Abstract
OBJECTIVE
In animal studies, hyperglycemia during fetal development reduces nephron numbers. We tested whether this observation translates into renal dysfunction in humans by studying renal functional reserve in adult offspring exposed in utero to maternal type 1 diabetes.
RESEARCH DESIGN AND METHODS
We compared 19 nondiabetic offspring of type 1 diabetic mothers with 18 offspring of type 1 diabetic fathers (control subjects). Glomerular filtration rate (51Cr-EDTA clearance), effective renal plasma flow (123I-hippurate clearance), mean arterial pressure, and renal vascular resistances were measured at baseline and during amino acid infusion, which mobilizes renal functional reserve.
RESULTS
Offspring of type 1 diabetic mothers were similar to control subjects for age (median 27, range 18–41, years), sex, BMI (23.1 ± 3.7 kg/m2), and birth weight (3,288 ± 550 vs. 3,440 ± 489 g). During amino acid infusion, glomerular filtration rate and effective renal plasma flow increased less in offspring of type 1 diabetic mothers than in control subjects: from 103 ± 14 to 111 ± 17 ml/min (8 ± 13%) vs. from 108 ± 17 to 128 ± 23 ml/min (19 ± 7%, P = 0.009) and from 509 ± 58 to 536 ± 80 ml/min (5 ± 9%) vs. from 536 ± 114 to 620 ± 140 ml/min (16 ± 11%, P = 0.0035). Mean arterial pressure and renal vascular resistances declined less than in control subjects: 2 ± 5 vs. −2 ± 3% (P = 0.019) and 3 ± 9 vs. −14 ± 8% (P = 0.001).
CONCLUSIONS
Reduced functional reserve may reflect a reduced number of nephrons undergoing individual hyperfiltration. If so, offspring of type 1 diabetic mothers may be predisposed to glomerular and vascular diseases.
A reduced number of nephrons may cause hypertension and favor renal and cardiovascular risks in humans (1). Autopsy findings support this assumption (2). In addition, birth weight is a determinant of nephron numbers in humans (3). In animal models, moderate hyperglycemia during pregnancy affects birth weight and nephron numbers in offspring (4), and favors the development of hypertension in adulthood (5). In addition, angiogenesis affects kidney development (6,7). In this respect, moderate hyperglycemia induces a defect in angiogenesis as reported in experimental conditions (8).
We hypothesized that the effects of moderate hyperglycemia on kidney development reported in animal studies might have clinical relevance in humans. Thus, we studied kidney function in subjects who had been exposed to hyperglycemia during their fetal development. For this purpose, we investigated, as previously (9), adults whose mothers had type 1 diabetes at the time of their conception and used the offspring of type 1 diabetic fathers as control subjects to minimize potential genetic heterogenicity between groups. Type 1 diabetes as a source of hyperglycemia during fetal development also minimizes confounding factors associated with type 2 diabetes such as hypertension. Counting nephron numbers and/or visualizing glomerular size by noninvasive methods is not currently feasible in humans. Thus, we measured global kidney function at baseline and during vasodilatation produced by amino acid infusion, i.e., renal functional reserve. Reduction in renal functional reserve can be interpreted as reflecting a reduced surface available for filtration, suggesting that the number of functional nephrons is reduced. As a result, the global hemodynamic load provokes hyperfiltration at the single nephron level (1). This disturbance in renal hemodynamics was associated with renal and vascular diseases, both in experimental models (1,4,5) and clinical settings (10–13). We report here that renal functional reserve is reduced in offspring of type 1 diabetic mothers.
RESEARCH DESIGN AND METHODS
Case subjects were the direct offspring of type 1 diabetic subjects attending specialized clinics in five hospitals in France: Hôpital Saint-Louis, Hôpital Bichat–Claude Bernard, Hôtel-Dieu (all in Paris), Centre Hospitalier Universitaire in Poitiers, and Centre Hospitalier Sud Francilien in Corbeil Essonne.
