Abstract
Background:
Fine needle aspiration (FNA) is a quick, minimally invasive procedure for evaluation of breast tumors. The Scarff-Bloom-Richardson (SBR) grade on histological sections is a well-established tool to guide selection of adjuvant systemic therapy. Grade evaluation is possible on cytology smears to avoid and minimize the morbidity associated with overtreatment of lower grade tumors.
Aim:
The aim was to test the hypothesis whether breast FNA from the peripheral portion of the lesion is representative of Scarff-Bloom-Richardson grade on histopathology as compared to FNA from the central portion.
Materials and Methods:
Fine-needle aspirates and subsequent tissue specimens from 45 women with ductal carcinoma (not otherwise specified) were studied. FNAs were performed under ultrasound guidance from the central as well as the peripheral third of the lesion for each case avoiding areas of necrosis/calcification. The SBR grading was compared on alcohol fixed aspirates and tissue sections for each case.
Results:
Comparative analysis of SBR grade on aspirates from the peripheral portion and histopathology by the Pearson chi-square test (χ2 =78.00) showed that it was statistically significant (P<0.001) with 93% concordance. Lower mitotic score on aspirates from the peripheral portion was observed in only 4 out of 45 (9%) cases. The results of the Pearson chi-square test (χ2 = 75.824) with statistically significant (P=0.000).
Conclusion:
This prospective study shows that FNA smears from the peripheral portion of the lesion are representative of the grading performed on the corresponding histopathological sections. It is possible to score and grade by SBR system on FNA smears.
Keywords: Breast carcinoma, fine-needle aspiration cytology, tumor grading
INTRODUCTION
Elston and Ellis modified Scarff-Bloom-Richardson (SBR) grading is one of the important pathological parameters to be evaluated for the management of breast carcinoma. The SBR grade is a useful, sensitive guide for selecting adjuvant systemic therapy and the method should be standardized for cytology specimens.[1–3] For the application of neoadjuvant therapy as primary medical treatment, assessment of the tumor grade is crucial. Fine-needle aspiration (FNA) can grade the tumor and avoid the morbidity associated with overtreatment of lower grade tumors.[4,5] This is essentially true in underdeveloped/developing countries, where the tissue core needle biopsy still is not used as a standard practice to sample newly diagnosed breast carcinoma cases.
Compared to the wide, well-established practice of the application of SBR grade to histopathology sections, grading on cytology is sparingly applied and reported. Different studies have implied varied methods for the grading and scoring on FNA smears. Literature shows that the main hurdle to apply the SBR score on cytology was encountered in scoring mitoses and tubule formation.[6–8] As the mitotic count is high at the growing edge (i.e., periphery), the present study has evaluated these scores and compared them with histopathology-based results by performing the aspirations from the central and peripheral portions.
MATERIALS AND METHODS
We included 45 consecutive cases of ductal carcinoma (not otherwise specified) with available fine-needle aspirate and subsequent tumor tissue specimens. None of the patients received preoperative neoadjuvant chemotherapy, hormonal therapy, or radiotherapy prior to the biopsies. FNAs were performed using 22- to 25-gauge needles under ultrasound guidance from the central and peripheral third of the lesion for each case. Areas of necrosis/calcification were avoided during FNA sampling. Cytologic smears were immediately alcohol fixed and subsequently stained with Papanicolaou stain and Hematoxylin-Eosin stain by standard procedure.
Scarff-Bloom-Richardson grading system
The SBR tumor grading was performed independently by two pathologists on fine-needle aspirates and tissue sections for each case. The results were reproducible in the majority of the cases. The discrepant findings were discussed and a final consensus was reached on a multiheader microscope. Each of the three features, i.e., tubule formation, nuclear pleomorphism, and mitotic count were scaled as 1, 2, or 3 and the final SBR score ranged between 3 and 9, which was divided into three grades (I-III). For grade I, the score varied from 3 to 5, for grade II the score was 6-7, and for grade III the score was 8-9.
