techniques. Rats were ovariectomized and given 5 days to recover before drug treatments were initiated.
Two 17β-estradiol-3-benzoate (EB) dose-response experiments were performed, one in a low dose range and a second experiment in a higher dose range of EB. In the low dose EB dose-response experiment, rats were treated with 0.2, 2.0 or 10 μg/kg/day EB or the sesame oil vehicle s.c. for 2 days. In the high dose EB dose-response experiment, rats were treated with 10, 40 or 80 μg/kg/day EB or the sesame oil vehicle s.c. for 2 days. The next day, a challenge injection of (+)8-OH-DPAT (40 or 200 μg/kg) or saline (0.9%) was given s.c. and 15 minutes later rats were sacrificed by decapitation. Trunk blood was collected and stored at −80°C.
A time course experiment was performed to determine whether a single dose of EB would result in changes in the hormone response to (+)8-OH-DPAT. Rats were treated with 10 μg/kg EB s.c. 1, 2, 4, or 24 hours before a challenge dose of 200 μg/kg (+)8-OH-DPAT or saline (0.9%) s.c. and rats were decapitated 5 minutes later. Trunk blood was collected and stored at −80°C.
In the last experiment, each rat received daily injections of EB (10 μg/kg) or vehicle (sesame oil) s.c. for 2 days. On the third day, a challenge injection of 200 μg/kg (+)8-OH-DPAT or saline (0.9%) was given s.c. and 15 minutes later rats were sacrificed by decapitation. Trunk blood was collected and stored at −80°C. Brains were removed and stored at −80°C.
4. RESULTS
4.1 Hormone Response to 8-OH-DPAT
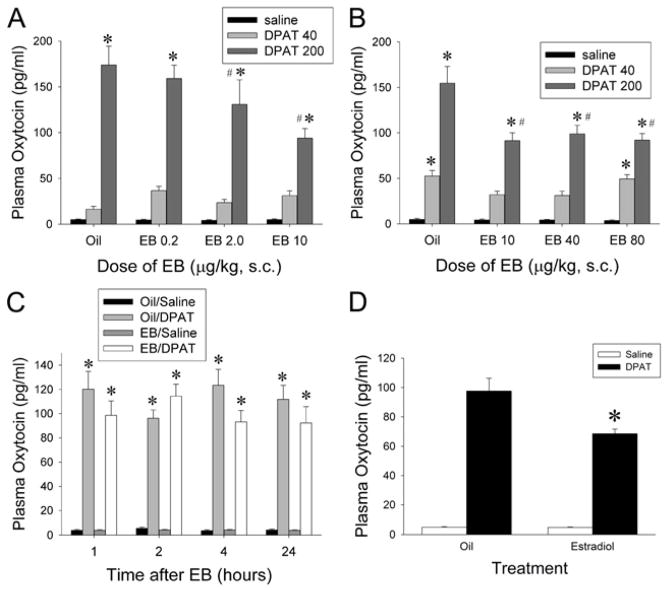
Dose-response experiments were conducted to determine the dose of EB that would result in maximal effects on 5-HT1A receptor signaling in the PVN. In the low dose dose-response experiment, rats were treated with 0.2, 2.0 or 10 μg/kg/day of EB or vehicle for two days and then challenged with 40 or 200 μg/kg 8-OH-DPAT or vehicle. Basal concentrations of oxytocin were not significantly affected by any of the doses of EB (Figure 1A). Although 40 μg/kg (+)8-OH-DPAT did not significantly alter plasma oxytocin concentrations, administration of 200 μg/kg of(+)8-OH-DPAT significantly increased plasma oxytocin levels in all of the treatment groups. Treatment with 2.0 or 10 μg/kg/day of EB for 2 days significantly reduced the plasma oxytocin levels in rats challenged with 200 μg/kg (+)8-OH-DPAT compared to rats treated with oil for 2 days. Two-way ANOVA for plasma oxytocin indicated a significant effect of EB (F(3,81) = 2.93, p < 0.05), a significant effect of (+)8-OH-DPAT (F(2,81) = 166.4, p < 0.0001) and a significant interaction between EB and (+)8-OH-DPAT (F(6,81) = 3.7, p < 0.005).
Figure 1.
Oxytocin responses to (+)8-OH-DPAT stimulation following treatment with EB. A) Results from a low dose dose-response experiment demonstrate that 2.0 and 10.0 μg/kg/day EB, but not 0.2 μg/kg/day EB, causes desensitization of the oxytocin response to stimulation of 5-HT1A receptors. B) Results from the high dose dose-response experiment demonstrate that 10.0, 40 and 80 μg/kg/day EB for two days cause similar levels of partial desensitization of the oxytocin response to stimulation of 5-HT1A receptors. Treatment with 10.0 μg/kg/day EB for 2 days causes the maximal desensitization response; further increases in the dose of EB above 10.0 μg/kg/day do not induce further decreases in the oxytocin response to 5-HT1A receptor stimulation. C) Results from the time course experiment demonstrate that 1, 2, 4 or 24 hours after a single injection of EB the oxytocin response to stimulation of 5-HT1A receptors is not altered. D) We verified the desensitization of 5-HT1A receptor signaling after treatment with 10.0 μg/kg/day EB for two days in the rats used for the western blot and co-immunoprecipiatation assays by measuring the plasma oxytocin concentrations after challenge with either (+)8-OH-DPAT or vehicle control. The * indicates significantly different from the saline-challenged group receiving the same pre-treatment at p < 0.05, # indicates significantly different from the group treated with oil and challenged with (+)8-OH-DPAT at p < 0.05.