We selected type 1 diabetic parents and then their offspring. Parents had been diagnosed with type 1 diabetes (as defined by the American Diabetes Association) at least 2 years before the conception of offspring. Participants were eligible if their spouses had neither type 1 nor type 2 diabetes at the time of the study. Mothers were asked if they had smoked during their pregnancies. Offspring were men or women age 18 years or older with no diabetes. Women who were pregnant at the time of the investigation were excluded. They were checked as having no immune marker of type 1 diabetes (anti-islet antibodies; antibodies against GAD, IA2, and IA2 β; and anti-insulin antibodies). Chronic drug intake, acute infection, any chronic disease, and personal or family history of kidney disease, other than possible diabetic nephropathy in their diabetic parents, were reasons for exclusion. Case subjects were offspring of type 1 diabetic mothers, and control subjects were offspring of type 1 diabetic fathers.
The current study is part of a program investigating the physiologic consequences of fetal exposure to maternal type 1 diabetes at adult age. We report here data on kidney function.
At the first visit, plasma glucose, insulin, and glucagon concentrations were measured after an overnight fast with an oral glucose tolerance test. Renal volume was measured using ultrasounds. Urine was collected for albumin measurement during 2 h before and after a 20-min exercise on an ergocycle at an intensity of 80% of maximal heart rate. Then, ambulatory blood pressures were monitored during the following 24 h.
On a separate occasion, the participants brought 24-h urine for albumin and sodium measurements, and their kidney function was investigated after an overnight fast. Glomerular filtration rate (GFR) and renal plasma flow were measured at baseline and during amino acid infusion using the renal clearances of two radiopharmaceuticals: 51Cr-EDTA (GE Health Care, France), as an estimate of GFR, and 123I-hippurate (Covidien Imaging, France), as a measure of effective renal plasma flow (ERPF). A catheter was inserted in a forearm vein for infusion and another one contralaterally for sampling. The subjects remained supine for 1 h, after which blood was sampled for plasma renin and aldosterone measurements. They were given tap water (100 ml every 15 min) throughout the study to induce a forced water diuresis (mean 10 ± 2 [SD] ml/min). Once diuresis was adequate, the participants received a primed infusion of both 51Cr-EDTA (bolus 1.8 MBq, then 0.9 MBq/h) and 123I-hippurate (bolus 4 MBq, then 1.5 MBq/h) throughout the study. Two hours were necessary to obtain a radioisotope plateau and to calculate baseline clearances of 51Cr-EDTA and 123I-hippurate (the baseline period). Amino acids (vamine 10%, Fresenius-Kabi, France) were infused at a rate of 5 mg/kg/min (previously reported as providing maximal renal vasodilatation) (14) afterward for the following 90 min (the stimulation period). Plasma and urine samples were processed to calculate urinary clearances of radiopharmaceuticals. Along the experiment, participants remained supine except when they voided their bladder every 30 min for urinary sampling. Blood samples were collected for isotope counting and hematocrit every 30 min, three times before the end of each period. Mean arterial pressure (MAP) was recorded using an automatic device (Dinamap, France).
All aspects of the study were approved by the Ethical Committee of Paris Saint-Louis. Each participant signed a written informed consent to participate.
Measurements.
Kidney volume was measured with ultrasounds (Siemens Elegra SSN 7083, Germany) using a previously published method (15).
Urinary albumin was measured using a nephelometric method (Behring test, Marburg, Germany, assay sensitivity 2 mg/l and intra- and interassay variability of 5 and 7%, respectively). Glucose was measured with glucose oxidase and insulin, glucagon, renin, and aldosterone with radioimmunoassays.
Isotope counting was performed using a gamma counter (1480 Wizard 3, Perkin Elmer).
Calculations.
Renal clearances of isotopes were calculated as urine volume multiplied by urine isotope concentration, divided by plasma isotope concentration. The first 60 min of the basal period and the first 30 min of the stimulation period were not used for calculations because a steady state could not be assumed for plasma isotopes and amino acids. Thus, renal clearances of isotopes were calculated from the mean of the two measurements performed 30 min apart at the end of each period. The GFR and ERPF values were not corrected for body surface. Filtration fraction (FF) was calculated as GFR divided by ERPF. To determine MAP values, blood pressures were recorded every 5 min and their mean for each 30-min clearance period. Renal vascular resistances (RVRs) were calculated from MAP/(ERPF/[1 − hematocrit]). The mean intrasubject variability for the basal periods were 4.2% for GFR, 3.5% for ERPF, 4.3% for FF, 2.2% for MAP, and 4.9% for RVR. For the stimulated periods, they were, respectively, 4.9% for GFR, 4.2% for ERPF, 3.9% for FF, 1.8% for MAP, and 4.9% for RVR. The renal functional reserve was calculated as the percentage change in each of the studied variables from the basal period to the stimulated period.