Criteria on cytology for identifying tubule formation and mitotic counts were modified from the standard histological criteria. Scaling of nuclear pleomorphism on cytology was the same as in tissue sections. Tubule formation in FNA smears was identified as microacini and/or as branching, elongated, three-dimensional tubular structures.
Mitotic figure counting method
Mitotic figures were counted in 10 consecutive high power fields with evenly spread cells starting at the point with highest density of mitotic figures. The fields containing necrosis or inflammation with less than 50% tumor cells, and thick portion of the smear with overlapping cells were excluded. The mitoses were counted separately in the smears made from the central and peripheral part of the same lesion. For both, the surgical and cytology slides, mitoses were counted in ten 40× fields using a Nikon microscope (field width of 0.65 mm). The threshold for the number of mitoses for each point in the scoring system was lowered in cytologic application of the SBR system,[9] as mentioned below:
0-5 (histopathology) or 0-1 (cy topathology) was scored 1, 6-10 (histopathology) or 2-4 (cytopathology) was scored 2, and ≥11 (histopathology) or ≥5 (cytopathology) was scored 3.
The criteria for recognizing and counting mitotic figures were as follows:
Only the mitotic figures in metaphase, anaphase, and telophase (with absence of nuclear membranes-to be sure that the cells have passed the prophase) were counted.
Clear, hairy extensions of nuclear material (condensed chromosomes) were present either as tightly clumped chromosomes (beginning metaphase), or in one plane (metaphase/anaphase), or in separate groups (telophase). Regular extensions with an empty central zone were not counted.
Two parallel, clear separate chromosome groups were counted as one mitotic figure. (It was considered so, because automated mitotic figure recognition with image analysis was not performed).
Statistical analysis
Statistical analysis was done by using SPSS version 13. The Pearson chi-square χ2 test was done and a probability value of P<0.001 was considered significant. The results of SBR grade and mitotic score on aspirations were compared with the corresponding histopathological findings.
RESULTS
The mean age for 45 cases of breast carcinoma classified as infiltrating ductal carcinoma (NOS) was 55 years. The tumor size ranged from 2.3 to 7.2 cm (mean tumor size 5.3 cm). On histopathological grading, 5 (11%) cases were grade I, 15 (33%) cases were grade II, and 25 (56%) cases were grade III. Over half of the cases were scored as high grade. This may be due to selection bias as the study included cases from a tertiary care center. Results of cytological grading done on aspirates from the peripheral portion of the tumor had higher concordance rates than the central portion when compared with the histopathological grade [Table 1].
Table 1.
Distribution of cases in different tumor grades on cytology and histopathology
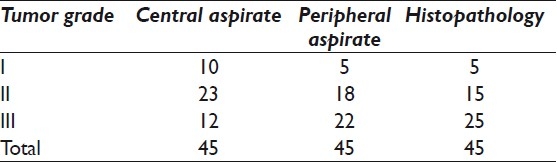
SBR grade on aspirates from central portion was falsely low in 5 grade II tumors and 13 grade III tumors. This shows that 18 out of 45 (40%) tumors were falsely down graded on cytomorphology from the central portion as compared to the respective histopathology grades.
Comparison of SBR grade on aspirates from the peripheral portion and histopathology showed discrepancy in only 3 out of 45 (7%) cases with 93% concordance and statistically significant (p<0.001) Pearson chi-square test (χ2=78.00). These 3 cases were assigned a false lower grade II on aspirates.
Comparison of grades on aspirates from the central and the peripheral portions showed greater discrepancy in 10 out of 22 (45%) cases for grade III tumors as compared to grade II tumors with discrepant grading in 5 out of 18 (28%) cases.
Table 2 shows that the mitotic score on aspirate from the central portion matched the score on histopathology in only 13 out of 45 (29%) cases. A false low mitotic score of 1 was given in 13 cases (12 cases of mitotic score 2 and 1 case of mitotic score 3). Nineteen cases of actual mitotic score 3 were given a false low score of 2 on aspirates when compared with histopathology.
Table 2.