In the high dose-dose-response experiment, rats were treated with 10, 40 or 80 μg/kg/day of EB or vehicle for two days and then challenged with 40 or 200 μg/kg (+)8-OH-DPAT or vehicle. Treatment with EB did not significantly change the baseline levels of oxytocin (Figure 1B). The challenge injection of 200 μg/kg (+)8-OH-DPAT significantly increased the levels of oxytocin in the plasma. Injection of 40 μg/kg 8-OH-DPAT significantly increased the levels of oxytocin in the oil and 80 μg/kg EB treatment groups but not the groups of rats treated with 10 or 40 μg/kg EB. Treatment with either 10, 40 or 80 μg/kg/day of EB significantly reduced the increase in plasma oxytocin levels in response to 200 μg/kg (+)8-OH-DPAT by approximately 40% compared to vehicle control. Two-way ANOVA demonstrated a significant main effect of EB (F(3,71) = 7.81, p = 0.001), and (+)8-OH-DPAT (F(2,71) = 171.8, p < 0.001), and a significant interaction between EB and (+)8-OH-DPAT (F(6,71) = 4.73, p < 0.005).
In the time course experiment, rats were treated with a single injection of 10 μg/kg of EB or oil and then given a challenge injection of 200 μg/kg (+)8-OH-DPAT or saline 1, 2, 4, or 24 hours later. EB did not significantly alter the baseline levels of oxytocin at any of the time points examined (Figure 1C). Administration of 200 μg/kg of (+)8-OH-DPAT significantly increased plasma oxytocin levels in all of the treatment groups. A single injection of 10 μg/kg of EB at any of the time points examined did not significantly change the increase in plasma oxytocin levels in response to the challenge injection of (+)8-OH-DPAT. A three-way ANOVA demonstrated a significant main effect of the (+)8-OH-DPAT injection (F(1,113) = 595.8, p < 0.001), but no significant effects of EB (F(1,113) = 2.67, p = 0.10), or time (F(1,113) = 0.151, p = 0.93). There were also no significant interaction effects.
To verify the effect of 10 μg/kg/day of EB treatment for 2 days on 5-HT1A receptor function in the rats used to examine the effects of EB on Gαz and RGSZ1 protein levels and interaction, 10 μg/kg/day of EB or vehicle was given for two days and 18 hours after the last dose rats were challenged with 200 μg/kg (+)8-OH-DPAT or vehicle. Basal oxytocin levels did not differ between animals treated with vehicle and EB (Figure 1D). Administration of 200 μg/kg (+)8-OH-DPAT significantly increased plasma oxytocin levels 21-fold over baseline. EB treatment significantly diminished the increase in plasma oxytocin induced by (+)8-OH-DPAT. The two-way ANOVA for plasma oxytocin indicated a significant effect of (+)8-OH-DPAT (F(1,28) = 231, p < 0.0001), a significant effect of EB (F(1,28) = 8.10, p < 0.01) and a significant interaction between (+)8-OH-DPAT and EB (F(1,28) = 8.02, p = 0.01).
4.2 Effect of (+)8-OH-DPAT and EB on Gαz and RGSZ1 Protein Levels
Western blot analysis was used to examine the effects of EB and 8-OH-DPAT on Gαz and RGSZ1 protein levels in the PVN, hypothalamus without PVN, hippocampus and amygdala. After separation of the proteins, the proteins were transferred to PVDF membranes. The PVDF membranes were first examined with an antibody for Gαz and then were washed and re-probed using an antibody directed against RGSZ1. The membrane and cytosol fractions of these brain regions were analyzed separately on western blots. In membrane and cytosol fractions, a 35 kD Gαz protein and a 40 kD RGSZ1 protein band were analyzed. In the membrane fractions, a 55 kD RGSZ1 protein band was also measured; this band was present in the membrane fraction but rarely detectable in the cytosol fractions of these brain regions. Other RGSZ1 protein bands were detectable in these brain regions of rats in preliminary experiments on untreated rats. However, RGSZ1 protein bands such as the 45 kD band overlapped bands detected by the Gαz protein antibody which was used prior to the RGSZ1 antibody on the western blots prepared with tissue from EB- and (+)8-OH-DPAT-treated rats. The limited amount of tissue samples from these brain regions did not permit analysis of RGSZ1 and Gαz protein on separate western blots.
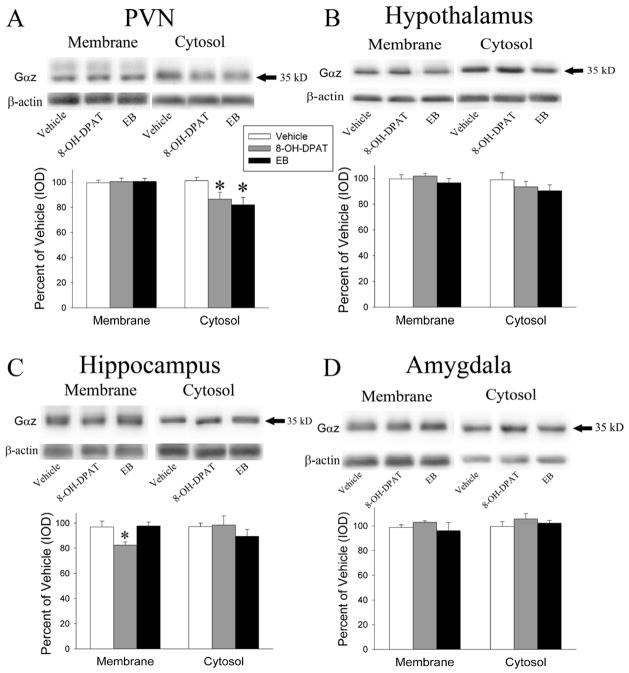
Two days of EB did not change Gαz levels in either the membrane or cytosol fractions of the hypothalamus, the hippocampus, and the amygdala or the membrane fraction of the PVN (Figure 2). However, EB treatment resulted in a significant decrease in Gαz protein levels in the cytosolic fraction of the PVN (F(1,13) = 9.78, p < 0.05).
Figure 2.
Representative images and quantitation of the Gαz protein examined on western blots. Gαz protein levels were examined in the membrane and cytosol fractions of the PVN(A), rest of the hypothalamus(B), hippocampus(C) and amygdala(D) after treatment with 10.0 μg/kg/day EB for 2 days, 15 minutes after 200 μg/kg (+)8-OH-DPAT, or vehicle. EB and (+)8-OH-DPAT caused a reduction in Gαz protein in the cytosol fraction of the PVN and in the membrane fraction of the hippocampus. The * indicates significantly different from the saline-treated group at p < 0.05.