Statistical analysis.
Data are presented as mean ± SD, median (range), or percentages. The primary end point was the intergroup difference in GFR changes produced by amino acid infusion. The assumption was that a 20% increase in GFR (SD 10%) would be observed during infusion in control subjects and that the mean increase would be 50% less in offspring of type 1 diabetic mothers. With a type 1 error rate of 5%, 21 subjects per group were required to test the hypothesis with a 90% power. For technical reasons, only experiments performed in 19 case subjects and 18 control subjects could be analyzed.
Intragroup changes were analyzed by paired t tests or signed Wilcoxon rank-sum tests and intergroup comparisons by modified Welsh t tests or Mann-Whitney tests. Parametric or nonparametric tests were chosen by assessing whether the data distribution was skewed. The correlation between renal hemodynamic changes and birth weight was assessed by the Pearson correlation coefficient. The hypothesis of a different correlation in case subjects and control subjects was tested by an interaction test in a linear model. The threshold for P value significance was set at 0.05.
Statistical analysis was performed using R 2.6.2 (The R Foundation for Statistical Computing, Vienna, Austria) statistical software.
RESULTS
Participant recruitment and characteristics.
Among approximately 2,000 type 1 diabetic subjects attending the five clinics, 102 were eligible, 83 agreed to contact their offspring, and 62 offspring agreed to participate in our program investigating the physiologic consequences of fetal exposure to maternal type 1 diabetes in adulthood. A total of 41 subjects (21 case subjects, 20 control subjects) agreed to undergo renal tests. They did not differ from the 21 other subjects. Four participants (2 in each group) were excluded from analyses for technical reasons (they vomited during amino acid infusions).
The characteristics of the participants' parents are given in Table 1. None of the mothers smoked during their pregnancies.
TABLE 1.
Characteristics of the parents of the case subjects
| Type 1 diabetic mothers | Type 1 diabetic fathers | |
|---|---|---|
| n | 19 | 18 |
| Current age (years) | 53 (50–65) | 55 (45–65) |
| Age at diabetes onset (years) | 12 (4–24) | 16 (3–33) |
| Age at offspring conception (years) | 28 (19–35) | 29 (25–41) |
| Number of patients with nephropathy | 3 | 4 |
Data are medians (range). Nephropathy was defined as present or past clinical proteinuria.
The characteristics of participants are given in Table 2. There was no intergroup difference for age or sex distribution. As anticipated, gestational age at delivery was lower in case subjects than in control subjects. Conversely, birth weights did not differ. Birth weights did not differ after adjustment for gestational age: 3,535 ± 516 g in case subjects vs. 3,404 ± 430 g in control subjects. The BMIs were identical. Fasting plasma glucose, insulin, and glucagon were similar and within normal range. One case subject and two control subjects displayed impaired glucose tolerance during the oral glucose tolerance test.
TABLE 2.
Characteristics of the case subjects (part one)
| Offspring of type 1 diabetic mothers | Offspring of type 1 diabetic fathers | P | |
|---|---|---|---|
| n | 19 | 18 | |
| Sex (male/female) | 5/14 | 9/9 | 0.18 |
| Age (years)* | 24 (18–41) | 25 (18–37) | 0.96 |
| Gestational age at delivery (weeks)* | 37 (33–40) | 40 (37–40) | 0.0004 |
| Birth weight (g)* | 3,200 (2,750–4,270) | 3,300 (2,700–4,540) | 0.29 |
| BMI (kg/m2)* | 22.6 (17.9–29.9) | 22.4 (19–27.7) | 0.58 |
| Fasting plasma glucose (mmol/l)† | 4.5 ± 0.4 | 4.5 ± 0.3 | 0.86 |
| Fasting plasma insulin (μU/ml)† | 5.0 ± 1.6 | 4.6 ± 2.2 | 0.56 |
| Fasting plasma glucagon (pg/ml)† | 130.4 ± 28.1 | 151.1 ± 41.1 | 0.13 |
| Kidney volume (ml)† | |||
| Right kidney | 157.0 ± 35.5 | 165.0 ± 42.2 | 0.65 |
| Left kidney | 155.9 ± 34.0 | 151.1 ± 18.6 | 0.71 |
Data are counts,
*medians (range), or
†means ± SD.
