Abstract
OBJECTIVE
We characterized fluctuations between states of glycemia in progressors to type 1 diabetes and studied whether those fluctuations are related to the early C-peptide response to oral glucose.
RESEARCH DESIGN AND METHODS
Oral glucose tolerance tests (OGTTs) from differing states of glycemia were compared within individuals for glucose and C-peptide. Dysglycemic OGTTs (DYSOGTTs) were compared with normal OGTTs (NLOGTT), while transient diabetic OGTTs (TDOGTTs) were compared with subsequent nondiabetic OGTTs and with OGTTs performed at diagnosis.
RESULTS
Of 135 progressors with four or more OGTTs, 30 (22%) went from NLOGTTs to DYSOGTTs at least twice. Area under the curve (AUC) glucose values from the second NLOGTT were higher (P < 0.001) than values from the first NLOGTT. Among 98 progressors whose DYSOGTTs and NLOGTTs were synchronized for the time before diagnosis, despite higher glucose levels (P < 0.01 at all time points) in the DYSOGTTs, 30- to 0-min C-peptide difference values changed little. Likewise, 30- to 0-min C-peptide difference values did not differ between TDOGTTs and subsequent (within 3 months) nondiabetic OGTTs in 55 progressors. In contrast, as glucose levels increased overall from the first to last OGTTs before diagnosis (P < 0.001 at every time point, n = 207), 30- to 0-min C-peptide difference values decreased (P < 0.001).
CONCLUSIONS
Glucose levels fluctuate widely as they gradually increase overall with progression to type 1 diabetes. As glucose levels increase, the early C-peptide response declines. In contrast, glucose fluctuations are not related to the early C-peptide response. This suggests that changes in insulin sensitivity underlie the glucose fluctuations.
Glucose levels can fluctuate substantially during the progression to type 1 diabetes (1). In this report, we examine glucose variability and explore its basis by comparing the C-peptide response to oral glucose between states of glycemia. The findings provide additional insights into the metabolic progression to type 1 diabetes.
RESEARCH DESIGN AND METHODS
Diabetes Prevention Trial–Type 1 (DPT-1) participants and procedures have been described in detail (2,3). All participants were islet cell autoantibody (ICA)–positive relatives of type 1 diabetes patients. Those analyzed for this report participated in either the parenteral insulin (2) or oral insulin (3) trials.
The interventions for the parenteral and oral trials were recombinant human ultralente insulin and recombinant human insulin crystals, respectively. Visit intervals were 6 months. Diagnoses of type 1 diabetes were frequently determined from the oral glucose tolerance tests (OGTTs) at routine visits. OGTTs in the diabetic range were confirmed with another OGTT (unless symptoms occurred or there was marked fasting hyperglycemia).
Laboratory measures.
Plasma glucose was measured by the glucose oxidase method. Insulin and C-peptide were measured by radioimmunoassay. The interassay coefficient of variation for the C-peptide assay was 6.9% in a reference pool with relatively high values and 7.8% in a reference pool with relatively low values. Fasting C-peptide values <0.2 ng/ml were assigned a value of 0.1 ng/ml for the analyses. OGTTs included in the analysis had complete glucose and C-peptide measurements at all time points.
Data analysis.
A dysglycemic OGTT (DYSOGTT) was defined as any of the following: impaired fasting glucose (fasting glucose value 100–125 mg/dl); indeterminate (30-, 60-, and/or 90-mm glucose values ≥200 mg/dl); and impaired glucose tolerance (2-h glucose value 140–199 mg/dl). The thresholds for a diabetic range OGTT were a fasting glucose value ≥126 mg/dl and/or a 2-h glucose value ≥200 mg/dl. The OGTTs that were not dysglycemic and not in the diabetic range were considered to be normal (NLOGTT). The 30- to 0-min C-peptide difference, defined as the fasting C-peptide value subtracted from the 30-min C-peptide value, was used to indicate the early C-peptide response.
