Abstract
UV radiation targets the skin and is a primary cause of skin cancer (both melanoma and non-melanoma skin cancer). Exposure to UV also suppresses the immune response, and UV-induced immune suppression is a major risk factor for skin cancer induction. The efforts of Dermatologists and Cancer Biologists to understand how UV exposure suppresses the immune response and contributes to skin cancer induction led to the development of the sub-discipline we call photoimmunology. Advances in photoimmunology have generally paralleled advances in immunology. However, there are a number of examples where investigations into the mechanisms underlying UV-induced immune suppression reshaped our understanding of basic immunological concepts. Unconventional immune regulatory roles for Langerhans cells, mast cells, and NKT cells as well as the immune suppressive function of lipid mediators of inflammation and alarmins, are just some examples of how advances in immunodermatology have altered our understanding of basic immunology. In this anniversary issue celebrating 75 years of Cutaneous Science, we will provide examples of how concepts that grew out of efforts by Immunologists and Dermatologists to understand immune regulation by UV radiation impacted on immunology in general.
Introduction
Photoimmunology is defined as the study of the effects of non-ionizing radiation on the immune system. It grew from experiments designed to understand the mechanism(s) underlying UVB (290 to 320 nm)-induced skin carcinogenesis. In 1974 Kripke reported that skin cancers that arose in UV-irradiated mice could not be successfully transplanted into normal age-and sex-matched syngeneic recipient mice. The tumors only grew progressively when they were transplanted into immune compromised recipients (Kripke, 1974). This finding indicated that the skin tumors induced following cutaneous UV-irradiation were highly antigenic and suppressing the host’s immune system was required to allow the antigenic tumors to grow progressively in the recipient. Left unanswered was the question of how these tumors initially developed in the immune competent UV-irradiated host. Subsequent studies showed that in addition to being carcinogenic, UV exposure induced immune suppression (Fisher and Kripke, 1977) in part by activating a special class of immune regulatory cells, known in the 1970’s as suppressor T cells (Fisher and Kripke, 1982), but now known as T regulatory cells (Schwarz, 2008).
Another early observation that confirmed that UV radiation modulates immune function was provided by the realization that cutaneous UV exposure affects antigen presenting cell function (Greene et al., 1979). UV exposure destroys the dendritic cell network of Langerhans cells in the skin (Streilein et al., 1980). Hapten sensitization through UV-irradiated sites not only fails to induce contact hypersensitivity (CHS), but also induces immune tolerance (Toews et al., 1980), in part through the induction of T regulatory cells (Elmets et al., 1983). These findings provided the early foundation for the discipline we now call photoimmunology. In this anniversary issue celebrating 75 years of Cutaneous Science, we will provide examples of how concepts that grew out of efforts by Immunologists and Dermatologists to understand skin carcinogenesis eventually impacted immunology in general.
Impact of Photoimmunology on Immunology
Because the immune suppression induced by UV exposure is a major risk factor for skin cancer induction (Yoshikawa et al., 1990), many investigators have set out to determine the mechanisms involved. Photoimmunologists were not the first to describe an immunosuppressive role for T cells, but unlike mainstream immunologists, photoimmunologists never abandoned the concept that the T cells suppressed cutaneous immune reactions (i.e., contact and delayed type hypersensitivity) and facilitated the growth of sunlight induced skin cancers. (See, “Regulatory T cells-Banned Cells for Decades” in this issue for more details). On the other hand, some of the very first reports that Langerhans Cells have immune regulatory function came from cutaneous biologists studying the effect of UV on antigen presentation (Streilein et al., 1980; Toews et al., 1980). Although most textbooks define Langerhans cells as the antigen presenting cell in the skin responsible for immune surveillance, recent reports have suggested that dermal dendritic cells may have a more prominent role as the cutaneous antigen presenting cell (Fukunaga et al., 2008; Stein et al., 2011), and that Langerhans cells may be more important for the activation of regulatory cells (Fukunaga et al., 2010) (See “Changing Views of the role of Langerhans cells” in this issue for more details). Regardless, a role for Langerhans cells in immune regulation was pioneered by photoimmunologists 30 years ago.
Mast cells regulate adaptive immune reactions
Mast cells are bone marrow derived cells that circulate in the blood as immature progenitors. They migrate into peripheral tissues where they differentiate into mature, long-lived mast cells. Conventional wisdom suggests that tissue-resident mast cells primarily serve as effector cells in IgE-mediated allergic reactions, in part through the release of pre-formed mediators stored in the cell’s cytoplasmic granules. However, immunologists now realize that mast cells regulate adaptive immune responses via the release of cytokines and other immune modulatory factors (Galli et al., 2008). Some of the first reports indicating that mast cells suppress adaptive immune reactions were published by those studying the mechanisms by which UV exposure suppresses CHS. Wayne Streilein and colleagues found that UV-induced the release of calcitonin gene-related peptide (CGRP) from cutaneous nerve endings, which ultimately suppressed the induction of CHS. When determining the mechanism involved they found that CGRP induced mast cells to release tumor necrosis factor alpha and concluded, “CGRP or UV radiation impairs CHS via an effect on mast cells” (Niizeki et al., 1997). Hart and colleagues provided formal proof for the role of mast cells in UV-induced immune suppression. These studies employed c-Kit mutant, mast cell deficient, Wf/Wf mice. These mice are immune competent and are capable of generating a vigorous CHS reaction when a contact allergen is applied to the skin. However, when the mast cell deficient mice were first exposed to UV radiation and then sensitized with hapten, no immune suppression was noted. Susceptibility to the immunosuppressive effects of UV radiation was restored when the mice were first reconstituted with bone marrow derived mast cells, and then UV-irradiated (Hart et al., 1998). These results have been confirmed by others (Alard et al., 2001; Alard et al., 1999). In addition, mast cells play a critical role in suppressing secondary immune reactions by UVA (320–400 nm) radiation (Ullrich et al., 2007). Mast cell density in non-sun-exposed human skin correlates positively with the risk of melanoma (Grimbaldeston et al., 2004) and basal cell carcinoma (Grimbaldeston et al., 2000; Grimbaldeston et al., 2003), suggesting that the immune regulatory property of mast cells may contribute to skin cancer induction.
The skin absorbs UVB, yet UV exposure induces system wide immune suppression. How the immune suppressive signal is transmitted from the skin to the lymph nodes is not entirely clear, but migrating mast cells play a role. Following UV exposure, mast cell density in the skin quickly increases and peaks 6h post UV exposure (Figure 1). This was not too surprising because mast cell progenitors are recruited to areas of inflammation, such as UV-irradiated skin. What was surprising was that within 24h the numbers of mast cells in the skin draining lymph nodes, but not distant lymph nodes (i.e., popliteal) was significantly increased. This suggested that the mast cells were migrating from the skin to the draining lymph node. To determine that this was the case, skin from green fluorescent protein (GFP)-positive mice was grafted onto the backs of mast cell deficient mice. The animals were then exposed to UV radiation. The appearance of GFP+ mast cells in the lymph nodes of UV-irradiated mast cell-deficient mice, but not in the nodes of skin grafted, un-irradiated mice, confirmed the hypothesis that UV-irradiation triggers the migration of mast cell from the skin to the lymph nodes (Byrne et al., 2008). This UV-induced mast cell migration was dependent on the CXCR4-CXCL12 chemokine pathway as CXCL12 was significantly up regulated in draining lymph nodes (Figure 2) and CXCR4+ mast cells were blocked from migrating in vivo in the presence of the CXCR4 antagonist, AMD3100. This had immune implications because blocking mast cell migration in this way also prevented UV-induced immunosuppression (Byrne et al., 2008).
