Abstract
We evaluated safety and feasibility of the transvenous limb perfusion gene delivery method in muscular dystrophy. A dose escalation study of single limb perfusion with 0.9% saline starting with 5% of limb volume was carried out in adults with muscular dystrophies under intravenous analgesia/anesthesia. Cardiac, vascular, renal, muscle, and nerve functions were monitored. A tourniquet was placed above the knee with inflated pressure of 310 mm Hg. Infusion was carried out with a clinically approved infuser via an intravenous catheter inserted in the saphenous vein with a goal infusion rate of 80 ml/minute. Infusion volume was escalated stepwise to 20% limb volume in seven subjects. No subject complained of any post procedure pain other than due to needle punctures. Safety warning boundaries were exceeded only for transient depression of limb tissue oximetry and transient elevation of muscle compartment pressures; these were not associated with nerve, muscle, or vascular damage. Muscle magnetic resonant imaging (MRI) demonstrated fluid accumulation in muscles of the perfused lower extremity. High-pressure retrograde transvenous limb perfusion with saline up to 20% of limb volume at above infusion parameters is safe and feasible in adult human muscular dystrophy. This study will serve as a basis for future gene transfer clinical trials.
Introduction
Muscular dystrophies are a heterogeneous group of inherited disorders characterized by progressive muscle degeneration. No treatments have been shown to halt or reverse disease progression, despite substantial progress in understanding the genetic and molecular bases of these diseases.1 A variety of therapeutic options are in development. These include stem cell transfer and gene transfer or gene regulation using both viral vectors and nonviral delivery of plasmids, oligonucleotides, and RNAs. In human studies of muscular dystrophy, delivery of these therapeutic macromolecules to skeletal muscles has been limited to direct intramuscular injection.2,3,4 In experimental animals, high-pressure hydrodynamic retrograde transvenous limb perfusion has been successfully used to regionally deliver nucleic acids into skeletal muscles with subsequent protein expression.5,6,7,8,9,10,11 However, translating this promising gene delivery technique to human muscular dystrophy requires solving multiple logistical problems separate from the specific therapeutic agent, including vascular access, larger infusion volumes, and analgesia as well as safety and tolerability. To address these issues, we conducted a dose escalation study of high-pressure transvenous perfusion with 0.9% saline in the lower extremities of adult patients with muscular dystrophy.
Results
Seven subjects, aged 21–38 years, were studied. Five were female and two were male. Two had limb-girdle muscular dystrophy (LGMD) 2A, two had Emery-Dreifuss muscular dystrophy (EDMD) and one each had autosomal recessive LGMD, autosomal dominant LGMD and Becker muscular dystrophy. Subject 05, who received 20% limb perfusion, had prolonged elevated compartment pressures (details below) and the dose escalation was stopped at this level. Two subsequent subjects (06 and 07) received 20% limb perfusion and magnetic resonant imaging (MRI) studies to assess the efficacy and distribution of fluid delivery to muscles.
The perfusion procedure
Perfusion. Table 1 details the specific perfusion parameters. All subjects had visible limb volume expansion, firmness to touch, and enlargement of mid-calf circumference (Figure 1). There was no cardiac dysfunction or signs of systemic fluid overload as measured by echocardiogram, electrocardiogram, heart rate, respiratory rate, or cutaneous oxygen saturation. Subject 01 was the only patient who did not receive deep sedation, intravenous analgesia/anesthesia (IVA), thus she was conscious during the perfusion (~4 minutes). She commented that “the perfusion was quite uncomfortable, but tolerable”. The remaining subjects (02–07) received IVA without need for intubation. None reported discomfort or remembered the perfusion after recovering from anesthesia (~10–15 minutes postperfusion). Three subjects commented that they felt the perfused leg was little “tight” up to 1–2 hours postperfusion. Subject 05 had petechiae under and just below the tourniquet site which resolved by 3 days at the postperfusion visit. Although the original plan was to monitor each subject for up to 24 hours, all seven subjects had returned to baseline within 4 hours postperfusion requiring only acetaminophen or ibuprofen for pain.
Table 1. Infusion parameters.
