Abstract
Glucocorticoids profoundly influence immune responses, and synthetic glucocorticoids are widely used clinically for their potent antiinflammatory effects. Endogenous glucocorticoid action is modulated by the two isozymes of 11β-hydroxysteroid dehydrogenase (11β-HSD). In vivo, 11β-HSD1 catalyzes the reduction of inactive cortisone or 11-dehydrocorticosterone into active cortisol or corticosterone, respectively, thereby increasing intracellular glucocorticoid levels. 11β-HSD2 catalyzes the reverse reaction, inactivating intracellular glucocorticoids. Both enzymes have been postulated to modulate inflammatory responses. In the K/BxN serum transfer model of arthritis, 11β-HSD1-deficient mice showed earlier onset and slower resolution of inflammation than wild-type controls, with greater exostoses in periarticular bone and, uniquely, ganglion cysts, consistent with greater inflammation. In contrast, K/BxN serum arthritis was unaffected by 11β-HSD2 deficiency. In a distinct model of inflammation, thioglycollate-induced sterile peritonitis, 11β-HSD1-deficient mice had more inflammatory cells in the peritoneum, but again 11β-HSD2-deficient mice did not differ from controls. Additionally, compared with control mice, 11β-HSD1-deficient mice showed greater numbers of inflammatory cells in pleural lavages in carrageenan-induced pleurisy with lung pathology consistent with slower resolution. These data suggest that 11β-HSD1 limits acute inflammation. In contrast, 11β-HSD2 plays no role in acute inflammatory responses in mice. Regulation of local 11β-HSD1 expression and/or delivery of substrate may afford a novel approach for antiinflammatory therapy.
High doses of exogenous glucocorticoids exert potent antiinflammatory effects and remain an effective therapy for rheumatoid arthritis and other inflammatory disorders (1). Physiological variation in endogenous glucocorticoid levels also influences inflammatory responses but with both anti- and proinflammatory actions, depending on context and the target cells involved (2, 3).
Endogenous glucocorticoid action within cells and tissues is modulated by intracellular glucocorticoid metabolism by the two isozymes of 11β-hydroxysteroid dehydrogenase (11β-HSD), which interconvert active glucocorticoids (cortisol, corticosterone) and intrinsically inert 11-keto metabolites (cortisone, 11-dehydrocorticosterone) (4). In vivo, 11β-HSD1 predominantly reactivates glucocorticoids, amplifying their action (5), whereas 11β-HSD2 inactivates glucocorticoids (6).
11β-HSD1 is expressed in immune cells, notably macrophages, dendritic cells, and lymphocytes (7–10), with enzyme levels dependent on cellular activation state (e.g. during an inflammatory response) (11). In humans, synovial inflammation in rheumatoid arthritis, but not osteoarthritis, is positively associated with cortisone reactivation, suggesting increased synovial 11β-HSD1 activity (12). 11β-HSD1 is expressed in synovial macrophages and fibroblasts of patients with rheumatoid arthritis and is highly induced by the proinflammatory cytokines IL-1 and TNF-α in the latter cell type (13). Moreover, 11β-HSD1 is induced in cells recruited into the peritoneum during sterile peritonitis (9) and is increased in intestine in both human and rodent colitis (14, 15). Macrophages from mice deficient in 11β-HSD1 show delayed acquisition of phagocytic capacity and exaggerated production of IL-6 and other cytokines after activation with lipopolysaccharide (9, 16).
Although 11β-HSD2 is not normally expressed in immune cells in either humans or mice (7, 9), 11β-HSD2 has been reported in immune cells in joints of patients with rheumatoid arthritis (12, 17). The nonselective 11β-HSD inhibitor, glycyrrhetinic acid, exerts antiinflammatory activity in rheumatoid arthritis, an effect interpreted as due to 11β-HSD2 inhibition (18). Reciprocal regulation of 11β-HSD1 and 11β-HSD2 by IL-1 and TNF-α has been reported in several nonimmune tissues (19, 20). It has therefore been suggested that targeting 11β-HSD2 may be an effective treatment in rheumatoid arthritis. However, the effects of 11β-HSD2 deficiency on inflammatory responses have not been reported. Moreover, 11β-HSD1 inhibitors are in clinical trials for metabolic disease and understanding the role of 11β-HSD in arthritis and other clinically relevant inflammatory conditions may be critical to their exploitation.
A rheumatoid arthritis-like disease develops spontaneously in offspring of KRN-transgenic mice bred with NOD mice due to circulating anti-glucose-6-phosphate-isomerase autoantibodies (K/BxN serum) (21). Injection of K/BxN serum into naive mice reliably and reproducibly induces a self-resolving passive arthritis in recipient mice (22). Here we have used mice deficient in either 11β-HSD1 (23) or 11β-HSD2 (24) to investigate the role of these enzymes in K/BxN serum-induced inflammatory arthritis and other mouse models of inflammation.