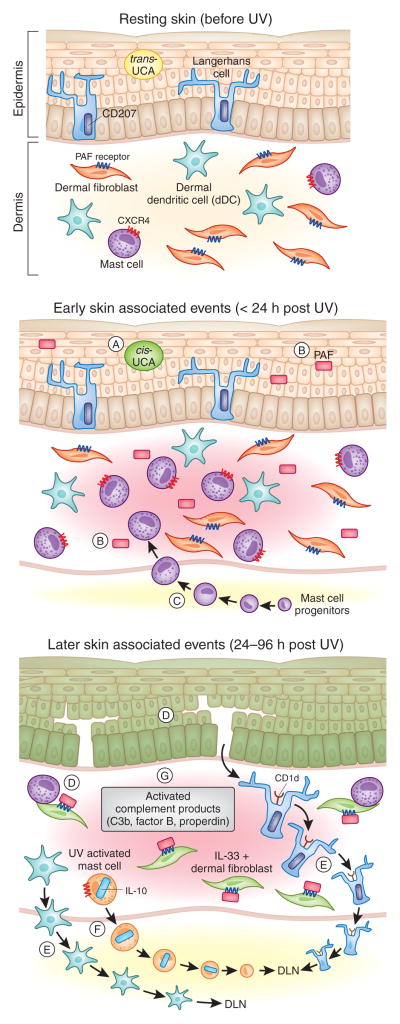
Figure 1. Cutaneous Photoimmunological Events.
A number of early/immediate immune modulating events (middle panel) occur in the skin following exposure to UV including: (A) isomerization of trans-urocanic acid (UCA) to the immune suppressive cis-UCA isoform (De Fabo and Noonan, 1983; Gibbs et al., 2008), (B) production of the biologically active lipid mediator, PAF (Alappatt et al., 2000; Travers et al., 2010) which contributes to skin cancer development (Sreevidya et al., 2008) by suppressing both adaptive immunity (Walterscheid et al., 2002) and DNA repair (Sreevidya et al., 2010), and (C) recruitment of immune modulating mast cells into the dermis peaking at 6h post UV (Byrne et al., 2008). These early events precipitate a number of later photoimmunological events (bottom panel) including: (D) The production of immune modulating IL-33 in keratinocytes and dermal fibroblasts (Byrne et al., 2011), (E) the migration of epidermal Langerhan’s Cells (Streilein et al., 1980), dermal dendritic cells (dDC) (Fukunaga et al., 2010) and (F) CXCR4+ dermal mast cells to the local draining lymph nodes (DLN) (Byrne et al., 2008) (G) Activation of complement components in the skin (including those associated with the alternative pathway; Factor B and Properdin (Stapelberg et al., 2009) also contributes to UV-induced immune suppression (Hammerberg et al., 1998; Yoshida et al., 1998).
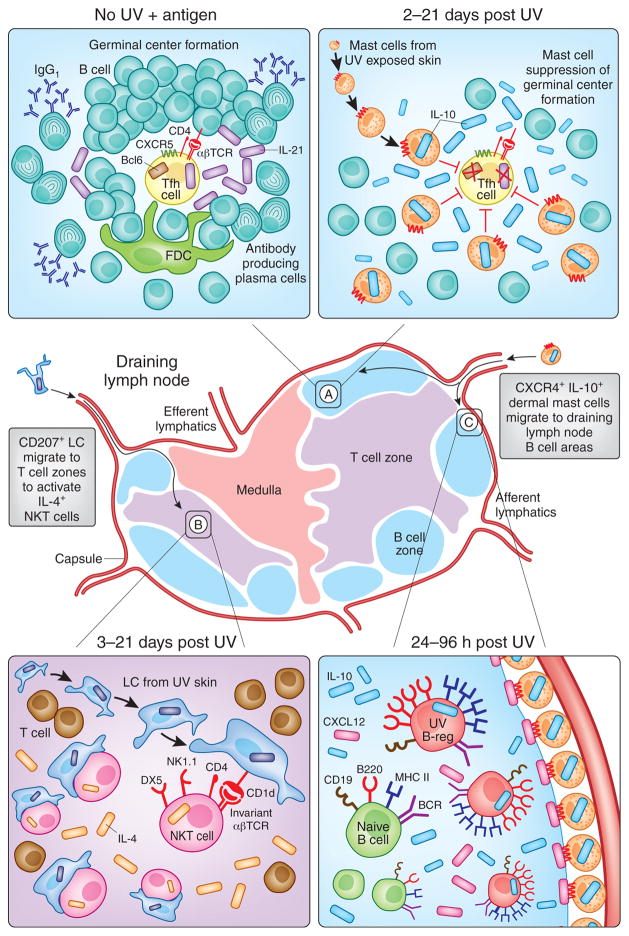
Figure 2. Photoimmunological Events Occurring in Skin Draining Lymph Nodes.
Immunosuppressive signals generated in the skin are transmitted by Langerhans Cells (LC) and mast cells to the local skin-draining lymph nodes to regulate both humoral and cell-mediated immune responses. (A: left hand panel) In response to immunization with protein antigens, B are activated by IL-21-expressing T follicular helper (Tfh) cells in germinal centers to produce IgG1 antibodies. (A: right hand panel) Mast cells that have migrated from UV-irradiated skin suppress this humoral arm of adaptive immunity by homing to B cell areas and producing IL-10 (Chacon-Salinas et al., 2011). At the same time, (B) CD207 (Langerin)+CD1d+ epidermal Langerhan’s Cells (LC) migrate from UV irradiated skin to the T cell zones where they activate IL-4-producing immunosuppressive NK-T cells (Fukunaga et al., 2010). Meanwhile, (C) UV-induced upregulation of CXCL12 (SDF1α) in B cell follicles attracts CXCR4+ dermal mast cells to the draining lymph nodes (Byrne et al., 2008). It is at this time that IL-10 producing UV-activated B regulatory cells, or “UV-B-regs” are induced (Byrne and Halliday, 2005) via a PAF and serotonin dependent mechanism (Matsumura et al., 2006).