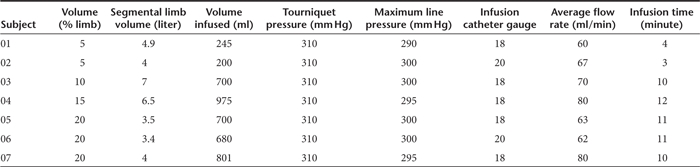
Figure 1.
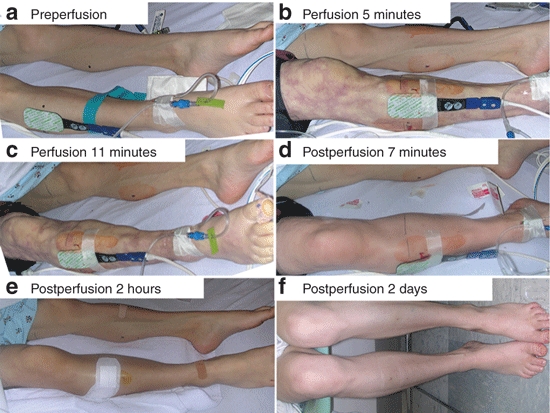
Photographs of subject 05.
Compartment pressure and tissue oxygen saturation monitoring. The first three subjects (5–10% of limb volume) did not have elevated compartment pressures. Subjects 04–07 had variable degree of elevation of compartment pressures. Compartment pressures return below the safety cut-off of 35 mm Hg within 10 minutes postperfusion in all subjects other than subject 05. Subject 05 (20% limb volume) had persistently elevated compartment pressures above 35 mm Hg with times to recovery below this value of 80, 30, and 10 minutes for the lateral, posterior, and anterior compartments respectively. During this time, tissue oxygen saturation was not reduced and she reported no pain.
During perfusion, subjects 06 and 07 (both 20% limb volume) showed peak compartment pressures of 155 and 144 mm Hg respectively. Pressures started to decrease immediately after the perfusion and returned to normal within 10 minutes (Figure 2a). Limb tissue oxygen saturation measurements showed a marked decline at the end of the perfusion, but rapidly recovered after tourniquet release and then rebounded above baseline (Figure 2b).
Figure 2.
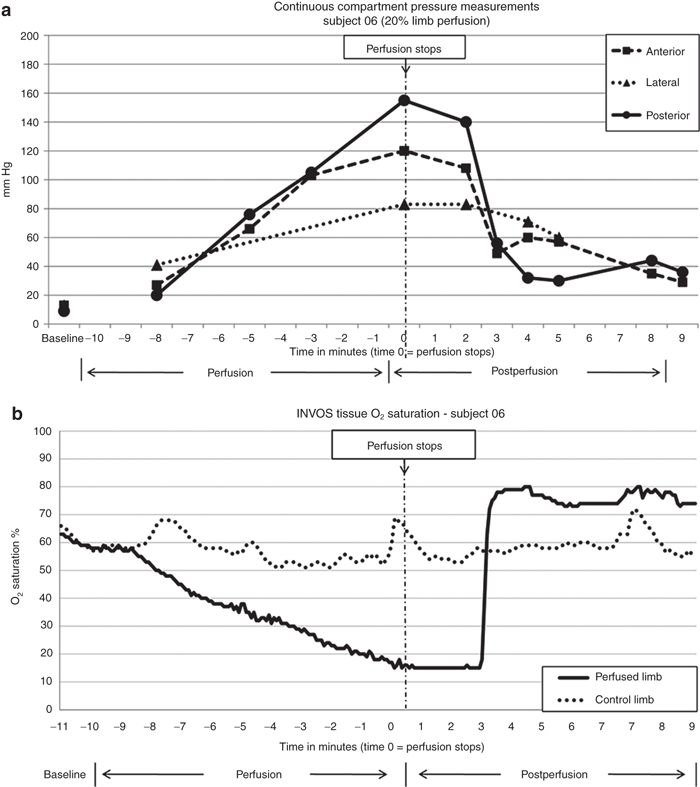
Compartment pressure and limb tissue oxygen saturation monitoring in subject 06. (a) Continuous compartment pressure monitoring. Note that the compartment pressure peaked at the end of perfusion and started to recover after the perfusion stopped while tourniquet was still inflated for 1 minute. The pressures rapidly recovered once the tourniquet was deflated. (b) continuous INVOS tissue oxygen saturation monitoring. The device has a lower reading limit of 15%. Note the rapid recovery to baseline was within 5 minutes postperfusion.
