SUMMARY
Loss of retinal ganglion cells (RGCs) accounts for visual function deficits after optic nerve injury, but how axonal insults leading to neuronal death remains elusive. By using an optic nerve crush model which results in the death of the majority of RGCs, we demonstrate that axotomy induces differential activation of distinct pathways of the unfolded protein response (UPR) in axotomized RGCs. Optic nerve injury provokes a sustained CCAAT/enhancer binding protein homologous protein (CHOP) up-regulation, and deletion of CHOP promotes RGC survival. In contrast, IRE/XBP-1 is only transiently activated, and forced XBP-1 activation dramatically protects RGCs from axon injury-induced death. Importantly, such differential activations of CHOP and XBP-1 and their distinct effects on neuronal cell death are also observed in RGCs with other types of axonal insults, such as vincristine treatment and intraocular pressure (IOP) elevation, suggesting a new protective strategy for neurodegeneration associated with axonal damage.
INTRODUCTION
RGCs are the only neurons that relay visual information from the retina to the brain. These neurons are highly vulnerable when their axons, which collectively form the optic nerve, are damaged (Levin, 1997). For example, traumatic optic nerve injury and subsequent loss of RGCs occur often in the setting of head injury, as a consequence of road traffic accidents or falls. In rodents, the majority of RGCs undergo cell death around 2 weeks after intraorbital optic nerve injury (Berkelaar et al., 1994; McKernan and Cotter, 2007; Selles-Navarro et al., 2001), creating a first hurdle for successful neural repair. In addition to optic nerve trauma, the retinal pathology of different types of optic neuropathy, in particular glaucoma, is also characterized by selective RGC loss (Howell et al., 2007; Kerrigan et al., 1997; Libby et al., 2005; Quigley, 1993; Quigley et al., 1995; Weinreb and Khaw, 2004). In these conditions, RGC loss has been attributed to apoptotic death (Howell et al., 2007; Kerrigan et al., 1997; Libby et al., 2005; Quigley, 1993; Quigley et al., 1995; Weinreb and Khaw, 2004; Qu et al., 2010). However, RGC apoptosis may occur as the last step in these diseases so that targeting apoptotic effectors may not be an efficient strategy for therapy. Thus, deciphering the key upstream signals that trigger the apoptotic cascade in RGCs should provide important targets for therapeutic interventions.
Multiple stimuli, such as hypoxia, nutrient deprivation, viral infection and disturbance of calcium levels, can directly or indirectly cause accumulation of unfolded or misfolded proteins in the endoplasmic reticulum (ER), triggering an ER stress condition which leads to an evolutionarily conserved UPR (Ron and Walter, 2007). The UPR has been proposed to be a protective mechanism that limits ER protein loading by inhibiting protein translation, facilitates protein folding through increasing the expression of ER chaperones and removes misfolded proteins from the ER through degradation. However, prolonged and unrestrained ER stress could lead to the activation of pro-apoptotic signaling pathways.
In mammals, the UPR includes three signal transduction pathways initiated by three ER-resident stress-sensing proteins: protein kinase RNA-like ER kinase (PERK), inositol-requiring protein-1 (IRE1) and activating transcription factor-6 (ATF6). PERK activation leads to the phosphorylation of the eukaryotic inactivation factor 2α (eIF2α). While suppressing general protein translation, eIF2α phosphorylation also promotes the selective translation of some mRNAs such as ATF4, a transcription factor that induces CHOP expression, which has been generally used as a ER stress marker (Fels and Koumenis, 2006; Harding et al., 2003). On the other hand, the site-specific endoribonuclease function of IRE1 mediates the specific splicing of XBP-1 mRNA to generate an active (spliced) form XBP-1s (Calfon et al., 2002; Sidrauski and Walter, 1997; Yoshida et al., 2001). XBP-1s targets a set of genes that increase the ER protein-folding capacity and facilitate degradation of misfolded proteins (Lee et al., 2003; Shaffer et al., 2004). Although IRE/XBP-1 has been proposed to be protective, the in vivo effect of XBP-1 on neuroprotection is less clear. In fact, it was shown that XBP-1 deletion in the nervous system (XBP-1flox/flox mice crossing with nestin-Cre mice) could extend lifespan of transgenic mice expressing a mutant SOD1, an amyotrophic lateral sclerosis model, by enhancing autophagy and thus degradation of the mutant SOD1 protein in vivo (Hetz et al., 2009).
In our analysis of the mechanisms for reduced protein synthesis ability in axotomized RGCs in the adult mice (Park et al., 2008), we found that axotomized RGCs showed signs of UPR, indicating that ER stress is induced in these neurons. In fact, ER structures that are distributed along entire lengths of axons and connected with those in the neuronal somas might possess the unique properties of transducing the local axonal signals to the soma of individual neurons. However, despite previous reports about ER stress responses in neurons (Aoki et al., 2002; Saxena et al., 2009), it is unknown how these pathways are activated and more importantly what the functional consequences are. Thus, we decided to assess axotomy-triggered UPR in depth using in vivo mouse models.
