Abstract
The caridean shrimp Rimicaris exoculata dominates the fauna at several Mid-Atlantic Ridge hydrothermal vent sites. This shrimp has an enlarged gill chamber, harboring a dense ectosymbiotic community of chemoautotrophic bacteria associated with mineral oxide deposits. Until now, their acquisition is not fully understood. At three hydrothermal vent sites, we analyzed the epibionts diversity at different moult stages and also in the first stages of the shrimp life (eggs, hatched eggs (with larvae) and juveniles). Hatched eggs associated with young larvae were collected for the first time directly from gravid females at the Logachev vent site during the Serpentine cruise. An approach using 16S rRNA clone libraries, scanning and transmission electron microscopy, and fluorescent in situ hybridization was used. Molecular results and microscope observations indicated a switch in the composition of the bacterial community between early R. exoculata life cycle stage (egg libraries dominated by the Gammaproteobacteria) and later stages (juvenile/adult libraries dominated by the Epsilonproteobacteria). We hypothesized that the epibiotic phylotype composition could vary according to the life stage of the shrimp. Our results confirmed the occurrence of a symbiosis with Gammaproteobacteria and Epsilonproteobacteria, but more complex than previously assumed. We revealed the presence of active type-I methanotrophic bacteria colonizing the cephalothorax of shrimps from the Rainbow site. They were also present on the eggs from the Logachev site. This could be the first ‘epibiotic' association between methanotrophic bacteria and hydrothermal vent crustacean. We discuss possible transmission pathways for epibionts linked to the shrimp life cycle.
Keywords: symbiosis, larvae, methanotrophic symbiont, Rimicaris exoculata, transmission pathways
Introduction
Trophic symbioses are common in deep-sea hydrothermal ecosystems. In these environments, symbiosis between chemosynthetic bacteria and invertebrates supports a strikingly diversified fauna and significantly more biomass than in surrounding seawater (Goffredi, 2010; Ruehland and Dubilier, 2010; Bates et al., 2011). One of these invertebrates is the shrimp Rimicaris exoculata (Williams and Rona, 1986). This crustacean, belonging to the family Alvinocarididae, is part of the dominant megafauna at several Mid-Atlantic Ridge (MAR) vent sites (Desbruyères et al., 2001), where it forms dense and motile aggregates around the chimney walls (Segonzac, 1992; Gebruk et al., 1993). R. exoculata harbors a rich community of epibiotic bacteria on the inner side of its enlarged gill chamber (also called cephalothorax) and on its mouthparts (scaphognathites and exopodites of the first maxillipeds, both covered with abundant bacteriophore setae). These characteristics were encountered in all R. exoculata specimens regardless of the site (Van Dover et al., 1988; Casanova et al., 1993; Segonzac et al., 1993; Zbinden et al., 2004), highlighting a possible obligate relationship between the shrimp and its epibionts. A δ13C stable isotope study showed that the predominant source of dietary carbon for the shrimp was the gill chamber epibionts (Rieley et al., 1999), but the bacterial community in the shrimp gut has also been proposed as an alternative nutritional source (Polz et al., 1998; Pond et al., 2000; Zbinden and Cambon-Bonavita, 2003; Durand et al., 2010).
Recent studies have shown that the diversity of R. exoculata epibionts was higher than previously reported (Zbinden et al., 2004, 2008; Petersen et al., 2009; Hügler et al., 2011). Based on in vivo experiments (IPOCAMP (Shillito et al., 2004)), microscopic and molecular analyses, the co-occurrence of three metabolisms (iron, sulfur and methane oxidation) among gill chamber epibiont communities has been proposed (Zbinden et al., 2008). Moreover the pmoA and aps genes were amplified. It was also suggested that the relative contribution of each metabolism might differ according to fluid chemical composition (Schmidt et al., 2008; Zbinden et al., 2008). Two filamentous epibiont phylotypes (Gammaproteobacteria and Epsilonproteobacteria) dominated the R. exoculata epibiosis and the sequences clustered spatially across the different vent sites along the MAR (Petersen et al., 2009). Finally, the occurrence of autotrophic carbon fixation (rTCA cycle) through sulfur and hydrogen oxidation, and sulfur reduction, was suggested on the Snake Pit site (Hügler et al., 2011).
Like all arthropods, R. exoculata undergoes moults, which regularly eliminate the bacterial community settled on the cuticle. The moult cycle seemed to be shortened (10 days) compared with coastal shrimps (Penaeus japonicus (21 days), Macrobrachium rosenbergii (41–98 days)) (Corbari et al., 2008). Briefly, the shrimps are white after moulting, turn gray or light red in the mid phase, and black or red in the late phase according to the sulfur or iron fluid concentration, respectively (Corbari et al., 2008). Microscopic observations showed that a new epibiotic community started to form on free surfaces of the new cuticle within 2 days after exuviations (Corbari et al., 2008).