Since the early C-peptide response diminishes with progression to type 1 diabetes (4), it was necessary to take into account the influence of the time from diagnosis that the DYSOGTTs and NLOGTTs were performed. Thus, OGTTs were paired according to the first NLOGTT to the subsequent last DYSOGTT, the first DYSOGTT to the subsequent last NLOGTT, and a NLOGTT and a DYSOGTT that were synchronized to nearly the same time (on average) before diagnosis. The synchronization was performed by pairing the first DYSOGTT with the last NLOGTT (both after randomization), as long as those OGTTs were within 2 years of each other. A transient diabetic OGTT (TDOGTT) was defined as an OGTT in the diabetic range that was followed by an OGTT that was not in the diabetic range (NDOGTT). If a participant had more than one transient OGTT, the first was used for the analysis.
Paired and unpaired t tests and the Wilcoxon rank-sum test were used to assess differences. Nonparametric testing was utilized, particularly in analyses that involved OGTTs in the diabetic range because of their glucose distributions. The trapezoidal rule was used to calculate OGTT areas under the curve (AUCs). SAS version 9.1.3 was used for the analyses. All P values are two sided.
RESULTS
There were 258 progressors to type 1 diabetes in the DPT-1 trials, of whom 207 ([mean ± SD] 11.4 ± 7.8 years, 58% male) were studied. All had a baseline OGTT and at least one OGTT during follow-up.
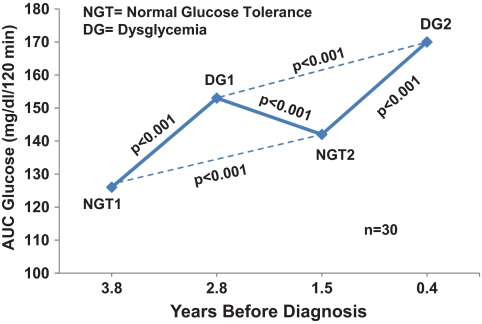
Of 135 progressors from both the parenteral and oral insulin trials with a minimum of four OGTTs, 30 (22%) had an alternating OGTT pattern (not necessarily consecutive) of normal, dysglycemic, normal again, and then dysglycemic again over their course of progression to type 1 diabetes. AUC glucose values from those OGTTs are shown in Fig. 1. As expected, there were significant increases in AUC glucose values when the DYSOGTTs were compared with their prior NLOGTTs (P < 0.001 for each difference). AUC glucose values from the second NLOGTTs were significantly higher than those from the first NLOGTTs (P < 0.001). Also, AUC glucose values from the second DYSOGTTs were significantly higher than those from the first DYSOGTTs (P < 0.01).
FIG. 1.
Shown is a sequence of alternating NLOGTTs and DYSOGTTs in progressors to type 1 diabetes. Each point represents the mean AUC glucose from the OGTTs. The mean time before diagnosis is shown for each of the OGTTs. There were significant increases in the AUC glucose from each of the NLOGTTs to their subsequent respective DYSOGTTs. There were also significant increases from the first NLOGTTs to the second NLOGTTs and from the first DYSOGTTs to the second DYSOGTTs.
We assessed whether the state of glycemia was related to the early insulin response to an oral glucose challenge. For this purpose, we used the 30- to 0-min C-peptide difference as a measure of early insulin secretion (4). Table 1 shows early C-peptide response values according to pairings of DYSOGTTs and NLOGTTs (see research design and methods). The early C-peptide response was substantially lower (P < 0.001) in the DYSOGTTs (P < 0.01 for both the parenteral and oral trials separately) when they occurred after the NLOGTTs (NL→DYSGLY; n = 146). However, when the DYSOGTTs preceded the NLOGTTs (DYSGLY→NL; n = 70), the early C-peptide response from the DYSOGTTs did not differ significantly from that of the NLOGTTs (nor did they differ when the trials were analyzed separately). In fact, the early C-peptide response tended to be higher in the DYSOGTTs. When the DYSOGTTs and the NLOGTTs were synchronized for the time before diagnosis (SYNCH; n = 98), the early C-peptide response was similar between the NLOGTTs and the DYSOGTTs (also when the trials were analyzed separately). Thus, when the time to diagnosis was minimized as a factor, the early C-peptide response did not vary between the normal and dysglycemic states. In addition, no significant difference was found between the normal and dysglycemic states in the ratio of the C-peptide response over the glucose response from 0 to 30 min.