RESULTS
Axotomy Triggers UPR in RGCs
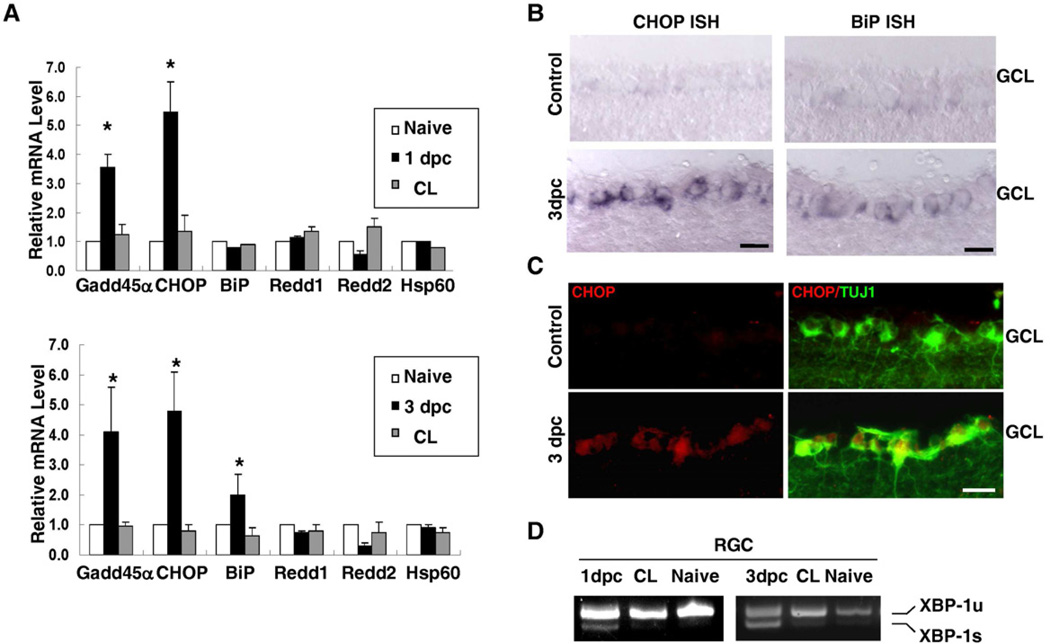
CHOP, a key downstream target of PERK pathway, has been linked to apoptosis after ER stress in multiple disease models (Pennuto et al., 2008; Puthalakath et al., 2007; Silva et al., 2005; Song et al., 2008; Zinszner et al., 1998). To assess the expression of CHOP in intact and axotomized RGCs, we purified the RGCs from wild type rats with or without optic nerve crush by retrograde labeling and fluorescence-activated cell sorting (FACS) (Park et al., 2008). Using the mRNA isolated from purified RGCs, quantitative real-time PCR (q-PCR) analysis showed increased expression of CHOP and other ER stress markers such as GADD45α (Lee et al., 2003), in axotomized RGCs (Figure 1A), which was further confirmed by both in-situ hybridization and immunohistochemical analysis in retina sections (Figures 1B,C). In contrast, Redd1/2, the inhibitors of the mTOR pathway induced by hypoxia (Brugarolas et al., 2004), and Hsp60, a mitochondria stress chaperone (Deocaris et al., 2006), were not induced by axotomy in RGCs (Figure 1A).
Figure 1. Optic Nerve Injury Induces UPR in Adult RGCs.
(A) Quantification of expression of different molecules in RGCs detected by q-PCR. Each sample was run in quadruplicate in each assay. GADPH was used as the endogenous control. CL: contralateral uninjured eye. *: p <0.01, paired Student’s t-test. Data are presented as means ± s.e.m and n=6. (B) In situ hybridization (ISH) results showing the expression of CHOP and BiP in the ganglion cell layer of adult mouse retinas. Scale bar: 20 µm. 3dpc: 3 days post-crush. GCL: ganglion cell layer. (C) Immunohistochemical analysis for CHOP or TUJ1 immunoreactivity in retinal sections. Scale bar: 20 µm. GCL: ganglion cell layer. (D) Detection of un-spliced and spliced XBP-1 mRNA (XBP-1u or XBP-1s) by RT-PCR. The mRNAs were prepared from retrograde-labeled and FACS-purified adult RGCs from retinas one day, three days post-crush (1dpc, 3dpc), contralateral (CL) eyes and naïve eyes. See also Figure S2.
We also examined the activation of other UPR targets in axotomized RGCs. As XBP-1 splicing has been considered as a hallmark of UPR (Calfon et al., 2002; Sidrauski and Walter, 1997; Yoshida et al., 2001), we examined whether the splicing product of XBP-1 (XBP-1s) could be detected in RGCs after optic nerve injury. By RT-PCR with mRNAs from purified RGCs, we found that a small amount of XBP-1s appeared in the RGCs obtained at both 1 and 3 days after optic nerve crush but not in those of naïve mice (Figure 1D). Consistently, modest up-regulation of BiP, a XBP-1 target (Lee et al., 2003), was seen at 3 day post-injury (Figure 1A, B), consistent with a modest activation of the IRE1/XBP-1 pathway in axotomized RGCs. These results suggested that optic nerve injury triggers robust CHOP induction and modest XBP-1 activation in axotomized RGCs.