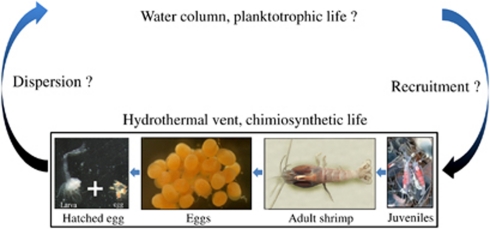
The R. exoculata life cycle is still unknown. It produces lipid-rich orange eggs (Llodra et al., 2000), which suggested the occurrence of planktotrophic larvae. Usually, egg size is about 300–400 μm (up to 836 eggs per female) (Tyler and Young, 1999). Gametogenic synchrony has never been observed (Tyler and Young, 1999), but a polymodal population structure for this shrimp suggested periodic recruitment (Copley et al., 1998). Up to now, only very few gravid females have been collected and no larvae have ever been collected around the vent sites. Only juveniles above 1.2 cm were collected at the aggregates' periphery and are orange (Komai and Segonzac, 2008). Wax esters, fatty acids and fatty alcohols found in the juveniles indicated that they might feed for extended periods in the euphotic zone, allowing dispersion (Pond et al., 1997). This was supported by genetic data that suggested high gene flow in R. exoculata populations (Teixeira et al., 2011).
In this study, we analyzed the diversity and development of epibionts in R. exoculata gill chamber at different moult stages and also in the first stages of shrimp life (eggs, hatched eggs and 2-cm juveniles). An approach using 16S rRNA clone libraries, transmission and scanning electron microscopy (TEM/SEM) and fluorescent in situ hybridization (FISH) was used. Our aims were to examine when the first acquisition of epibionts occurs and to determine whether the epibiont community differs between early life stages and adults, and also between moult stages.
Materials and methods
Collection/selection/pretreatment
Specimens of R. exoculata were collected at several hydrothermal vents sites along the MAR: at Logachev (14°45′N; 44°57′W; 3037 m depth) and Ashadze (12°58′N; 44°51′W; 4088 m depth) during the Serpentine cruise (March 2007), and at Rainbow (36°13′N; 33°54′W; 2350 m depth) during the MoMARDREAM-Naut cruise (June 2007). Shrimps were collected using the suction sampler of the remoted operated vehicle ‘Victor 6000' or the Nautile operated from the R/V ‘Pourquoi pas?'. Once on board, living individuals were dissected into body parts (branchiostegites (LB), scaphognathites (Sc), exopodites, gills, stomach and digestive tract). For molecular studies, animal tissues and eggs, hatched eggs (still associated with young larvae) and orange juveniles (2 cm) were directly frozen (−80 °C) and DNA extractions were performed in the laboratory. For TEM and SEM, samples were fixed as described previously (Zbinden et al., 2008), as well as for FISH (Durand et al., 2010). Shrimps were sorted according to moulting stages (corresponding to a color gradient: white (first stage), light red or gray (middle stage), red or black (last stage)). Only black shrimps were collected at the Ashadze site. Eggs and hatched eggs were only found at the Logachev site. Seawater near shrimp aggregates (pH 7.3 and T°C=13 °C) was also sampled at the Rainbow site.
DNA extraction and PCR amplification
DNA from Rainbow seawater, adult LB/Sc, eggs, hatched eggs and juveniles (Sc) was extracted using the Fast DNA Pro Soil-Direct Kit (Qbiogen, Santa Ana, CA, USA) (Supplementary Table S2). Extracted DNA was then purified with Quick-Clean Spin Filters (Qbiogen). Bacterial 16S rRNA gene fragments were PCR-amplified in 30 cycles at an annealing temperature of 49 °C, using the general bacterial primer set 8F and 1492R (Lane, 1991). They were then purified with a QIAquick PCR purification kit (Qiagen, Hilden, Germany).
Cloning and sequencing
The pooled amplified and purified PCR products were cloned using the TOPO XL Cloning kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. The plasmid inserts were controlled by amplification using M13F and M13R primers. Positive clones were then cultured and treated for sequencing at the Biogenouest Platform (Roscoff, France; http//www.sb-roscoff.fr/SG/) on an ABI prism 3130xl (Applied Biosystems, Foster City, CA, USA), using the Big-Dye Terminator V3.1 (Applied Biosystems).
