“I am accordingly of the opinion that the normal regulation of islet content in the pancreas is by interstitial growth of pre-existing islets and by the formation of new islets from the duct epithelium, and not at all by the formation of new islets out of acini.”
R.R. Bensley Am J Anatomy 1911;12:297–388
For almost a century (for historical review, see 1) both β-cell replication and neogenesis (the differentiation of new islet cells from progenitors or stem cells) have been thought to be responsible for postnatal growth of the endocrine pancreas. Even though doubts have been raised in recent years about the existence and importance of neogenesis, this skepticism may be subsiding. Replication and neogenesis are not mutually exclusive. Both processes often occur simultaneously, as seen during the regeneration that follows pancreatic injury. However, there are important differences in the balance of these two pathways that depend upon species and age. Replication of β-cells is an important mechanism particularly in adult rodents, but there are compelling data that after-birth progenitors also have a role in renewal and growth of islets. Eventually one or both of these pathways may be manipulated for therapeutic treatment of diabetes. Since we and others have extensively reviewed the regulation of β-cell mass (2,3), this Perspective will specifically address the contributions of the neogeneic pathway to new β-cell formation, considering whether postnatal neogenesis occurs, to what extent; possible differences between mammalian species; and whether it might be exploited therapeutically.
β-Cell expansion in normal growth.
The concept that β-cell mass is dynamic and increases and decreases both in function and mass to maintain the glycemic level within a very narrow physiological range (4) is now generally accepted. In both normal and pathophysiological states, the mechanisms responsible are changes in replication and neogenesis, changes in individual cell volume, and changes in cell loss or death rates. In rodents, β-cell replication is significantly higher during late gestation and the neonatal period than after weaning and changes little beyond 30–40 days of age except in response to physiological/pathological changes (2,3,5). Other than during the neonatal remodeling of the endocrine pancreas that occurs before weaning (6), the frequency of apoptosis is low and unchanging (7). In the initial months of life, β-cell mass increases with body weight through increases in both β-cell number and size, but in old (15–20 months) rats the increment in mass is largely accounted for by increased β-cell size (7). While replication and apoptosis of the β-cell have been the focus of many studies in the last decade, changes in cell volume are usually not measured; however, changes have been well documented in a number of other models (8–10). In the case of neogenesis, there is strong evidence that the process occurs normally and can be activated in mouse, rat, pig, and human.
The concept of neogenesis or the formation of new islet cells from pancreatic progenitor/stem cells after birth has been built upon many observations from different models and species over many years. An appealing assumption has been that the islet hormone-positive cells within the ductal epithelium or that appear to be “budding” from the ducts represent neogenesis (Figs. 1 and 2). However, it can be argued that they are static with no active “budding” or neogenesis (11). Other than lineage tracing, no direct means of showing a dynamic process of neogenesis exists, but with the limited labeling efficiency of current lineage-tracing approaches, detection of marked cells rather than the lack of such cells represents stronger evidence. Many studies have quantified neogenesis as increased hormone-positive cells within ducts or small clusters of hormone-positive cells in the parenchyma that accompany growth whether normal growth, after surgical perturbation or addition of various growth factors and/or cytokines. These data have strengthened the hypothesis that this budding appearance actually represents the dynamic process of neogenesis, such that hormone-positive cells within ducts may be used as quantifiable markers. Table 1 shows more quantitative studies over the past several decades; more detail is provided below about some of these models. It should be noted that not all rodent models of β-cell growth show neogenesis: no increases in islet number were seen in pregnancy in mice (12) or in adult ob/ob mice compared with ob/+ controls (13); both of these studies showed increased β-cell mass with larger islets, supporting replication as the mechanism of expansion. One must be aware of possible species differences, as illustrated by neogenesis not being reported in adult mice fed a high-fat diet but reported in adult pigs that became obese after ad libitum feeding for 1–2 years (14). Additionally, in contrast to the data from rodents, the adaptive increase in β-cells during pregnancy in humans is accompanied by decreased mean islet size, increased number of β-cells in “apparently new small islets” with no change in β-cell size, replication, or apoptosis frequency (15).
FIG. 1.
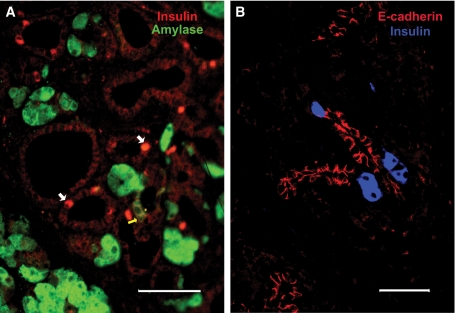
Neogenesis in mouse pancreas. A: The metaplastic ducts seen in the zinc-treated metallothionen. TGF-α transgenic mice have 6% insulin+ (red, white arrows) cells and some amylase+ (green, yellow arrow) cells. Tissue from study reported in (37); the background in the red channel was enhanced to see the context of the ducts. Magnification bar = 100 μm. B: After partial (70–80%) pancreatectomy in adult mice, insulin+ (blue) cells “budding” from proliferating ductules labeled with E-cadherin (red) are found in well-defined areas termed foci of regeneration that further differentiate into new lobes of pancreas (40). Magnification bar = 50 μm. (A high-quality digital representation of this figure is available in the online issue.)
FIG. 2.
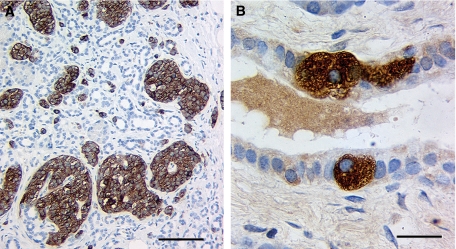
Neogenesis in adult human pancreas. Insulin+ (brown) cells seen in the ducts in adult human pancreas. A: Surgical sample from patient who had partial pancreatectomy due to recurrent postprandial hypoglycemia after gastric bypass as reported in Patti et al. (79). While no Ki67+ islet cells were seen in sections from this pancreas, many ductal cells were Ki67+ (not shown) and many insulin+ cells are seen within the ductules. Magnification bar = 100 μm. B: Insulin+ cells are found within the ductal epithelium in human pancreas. Magnification bar = 20 um. (A high-quality digital representation of this figure is available in the online issue.)
TABLE 1.