Materials and Methods
Animals
Mice homozygous for a targeted disruption of the Hsd11b1 gene (Hsd11b1−/−) or the Hsd11b2 gene (Hsd11b2−/−), both congenic on the C57BL/6J background (more than eight backcrosses), have been previously described (23–25). Controls were age-matched C57BL/6J mice. All experimental mice were males, aged 8–12 wk, housed in groups of two to five mice per cage under controlled conditions (12 h light, 12 h dark cycle, 21 C) with ad libitum access to water and standard rodent chow. All experimentation was conducted in strict accord with accepted standards of humane animal care under the auspices of the U.K. Animals (Scientific Procedures) Act, 1986, after the prior approval by the local ethical committee.
Induction and assessment of inflammatory arthritis
Mice were injected ip with arthritic K/BxN serum, generated in-house from K/BxN mice [mice expressing the transgenic T cell receptor KRN and the MHC class II allele Ag7] as previously described (26) using either the standard dose (7.5 μl/g of body weight on d 0 and d 2) or a reduced dose (single injection of 5.6 μl/g of body weight on d 0). The standard dose produces a maximal response in control mice; thus, the reduced dose was used to test the hypothesis that the inflammatory response would be greater in Hsd11b1−/− mice. Inflammation was scored by visual examination according to a clinical index in which each joint was assigned a score of 0–3 as described (38). The mean cumulative scores were calculated (maximum score per animal of 12) for each group. Right tarsal joint swelling was measured using a caliper (Kroeplin POCO-2T; Wessex Metrology, Poole, UK) and was defined as the cumulative difference in joint diameter (millimeters) after induction of arthritis from that measured at the start of the experiment, reported as mean area under the curve (AUC) per group. For histology, joints were fixed in 10% formalin, decalcified in 10% EDTA in neutral buffered formalin, and paraffin embedded. Deparaffinized and hydrated joint sections (4 μm) were stained with hematoxylin and eosin for histopathological examination.
Corticosterone RIA
Peripheral blood was collected from Hsd11b1−/− and wild-type mice 2 d and 21 d after induction of K/BxN arthritis under nonstressed conditions by tail nick at 0800 h for measurement of circulating corticosterone by RIA as described previously (27).
Thioglycollate-induced sterile peritonitis
Peritonitis was induced in mice by ip injection of 1 ml of 3% thioglycollate as previously described (9). Peritoneal cells were collected by lavage with 5 ml cold PBS and counted by hemocytometer.
Carrageenan-induced pleurisy
Pleural inflammation was induced in mice by intrapleural injection of 100 μl of 0.1% λ-carrageenan (Marine Colloids Inc., FMC BioPolymer, Philadelphia, PA) as previously described (28). The pleural exudates were collected by lavage with 1 ml cold PBS containing 3.15% sodium citrate and recovered cells counted by hemocytometer. The thorax was fixed in 10% formalin, paraffin embedded, and hematoxylin and eosin (H&E) staining was performed on 4- to 5-μm sections.
Statistics
The effects of genotype and treatment were assessed by two-factor, repeated-measures ANOVA followed by post hoc Bonferroni tests. A Student's t test was used for comparisons between genotypes. Significance was set at P < 0.05. Values are means ± sem.