Preperfusion and postperfusion studies
None of the postperfusion assessments showed any detectable injuries related to perfusion. All subjects returned to baseline except for healing needle puncture sites at the postperfusion visit and all subjects have had telephone follow-up within 2 weeks of postperfusion. There were no reported problems in the perfused leg. No serious adverse events occurred during this study.
Laboratory studies. The range of fluctuation in serum creatine kinase (CK) was consistent with normal variation described in muscular dystrophies attributed to normal activity.12,13 Subjects 02 and 06 each had one occasion when a potassium level was more than 0.5 mmol/l higher than baseline. Each was a difficult blood draw and there were no associated electrocardiogram changes. The levels were within normal limits on repeated testing within ten minutes and were attributed to a partially hemolyzed blood samples.
Venous and arterial Doppler study. Subject 03 had pre-existing venous insufficiency that did not worsen (Supplementary Table S1). All other subjects had normal venous and arterial studies preperfusion and postperfusion.
Muscle strength test and timed walk. There was no decrease in the distance of the timed walk (Supplementary Table S1). Subject 03 had decreased muscle strength bilaterally, 54–60% decline in hip extension and hip adduction in the perfused leg (Supplementary Table S1) and 57–65% decline in the control leg (control leg data was not included in Supplementary Table S1). We believe that this was probably due to generalized fatigue as the postperfusion study was done in the afternoon versus in the morning for the preperfusion study. Subject 07 also had a 40% decrease of muscle strength in hip flexion in the perfused leg and a 34% decrease of ankle dorsi-flexion (Supplementary Table S1). The decrease in strength occurred in both legs with the biggest decrease in hip flexion which could not be directly affected by this perfusion procedure as tourniquet placement was just above the knee.
Neurography. Study values were all within normal reference values. Subject 07 had a 22–23% reduction in compound motor action potential (CMAP) amplitudes of peroneal nerve in the perfused leg. (Supplementary Table S1), this magnitude of changes in peroneal CMAP can be observed simply due to differences in electrode placement.14,15
MRI studies
At baseline, subjects 06 and 07 had variable patterns of low signal intensity on T2-weighted fat-saturation (T2FS) images and high signal intensity on the T2 weighted images as expected for fatty degeneration. Postperfusion MRI studies were started 30 minutes after perfusion was stopped. Figure 3 shows examples of MRI studies in subjects 06. Subject 06 had a combined muscle mass volume expansion of 20% (630 ml preperfusion to 757 ml postperfusion) and 07 had an expansion of 19% (780 ml preperfusion to 929 ml postperfusion) by muscle manual segmentation analysis in the segmented muscles. Figure 4a shows the percent postperfusion volumetric change of individual muscle groups, identified by their compartment location. The normalized T2FS intensity was also markedly increased postperfusion, especially in the muscle groups in posterior compartments reflecting increased water content (Figure 4b). The volume expansion and fluid signal (T2FS intensity) increase in postperfusion MRI studies further confirmed the fluid entry into muscles in the perfused limb segment.
Figure 3.
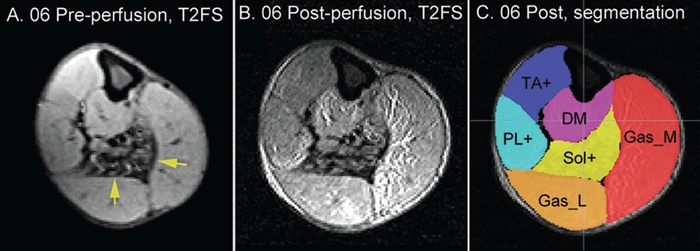
Examples of axial view of T2FS images at mid-calf level of preperfusion. (a) postperfusion (b) and postperfusion with manual segmentation (c) for subject 06. Yellow arrows: muscles with severe degenerative changes. DM, deep muscles that includes flexor hallucis longus and flexor digitorium longus; Gas_L, gastricnemius lateral head; Gas_M, gastrocnemius medial head; PL+, peroneus longus, peroneus brevis, and peroneus tertius; Sol, soleus muscle; TA+, tibialis anterior, extensor hallucis longus, and extensor digitorum longus; T2FS, T2-weighted fat-saturation.