Studies providing evidence of in vivo neogenesis
| Species | Observation | References |
|---|---|---|
| Rat | ||
| Neonates | Islet mass growth from cytokeratin+ cells at periphery of islets; β-cell mass determinants measured, and mathematical modeling | Bouwens et al. (21); Wang et al. (89); Finegood et al. (5); Scaglia et al. (6); Bonner-Weir et al. (22) |
| Neonatal STZ | Appearance of small islet clusters associated with ducts | Portha and colleagues (90) |
| Duct ligation | Appearance of small islet clusters associated with ducts | Edstrom (50) |
| Wang et al. (32) | ||
| Duct ligation + gastrin | Appearance of small islet clusters associated with ducts | Rooman et al. (52) |
| Pancreatectomy (90%) | New lobe formation with new islets, enhanced replication too | Bonner-Weir and colleagues (31, 38) |
| Zucker fatty and Zucker fatty diabetic | Increased small islet clusters associated with ducts | Pick et al. (91) |
| Zucker fatty rats | Increased small islets associated with ducts, increased number islets | Jetton et al. (44) |
| 20% glucose infusion (48 h) | Increased small islet clusters associated with ducts | Jetton et al. (92) |
| Exendin 4 | Increased hormone+ cells in ducts | Xu et al. (8) |
| Soybean trypsin inhibitor | Increased volume of insulin+ cells in ducts | Weaver et al. (33) |
| Mouse | ||
| Early postnatal | Increased number of islets from 1 week to 2 months; lineage tracing of duct-specific promoter birth to 4 weeks | Inada et al. (20); Peng et al. (26) |
| Ductal ligation | Ngn3+ cells in and adjacent to ducts | Xu et al. (19) |
| CAIICreERT lineage tracing | Inada et al. (20) | |
| Alloxan perfusion to part of pancreas | Replication in nonperfused part and neogenesis in perfused | Waguri et al. (34) |
| Alloxan + betacellulin | Increased ICC/mm2 pancreas | Yamamoto et al. (93) |
| Alloxan + adv-betacellulin | Significantly increased insulin+ duct cells | Tokui et al. (94) |
| Alloxan + EGF and gastrin | Significantly increased insulin+ duct cells | Rooman and Bouwens (95) |
| Pancreatectomy (60%) | Increased small clusters before β-cell proliferation; FOXM1 necessary for proliferation but for not neogenesis | Peshavaria et al. (46); Ackermann Misfeldt et al. (47) |
| Retroductal adv-GFP (neonatal) | GFP in islets over first 2 weeks | Peng et al. (26) |
| Metallothionen:TGF-α × Ins:gastrin | Insulin+ cells in metaplastic ducts, increased islets | Wang et al. (37) |
| RIP:interferon-ψ | Increased insulin+ clusters in ducts | Gu and Sarvetnick (36) |
| NeuroD-null mice | 10% duct in null mice had “budding” insulin+ cells | Huang et al. (96) |
| Glucagon:Pax4 | Reprogramming of α- to β-cells and replenishment of α from ducts | Collombat et al. (30) |
| Pig | ||
| Obese minipig, after 1 of year age | Increased islet numbers but same mean volume of islets | Larsen et al. (14) |
| Human | ||
| Autopsied pancreas, birth to age 20 years | After 12 years, most 0.5–1.2% insulin+ duct cells, some none | Meier et al. (62) |
| Donor pancreas, aged 7–70 years | Unchanged low level of neogenesis from 7 to 70 years | Reers et al. (65) |
| Autopsied, control subjects | Obese 1.2% insulin+ duct cells but lean 0.6 ± 0.2% insulin+ duct cells | Meier and colleagues (66, 74) |
| Autopsied, chronic pancreatitis | Significantly increased glucagon+ or insulin+ duct cells | Phillips et al. (76) |
| Biopsied failed pancreatic transplant | Increased insulin+ ducts in transplants with recurrent autoimmunity | Martin-Pagola et al. (78) |
| Pregnancy | Increased β-cells with no change in replication, cell size, or apoptosis frequency; increased insulin+ duct cells and very small islets | Butler et al. (15) |
However, largely based on Dor et al. in 2004 (11), the occurrence of neogenesis has been questioned (16,17). The Dor et al. study, in which genetic marking of β-cells in rat insulin promoter:CreER mice was not diluted over several months of chase in adult mice, showed that replication as a major mechanism of β-cell expansion in adult mice. However, with possible low levels of leakiness of the Cre-lox system over time with the strong insulin promoter (18), the small proportion of the islets counted, and only 30% labeling of the β-cells, neogenesis in the adult cannot be ruled out by negative data of a lack of dilution of labeled β-cells. Furthermore, the common extrapolation of these data that neogenesis does not occur after birth is flawed since neither the neonatal period nor the new lobes after pancreatectomy were examined.
In building a case for a given hypothesis, it has become fashionable to rely more heavily upon genetic methodology than correlative observations. However, each approach has its strengths and limitations. In the case of neogenesis, years of strong observational support had now been supplemented by molecular tracing experiments, with two strong studies (19,20) in 2008 that complement past evidence for postnatal neogenesis.
Neogenesis in normal growth during the neonatal period.
During the fetal stage, differentiation is the major mechanism for forming new β-cells, but replication or self-duplication is enhanced during the perinatal period. During the neonatal period, β-cell replication continues and significant neogenesis occurs under these normal physiological conditions, as has been demonstrated using various methods, including Cre-lox lineage tracing experiments.
Bouwens et al. (21) studying neonatal rat pancreas in the first 2 weeks after birth suggested that cytokeratin 20+ (a marker of rat duct epithelium) cells at the periphery of islets served as islet progenitors. Between 2 and 5 days of age, the β-cell mass more than doubled, but the cross-sectional area of the β-cells was unchanged, indicating that new cells rather than larger cells accounted for the increased mass. The BrdU incorporation in β-cells of 2.4% at day 2 could account for only 12% of the observed growth by day 5. It was concluded that most of the new β-cells originated from neogenesis. Their observation that CK20+ cells had higher BrdU incorporation than hormone-positive cells is consistent with the finding that duct cell replication precedes neogenesis (22).
Our mathematical modeling study (5) predicted two waves of neogenesis: one immediately after birth and the other 2–3 weeks after birth. This model used existing data on β-cell mass and its determinants (cell volume, replication, and apoptosis frequency) to estimate turnover of β-cells. In our subsequent longitudinal study of β-cell mass and its determinants over the first month after birth (6), we documented the increased appearance of islets budding from the ducts at the same times (shortly after birth and just before weaning), confirming the predicted waves of neogenesis. Using these data to estimate the number of β-cells at each time point, we estimated that ∼70% of the β-cells seen at day 31 could be accounted for by replication of preexisting β-cells, while the remaining 30% were from neogenesis (22). This estimate is consistent with the findings from our duct-specific lineage tracing experiments described next (20).