Results
11β-HSD1 deficiency, but not 11β-HSD2 deficiency, worsens the course of experimental arthritis
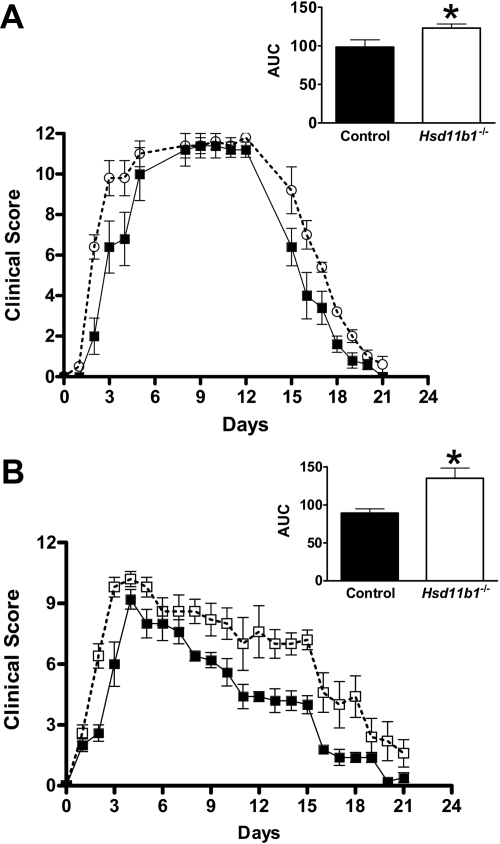
Inflammatory arthritis was induced in all mice after injection of arthritogenic K/BxN serum, whether at the standard dose (Fig. 1A) or the submaximal (37% of the standard) dose (Figs. 1B and 2). However, in Hsd11b1−/− mice, the onset of inflammation was earlier than in wild-type controls, irrespective of dose, and resolution was impaired, producing a greater AUC for the clinical score of inflammation (Fig. 1). Cumulative joint swelling (measured as joint thickness) induced by the standard dose of K/BxN serum was also greater in Hsd11b1−/− than in control mice (Hsd11b1+/+, 8.5 ± 0.4 mm AUC vs. controls, 6.9 ± 1.1 mm AUC, P < 0.05). Plasma corticosterone levels were elevated to a similar extent in Hsd11b1−/− and control mice 2 d after K/BxN serum injection (Hsd11b1−/−, 218 ± 61 nm vs. controls, 253 ± 39 nm), showing that inadequate hypothalamic-pituitary-adrenal (HPA) axis activation does not underlie the exacerbated acute inflammation at 2 d. However, at 21 d (when inflammation had resolved in control mice), although plasma corticosterone levels had reduced in control mice, they remained elevated in Hsd11b1−/− mice (Hsd11b1−/−, 227 ± 76 nm vs. controls, 86 ± 27 nm). This latter finding suggests the persistent activation of the HPA axis in Hsd11b1−/− mice, plausibly due to the ongoing inflammation because the negative feedback by corticosterone on the HPA axis in Hsd11b1−/− mice is normal on the C57BL/6 strain background (29).
Fig. 1.
11β-HSD1 deficiency worsens arthritis in mice. K/BxN arthritis was induced in Hsd11b1−/− (white squares, white bar) and control mice (black squares, black bar) by ip injection of arthritogenic K/BxN serum, using a standard dose of 7.5 μl/g body weight on d 0 and d 2 (A) and a reduced dose of 5.6 μl/g body weight on d 0 only (B). Clinical score (based on edema and redness of carpal and hock joints) was measured over 21 d. Insets show AUC. Data are mean ± sd (n = 5/group), P < 0.05 by repeated-measures ANOVA or (for AUC) by unpaired Student's t test. *, P < 0.05.
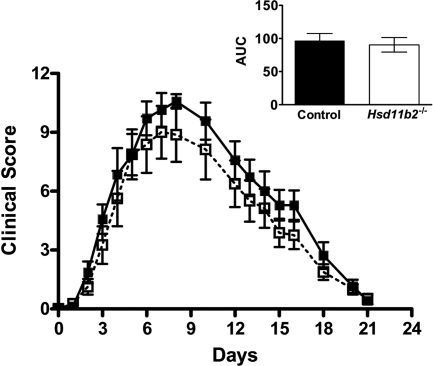
Fig. 2.
11β-HSD2-deficiency has no effect on K/BxN arthritis in mice. K/BxN arthritis was induced in Hsd11b2−/− (white squares, white bar) and control mice (black squares, black bar) by a single ip injection of arthritogenic K/BxN serum (5.6 μl/g body weight) on d 0. Clinical score was measured over 21 d. Inset shows AUC. Data are mean ± sd (n = 7–8/group); P = 0.96 by repeated-measures ANOVA or (for AUC) by unpaired Student's t test; P = 0.69.
In contrast, there was no difference in the clinical score of inflammation between Hsd11b2−/− and control mice during the course of the inflammatory arthritis, with a similar AUC for both genotypes (Fig. 2). These data suggest that 11β-HSD1 limits joint inflammation in vivo, whereas 11β-HSD2 has no effect on the development or progression of K/BxN arthritis.
11β-HSD1 deficiency, but not 11β-HSD2 deficiency, worsens joint histopathology
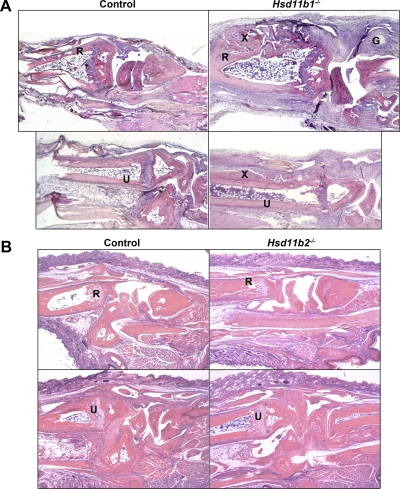
After inflammatory arthritis (21 d after injection of K/BxN serum, when the clinical signs of inflammation had largely subsided), carpal joints from all mice showed periarticular inflammation, capsular thickening, tenosynovitis, and exostoses of distal radius and ulna and proximal metacarpals (Fig. 3). Joints of Hsd11b1−/− mice, however, had more exuberant and extensive exostoses, greater periarticular fibrosis, and, uniquely, ganglion cyst formation, suggestive of more severe inflammation (Fig. 3). In contrast, there was no histopathological difference between the joints of Hsd11b2−/− mice and controls (Fig. 3).