CHOP Deficiency Promotes RGC Survival
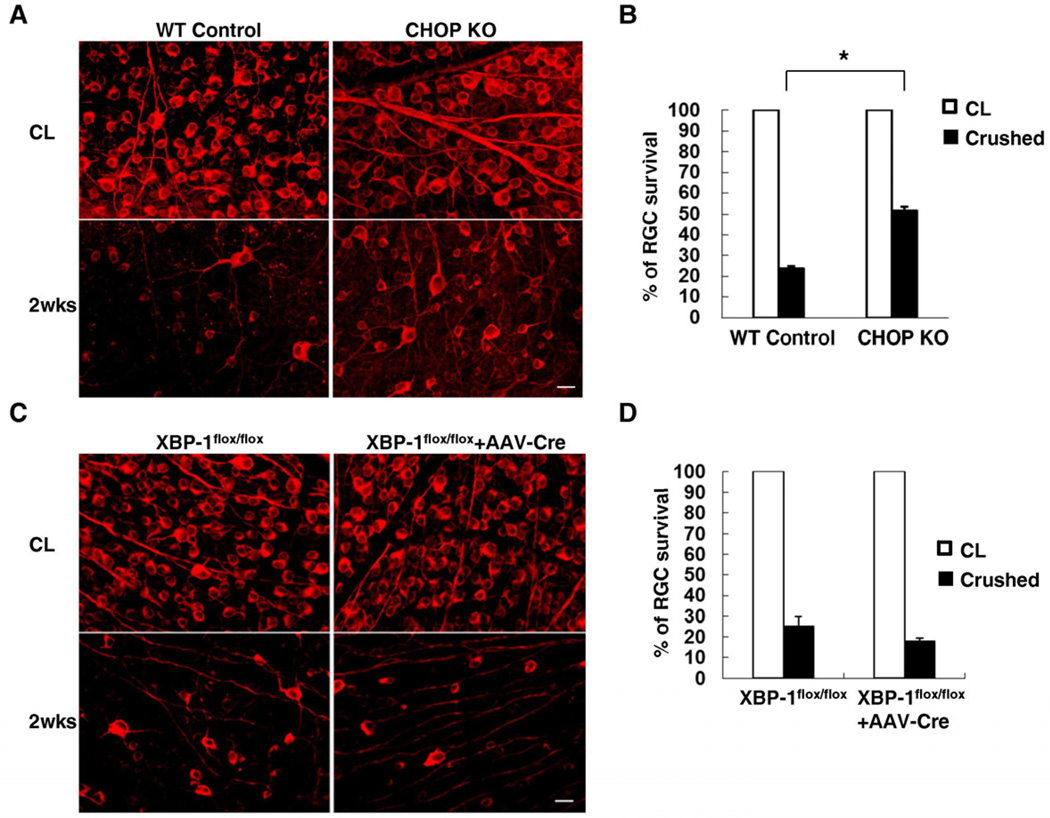
We next examined whether UPR activation contributes to RGC cell death after axotomy. We thus performed optic nerve crush in CHOP knockout (KO) mice (Marciniak et al., 2004) and control mice, and analyzed the extents of RGC survival by counting survived TUJ1-positive RGCs at different post-injury points (Park et al., 2008). Consistent with the notion that CHOP could act as a pro-apoptotic molecule, we found significant increases of RGC survival in CHOP KO mice, compared to wild-type (WT) control mice after injury (Figure 2A). As shown in Figure 2B, 52% of RGC survived in CHOP KO mice 2 weeks after optic nerve crush, compared to 24% RGC survival in WT mice. Therefore these results suggest that CHOP activation is a critical mechanism that mediates axotomy-induced RGC death.
Figure 2. CHOP KO, but not XBP-1 KO, Increases RGC survival after Optic Nerve Injury.
Representative images (A, C) of fluorescent photomicrographs of flat-mounted retinas showing surviving TUJ1+ RGCs at 2 weeks after injury in WT mice and CHOP KO mice (A), and XBP-1flox/flox control mice (no AAV-Cre) and XBP-1flox/flox mice injected with AAV-Cre (C). CL: contralateral uninjured eye. Scale bar, 20 µm. (B, D) Quantification of surviving RGCs, represented as percentage of TUJ1+ RGCs, compared to the uninjured contralateral retinas, in CHOP KO mice (B) or XBP1 KO mice (D). *: p<0.01, Student’s t-test. Data are presented as means ± s.e.m and n = 6. See also Figure S1.
Lack of Significant Effects of XBP-1 Deletion on RGC Survival
Based on the observation of XBP-1 activation, albeit to a modest level, in axotomized RGCs (Figure 1D), we examined the effects of genetic deletion of XBP-1 in RGCs on RGC survival after optic nerve injury. Because XBP-1 germline KO is embryonic lethal (Reimold et al., 2000), we utilized an AAV-Cre-assisted conditional knockout strategy (Park et al., 2008) to delete XBP-1 in adult RGCs of XBP-1flox/flox mice (Hetz et al., 2008). Intravitreal injection of AAV-Cre has previously been shown to delete a floxed gene in most RGC (Park et al., 2008). By in situ hybridization, we further verified the lack of XBP-1 expression in the RGCs of XBP-1flox/flox mice with AAV-Cre injection (Figure S1A). As shown in Figure 2C and 2D, there was no significant difference in RGC survival between XBP-1-deleted mice and control mice after injury, suggesting that XBP-1 deletion does not affect axotomy-triggered RGC death.
Differential Activation of CHOP and XBP-1 in RGCs after Axonal Insults
To explore possible mechanisms for differential effects of CHOP and XBP-1 deletion on RGC death, we monitored the temporal expression levels of XBP-1s and CHOP in axotomized RGCs during the first week after axotomy (because of difficulty in collecting RGCs at later time points due to massive RGC loss). XBP-1s level was elevated in RGCs isolated from animals at 3 and 5 days after optic nerve crush, but reduced at 7 days post-injury (Figure S1B). In addition to being transient, XBP-1 activation in axotomized RGCs is also modest, when comparing with the amount of XBP-1s in cells treated with thapsigargin, an agent that induces ER stress by depletion of luminal calcium stores (Figure S1B). In contrast, CHOP expression was persistently high during the time course studied (Figure S1C). These results suggest that an optic nerve injury triggers differential activation of different UPR pathways: while CHOP is robustly and persistently activated, XBP-1 is only transiently and modestly activated. As previous studies in cultured non-neuronal cells suggested that the duration of IRE1/XBP-1 activation correlates with its protective effects (Lin et al., 2007), the transient activation of IRE1/XBP-1 in axotomized RGCs might explain the failure of XBP-1 knockout in affecting RGC survival.
To assess whether differential activation of CHOP and XBP-1 occurs in other types of axonal damage, we intravitreously injected vincristine, a microtubule destabilizer which preferentially induces axonal degeneration (Silva et al., 2006; Vohra et al., 2010). As shown in Figure S2, vincristine triggered CHOP up-regulation, but not XBP-1 splicing (detected by RT-PCR using mRNAs of whole retina or isolated RGCs), at 1 day post-injection. In contrast, both CHOP up-regulation (Figure S2A) and XBP-1 splicing (Figure S2B, detected by RT-PCR using mRNAs from whole retinas) were induced by similarly applied thapsigargin, which presumably acts on both axons and cell bodies. Thus, instead of simultaneous activation of all UPR pathways that occurs in non-neuronal cells, axonal insults preferentially lead to the activation of CHOP, but not XBP-1, in RGCs. In comparison with other cell types, a striking feature of neurons is the unique compartmentation in which the axon is separated from the soma. It is conceivable that certain unique properties of the axonal compartment, such as the lack of detectable mRNAs and long distance to the soma, might contribute to the limited XBP-1 activation in axotomized adult RGCs.