Phylogenetic analyses
Sequences (16S rDNA) were compared to those available in databanks using the BLAST online service (Altschul et al., 1990). Unstable (for example, chimeras) and short sequences were excluded; others were cleaned manually with ‘EDITSEQ' (DNA STAR, Madison, WI, USA). Sequences were aligned using the CLUSTALW program (Thompson et al., 1994), further refined manually using the SEAVIEW program (Galtier et al., 1996). All trees were built using PHYLO-WIN (Galtier et al., 1996). Phylogenetic analyses were performed on the basis of evolutionary distance (Neighbor-Joining; (Saitou and Nei, 1987)) using the Kimura two-parameters correction matrix. The robustness of phylogenetic reconstructions was tested by bootstrap re-sampling (500) (Felsenstein, 1985). Sequences showing more than 97% similarity were considered to be sufficiently related and were grouped in the same phylotype.
The rarefaction curves and Simpson indices were performed using DOTUR (at 97% similarity) for all libraries (Schloss and Handelsman, 2005). Simpson index was calculated as follows:
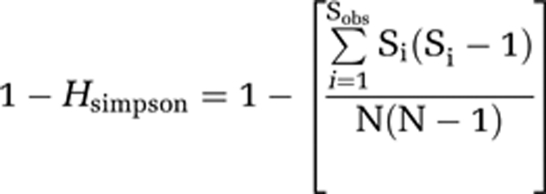 |
where Sobs representing the number of OTUs observed, Si the number of individuals for one OTUs and N the total number of OTUs. Good's coverage was calculated as a percentage, according to the following relation: C=[1−(n/N)] × 100, where n represented the number of phylotypes appearing only once in a library and N being the library size (Good, 1953; Ravenschlag et al., 1999).
Fluorescence in situ hybridization
The FISH protocol used was described previously (Durand et al., 2010). Whole LB/Sc (adult and juvenile), eggs and hatched eggs (Supplementary Table S2) were hybridized using several published probes (Table 2). The hybridization temperature was the same for all samples treated (46 °C). Observations and imaging were performed using an Apotome Axio Imager Z2 with a COLIBRI system (Zeiss, Jena, Germany).
Scanning electron microscopy
LB/Sc, eggs and hatched eggs were dehydrated in ethanol series (30, 50, 70, 95 and 100% ethanol) and for 5 h in a critical point dryer CPD 020 (Balzers Union, Balzers, Liechtenstein). Finally, samples were gold-coated with an SCD 040 (Balzers Union). Observations and imaging were performed using a Quanta 200 MK microscope (FEI, Hillsboro, OR, USA) and the SCANDIUM acquisition program (Soft Imaging System, Munster, Germany).
Transmission electron microscopy
Samples were dehydrated in ethanol and propylene oxide series, and then embedded in an epoxy resin (Serlabo, Paris, France). Semi-thin and ultra-thin sections were made using a Reichert-Jung Ultramicrotome (Ultracut R, Depew, NY, USA) with a diamond knife. Semi-thin sections were stained with toluidine blue for observations by light microscopy (using an Olympus (Tokyo, Japan) BX61 microscope). Thin sections were laid on copper grids and stained with uranyl acetate and lead citrate. Observations were performed on a LEO 912 electron microscope (LEO Electron Optics GmbH, Oberkochen, Germany) equipped with a LaB6 source and operated at 80 kV.
Nucleotide sequence accession numbers
The sequences from this study are available through GenBank under the following accession numbers: FR797908 to FR797966 (16S rRNA sequences).
Results
Sample description
Seawater collected inside Rainbow shrimp aggregates was slightly orange. Shrimps collected at the three sites (Rainbow, Logachev and Ashadze) were sorted at different moult stages, according to their color, from white (no minerals or bacteria) to dark red or black (mineral oxide deposits). At the ultramafic Rainbow vent field, the end-member was characterized by extremely high concentrations of ferrous iron (Charlou et al., 2002; Douville et al., 2002), explaining the reddish color of the majority of shrimps (Zbinden et al., 2004). At the Logachev vent site, there was a majority of gray/black shrimps, corresponding probably to iron sulfate deposits (Gebruk et al., 1993). For Ashadze, only six black specimens were retrieved. Surprisingly, the Ashadze fauna was dominated by species usually recovered at the periphery of hydrothermal communities (Maractis rimicarivora and Phyllochaetopterus sp. nov.) (Fabri et al., 2011). All the collected shrimps were alive and active when recovered from the slurp gun bowls, but the Ashadze specimens were less active than the other sites. It should be noted that Ashadze is the deepest hydrothermal site (4080 m) where R. exoculata has been identified.