TABLE 1.
The early C-peptide (ng/ml) response (30–0 minutes) according to the sequence of NLOGTT and DYSOGTT pairs before diagnosis
| NL→DYSGLY (n = 146) |
DYSGLY→NL (n = 70) |
SYNCH (n = 98)** |
||||
|---|---|---|---|---|---|---|
| NLOGTT | DYSOGTT | NLOGTT | DYSOGTT | NLOGTT | DYSOGTT | |
| 30–0 minutes* | 2.45 ± 1.25 | 2.01 ± 1.64† | 1.96 ± 1.11 | 2.25 ± 2.00 | 2.24 ± 1.38 | 2.27 ± 1.87 |
| Years to diabetes* | 2.97 ± 1.32 | 0.63 ± 0.45 | 1.31 ± 0.81 | 2.90 ± 1.22 | 1.66 ± 0.83 | 1.72 ± 0.97 |
*Data are mean ± SD.
**OGTTs synchronized (SYNCH) to the time before diagnosis.
†P < 0.001 vs. NLOGTT.
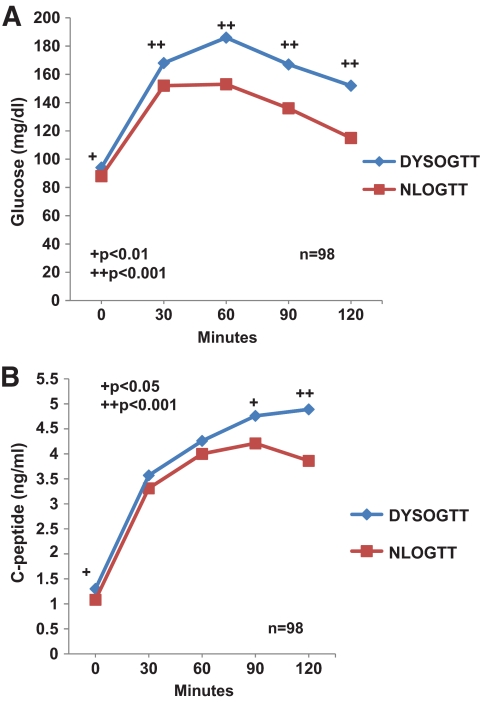
We examined the entire glucose and C-peptide curves from the OGTTs among SYNCH, the group whose DYSOGTTs and NLOGTTs were synchronized to the same time before diagnosis (Fig. 2). As expected, glucose levels (Fig. 2A) from the DYSOGTTs were significantly higher (P < 0.01) at every OGTT time point. The differences were especially apparent from 60 to 120 min. Even though glucose levels were higher from the DYSOGTTs, C-peptide levels (Fig. 2B) were also significantly higher in the fasting state (P < 0.05), at 90 min (P < 0.05), and at 120 min (P < 0.001). The sum of the differences between the 30-min C-peptide value and the subsequent values during the OGTT was significantly higher in the DYSOGTTs than in the NLOGTTs (3.20 ± 3.47 ng/ml vs. 2.14 ± 3.55 ng/ml, P = 0.008). BMI values did not differ between DYSOGTTs and NLOGTTs in 49 paired measurements concurrent with the paired DYSOGTTs and NLOGTTs (19.9 ± 5.2 kg/m2 vs. 19.6 ± 4.9 kg/m2, respectively).