Intercomparison of mitotic score on aspirate (central, peripheral) with histopathology
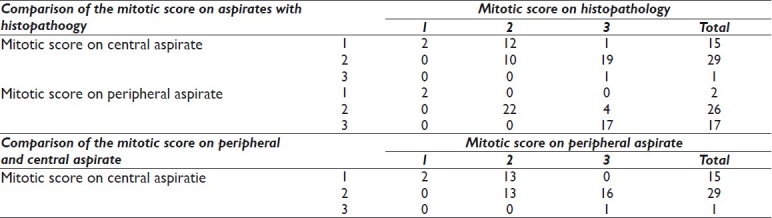
The mitotic score on aspirate from the peripheral portion was given a false low value in only 4 out of 45 (9%) cases. Results of the Pearson chi-square test (χ2 = 75.824) showed the finding to be statistically significant with P=0.000.
Comparison of the mitotic score in aspirates from the central and the peripheral portion shows that 29 out of 45 (64%) tumors were given a lower score in cytology from the central portion.
Figures 1, 2, and 3 show the difference in cytomorphology for grades I, II and III ductal carcinoma respectively.
Figure 1.
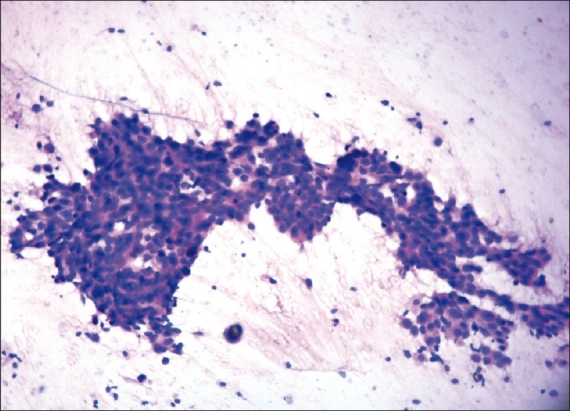
Photomicrograph of nuclear grade I duct carcinoma. The cells are arranged in elongated, branching tubular configuration. The nuclei are enlarged, with mild pleomorphism, smooth nuclear margin, and inconspicuous nucleoli. (H & E, ×100)
Figure 2.
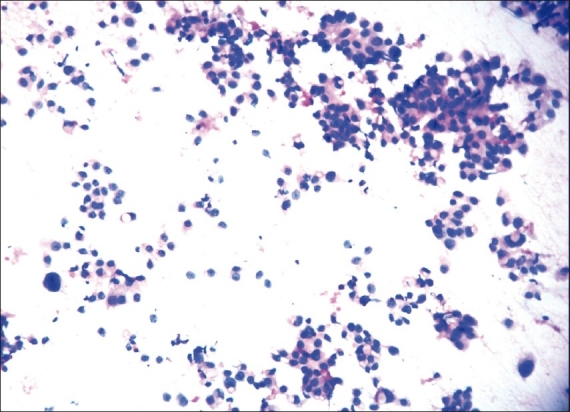
Photomicrograph showing nuclear grade II duct carcinoma. The cells are forming microacini and loose clusters. Nuclei are three to four times the erythrocytes, with granular nuclear chromatin and smooth contour. (H & E, ×100)
Figure 3.
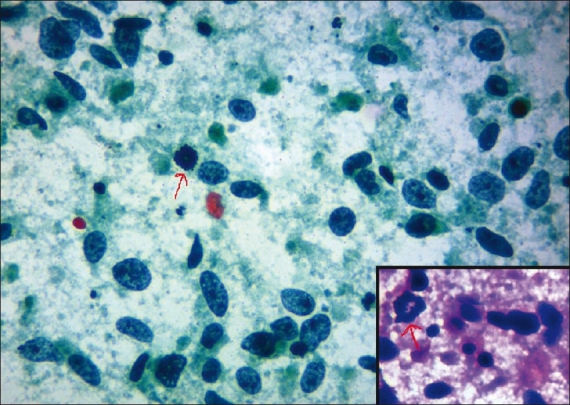
Photomicrograph showing nuclear grade III duct carcinoma. The cells are predominantly singly dispersed on a necrotic background with coarsely granular chromatin, irregular nuclear margin, and prominent nucleoli. Atypical mitotic figure is pointed with arrow. (Pap, ×400); inset (H & E, × 400)
DISCUSSION
Pathological grade of breast carcinoma is a prognostic marker which has role in deciding appropriate therapy according to the tumor characteristics in a particular patient.[10] Grading on cytology provides prognostic evaluation in addition to diagnosis without additional morbidity or expense of core or excision biopsy to the patient especially in resource limited situations. Others have studied varied cytologic features for grading purpose, with different conclusions.[11,12] Most researchers[13–17] have attempted to grade cytologic features based on nuclear morphology and have not taken into account the pattern of cell distribution or mitotic counts. We studied the cytologic features comparable to those applied to SBR grading in histopathology.