Orange juveniles (2 cm stage-A larvae (Komai and Segonzac, 2008)) were sampled at the Rainbow and Logachev sites, in the periphery of adults but close to the aggregates (Supplementary Figure S3). For the first time, eggs and hatched eggs were collected at Logachev during the Serpentine cruise in March 2007 from females collected among the shrimp aggregates (Figures 1a and b). The eggs, orange, were at different maturity stages, from small young eggs (approximately 200 μm) to mature eggs (approximately 400 μm), with only one stage per female. The eggs were always hatched beneath the female abdomen so that only free larvae would be released into the environment. The collected larvae were just hatched eggs, probably at a zoeal stage (Figure 1b). The rostrum was absent. Eyes were present, ovoid, and seemed to be borne on short eyestalks. Orange pigmented spots were observed in the eyes. Four pairs of pereiopods were visible, three of which were bifid and had three setae at their tip; the fourth was just a bud. Cephalothorax, covered by a loose carapace, contained the same orange lipid droplets observed in eggs. The abdomen was composed of five well-delimited short segments. A long terminal segment ended with two blades provided with six setae. No pleopods were observed.
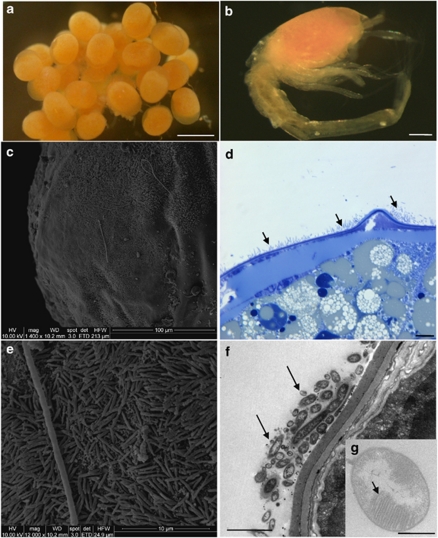
Figure 1.
Microscopic observations of eggs and larvae (SEM, c and e; TEM, f and g). (a) R. exoculata eggs from Logachev. Scale bar=500 μm. (b) R. exoculata larvae, which just hatched from Logachev (manually separated from egg). Scale bar=200 μm. (c) Egg surface from Logachev covered by thin rod-shaped bacterial mat. (d) Egg thin section from Logachev, showing bacterial mat (indicated by dark arrows) on R. exoculata egg membrane. Scale bar=10 μm. (e) Magnified view of the picture in panel c, showing thin rod-shaped bacterial mat. (f) Egg thin section, showing thin rod-shaped bacteria (indicated by dark arrows) on the egg membrane. Scale bar=2 μm. (g) Methanotrophic-like bacteria (with intracytoplasmic membranes, indicated by a dark arrow) retrieved in the thin rod-shaped bacterial mat associated with the egg membrane. Scale bar=500 nm.
Microscopic observations
Adult/juvenile gill chamber
Scanning electron microscopic observations of cephalothorax pieces (LB, branchiostegite and Sc, scaphognathite) along the moult cycle confirmed the different epibiont morphologies (for example, rod-shaped, thin and thick long filaments) observed before (Zbinden et al., 2008). Their development seemed to follow a chronological order along the moult cycle: rod-shaped bacterial mat, followed by long filamentous bacteria, as described previously (Corbari et al., 2008). These morphologies were observed at all sites studied (Rainbow, Logachev and Ashadze). FISH observations of LB and Sc along the moult cycle, whatever the hydrothermal site, indicated the predominance of Epsilonproteobacteria with thick and thin filamentous morphologies (Figures 2a and b). This was congruent with molecular studies (Table 1) (Polz and Cavanaugh, 1995; Zbinden et al., 2008; Petersen et al., 2009; Hügler et al., 2011). Gammaproteobacteria signals were also detected to a lesser extent, and were related exclusively to some thin filamentous morphologies (Figures 2a and b), confirming previous results (Petersen et al., 2009; Hügler et al., 2011). Type-I methanotrophic Gammaproteobacteria morphologies were observed by TEM (Zbinden et al., 2008). For the first time we confirmed it with the typical circular positive FISH signal (Duperron et al., 2005) using both the GAM42a probe and the LBl32/130 probe (Table 2 and Figure 2c). At the Rainbow site, these methanotrophic-like bacteria were clearly at the basis of long filaments affiliated to Epsilonproteobacteria, directly fixed on the R. exoculata tissues (LB and Sc) (Supplementary Figure S1). This specific localization seemed to confirm that the type-I methanotrophic Gammaproteobacteria were not opportunistic. This morphology was observed only to be associated with Rainbow juveniles and adults, whatever the moult stage. The other phylogenetic groups (Table 1) were not detected in the gill chamber (using FISH analyses; Table 2).