Acute (+)8-OH-DPAT treatment significantly decreased Gαz protein levels in the cytosol fraction of the PVN (F(1,13) = 6.53, p < 0.05) and the membrane fraction of the hippocampus (F=5.68, p < 0.05) but did not significantly alter Gαz protein levels in the membrane or cytosol fraction of the hypothalamus and the amygdale, the membrane fraction of the PVN or the cytosol fraction of the hippocampus (Figure 2).
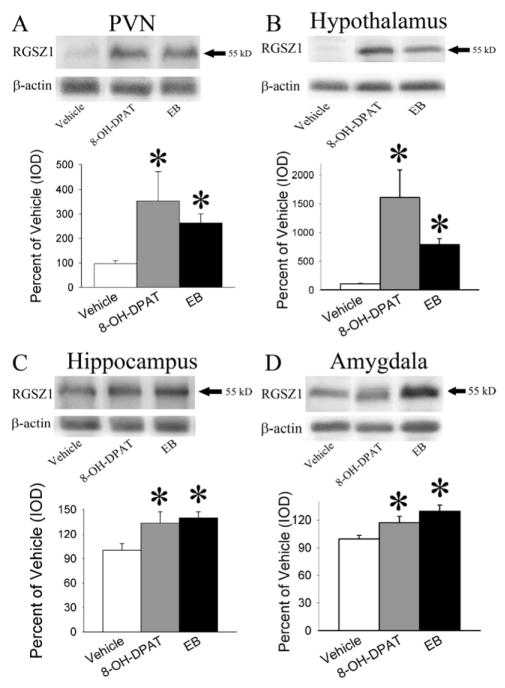
EB treatment resulted in a significant increase in the 55 kD RGSZ1 protein levels (Figure 3) in the membrane fraction of the hypothalamus (F(1,15) = 38.88, p < 0.001); hippocampus (F(1,13)= 11.75, p < 0.01), PVN (F(1,9) = 15.11, p < 0.01) and amygdala (F(1,14) = 14.25, p < 0.01). (+)8-OH-DPAT treatment also resulted in a significant increase in the 55 kD RGSZ1 protein levels in the membrane fraction of the hypothalamus (F(1,14) = 10.00, p < 0.01), hippocampus (F(1,12) = 5.06, p < 0.05), PVN (F(1,7) = 5.86, p < 0.05) and the amygdala (F(1,13) = 5.75, p < 0.05).
Figure 3.
Representative images and quantitation of the 55 kD RGSZ1 protein examined on western blots. The 55 kD RGSZ1 protein was measured in the membrane fraction of the PVN(A), rest of the hypothalamus(B), hippocampus(C) and amygdala(D) after treatment with 10.0 μg/kg/day EB for 2 days, 15 minutes after 200 μg/kg (+)8-OH-DPAT, or vehicle. Both EB and (+)8-OH-DPAT increased the levels of the 55kD RGSZ1 protein compared to vehicle controls in all of the brain regions examined. The * indicates significantly different from the saline-treated group at p < 0.05.
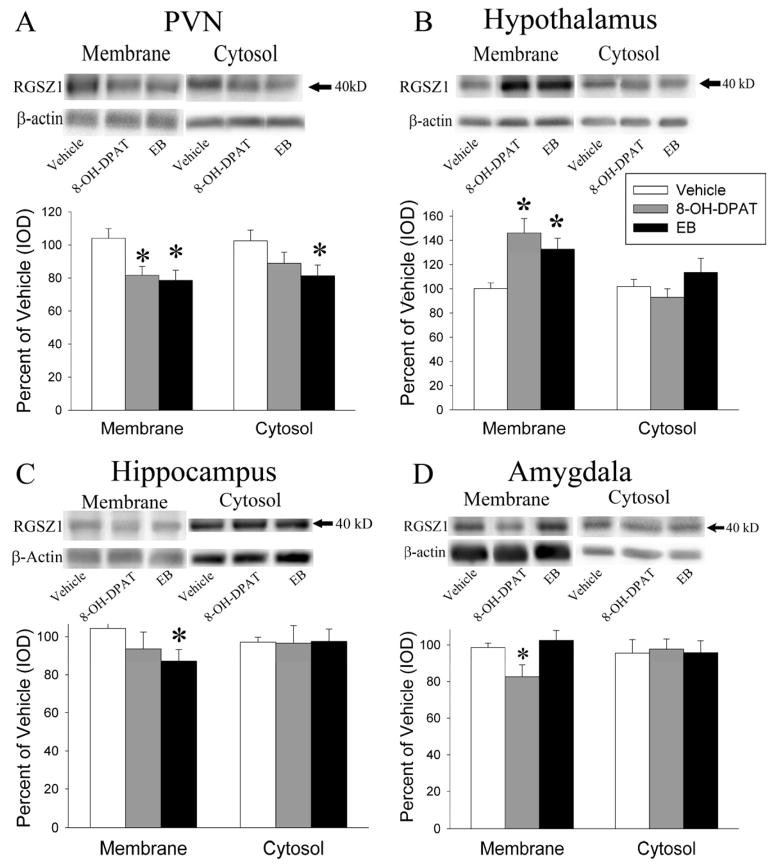
EB significantly increased the levels of the 40 kD RGSZ1 protein (Figure 4) only in the membrane fraction of the hypothalamus (F(1,15) = 10.58, p < 0.01). In contrast EB caused a significant decrease the levels of the 40 kD RGSZ1 protein in the membrane fraction of the hippocampus (F(1,13) = 5.78, p < 0.05) and the PVN (F(1,14) = 8.90, p < 0.01) and the cytosol fraction of the PVN (F(1,13) = 5.24, p < 0.05)
Figure 4.
Representative images and quantitation of the 40 kD RGSZ1 protein examined on western blots. The 40 kD RGSZ1 protein was examined in the membrane and cytosol fractions of the PVN(A), rest of the hypothalamus(B), hippocampus(C) and amygdala(D) after treatment with 10.0 μg/kg/day EB for 2 days, 15 minutes after 200 μg/kg (+)8-OH-DPAT, or vehicle. Each brain region examined displayed a unique pattern of changes in the expression of the 40 kD RGSZ1 protein. The * indicates significantly different from the saline-treated group at p < 0.05.