Carbonic anhydrase II (CAII), which only starts to be expressed in ducts at the very end of gestation (23), has been considered a marker for ducts to distinguish the mature ductal phenotype from the embryonic tubular structures often called embryonic ducts. Thus, bigenic CAIICre:ROSA26R mice can provide duct-specific lineage tracing with only genetically marked ducts expressing the reporter at birth with no CAII or Cre expressed in β-cells (20). We showed that in the 4 weeks after birth, both islets and acini were formed from cells that once expressed CAII: 38% of the islets were marked (17% of all insulin+ cells) as well as a number of acinar cells; some lobes were marked and others not. Since the pancreatic weight increases fourfold between day 17 and day 31 (6), we interpret the lobular pattern of marked islets and acini as evidence of new lobe formation in the neonatal period. It should be noted that two recent lineage-tracing studies using inducible Cre-ER driven by either the hepatocyte nuclear factor (Hnf) 1β (24) or mucin1 promoter (25) found no marked islet nor acinar tissue in neonates when tamoxifen was administered at the end of gestation (24) or birth (25). These studies found only marked ducts during this time of rapid pancreatic expansion, but their negative data may be due to 1) their low efficiency of marking ducts (20 and 7.6%, respectively) and only ∼1,000 insulin+ cells counted per animal and 2) a marked heterogeneity within the ducts of the expressed product of their driver gene.
Recently, Peng et al. (26) published studies that showed an increasing number of islets from 1 week to 2 months of age. The obvious caveat for enumeration of islets over time in that small clusters of islet cells, which initially may have been below the measurable limit, may increase in size due to proliferation and be counted as newly formed. Interestingly, they also report that Ki67 mRNA and telomere length were significantly correlated in single islets at either 2 weeks or 4 months but differed for islets within the same animal, suggesting islets of differing ages in adult mice.
If new islets were generated from preexisting ductal tissue, one might expect expression of residual duct markers for a short period of time after hormone expression. Indeed, transient expression of markers of duct cells has been demonstrated in β-cells of newborn rats (27) as well as in regenerated islets after partial pancreatectomy (22,28), in grafts of purified human duct cells (29), and in islets of mice conditionally expressing Pax4 in glucagon-producing cells (POE::GluCre) (30), suggesting their recent passage through the ductal phenotype.
Models of regeneration associated with neogenesis.
Both partial pancreatectomy (31) and partial duct ligation (32), classic models of regeneration in rodents, are followed by both β-cell replication and neogenesis. Adding to the weight of evidence, newly formed or “budding” islets have been found in a number of other experimental conditions (Table 1), including dietary treatment with soybean trypsin inhibitor (33), after glucagon-like peptide (GLP)-1/exendin-4 treatment (8), treatment with betacellulin (34), and after cellophane wrapping of the head of the pancreas (a partial duct obstruction) (35). Additional strong evidence of formation of new β-cells from differentiation of progenitors comes from a number of transgenic models, including overexpression of interferon-γ in the β-cells of transgenic mice (36), overexpression of transforming growth factor (TGF)-α in pancreatic ducts (37), and Pax4 ectopic expression in glucagon-positive cells (30). These regeneration models may not be normal physiology but may show pathways that can be exploited, as much as transgenic and knockout mice are hardly physiological but quite informative.
Partial pancreatectomized rodents.
A 90% pancreatectomy in rats demonstrates the substantial regenerative capacity of the adult pancreas (31,38,39). By 8 weeks after surgery, the 10% remnant had regenerated such that the pancreatic weight was 27% and the β-cell mass was 45% of the sham-operated pancreas. This was accounted for by both increased β-cell proliferation and formation of whole-new lobes of pancreas that contained newly formed islets (31,40). Expansion of the ductal tree by replication could be demonstrated with BrdU incorporation and subsequent appearance of branching ductules in well-defined areas (foci of regeneration) that correspond to new lobes. The pancreatic duct cells after replication transiently express pancreatic duodenal homeobox (PDX)-1 protein (41) and lose expression of key markers of the mature duct phenotype (40). The appearance of these focal regions and their disappearance with maturation coincide with the marked growth of the pancreatic remnant. Within a single pancreas, there are multiple focal areas of varying stages of maturation, as assessed by their histological appearance and gene expression profiles. Ductules in these foci express many markers of embryonic pancreatic progenitors, including transient expression of the endocrine lineage-specific transcription factor neurogenin 3 (40). Islets in these foci resemble embryonic islets with higher proportion of insulin+ cells lacking MafA expression; the proportion of MafA+/insulin+ cells increases as foci differentiate further, but even in mature foci the proportion of MafA-expressing β-cells was lower than in the remnant pancreas of the same animals (40). We conclude that in response to pancreatectomy, pancreatic duct cells recapitulate aspects of embryonic pancreas differentiation and contribute to the regenerating pancreas.
After partial pancreatectomy, regeneration is not limited to young animals. Both replication and neogenesis could be demonstrated in retired breeder (500 g) rats subjected to the same surgery (42). Whole-new lobes of pancreas may even be formed spontaneously in normal adult animals; occasional pancreatic lobes with high BrdU incorporation were seen in 6-month-old rats when most of the pancreas had almost none (5,22). When the extent of resection of pancreas is less, there is less regeneration (43). Even so, Jetton et al. (44) have utilized the 60% pancreatectomy in adult rats extensively and reported enhanced β-cell proliferation and neogenesis.
The partial pancreatectomy model has been transferred to mice using less extensive resection, often only 50%. In several reports, only enhanced replication was reported (11,16,45), but two groups using 60% pancreatectomy reported neogenesis in addition to enhanced replication (46,47). These latter studies have even defined some of the regulatory pathways involved in neogenesis. Using 70–80% pancreatectomy in C57BL/6 mice, we obtain mild to moderate hyperglycemia and have found the same regeneration pattern as in 90% pancreatectomy rats (Fig. 1B) (48).
Partial duct ligation in rodents.
The partial duct ligation has been used for several decades in rats (32,49–52). Wang et al. (32) did a comprehensive study of this model and showed that the β-cell population distal to the ligation doubled in 1 week and that the labeling index with BrdU pulse labeling in β-cells could not account for this increase. With increased number of small islets and islet cell clusters in the distal ligated portion, they concluded that there was islet neogenesis. A value of this model is that regeneration is limited to the portion distal to the ligation, allowing an internal control. In later experiments, this model was used to show that gastrin would stimulate β-cell neogenesis in the distal portion but not in the pancreas proximal to the ligation (52).