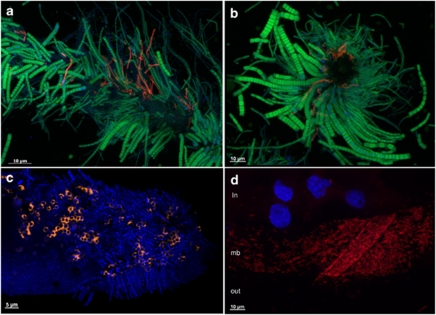
Figure 2.
FISH. (a) Longitudinal view of Scaphognathite setae from Logachev black moult shrimp with epibionts. Gammaproteobacteria (red) were hybridized with a GAM42a probe and Epsilonproteobacteria (green) were hybridized by an EPSY549 probe. (b) Transversal view of Scaphognathite setae from Logachev black moult shrimp with epibionts. Gammaproteobacteria (red) were hybridized with a GAM42a probe and Epsilonproteobacteria (green) were hybridized by an EPSY549 probe. (c) Longitudinal view of Scaphognathite setae from Rainbow red moult shrimp with epibionts. The methanotrophic Gamma symbionts (orange) were hybridized with both LBl32/130 and GAM42a probes. DAPI staining is shown in blue. (d) Egg membrane (mb) with epibionts, egg content (in) and outer environment (out). Gammaproteobacteria (red) were hybridized with a GAM42a probe. DAPI staining is shown in blue, showing eukaryotic nucleus of the egg.
Table 1. Clone library results (based on partial 16S rRNA sequences).
| Phylogenetics groups |
Logachev |
Rainbow |
Ashadze | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eggs | Hatched eggs | Juvenile | White moult | Gray moult | Black moult | White moult | Light Red moult | Red moult* | Hydrothermal fluid | Black moult | ||
| Alphaproteobacteria | 3 | 2 | 2 | 1 | 3 | 11 | ||||||
| Betaproteobacteria | 4 | 1 | 5 | |||||||||
| Gammaproteobacteria | 53 | 30 | 1 | 1 | 10 | 1 | 25 | 5 | 126 | |||
| Deltaproteobacteria | 1 | 2 | 3 | 15 | 21 | 3 | 1 | 46 | ||||
| Epsilonproteobacteria | 6 | 3 | 50 | 71 | 84 | 83 | 38 | 67 | 45 | 54 | 95 | 596 |
| Bacteroidetes | 4 | 7 | 7 | 1 | 3 | 5 | 1 | 4 | 1 | 33 | ||
| Total | 67 | 44 | 61 | 87 | 84 | 108 | 59 | 71 | 76 | 64 | 96 | 817 |
The main phylogenetic group per sample is shown in bold (*by Zbinden et al., 2008).
Table 2. Fluorescent probes (the probes sequences have been compared by BLAST to our sequences to check their specificity and determine their mismatches).
| Specificity | Probe name | Sequence (5′–3′) | Fluorescent dye | % Formamide | References |
|---|---|---|---|---|---|
| Archaea | Arch915 | GTGCTCCCCCGCCAATTCCT | Cy3 | 10-20-30 | Stahl and Amann (1991) |
| Eubacteria | Eub338 | GCTGCCTCCCGTAGGAGT | Cy3 or Cy5 or ATTO488 | 10-20-30-40 | Amann et al. (1990) |
| Alphaproteobacteria | ALF968 | GGTAAGGTTCTGCGCGTT | Cy3 | 10-20-30-40 | Manz et al. (1992) |
| Betaproteobacteria | BET42a | GCCTTCCCACTTCGTTT | Cy3 | 10-20-30-40 | Manz et al. (1992) |
| Deltaproteobacteria | DELTA495b | AGTTAGCCGGCGCTTCCT | Cy3 | 10-20-30-40 | Loy et al. (2002) |
| Gammaproteobacteria | GAM42a | GCCTTCCCACATCGTTT | Cy3 | 10-20-30-40 | Manz et al. (1992) |
| Epsilonproteobacteria | EPSY549 | CAGTGATTCCGAGTAACG | Cy3 | 20-30 | Lin et al. (2006) |
| Bacteroidetes | CF319 | TGGTCCGTGTCTGAGTAC | ATTO488 | 10-20-30-40 | Manz et al. (1996) |
| Gammaproteobacteria R. exoculata cephalothoracic clones | LBl32/130 | TCCTGGCTATCCCCCACTAC | ATTO488 | 10-20-30 | Durand et al. (2010) |
Eggs
Scanning electron microscopic and semi-thin observations showed the presence of a mat of thin rod-shaped bacteria (around 2.5 μm length and 0.3 μm diameter) settled on the egg surface for the majority of eggs observed (Figures 1c–e). This microbial mat hybridized only with the GAM42a probe, but no methanotrophic-like bacteria were revealed by FISH analyses (Figure 2d). TEM observations confirmed the presence of bacteria embedded in a mucus covering the eggs (Figure 1f) and some had intracytoplasmic membranes like type I methanotroph (Figure 1g). They were smaller (1 μm) than the one observed on the Rainbow adult shrimps (2 μm). No bacteria were observed inside the eggs (TEM and FISH).