Acute (+)8-OH-DPAT treatment resulted in a significant increase in 40 kD RGSZ1 protein levels (Figure 4) in the membrane fraction of the hypothalamus (F(1,15)=14.13, p < 0.01) but a significant decrease in the membrane fraction of the PVN (F(1,14) = 7.90, p < 0.05) and the membrane fraction of the amygdala (F(1,12) = 5.24, p < 0.05)
4.3 Co-immunoprecipitation
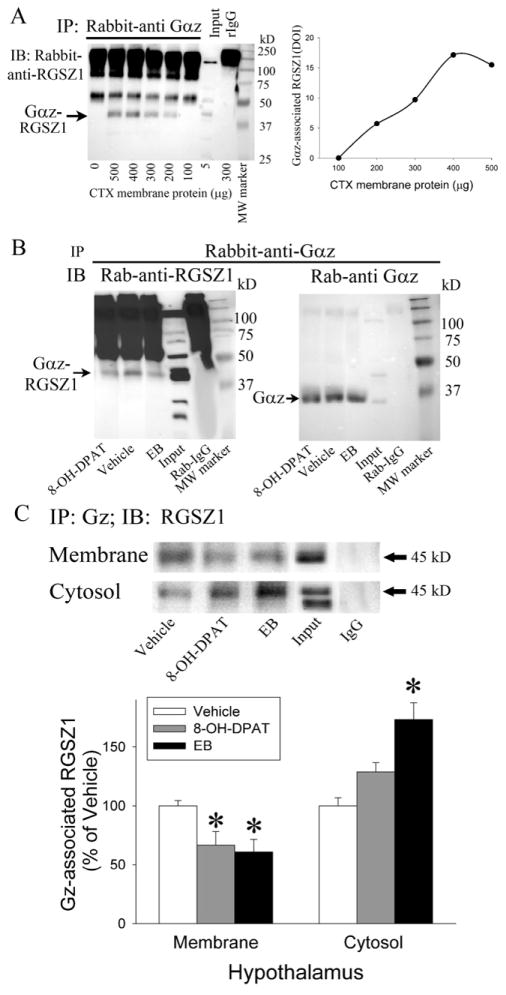
To examine the interaction of Gαz and RGSZ1, co-immunoprecipitation of Gαz and RGSZ1 was performed. To determine the linearity of the co-immunoprecipitation, five different starting protein amounts from 100 μg to 500 μg were used, as well as an antibody control without tissue homogenate. Gαz protein was immunoprecipitated from the membrane fraction of the frontal cortex and the proteins were examined on western blots using an RGSZ1 antibody. Two bands at 55 kD and 45 kD were detected on the western blots as well as a large dark area above 75 kD (Figure 5A). In a control sample, immunoprecipitation was performed without tissue homogenate, i.e., with only the Gαz antibody present. In this control sample, the large dark area above 75kD and a 55 kD band were detectable and assumed to be IgG protein from the Gαz antibody. Since the 55 kD IgG band obscures the ability to independently detect the 55 kD RGSZ1 protein band (Figure 5A), only the 45 kD band was analyzed in the co-immunoprecipitation assays. Immunoprecipitation of RGSZ1 with Gαz protein resulted in increases in the density of the RGSZ1 protein band with increasing amounts of starting protein in the samples from 100 μg to 400 μg of homogenate protein, however the assay reached a plateau with 500 μg of starting protein (Figure 5A). Since 200 μg of protein in the tissue homogenates are sufficient to co-immunoprecipitate Gαz and RGSZ1, 200 μg of protein was used in all further immunoprecipitation assays. To further verify the immunoprecipitation of Gαz protein and co-immunoprecipitation of RGSZ1, a control experiment was performed using the membrane fraction of the hippocampus (Figure 5B). After immunoprecipitation of Gαz protein, the proteins were examined on a western blot with the RGSZ1 antibody (Figure 5B right) and an antibody against Gαz (Figure 5B left).
Figure 5.
Co-immunoprecipitation of RGSZ1 with Gαz protein. A) A concentration response experiment was performed to determine the optimum amount of protein to use in the co-immunoprecipitation assays and to determine the linearity of the assay. B) A control experiment was performed to determine if RGSZ1 protein would co-immunoprecipitate with the Gαz protein. Proteins were immunoprecipitated using an antibody directed against Gαz protein (Rab-anti-Gαz) and western blots were performed using the RGSZ1 antibody (left, Rab-anti-RGSZ1) and the Gαz antibody (right). C) Representative image and quantitation of RGSZ1 protein co-immunoprecipitated with Gαz protein in the membrane and cytosol fractions of the PVN after treatment with 10.0 μg/kg/day EB for 2 days, 15 minutes after 200μg/kg (+)8-OH-DPAT, or vehicle. EB and (+)8-OH-DPAT decreased the association of RGSZ1 with Gαz protein in the membrane fraction and EB increased the association in the cytosol fraction of the PVN. The * indicates significantly different from the saline-treated group at p < 0.05. MW marker indicates the lane loaded with molecular weight standard markers, input indicates the lanes loaded with tissue homogenate not subjected to immunoprecipitation, IgG indicates the lanes loaded with homogenate immunoprecipitated with a non-specific rabbit IgG protein as a control.
4.4 Gαz and RGSZ1 interaction in the hypothalamus
Co-immunoprecipitation of Gαz and RGSZ1 was evaluated in both the membrane and cytosol fractions of the hypothalamus (excluding the PVN) after daily injections of EB for 2 days, acute (+)8-OH-DPAT injection or vehicle treatments (Fig. 5C). EB treatment caused a significant decrease in co-immunoprecipitation of Gαz and RGSZ1 in the membrane fraction of the hypothalamus (F(1,10) = 11.60, p < 0.01) and a significant increase in co-immunoprecipitation in the cytosol fraction of the hypothalamus (F(1,14) = 21.91, p < 0.001). Similarly, acute (+)8-OH-DPAT treatment significantly decreased co-immunoprecipitation of Gαz and RGSZ1 in the membrane (F(1,9) = 8.38, p < 0.05), but significantly increased in the cytosol (F(1,14) = 7.83, p < 0.05) of the hypothalamus.