Importantly, this model transferred to mice has been useful in lineage-tracing studies. For example, with duct ligation on acinar-specific elastase 1 promoter:ROSA 26R bigenic mice, Desai et al. (53) found no marked islets after ductal ligation and concluded that acinar cells did not contribute to in vivo new islet formation after pancreatic ligation. Then, in a series of elegant experiments with various neurogenin 3 reporter mice, Heimberg and colleagues (19) showed that neurogenin 3 was induced in cells in or adjacent to the pancreatic ducts after partial duct ligation. By isolating these cells with flow cytometry and subsequent transplanting them into explants of embryonic pancreas from neurogenin 3–null mice, they showed that these cells gave rise to islet cells including β-cells. While lineage tracing was not performed, these data provide strong support for the concept that multipotent progenitor cells can be activated to increase the islet mass. Complementing these experiments, we tested the hypothesis that duct cells could give rise to β-cells by doing partial duct ligation in inducible duct-specific carbonic anhydrase II CreERT:Rosa26R bigenic mice (20). In the distal, regenerated pancreas, 42% of the islets (24% β-cells) were marked compared with 12% (5.5% β-cells) in the nonligated pancreas of the same animals. Thus, these studies using duct ligation in adult mice have clearly shown that new islet cells can be formed from progenitors activated in the ducts.
Transgenic mice overexpressing cytokines/growth factors/transcription factors.
A number of transgenic mice have reported increased neogenesis, but only three will be described herein. The most studied transgenic model of neogenesis is the human insulin promoter, interferon-γ mouse (54). These mice were found to have continual inflammatory destruction of islets associated with continued proliferation of ductal epithelium and “budding” of new islets from the ducts. Originally the insulitis seen in this model was thought to trigger the neogenesis, but similar results were found with this transgene in immunocompromised mice. In these proliferating ducts, the expression of PDX-1 protein and other transcription factors seen in embryonic pancreatic development suggested that this regeneration recapitulates the embryonic process (55,56).
A second transgenic model, the metallothionein-1–TGF-α mouse, also provides evidence of neogenesis from ducts. The induction of TGF-α in the exocrine pancreas by zinc in the drinking water (57) resulted in sustained proliferation of the ductal epithelium and metaplastic ducts. In these metaplastic ducts, 6% of the cells were immunostained for insulin (37) (Fig. 1A). Moreover, enhanced PDX-1 expression and focal expression of Pax6 suggested the initiation of islet neogenesis (58). When these mice were crossed with mice in which gastrin expression was driven by the insulin promoter, the number of metaplastic ducts was reduced and islet mass increased, leading to the hypothesis that gastrin could drive the differentiation of progenitors to islets (37).
More recently, another variation of neogenesis of islet cells from ducts has been shown in bigenic Pax4:glucagon Cre (POE::gluCre) transgenic mice (30), in which Pax4 is expressed in glucagon-expressing α-cells. This ectopic expression of Pax4 reprogrammed the α-cells to insulin-producing β-cells. However, even with continuing conversion to β-cells, there were still α-cells, suggesting a renewal of the α-cell population. The authors concluded that α-cell neogenesis occurred since 1) BrdU incorporation was mainly in cells within or adjacent to the duct epithelium rather than in hormone-expressing cells; 2) using a lentiviral reporter injected into the ductal tree, and both glucagon and insulin-positive cells were labeled in the induced transgenic mice but not in noninduced controls, suggesting that the hormone+ reporter+ cells were derived from the labeled ducts; and 3) knockdown of neurogenin 3 with shRNA blocked the replenishment of the α-cells.
β-Cell compensation in human pancreas.
Our main interest in studying rodent pancreas is to understand what happens in the human pancreas with which we cannot do the same experiments. We know that the pancreatic β-cell mass is increased in human obesity albeit less than in rodents. Whereas mice can have a 30-fold increase in β-cell mass with insulin resistance or obesity (59), the estimate for the increase in humans is more like 30–40% (60,61). Yet, β-cell proliferation in adult humans is extremely low, and greatly enlarged islets are rarely found. In autopsied human pancreas, β-cell replication (Ki67+ β-cells) drops to <0.2% already by 5 years of age (62) and can be almost negligible in adults (63,64). However, this low level of detection may result from the tissue being only retrieved after death. In a recent study on human organ donor pancreases, all pancreases had Ki67+ β-cells, with a stable, albeit low, percentage in those donors 40–65 years of age (65). An intriguing report documents increased Ki67+ β-cells (0.69 ± 0.15%) in a surgically resected pancreas from an 89-year-old with recent-onset type 1 diabetes (66), suggesting that β-cell replication could still be stimulated.
A major difference between mice and humans is that in humans telomere shortening limits replication and leads to senescence, a process described as replicative aging (67). In contrast, mice have long telomeres and do not show impairment of replication for several generations after ablation of telomerase (68,69). Similarly rats do not show replicative aging (70,71). This difference in replicative aging could account for the differential response (proliferation versus differentiation from stem cells/progenitors) in β-cell compensation (72).
Evidence of neogenesis in human pancreas.
Due to the limits of experimentation possible on human pancreas, our knowledge is based mainly on observations made from pancreases obtained at autopsy, pancreas donation, and surgical resection. A number of pathological reports cite increased hormone-positive cells within the ducts in some human diseases, such as recent-onset type 1 diabetes and severe liver disease (73). In a recent study on pancreata harvested from organ donors (65), insulin+ cells in pancreatic ducts were usually present at low levels (0.4%) across the age span of 7–70 years. Similarly, in autopsied pancreata after the age of 12, 0.5–1.2% of the duct cells were insulin+, although in some pancreata none could be found (62). In other studies by Meier and colleagues (74,75) in lean controls, 0.6 ± 0.2% of duct cells were insulin+ but in pancreata from obese patients, 1.2%. In 10 pancreata from patients with chronic pancreatitis, there were significant increases in the percentage of duct cells positive for glucagon, amylase, Ki67, Pdx1, or insulin compared with control pancreata (76). It is of interest that in a small series of patients with 50% pancreatectomy, there was no evidence of pancreatic or β-cell regeneration and no change in Ki67+ or insulin+ cells in ducts (77), but this is not surprising because replication would only be expected shortly after the surgery. Further evidence that these hormone-positive cells within the ducts were dynamic comes from the comparison of 16 donor pancreata and biopsied pancreata from eight simultaneous pancreas and kidney transplantations (78). In the donor pancreata, the frequency of 0.45% insulin+ duct cells was similar to the other above-mentioned studies and in three transplants without autoimmune recurrence (0.5%). However, in five pancreatic transplants with recurrent autoimmunity, 57.5% of the duct cells expressed insulin protein. This unexpectedly high value needs to be confirmed. Still more evidence suggesting neogenesis in humans comes from a small group patients who develop hypoglycemia after gastric bypass surgery (79). They have been found to have increased β-cell mass and impressive numbers of islet cells within the ducts (Fig. 2A), accompanied by high circulating levels GLP-1, which has been shown to stimulate neogenesis in rodents (8). From these accumulating circumstantial data, one can be more confident that neogenesis is an important process in humans.
Evidence of neogenesis from human tissue in vitro or transplanted into mice.