Overexpressing XBP-1s Promotes RGC Survival after Optic Nerve Injury
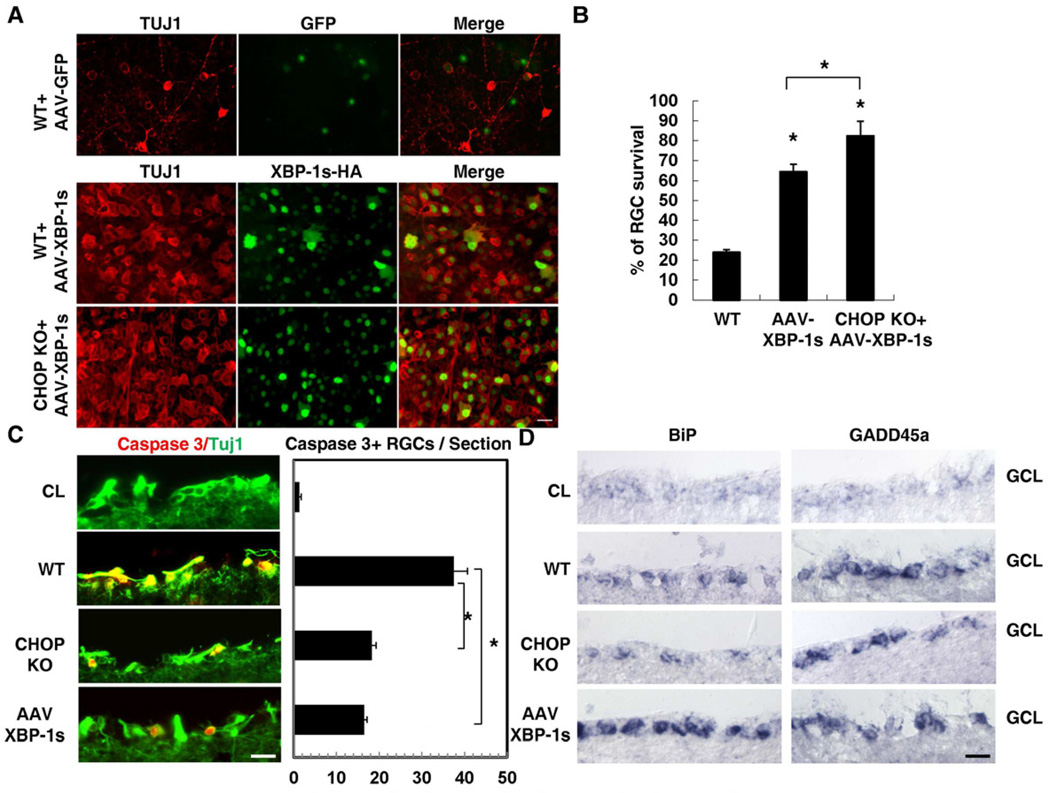
Limited XBP-1 activation in axotomized RGCs suggests the possibility that forced XBP-1 activation might alter RGC survival after optic nerve injury. To test this, we over-expressed an active HA-tagged XBP-1s in RGCs using recombinant AAVs in WT and CHOP KO mice. As shown in Figure S3A, approximately 50% and 80% of TUJ1-positive RGCs were stained with an anti-HA antibody 1 or 2 weeks after injection of AAV-XBP-1s-HA, respectively. Then we performed optic nerve injury at two weeks after the intravitreal injection of AAVs. As shown in Figures 3A and 3B, AAV-XBP-1s dramatically increased RGC survival in both WT mice and CHOP KO mice. In comparison with approximately 20% RGC survival in AAV-GFP injected control mice, WT mice with XBP-1s overexpression showed approximately 64% RGCs survival at 2 weeks after injury (Figures 3B). CHOP KO could further enhance the survival effects of XBP-1s overexpression, as 82% RGCs survived in CHOP KO mice with XBP-1s overexpression 2 weeks after optic nerve crush (Figures 3B). Taken together, these results suggest that XBP-1 and CHOP play opposite roles in controlling neuronal survival after axonal injury.
Figure 3. The Effects of XBP-1s Overexpression on RGC Survival after Optic Nerve Injury.
(A) Representative images of flat-mounted retinas showing surviving TUJ1+ RGCs at 2 weeks post-injury in WT control mice injected with AAV-GFP or AAV-XBP-1s or CHOP KO mice injected with AAV-XBP-1s. Anti-HA antibody is used to indicate RGCs injected with AAVs expressing his-tagged XBP-1s. Scale bar, 20 µm (B) Quantification of TUJ1+ RGCs represented as percentage of TUJ1+ RGCs compared to the uninjured contralateral retinas. Data are presented as means ± s.e.m and n=6. * p<0.01, One way ANOVA and Tukey's Multiple Comparison Test. (C) Left panel: immunostaining of active caspase-3 at 3 days post-injury in WT, CHOP KO mice and AAV-XBP-1s injected mice. CL: uninjured contralateral eye. Scale bar: 20 µm. Right panel: quantification of caspase-3+ RGCs per section. Data are presented as means ± s.e.m and n=4. * p<0.01, One way ANOVA and Tukey's Multiple Comparison Test. (D) In situ hybridization results showing the expression of BiP and GADD45αin the ganglion cell layer (GCL) of WT, CHOP KO mice and AAV-XBP-1s injected mouse retinas 3 days post-injury. CL: contralateral uninjured eye. Scale bar: 20 µm. See also Figure S3.