5. DISCUSSION
Hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis is characteristic of depressed patients. Normalization of HPA axis responses coincides with clinical responsiveness to antidepressant medications including SSRIs such as fluoxetine and citalopram, and the non-selective tricyclic antidepressant imipramine (Gomez-Gil et al., 2010; Navines et al., 2007; Nikisch et al., 2005). For example, hormone responses to a challenge with the 5-HT1A receptor agonists buspirone or ipsapirone are reduced after chronic treatment with antidepressant therapy in clinical studies (Gomez-Gil et al., 2010; Lerer et al., 1999; Navines et al., 2007; Nikisch et al., 2005). This diminished responsiveness in the hormone response to a challenge dose of a 5-HT1A receptor agonist indicates that desensitization of 5-HT1A receptor signaling in the PVN can play an important role in the clinical response to antidepressant therapy. These clinical responses in human trials can be modeled in rodents. Treatment of rats with SSRIs for 7 to 14 days results in desensitization of the hormone responses to a challenge dose of a 5-HT1A receptor agonist (Li et al., 1997; Li et al., 1996) suggesting that this rat model can be used to examine other potential therapeutic approaches for depression as well as possible adjuvant therapies such as estrogens to reduce the response time to antidepressant treatments.
Our previous studies demonstrated that a partial desensitization of the oxytocin and ACTH responses to 5-HT1A receptor stimulation could be produced by two once daily injections of EB at doses of 2 μg/kg (Rossi et al., 2010), 10 μg/kg (Xu et al., 2009) and 10 μg/rat i.e., approximately 42 μg/kg (Raap et al., 2000). However, a direct comparison of the EB dose and the desensitization response had not been established. In the current study, we performed two dose response experiments, one using low doses of EB (0.2 – 10 μg/kg) and the other using higher doses (10 – 80 μg/kg). The results from these experiments demonstrate that EB is only capable of producing a partial desensitization of the oxytocin response to 5-HT1A receptor stimulation and this response reaches a maximum of approximately a 40% reduction at the 10 μg/kg dose. Further increases in the dose of EB did not result in further reductions in the plasma oxytocin concentrations induced by 5-HT1A receptor stimulation. These experiments also demonstrated that 2.0 μg/kg/day EB for 2 days resulted in a lower 25% reduction in the plasma concentrations of oxytocin induced by 5-HT1A receptor stimulation but 0.2 μg/kg EB had no significant effects on plasma oxytocin concentrations.
Although the data clearly demonstrate that two days of administration of EB is sufficient to produce a partial desensitization of the oxytocin response to 5-HT1A receptor stimulation, we did not know whether a single dose of EB was capable of producing desensitization. To address this question, we performed a time course experiment to determine whether a single dose of 10 μg/kg EB would produce desensitization of 5-HT1A receptor signaling in the PVN. A single dose of EB, examined from 1 to 24 hours after administration, did not significantly reduce the oxytocin response to 5-HT1A receptor stimulation. From this experiment, we can conclude that at least 2 days of administration of 10 μg/kg EB are necessary for desensitization of the oxytocin response to 5-HT1A receptor stimulation. However, it is also possible that a single higher dose of EB could produce desensitization of 5-HT1A receptor signaling after 24 hours and that a single dose of 10 μg/kg EB could produce a desensitization response after 48 hours.
The mechanisms underlying the EB-induced desensitization of 5-HT1A receptor signaling are beginning to be elucidated. Several studies have focused on identifying the estrogen receptor mediating the EB-induced desensitization of 5-HT1A receptor signaling in the PVN (Rossi et al., 2010; Xu et al., 2009). The results from these studies suggest that the G protein coupled estrogen receptor GPR30 (also known as GPER) but not estrogen receptor β in the PVN is necessary for EB-induced desensitization of 5-HT1A receptor signaling. Further studies are needed to determine if there is a role for estrogen receptor a in the desensitization of 5-HT1A receptor signaling in the PVN.
The impact of EB on components of the 5-HT1A receptor system in the PVN has been previously examined and was further examined in the current study. Previous studies demonstrated an increase in the expression of a 30kD RGSZ1 protein in the membrane fraction of the PVN (Carrasco et al., 2004) However, there was no change in the levels of Gαz in the membrane fraction of the PVN (D’Souza et al., 2004). Similar to our findings in the previous study, there was no change in the expression of Gαz in the membrane fraction of the PVN in the present study. However, in the current study, we also examined the cytosol fraction of the PVN and now found that the expression of Gαz was decreased by EB treatment. In the present study, we examine the effects of EB on two RGSZ1 isoforms, a 40kD and a 55kD protein. EB reduced the 40kD RGSZ1 protein in both the membrane and cytosol fraction of the PVN by approximately 20% but increased the 55kD RGSZ1 protein in the membrane fraction to over 250% of control values for a net increase in the expression of these RGSZ1 proteins in the membrane of the PVN. This increase in RGSZ1 in the membrane of the PVN could play a role in the desensitization response via the GTPase accelerating protein (GAP) function of RGS proteins.
The RGS20 gene encodes both RGSZ1 and Ret RGS (Barker et al., 2001). Multiple start sites for transcription of RGSZ1 likely contribute to the heterogeneity in protein size in the lower molecular weight range (Barker et al., 2001) and could account for the multiple protein bands between 25 and 30 kD identified in rat brain tissue on western blots. RGSZ1 undergoes multiple posttranslational modifications including N- and O-linked glycosylation (Garzon et al., 2004), sumoylation by sumo1 and sumo2 and serine phosphorylation (Rodriguez-Munoz et al., 2007) which contribute to the presence of multiple higher molecular weight bands detected on western blots. RGSZ1 contains a cysteine string region which is a likely region for palmitoylation and regulation of membrane association (Barker et al., 2001; De Vries et al., 1996). The post-translational modifications resulting in the 40kD and 55kD RGSZ1 protein bands are unknown but since the 55kD band predominantly exists in the membrane fraction, modifications to the 55kD band are likely to include those that increase hydrophobicity and thereby membrane association such as palmitoylation. Further studies are needed to determine alternative mRNA splicing, post-translational modifications, dimer or polymer formation and the functional differences among these isoforms of RGSZ1.