In addition to cell-mediated immune reactions, UV-irradiation suppresses antibody formation (Spellman et al., 1984; Brown et al., 1995; El-Ghorr et al., 1998). Mast cells play a prominent role in suppressing antibody formation in vivo. Wild type mice were first exposed to UV radiation and 3 days later immunized with a T-dependent antigen (DNP-KLH). Germinal center formation, antibody secretion and T follicular helper cell generation were all suppressed by prior UV exposure (Figure 2). Injecting the UV-irradiated mice with cromolyn, a well-known inhibitor of mast cell function, blocked the suppression of antibody formation. Moreover, when mast cell-deficient mice were UV-irradiated and immunized with DNP-KLH, antibody formation, germinal center formation and T follicular helper cell generation were not different from what was seen in un-irradiated controls. We were able to restore UV-induced suppression of antibody formation by reconstituting mast cell deficient mice with mast cells derived from the bone marrow of wild type mice. Reconstituting the mast cell deficient mice with mast cells derived from interleukin (IL)-10-deficient mice failed to restore the ability of UV to suppress antibody formation, indicating the importance of mast cell-derived IL-10 in UV-induced immune suppression. These findings are among the first to demonstrate that mast cells, and mast cell-derived IL-10 inhibits antibody formation by suppressing T follicular helper function (Chacon-Salinas et al., 2011).
Experiments demonstrating an immune regulatory role for mast cells depend heavily on the use of mast cell deficient mice (Grimbaldeston et al., 2005). Immune function and inflammation is often enhanced in mast cell-deficient mice and resored to normal when bone marrow derived mast cells are transplanted into the mice (see as an example Li et al., 2011). These findings indicate an immune regulatory role for mast cells. Data presented in a recent paper, using new strains of mast cell deficient mice, are challenging the concept that mast cells have immunosuppressive potential (Dudeck et al., 2011). Mice were genetically engineered with the mast cell protease 5 promoter driving the expression of a simian dipthteria toxin receptor (Mcpt5-DTR). Because mice do not normally express the dipthteria toxin receptor, using a mast cell specific promoter to drive expression of the dipthteria toxin receptor results in mice in which the dipthteria toxin receptor is only expressed by mast cells. Injecting these mice with dipthteria toxin results in a selective depletion of mast cells. Unlike the situation found in Kit mutant mice, where CHS was enhanced in the absence of mast cells (Grimbaldeston et al., 2007), when Mcpt-5-DTR were first injected with dipthteria toxin, and then sensitized with hapten, a depressed CHS reaction (approximately 50% of the response found in the controls) was observed. Similar results were obtained in constitutively mast cell deficient mice (mice in which both the toxin receptor and the toxin producing genes are expressed only in mast cells; the mast cells commit suicide). Because these results challenge the paradigm that mast cells are immune reagulatory, it will be important to use these mice in models of UV-induced immune suppression to validate or challenge previous results.
Solar activated suppressor B cells
Although they do not receive as much attention as T regulatory cells, B cells can also suppress the immune response, primarily by secreting immune regulatory cytokines, such as IL-10. Following cutaneous UV-irradiation, the size and cellularity of the lymph nodes that drain the skin increases significantly. Byrne and Halliday (2005) studied the identity and function of the cells in the draining lymph nodes to gain a better understanding of the mechanisms driving immune suppression. They observed increased numbers of dendritic cells and B cells in the draining lymph nodes of UV-irradiated mice. The migration of dendritic cells to the lymph node was not too surprising, since UV-induced migration of Langerhans cells is a well-described phenomenon. In addition, they noted no alteration in lymph node dendritic cell phenotype, activation and function was not affected. Furthermore, injecting hapten-conjugated dendritic cells from normal or UV-irradiated mice into recipient animals activated CHS. This was somewhat surprising because conventional wisdom suggests UV-irradiation imparts a defect in draining lymph node dendritic cell function (Okamoto and Kripke, 1987), although it must be kept in mind that others have reported that dendritic cells function in the lymph nodes of UV-irradiated mice was normal (Lappin et al., 1996; Gorman et al., 2005). On the other hand, the B cells found in the lymph nodes expressed an activated phenotype (i.e., increased Major histocompatibility (MHC) Class II expression, B220 up-regulation and increased IL-10 secretion) (Figure 2). When the B cells from UV-irradiated mice were mixed with hapten-conjugated dendritic cells isolated from either normal or UV-irradiated mice, and injected into recipient mice, the subsequent CHS reaction was significantly suppressed (Byrne and Halliday, 2005). These data indicate that lymph nodes draining UV-irradiated skin contain a population of B cells that can suppress dendritic cell function. The authors suggest that B cell-derived IL-10 is involved.
These findings were subsequently confirmed by Matsumura et al. (2006). Transferring lymph node cells from the UV-irradiated mice into normal recipients suppressed the induction of CHS in the recipient mice, and induced long-lasting immune tolerance. The fluorescein isothiocyanate (FITC) positive cells that transferred immune suppression and induced tolerance were CD19+, B220+, B cells. Transferring cells from FITC-immunized B cell-deficient mice failed to induce immune suppression or induce tolerance. Similarly, no immune suppression resulted when lymph node cells from FITC-immunized, IL-10-deficient mice were transferred, indicating that IL-10-producing B cells were involved. In the gut, chronic inflammation induces IL-10-secreting immune suppressive B cells (Mizoguchi et al., 2002). It is now clear that UV-induced inflammation also plays a role in the induction of immune suppressive B cells. Two important UV-induced mediators of inflammation found in the skin are platelet activating factor (PAF) (Marathe et al., 2005) and serotonin (Slominski et al., 2005). Using a combination of selective PAF and serotonin receptor antagonists, and receptor-deficient mice, Matsumura et al. (2006) demonstrated that both PAF and serotonin were involved in the generation of UV-activated B regulatory cells, or “UV-B-rags”. B cells play a key role in skin cancer promotion (de Visser et al., 2005) and activation of UV-B-regs is likely to be an important contributor to the ability of UV radiation to induce skin cancer as blocking both the PAF and serotonin pathways not only inhibits the generation of UV-B-regs but protects mice from developing UV-induced skin cancer (Sreevidya et al., 2008).
An unconventional role for an unconventional T cell
A unique class of T cells, known as Natural Killer T (NKT) was first described in the late 1980’s (reviewed by Vicari and Zlotnik, 1996). They were called NKT cells because this unconventional T cell subset expresses surface markers normally found on T cells (CD4, αβ T cell antigen receptor) as well as receptors normally expressed on natural killer cells (NK1.1, DX5). Unlike conventional T cells, NKT cells do not recognize peptide antigens presented by MHC, but rather recognize lipid antigens presented by CD1. Although it is now understood that NKT cells can either enhance or suppress immune reactions, initially it was believed that NKT cells, by virtue of their ability to rapidly secrete large amounts of interferon-γ upon activation, only enhanced immune reactions Kronenberg and Gapin, 2002). One of the first reports that NKT cells can have suppressive activity came from a study investigating the mechanisms responsible for UV-induced immune suppression. As shown previously, transferring T cells from UV-irradiated mice exposed to a chronic sub-carcinogenic dose of UV radiation into recipient mice, suppressed the immune response of the recipient mouse and allowed for the progressive growth of highly antigenic UV-induced skin cancers (Fisher and Kripke, 1978). Moodycliffe et al. (2000) found that transferring NKT cells (CD4+, DX5+) from the spleens of mice exposed to a chronic sub-carcinogenic dose of UV to normal recipients, suppressed the immune response and allowed for the progressive growth of the skin cancers. In addition, when NKT cells were transferred from mice that were exposed to UV and then immunized with Candida albicans, delayed type hypersensitivity in the recipient mice was suppressed (Moodycliffe et al., 2000). Failure to induce immune regulatory NKT cells when CD1-deficient mice were UV irradiated provided additional evidence indicating that NKT cells were responsible.