Figure 4.
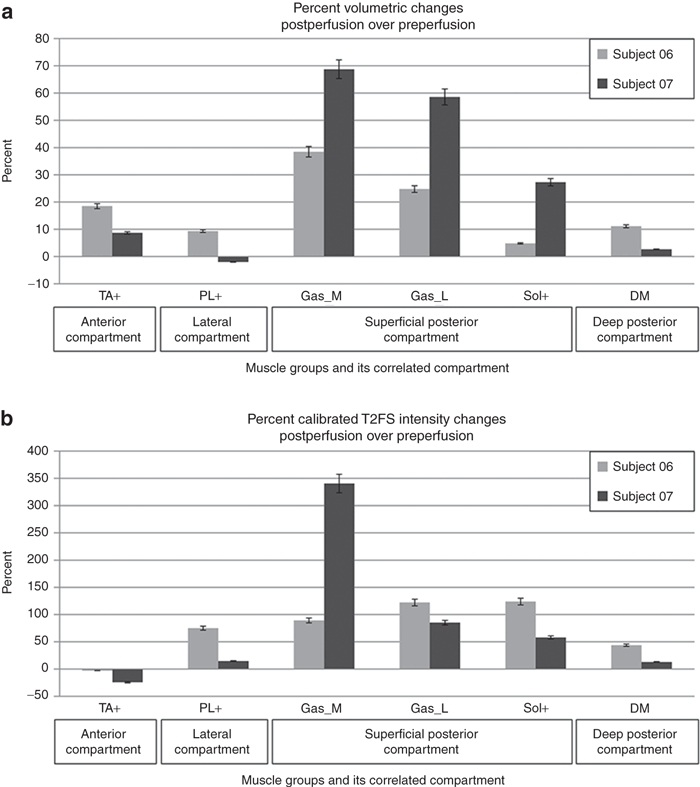
Postperfusion percent changes of muscle groups in subjects 06 and 07. (a) volumetric changes. (b) T2FS intensity changes. DM, deep muscles that includes flexor hallucis longus and flexor digitorium longus; Gas_L, gastricnemius lateral head; Gas_M, gastricnemius medial head; PL+, peroneus longus, peroneus brevis, and peroneus tertius; Sol, soleus muscle; TA+, tibialis anterior, extensor hallucis longus, and extensor digitorum longus; T2FS, T2-weighted fat-saturation.
Discussion
Our study demonstrates that it is safe and feasible to perform high-pressure transvenous perfusion with 0.9% saline up to 20% of limb volume in the lower extremities of young adults with muscular dystrophy. This study used lower cuff pressures (310 mm Hg versus 450–700 mm Hg in primate) and lower limb volumes (20% versus 35–45% in primate) than in nonhuman primate studies,9,16 but achieved comparable peak tissue/compartment pressures during the perfusion (155 mm Hg versus 175 mm Hg in primate). This study also found that deep IVA without intubation is sufficient for pain control in this age group of subjects. The large volume infused (~1 liter) can be delivered in less than 15 minutes, a time frame that is well within the established safety time limit of 1.5–2 hours of tourniquet time in a Bier block regional anesthesia.17 The rate of infusion used in this study was up to 80 ml/minute which is comparable to that used in large animal studies of 60–120 ml/minute.18,19,20 In contrast to arterial limb perfusion methods, no surgical procedure such as femoral artery catheterization or cutdown is necessary.21
With the infusion parameters used, there were no detectable regional injury to the arteries, veins, muscles or nerves in the limb nor were systemic cardiac or renal toxicity observed. Subjects recovered within 4 hours and were discharged home. Furthermore, a phone follow-up in 2 weeks revealed no self-reported concerns of the subjects. Although the compartment pressures in subject 05 were initially high, the pressures in all three compartments steadily returned to below threshold level without any intervention, and there was no evidence of tissue damage related to the transient elevation of compartment pressure. For the two subjects (06 and 07) who had muscle MRI, the majority of muscles had marked volume enlargement. The muscles in posterior compartment, the largest compartment, had the most muscle volume enlargement and most T2FS intensity increase (Figure 4). This correlated with the observation that the posterior compartment had the greatest pressure increases during perfusion (Figure 2a). In primate studies, increased T2FS signal was associated with gene expression.18 It has been postulated that postcapillary venules may be the site of fluid entry into the muscle.22,23
Our study is limited by the small number of subjects. We noticed variations in the compartmental pressure in the three subjects who received 20% of limb volume perfusions. It was not certain why it took 05 longer to recover compartment pressures to normal. However, this subject was a very slim individual with a body mass index of 19. It is likely that this subject had relatively less expandable soft tissues (muscles and subcutaneous tissue) in proportion to nonexpandable tissue (bones); thus 20% limb perfusion may have been a relatively higher perfusion volume relative to soft tissue volume compared to the other subjects.