Acute (+)8-OH-DPAT treatment resulted in a similar pattern of changes in the expression of Gαz and RGSZ1 in the PVN, though several of the changes that resulted from EB treatment did not reach significance with (+)8-OH-DPAT treatment. The most striking effect of (+)8-OH-DPAT was an over 350% increase in the 55kD RGSZ1 protein which could contribute to a possible tachypylaxis response in the 5-HT1A receptor signaling system.
In the other brain regions examined, the 55kD RGSZ1 protein also increased with EB or (+)8-OH-DPAT treatment; the magnitude of the increases were large in the rest of the hypothalamus, similar to the PVN but were much more modest in the hippocampus and amygdala. In the rest of the hypothalamus, hippocampus and amygdala, there were no effects of either EB or (+)8-OH-DPAT on Gαz protein and there were region specific changes in the expression of the 40kD RGSZ1 protein.
We examined the binding of RGSZ1 to Gαz following treatment with either EB or (+)8-OH-DPAT in the hypothalamus (without the PVN). Both treatments resulted in decreased binding in the membrane fraction and increased binding in the cytosol fraction of the hypothalamus. RGSZ proteins bind to active Gα proteins and transition state Gα proteins in contrast to other RGS proteins which only bind to the transition state (Wang et al., 1997). Binding of RGSZ1 to activated Gαz could act to sequester the activated Gαz from down-stream effectors resulting in effector antagonism in the membrane fraction. The decreased binding of RGSZ1 to Gαz in the membrane fraction of the hypothalamus could result in increased responsiveness to 5-HT1A receptor stimulation. Although there is a rich literature demonstrating the effects of RGSZ1 on opioid receptor signaling, the effects of 5-HT1A receptor signaling (Ghavami et al., 2004) are not as well documented. Stimulation of mu opioid receptors rapidly (within 45min) increases binding of RGSZ2 to Gαi2 in synaptic membrane (Sanchez-Blazquez et al., 2010). Similarly, the sequestration of receptor activated Gαz by RGSZ2 proteins was thought to contribute to desensitization of mu opioid receptors (Garzon et al., 2005). Since there is both a decrease in the binding of RGSZ1 to Gαz which could increase signaling and an large increase in the amount of the 55kD and 40 kD RGSZ1 in the membrane fraction after treatment with EB or (+)8-OH-DPAT, which could decrease signaling, the net effect on 5-HT1A receptor- Gαz signaling in the hypothalamus (without the PVN) is unclear.
5.1 Conclusions
The present study provided important information regarding the dose and time course of EB administration that produces maximal effects on desensitization of 5-HT1A receptor signaling in the PVN. Since normalization of HPA axis responses especially to 5-HT1A receptor stimulation coincides with anti-depressive responses clinically, a brief initial adjuvant treatment with EB in combination with SSRIs has the potential to induce a more rapid clinical response especially for the treatment of post-menopausal depression and should be tested in this rat model. Estrogen clearly plays an important role in depression and the treatment of depression. Understanding the mechanisms underlying the more rapid estrogen-dependent desensitization of 5-HT1A receptor signaling in the PVN may also lead to the identification of novel targets for the treatment of depression. The additional changes in RGSZ1 protein with EB treatment identified in the current study further strengthen the importance of this protein as a target for the treatment of depression. Further studies are needed to clarify the role of RGSZ1 protein isoforms in 5-HT1A receptor signaling and their involvement in the mechanisms by which estrogen impacts on receptor systems mediating depression and anti-depressive responses.
Highlights.
2 once daily doses of EB is the minimum to induce desensitization of 5-HT1A receptors
10 μg/kg/day EB produces the Emax response partial desensitization of 5-HT1A receptors
EB greatly increased a 55 kD membrane-associate RGSZ1 protein in hypothalamus
EB increased a 55 kD membrane-associate RGSZ1 protein in amygdala & hippocampus
EB is a candidate for adjuvant therapy with SSRIs to hasten the therapeutic response
Acknowledgments
This work was supported by a grant, R01MH058448, to NAM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barker SA, Wang J, Sierra DA, Ross EM. RGSZ1 and Ret RGS: two of several splice variants from the gene RGS20. Genomics. 2001;78:223–229. doi: 10.1006/geno.2001.6659. [DOI] [PubMed] [Google Scholar]
- Bosker FJ, Cremers TI, Jongsma ME, Westerink BH, Wikstrom HV, den Boer JA. Acute and chronic effects of citalopram on postsynaptic 5-hydroxytryptamine(1A) receptor-mediated feedback: a microdialysis study in the amygdala. J Neurochem. 2001;76:1645–1653. doi: 10.1046/j.1471-4159.2001.00194.x. [DOI] [PubMed] [Google Scholar]
- Bromberger JT, Kravitz HM, Chang YF, Cyranowski JM, Brown C, Matthews KA. Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN) Psychol Med. 2011;41:1879–1888. doi: 10.1017/S003329171100016X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco GA, Barker SA, Zhang Y, Damjanoska KJ, Sullivan NR, Garcia F, D’Souza DN, Muma NA, Van de Kar LD. Estrogen treatment increases the levels of regulator of G protein signaling-Z1 in the hypothalamic paraventricular nucleus: possible role in desensitization of 5-hydroxytryptamine(1A) receptors. Neuroscience. 2004;127:261–267. doi: 10.1016/j.neuroscience.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63:385–390. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- D’Souza DN, Zhang Y, Damjanoska KJ, Carrasco GA, Sullivan NR, Garcia F, Battaglia G, Doncarlos LL, Muma NA, Van de Kar LD. Estrogen reduces serotonin-1A receptor-mediated oxytocin release and Galpha(i/o/z) proteins in the hypothalamus of ovariectomized rats. Neuroendocrinology. 2004;80:31–41. doi: 10.1159/000080795. [DOI] [PubMed] [Google Scholar]