During the exercise test, systolic/diastolic blood pressures increased similarly in the two groups: from 116 ± 14/71 ± 14 mmHg to 127 ± 15/71 ± 12 mmHg in case subject vs. from 118 ± 16/68 ± 12 mmHg to 126 ± 14/70 ± 10 mmHg in control subjects. Urinary albumin excretions varied similarly: from 2 (range 2–20) to 35 (5–810) μg/min in case subjects vs. from 2 (2–3) to 64 (4–312) μg/min in control subjects. The 24-h ambulatory blood pressures were not different, showing diurnal and nocturnal blood pressures, respectively, at 117 ± 10/71 ± 4 mmHg and 114 ± 4/64 ± 8 mmHg in case subjects and 118 ± 8/72 ± 7 mmHg and 113 ± 8/66 ± 7 mmHg in control subjects.
Kidney volumes were similar in both groups (Table 2). No morphologic anomaly was detected in the kidneys and urinary tracts during ultrasound examination.
Renal tests.
Systolic blood pressures and urinary albumin excretions were slightly but not statistically higher in case subjects than in control subjects. Although urinary excretions of sodium, potassium, and plasma renin were similar between groups, plasma aldosterone was higher in case subjects than in control subjects. However, the plasma renin/aldosterone ratio was similar (Table 3).
TABLE 3.
Characteristics of the case subjects at the time of renal tests (part two)
| Offspring of type 1 diabetic mothers | Offspring of type 1 diabetic fathers | P | |
|---|---|---|---|
| n | 19 | 18 | |
| Systolic BP/diastolic BP (mmHg)* | 118 ± 6/72 ± 7 | 114 ± 7 /70 ± 8 | 0.085/0.36 |
| Urinary sodium (mmol/24 h)* | 136 ± 78 | 131 ± 66 | 0.84 |
| Urinary potassium (mmol/24 h)* | 82 ± 58 | 72 ± 54 | 0.59 |
| Urinary albumin (mg/24 h)† | 9 (5–14) | 5 (4–8) | 0.11 |
| Plasma renin (pg/ml)† | 7 (3–78) | 7 (3–26) | 0.75 |
| Plasma aldosterone (pg/ml)† | 109.6 (56.5–138) | 63.1 (35.8–83) | 0.036 |
| Renin/aldosterone ratio† | 0.15 (0.01–0.78) | 0.15 (0.02–0.29) | 0.30 |
Data are
*means ± SD or
†medians (range). BP, blood pressure.
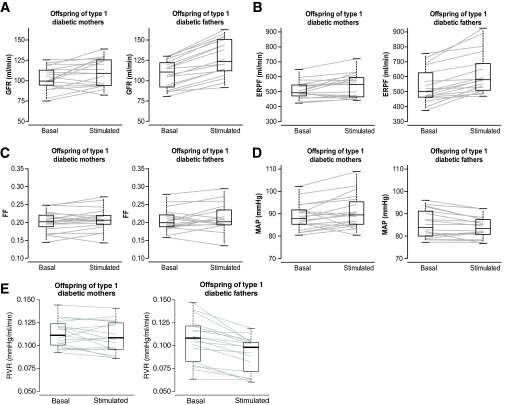
The individual changes in renal parameters are given in Fig. 1. Although the baseline GFR values did not differ, they increased less in response to amino acids in case subjects compared with control subjects: from 102 ± 14 to 111 ± 17 ml/min (8 ± 13%, P = 0.019) vs. from 108 ± 17 to 128 ± 23 ml/min (19 ± 7%, P = 0.002, intergroup difference P = 0.009, Fig. 1A). The ERPF changes also increased less in response to amino acids in case subjects compared with control subjects: from 509 ± 58 to 536 ± 114 ml/min (5 ± 9%, P = 0.016) vs. from 536 ± 114 to 620 ± 140 ml/min (16 ± 11%, P = 0.002; intergroup difference P = 0.0035, Fig. 1B). The baseline FFs (0.20 ± 0.03 in case subjects vs. 0.21 ± 0.03 in control subjects) were not altered: 3 ± 8% (P = 0.14) vs. 3 ± 11% (P = 0.26; intergroup difference P = 0.92, Fig. 1C). In case subjects, MAP varied from 88 ± 6 to 91 ± 8 mmHg (2 ± 5%, P = 0.061), whereas it declined from 86 ± 6 to 84 ± 5 mmHg in control subjects (−2 ± 3%, P = 0.029; intergroup difference P = 0.019, Fig. 1D). Consequently, RVR in case subjects did not vary: from 0.113 ± 0.015 to 0.110 ± 0.017 mmHg/ml/min (−3 ± 9%, P = 0.1), whereas they declined from 0.106 ± 0.024 to 0.091 ± 0.019 mmHg/ml/min in control subjects (−14 ± 8%, P = 0.002, intergroup difference P = 0.001, Fig. 1E).