The maximum fluid volume that we used was 20% of the perfused limb segment volume. This volume is similar to that used for whole pelvic limb perfusion in dogs with the AAV-mini-dystrophin construct which has produced robust mini-dystrophin expression.20 The typical dose in dogs is 15–20 ml/kg of body weight. Subjects 05–07 in our study received 12–15 ml/kg but this entire volume was perfused below the knee.
In conclusion, high-pressure retrograde transvenous perfusion of the lower extremity with 0.9% saline up to 20% of limb volume at the employed infusion parameters is safe and feasible in human muscular dystrophy. This trial serves as a basis for future human gene transfer clinical trials as a method of regionally delivering macromolecular therapeutics, such as gene transfer, in high doses to skeletal muscles.
Materials and Methods
Study subjects. Study inclusion criteria were: biopsy-confirmed or mutation proven Becker muscular dystrophy or other clinically diagnosed muscular dystrophies (including LGMD or EDMD), ability to stand for minimum of 10 minutes and to give informed consent. Exclusion criteria included positive pregnancy test, evidence of cardiomyopathy, pulmonary insufficiency, renal insufficiency, history of local injury to lower extremities such as neuropathy, vascular injury (including arterial or venous thrombosis), surgery, trauma, or compartment syndrome. The study was approved by the Office of Research Ethics of the University of North Carolina at Chapel Hill (IRB). Written informed consent was obtained from each subject. This study is registered on ClinicalTrials.gov with an identifier of NCT00873782.
Preperfusion and postperfusion studies. Outpatient preperfusion and postperfusion visits within 1 week of the perfusion include the following tests: venous Doppler study to assess venous valvular incompetency or venous thrombosis, arterial Doppler study,24 electro-diagnostic testing using standard neurographic techniques of three nerves in lower extremities performed in triplicate by the same examiner, quantitative muscle strength assessments with a handheld dynamometer (JTechAA104; JTech Medical, Salt Lake City, UT) and timed walk25 (performed by licensed physical therapists who are familiar with muscular dystrophy). Preperfusion studies were done bilaterally in the lower extremities. postperfusion neurography and Doppler studies were only performed in the perfused leg. Also obtained at these visits were detailed history, neurological examination, basic metabolic panel (Na+, K+, Cl–, CO2, BUN, and creatinine), CK, plasma and urine myoglobin, pregnancy test for women, and limb photos. Limb segmental volume (just above knee) was measured by water immersion.