Larvae
Larvae used in this study had just hatched (Figure 1b) and were still associated to their egg (Figures 1a and 4). Scanning electron microscopic and FISH observations showed no obvious bacterial mats on the larvae itself, but only single cells. No bacteria were observed inside the larvae gill chamber (TEM and FISH). Molecular surveys were therefore not undertaken on larvae alone but on larvae and its egg (hatched egg).
Bacterial diversity (16S rRNA) along the R. exoculata life cycle
Diversity studies using PCR amplification and cloning are known to underestimate genetic diversity because of faster amplification of some sequences and bias both in amplification and cloning (Qiu et al., 2001). Moreover, sampling methods introduce additional biases (Bent and Forney, 2008). The clone libraries obtained in this study can therefore be considered only partially quantitative. As all experiments were performed using the same protocols, they can nevertheless be compared. Moreover FISH analyses confirmed libraries' diversity. Phylogenetic diversity along the R. exoculata life cycle was completed by using rarefaction analyses and diversity indices (Supplementary Figure S2 and Supplementary Table S1). A total of 11 bacterial 16S rRNA gene clone libraries were analyzed, corresponding to 817 clone sequences (Table 1). Epsilonproteobacteria and Gammaproteobacteria dominated all the clone libraries (Table 1). This was consistent with recent studies (Zbinden et al., 2008; Petersen et al., 2009; Hügler et al., 2011). Deltaproteobacteria, Alphaproteobacteria, Betaproteobacteria and Bacteroidetes were poorly represented (Table 1), confirmed by absence of FISH signal. These sequences might represent opportunistic microorganisms embedded in the mat covering the appendages. Nevertheless, a recent Snake Pit site study showing recovery of one deltaproteobacterial phylotype in high frequency suggested that it might have a role in the epibiotic community (Hügler et al., 2011). The clone diversity coverage (Good's coverage) was high for all clone libraries, with an average of 93% (±5) (Supplementary Table S1), and rarefaction curves showed that the clone libraries correctly described the epibiotic communities, excepted for the hatched eggs library (Supplementary Figure S2).
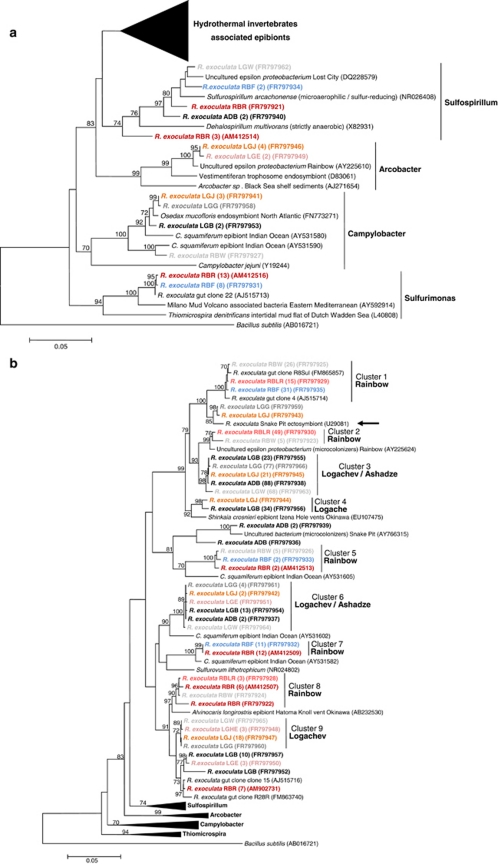
In this study, Epsilonproteobacteria sequences were overwhelmingly related to sequences usually retrieved from hydrothermal invertebrates (for example, Crysomallon squamiferum (Goffredi et al., 2004); Alvinocaris longirostris (Tokuda et al., 2008); Shinkaia crosnieri (unpublished); Rimicaris exoculata gut (Zbinden and Cambon-Bonavita, 2003) and gill chamber (Polz and Cavanaugh, 1995; Zbinden et al., 2008; Petersen et al., 2009)) and also to the MAR environment (Lost City (Brazelton et al., 2006); Rainbow (Lopez-Garcia et al., 2003); Snake Pit (unpublished)) (Figures 3a and b, and Supplementary Table S3). The main nine Epsilonproteobacteria clusters fell within the ‘hydrothermal invertebrates-associated epibionts' group (Marine Group-1) (Figure 3a). The closest cultivated relative was Sulfurovum lithotrophicum, a sulfur-oxidizing chemolithoautotroph isolated from a hydrothermal vent in the mid-Okinawa Trough (Inagaki et al., 2004) (94% similarity with R. exoculata RBR (AM412509)) (Figure 3b). Other Epsilonproteobacteria sequences were affiliated to known genera: Sulfurimonas, Campylobacter, Arcobacter and Sulfospirillum (Figure 3a). The three latter genera belong to the Campylobacteraceae family are known to show important metabolic diversity (including sulfur-oxidizing