Fig. 3.
Greater exostosis and inflammatory ganglion cysts in Hsd11b1−/− mice but not in Hsd11b2−/− mice, after arthritis. Representative images of H&E sections, showing left carpal joint (top panels) and left tarsal joint (bottom panels) from the following: A, Hsd11b1−/− mice (right panels) vs. control mice (left panels) and B, Hsd11b2−/− (right panels) vs. control mice (left panels). Joints were collected 21 d after induction of experimental arthritis with K/BxN serum (single injection of 5.6 μl/g body weight on d 0) (n = 5–8/genotype). Images captured at ×50 magnification. R, Anterior distal radius; U, anterior ulna; G, inflammatory ganglion cyst; X, exostosis.
11β-HSD1 deficiency, but not 11β-HSD2-deficiency, results in greater inflammation during sterile peritonitis
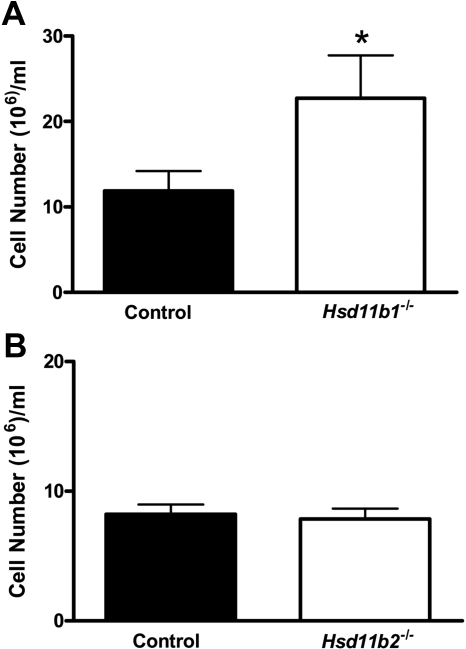
After ip injection of thioglycollate to induce sterile peritonitis, Hsd11b1−/− mice had more inflammatory cells within the peritoneum (Fig. 4A). However, despite the increase in inflammatory cells, there was no difference in resolution of thioglycollate-induced peritonitis in Hsd11b1−/− mice (data not shown), consistent with previous data (9). In contrast, inflammatory cell number within the peritoneum was similar in Hsd11b2−/− and control mice (Fig. 4B), with no difference in resolution, suggesting that inflammation was normal in this model.
Fig. 4.
Greater inflammation in Hsd11b1−/− mice, but not in Hsd11b2−/− mice, during sterile thioglycollate-induced peritonitis. Peritonitis was induced in Hsd11b1−/− (white bars) and control mice (black bars) (A) and in Hsd11b2−/− (white bars) and control mice (black bars) (B) by ip injection of 1 ml 3% thioglycollate. Twenty-four hours later, cells were lavaged (with 5 ml PBS) and counted. Data are mean ± sem, expressed as cells per milliliter (n = 5–8/group); *, P < 0.05 by unpaired Student's t test.
11β-HSD1 deficiency results in greater recruitment of inflammatory cells and more severe pathology in carrageenan-induced pleural inflammation
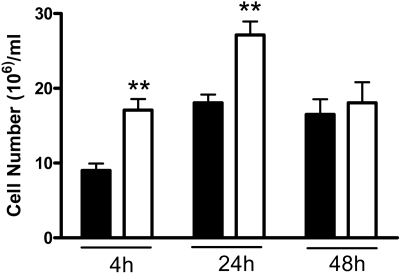
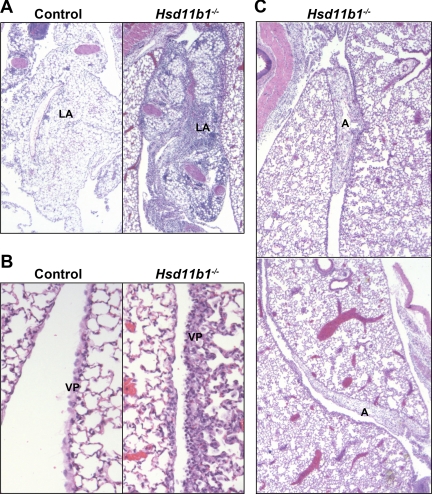
The antiinflammatory effects of 11β-HSD1 were confirmed in another model of inflammation, carageenan-induced pleurisy, in which both inflammatory cell number and resulting pathology can be assessed within a single model. Similar to sterile peritonitis, more inflammatory cells were recovered from Hsd11b1−/− mice than control mice 4 h and 24 h after intrapleural injection of carrageenan (Fig. 5). Edema (assessed by weighing the collected lavages) did not differ between genotypes at either time point (data not shown). Histological assessment showed that 48 h after injection of carrageenan, inflammation in control mice was resolving yet was still in progress in Hsd11b1−/− mice, with persistence of inflammation in the visceral pleura, the presence of lymphoid aggregates (comprised of plasma cells, lymphocytes, neutrophils, and macrophages) within the lung and periesophageal mediastinum, and, uniquely in Hsd11b1−/− mice, formation of fibrous adhesions between lung lobes 48 h after initiation of pleurisy (Fig. 6). No differences in lung histology were found between untreated mice (data not shown).