With the caveat that tissue in vitro may be released from a number of regulatory controls and thereby appear more plastic, there is strong evidence that neogenesis can occur in human pancreatic tissue. Starting with the islet-depleted pancreatic digest remaining after islet isolation, several groups were able to show increased islet cells and increased insulin mRNA (80–83). Indeed, in our own in vitro studies, collections of new islet cells could be seen budding from cysts of duct epithelium (80). Since all of these studies were confounded by the possible inclusion of β-cells in the starting material, we purified human ductal cells, using immunomagnetic beads and an antibody against the cell surface antigen CA19–9, and transplanted aggregates of expanded duct cells into immunocompromised mice (29). Here, too, we could show the induction of insulin+ cells and insulin mRNA. Some insulin+ cells in the grafts coexpressed duct markers (CK19 and CA19-9) and HSP27, a marker of nonislet cells, suggesting their transition from duct.
Suarez-Pinzon and colleagues (84,85) have provided further support to activation of neogenesis in human pancreatic ducts. First, using human pancreatic preparations that included islets, ducts and acinar cells treated 4 weeks in vitro with the combination of EGF and gastrin were found to have a doubling of β-cells with similar increases in the number of cells expressing the duct marker cytokeratin 19 and the transcription factor IPF-1/PDX-1 (84). Then, in a later study (85), similar low-purity human islet preparations were dispersed and transplanted under the kidney capsule of streptozotocin-induced diabetic NOD-scid mice, which then were treated with GLP-1 and gastrin for 5 weeks. In those treated with this combination, there was a fourfold increase in insulin+ cells compared with vehicle-treated controls from the same donor pancreas, with a high proportion of insulin+ cells also expressing cytokeratin 19.
What is the cell of origin of the neogeneic islet cells?
A major question about neogenesis under debate is, What is the cell of origin? Most reports have suggested a cell of origin in or adjacent to the pancreatic duct epithelium, but is it a true stem cell, a differentiated duct epithelial cell capable of multipotent redifferentiation, or some other cell type? If a duct cell, can any duct cell have the potential or just a subpopulation? Xu et al. (19) clearly showed neurogenin 3 activation in cells within or adjacent to the ducts, and our report (20) showed that carbonic anhydrase II was expressed in the cell of origin. However, the recent Solar et al. (24) study using Hnf1β promoter as a driver for the Cre recombinase excision did not find any marked β-cells after partial duct ligation. Their interpretation was that differentiated duct cells do not contribute to new β-cell formation but a more precise interpretation would be that duct cells with high enough levels of Hnf1β transcription to cause excision do not have this potential (86).
In light of the report of Solar et al. (24), a redefined question should be, Are there identifiable subpopulations within the ductal tree and which ones have progenitor potential? We asked whether there is a heterogeneity of expression of progenitor markers throughout the ductal tree (87) by immunostaining with titration of antibodies against Hnf1β and Sox9. Both proteins can be found throughout the pancreatic ductal tree, but the intensity and proportion of the expressing population varies within the various categories of ducts, and only some cells express both. Better understanding of this heterogeneity may define a progenitor population. The recent identification of a population of terminal ductule/centroacinar cells that express high activity of aldehyde dehydrogenase 1 and have the ability to self-renew and to differentiate into both endocrine and acinar cells (88) adds new exciting directions for study. Moreover, expansion of these cells was found in chronic caerulein-induced pancreatitis. Further characterization of this population of cells in various other models is now a clear priority.
In summary, considering the overall body of evidence, we can only conclude that both replication of preexisting β-cells and neogenesis are pathways that contribute to maintaining an adequate β-cell mass after birth. Data show that both pathways are functional in all mammalian species studied, but different contributions, which are quantitative rather than qualitative, are made in different species and under different conditions. Eventually, one or both of these pathways may be manipulated for therapeutic treatment of diabetes. Our next efforts should address important questions such as, What is the cell of origin? what are the pathways that drive the activation of the progenitors? can we selectively stimulate neogenesis and to what extent? how applicable will these treatments be for human therapy?
ACKNOWLEDGMENTS
This research has been supported by the National Institute of Diabetes and Digestive and Kidney Diseases, the Juvenile Diabetes Research Foundation, and an important group of private donors.
No potential conflicts of interest relevant to this article were reported.
S.B.-W. wrote the manuscript. W.-C.L., L.O.-Y., and L.G. researched data. G.C.W. and A.S. contributed to the discussion and reviewed/edited the manuscript.
The authors thank those past and present members of the laboratory who have contributed in many ways to exploring the formation of new islet cells and for helpful discussions.
REFERENCES
- 1. Granger A, Kushner JA. Cellular origins of beta-cell regeneration: a legacy view of historical controversies. J Intern Med 2009;266:325–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonner-Weir S. Perspective: postnatal pancreatic beta cell growth. Endocrinology 2000;141:1926–1929 [DOI] [PubMed] [Google Scholar]
- 3. Bouwens L, Rooman I. Regulation of pancreatic beta-cell mass. Physiol Rev 2005;85:1255–1270 [DOI] [PubMed] [Google Scholar]
- 4. Bonner-Weir S. Regulation of pancreatic β-cell mass in vivo. Recent Prog Horm Res 1994;49:91–104 [DOI] [PubMed] [Google Scholar]
- 5. Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of β-cell mass in the growing rat pancreas: estimation with a simple mathematical model. Diabetes 1995;44:249–256 [DOI] [PubMed] [Google Scholar]