FIG. 1.
Kidney function of offspring of type 1 diabetic mothers (case subjects, left) and of offspring of type 1 diabetic fathers (control subjects, right) in basal conditions and during infusion of amino acids (stimulated). Data are given as median values with box plots and individual values. A: Changes in glomerular filtration rate (GFR), intragroup changes in case subjects: 8 ± 13%, P = 0.019, and in control subjects: 19 ± 17%, P = 0.002, intergroup comparison: P = 0.009. B: Changes in effective renal plasma flow (ERPF), intragroup changes in case subjects: 5 ± 9%, P = 0.016, and in control subjects: 16 ± 11%, P = 0.002, intergroup comparison: P = 0.0035. C: Changes in FF, intragroup changes in case subjects: 3 ± 8%, P = 0.14, and in control subjects: 3 ± 8%, P = 0.26, intergroup comparisons: P = 0.92. D: Changes in mean arterial pressure (MAP), intragroup changes in case subjects: 2 ± 5%, P = 0.061, and in control subjects: −2 ± 3%, P = 0.029, intergroup comparison: P = 0.019. E: Changes in renal vascular resistances (RVR), intragroup changes in case subjects: −3 ± 9%, P = 0.1, and in control subjects: −14 ± 8%, P = 0.002, intergroup comparison: P = 0.001.
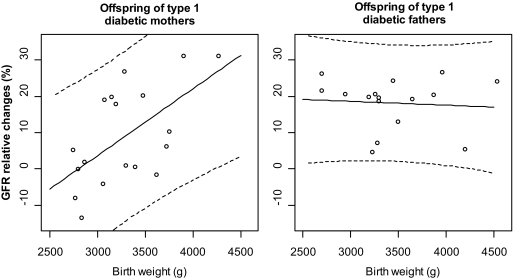
We examined the relationship between renal hemodynamic changes and birth weight. It was significantly different in case subjects compared with control subjects (P = 0.009): the relationship between changes in GFR during amino acid infusion and birth weight was highly significant in case subjects (r = 0.61, P = 0.006), but not in control subjects (r = −0.08, P = 0.78, Fig. 2). These analyses were not altered when birth weight was adjusted for gestational age. The relationship was not different according to sex (P = 0.70). There was no association between height and changes in GFR (r = 0.27; 95% CI −0.06 to 0.54, P = 0.11).
FIG. 2.
Relationship between birth weight and GFR relative changes during tests in the offspring of type 1 diabetic mothers (left panel) and of type 1 diabetic fathers (right panel). Solid lines represent the regression lines, and dashed lines represent the 95% prediction intervals. Pearson correlation coefficient in offspring of type 1 diabetic mothers: r = 0.61 (P = 0.006); in offspring of type 1 diabetic fathers: r = −0.08 (P = 0.76).