Fig. 5.
Greater inflammation in Hsd11b1−/− mice during carrageenan-induced pleurisy. Pleurisy was induced in Hsd11b1−/− (white bars) and control (black bars) mice by intrapleural injection of 100 μl 0.1% carrageenan. Four, 24, and 48 h later, cells were lavaged (with 1 ml PBS) and counted. Results are mean ± sd, expressed as cells per milliliter (n = 7/group); **, P < 0.01 by two-way ANOVA.
Fig. 6.
Ongoing inflammation in Hsd11b1−/− mice 48 h after carrageenan injection. Representative images of H&E-stained sections of lungs from Hsd11b1−/− and control mice 48 h after intrapleural injection of carrageenan (100 μl of 0.1% carrageenan) showing, more pronounced lymphoid aggregates (A), persistent thickening of visceral pleura (B), and more aggressive adhesion of lung lobes (C) in Hsd11b1−/− than in control mice. Images captured at ×50 (A and B) and ×500 (C) magnification. LA, Lymphoid aggregates; VP, visceral pleura; A, adhesions.
Discussion
Mice deficient in 11β-HSD1 showed worse inflammation in three different experimental models, with impaired resolution in two. In contrast, 11β-HSD2 deficiency had no effect on inflammation in either arthritis or peritonitis. 11β-HSD1 clearly plays a role in restraining the initial inflammatory response because greater inflammatory cell number was seen in 11β-HSD1-deficient mice in both pleurisy and peritonitis, and clinical signs of arthritic inflammation were visible in these mice a full day before control mice. Why the acute response is worse in these mice is currently unclear but could be due to increased recruitment of inflammatory cells or decreased clearance. The latter is supported by our previous finding of a delay in vivo in acquisition of macrophage phagocytic capacity in Hsd11b1−/− mice (9). In both the arthritis and the pleurisy models of inflammation, Hsd11b1−/− mice showed worse pathology. This could simply reflect the greater number of inflammatory cells present during inflammation in these mice (causing greater tissue damage) or may additionally reflect an altered tissue response to inflammation. Proinflammatory cytokines induce 11β-HSD1 expression in synovial fibroblasts (13) in which locally amplified glucocorticoid action is likely to influence both the response of fibroblasts to inflammation and the local inflammatory environment that prevails during the course of arthritis. Whether a similar induction of 11β-HSD1 in pleural and lung fibroblasts dampens local proinflammatory responses and reduces inflammatory cell number in pleurisy in control mice remains to be determined but is plausible.
In the case of bone, the role of endogenous glucocorticoid metabolism here, particularly during an inflammatory response, is likely to be complex, as demonstrated by the protective effect of transgenic 11β-HSD2 expression in osteoblasts in the K/BxN serum-transfer model of arthritis (30, 31). The increased exostosis seen here in the joints of Hsd11b1−/− mice suggest altered bone turnover and/or increased bone synthesis as a response to the local proinflammatory environment [because basal bone architecture is unaltered in this model (32]. The delay in the resolution of arthritis in Hsd11b1−/− mice may be due to lack of activity of this enzyme in osteoblasts or may reflect a normal response to the greater initial inflammation in Hsd11b1−/− mice.
