Abstract
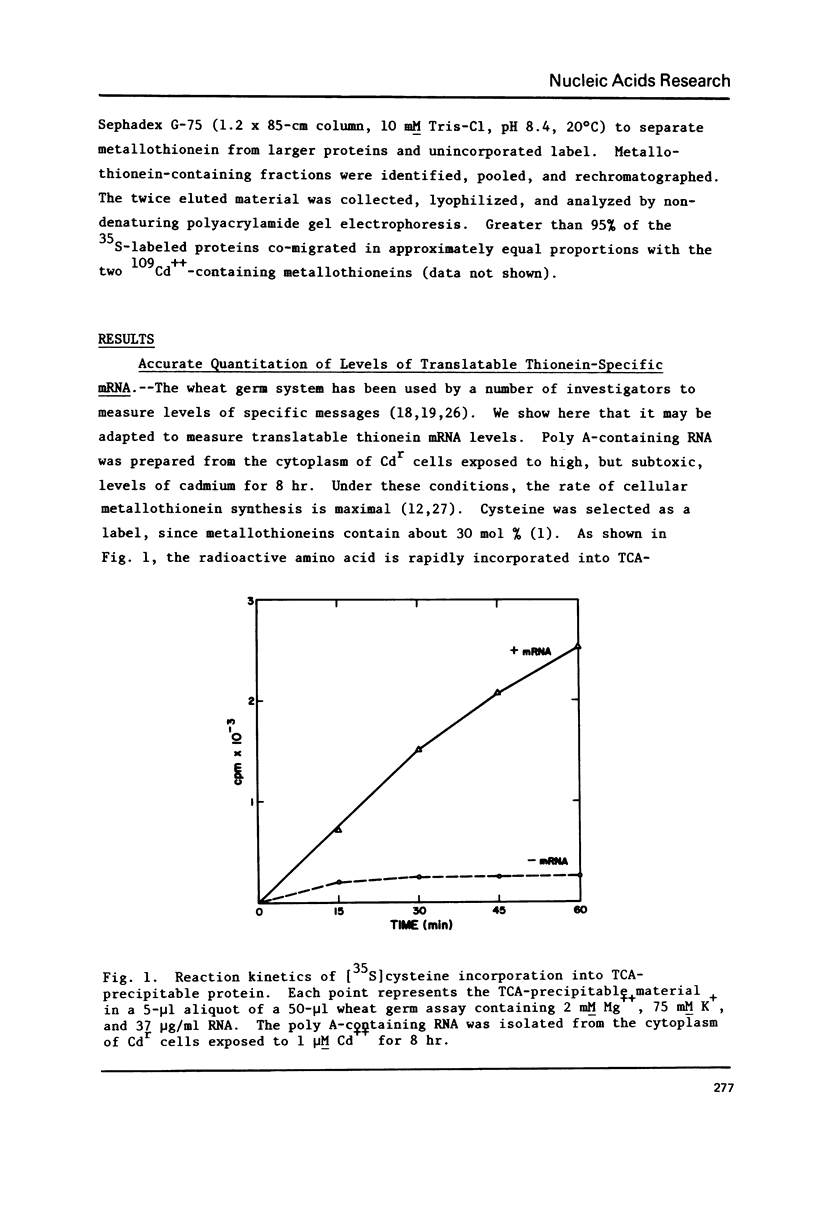
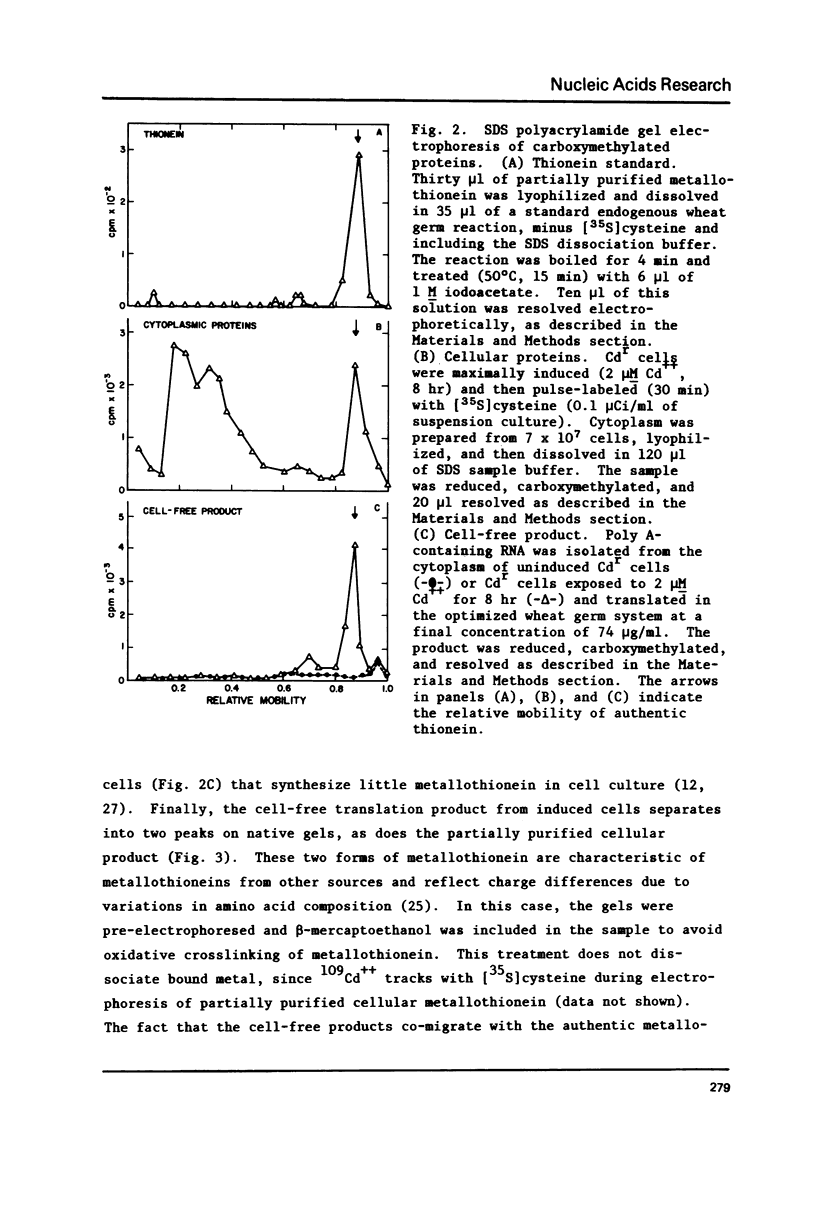
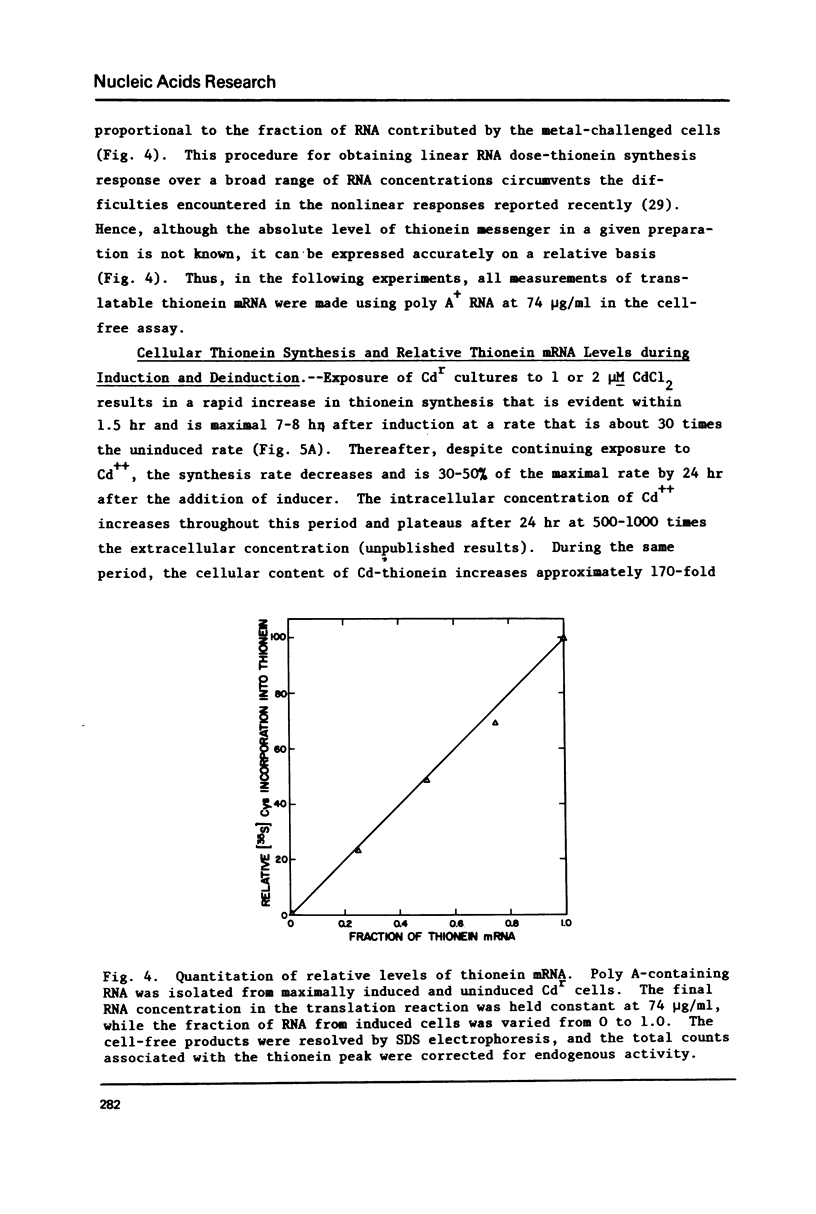
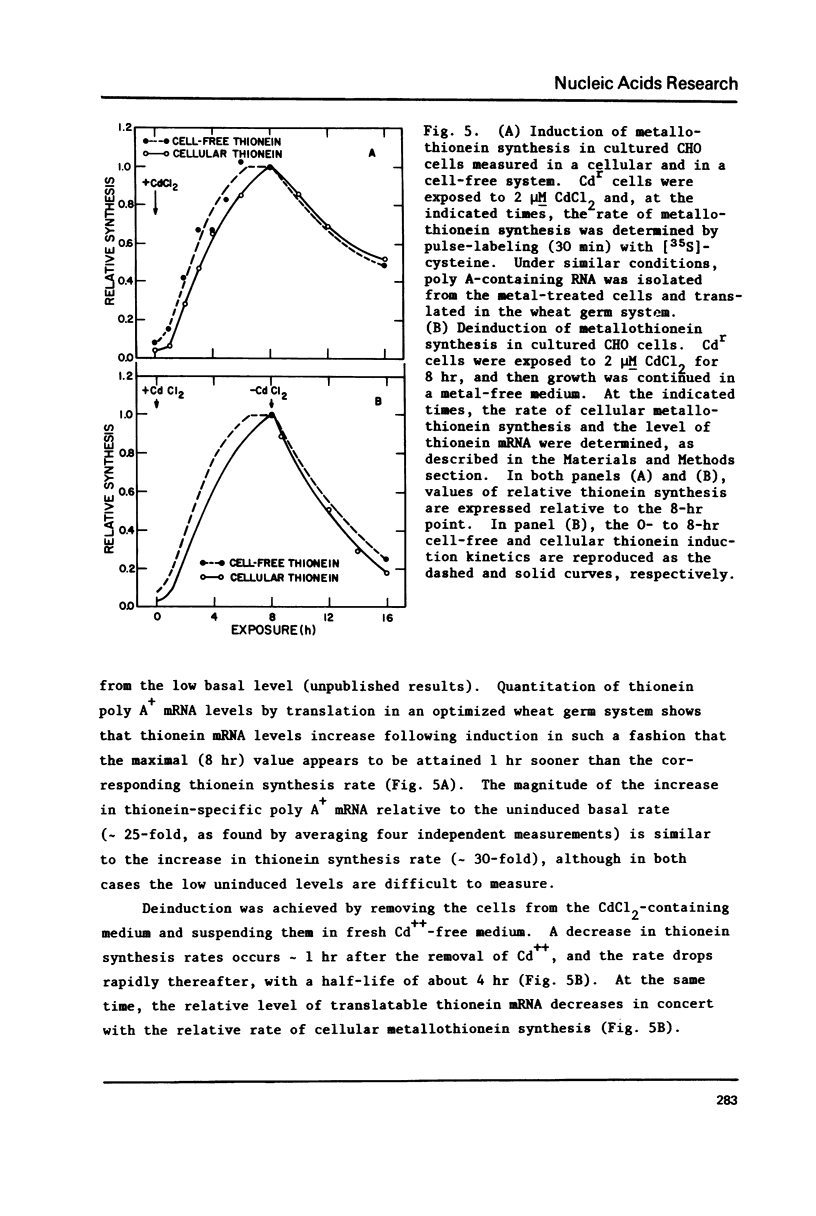
The relationship of thionein synthesis rates to translatable cytoplasmic thionein mRNA levels was investigated for the first time in a cultured cell system. Thionein synthesis was induced in Cdr, a cadmium-resistant variant of CHO, by exposure to 2 microM CdCl2. Following a short (1.5 hr) lag, thionein synthesis increases to a rate that is at least 30 times the uninduced rate 7-8 hr after addition of Cd++. This increase is blocked by the coincident addition of a actinomycin D. Cytoplasmic thionein mRNA levels, measured by translation in a modified wheat germ system, increase rapidly following induction to values approximately 25 times uninduced levels within 6-8 hr. The increase in thionein mRNA precede proportionate increases in thionein synthesis by 0.5-1.0 hr. Continued exposure to Cd++ results in a decreased thionein synthesis rate after 8 hr. By 30 hr, the rate is one-half that seen 6-8 hr after induction. Removal of Cd++ after 8 hr results in a rapid decrease in thionein synthesis (t 1/2 approximately 4 hr). Both decreases are inhibited by the addition of actinomycin. In all instances--induction, deinduction, and actinomycin-mediated "super-induction"--translatable thionein mRNA levels and thionein synthesis rates increase, decrease, or are maintained coordinately. The results suggest that thionein synthesis in Cdr is controlled primarily by the level of translatable cytoplasmic thionein mRNA.