Since failure of RGC axon regeneration is another major feature of optic nerve damage, we also determined if increase of RGC survival improves axon regeneration. We anterogradely labeled the RGC axons with neuronal tracer CTB; however, in all of these animals, we failed to observe any enhancement of optic nerve regeneration (Figure S3B), suggesting that UPR selectively affects neuronal survival, but not axon regeneration.
Independent Action of XBP-1 and CHOP on RGC Survival
We next examined possible interactions between XBP-1 and CHOP in their effects on neuronal survival. Although the promoter of CHOP contains a putative XBP-1 binding site (Roy and Lee, 1999; Urano et al., 2000), we failed to observe significant change of CHOP expression in intact or injured RGCs upon AAV-assisted XBP-1s over-expression (Figure S3C,D). Conversely, XBP-1s induction was not affected by CHOP knockout (Figure S3E), suggesting independent regulation of XBP-1 and CHOP activation or expression in neurons.
Both CHOP KO and XBP-1s over-expression reduced the extent of injury-induced RGC apoptosis, as indicated by TUNEL (data not shown) and active caspase 3 staining (Figure 3C). We then assessed whether similar down-stream effectors might contribute to the effects of CHOP KO and XBP-1s over-expression on neuronal survival. As shown in Figure 3D, neither CHOP KO nor XBP-1s over-expression altered axotomy-induced expression of GADD45α. However, XBP-1s over-expression, but not CHOP KO, significantly induced the expression of the ER chaperon BiP (Lee et al., 2003), suggesting that different downstream mechanisms might be involved in the effects of XBP-1s and CHOP KO on regulating RGC apoptosis after axon injury.
The Effect of CHOP and XBP-1 on Glaucomatous RGC Degeneration
Glaucoma is a common form of optic neuropathy that is characterized by progressive RGC degeneration (Howell et al., 2007; Kerrigan et al., 1997; Libby et al., 2005; Quigley, 1993; Quigley et al., 1995; Weinreb and Khaw, 2004). Elevated IOP is the most well-recognized risk factor for primary open-angle glaucoma (Quigley, 1993). Studies in primates demonstrate that experimentally elevated IOP results in axonal transport obstruction and nerve damage at the optic nerve head, followed by RGC loss (Minckler et al., 1977). Moreover, it was shown that elevated IOP induces CHOP expression in RGCs (Doh et al., 2010). We thus attempted to examine whether manipulation of the UPR pathways could protect RGCs in a mouse model of glaucoma in which IOP was elevated by injection of microbeads into the anterior chamber of adult mice to block aqueous outflow (the contralateral eyes with sham injection were served as controls) (Sappington et al., 2010). This established procedure has been shown to induce many features of glaucoma, such as optic nerve head cupping, optic nerve degeneration and RGC loss (Chen et al., 2010; Sappington et al., 2010).
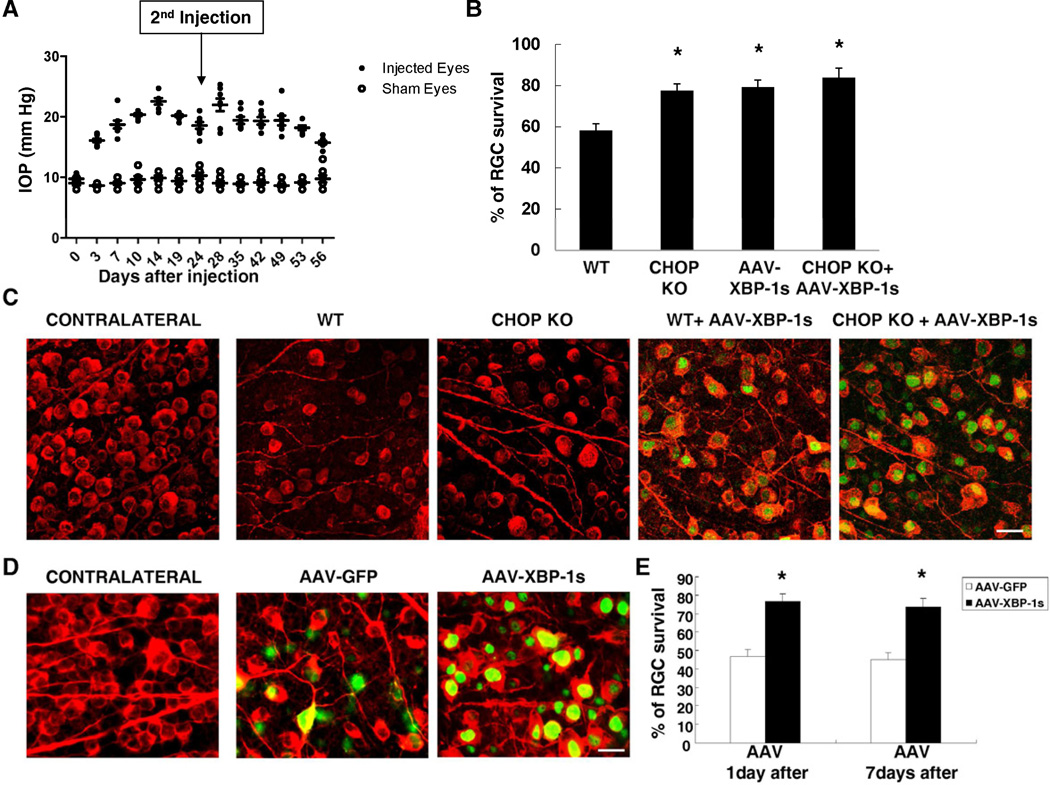
A first anterior chamber injection of microbeads induced IOP elevation to 20–25 mmHg, about two times higher than normal, for 4 weeks, and then maintained for another 4 weeks with a second microbeads injection, compared to a steady level of 10 mmHg of IOP in the control eyes (Figure 4A). As a result, approximately 42% RGCs are lost at 8 weeks after microbeads injection in WT mice (Figures 4B,C). In these animals, at 7 days after microbeads injection, there was a marked increase in CHOP expression in RGCs assessed by immunostaining (Figure S4A). However, we failed to detect the spliced form of XBP-1 at all the time points studied (3, 5 and 7 days after microbeads injection) (data not shown), suggesting that similar to optic nerve injury, IOP elevation triggers differential activation of different UPR pathways in RGCs.