Licorice and its active ingredients such as glycyrrhetinic acid are nonselective but potent 11β-HSD inhibitors that have long been known to exhibit antiinflammatory activity, including in rheumatoid arthritis (18). Whether this is due to increased plasma half-life of cortisol because of inhibition of renal 11β-HSD2-mediated clearance of glucocorticoids (33) or a direct intracrine effect on 11β-HSD activity at the site of inflammation is unclear. Our data do not suggest a direct effect of 11β-HSD2 deficiency on acute inflammation. Indeed, in contrast to 11β-HSD1, expression of 11β-HSD2 is down-regulated by proinflammatory cytokines (34, 35), inconsistent with any major role in the response to inflammation. Moreover, 11β-HSD2 is not expressed in mouse immune cells (11) or in human leukocytes (7). However, a caveat is raised by reports of 11β-HSD2 in synovial macrophages in rheumatoid arthritis, and it is possible that the chronic inflammatory environment with persistent disease may induce 11β-HSD2. It also remains possible that the renal phenotype of Hsd11b2−/− mice (hypertension, hypokalaemia, and hypernatraemia) compensates in some way an effect on inflammation, although it is difficult to envisage how this might come about.
Inhibition of 11β-HSD1, thus reducing intracellular glucocorticoid levels, shows promise as a drug target for treatment of metabolic disease, including type 2 diabetes mellitus (36). Our data suggest that caution should be exercised in the use of these inhibitors because they may worsen acute inflammation. Understanding how 11β-HSD1 deficiency exacerbates acute inflammation yet is protective against the low-grade inflammation associated with metabolic disease [Hsd11b1−/− mice show reduced inflammation in adipose tissue in obesity (37)] will be critical in the optimization of emerging therapies for metabolic disease. Moreover, it might facilitate development of new antiinflammatory therapies, for example, encapsulated delivery of cortisone to the site of inflammation to take advantage of local 11β-HSD1 activity to generate cortisol within the tissue in which it will be of most benefit, thus limiting the adverse metabolic effects of whole-body exposure to exogenous synthetic glucocorticoids.
Acknowledgments
We thank Ruth Andrew for help with the HPLC measurement of steroids, Katherine Miles for technical support, and Susan Harvey and Bob Morris for histology assistance.
This work was supported by Medical Research Council Project Grant G0800235 (to K.E.C., M.G., J.S.S., and J.R.S.) and Wellcome Trust Program Grants WT083184 (to J.R.S. and K.E.C.) and WT064497 (to J.S.S.). M.G. is supported by an Arthritis Research Campaign Clinician Scientist Fellowship.
Disclosure Summary: A.E.C., M.G., D.G.B., D.M.S., D.A.S., S.C., J.S.G., J.S.S., and K.E.C. have nothing to declare. J.R.S. has consulted for Boehringer-Ingleheim, Sanofi-Aventis, and Piramal.
Footnotes
- AUC
- Area under the curve
- H&E
- hematoxylin and eosin
- HPA
- hypothalamic-pituitary-adrenal
- 11β-HSD
- 11β-hydroxysteroid dehydrogenase
- K/BxN serum
- KRN-transgenic mice bred with NOD mice due to circulating anti-glucose-6-phosphate-isomerase autoantibodies.
References
- 1. Spies CM, Bijlsma JW, Burmester GR, Buttgereit F. 2010. Pharmacology of glucocorticoids in rheumatoid arthritis. Curr Opin Pharmacol 10:302–307 [DOI] [PubMed] [Google Scholar]
- 2. Coutinho AE, Chapman KE. 2011. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol 335:2–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL, Weiss JM. 1997. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res Brain Res Rev 23:79–133 [DOI] [PubMed] [Google Scholar]
- 4. Chapman KE, Kotelevtsev YV, Jamieson PM, Williams LJ, Mullins JJ, Seckl JR. 1997. Tissue-specific modulation of glucocorticoid action by the 11β-hydroxysteroid dehydrogenases. Biochem Soc Trans 25:583–587 [DOI] [PubMed] [Google Scholar]
- 5. Seckl JR, Morton NM, Chapman KE, Walker BR. 2004. Glucocorticoids and 11β-hydroxysteroid dehydrogenase in adipose tissue. Recent Prog Horm Res 59:359–393 [DOI] [PubMed] [Google Scholar]