DISCUSSION
We found that fetal exposure to maternal type 1 diabetes was associated with a reduced renal reserve in offspring of humans at adult age. Our results in humans are consistent with what has been observed in rats regarding the impact of moderate hyperglycemia during fetal development on adult kidney dysfunction (5). They suggest a reduced number of nephrons in offspring of type 1 diabetic mothers, even though the precise quantification was not performed, as noninvasive methods are not currently available in humans. We recognize that a reduced functional reserve does not mean a reduced nephron number in the absence of direct association in humans. However, a reduced functional reserve in humans indicates an already dilated renal vasculature resulting in global hyperfiltration, or an already established glomerular disease with reduced glomerular filtration rate (10). Mogensen and Christensen (12) reported that in type 1 diabetes, global hyperfiltration is predictive of clinical proteinuria, and Sackmann et al. (13) reported a reduced functional reserve both in normotensive, normoalbuminuric subjects with recent type 1 diabetes (those prone to nephropathy displayed global hyperfiltration), and in proteinuric subjects with long-lasting type 1 diabetes (those with an already reduced GFR). The participants with microalbuminuria studied by these authors were on ACE inhibitors, making data interpretation difficult, since ACE inhibitors can alter kidney function of such patients (16), and since renal functional reserve can be altered by these drugs (17). Studies conducted in animals (18,19) support the view that the glomeruli of subjects such as those with reduced functional reserve undergo individual hyperfiltration, a hemodynamic condition that favors glomerulosclerosis, hypertension, and high renovascular risk (1,11). The possibility that permanent hyperfiltration occurs within each nephron of the offspring of type 1 diabetic mothers is supported here by the lack of intergroup difference for baseline GFR, whereas the renal vasodilatation produced by amino acid infusion was severely reduced in case subjects compared with control subjects. In rats, a reduced number of nephrons provoked by hyperglycemia during fetal development favors salt-sensitive hypertension in adulthood (5). Salt-sensitive hypertension was related to glomerular dysfunction in human studies (20). Here we found no difference for blood pressures or urinary albumin excretions, but our study was not powered to determine such differences. Conversely, a large prospective study in Denmark reported that offspring of type 1 diabetic mothers displayed elevated systolic blood pressures (21), but their kidney function was not studied. Also, Pima Indians with type 2 diabetes exposed in utero to maternal diabetes developed microalbuminuria and higher blood pressure more frequently than those who were not exposed (22). Thus, large-scale studies are now required on blood pressure and albuminuria of the offspring of type 1 diabetic mothers. Note that we did not replicate our initial findings regarding glucose tolerance in the offspring of type 1 diabetic mothers (9). Since this earlier report, several public health interventions have been conducted in France to reduce the incidence of obesity and diabetes. The offspring we studied might have been especially sensitive to these measures (their mean BMI was 22.5 kg/m2, thereby reducing their risk for glucose intolerance). Also, the present study was not sized to address the issue of glucose intolerance in the participants.
The role of birth weight on kidney function in adulthood must be delineated. Hughson et al. (3) reported in an autopsy study an association between birth weight and glomerular number and size. Here we observed a positive correlation between renal functional reserve and birth weight in the offspring of type 1 diabetic mothers, but not in control subjects, despite similar birth weight. Thus, birth weight may influence kidney function in adulthood only in subjects exposed in utero to maternal diabetes. Moderate hyperglycemia during fetal development may be necessary for birth weight to affect adult kidney function.
We studied offspring of type 1, rather than type 2, diabetic mothers (22), taking offspring of type 1 diabetic fathers as control subjects as we did earlier (9), to avoid confusion because of the genetic background of hypertension, a condition linked both to kidney disease and to type 2 diabetes. Thus, our study supports a direct impact of hyperglycemia or its associated metabolic abnormalities (23–25) during fetal development on kidney dysfunction later in life.
We could not establish a relationship between the level of hyperglycemia during pregnancy and kidney dysfunction at adulthood. Uncontrolled hyperglycemia in type 1 diabetic mothers promotes renal malformations in offspring (26). None of our participants had morphologic kidney anomalies. The average term for delivery of type 1 diabetic mothers was 37 weeks (range 33–40) without frank macrosomia or microsomia, and birth weights were not different between the groups, suggesting optimized blood glucose control. However, we could not retrospectively quantify the level of glycemic control attained during these pregnancies. Half of them occurred before 1980, a time when neither glycated hemoglobin nor self-monitoring of blood glucose was available for diabetes care.
Hyperglycemia induces several biochemical and hemodynamic abnormalities (23–25) that may account for the renal changes reported here. In rats, exposure in utero to hyperglycemia and reduced nephron numbers in offspring (4) was associated with alterations of the expressions of insulin-like growth factors and their receptors in fetal kidney (27). Alterations in angiogenesis may also be plausible. In the model of chicken chorioallantoic membrane, we did not find that high glucose levels altered the expression of several growth factors involved in angiogenesis, but increased apoptosis and decreased proliferation of endothelial cells occurred (8). Whether hyperglycemia during fetal development provokes epigenetic alterations leading to renal dysfunction in adulthood remains to be elucidated (28).
Plasma aldosterone was higher in case subjects than in control subjects, whereas plasma renin did not differ significantly. These data are exploratory, and they may be due to a type 1 error. However, such a figure is often encountered in people with a volume-dependent hypertension (29). We found no relationship between plasma aldosterone, renin, and sodium or potassium urinary excretions or blood pressure values (data not shown). However, we did not measure extracellular volumes of the participants to this study, and sodium intake was not controlled. Further investigations are required in this respect. Interestingly, epigenetic modifications of the renin-angiotensin system were reported in experimental settings (30), and an inverse relationship was reported between birth weight and plasma aldosterone at adult age in humans (31).