Perfusion procedure. The subjects were instructed to take no solid food after midnight and no liquids after 6 . They were admitted to Pediatric Intensive Care Unit the morning of the perfusion study. A peripheral intravenous catheter was placed in the antecubital vein for blood draws and IVA. IVA was administered by a board-certified anesthesiologist with a combination of opioids, benzodiazepines, and propofol with monitoring following the American Society of Anesthesiology (ASA) guidelines. Blood pressure, heart rate, electrocardiogram, respiratory rate, and pulse oximetry were continuously monitored. A Somanetics INVOS near-infrared oxygen monitor sensor (Somanetics, Troy, MI) was placed on each calf to monitor distal limb tissue oxygenation,26 while a second pulse oximetry was placed onto the toe of the perfused limb. Basic metabolic panel, CK, urine, and serum myoglobin were checked at baseline (preperfusion) and hourly postperfusion until the patient was deemed ready for discharge. A portable transthoracic echocardiograph (Sonos 5500; Philips Healthcare, Andover, MA) was used to assess cardiac systolic function and measure systolic and diastolic dimensions. In addition, we continuously observed for microcavitation in the right atrium during infusion which would indicate saline leak due to loss of cuff occlusion. A lower extremity was chosen for the study for each subject. An intravenous catheter (1.5 inches long and 18 or 20 gauge) was placed percutaneously in the saphenous vein at the ankle. Using the premeasured segmental limb volume and the protocol specified perfusion dose, the volume of normal 0.9% saline to be infused as a percent of the segmental limb volume was determined. A pneumatic tourniquet (ATS 2000; Zimmer, Warsaw, IN) was placed above the knee at the line marked during limb volume measurements at the preperfusion visit. The inflation pressure was set to 310 mm Hg and tested briefly while the subject was awake. This is the pressure recommended for intravenous regional anesthesia.27 Saline was infused with an FMS 2000 Rapid Infuser (Belmont Instrument, Billerica, MA).28 The infusion rate was set at 60 ml/minute in the first subject and increased to 80 ml/minute for the others. The Belmont FMS 2000 Rapid Infuser will infuse to a maximum line pressure of 300 mm Hg. When a line pressure of 300 mm Hg is reached, the infusion rate is automatically decreased. Following completion of the infusion, the tourniquet remained inflated for 1 minute and was then deflated. Photos of the limb were taken before, during and after perfusion.
We measured pressures in three muscle compartments (anterior, lateral, and posterior) of the perfused limb in all subjects immediately after the tourniquet was released to evaluate for compartment syndrome. For the first five subjects, we used spot checks that required multiple needle punctures. For the last two subjects, we employed continuous monitoring by indwelling catheters (Indwelling Slit Catheter; Stryker Surgical, Kalamazoo, MI) before, during, and after the perfusion until the pressures returned to less than 35 mm Hg.
Safety boundaries. Safety boundaries were set conservatively in anticipation that these changes may not be clinically significant but may predict problems at the next higher dose. The safety boundaries for limb tissue oximetry (<31%) and compartment pressures (>35 mm Hg) were based on a study of chronic exertional compartment syndrome with transient pain during exercise without muscle damage, not traumatic compartment syndrome.26 For electro-diagnostic nerve testing, the postperfusion versus preperfusion changes was set to >1 msec for the distal motor latency, <75% of CMAP or sensory nerve action potential amplitude and velocity. Cardiac function and dimension were to remain within 90% of baseline. Quantitative muscle testing and timed walking were set to be within 85% baseline. Venous Doppler was used to evaluate for new valvular incompetence or thrombosis and arterial Doppler to detect stenosis. For laboratory values, we set criteria for serum creatinine of >0.5 mg/dl over baseline, and for serum potassium of >0.5 mmol/l over baseline or >5.0 mmol/l. We did not specify the safety boundary for CK, urine and serum myoglobin, as ascribing clinical significance to specific values with high baseline values and underlying muscle disease is problematic.
Safety monitoring. A Local Independent Safety Monitor reviewed the safety report prepared after the completion of each subject's study and approved the infusion parameters for the next subject.
An external independent Safety Officer was appointed by National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). Biannual safety reports were submitted via KAI Research (Rockville, MD).
MRI acquisition and data analysis. The final two subjects underwent T2FS MRI scan at baseline and immediately postperfusion to determine the distribution of fluid entry in the muscles. Images were acquired using a 3T Trio MR system (Siemens Medical, Erlangen, Germany) with a maximal gradient strength of 40 mT/m and a maximal slew rate of 200 mT/m/ms. A phase array body coil was used to acquire data. A turbo spin echo with variable flip angle sequence was utilized to acquire 3D high resolution T2 weighted images. The imaging parameters were set as follows: TR/TE = 2,500/390 msec, field of view (FOV) = 400 × 400 × 112 mm3, matrix size = 384 × 384 × 112, resulting in a nearly isotropic voxel size of 1 × 1 × 1 mm3; 38.5% oversampling in the slice select direction; fat saturation; two averages; total data acquisition time = 11 minutes 42 seconds.
T2FS images were manually segmented into several groups of muscles in whole muscle length using open source software ITK-SNAP 2.0 (ref. 29) (www.itksnap.org). The preperfusion and postperfusion images were aligned using the tibial bone as landmark. Volumetric data and T2FS intensity data were obtained after the segmentation was finalized. The average signal intensity from the whole length of the peroneus muscles in the control leg was used to normalize the signal intensities of the preperfusion and postperfusion scans.