We do not intend to compare the different cytological grading methods in the literature but to test the hypothesis whether FNA from the peripheral portion of the lesion corresponds to SBR grade on histopathology.
Our study evaluated cases on wet fixed Hematoxylin-Eosin stained as well as Papanicolaou stained smears. A study by Dabbs et al.[17] has shown that comparable and adequate cytomorphological information can be obtained by using any of the staining methods such as Papanicolaou stain or air-dried Diff-Quik stain. The minor differences noted were in the appearance of nucleoli and nuclear chromatin. Nucleoli were rather inconspicuous on air-dried Diff-Quik stained material; however, it allowed greater appreciation of nuclear size variation. Nuclear chromatin was better assessed in the Papanicolaou stained smears.[18,19]
Various investigators have applied different methods for cytology grading. The major deficiency in these studies is the dissimilarity in the parameters considered and compared on FNA and histopathology material. A study by Dabbs et al.[13] showed that unlike tubule formation and mitotic counts, nuclear grading on cytological specimens correlates well with that of histological sections. Robinson and Mckee[12] proposed a cytologic grading system on breast FNAs which included six cytologic features: cell dissociation, nuclear size, cell uniformity, nucleoli, nuclear margins, and chromatin pattern. The single feature of bare atypical nuclei in the background was studied by Ozkutlu.[20] Later on a study was done by Taniguchi E et al. with its main focus on detailed nuclear features.[11] Cellular dyscohesion alone was studied in FNA smears and the scoring method was shown to be applicable and reproducible.[21,22] Recently, the cytoprognostic score has been proposed by studying combined features of nuclear grade, cellular dyscohesion, and bare atypical nuclei.[23]
It is difficult to compare the cytology and histopathology if all the three features for the SBR grade are not analyzed on FNA smears.[24,25] The SBR grading system is a multiparameter grading based on cell dyscohesion with formation of tubules, mitotic rate, and nuclear characteristics. Other authors have attempted to apply the Elston and Ellis modified SBR grading system to FNAs of the breast; however, with little success, mainly because of difficulty in detecting tubule formation and counting mitoses in cytologic smears due to sampling errors and low mitotic count.[8,13,25] We performed USG guided aspirates from peripheral portion avoiding areas of necrosis as the mitotic count is highest at the growing edge of tumor (i.e., peripheral portion).[9] No study has been attempted to apply all the three components used for SBR grading on cytology after the difficulties faced by initial workers, leading to compromises with approaches utilizing only a few components of the SBR scoring system with different modifications.[8,13,25] Comparing only the nuclear features without consideration to tubule formation and mitotic count is not equivalent to SBR grading in histopathology.
Based on the cytologic grade, stratification of patients into prognostic groups has been found to be distinct[12,24] (grade I, 95% 7-year disease free survival versus 45% for grade III). Thus, the literature[1] supports the role of assigning cytological grades for prognostication, and the present study shows that the aspirates from the peripheral portion correlates with SBR grade on histopathology with P<0.001. In the current study, we evaluated and analyzed the results of the grading system on FNA smears from the central as well as the peripheral portion of the lesion and compared with individual parameters of SBR scoring in their respective surgical specimens. Larger number with inclusion of increased number of low and intermediate grade tumors is recommended to further support our findings. To explore the experience with the application of SBR grade on breast aspirates, further cytologic grading studies with larger number of cases with inclusion of other categories of breast carcinomas, like lobular carcinoma, medullary carcinoma, etc., that were not represented in this study is also recommended.