- 6. Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinol 1997;138:1736–1741 [DOI] [PubMed] [Google Scholar]
- 7. Montanya E, Nacher V, Biarnes M, Soler J. Linear correlation between beta cell mass and body weight throughout life in Lewis rats: role of β-cell hyperplasia and hypertrophy. Diabetes 2000;49:1341–1346 [DOI] [PubMed] [Google Scholar]
- 8. Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both β-cell replication and neogenesis, resulting in increased β-cell mass and improved glucose tolerance in diabetic rats. Diabetes 1999;48:2270–2276 [DOI] [PubMed] [Google Scholar]
- 9. Scaglia L, Smith FE, Bonner-Weir S. Apoptosis contributes to the involution of β cell mass in the post partum rat pancreas. Endocrinol 1995;136:5461–5468 [DOI] [PubMed] [Google Scholar]
- 10. Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic β-cells in adult rats after short-term glucose infusion. Diabetes 1989;38:49–53 [DOI] [PubMed] [Google Scholar]
- 11. Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41–46 [DOI] [PubMed] [Google Scholar]
- 12. Parsons JA, Bartke A, Sorenson RL. Number and size of islets of langerhans in pregnant human growth hormone-expressing transgenic, and pituitary dwarf mice: effect of lactogenic hormones. Endocrinol 1995;136:2013–2021 [DOI] [PubMed] [Google Scholar]
- 13. Bock T, Pakkenberg B, Buschard K. Increased islet volume but unchanged islet number in ob/ob mice. Diabetes 2003;52:1716–1722 [DOI] [PubMed] [Google Scholar]
- 14. Larsen MO, Rolin B, Raun K, Bjerre Knudsen L, Gotfredsen CF, Bock T. Evaluation of beta-cell mass and function in the Gottingen minipig. Diabetes Obes Metab 2007;9(Suppl. 2):170–179 [DOI] [PubMed] [Google Scholar]
- 15. Butler AE, Cao-Minh L, Galasso R, Rizza RA, Corradin A, Cobelli C, Butler PC. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia, 2010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult Beta cells does not involve specialized progenitors. Dev Cell 2007;12:817–826 [DOI] [PubMed] [Google Scholar]
- 17. Dor Y, Melton DA. Facultative endocrine progenitor cells in the adult pancreas. Cell 2008;132:183–184 [DOI] [PubMed] [Google Scholar]
- 18. Guo C, Yang W, Lobe CG. A Cre recombinase transgene with mosaic, widespread tamoxifen-inducible action. Genesis 2002;32:8–18 [DOI] [PubMed] [Google Scholar]
- 19. Xu X, D'Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van De Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 2008;132:197–207 [DOI] [PubMed] [Google Scholar]
- 20. Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A 2008;105:19915–19919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bouwens L, Wang RN, De Blay E, Pipeleers DG, Kloppel G. Cytokeratins as markers of ductal cell differentiation and islet neogenesis in the neonatal rat pancreas. Diabetes 1994;43:1279–1283 [DOI] [PubMed] [Google Scholar]
- 22. Bonner-Weir S, Toschi E, Inada A, Reitz P, Fonseca SY, Aye T, Sharma A. The pancreatic ductal epithelium serves as a potential pool of progenitor cells. Ped Diabetes 2004;5:15–22 [DOI] [PubMed] [Google Scholar]
- 23. Inada A, Nienaber C, Fonseca S, Bonner-Weir S. Timing and expression pattern of carbonic anhydrase II in pancreas. Dev Dyn 2006;235:1571–1577 [DOI] [PubMed] [Google Scholar]
- 24. Solar M, Cardalda C, Houbracken I, Martin M, Maestro MA, De Medts N, Xu X, Grau V, Heimberg H, Bouwens L, Ferrer J. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell 2009;17:849–860 [DOI] [PubMed] [Google Scholar]
- 25. Kopinke D, Murtaugh LC. Exocrine-to-endocrine differentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC Dev Biol 2010;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peng SW, Zhu LY, Chen M, Zhang M, Li DZ, Fu YC, Chen SR, Wei CJ. Heterogeneity in mitotic activity and telomere length implies an important role of young islets in the maintenance of islet mass in the adult pancreas. Endocrinology 2009;150:3058–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aye T, Toschi E, Sharma A, Sgroi D, Bonner-Weir S. Identification of markers for newly formed beta-cells in the perinatal period: a time of recognized beta-cell immaturity. J Histochem Cytochem 2010;58:369–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Toschi EAT, Fuller A, Gaudet J, Sharma A, Weir GC, Sgroi D, Bonner-Weir S. Gene expression profiling of new b cells: evidence for differentiating β cells passing through a ductal phenotype. Diabetes 53, 2004 [Google Scholar]
- 29. Yatoh S, Dodge R, Akashi T, Omer A, Sharma A, Weir GC, Bonner-Weir S. Differentiation of affinity-purified human pancreatic duct cells to β-cells. Diabetes 2007;56:1802–1809 [DOI] [PubMed] [Google Scholar]
- 30. Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell 2009;138:449–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of the adult exocrine and endocrine pancreas: a possible recapitulation of embryonic development. Diabetes 1993;42:1715–1720 [DOI] [PubMed] [Google Scholar]
- 32. Wang RN, Kloppel G, Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia 1995;38:1405–1411 [DOI] [PubMed] [Google Scholar]
- 33. Weaver CV, Sorenson RL, Kaung HC. Immunocytochemical localization of insulin-immunoreactive cells in the ducts of rats treated with trypsin inhibitor. Diabetologia 1985;28:781–785 [DOI] [PubMed] [Google Scholar]
- 34. Waguri M, Yamamoto K, Miyagawa Ji, Tochino Y, Yamamori K, Kajimoto Y, Nakajima H, Watada H, Yoshiuchi I, Itoh N, Imagawa A, Namba M, Kuwajima M, Yamasaki Y, Hanfusa T, Matsuzawa Y. Demonstration of two different processes of β-cell regeneration in a new diabetic mouse model induced by selective perfusion of alloxan. Diabetes 1997;46:1281–1290 [DOI] [PubMed] [Google Scholar]
- 35. Rosenberg L, Duguid WP, Vinik AI. The effect of cellophane wrapping of the pancreas in the Syrian golden hamster: autoradiographic observations. Pancreas 1989;4:31–37 [DOI] [PubMed] [Google Scholar]
- 36. Gu D, Sarvetnick N. Epithelial cell proliferation and islet neogenesis in IFN-γ transgenic mice. Development 1993;118:33–46 [DOI] [PubMed] [Google Scholar]
- 37. Wang TC, Bonner-Weir S, Oates PS, Chulak M, Simon B, Merlino GT, Schmidt EV, Brand SJ. Pancreatic gastrin stimulates islet differentiation of transforming growth factor alpha-induced ductular precursor cells. J Clin Invest 1993;92:1349–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bonner-Weir S, Trent DF, Weir GC. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest 1983;71:1544–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brockenbrough JS, Weir GC, Bonner-Weir S. Discordance of exocrine and endocrine growth after 90% pancreatectomy in rats. Diabetes 1988;37:232–236 [DOI] [PubMed] [Google Scholar]