SUPPLEMENTARY MATERIAL Table S1. Summary of demographics of study subjects and testing results.
Acknowledgments
This study was supported by USPHS grant 1U54AR056953-01 and the University of North Carolina at Chapel Hill. No authors have any financial conflict of interest related to the submitted manuscript. We are indebted to our study subjects and their families for their dedication to making a difference in the battle against muscular dystrophy. We thank the following people for their assistance in carrying out the trial: Doreen Marlowe, RN; Sabrina Thompson, RN; Spencer Weig, MD; Jesse Thomas, RVT; Niki Baker, RVT; Diane Meyer, RPT; Thomas Delviscio, RPT; Susan Gisler, RPT; Tom Beckman, RT(R); Lewis Daughtry, RT(R); Kathy Wilber; Jon Wolff, MD, PhD; Julia Hegge, PhD; Yasheng Chen, PhD; Joe Kornegay, PhD, DVM; Richard Jude Samulski, PhD; Xiao Xiao, PhD and Robert Leshner, MD.
Supplementary Material
Summary of demographics of study subjects and testing results.
REFERENCES
- Mendell JR, Moxley RT, Griggs RC, Brooke MH, Fenichel GM, Miller JP.et al. (1989Randomized, double-blind six-month trial of prednisone in Duchenne's muscular dystrophy N Engl J Med 3201592–1597. [DOI] [PubMed] [Google Scholar]
- Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A.et al. (2000Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector Nat Genet 24257–261. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, Kota J, Coley BD, Galloway G.et al. (2009Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins Ann Neurol 66290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman H, Wilson JM, Finke R, Kleckner AL., and, Mendell J. Phase I clinical trial utilizing gene therapy for limb girdle muscular dystrophy: alpha-, beta-, gamma-, or delta-sarcoglycan gene delivered with intramuscular instillations of adeno-associated vectors. Hum Gene Ther. 2000;11:777–790. doi: 10.1089/10430340050015671. [DOI] [PubMed] [Google Scholar]
- Hagstrom JE, Hegge J, Zhang G, Noble M, Budker V, Lewis DL.et al. (2004A facile nonviral method for delivering genes and siRNAs to skeletal muscle of mammalian limbs Mol Ther 10386–398. [DOI] [PubMed] [Google Scholar]
- Davis HL, Demeneix BA, Quantin B, Coulombe J., and, Whalen RG. Plasmid DNA is superior to viral vectors for direct gene transfer into adult mouse skeletal muscle. Hum Gene Ther. 1993;4:733–740. doi: 10.1089/hum.1993.4.6-733. [DOI] [PubMed] [Google Scholar]
- Kessler PD, Podsakoff GM, Chen X, McQuiston SA, Colosi PC, Matelis LA.et al. (1996Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein Proc Natl Acad Sci USA 9314082–14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragot T, Vincent N, Chafey P, Vigne E, Gilgenkrantz H, Couton D.et al. (1993Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice Nature 361647–650. [DOI] [PubMed] [Google Scholar]
- Kamimura K, Zhang G., and, Liu D. Image-guided, intravascular hydrodynamic gene delivery to skeletal muscle in pigs. Mol Ther. 2010;18:93–100. doi: 10.1038/mt.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichavant C, Chapdelaine P, Cerri DG, Dominique JC, Quenneville SP, Skuk D.et al. (2010Expression of dog microdystrophin in mouse and dog muscles by gene therapy Mol Ther 181002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Li J, Qiao C, Chen C, Hu P, Zhu X.et al. (2008A canine minidystrophin is functional and therapeutic in mdx mice Gene Ther 151099–1106. [DOI] [PubMed] [Google Scholar]
- Florence JM, Fox PT, Planer GJ., and, Brooke MH. Activity, creatine kinase, and myoglobin in Duchenne muscular dystrophy: a clue to etiology. Neurology. 1985;35:758–761. doi: 10.1212/wnl.35.5.758. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, Round JM, Newham DJ., and, Edwards RH. An examination of some factors influencing creatine kinase in the blood of patients with muscular dystrophy. Muscle Nerve. 1987;10:15–21. doi: 10.1002/mus.880100105. [DOI] [PubMed] [Google Scholar]
- Bromberg MB., and, Spiegelberg T. The influence of active electrode placement on CMAP amplitude. Electroencephalogr Clin Neurophysiol. 1997;105:385–389. doi: 10.1016/s0924-980x(97)00037-4. [DOI] [PubMed] [Google Scholar]
- Kong X, Lesser EA., and, Gozani SN. Repeatability of nerve conduction measurements derived entirely by computer methods. Biomed Eng Online. 2009;8:33. doi: 10.1186/1475-925X-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegge JO, Wooddell CI, Zhang G, Hagstrom JE, Braun S, Huss T.et al. (2010Evaluation of hydrodynamic limb vein injections in nonhuman primates Hum Gene Ther 21829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry LA, Balliana BS, Galeppi AC.Intravenous regional anesthesia (Bier block) Techniques in Regional Anesthesia and Pain ManagementVolume 10, Issue 3, 2006123–131.