- 6. Brown RW, Chapman KE, Kotelevtsev Y, Yau JL, Lindsay RS, Brett L, Leckie C, Murad P, Lyons V, Mullins JJ, Edwards CR, Seckl JR. 1996. Cloning and production of antisera to human placental 11β-hydroxysteroid dehydrogenase type 2. Biochem J 313:1007–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thieringer R, Le Grand CB, Carbin L, Cai TQ, Wong B, Wright SD, Hermanowski-Vosatka A. 2001. 11β-Hydroxysteroid dehydrogenase type 1 is induced in human monocytes upon differentiation to macrophages. J Immunol 167:30–35 [DOI] [PubMed] [Google Scholar]
- 8. Freeman L, Hewison M, Hughes SV, Evans KN, Hardie D, Means TK, Chakraverty R. 2005. Expression of 11β-hydroxysteroid dehydrogenase type 1 permits regulation of glucocorticoid bioavailability by human dendritic cells. Blood 106:2042–2049 [DOI] [PubMed] [Google Scholar]
- 9. Gilmour JS, Coutinho AE, Cailhier JF, Man TY, Clay M, Thomas G, Harris HJ, Mullins JJ, Seckl JR, Savill JS, Chapman KE. 2006. Local amplification of glucocorticoids by 11β-hydroxysteroid dehydrogenase type 1 promotes macrophage phagocytosis of apoptotic leukocytes. J Immunol 176:7605–7611 [DOI] [PubMed] [Google Scholar]
- 10. Zhang TY, Ding X, Daynes RA. 2005. The expression of 11β-hydroxysteroid dehydrogenase type I by lymphocytes provides a novel means for intracrine regulation of glucocorticoid activities. J Immunol 174:879–889 [DOI] [PubMed] [Google Scholar]
- 11. Chapman KE, Coutinho A, Gray M, Gilmour JS, Savill JS, Seckl JR. 2006. Local amplification of glucocorticoids by 11β-hydroxysteroid dehydrogenase type 1 and its role in the inflammatory response. Ann NY Acad Sci 1088:265–273 [DOI] [PubMed] [Google Scholar]
- 12. Schmidt M, Weidler C, Naumann H, Anders S, Schölmerich J, Straub RH. 2005. Reduced capacity for the reactivation of glucocorticoids in rheumatoid arthritis synovial cells: possible role of the sympathetic nervous system? Arthritis Rheum 52:1711–1720 [DOI] [PubMed] [Google Scholar]
- 13. Hardy RS, Filer A, Cooper MS, Parsonage G, Raza K, Hardie DL, Rabbitt EH, Stewart PM, Buckley CD, Hewison M. 2006. Differential expression, function and response to inflammatory stimuli of 11β-hydroxysteroid dehydrogenase type 1 in human fibroblasts: a mechanism for tissue-specific regulation of inflammation. Arthritis Res Ther 8:R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zbánkov á S, Bryndov á J, Leden P, Kment M, Svec A, Pácha J. 2007. 11β-Hydroxysteroid dehydrogenase 1 and 2 expression in colon from patients with ulcerative colitis. J Gastroenterol Hepatol 22:1019–1023 [DOI] [PubMed] [Google Scholar]
- 15. Ergang P, Vytáčkov á K, Svec J, Bryndov á J, Mikšik I, Pácha J. 2011. Upregulation of 11β-hydroxysteroid dehydrogenase 1 in lymphoid organs during inflammation in the rat. J Steroid Biochem Mol Biol 126:19–25 [DOI] [PubMed] [Google Scholar]
- 16. Zhang TY, Daynes RA. 2007. Macrophages from 11β-hydroxysteroid dehydrogenase type 1-deficient mice exhibit an increased sensitivity to lipopolysaccharide stimulation due to TGF-β-mediated up-regulation of SHIP1 expression. J Immunol 179:6325–6335 [DOI] [PubMed] [Google Scholar]
- 17. Haas CS, Creighton CJ, Pi X, Maine I, Koch AE, Haines GK, Ling S, Chinnaiyan AM, Holoshitz J. 2006. Identification of genes modulated in rheumatoid arthritis using complementary DNA microarray analysis of lymphoblastoid B cell lines from disease-discordant monozygotic twins. Arthritis Rheum 54:2047–2060 [DOI] [PubMed] [Google Scholar]
- 18. Davis EA, Morris DJ. 1991. Medicinal uses of licorice through the millennia: the good and plenty of it. Mol Cell Endocrinol 78:1–6 [DOI] [PubMed] [Google Scholar]
- 19. Ergang P, Leden P, Bryndov á J, Zbánkov á S, Miksík I, Kment M, Pácha J. 2008. Glucocorticoid availability in colonic inflammation of rat. Dig Dis Sci 53:2160–2167 [DOI] [PubMed] [Google Scholar]
- 20. Abbott AN, Guidry TV, Welsh KJ, Thomas AM, Kling MA, Hunter RL, Actor JK. 2009. 11β-Hydroxysteroid dehydrogenases are regulated during the pulmonary granulomatous response to the mycobacterial glycolipid trehalose-6,6′-dimycolate. Neuroimmunomodulation 16:147–154 [DOI] [PubMed] [Google Scholar]
- 21. Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. 1996. Organ-specific disease provoked by systemic autoimmunity. Cell 87:811–822 [DOI] [PubMed] [Google Scholar]