CONCLUSION
This prospective study shows that FNA smears from the peripheral portion of the lesion are representative of the corresponding histopathological sections. SBR grading on FNA smears from the peripheral third of the tumor yield results comparable to the histopathology grading.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
No competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author acknowledges that this final version was read and approved.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from Institutional Review Board (IRB) of all the institutions associated with this study. All authors take responsibility to maintain relevant documentation in this respect.
EDITORIAL / PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
Footnotes
Available FREE in open access from: http://www.cytojournal.com/text.asp?2012/9/1/4/92550.
Contributor Information
Cherry Bansal, Email: drcherrybansal@gmail.com.
U. S. Singh, Email: ussinghjyotsna@yahoo.com.
Sanjeev Misra, Email: misralko@gmail.com.
Kiran Lata Sharma, Email: helloji.kiran@gmail.com.
Vandana Tiwari, Email: drvandana2166@yahoo.com.
A. N. Srivastava, Email: ans4csmmu@gmail.com.
REFERENCES
- 1.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 2.Ruibal A, Arias JI, Del Río MC, Lapeña G, Schneider J, Tejerina A. Histological grade in breast cancer: association with clinical and biological features in a series of 229 patients. Int J Biol Markers. 2001;16:56–61. doi: 10.1177/172460080101600108. [DOI] [PubMed] [Google Scholar]
- 3.Morgan D, Sibbering D, Galea M, Ellis I, Elston C, Blamey R. Selection for adjuvant therapy using the Nottinghams prognostic index. Breast. 1993;2:187. [Google Scholar]
- 4.Schwartz GF, Hortobagyi GN, Masood S, Palazza J, Holland R, Page D. Proceedings of the consensus conference on neoadjuvant chemotherapy in carcinoma of the breast, April 26-28, 2003, Philadelphia, PA. Hum Pathol. 2004;35:781–4. doi: 10.1016/j.humpath.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:966–78. doi: 10.5858/2000-124-0966-PFIBC. [DOI] [PubMed] [Google Scholar]
- 6.Kocjan G. Needle aspiration cytology of the breast: current perspective on the role in diagnosis and management. Acta Med Croatica. 2008;62:391–401. [PubMed] [Google Scholar]
- 7.Robles-Frías A, González-Cámpora R, Martínez-Parra D, Robles-Frías MJ, Vázquez-Cerezuela T, Otal-Salaverri C, et al. Robinson cytologic grading of invasive ductal breast carcinoma: correlation with histologic grading and regional lymph node metastasis. Acta Cytol. 2005;49:149–53. doi: 10.1159/000326123. [DOI] [PubMed] [Google Scholar]
- 8.Howell LP, Gandour-Edwards R, O’Sullivan D. Application of the Scarff-Bloom-Richardson tumor grading system to fine-needle aspirates of the breast. Am J Clin Pathol. 1994;101:262–5. doi: 10.1093/ajcp/101.3.262. [DOI] [PubMed] [Google Scholar]
- 9.Laroye GJ, Minkin S. The impact of mitotic index on predicting outcome in breast carcinoma: a comparison of different counting methods in patients with different lymph node status. Mod Pathol. 1991;4:456–60. [PubMed] [Google Scholar]
- 10.Latinovie L, Heinze G, Birner P, Samonigy H, Hausmaninger H, Kubista E, et al. Prognostic relevance of three histological grading methods in breast cancer. Int J Oncol. 2001;19:1271–7. [PubMed] [Google Scholar]
- 11.Taniguchi E, Yang Q, Tang W, Nakamura Y, Shan L, Nakamura M, et al. Cytological grading of invasive breast carcinoma: Correlation with clinicopathologic variables and predictive value of nodal metastasis. Acta Cytol. 2000;44:587–91. doi: 10.1159/000328533. [DOI] [PubMed] [Google Scholar]