In conclusion, this study supports that moderate hyperglycemia (or its related metabolic alterations) during fetal development may alter kidney function in human adults. As pregnancy is now common in type 1 diabetic women, we will have to pay attention to the renal and vascular status of their offspring during adulthood.
ACKNOWLEDGMENTS
This study was supported by an institutional grant (PHRC AOR 04032, principal investigator: J.-F.G.) from Assistance Publique–Hôpitaux de Paris. C.A.K. was supported by grants from the International Society of Endocrinology and by the Association Diabète Risque Vasculaire. F.T. was supported by the Association pour l'Etude et la Recherche de l'Amélioration du Traitement du Diabète.
No potential conflicts of interest relevant to this article were reported.
C.A.K., J.-F.G., and M.M. researched data, contributed to discussion, and wrote and reviewed the manuscript. F.T., R.P., S.H., and E.L. researched data, contributed to discussion, and reviewed the manuscript. S.F. researched data and contributed to discussion. F.R. and D.L.G. researched data and reviewed the manuscript. J.-P.R. researched data. R.R. contributed to discussion and reviewed the manuscript. P.V. contributed to discussion.
Parts of this study were presented in abstract form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
The authors thank the participants in this study, their parents, and their doctors (Hervé Leblanc, Thierry Gabreau, and Isabelle Morbois at Hôpital Saint-Louis, Paris). They also thank Dr. Philippe Boudou (Hôpital Saint-Louis, Paris) for all hormone dosages used in the study and Prof. Pascal Ferré (University Paris VI) and Prof. Jean-Michel Halimi (Université de Tours) for their expert advice.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med 1982;307:652–659 [DOI] [PubMed] [Google Scholar]
- 2. Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. N Engl J Med 2003;348:101–108 [DOI] [PubMed] [Google Scholar]
- 3. Hughson M, Farris AB, III, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int 2003;63:2113–2122 [DOI] [PubMed] [Google Scholar]
- 4. Amri K, Freund N, Vilar J, Merlet-Benichou C, Lelievre-Pegorier M. Adverse effects of hyperglycemia on kidney development in rats: in vivo and in vitro studies. Diabetes 1999;48:2240–2245 [DOI] [PubMed] [Google Scholar]
- 5. Nehiri T, Duong Van Huyen JP, Viltard M, Fassot C, Heudes D, Freund N, Deschênes G, Houillier P, Bruneval P, Lelièvre-Pégorier M. Exposure to maternal diabetes induces salt-sensitive hypertension and impairs renal function in adult rat offspring. Diabetes 2008;57:2167–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernhardt WM, Schmitt R, Rosenberger C, Münchenhagen PM, Gröne HJ, Frei U, Warnecke C, Bachmann S, Wiesener MS, Willam C, Eckardt KU. Expression of hypoxia-inducible transcription factors in developing human and rat kidneys. Kidney Int 2006;69:114–122 [DOI] [PubMed] [Google Scholar]
- 7. Carev D, Saraga M, Saraga-Babic M. Involvement of FGF and BMP family proteins and VEGF in early human kidney development. Histol Histopathol 2008;23:853–862 [DOI] [PubMed] [Google Scholar]
- 8. Larger E, Marre M, Corvol P, Gasc JM. Hyperglycemia-induced defects in angiogenesis in the chicken chorioallantoic membrane model. Diabetes 2004;53:752–761 [DOI] [PubMed] [Google Scholar]
- 9. Sobngwi E, Boudou P, Mauvais-Jarvis F, Leblanc H, Velho G, Vexiau P, Purcher R, Hadjadj S, Pratley R, Tataranni PA, Calvo F, Gautier JF. Effect of a diabetic environment in utero on predisposition to type 2 diabetes. Lancet 2003;361:1861–1865 [DOI] [PubMed] [Google Scholar]
- 10. Bosch JP, Lew S, Glabman S, Lauer A. Renal hemodynamic changes in humans. Response to protein loading in normal and diseased kidneys. Am J Med 1986;81:809–815 [DOI] [PubMed] [Google Scholar]