- De Vries L, Elenko E, Hubler L, Jones TL, Farquhar MG. GAIP is membrane-anchored by palmitoylation and interacts with the activated (GTP-bound) form of G alpha i subunits. Proc Natl Acad Sci U S A. 1996;93:15203–15208. doi: 10.1073/pnas.93.26.15203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Camarena E, Fernandez-Guasti A, Lopez-Rubalcava C. Participation of the 5-HT1A receptor in the antidepressant-like effect of estrogens in the forced swimming test. Neuropsychopharmacology. 2006a;31:247–255. doi: 10.1038/sj.npp.1300821. [DOI] [PubMed] [Google Scholar]
- Estrada-Camarena E, Lopez-Rubalcava C, Fernandez-Guasti A. Facilitating antidepressant-like actions of estrogens are mediated by 5-HT1A and estrogen receptors in the rat forced swimming test. Psychoneuroendocrinology. 2006b;31:905–914. doi: 10.1016/j.psyneuen.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Garzon J, Rodriguez-Munoz M, de la Torre-Madrid E, Sanchez-Blazquez P. Effector antagonism by the regulators of G protein signalling (RGS) proteins causes desensitization of mu-opioid receptors in the CNS. Psychopharmacology (Berl) 2005;180:1–11. doi: 10.1007/s00213-005-2248-9. [DOI] [PubMed] [Google Scholar]
- Garzon J, Rodriguez-Munoz M, Lopez-Fando A, Garcia-Espana A, Sanchez-Blazquez P. RGSZ1 and GAIP regulate mu- but not delta-opioid receptors in mouse CNS: role in tachyphylaxis and acute tolerance. Neuropsychopharmacology. 2004;29:1091–1104. doi: 10.1038/sj.npp.1300408. [DOI] [PubMed] [Google Scholar]
- Ghavami A, Hunt RA, Olsen MA, Zhang J, Smith DL, Kalgaonkar S, Rahman Z, Young KH. Differential effects of regulator of G protein signaling (RGS) proteins on serotonin 5-HT1A, 5-HT2A, and dopamine D2 receptor-mediated signaling and adenylyl cyclase activity. Cell Signal. 2004;16:711–721. doi: 10.1016/j.cellsig.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Glick JL, Meigs TE, Miron A, Casey PJ. RGSZ1, a G z -selective regulator of G protein signaling whose action is sensitive to the phosphorylation state of G z à. Journal of Biological Chemistry. 1998;273:26008–26013. doi: 10.1074/jbc.273.40.26008. [DOI] [PubMed] [Google Scholar]
- Gomez-Gil E, Navines R, Martinez De Osaba MJ, Diaz-Ricart M, Escolar G, Salamero M, Martin-Santos R, Galan A, Gasto C. Hormonal responses to the 5-HT1A agonist buspirone in remitted endogenous depressive patients after long-term imipramine treatment. Psychoneuroendocrinology. 2010;35:481–489. doi: 10.1016/j.psyneuen.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Lerer B, Gelfin Y, Gorfine M, Allolio B, Lesch KP, Newman ME. 5-HT 1A receptor function in normal subjects on clinical doses of fluoxetine: Blunted temperature and hormone responses to ipsapirone challenge. Neuropsychopharmacology. 1999;20:628–639. doi: 10.1016/S0893-133X(98)00106-7. [DOI] [PubMed] [Google Scholar]
- Li Q, Levy AD, Cabrera TM, Brownfield MS, Battaglia G, Van de Kar LD. Long-term fluoxetine, but not desipramine, inhibits the ACTH and oxytocin responses to the 5-HT 1A agonist 8-OH-DPAT in male rats. Brain Res. 1993;630:148–156. doi: 10.1016/0006-8993(93)90652-4. [DOI] [PubMed] [Google Scholar]
- Li Q, Muma NA, Battaglia G, Van de Kar LD. A desensitization of hypothalamic 5-HT1A receptors by repeated injections of paroxetine: reduction in the levels of G(i) and G(o) proteins and neuroendocrine responses, but not in the density of 5-HT1A receptors. J Pharmacol Exp Ther. 1997;282:1581–1590. [PubMed] [Google Scholar]
- Li Q, Muma NA, Van de Kar LD. Chronic fluoxetine induces a gradual desensitization of 5-HT 1A receptors: reductions in hypothalamic and midbrain G i and G o proteins and in neuroendocrine responses to a 5-HT 1A agonist. Journal of Pharmacology and Experimental Therapeutics. 1996;279:1035–1042. [PubMed] [Google Scholar]
- Mize AL, Alper RH. Acute and long-term effects of 17beta-estradiol on G(i/o) coupled neurotransmitter receptor function in the female rat brain as assessed by agonist-stimulated [35S]GTPgammaS binding. Brain Res. 2000;859:326–333. doi: 10.1016/s0006-8993(00)01998-3. [DOI] [PubMed] [Google Scholar]
- Mize AL, Poisner AM, Alper RH. Estrogens act in rat hippocampus and frontal cortex to produce rapid, receptor-mediated decreases in serotonin 5-HT(1A) receptor function. Neuroendocrinology. 2001;73:166–174. doi: 10.1159/000054633. [DOI] [PubMed] [Google Scholar]
- Navines R, Martin-Santos R, Gomez-Gil E, Martinez de Osaba MJ, Imaz ML, Gasto C. Effects of citalopram treatment on hypothermic and hormonal responses to the 5-HT1A receptor agonist buspirone in patients with major depression and therapeutic response. Psychoneuroendocrinology. 2007;32:411–416. doi: 10.1016/j.psyneuen.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Nikisch G, Mathe AA, Czernik A, Thiele J, Bohner J, Eap CB, Agren H, Baumann P. Long-term citalopram administration reduces responsiveness of HPA axis in patients with major depression: relationship with S-citalopram concentrations in plasma and cerebrospinal fluid (CSF) and clinical response. Psychopharmacology (Berl) 2005;181:751–760. doi: 10.1007/s00213-005-0034-3. [DOI] [PubMed] [Google Scholar]