Langerhans cells that migrate from the skin of UV-irradiated mice to the draining lymph nodes are essential for activating these NKT cells in vivo (Fukunaga et al., 2010) (Figure 1 and 2). The induction of CHS was significantly depressed when the mice received Langerhans cells (CD207+, Ep-Cam+, CD24a+, CD103−) isolated from the lymph nodes of UV-irradiated mice. This was in direct contrast to what was found when hapten-conjugated Langerhans cells from normal mice were used or when hapten-conjugated dermal dendritic cells isolated from UV-irradiated mice were injected into normal recipients. Microscopic examination of the draining lymph nodes indicated that the Langerhans cells migrated to the T cell area of the node, and were in close approximation to NKT cells (Figure 2). No immune suppression was observed when hapten-conjugated Langerhans cells from UV-irradiated mice were injected into NKT-deficient animals (CD1−/− or Jα18−/− mice). NKT cells isolated from the lymph nodes secreted IL-4, and anti-IL-4 monoclonal antibody blocked immune suppression. These data indicate that Langerhans cells transmit an immune suppressive signal from the skin to lymph nodes, where they activate NKT cells to secrete immune regulatory cytokines.
Cutaneous Mediators of Immune Suppression
As mentioned above, UVB radiation, which is absorbed in the skin, induces system wide immune regulation. We know that migrating mast cells and Langerhans cells play an important role in activating systemic immune suppression, but a great deal of attention has also been addressed at determining the cutaneous signals that activate the process. Keratinocytes are activated to release a wide variety of immune modulatory factors following UV irradiation (reviewed by Ullrich, 2005). In the course of those studies the investigators have often discovered novel immune regulatory processes (Figure 1).
Cis-urocanic acid (cis-UCA)
Trans-urocanic acid is found in the stratum corneum, and upon UV exposure, it isomerizes to the cis-isoform (Anglin et al., 1961). Cis-UCA has potent immune suppressive properties (as initially demonstrated by De Fabo and Noonan, 1983 and recently reviewed by Gibbs et al., 2008). Cis-UCA mediates immune suppression by binding to the serotonin (5HT-2A) receptor (Walterscheid et al., 2006; Shen and Ji, 2009). A monoclonal antibody to cis-UCA significantly reduced the numbers of skin cancers induced in UV-irradiated mice (Beissert et al., 2001). It is interesting to note that Hart and colleagues demonstrated that cis-UCA activates nerve endings in the skin to release neuropeptides that activate mast cells (Hart et al., 2002); so cis-UCA production may activate mast cell migration.
Some recent findings, however, may cause a re-evaluation of the role of cis-UCA in cancer induction. Laihia and colleagues found that applying cis-UCA to melanoma cells in culture, at pH 6.5, promotes intracellular acidification that results in apoptotic cell death. In addition, injecting cis-UCA into melanoma tumor xenografts, suppressed tumor growth in vivo, demonstrating a direct anti-cancer effect for cis-UCA (Laihia et al., 2010). The photoprotective role of cis-UCA may also play a role. Urocanic acid is produced by histidase. The stratum corneum of mice with a mutation in the histidase gene are deficient in urocanic acid, and these mice demonstrate a decreased ability to absorb UVB. When the dorsal skin of the urocanic acid-deficient mice was exposed to UVB radiation, pyrimidine dimer formation and apoptosis was increased. Applying cis-UCA to the skin of the urocanic acid-deficient mice reduced UVB-induced pyrimidine dimer formation (Barresi et al., 2010). These findings indicate that cis-UCA modulates UV-induced DNA damage in the skin and kills melanoma cells.
Inflammatory mediators with immune suppressive properties that unexpectedly affect DNA repair
Immunologists are well aware that cytokines and chemokines can activate and regulate the immune response. Perhaps not as well appreciated are the immune modulatory functions of biologically active lipid mediators, such as PAF. They play important roles in multiple organ systems (i.e., gastrointestinal, cardiovascular, reproductive, pulmonary) as regulators of inflammation, cell migration, differentiation and cell proliferation. The PAF receptor is found on platelets and a wide variety of immune cells, including neutrophils, macrophages, eosinophils, and mast cells, as well as T and B cells (Shimizu, 2009). Acute skin damage caused by UV radiation activates keratinocytes to secrete PAF (Alappatt et al., 2000; Travers et al., 2010) (Figure 1). Evidence indicting that PAF mediates UV-induced immune suppression was presented by Walterscheid and co-workers who found that selective PAF-receptor antagonists blocked UV-induced IL-10 and prostaglandin E-2 transcription in vitro and immune suppression in vivo. Moreover, injecting PAF into mice mimicked the effect of UV and activated immune suppression (Walterscheid et al., 2002). The failure to induce immune suppression in UV-irradiated PAF receptor-deficient mice provided the ultimate proof that PAF receptor binding is a critical step in the immune suppressive pathway (Wolf et al., 2006; Zhang et al., 2008). PAF also plays a role in UV-induced hyperalgesia (Zhang et al., 2009), and has been reported to be an essential factor in the generation of immune suppression observed following the application of aromatic hydrocarbons (i.e., jet fuel) to the skin (Ramos et al., 2004).
Using a microarray analysis, Travers and colleagues reported that PAF augments UV-induced keratinocyte gene expression, suggesting a potential mechanism of action (Travers et al., 2008). Although PAF is a well-known transcriptional activator, which is consistent with its augmentation of gene expression, its mechanism of action maybe related to its ability to affect DNA repair. When UV-irradiated hairless mice were injected with either a PAF or a serotonin receptor antagonist, tumor incidence and tumor progression was significantly suppressed. In addition, the two drugs, when injected in sub-optimal dose worked synergistically to suppress tumor induction in that only 25% of irradiated mice developed a skin cancer (Sreevidya et al., 2008). Presumably, blocking the binding of PAF and cis-UCA to their receptors was blocking the generation of UV-B-regs and the induction of immune suppression thus depressing skin cancer induction. However, a subsequent study by Sreevidya and co-workers indicated that PAF and serotonin receptor antagonists have an additional function in vivo: acceleration of DNA repair (Sreevidya et al., 2010). The investigators noted that treating UV-irradiated mice with PAF or serotonin antagonists did not prevent cyclobutane pyrimidine dimer (CPD) formation in the skin (a control to ensure the drugs were not sunscreens). However, they observed that the repair of CPD was significantly accelerated in mice injected with a PAF or a serotonin receptor antagonist. Nucleotide excision repair (NER) was accelerated in the skin of UV-irradiated PAF or serotonin receptor antagonist-injected mice, but the drugs had no effect on DNA repair in Xeroderma pigmentosum (XP)-deficient animals, which lack the genes needed to carry out NER. The formation of reactive oxygen species and 8-oxo-deoxyguanosine was also suppressed in the skin of UV-irradiated receptor antagonist injected mice. These data indicate that inflammatory mediators such as PAF depress DNA repair, which provides a mechanistic link between inflammation, immune suppression and cancer induction.