and reducing bacteria). The closest Sulfurimonas species, Thiomicrospira denitrificans (Figure 3a, 89% similarity with R. exoculata RBR (AM412516)), is an obligate chemolithotroph oxidizing sulfide and thiosulfate, and is also a denitrifier (Muyzer et al., 1995). The Logachev and Ashadze Epsilonproteobacteria-related sequences clustered together (Figure 3b, clusters 3 and 6) but not with the Rainbow sequences (Figure 3b, clusters 1, 2, 5, 7 and 8). Phylogenetic analyses showed that the same epibiont sequences were retrieved all along the shrimp life cycle, from eggs to adult on Logachev (Figure 3b, clusters 6 and 9). At the Rainbow site, some seawater sequences (R. exoculata RBF (FR797932)) were almost identical (99.9% similarity) to shrimp epibiont sequences (R. exoculata RBR (AM412509)) (Figure 3b, cluster-7).
Figure 3.
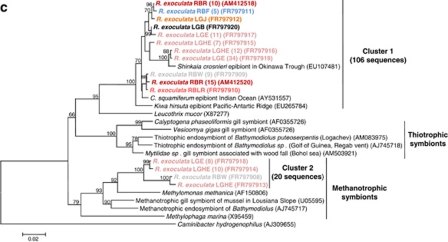
16S rRNA phylogeny of the Epsilonproteobacteria (a, b; calculated on 817 bp) and Gammaproteobacteria (c; calculated on 804 bp) associated with the R. exoculata gill chamber. Robustness was tested using 500 bootstraps re-sampling of the tree calculated by using the Neighbor-Joining algorithm, using the Kimura two-parameter correction matrix (only bootstrap values over 70 are shown). Sequences names have been resumed as follows: AD, LG or RB for Ashadze, Logachev or Rainbow specimens, respectively, and E, HE, J, W, G, B, LR, R and F for Eggs, Hatched Eggs, Juvenile, White moult, Gray moult, Black moult, Light Red moult, Red moult and Fluid, respectively, and finally the numbers in parentheses refer to the number assigned to each individual. Our clones are shown in color. (a) Global tree representing the R. exoculata epsilon symbiont and their close relatives. (b) Secondary tree showing the ‘Hydrothermal invertebrate-associated epibionts' (Marine Group-1) (see Figure 3a). The black arrow indicates the first R. exoculata epibiont sequence discovered in the Snake Pit site. (c) 16S rRNA phylogeny of the Gammaproteobacteria associated with the R. exoculata gill chamber.
The Gammaproteobacteria were mostly affiliated to bacteria associated with hydrothermal vent invertebrates (for example, C. squamiferum and Kiwa hirsuta (Goffredi et al., 2004); Shinkaia crosnieri (unpublished); R. exoculata (Zbinden et al., 2008)) (Figure 3c and Supplementary Table S3). The closest cultured relative to the cluster-1 Gammaproteobacteria epibionts (90.6% similarity) was Leucothrix mucor (Grabovich et al., 1999), a filamentous sulfur oxidizer (Figure 3c). The closest cultivated relative to the cluster-2 Gammaproteobacteria epibionts (92.5% similarity) was Methylomonas methanica (Costello and Lidstrom, 1999), a rod-shaped methanotrophic bacterium (Figure 3c).
All adult, juvenile and seawater libraries were dominated by the Epsilonproteobacteria-related sequences (Table 1). Epsilonproteobacteria sequences dominated Logachev gray, Rainbow light red and Ashadze black moult stages libraries as compared with others (Table 1). The Gammaproteobacteria were more represented in the Rainbow red moult library (Table 1).
Egg and hatched egg clone library distribution at Logachev was clearly different as compared with adult, juvenile and seawater libraries (Table 1). They were dominated by sequences related to the Gammaproteobacteria (Table 1), confirmed by FISH observations (Figure 2d). For cluster-1, most of the eggs and hatched eggs sequences were closely related (99% similarity) to a Shinkaia crosnieri epibiont (Figure 3c). For cluster-2, the eggs and hatched eggs sequences were closely related to M. methanica (Figure 4).