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen R. D., Weser U. Partial purification, characterization and translation in vitro of rat liver metallothionein messenger ribonucleic acid. Biochem J. 1978 Dec 1;175(3):841–852. doi: 10.1042/bj1750841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astell C. R., Ganoza M. C. Translation of hepatic mRNA in extracts from wheat germ embryos. Mol Biol Rep. 1974 Dec;1(8):483–491. doi: 10.1007/BF00360676. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Beaudet A., Nirenberg M. RNA codons and protein synthesis. 15. Dissimilar responses of mammalian and bacterial transfer RNA fractions to messenger RNA codons. J Mol Biol. 1968 Oct 14;37(1):99–118. doi: 10.1016/0022-2836(68)90076-4. [DOI] [PubMed] [Google Scholar]
- Cempel M., Webb M. The time-course of cadmium-thionein synthesis in the rat. Biochem Pharmacol. 1976 Sep 15;25(18):2067–2071. doi: 10.1016/0006-2952(76)90431-7. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Davies J. W., Kaesberg P. Translation of virus mRNA: synthesis of bacteriophage Q beta proteins in a cell-free extract from wheat embryo. J Virol. 1973 Dec;12(6):1434–1441. doi: 10.1128/jvi.12.6.1434-1441.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day F. A., Coles B. J., Brady F. O. Postinductive actinomycin D effects on the concentrations of cadmium thionein, zinc thionein, and copper chelatin in rat liver. Bioinorg Chem. 1978;8(2):93–105. doi: 10.1016/s0006-3061(00)80236-7. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Hidalgo H. A., Koppa V., Bryan S. E. Induction of cadmium-thionein in isolated rat liver cells. Biochem J. 1978 Feb 15;170(2):219–225. doi: 10.1042/bj1700219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House W., Waddell A. Detection of mycoplasma in cell cultures. J Pathol Bacteriol. 1967 Jan;93(1):125–132. doi: 10.1002/path.1700930112. [DOI] [PubMed] [Google Scholar]
- Huang I. Y., Yoshida A. Mouse liver metallothioneins. Complete amino acid sequence of metallothionein-I. J Biol Chem. 1977 Nov 25;252(22):8217–8221. [PubMed] [Google Scholar]
- Killewich L., Schutz G., Feigelson P. Functional level of rat liver tryptophan 2,3-dixoygenase messenger RNA during superinduction of enzyme with actinomycin D. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4285–4287. doi: 10.1073/pnas.72.11.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane L. C. A simple method for stabilizing protein-sulfhydryl groups during SDS-gel electrophoresis. Anal Biochem. 1978 Jun 1;86(2):655–664. doi: 10.1016/0003-2697(78)90792-3. [DOI] [PubMed] [Google Scholar]
- Lucis O. J., Shaikh Z. A., Embil J. A., Jr Cadmium as a trace element and cadmium binding components in human cells. Experientia. 1970 Oct 15;26(10):1109–1110. doi: 10.1007/BF02112704. [DOI] [PubMed] [Google Scholar]
- Nickol J. M., Lee K. L., Kenney F. T. Changes in hepatic levels of tyrosine aminotransferase messenger RNA during induction by hydrocortisone. J Biol Chem. 1978 Jun 10;253(11):4009–4015. [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Inhibition of RNA synthesis by actinomycin D: characteristic dose-response of different RNA species. J Cell Physiol. 1970 Oct;76(2):127–139. doi: 10.1002/jcp.1040760202. [DOI] [PubMed] [Google Scholar]