These findings were reminiscent of the data regarding the dual effects of the classic immune stimulatory cytokine, IL-12. UV-induced immune suppression, the induction of T regulatory cells, and the induction of immune tolerance are all reversed by recombinant IL-12 (Schmitt et al., 1995; Schwarz et al., 1996). Undoubtedly, the immune promoting effects of IL-12 are critical, but as described by Schwarz and colleagues, IL-12 has dual effects (Schwarz et al., 2002). Treating UV-irradiated keratinocytes with IL-12 inhibits UV-induced apoptosis. UV-induced CPD formation and sunburn cell formation was reduced by IL-12 treatment. No reversal of sunburn cell formation was noted when IL-12 was injected into UV-irradiated XP-deficient mice, indicating that IL-12 activated NER. Similarly, CPD formation induced by in vitro irradiation of human peripheral blood mononuclear cells was suppressed by IL-12, but no suppression of CPD formation was observed when peripheral blood mononuclear cells from XP patients were exposed to UV and treated with IL-12. The findings from these studies confirm the important role that DNA damage, and its repair has in UV-induced immune suppression (Kripke et al., 1992). They also highlight the novel and unexpected dual role that immune modulatory factors (IL-12, PAF, serotonin, cis-UCA) have on DNA repair.
As mentioned above, cis-UCA is immunosuppressive and photoprotective. These findings at first glance appear to be counterintuitive and raise the larger question of why UV radiation, a common daily event, induces immune suppression. A number of possible explanations have been proposed. UV-induced immune suppression may serve to prevent an autoimmune reaction to neo-antigens that may arise in the skin following UV exposure (reviewed by Kripke, 1994). Another point of view suggests that UV-induced immune suppression is a transient side effect triggered by the attempt to maintain genomic integrity (Ullrich, 2008). Following UV exposure the cell must either repair the damage, or if the damage is too extensive, undergo apoptosis. To repair DNA damage, the cell must arrest at the G2/M checkpoint. UV-induced activation of MAP Kinase p38 is critical for the initiation of cell cycle arrest (Bulavin et al., 2001). Activated MAP Kinase p38 promotes the activation of PLA2 the first enzymatic step in the synthesis of PAF (Ishii and Shimizu, 2000). Activated MAP Kinase p38 also promotes IL-10 transcription (Ma et al., 2001). The photoprotective role of UCA is consistent with the genomic integrity model, and consistent with the idea that evolution permitted transient immune suppression in exchange for a mechanism to promote genomic integrity following a common environmental insult (sunlight exposure).
A cutaneous alarmin that un-characteristically induces immunosuppression
Alarmins are endogenous messengers that are released following cellular damage and serve to activate innate and adaptive immune reactions to deal with infection or tissue damage. Some prominent examples are high mobility group box 1 proteins, heat shock proteins, uric acid, IL-1α, defensins and cathelicidins (see “Antimicrobial Peptides” in this issue for more details). Alarmins are chemo attractants; they induce the migration of antigen-presenting cells to sites of damage. Alarmins activate antigen-presenting cells and generally stimulate the immune response by enhancing immune function (reviewed by Oppenheim and Yang, 2005).
A newly described alarmin is IL-33. In the gut its conventional function is to enhance the immune response and attenuate sepsis and parasitic infection (Alves-Filho et al., 2010; Neill et al., 2010). However a recent report indicates that keratinocyte-derived IL-33 depresses immune function (Byrne et al., 2011). Exposing skin (either intact mouse or human skin and murine or human keratinocyte cultures) to UVB, but not UVA radiation up-regulates IL-33 expression (Figure 1). Injecting mice with recombinant IL-33 suppresses the induction of CHS, and treating UV-irradiated mice with antibodies to IL-33 blocks UV-induced immune suppression. An indirect mechanism appears to be involved. Treating fibroblast cultures with PAF, but not cis-UCA induced IL-33 expression, indicating a role for PAF in alarmin production. To the best of our knowledge, this is the first report to show that alarmins can down-regulate the immune response.
Is complement an environmental sensor for UV damage?
A number of years ago, Hammerberg and colleagues observed that immune suppression and immune tolerance was not induced in Complement (C3)-deficient mice (Hammerberg et al., 1998). Subsequent studies indicated that products of complement activation are expressed in UV-irradiated human skin, and that binding of the activated C3 fragment, iC3b to its receptor (CD11b) on monocytes results in increased IL-10 secretion with concomitant suppression in IL-12 secretion (Yoshida et al., 1998). These results were somewhat surprising, but do fit in with the known role of inflammation, and inflammatory products, in UV-induced immune suppression. Recently this issue was revisited by Stapelberg et al (Stapelberg et al., 2009), who were interested in discerning the molecular triggers for UVA-induced immune suppression. UVA exposure is immunosuppressive, but unlike UVB, where the dose-response curve for the induction of immune suppression is linear, the dose response curve for UVA is bell-shaped both in mice (Byrne et al., 2002) and humans (Matthews et al., 2010). Moreover, exposure to high doses of UVA given before an immunosuppressive dose of UVB is immunoprotective (Reeve et al., 1998). A microarray analysis was done using RNA isolated from the skin of mice exposed to a relatively low (immunosuppressive) dose of UVA. Only genes comprising the alternative complement pathway (C3, complement factor B and properdin) were activated (Figure 1G). This pathway was not activated by high dose UVA or low dose UVB. The observation that complement may serve as an environmental sensor is novel, but whether other skin damaging agents activate complement to influence immunity remain to be seen.
Conclusions
The UV radiation in sunlight is a major environmental carcinogen and because UV-induced immune suppression is a major risk factor for skin cancer induction, dermatologists and cancer immunologists have a long history of studying the mechanisms involved. Advances in photoimmunology have generally paralleled advances in immunology (i.e., important role of antigen presentation, role of cytokines in regulating the response, critical role of regulatory cells in dampening immune reactions). However, there are a number of examples where investigations into the mechanisms underlying UV-induced immune suppression have led to new immunologic insights. This review was not meant to be all-inclusive (and we apologize to the many investigators whose work was not mentioned here due to space limitations), but rather to show examples of how advances made by photoimmunologists eventually became well accepted in the mainstream immunologic literature (For readers who are interested in a more comprehensive treatment of the subject we suggest three recent reviews; Norval and Halliday, 2011; Norval and Woods, 2011; Ullrich, 2011). The idea that Langerhans cells, Mast cells and NKT cells can also serve in an unconventional fashion to regulate the immune response is now well-accepted in the general immunological community; it had its first acceptance in the photoimmunological community. The realization that bioactive lipids can suppress the immune response, and the understanding of the unique role of IL-12, cis-UCA and PAF in modulating DNA repair came from studies by investigators whose focus on skin, sunlight, carcinogenesis and immune suppression equipped them to recognize unconventional roles for conventional molecules. It appears the same can be said for IL-33; its role as a mediator for UV-induced immune suppression is certainly outside the normal described function of alarmins. None of this should be too surprising. One constant of science is that once the community thinks we really understand something; new findings generally turn the existing dogma up side down. What we have tried to do here is briefly list some examples of advances made by cutaneous immunologists that have helped to alter conventional immunologic wisdom.