- 40. Li WC, Rukstalis JM, Nishimura W, Tchipashvili V, Habener JF, Sharma A, Bonner-Weir S. Activation of pancreatic duct-derived progenitor cells during pancreatic regeneration in adult rats. J Cell Science 2010;123:2792–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sharma A, Zangen GH, Reitz P, Taneja M, Lissauer ME, Miller CP, Weir GC, Habener JF, Bonner-Weir S. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes 1999;48:507–513 [DOI] [PubMed] [Google Scholar]
- 42. Bonner-Weir S, Stubbs M, Reitz P, Taneja M, Smith FE. Partial pancreatectomy as a model of pancreatic regeneration. In Pancreas regeneration. Sarvetnick N. Ed. Karger Landes Systems, 1997, p. 138–153 [Google Scholar]
- 43. Bonner-Weir S, Leahy JL, Weir GC. Induced rat models of non-insulin-dependent diabetes mellitus. In Lessons From Animal Diabetes II. Shapiro E, Renold AE. Eds. London, John Libbey, 1988, p. 295–300 [Google Scholar]
- 44. Jetton TL, Lausier J, LaRock K, Trotman WE, Larmie B, Habibovic A, Peshavaria M, Leahy JL. Mechanisms of compensatory β-cell growth in insulin-resistant rats: roles of Akt kinase. Diabetes 2005;54:2294–2304 [DOI] [PubMed] [Google Scholar]
- 45. Lee CS, De Leon DD, Kaestner KH, Stoffers DA. Regeneration of pancreatic islets after partial pancreatectomy in mice does not involve the reactivation of neurogenin-3. Diabetes 2006;55:269–272 [PubMed] [Google Scholar]
- 46. Peshavaria M, Larmie BL, Lausier J, Satish B, Habibovic A, Roskens V, Larock K, Everill B, Leahy JL, Jetton TL. Regulation of pancreatic beta-cell regeneration in the normoglycemic 60% partial-pancreatectomy mouse. Diabetes 2006;55:3289–3298 [DOI] [PubMed] [Google Scholar]
- 47. Ackermann Misfeldt A, Costa RH, Gannon M. β-Cell proliferation, but not neogenesis, following 60% partial pancreatectomy is impaired in the absence of FoxM1. Diabetes 2008;57:3069–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guo L, Li WC, Pittenger G, Fishwick-Taylor D, Vinik AI, Bonner-Weir S. Pancreatic regeneration after partial pancreatectomy (Px) in mice mirrors that in rats with both enhanced replication and neogenesis. Diabetes 2010;59(Suppl. 1):A676 [Google Scholar]
- 49. Hultquist GT, Karlsson U, Hallner AC. The regenerative capacity of the pancreas in duct-ligated rats. Exp Pathol 1979;17:44–52 [DOI] [PubMed] [Google Scholar]
- 50. Edstrom C. Further quantitative structural studies of the pancreatic islet parenchyma in rats with duct ligation. Acta Soc Med Uppsala 1971;76:127–138 [PubMed] [Google Scholar]
- 51. Lardon J, Huyens N, Rooman I, Bouwens L. Exocrine cell transdifferentiation in dexamethasone-treated rat pancreas. Virchows Arch 2004;444:61–65 [DOI] [PubMed] [Google Scholar]
- 52. Rooman I, Lardon J, Bouwens L. Gastrin stimulates β-cell neogenesis and increases islet mass from transdifferentiated but not from normal exocrine pancreas tissue. Diabetes 2002;51:686–690 [DOI] [PubMed] [Google Scholar]
- 53. Desai BM, Oliver-Krasinski J, De Leon DD, Farzad C, Hong N, Leach SD, Stoffers DA. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest 2007;117:971–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sarvetnick NE, Gu D. Regeneration of pancreatic endocrine cells in interferon-gamma transgenic mice. Adv Exp Med Biol 1992;321:85–89 [DOI] [PubMed] [Google Scholar]
- 55. Kritzik MR, Krahl T, Good A, Krakowski M, St-Onge L, Gruss P, Wright C, Sarvetnick N. Transcription factor expression during pancreatic islet regeneration. Mol Cell Endocrinol 2000;164:99–107 [DOI] [PubMed] [Google Scholar]
- 56. Kritzik MR, Jones E, Chen Z, Krakowski M, Krahl T, Good A, Wright C, Fox H, Sarvetnick N. PDX-1 and Msx-2 expression in the regenerating and developing pancreas. J Endocrinol 1999;163:523–530 [DOI] [PubMed] [Google Scholar]
- 57. Jhappan C, Stahle C, Harkins RN, Fausto N, Smith GH, Merlino GT. TGFa overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell 1990;61:1137–1146 [DOI] [PubMed] [Google Scholar]
- 58. Song SY, Gannon M, Washington MK, Scoggins CR, Meszoely IM, Goldenring JR, Marino CR, Sandgren EP, Coffey RJ, Jr, Wright CV, Leach SD. Expansion of Pdx1-expressing pancreatic epithelium and islet neogenesis in transgenic mice overexpressing transforming growth factor alpha. Gastroenterology 1999;117:1416–1426 [DOI] [PubMed] [Google Scholar]
- 59. Bruning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell 1999;88:561–572 [DOI] [PubMed] [Google Scholar]
- 60. Kloppel G, Lohr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res 1985;4:110–125 [DOI] [PubMed] [Google Scholar]
- 61. Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 2008;10(Suppl. 4):32–42 [DOI] [PubMed] [Google Scholar]
- 62. Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. β-Cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes 2008;57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tyrberg B, Ustinov J, Otonkoski T, Andersson A. Stimulated endocrine cell proliferation and differentiation in transplanted human pancreatic islets: effects of the ob gene and compensatory growth of the implantation organ. Diabetes 2001;50:301–307 [DOI] [PubMed] [Google Scholar]
- 64. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 65. Reers C, Erbel S, Esposito I, Schmied B, Buchler MW, Nawroth PP, Ritzel RA. Impaired islet turnover in human donor pancreata with aging. Eur J Endocrinol 2009;160:185–191 [DOI] [PubMed] [Google Scholar]
- 66. Meier JJ, Lin JC, Butler AE, Galasso R, Martinez DS, Butler PC. Direct evidence of attempted beta cell regeneration in an 89-year-old patient with recent-onset type 1 diabetes. Diabetologia 2006;49:1838–1844 [DOI] [PubMed] [Google Scholar]
- 67. Wright WE, Shay JW. Historical claims and current interpretations of replicative aging. Nat Biotechnol 2002;20:682–688 [DOI] [PubMed] [Google Scholar]
- 68. Forsyth NR, Wright WE, Shay JW. Telomerase and differentiation in multicellular organisms: turn it off, turn it on, and turn it off again. Differentiation 2002;69:188–197 [DOI] [PubMed] [Google Scholar]
- 69. Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 1997;91:25–34 [DOI] [PubMed] [Google Scholar]