Figure 4. CHOP Deletion and XBP-1s Over-expression Protect RGCs after IOP Elevation.
(A) Measured IOP levels in the eyes of the wild type mice with saline injection (sham eyes) or microbeads injection (injected eyes). As the sham eyes exhibited a steady level of IOP at 10.0 ± 1.5 mmHg (n = 8), a single injection induced IOP elevation which lasts for 4 weeks and a second dose of microbeads injection on day 24 (arrow) could maintain elevated IOP levels to 8 weeks (n=8). (B) Quantification of surviving RGCs, represented as percentage of TUJ1+ RGCs, compared to the uninjured contralateral retinas, in each group at 8 weeks after IOP elevation. Data are presented as means ± s.e.m and n = 8. * p<0.01; One way ANOVA and Tukey's Multiple Comparison Test. (C) Representative images of TUJ1 (red) and HA (green) labeling in flat-mounted retinas from uninjected contralateral eyes, and from those eyes 8 weeks after IOP elevation. AAV-XBP-1s were injected two weeks before IOP elevation. Scale bar, 20 µm. (D) Representative images of TUJ1 (red) and GFP or HA (green) labeling in flat-mounted retinas from uninjected contralateral eyes, and from those eyes 8 weeks after IOP elevation. AAV-XBP-1s were injected 1 day after IOP elevation. Scale bar, 20 µm. (E) Quantification of surviving RGCs, represented as percentage of TUJ1+ RGCs, compared to the uninjured contralateral retinas, in each group at 8 weeks after IOP elevation. AAV-XBP-1s were injected 1 day or 7 days after IOP elevation. Data are presented as means ± s.e.m and n = 8. * p<0.01; Student’s t-test. See also Figure S4.
Importantly, both CHOP KO and XBP-1s over-expression significantly reduced RGC death. The combination of CHOP KO and XBP-1s over-expression showed a trend of further protection, but the extents did not reach the level of statistical significance as compared to CHOP KO or XBP-1s over-expression alone (Figures 4B,C). These protective effects are not due to the alteration of the IOP levels, because microbeads injection induced similar degrees of IOP elevation in all experimental groups (Figure S4B). As BDNF has been shown to be protective for RGCs (Cohen-Cory and Fraser, 1994; Mansour-Robaey et al., 1994), we simultaneously applied BDNF and XBP-1s to the eyes of animals received an optic nerve crush injury (Figure S4C) or subjected to IOP elevation (Figure S4D). While BDNF alone protected RGCs to some extents, it did not lead to a significant further enhancement of RGC survival in any of these models when was combined with XBP-1s over-expression. The mechanistic interactions between UPR and neurotrophin pathways remain to be further elucidated.
To mimic a clinically relevant scenario, we also examined whether a delayed expression of XBP-1s can be protective for RGCs in the IOP elevated model. We thus increased IOP by microbeads injection followed by introduction of AAV-XBP-1s 1 or 7 days later. Since AAV-mediated gene expression in RGCs is normally peaked at 2 weeks after infection (Martin et al., 2002, Park et al., 2008), XBP-1s expression in RGCs is likely to occur 2–3 weeks after IOP elevation. Interestingly, such delayed AAV-XBP-1s expression still showed significant protective effects on RGCs (Figure 4D and E), suggesting that forced XBP-1s expression might be a promising therapeutic approach for RGC degeneration in glaucoma.
DISCUSSION
A predominant hypothesis holds that ER stress activates all UPR pathways, thereby simultaneously producing antagonistic outputs that can be both protective and harmful to cells; only unresolved ER stress results in cell death (Ron and Walter, 2007). While these principles might be applicable to non-neuronal cells, the results from this study suggest a different scenario for neurons, at least adult RGCs. By using different axonal damage models, we demonstrate that diverse UPR pathways are differentially activated in the affected RGCs and in fact have opposite effects on neuronal survival. These results reveal a potentially important logic of protecting RGCs by differentially manipulating the UPR pathways.
In all models, we observed robust and persistent CHOP induction. Consistent with previous studies (Pennuto et al., 2008; Puthalakath et al., 2007; Silva et al., 2005; Song et al., 2008; Zinszner et al., 1998), CHOP induction might be an important contributor to RGC loss in these conditions. In contrast, in these same models, IRE/XBP-1 pathway is either not or only transiently activated, consistent with the lack of phenotypes of XBP-1 deletion on neuronal death. Directly over-expressing an active XBP-1 in the adult RGCs protects RGCs from apoptotic death after both acute and chronic insults, indicating a neuroprotective role of XBP-1 in RGC survival.
Likely, all of the ER stress sensors, including IRE1, become activated when axon injury occurs. The unique properties of the axonal compartments, such as length and limited mRNAs localization, might explain the different UPR activation patterns in adult RGCs (this study) and non-neuronal cells (Ron and Walter, 2007). For example, as activation of XBP-1, a protective arm of UPR pathways, requires IRE1-mediated xbp-1 mRNA splicing (Yoshida et al., 2001), little XBP-1 mRNAs in the axonal compartment in adult neurons might limit the activation of this pathway in the axon. As a consequence, axonal insults result in the overweight of pro-apoptotic UPR activation, which might contribute to irreversible neuronal death associated with traumatic optic nerve injury, glaucoma and perhaps other types of neuropathies. In light of recent successes in AAV-mediated gene therapy in retinal diseases (Busskamp et al., 2010; Tan et al., 2009), our results may provide potentially important molecular targets for neuroprotective strategies for optic nerve injury and diseases.