Acknowledgments
Work performed in our laboratories was supported by a Cancer Institute NSW Career Development and Support Fellowship (SNB) and by a Grant from the National Cancer Institute (CA131207; SEU)
Footnotes
Conflict of Interest: The authors declare no conflict of interest
References
- Alappatt C, Johnson CA, Clay KL, Travers JB. Acute keratinocyte damage stimulates platelet-activating factor production. Arch Dermatol Res. 2000;292:256–9. doi: 10.1007/s004030050483. [DOI] [PubMed] [Google Scholar]
- Alard P, Kurimoto I, Niizeki H, Doherty JM, Streilein JW. Hapten-specific tolerance induced by acute, low-dose ultraviolet B radiation of skin requires mast cell degranulation. Eur J Immunol. 2001;31:1736–46. doi: 10.1002/1521-4141(200106)31:6<1736::aid-immu1736>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Alard P, Niizeki H, Hanninen L, Streilein JW. Local ultraviolet B irradiation impairs contact hypersensitivity induction by triggering release of tumor necrosis factor-alpha from mast cells. Involvement of mast cells and Langerhans cells in susceptibility to ultraviolet B. J Invest Dermatol. 1999;113:983–90. doi: 10.1046/j.1523-1747.1999.00772.x. [DOI] [PubMed] [Google Scholar]
- Alves-Filho JC, Sonego F, Souto FO, Freitas A, Verri WA, Jr, Auxiliadora-Martins M, et al. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med. 2010;16:708–12. doi: 10.1038/nm.2156. [DOI] [PubMed] [Google Scholar]
- Anglin JH, Jr, Bever AT, Everett MA, Lamb JH. Ultraviolet-light-induced alterations in urocanic acid in vivo. Biochim Biophys Acta. 1961;53:408–9. doi: 10.1016/0006-3002(61)90454-1. [DOI] [PubMed] [Google Scholar]
- Barresi C, Stremnitzer C, Mlitz V, Kezic S, Kammeyer A, Ghannadan M, et al. Increased sensitivity of histidinemic mice to UVB radiation suggests a crucial role of endogenous urocanic acid in photoprotection. J Invest Dermatol. 2010;131:188–94. doi: 10.1038/jid.2010.231. [DOI] [PubMed] [Google Scholar]
- Beissert S, Ruhlemann D, Mohammad T, Grabbe S, El-Ghorr A, Norval M, et al. IL-12 prevents the Inhibitory effects of cis-Urocanic acid on tumor antigen presentation by Langerhans cells: Implications for photocarcinogenesis. J Immunol. 2001;167:6232–8. doi: 10.4049/jimmunol.167.11.6232. [DOI] [PubMed] [Google Scholar]
- Brown EL, Rivas JM, Ullrich SE, Young CR, Norris SJ, Kripke ML. Modulation of immunity to Borrelia burgdorferi by ultraviolet irradiation: differential effect on Th1 and Th2 immune responses. Eur J Immunol. 1995;25:3017–22. doi: 10.1002/eji.1830251105. [DOI] [PubMed] [Google Scholar]
- Bulavin DV, Higashimoto Y, Popoff IJ, Gaarde WA, Basrur V, Potapova O, et al. Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature. 2001;411:102–7. doi: 10.1038/35075107. [DOI] [PubMed] [Google Scholar]
- Byrne SN, Beaugie C, O’Sullivan C, Leighton S, Halliday GM. The Immune-Modulating Cytokine and Endogenous Alarmin Interleukin-33 Is Upregulated in Skin Exposed to Inflammatory UVB Radiation. Am J Pathol. 2011;179:211–22. doi: 10.1016/j.ajpath.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne SN, Halliday GM. B cells activated in lymph nodes in response to ultraviolet irradiation or by interleukin-10 inhibit dendritic cell induction of immunity. J Invest Dermatol. 2005;124:570–8. doi: 10.1111/j.0022-202X.2005.23615.x. [DOI] [PubMed] [Google Scholar]
- Byrne SN, Limon-Flores AY, Ullrich SE. Mast cell migration from the skin to the draining lymph nodes upon ultraviolet irradiation represents a key step in the induction of immune suppression. J Immunol. 2008;180:4648–55. doi: 10.4049/jimmunol.180.7.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne SN, Spinks N, Halliday GM. Ultraviolet A irradiation of C57BL/6 mice suppresses systemic contact hypersensitivity or enhances secondary immunity depending on dose. J Invest Dermatol. 2002;119:858–64. doi: 10.1046/j.1523-1747.2002.00261.x. [DOI] [PubMed] [Google Scholar]
- Chacon-Salinas R, Limon-Flores AY, Chavez-Blanco AD, Gonzalez-Estrada A, Ullrich SE. Mast cell-derived IL-10 suppresses germinal center formation by affecting T follicular helper cell function. J Immunol. 2011;186:25–31. doi: 10.4049/jimmunol.1001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Fabo EC, Noonan FP. Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. J Exp Med. 1983;157:84–98. doi: 10.1084/jem.158.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–23. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Dudeck A, Dudeck J, Scholten J, Petzold A, Surianarayanan S, Kohler A, et al. Mast Cells Are Key Promoters of Contact Allergy that Mediate the Adjuvant Effects of Haptens. Immunity. 2011;34:973–84. doi: 10.1016/j.immuni.2011.03.028. [DOI] [PubMed] [Google Scholar]
- El-Ghorr AA, Horsburgh G, Norval M. The effect of UVB irradiation on antibody responses during herpes simplex virus type 1 (HSV-1) infections of mice. Photodermatol Photoimmunol Photomed. 1998;14:17–25. doi: 10.1111/j.1600-0781.1998.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Elmets CA, Bergstresser PR, Tigelaar RE, Wood PJ, Streilein JW. Analysis of the mechanism of unresponsiveness produced by haptens painted on skin to low dose UV radiation. J Exp Med. 1983;158:781–94. doi: 10.1084/jem.158.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MS, Kripke ML. Systemic alteration induced in mice by ultraviolet light irradiation and its relationship to ultraviolet carcinogenesis. Proc Natl Acad Sci U S A. 1977;74:1688–92. doi: 10.1073/pnas.74.4.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MS, Kripke ML. Further studies on the tumor-specific suppressor cells induced by ultraviolet radiation. J Immunol. 1978;121:1139–44. [PubMed] [Google Scholar]
- Fisher MS, Kripke ML. Suppressor T lymphocytes control the development of primary skin cancers in ultraviolet-irradiated mice. Science. 1982;216:1133–4. doi: 10.1126/science.6210958. [DOI] [PubMed] [Google Scholar]
- Fukunaga A, Khaskhely NM, Ma Y, Sreevidya CS, Taguchi K, Nishigori C, et al. Langerhans cells serve as immunoregulatory cells by activating NKT cells. J Immunol. 2010;185:4633–40. doi: 10.4049/jimmunol.1000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga A, Khaskhely NM, Sreevidya CS, Byrne SN, Ullrich SE. Dermal dendritic cells, and not Langerhans cells, play an essential role in inducing an immune response. J Immunol. 2008;180:3057–64. doi: 10.4049/jimmunol.180.5.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–86. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs NK, Tye J, Norval M. Recent advances in urocanic acid photochemistry, photobiology and photoimmunology. Photochem Photobiol Sci. 2008;7:655–67. doi: 10.1039/b717398a. [DOI] [PubMed] [Google Scholar]