- Rether B., Belarbi A., Beck G. Translation of tyrosine aminotransferase mRNA from hepatoma cells in a wheat germ cell-free system. FEBS Lett. 1978 Sep 15;93(2):194–195. doi: 10.1016/0014-5793(78)81103-x. [DOI] [PubMed] [Google Scholar]
- Richards M. P., Cousins R. J. Mammalian zinc homeostasis: requirement for RNA and metallothionein synthesis. Biochem Biophys Res Commun. 1975 Jun 16;64(4):1215–1223. doi: 10.1016/0006-291x(75)90822-0. [DOI] [PubMed] [Google Scholar]
- Rudd C. J., Herschman H. R. Metallothionein accumulation in response to cadmium in a clonal rat liver cell line. Toxicol Appl Pharmacol. 1978 Jun;44(3):511–521. doi: 10.1016/0041-008x(78)90259-4. [DOI] [PubMed] [Google Scholar]
- Rugstad H. E., Norseth T. Cadmium resistance and content of cadmium-binding protein in two enzyme-deficient mutants of mouse fibroblasts (L-cells). Biochem Pharmacol. 1978 Mar 1;27(5):647–650. doi: 10.1016/0006-2952(78)90499-9. [DOI] [PubMed] [Google Scholar]
- Rugstad N. E., Norseth T. Cadmium resistance and content of cadmium-binding protein in cultured human cells. Nature. 1975 Sep 11;257(5522):136–137. doi: 10.1038/257136a0. [DOI] [PubMed] [Google Scholar]
- Shapiro S. G., Squibb K. S., Markowitz L. A., Cousins R. J. Cell-free synthesis of metallothionein directed by rat liver polyadenylated messenger ribonucleic acid. Biochem J. 1978 Dec 1;175(3):833–840. doi: 10.1042/bj1750833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squibb