EXPERIMENTAL PROCEDURES
Detail methods and materials are in the Supplemental Information.
Mice
CHOP KO and C57BL/6 mice and Sprague-Dawley rats were purchased from the Jackson Laboratory. XBP-1flox/flox mice were described before (Hetz et al., 2008). All experimental procedures were performed in compliance with animal protocols approved by the IACUC at Children's Hospital, Boston.
Intravitreal injection and optic nerve crush
For each intravitreal injection, the micropipette was inserted in peripheral retina just behind the ora serrata, and was deliberately angled to avoid damage to the lens. The left optic nerve was exposed intraorbitally and crushed with forceps for 5 seconds approximately 1 mm behind the optic disc, as described previously (Park et al., 2008).
RGC purification
For retrograde labeling of RGCs, the superior colliculi of adult Sprague-Dawley rats were injected with DiI (2% in DMF). 5–7 days after surgery, eyes were removed and retinas were prepared for the cell dissociation procedures. Dissociated retinal cells were for FACS sorting to collect DiI positive RGC cells.
RT-PCR and q-PCR
Total RNA was extract from purified RGCs and was reverse-transcribed to cDNA, which was amplified by PCR using specific primers for XBP-1u or XBP-1s. For q-PCR, total RNA (50–100 ng) was reverse transcribed and amplified with TagMan pre-designed real-time PCR assays. Each sample was run in quadruplicate in each assay. GADPH was used as the endogenous control.
Immunohistochemistry and in situ hybridization
Immunostaining and in situ hybridization were performed following standard protocols (Park et al., 2008). Retinal sections were incubated with primary antibodies overnight at 4°C and washed three times for 15minutes each with PBS. Secondary antibodies were then applied and incubated for 1 hour at room temperature. Sections were again washed three times for 15 minutes each with PBS before a cover slip was attached with Fluoromount-G. For RGC counting, whole-mount were immunostained with the TUJ1 antibody and 6–9 fields were randomly sampled from peripheral region per retina to estimate RGC survival. The people who counted the cells were blinded with the treatment of the samples.
AAV production
For making AAV2-XBP-1s, the cDNA of XBP-1s-3HA was inserted downstream of the CMV promoter/β-globin intron enhancer in the vector pAAVsc CB6.RBG and viral preparation was made by UMass Gene Therapy Center. The titer determined by silver staining is 1.85×1012.
Induction of chronic IOP elevation in mice
The procedure in detail has been described recently (Chen et al., 2010; Sappington et al., 2010). Briefly, in anesthetized mice, elevation of IOP was induced unilaterally in adult mice by anterior chamber injection of 2 µl fluorescent polystyrene microspheres. The control group received 2µl saline to the anterior chamber. Mice received a second injection of microbeads at 4 week after the first injection. The mice with corneal opacity or signs of inflammation in the anterior chamber (e.g. cloudy anterior chamber) were excluded from further analysis. IOP was measured every other day in both eyes using a TonoLab tonometer.
Statistical analyses
Data are presented as means ± s.e.m. We used Student’s t-test for two group comparison and One way ANOVA and Tukey's Multiple Comparison Test for multiple comparisons.
Supplementary Material
ACKNOWLEDGEMENTS
We thank B. Xu and L. Connolly for technical supports. Supported by grants from NEI (ZH), Miami Project to Cure paralysis (KKP), NIH grant AI32412 and a grant from an anonymous foundation (LHG). YH was supported by a NIH NRSA Postdoctoral Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
YH, KKP, LY, QY, XW, PT and AHL performed the experiments and analyzed the data. YH, LHG, DFC, and ZH designed experiments and prepared the manuscript.
REFERENCES
- Aoki S, Su Q, Li H, Nishikawa K, Ayukawa K, Hara Y, Namikawa K, Kiryu-Seo S, Kiyama H, Wada K. Identification of an axotomy-induced glycosylated protein, AIGP1, possibly involved in cell death triggered by endoplasmic reticulum-Golgi stress. J Neurosci. 2002;22:10751–10760. doi: 10.1523/JNEUROSCI.22-24-10751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci. 1994;14:4368–4374. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG., Jr Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busskamp V, Duebel J, Balya D, Fradot M, Viney TJ, Siegert S, Groner AC, Cabuy E, Forster V, Seeliger M, et al. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science. 2010;329:413–417. doi: 10.1126/science.1190897. [DOI] [PubMed] [Google Scholar]
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- Chen H, Wei X, Cho KS, Chen G, Sappington RM, Calkins D, Chen DF. Optic Neuropathy Due to Microbead-Induced Elevated Intraocular Pressure in the Mouse. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.09-5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Cory S, Fraser SE. BDNF in the development of the visual system of Xenopus. Neuron. 1994;12:747–761. doi: 10.1016/0896-6273(94)90328-x. [DOI] [PubMed] [Google Scholar]
- Deocaris CC, Kaul SC, Wadhwa R. On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60. Cell Stress Chaperones. 2006;11:116–128. doi: 10.1379/CSC-144R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doh SH, Kim JH, Lee KM, Park HY, Park CK. Retinal ganglion cell death induced by endoplasmic reticulum stress in a chronic glaucoma model. Brain Res. 2010;1308:158–166. doi: 10.1016/j.brainres.2009.10.025. [DOI] [PubMed] [Google Scholar]