- Gorman S, Tan JW, Thomas JA, Townley SL, Stumbles PA, Finlay-Jones JJ, et al. Primary defect in UVB-induced systemic immunomodulation does not relate to immature or functionally impaired APCs in regional lymph nodes. J Immunol. 2005;174:6677–85. doi: 10.4049/jimmunol.174.11.6677. [DOI] [PubMed] [Google Scholar]
- Greene MI, Sy MS, Kripke M, Benacerraf B. Impairment of antigen-presenting cell function by ultraviolet radiation. Proc Natl Acad Sci U S A. 1979;76:6591–5. doi: 10.1073/pnas.76.12.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–48. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbaldeston MA, Green A, Darlington S, Robertson BO, Marshman G, Finlay-Jones JJ, et al. Susceptibility to basal cell carcinoma is associated with high dermal mast cell prevalence in non-sun-exposed skin for an Australian populations. Photochem Photobiol. 2003;78:633–9. doi: 10.1562/0031-8655(2003)078<0633:stbcci>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- Grimbaldeston MA, Pearce AL, Robertson BO, Coventry BJ, Marshman G, Finlay-Jones JJ, et al. Association between melanoma and dermal mast cell prevalence in sun-unexposed skin. Br J Dermatol. 2004;150:895–903. doi: 10.1111/j.1365-2133.2004.05966.x. [DOI] [PubMed] [Google Scholar]
- Grimbaldeston MA, Skov L, Baadsgaard O, Skov BG, Marshman G, Finlay-Jones JJ, et al. Communications: high dermal mast cell prevalence is a predisposing factor for basal cell carcinoma in humans. J Invest Dermatol. 2000;115:317–20. doi: 10.1046/j.1523-1747.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- Hammerberg C, Katiyar SK, Carroll MC, Cooper KD. Activated complement component 3 (C3) is required for ultraviolet induction of immunosuppression and antigenic tolerance. J Exp Med. 1998;187:1133–8. doi: 10.1084/jem.187.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart PH, Grimbaldeston MA, Swift GJ, Jaksic A, Noonan FP, Finlay-Jones JJ. Dermal mast cells determine susceptibility to Ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J Exp Med. 1998;187:2045–53. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart PH, Townley SL, Grimbaldeston MA, Khalil Z, Finlay-Jones JJ. Mast cells, neuropeptides, histamine, and prostaglandins in UV-induced systemic immunosuppression. Methods. 2002;28:79–89. doi: 10.1016/s1046-2023(02)00201-3. [DOI] [PubMed] [Google Scholar]
- Ishii S, Shimizu T. Platelet-activating factor (PAF) receptor and genetically engineered PAF receptor mutant mice. Prog Lipid Res. 2000;39:41–82. doi: 10.1016/s0163-7827(99)00016-8. [DOI] [PubMed] [Google Scholar]
- Kripke ML. Antigenicity of murine skin tumors induced by ultraviolet light. J Natl Cancer Inst. 1974;53:1333–6. doi: 10.1093/jnci/53.5.1333. [DOI] [PubMed] [Google Scholar]
- Kripke ML. Ultraviolet radiation and immunology: something new under the sun--presidential address. Cancer Res. 1994;54:6102–5. [PubMed] [Google Scholar]
- Kripke ML, Cox PA, Alas LG, Yarosh DB. Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci U S A. 1992;89:7516–20. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- Laihia JK, Kallio JP, Taimen P, Kujari H, Kahari VM, Leino L. Protodynamic intracellular acidification by cis-urocanic acid promotes apoptosis of melanoma cells in vitro and in vivo. J Invest Dermatol. 2010;130:2431–9. doi: 10.1038/jid.2010.151. [DOI] [PubMed] [Google Scholar]
- Lappin MB, Kimber I, Dearman RJ, Norval M. Exposure of UVB-sensitive mice to immunosuppressive doses of UVB in vivo fails to affect the accessory function or the phenotype of draining lymph node dendritic cells. Exp Dermatol. 1996;5:286–94. doi: 10.1111/j.1600-0625.1996.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Li H, Nourbakhsh B, Safavi F, Li K, Xu H, Cullimore M, et al. Kit (W-sh) Mice Develop Earlier and More Severe Experimental Autoimmune Encephalomyelitis Due to Absence of Immune Suppression. J Immunol. 2011;187:274–82. doi: 10.4049/jimmunol.1003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Lim W, Gee K, Aucoin S, Nandan D, Kozlowski M, et al. The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. J Biol Chem. 2001;276:13664–74. doi: 10.1074/jbc.M011157200. [DOI] [PubMed] [Google Scholar]
- Marathe GK, Johnson C, Billings SD, Southall MD, Pei Y, Spandau D, et al. Ultraviolet B Radiation Generates Platelet-activating Factor-like Phospholipids underlying Cutaneous Damage. J Biol Chem. 2005;280:35448–57. doi: 10.1074/jbc.M503811200. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Byrne SN, Nghiem DX, Miyahara Y, Ullrich SE. A role for inflammatory mediators in the induction of immunoregulatory B cells. J Immunol. 2006;177:4810–7. doi: 10.4049/jimmunol.177.7.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews YJ, Halliday GM, Phan TA, Damian DL. Wavelength dependency for UVA-induced suppression of recall immunity in humans. J Dermatol Sci. 2010;59:192–7. doi: 10.1016/j.jdermsci.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–30. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- Moodycliffe AM, Nghiem D, Clydesdale G, Ullrich SE. Immune suppression and skin cancer development: regulation by NKT cells. Nat Immunol. 2000;1:521–5. doi: 10.1038/82782. [DOI] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niizeki H, Alard P, Streilein JW. Cutting edge: Calcitonin gene-related peptide is necessary for ultraviolet B-impaired induction of contact hypersensitivity. J Immunol. 1997;159:5183–6. [PubMed] [Google Scholar]
- Norval M, Halliday GM. The Consequences of UV-induced Immunosuppression for Human Health. Photochemistry and Photobiology. 2011 doi: 10.1111/j.1751-1097.2011.00969.x. Accepted Article. [DOI] [PubMed] [Google Scholar]
- Norval M, Woods GM. UV-induced immunosuppression and the efficacy of vaccination. Photochemical & Photobiological Sciences. 2011 doi: 10.1039/C1PP05105A. Accepted Article. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Kripke ML. Effetor and suppressor circuits of the immune response are activated in vivo by different mechanims. Proc Natl Acad Sci U S A. 1987;84:3841–45. doi: 10.1073/pnas.84.11.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Current Opinion in Immunology. 2005;17:359–65. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Ramos G, Kazimi N, Nghiem DX, Walterscheid JP, Ullrich SE. Platelet activating factor receptor binding plays a critical role in jet fuel-induced immune suppression. Toxicol Appl Pharmacol. 2004;195:331–8. doi: 10.1016/j.taap.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Reeve VE, Bosnic M, Boehm-Wilcox C, Nishimura N, Ley RD. Ultraviolet A radiation (320–400 nm) protects hairless mice from immunosuppression induced by ultraviolet B radiation (280–320 nm) or cis-urocanic acid. Int Arch Allergy Immunol. 1998;115:316–22. doi: 10.1159/000069463. [DOI] [PubMed] [Google Scholar]