FIG. 2.
Shown are glucose (A) and C-peptide (B) values (mean ± SD) for paired NLOGTTs and DYSOGTTs that were synchronized on average to the time before diagnosis in progressors to type 1 diabetes. As expected, the glucose values from the DYSOGTTs were substantially higher at every time point. Even though glucose levels were higher from the DYSOGTTs, C-peptide values were also significantly higher in the fasting state and at 90 and 120 min.
Among 60 progressors with TDOGTTs, we analyzed data from 55 who had a nondiabetic OGTT (NDOGTT) within 3 months ([mean ± SD] 36 ± 16 days) of the TDOGTT. Glucose levels (online appendix Fig. 1A, available at http://diabetes.diabetesjournals.org/cgi/content/full/db10-0534/DC1) were higher in the TDOGTTs at the later time points (P < 0.01 at 60 min; P < 0.001 at both 90 and 120 min). Despite those higher glucose levels in the TDOGTTs, C-peptide levels (online appendix Fig. 1B) were similar at all time points, except for higher 120-min C-peptide levels (P < 0.01) in the TDOGTTs.
Similar to the findings from the comparison between the DYSOGTTs and the NLOGTTs, the early C-peptide response did not significantly differ between the TDOGTTs and the NDOGTTs. Also, there was no significant difference in the ratio of the C-peptide response over the glucose response from 0 to 30 min.
Of the 55 TDOGTTs analyzed above, 38 also had a subsequent OGTT at the time of diagnosis (DOGTT). The mean ± SD difference in time from the TDOGTTs to the DOGTTs was 0.9 ± 0.8 years. Glucose levels (online appendix Fig. 2A) were significantly higher at every time point in the DOGTTs than in the TDOGTTs, especially postchallenge (P < 0.001 for all time points ≥30 min). C-peptide levels (online appendix Fig. 2B) were significantly lower in the DOGTTs at every postchallenge time point (P < 0.01) after 30 min. The early C-peptide response was also significantly lower in the DOGTTs (P < 0.001) than in the TDOGTTs.
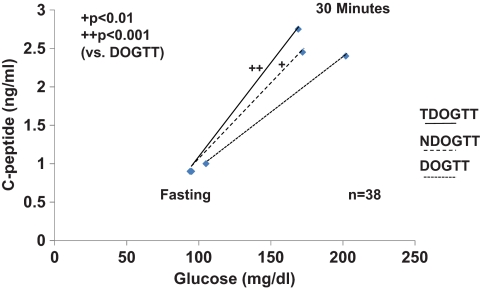
Figure 3 shows the C-peptide response relative to the glucose response from the fasting state to 30 min in the serial TDOGTTS, NDOGTTs, and DOGTTs from the 38 progressors. The C-peptide response relative to the glucose response was greater in both the TDOGTTs (P < 0.001) and the NDOGTTs (P < 0.017) than in the DOGTTs. There was no significant difference between the TDOGTTs and the NDOGTTs.
FIG. 3.
Shown are C-peptide responses in relation to glucose responses from 0 to 30 min in the TDOGTTs, the subsequent NDOGTTs (within 3 months), and the DOGTTs in progressors to type 1 diabetes. The ratio of the C-peptide response over the glucose response from 0 to 30 min was significantly higher in both the TDOGTTs and the NDOGTTs than in the DOGTTs (median values are shown).
In 207 progressors who had two OGTTs, glucose levels increased overall from the first ([mean ± SD] 2.8 ± 1.4 years before diagnosis) to the last OGTTs (0.6 ± 0.5 years before diagnosis) at every time point. In contrast with the lack of change in the early C-peptide response between states of glycemia, the early C-peptide response declined markedly from the first OGTT to the last OGTT ([mean ± SD] 2.38 ± 1.25 ng/dl to 1.87 ± 1.11 ng/dl; P < 0.001).