- Fels DR, Koumenis C. The PERK/eIF2alpha/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol Ther. 2006;5:723–728. doi: 10.4161/cbt.5.7.2967. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Hetz C, Lee AH, Gonzalez-Romero D, Thielen P, Castilla J, Soto C, Glimcher LH. Unfolded protein response transcription factor XBP-1 does not influence prion replication or pathogenesis. Proc Natl Acad Sci U S A. 2008;105:757–762. doi: 10.1073/pnas.0711094105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C, Thielen P, Matus S, Nassif M, Court F, Kiffin R, Martinez G, Cuervo AM, Brown RH, Glimcher LH. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009;23:2294–2306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Libby RT, Jakobs TC, Smith RS, Phalan FC, Barter JW, Barbay JM, Marchant JK, Mahesh N, Porciatti V, et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol. 2007;179:1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrigan LA, Zack DJ, Quigley HA, Smith SD, Pease ME. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol. 1997;115:1031–1035. doi: 10.1001/archopht.1997.01100160201010. [DOI] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin LA. Mechanisms of optic neuropathy. Curr Opin Ophthalmol. 1997;8:9–15. doi: 10.1097/00055735-199712000-00003. [DOI] [PubMed] [Google Scholar]
- Libby RT, Li Y, Savinova OV, Barter J, Smith RS, Nickells RW, John SW. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet. 2005;1:17–26. doi: 10.1371/journal.pgen.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci U S A. 1994;91:1632–1636. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KR, Klein RL, Quigley HA. Gene delivery to the eye using adeno-associated viral vectors. Methods. 2002;28:267–275. doi: 10.1016/s1046-2023(02)00232-3. [DOI] [PubMed] [Google Scholar]
- McKernan DP, Cotter TG. A Critical role for Bim in retinal ganglion cell death. J Neurochem. 2007;102:922–930. doi: 10.1111/j.1471-4159.2007.04573.x. [DOI] [PubMed] [Google Scholar]
- Minckler DS, Bunt AH, Johanson GW. Orthograde and retrograde axoplasmic transport during acute ocular hypertension in the monkey. Invest Ophthalmol Vis Sci. 1977;16:426–441. [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennuto M, Tinelli E, Malaguti M, Del Carro U, D'Antonio M, Ron D, Quattrini A, Feltri ML, Wrabetz L. Ablation of the UPR-mediator CHOP restores motor function and reduces demyelination in Charcot-Marie-Tooth 1B mice. Neuron. 2008;57:393–405. doi: 10.1016/j.neuron.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Qu J, Wang D, Grosskreutz CL. Mechanisms of retinal ganglion cell injury and defense in glaucoma. Exp Eye Res. 2010;91:48–53. doi: 10.1016/j.exer.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA. Open-angle glaucoma. N Engl J Med. 1993;328:1097–1106. doi: 10.1056/NEJM199304153281507. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–786. [PubMed] [Google Scholar]
- Reimold AM, Etkin A, Clauss I, Perkins A, Friend DS, Zhang J, Horton HF, Scott A, Orkin SH, Byrne MC, et al. An essential role in liver development for transcription factor XBP-1. Genes Dev. 2000;14:152–157. [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Roy B, Lee AS. The mammalian endoplasmic reticulum stress response element consists of an evolutionarily conserved tripartite structure and interacts with a novel stress-inducible complex. Nucleic acids research. 1999;27:1437–1443. doi: 10.1093/nar/27.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappington RM, Carlson BJ, Crish SD, Calkins DJ. The microbead occlusion model: a paradigm for induced ocular hypertension in rats and mice. Invest Ophthalmol Vis Sci. 2010;51:207–216. doi: 10.1167/iovs.09-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S, Cabuy E, Caroni P. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat Neurosci. 2009;12:627–636. doi: 10.1038/nn.2297. [DOI] [PubMed] [Google Scholar]
- Selles-Navarro I, Ellezam B, Fajardo R, Latour M, McKerracher L. Retinal ganglion cell and nonneuronal cell responses to a microcrush lesion of adult rat optic nerve. Exp Neurol. 2001;167:282–289. doi: 10.1006/exnr.2000.7573. [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, Lee AH, Qian SB, Zhao H, Yu X, Yang L, Tan BK, Rosenwald A, et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity. 2004;21:81–93. doi: 10.1016/j.immuni.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Sidrauski C, Walter P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell. 1997;90:1031–1039. doi: 10.1016/s0092-8674(00)80369-4. [DOI] [PubMed] [Google Scholar]
- Silva A, Wang Q, Wang M, Ravula SK, Glass JD. Evidence for direct axonal toxicity in vincristine neuropathy. Journal of the peripheral nervous system. JPNS. 2006;11:211–216. doi: 10.1111/j.1529-8027.2006.0090.x. [DOI] [PubMed] [Google Scholar]
- Silva RM, Ries V, Oo TF, Yarygina O, Jackson-Lewis V, Ryu EJ, Lu PD, Marciniak SJ, Ron D, Przedborski S, et al. CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J Neurochem. 2005;95:974–986. doi: 10.1111/j.1471-4159.2005.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MH, Smith AJ, Pawlyk B, Xu X, Liu X, Bainbridge JB, Basche M, McIntosh J, Tran HV, Nathwani A, et al. Gene therapy for retinitis pigmentosa and Leber congenital amaurosis caused by defects in AIPL1: effective rescue of mouse models of partial and complete Aipl1 deficiency using AAV2/2 and AAV2/8 vectors. Hum Mol Genet. 2009;18:2099–2114. doi: 10.1093/hmg/ddp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano F, Bertolotti A, Ron D. IRE1 and efferent signaling from the endoplasmic reticulum. Journal of cell science. 2000;113(Pt 21):3697–3702. doi: 10.1242/jcs.113.21.3697. [DOI] [PubMed] [Google Scholar]
- Vohra BP, Sasaki Y, Miller BR, Chang J, DiAntonio A, Milbrandt J. Amyloid precursor protein cleavage-dependent and -independent axonal degeneration programs share a common nicotinamide mononucleotide adenylyltransferase 1-sensitive pathway. J Neurosci. 2010;30:13729–13738. doi: 10.1523/JNEUROSCI.2939-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.