K. S., Cousins R. J., Feldman S. L. Control of zinc-thionein synthesis in rat liver. Biochem J. 1977 Apr 15;164(1):223–228. doi: 10.1042/bj1640223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E. B., Granner D. K., Tomkins G. M. Superinduction of tyrosine aminotransferase by actinomycin D in rat hepatoma (HTC) cells. J Mol Biol. 1970 Dec 14;54(2):159–175. doi: 10.1016/0022-2836(70)90424-9. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Gelehrter T. D., Granner D., Martin D., Jr, Samuels H. H., Thompson E. B. Control of specific gene expression in higher organisms. Expression of mammalian genes may be controlled by repressors acting on the translation of messenger RNA. Science. 1969 Dec 19;166(3912):1474–1480. doi: 10.1126/science.166.3912.1474. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Levinson B. B., Baxter J. D., Dethlefsen L. Further evidence for posttranscriptional control of inducible tyrosine aminotransferase synthesis in cultured hepatoma cells. Nat New Biol. 1972 Sep 6;239(88):9–14. doi: 10.1038/newbio239009a0. [DOI] [PubMed] [Google Scholar]
- Webb M., Daniel M. Induced synthesis of metallothionein by pig kidney cells in vitro in response to cadmium. Chem Biol Interact. 1975 Apr;10(4):269–276. doi: 10.1016/0009-2797(75)90091-5. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Premakumar R., Rajagopalan K. V. Metal-induced formation of metallothionein in rat liver. Arch Biochem Biophys. 1975 Sep;170(1):242–252. doi: 10.1016/0003-9861(75)90115-0. [DOI] [PubMed] [Google Scholar]