DISCUSSION
We have previously shown that on average glucose levels increase over time with progression to type 1 diabetes (5). However, the data in this report suggest that within the individual, glucose levels do not necessarily increase in a simple, linear manner; rather there can be wide fluctuations that occur on a background of gradually increasing glucose levels. The overall picture can perhaps best be described as a kind of ratcheting, as is evident in Fig. 1. The second normal OGTT did not have the same degree of “normalcy” as the first normal OGTT. The data indicate that this pattern extends even into the higher ranges of glycemia as the onset of type 1 diabetes approaches.
There appear to be at least two distinct patterns of change in glucose levels during the course of progression to type 1 diabetes, each occurring through a different mechanism. In one pattern, glucose levels increase over time as the early C-peptide response decreases. This pattern was evident when the first and last OGTTs were compared. The data suggest that the increasing glucose is at least in part attributable to a decline in early insulin secretion.
The second pattern, characterized by wide fluctuations of glucose levels, contrasts with the first pattern in that the excursions into the higher glucose range do not appear to be associated with a decrease in the early C-peptide response. The early C-peptide response was similar between the DYSOGTTs and NLOGTTs when they were synchronized to the time before diagnosis. Also, the early C-peptide response did not differ between the TDOGTTs and their subsequent NDOGTTs. Moreover, there were no significant differences in the ratio of the C-peptide response over the glucose response from 0 to 30 min between the DYSOGTTs and the NLOGTTS and between the TDOGTTs and the NDOGTTs. Thus, two separate analyses at different ranges of glycemia were consistent in showing a lack of association between glucose fluctuations and the early C-peptide response.
The data appear to indicate that differences in the early C-peptide response between DYSOGTTs and NLOGTTs are a function of the time before diagnosis when the OGTT is performed. The fact that the early C-peptide response tended to be higher in the OGTT that came first, and was independent of the state of glycemia, is consistent with the decline in the early C-peptide response with progression to type 1 diabetes.
Since glucose excursions were not related to the early C-peptide response, variation in glucose sensitivity could have been a factor. Although BMI values were not significantly higher when they were associated with the DYSOGTTs, the higher later OGTT C-peptide values in the DYSOGTTs is consistent with insulin data in nonobese adults with impaired glucose tolerance (6) and adults with diabetes (6,7). Data from other studies lend some support to the view that insulin resistance could be involved in the pathogenesis of type 1 diabetes (8–12). Interestingly, it appears that increased insulin sensitivity could contribute to the remissions that occur following the diagnosis of type 1 diabetes (13,14).
The DOGTTs had much higher glucose levels and much lower C-peptide levels and early C-peptide responses than did the TDOGTTs. Thus, β-cell function is much more impaired when an OGTT is diagnostic of type 1 diabetes than when it is transiently in the diabetic range. However, those with TDOGTTs represent a potential high risk target population for type 1 diabetes prevention trials.
Excursions into higher glucose ranges could exacerbate the loss of β-cell function through factors such as glucotoxicity (15). Therefore, it seems reasonable to consider interventions that would decrease glucose variability, and perhaps ultimately, preserve β-cell function.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Allergy and Infectious Diseases, National Institute of Child Health and Human Development, and General Clinical Research Centers Program; the Juvenile Diabetes Research Foundation International; and the American Diabetes Association.
No potential conflicts of interest relevant to this article were reported.
J.M.S. analyzed data and wrote the manuscript. J.S.S. conducted the study and reviewed the manuscript. J.P.K. conducted the study and reviewed the manuscript. C.J.G. conducted the study and reviewed the manuscript. J.M. reviewed the manuscript. L.E.R. conducted the study and reviewed the manuscript. D.C. programmed the study and reviewed the manuscript. C.C. conducted the study and reviewed the manuscript. K.H. reviewed the manuscript. G.E. conducted the study and reviewed the manuscript. J.P.P. conducted the study, reviewed the manuscript, and assisted in writing the manuscript.