- 12.Robinson IA, McKee G, Kissin MW. Typing and grading breast carcinoma on fine-needle aspiration: is this clinically useful information? Diagn Cytopathol. 1995;13:260–5. doi: 10.1002/dc.2840130315. [DOI] [PubMed] [Google Scholar]
- 13.Dabbs DJ, Silverman JF. Prognostic factors from the fine-needle aspirate: breast carcinoma nuclear grade. Diagn Cytopathol. 1994;10:203–8. doi: 10.1002/dc.2840100302. [DOI] [PubMed] [Google Scholar]
- 14.Fisher ER, Redmond C, Fisher B, Bass G. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Projects (NSABP).Prognostic discriminants for 8-year survival for node-negative invasive breast cancer patients. Cancer. 1990;65(9 Suppl):2121–8. doi: 10.1002/1097-0142(19900501)65:9+<2121::aid-cncr2820651408>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15.van Dienst PJ, Mouriquand J, Schipper NW, Baak JP. Prognostic value of nucleolar morphometric variables in cytological breast cancer specimens. J Clin Pathol. 1990;43:157–9. doi: 10.1136/jcp.43.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciatto S, Bonardi R, Herd-Smith A, Cariaggi P, Confortini M, Bulgaresi P. Prognostic value of breast cancer cytologic grading: A retrospective study of 213 cases. Diagn Cytopathol. 1993;9:160–3. doi: 10.1002/dc.2840090210. [DOI] [PubMed] [Google Scholar]
- 17.Dabbs DJ. Role of nuclear grading of breast carcinomas in fine needle aspiration specimens. Acta Cytol. 1993;37:361–6. [PubMed] [Google Scholar]
- 18.Skrbinc B, Babic A, Cufer T, Us-Krasovec M. Cytological grading of breas cancer in Giemsa-stained fine needle aspiration smears. Cytopathology. 2001;12:15–25. doi: 10.1046/j.1365-2303.2001.00297.x. [DOI] [PubMed] [Google Scholar]
- 19.Khan MZ, Haleem A, Hassani HA, Kfoury H. Cytopathological grading, as a predictor of histopathological grade, in ductal carcinoma (NOS) of breast, on air-dried Diff-Quick smears. Diagn Cytopathol. 2003;29:185–93. doi: 10.1002/dc.10285. [DOI] [PubMed] [Google Scholar]
- 20.Ozkutlu D, Raab SS, Level JC, Thomas PA. Selection of key cytology criteria to separate proliferative breast disease from carcinoma in breast fine needle aspiration using logistic regression analysis. Mod Pathol. 1995;8:43A. [Google Scholar]
- 21.Yu GH, Cajulis RS, De Frias DV. Tumor cell (dys)cohesion as a prognostic factor in aspirate smears of breast carcinoma. Am J Clin Pathol. 1998;109:315–9. doi: 10.1093/ajcp/109.3.315. [DOI] [PubMed] [Google Scholar]
- 22.Schiller AB, Tadros TS, Birdsong GG, Grossl NA. Cellular dyscohesion in fine-needle aspiration of breast carcinoma. Prognostic indicator for axillary lymph node metastases? Am J Clin Pathol. 2001;115:219–23. doi: 10.1309/PR6K-7RXQ-NJUD-443Q. [DOI] [PubMed] [Google Scholar]
- 23.Fan F, Namiq AL, Tawfik OW, Thomas PA. Proposed prognostic score for breast carcinoma on fine needle aspiration based on nuclear grade, cellular dyscohesion and bare atypical nuclei. Diagn Cytopathol. 2006;34:542–6. doi: 10.1002/dc.20529. [DOI] [PubMed] [Google Scholar]
- 24.Robinson IA, McKee G, Nicholson A, D’Acry J, Jackson PA, Cook MG, et al. Prognostic value of cytological grading of fine-needle aspirates from breast carcinomas. Lancet. 1994;343:947–9. doi: 10.1016/s0140-6736(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 25.Masood S. Prognostic factors in breast cancer: use of cytologic preparations. Diagn Cytopathol. 1995;13:388–95. doi: 10.1002/dc.2840130507. [DOI] [PubMed] [Google Scholar]