- 11. Luyckx VA, Brenner BM. Low birth weight, nephron number, and kidney disease. Kidney Int Suppl 2005;S68–S77 [DOI] [PubMed] [Google Scholar]
- 12. Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med 1984;311:89–93 [DOI] [PubMed] [Google Scholar]
- 13. Sackmann H, Tran-Van T, Hanaire-Broutin H, Tauber JP, Ader JL. Renal functional reserve in IDDM patients. Diabetologia 1998;41:86–93 [DOI] [PubMed] [Google Scholar]
- 14. Giordano M, Castellino P, McConnell EL, DeFronzo R. Effect of amino acid infusion on renal hemodynamics in humans: a dose-response study. Am J Physiol 1994;267:F703–F708 [DOI] [PubMed] [Google Scholar]
- 15. Lewis E, Ritchie WG. A simple ultrasonic method for assessing renal size. J Clin Ultrasound 1980;8:417–420 [DOI] [PubMed] [Google Scholar]
- 16. Marre M, Leblanc H, Suarez L, Guyenne T, Ménard J, Passa P. Converting enzyme inhibition and kidney function in normotensive diabetic patients with persistent microalbuminuria. Br Med J 1987;294:1448–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rondeau E, Paillard F, Peraldi MN, Violet I, Tasse S, Dussaule JC, Ardaillou R, Sraer JD. Role of the renin-angiotensin system on the renal functional reserve in renal transplant recipients. Kidney Int 1993;44:165–172 [DOI] [PubMed] [Google Scholar]
- 18. Anderson S, Meyer TW, Rennke HG, Brenner BM. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest 1985;76:612–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zatz R, Dunn BR, Meyer TW, Anderson S, Rennke HG, Brenner BM. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest 1986;77:1925–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bigazzi R, Bianchi S, Baldari D, Sgherri G, Baldari G, Campese VM. Microalbuminuria in salt-sensitive patients. A marker for renal and cardiovascular risk factors. Hypertension 1994;23:195–199 [DOI] [PubMed] [Google Scholar]
- 21. Clausen TD, Mathiesen ER, Hansen T, Pedersen DM, Lauenborg J, Schmidt L, Damm P. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab 2009;94:2464–2470 [DOI] [PubMed] [Google Scholar]
- 22. Nelson RG, Morgenstern H, Bennett PH. Intrauterine diabetes exposure and the risk of renal disease in diabetic Pima Indians. Diabetes 1998;47:1489–1493 [DOI] [PubMed] [Google Scholar]
- 23. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813–820 [DOI] [PubMed] [Google Scholar]
- 24. Cooper ME. Interaction of metabolic and haemodynamic factors in mediating experimental diabetic nephropathy. Diabetologia 2001;44:1957–1972 [DOI] [PubMed] [Google Scholar]
- 25. Fetita LS, Sobngwi E, Serradas P, Calvo F, Gautier JF. Consequences of fetal exposure to maternal diabetes in offspring. J Clin Endocrinol Metab 2006;91:3718–3724 [DOI] [PubMed] [Google Scholar]
- 26. Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes 1980;29:1023–1035 [DOI] [PubMed] [Google Scholar]
- 27. Amri K, Freund N, Van Huyen JP, Merlet-Benichou C, Lelievre-Pegorier M. Altered nephrogenesis due to maternal diabetes is associated with increased expression of IGF-II/mannose-6-phosphate receptor in the fetal kidney. Diabetes 2001;50:1069–1075 [DOI] [PubMed] [Google Scholar]
- 28. Dressler GR. Epigenetics, development, and the kidney. J Am Soc Nephrol 2008;19:2060–2067 [DOI] [PubMed] [Google Scholar]
- 29. Blumenfeld JD, Laragh JH. Renin system analysis: a rational method for the diagnosis and treatment of the individual patient with hypertension. Am J Hypertens 1998;11:894–896 [DOI] [PubMed] [Google Scholar]
- 30. Bogdarina I, Welham S, King PJ, Burns SP, Clark AJ. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ Res 2007;100:520–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reynolds RM, Walker BR, Phillips DI, Dennison EM, Fraser R, Mackenzie SM, Davies E, Connell JM. Programming of hypertension: associations of plasma aldosterone in adult men and women with birthweight, cortisol, and blood pressure. Hypertension 2009;53:932–936 [DOI] [PubMed] [Google Scholar]