Parts of this study were presented at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. Sosenko JM, Palmer JP, Rafkin-Mervis L, Krischer JP, Cuthbertson D, Mahon J, Greenbaum CJ, Cowie CC, Skyler JS. the Diabetes Prevention Trial–Type 1 Study Group Incident dysglycemia and the progression to type 1 diabetes among participants in the Diabetes Prevention Trial–Type 1. Diabetes Care 2009;32:1603–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diabetes Prevention Trial–Type 1 Diabetes Study Group Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002;346:1685–1691 [DOI] [PubMed] [Google Scholar]
- 3. Diabetes Prevention Trial–Type 1 Diabetes Study Group Effects of oral insulin in relatives of patients with type 1 diabetes. Diabetes Care 2005;28:1068–1076 [DOI] [PubMed] [Google Scholar]
- 4. Sosenko JM, Palmer JP, Rafkin-Mervis L, Krischer JP, Cuthbertson D, Greenbaum CJ, Eisenbarth G, Skyler JS. Trends of earlier and later responses of C-peptide to oral glucose challenges with progression to type 1 diabetes in Diabetes Prevention Trial–Type 1 participants. Diabetes Care 2010;33:620–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sosenko J, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS. the Diabetes Prevention Trial–Type 1 Study Group Patterns of metabolic progression to type 1 diabetes in the diabetes prevention trial-type 1. Diabetes Care 2006;29:643–649 [DOI] [PubMed] [Google Scholar]
- 6. Yalow RS, Glick SM, Roth J, Berson SA. Plasma insulin and growth hormone levels in obesity and diabetes. Ann N Y Acad Sci 1965;131:357–373 [DOI] [PubMed] [Google Scholar]
- 7. Bagdade JD, Bierman EL, Porte D., Jr The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest 1967;41:1549–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greenbaum CJ. Insulin resistance in type 1 diabetes. Diabetes Metab Res Rev 2002;18:192–200 [DOI] [PubMed] [Google Scholar]
- 9. Xu P, Cuthbertson D, Greenbaum C, Palmer JP, Krischer JP. the Diabetes Prevention Trial–Type 1 Study Group Role of insulin resistance in predicting progression to type 1 diabetes. Diabetes Care 2007;30:2314–2320 [DOI] [PubMed] [Google Scholar]
- 10. Fourlanos S, Narendran P, Byrnes GB, Colman PG, Harrison LC. Insulin resistance is a risk factor for progression to type 1 diabetes. Diabetologia 2004;47:1661–1667 [DOI] [PubMed] [Google Scholar]
- 11. Mrena S, Virtanen SM, Laippala P, Kulmala P, Hannila ML, Åkerblom HK, Knip M. the Childhood Diabetes in Finland Study Group Models for predicting type 1 diabetes in siblings of affected children. Diabetes Care 2006;29:662–667 [DOI] [PubMed] [Google Scholar]
- 12. Bingley PJ, Mahon JL. Gale EAM for the European Nicotinamide Diabetes Intervention Trial (ENDIT) Group Insulin resistance and progression to type 1 diabetes in the European Nicotinamide Diabetes Intervention Trial (ENDIT). Diabetes Care 2008;31:146–150 [DOI] [PubMed] [Google Scholar]
- 13. Yki-Yarvinen H, Koivisto VA. Natural course of insulin resistance in type 1 diabetes. N Engl J Med 1986;315:224–230 [DOI] [PubMed] [Google Scholar]
- 14. Martin S, Pawlowski B, Greulich B, Ziegler AG, Mandrup-Poulsen T, Mahon J. Natural course of remission in IDDM during 1st year after diagnosis. Diabetes Care 1992;15:66–74 [DOI] [PubMed] [Google Scholar]
- 15. Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in β-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes 2003;52:581–587 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.