- 70. Seluanov A, Chen Z, Hine C, Sasahara TH, Ribeiro AA, Catania KC, Presgraves DC, Gorbunova V. Telomerase activity coevolves with body mass not lifespan. Aging Cell 2007;6:45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Seluanov A, Hine C, Bozzella M, Hall A, Sasahara TH, Ribeiro AA, Catania KC, Presgraves DC, Gorbunova V. Distinct tumor suppressor mechanisms evolve in rodent species that differ in size and lifespan. Aging Cell 2008;7:813–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hanley NA, Hanley KP, Miettinen PJ, Otonkoski T. Weighing up beta-cell mass in mice and humans: self-renewal, progenitors or stem cells? Mol Cell Endocrinol 2008;288:79–85 [DOI] [PubMed] [Google Scholar]
- 73. Gepts W. Pathological anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 1965;14:619–633 [DOI] [PubMed] [Google Scholar]
- 74. Meier JJ, Butler AE, Galasso R, Butler PC. Hyperinsulinemic hypoglycemia after gastric bypass surgery is not accompanied by islet hyperplasia or increased β-cell turnover. Diabetes Care 2006;29:1554–1559 [DOI] [PubMed] [Google Scholar]
- 75. Meier JJ, Butler AE, Galasso R, Rizza RA, Butler PC. Increased islet beta cell replication adjacent to intrapancreatic gastrinomas in humans. Diabetologia 2006;49:2689–2696 [DOI] [PubMed] [Google Scholar]
- 76. Phillips JM, O'Reilly L, Bland C, Foulis AK, Cooke A. Patients with chronic pancreatitis have islet progenitor cells in their ducts, but reversal of overt diabetes in NOD mice by anti-CD3 shows no evidence for islet regeneration. Diabetes 2007;56:634–640 [DOI] [PubMed] [Google Scholar]
- 77. Menge BA, Tannapfel A, Belyaev O, Drescher R, Muller C, Uhl W, Schmidt WE, Meier JJ. Partial pancreatectomy in adult humans does not provoke beta-cell regeneration. Diabetes 2008;57:142–149 [DOI] [PubMed] [Google Scholar]
- 78. Martin-Pagola A, Sisino G, Allende G, Dominguez-Bendala J, Gianani R, Reijonen H, Nepom GT, Ricordi C, Ruiz P, Sageshima J, Ciancio G, Burke GW, Pugliese A. Insulin protein and proliferation in ductal cells in the transplanted pancreas of patients with type 1 diabetes and recurrence of autoimmunity. Diabetologia 2008;51:1803–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Patti ME, McMahon G, Mun EC, Bitton A, Holst JJ, Goldsmith J, Hanto DW, Callery M, Arky R, Nose V, Bonner-Weir S, Goldfine AB. Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia 2005;48:2236–2240 [DOI] [PubMed] [Google Scholar]
- 80. Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O'Neil JJ. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci U S A 2000;97:7999–8004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gao R, Ustinov J, Korsgren O, Otonkoski T. In vitro neogenesis of human islets reflects the plasticity of differentiated human pancreatic cells. Diabetologia 2005;48:2296–2304 [DOI] [PubMed] [Google Scholar]
- 82. Gao R, Ustinov J, Pulkkinen MA, Lundin K, Korsgren O, Otonkoski T. Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes 2003;52:2007–2015 [DOI] [PubMed] [Google Scholar]
- 83. Hao E, Tyrberg B, Itkin-Ansari P, Lakey JR, Geron I, Monosov EZ, Barcova M, Mercola M, Levine F. Beta-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat Med 2006;12:310–316 [DOI] [PubMed] [Google Scholar]
- 84. Suarez-Pinzon WL, Lakey JR, Brand SJ, Rabinovitch A. Combination therapy with epidermal growth factor and gastrin induces neogenesis of human islet b-cells from pancreatic duct cells and an increase in functional β-cell mass. J Clin Endocrinol Metab 2005;90:3401–3409 [DOI] [PubMed] [Google Scholar]
- 85. Suarez-Pinzon WL, Lakey JR, Rabinovitch A. Combination therapy with glucagon-like peptide-1 and gastrin induces beta-cell neogenesis from pancreatic duct cells in human islets transplanted in immunodeficient diabetic mice. Cell Transplant 2008;17:631–640 [DOI] [PubMed] [Google Scholar]
- 86. Kushner JA, Weir GC, Bonner-Weir S. Ductal origin hypothesis of pancreatic regeneration under attack. Cell Metab 2010;11:2–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ouziel-Yahalom L, Li WC, Straussman S, Guo L, Sharma A, Weir GC, Bonner-Weir S. Ductal heterogeneity suggests a subpopulation serves as postnatal pancreatic progenitors. Diabetes 2010;59(Suppl. 1):A25 [Google Scholar]
- 88. Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci U S A 2010;107:75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wang RN, Bouwens L, Kloppel G. Beta-cell proliferation in normal and streptozotocin-treated newborn rats: site, dynamics and capacity. Diabetologia 1994;37:1088–1096 [DOI] [PubMed] [Google Scholar]
- 90. Cantenys D, Portha B, Dutrillaux MC, Hollande E, Roze C, Picon L. Histogenesis of the endocrine pancreas in newborn rats after destruction by streptozotocin an immunocytochemical study. Virchows ArchB 1981;35:109–122 [DOI] [PubMed] [Google Scholar]
- 91. Pick A, Clark J, Kubstrup C, Pugh W, Bonner-Weir S, Polonsky K. Role of apoptosis in failure of β cell mass compensation for insulin resistance and β cell defects in the male Zucker diabetic fatty (ZDF) rat. Diabetes 1998;47:358–364 [DOI] [PubMed] [Google Scholar]
- 92. Jetton TL, Everill B, Lausier J, Roskens V, Habibovic A, LaRock K, Gokin A, Peshavaria M, Leahy JL. Enhanced beta-cell mass without increased proliferation following chronic mild glucose infusion. Am J Physiol Endocrinol Metab 2008;294:E679–E687 [DOI] [PubMed] [Google Scholar]
- 93. Yamamoto K, Miyagawa J, Waguri M, Sasada R, Igarashi K, Li M, Nammo T, Moriwaki M, Imagawa A, Yamagata K, Nakajima H, Namba M, Tochino Y, Hanafusa T, Matsuzawa Y. Recombinant human betacellulin promotes the neogenesis of β-cells and ameliorates glucose intolerance in mice with diabetes induced by selective alloxan perfusion. Diabetes 2000;49:2021–2027 [DOI] [PubMed] [Google Scholar]
- 94. Tokui Y, Kozawa J, Yamagata K, Zhang J, Ohmoto H, Tochino Y, Okita K, Iwahashi H, Namba M, Shimomura I, Miyagawa J. Neogenesis and proliferation of beta-cells induced by human betacellulin gene transduction via retrograde pancreatic duct injection of an adenovirus vector. Biochem Biophys Res Commun 2006;350:987–993 [DOI] [PubMed] [Google Scholar]
- 95. Rooman I, Bouwens L. Combined gastrin and epidermal growth factor treatment induces islet regeneration and restores normoglycaemia in C57BL6/J mice treated with alloxan. Diabetologia 2004;47:259–265 [DOI] [PubMed] [Google Scholar]
- 96. Huang HP, Chu K, Nemoz-Gaillard E, Elberg D, Tsai MJ. Neogenesis of beta-cells in adult BETA2/NeuroD-deficient mice. Mol Endocrinol 2002;16:541–551 [DOI] [PubMed] [Google Scholar]