- Schmitt DA, Owen-Schaub L, Ullrich SE. Effect of IL-12 on immune suppression and suppressor cell induction by ultraviolet radiation. J Immunol. 1995;154:5114–20. [PubMed] [Google Scholar]
- Schwarz A, Grabbe S, Aragane Y, Sandkuhl K, Riemann H, Luger TA, et al. Interleukin-12 prevents ultraviolet B-induced local immunosuppression and overcomes UVB-induced tolerance. J Invest Dermatol. 1996;106:1187–91. doi: 10.1111/1523-1747.ep12347944. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Stander S, Berneburg M, Bohm M, Kulms D, van Steeg H, et al. Interleukin-12 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Nat Cell Biol. 2002;4:26–31. doi: 10.1038/ncb717. [DOI] [PubMed] [Google Scholar]
- Schwarz T. 25 years of UV-induced immunosuppression mediated by T cells-from disregarded T suppressor cells to highly respected regulatory T cells. Photochem Photobiol. 2008;84:10–8. doi: 10.1111/j.1751-1097.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- Shen L, Ji HF. Molecular basis for cis-urocanic acid as a 5-HT(2A) receptor agonist. Bioorg Med Chem Lett. 2009;19:5307–9. doi: 10.1016/j.bmcl.2009.07.143. [DOI] [PubMed] [Google Scholar]
- Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol. 2009;49:123–50. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. Faseb J. 2005;19:176–94. doi: 10.1096/fj.04-2079rev. [DOI] [PubMed] [Google Scholar]
- Spellman CW, Anderson WL, Bernhard EJ, Tomasi TB. Suppression of antibody responses to topically applied antigens by ultraviolet light irradiation. Induction of phototolerance. J Exp Med. 1984;160:1891–900. doi: 10.1084/jem.160.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreevidya CS, Fukunaga A, Khaskhely NM, Masaki T, Ono R, Nishigori C, et al. Agents that reverse UV-Induced immune suppression and photocarcinogenesis affect DNA repair. J Invest Dermatol. 2010;130:1428–37. doi: 10.1038/jid.2009.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreevidya CS, Khaskhely NM, Fukunaga A, Khaskina P, Ullrich SE. Inhibition of photocarcinogenesis by platelet-activating factor or serotonin receptor antagonists. Cancer Res. 2008;68:3978–84. doi: 10.1158/0008-5472.CAN-07-6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapelberg MP, Williams RB, Byrne SN, Halliday GM. The alternative complement pathway seems to be a UVA sensor that leads to systemic immunosuppression. J Invest Dermatol. 2009;129:2694–701. doi: 10.1038/jid.2009.128. [DOI] [PubMed] [Google Scholar]
- Stein P, Rechtsteiner G, Warger T, Bopp T, Fuhr T, Prufer S, et al. UV exposure boosts transcutaneous immunization and improves tumor immunity: cytotoxic T-cell priming through the skin. J Invest Dermatol. 2011;131:211–9. doi: 10.1038/jid.2010.254. [DOI] [PubMed] [Google Scholar]
- Streilein JW, Toews GT, Gilliam JN, Bergstresser PR. Tolerance or Hypersensitivity to 2,4-dinitro-1-fluorobenzene: The Role of Langerhans Cell Density within Epidermis. J Invest Dermatol. 1980;74:319–22. doi: 10.1111/1523-1747.ep12543557. [DOI] [PubMed] [Google Scholar]
- Toews GB, Bergstresser PR, Streilein JW. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980;124:445–9. [PubMed] [Google Scholar]
- Travers JB, Berry D, Yao Y, Yi Q, Konger RL. Ultraviolet B radiation of human skin generates platelet-activating factor receptor agonists. Photochem Photobiol. 2010;86:949–54. doi: 10.1111/j.1751-1097.2010.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers JB, Edenberg HJ, Zhang Q, Al-Hassani M, Yi Q, Baskaran S, et al. Augmentation of UVB radiation-mediated early gene expression by the epidermal platelet-activating factor receptor. J Invest Dermatol. 2008;128:455–60. doi: 10.1038/sj.jid.5701083. [DOI] [PubMed] [Google Scholar]
- Ullrich SE. Mechanisms underlying UV-induced immune suppression. Mutat Res. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- Ullrich SE. Immune Modulation by phototherapy: Why Immune Suppression? Expert Rev Dermatol. 2008;3:7–9. [Google Scholar]
- Ullrich SE. Ultraviolet carcinogenesis and immune suppression. In: Weber RS, Moore BA, editors. Cutaneous Malignancy of the Head and Neck: A multidisciplinary Approach. San Diego, CA: Plural Publishing; 2011. pp. 57–78. [Google Scholar]
- Ullrich SE, Nghiem DX, Khaskina P. Suppression of an established immune response by UVA--a critical role for mast cells. Photochem Photobiol. 2007;83:1095–100. doi: 10.1111/j.1751-1097.2007.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari AP, Zlotnik A. Mouse NK1.1+ T cells: a new family of T cells. Immunol Today. 1996;17:71–6. doi: 10.1016/0167-5699(96)80582-2. [DOI] [PubMed] [Google Scholar]
- Walterscheid JP, Nghiem DX, Kazimi N, Nutt LK, McConkey DJ, Norval M, et al. Cis-urocanic acid, a sunlight-induced immunosuppressive factor, activates immune suppression via the 5-HT2A receptor. Proc Natl Acad Sci U S A. 2006;103:17420–5. doi: 10.1073/pnas.0603119103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walterscheid JP, Ullrich SE, Nghiem DX. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J Exp Med. 2002;195:171–9. doi: 10.1084/jem.20011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf P, Nghiem DX, Walterscheid JP, Byrne S, Matsumura Y, Matsumura Y, et al. Platelet-activating factor is crucial in psoralen and ultraviolet A-induced immune suppression, inflammation, and apoptosis. Am J Pathol. 2006;169:795–805. doi: 10.2353/ajpath.2006.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Kang K, Berger M, Chen G, Gilliam AC, Moser A, et al. Monocyte induction of IL-10 and down-regulation of IL-12 by iC3b deposited in ultraviolet-exposed human skin. J Immunol. 1998;161:5873–9. [PubMed] [Google Scholar]
- Yoshikawa T, Rae V, Bruins-Slot W, Van den Berg JW, Taylor JR, Streilein JW. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol. 1990;95:530–6. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Sitzman LA, Al-Hassani M, Cai S, Pollok KE, Travers JB, et al. Involvement of Platelet-Activating Factor in Ultraviolet B-Induced Hyperalgesia. J Invest Dermatol. 2009;129:167–74. doi: 10.1038/jid.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Yao Y, Konger RL, Sinn AL, Cai S, Pollok KE, et al. UVB radiation-mediated inhibition of contact hypersensitivity reactions is dependent on the platelet-activating factor system. J Invest Dermatol. 2008;128:1780–7. doi: 10.1038/sj.jid.5701251. [DOI] [PMC free article] [PubMed] [Google Scholar]