- Osei-Owusu P, Sullivan NR, Van de Kar LD, Scrogin KE. 5-HT1A receptors in the paraventricular nucleus of the hypothalamus mediate endocrine and behavioral but not cardiovascular responses to 8-OH-DPT. FASEB J. 2003;17:A445–A445. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 2007. [Google Scholar]
- Raap DK, DonCarlos LL, Garcia F, Muma NA, Wolf WA, Battaglia G, Van de Kar LD. Estrogen desensitizes 5-HT 1A receptors and reduces levels of G z, G i1 and G i3 proteins in the hypothalamus. Neuropharmacology. 2000;39:1823–1832. doi: 10.1016/s0028-3908(99)00264-6. [DOI] [PubMed] [Google Scholar]
- Rasgon NL, Altshuler LL, Fairbanks LA, Dunkin JJ, Davtyan C, Elman S, Rapkin AJ. Estrogen replacement therapy in the treatment of major depressive disorder in perimenopausal women. J Clin Psychiatry. 2002;63(Suppl 7):45–48. [PubMed] [Google Scholar]
- Rasgon NL, Dunkin J, Fairbanks L, Altshuler LL, Troung C, Elman S, Wroolie TE, Brunhuber MV, Rapkin A. Estrogen and response to sertraline in postmenopausal women with major depressive disorder: a pilot study. J Psychiatr Res. 2007;41:338–343. doi: 10.1016/j.jpsychires.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Munoz M, Bermudez D, Sanchez-Blazquez P, Garzon J. Sumoylated RGS-Rz proteins act as scaffolds for Mu-opioid receptors and G-protein complexes in mouse brain. Neuropsychopharmacology. 2007;32:842–850. doi: 10.1038/sj.npp.1301184. [DOI] [PubMed] [Google Scholar]
- Rossi DV, Dai Y, Thomas P, Carrasco GA, DonCarlos LL, Muma NA, Li Q. Estradiol-induced desensitization of 5-HT1A receptor signaling in the paraventricular nucleus of the hypothalamus is independent of estrogen receptor-beta. Psychoneuroendocrinology. 2010;35:1023–1033. doi: 10.1016/j.psyneuen.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Blazquez P, Rodriguez-Munoz M, Garzon J. Mu-opioid receptors transiently activate the Akt-nNOS pathway to produce sustained potentiation of PKC-mediated NMDAR-CaMKII signaling. PLoS One. 2010;5:e11278. doi: 10.1371/journal.pone.0011278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, Rubinow DR. Estrogen replacement in perimenopause-related depression: A preliminary report. American Journal of Obstetrics and Gynecology. 2000;183:414–420. doi: 10.1067/mob.2000.106004. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393–399. [PubMed] [Google Scholar]
- Schneider LS, Small GW, Hamilton SH, Bystritsky A, Nemeroff CB, Meyers BS. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97–106. [PubMed] [Google Scholar]
- Serres F, Li Q, Garcia F, Raap DK, Battaglia G, Muma NA, Van de Kar LD. Evidence that G z proteins couple to hypothalamic 5-HT 1A receptors in vivo. J Neurosci. 2000;20:3095–3103. doi: 10.1523/JNEUROSCI.20-09-03095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Li H, Meller E. Repeated treatment with antidepressants differentially alters 5-HT 1A agonist-stimulated [35 S]GTPgammaS binding in rat brain regions. Neuropharmacology. 2002;42:1031–1038. doi: 10.1016/s0028-3908(02)00064-3. [DOI] [PubMed] [Google Scholar]
- Silva M, Veiga I, Ribeiro FR, Vieira J, Pinto C, Pinheiro M, Mesquita B, Santos C, Soares M, Dinis J, Santos L, Lopes P, Afonso M, Lopes C, Teixeira MR. Chromosome copy number changes carry prognostic information independent of KIT/PDGFRA point mutations in gastrointestinal stromal tumors. BMC Med. 2010;8:26. doi: 10.1186/1741-7015-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares CD, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women - A double-blind, randomized, placebo-controlled trial. Archives of General Psychiatry. 2001;58:529–534. doi: 10.1001/archpsyc.58.6.529. [DOI] [PubMed] [Google Scholar]
- Soares CN. Can depression be a menopause-associated risk? BMC Med. 2010;8:79. doi: 10.1186/1741-7015-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uphouse L, Caldarola-Pastuszka M, Maswood S, Andrade M, Moore N. Estrogen-progesterone and 8-OH-DPAT attenuate the lordosis-inhibiting effects of the 5-HT1A agonist in the VMN. Brain Res. 1994;637:173–180. doi: 10.1016/0006-8993(94)91230-0. [DOI] [PubMed] [Google Scholar]
- Vicentic A, Li Q, Battaglia G, Van de Kar LD. WAY-100635 inhibits 8-OH-DPAT-stimulated oxytocin, ACTH and corticosterone, but not prolactin secretion. Eur J Pharmacol. 1998;346:261–266. doi: 10.1016/s0014-2999(97)01607-5. [DOI] [PubMed] [Google Scholar]
- Wang J, Ducret A, Tu Y, Kozasa T, Aebersold R, Ross EM. RGSZ1, a Gz-selective RGS protein in brain. Structure, membrane association, regulation by Galphaz phosphorylation, and relationship to a Gz gtpase-activating protein subfamily. J Biol Chem. 1998;273:26014–26025. doi: 10.1074/jbc.273.40.26014. [DOI] [PubMed] [Google Scholar]
- Wang J, Tu YP, Woodson J, Song XL, Ross EM. A GTPase-activating protein for the G protein Gà z - Identification, purification, and mechanism of action. Journal of Biological Chemistry. 1997;272:5732–5740. doi: 10.1074/jbc.272.9.5732. [DOI] [PubMed] [Google Scholar]
- Xu H, Qin S, Carrasco GA, Dai Y, Filardo EJ, Prossnitz ER, Battaglia G, Doncarlos LL, Muma NA. Extra-nuclear estrogen receptor GPR30 regulates serotonin function in rat hypothalamus. Neuroscience. 2009;158:1599–1607. doi: 10.1016/j.neuroscience.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]