- 22. Ji H, Gauguier D, Ohmura K, Gonzalez A, Duchatelle V, Danoy P, Garchon HJ, Degott C, Lathrop M, Benoist C, Mathis D. 2001. Genetic influences on the end-stage effector phase of arthritis. J Exp Med 194:321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kotelevtsev Y, Holmes MC, Burchell A, Houston PM, Schmoll D, Jamieson P, Best R, Brown R, Edwards CR, Seckl JR, Mullins JJ. 1997. 11β-Hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc Natl Acad Sci USA 94:14924–14929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kotelevtsev Y, Brown RW, Fleming S, Kenyon C, Edwards CR, Seckl JR, Mullins JJ. 1999. Hypertension in mice lacking 11β-hydroxysteroid dehydrogenase type 2. J Clin Invest 103:683–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morton NM, Ramage L, Seckl JR. 2004. Down-regulation of adipose 11β-hydroxysteroid dehydrogenase type 1 by high-fat feeding in mice: a potential adaptive mechanism counteracting metabolic disease. Endocrinology 145:2707–2712 [DOI] [PubMed] [Google Scholar]
- 26. Monach PA, Mathis D, Benoist C. 2008. The K/BxN arthritis model. Curr Protoc Immunol Chapter 15:Unit 15.22 [DOI] [PubMed] [Google Scholar]
- 27. Yau JL, Noble J, Kenyon CJ, Hibberd C, Kotelevtsev Y, Mullins JJ, Seckl JR. 2001. Lack of tissue glucocorticoid reactivation in 11β-hydroxysteroid dehydrogenase type 1 knockout mice ameliorates age-related learning impairments. Proc Natl Acad Sci USA 98:4716–4721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. 1999. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med 5:698–701 [DOI] [PubMed] [Google Scholar]
- 29. Carter RN, Paterson JM, Tworowska U, Stenvers DJ, Mullins JJ, Seckl JR, Holmes MC. 2009. Hypothalamic-pituitary-adrenal axis abnormalities in response to deletion of 11β-HSD1 is strain-dependent. J Neuroendocrinol 21:879–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Buttgereit F, Zhou H, Kalak R, Gaber T, Spies CM, Huscher D, Straub RH, Modzelewski J, Dunstan CR, Seibel MJ. 2009. Transgenic disruption of glucocorticoid signaling in mature osteoblasts and osteocytes attenuates K/BxN mouse serum-induced arthritis in vivo. Arthritis Rheum 60:1998–2007 [DOI] [PubMed] [Google Scholar]
- 31. Weber AJ, Li G, Kalak R, Street J, Buttgereit F, Dunstan CR, Seibel MJ, Zhou H. 2010. Osteoblast-targeted disruption of glucocorticoid signalling does not delay intramembranous bone healing. Steroids 75:282–286 [DOI] [PubMed] [Google Scholar]
- 32. Justesen J, Mosekilde L, Holmes M, Stenderup K, Gasser J, Mullins JJ, Seckl JR, Kassem M. 2004. Mice deficient in 11β-hydroxysteroid dehydrogenase type 1 lack bone marrow adipocytes, but maintain normal bone formation. Endocrinology 145:1916–1925 [DOI] [PubMed] [Google Scholar]
- 33. Stewart PM, Wallace AM, Valentino R, Burt D, Shackleton CH, Edwards CR. 1987. Mineralocorticoid activity of liquorice: 11β-hydroxysteroid dehydrogenase deficiency comes of age. Lancet 2:821–824 [DOI] [PubMed] [Google Scholar]
- 34. Cooper MS, Bujalska I, Rabbitt E, Walker EA, Bland R, Sheppard MC, Hewison M, Stewart PM. 2001. Modulation of 11β-hydroxysteroid dehydrogenase isozymes by proinflammatory cytokines in osteoblasts: an autocrine switch from glucocorticoid inactivation to activation. J Bone Miner Res 16:1037–1044 [DOI] [PubMed] [Google Scholar]
- 35. Cai TQ, Wong B, Mundt SS, Thieringer R, Wright SD, Hermanowski-Vosatka A. 2001. Induction of 11β-hydroxysteroid dehydrogenase type 1 but not -2 in human aortic smooth muscle cells by inflammatory stimuli. J Steroid Biochem Mol Biol 77:117–122 [DOI] [PubMed] [Google Scholar]
- 36. Wamil M, Seckl JR. 2007. Inhibition of 11β-hydroxysteroid dehydrogenase type 1 as a promising therapeutic target. Drug Discov Today 12:504–520 [DOI] [PubMed] [Google Scholar]
- 37. Wamil M, Battle JH, Turban S, Kipari T, Seguret D, de Sousa Peixoto R, Nelson YB, Nowakowska D, Ferenbach D, Ramage L, Chapman KE, Hughes J, Dunbar DR, Seckl JR, Morton NM. 2011. Novel fat depot-specific mechanisms underlie resistance to visceral obesity and inflammation in 11β-hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes 60:1158–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. 2002. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science 297:1689–1692 [DOI] [PubMed] [Google Scholar]