- Vigen KK, Hegge JO, Zhang G, Mukherjee R, Braun S, Grist TM.et al. (2007Magnetic resonance imaging-monitored plasmid DNA delivery in primate limb muscle Hum Gene Ther 18257–268. [DOI] [PubMed] [Google Scholar]
- Kornegay JN, Li J, Bogan JR, Bogan DJ, Chen C, Zheng H.et al. (2010Widespread muscle expression of an AAV9 human mini-dystrophin vector after intravenous injection in neonatal dystrophin-deficient dogs Mol Ther 181501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao C, Li J, Zheng H, Bogan J, Li J, Yuan Z.et al. (2009Hydrodynamic limb vein injection of adeno-associated virus serotype 8 vector carrying canine myostatin propeptide gene into normal dogs enhances muscle growth Hum Gene Ther 201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodino-Klapac LR, Montgomery CL, Mendell JR., and, Chicoine LG. AAV-mediated gene therapy to the isolated limb in rhesus macaques. Methods Mol Biol. 2011;709:287–298. doi: 10.1007/978-1-61737-982-6_19. [DOI] [PubMed] [Google Scholar]
- Petrov M, Malik A, Mead A, Bridges CR., and, Stedman HH. Gene transfer to muscle from the isolated regional circulation. Methods Mol Biol. 2011;709:277–286. doi: 10.1007/978-1-61737-982-6_18. [DOI] [PubMed] [Google Scholar]
- Plante GE, Chakir M, Ettaouil K, Lehoux S., and, Sirois P. Consequences of alteration in capillary permeability. Can J Physiol Pharmacol. 1996;74:824–833. doi: 10.1139/cjpp-74-7-824. [DOI] [PubMed] [Google Scholar]
- Labropoulos N, Tiongson J, Pryor L, Tassiopoulos AK, Kang SS, Ashraf Mansour M.et al. (2003Definition of venous reflux in lower-extremity veins J Vasc Surg 38793–798. [DOI] [PubMed] [Google Scholar]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories (2002) ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- van den Brand JG, Nelson T, Verleisdonk EJ., and, van der Werken C. The diagnostic value of intracompartmental pressure measurement, magnetic resonance imaging, and near-infrared spectroscopy in chronic exertional compartment syndrome: a prospective study in 50 patients. Am J Sports Med. 2005;33:699–704. doi: 10.1177/0363546504270565. [DOI] [PubMed] [Google Scholar]
- Marsch SC, Sluga M, Studer W, Barandun J, Scharplatz D., and, Ummenhofer W. 0.5% versus 1.0% 2-chloroprocaine for intravenous regional anesthesia: a prospective, randomized, double-blind trial. Anesth Analg. 2004;98:1789–93, table of contents. doi: 10.1213/01.ANE.0000116929.45557.CE. [DOI] [PubMed] [Google Scholar]
- Comunale ME. A laboratory evaluation of the level 1 rapid infuser (H1025) and the Belmont instrument fluid management system (FMS 2000) for rapid transfusion. Anesth Analg. 2003;97:1064–1069, table of contents.. doi: 10.1213/01.ANE.0000077078.53242.29. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC.et al. (2006User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability Neuroimage 311116–1128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of demographics of study subjects and testing results.