Figure 4.
Partial R. exoculata life cycle completed by this study.
Discussion
Female behavior and life cycle
Until now, there was no report of R. exoculata females carrying eggs inside shrimp aggregates close to the hydrothermal chimney walls at the MAR vent sites. One assumption was that gravid females were not inside the aggregates to avoid damaging the eggs (Vereshchaka et al., 1998), but only few gravid shrimps have been observed around the MAR vent sites (Tyler and Young, 1999). During the Serpentine cruise, gravid R. exoculata females were observed and collected from aggregates at the Logachev vent chimney Irina II. For the first time hatched eggs with larvae were collected, improving the knowledge of the shrimp life cycle (Figure 4). This cruise was held earlier in the season (March) than others did (from May to November). The small size and the composition (rich in lipids) of R. exoculata eggs could indicate short embryonic development, with larvae hatching at an early stage and undergoing a relatively long planktotrophic period (Llodra et al., 2000). R. exoculata could thus show seasonal reproduction, in which larvae hatch in early spring and undertake an as yet unspecified period of planktotrophic development in the water column. The lack of year-round data (absence of specimen between larvae and 1.2-cm juvenile) made it difficult to conclude on the full life cycle of this shrimp (Figure 4). All eggs on a given female were at the same maturity stage, but the stage differed from one female to another. This indicated that they were not sexually mature at the same time, and that the reproductive period would be longer than the egg development duration. Eggs were still associated with the gravid females when the hatching occurred, so only mature larvae would be released. To evaluate the egg development duration, pressured incubator (IPOCAMP) maintenance of gravid females would be necessary.
Epibiont diversity and acquisition
Some epibiont sequences were retrieved all along the shrimp life cycle (Figure 3b, clusters 6 and 9; Figure 3c, clusters 1 and 2). This result suggested a high specificity and the occurrence of an acute recognition mechanism such as in nematode ectosymbioses (Nussbaumer et al., 2004). Moreover, molecular surveys indicated a bacterial community switch occurring between the first stages of the R. exoculata life cycle (egg and hatched egg libraries dominated by the Gammaproteobacteria) and later stages (juvenile/adult libraries dominated by the Epsilonproteobacteria) (Table 1), confirmed by FISH observations (Figures 2a and b versus d). These results reinforced the occurrence of a complex stable symbiosis in R. exoculata with the same Gammaproteobacteria- and Epsilonproteobacteria-related sequences, and further showed that symbiont phylotype representativeness could vary according to the life stage of the host. Observations highlighted the presence of colorless mucus-like material surrounding the eggs. Mucus could be a ‘scaffolding' that provides anchorage and protection for the eggs (Davies and Viney, 1998), whereas epibionts embedded in the mucus could have a protective role in detoxication and also against potential pathogens (for example, bacteria and fungi). This was the case for epibiotic bacteria associated with the Homarus americanus embryo, which produce substances inhibiting pathogenic fungi growth (Gilturnes and Fenical, 1992). Bacteria within the gill chamber could have roles such as detoxication or nutrition for the host (Zbinden et al., 2004, 2008).
The Ashadze and Logachev sequences clustered together (Figure 3b, clusters 3 and 6), which might be explained by the very close proximity between these two sites (Fabri et al., 2011). A recent study showed a significant correlation between genetic (16S rRNA) and geographic distances for R. exoculata epibionts along the MAR (Petersen et al., 2009). The depth could also explain the clustering with the possible depth limit of 3000 m proposed previously (Priede et al., 2006). Some Epsilonproteobacteria and Gammaproteobacteria sequences retrieved from the Rainbow seawater sample were closely related (99% similarity) to epibiont sequences from the gill chamber of shrimps from the same site (Figure 3b, clusters 1, 5 and 7; Figure 3c, cluster-1). All of these results would indicate the existence of horizontal (environmental) transmission for the shrimp cephalothorax epibionts. Epibionts associated to egg mucus could also be a result of vertical transmission (from mother to offspring) through mucus secretion (Mira and Moran, 2002). Vertical transmission usually implies internalization of symbionts inside the egg or in oviducts (Bright and Bulgheresi, 2010). But microscopic observations (SEM, TEM and FISH) showed that (I) there were no active bacteria inside the eggs, but only associated with their outer surface (Figure 1c), and (II) no bacterial mat was observed associated with the young larvae just after hatching. The egg mucus interface probably facilitated attraction, accumulation and host recognition of epibionts for horizontal transmission. This epibiont transmission pathway is in adequacy with the large colonization of the MAR by R. exoculata because horizontal transmission is supposed to promote dispersal as compared with vertical transmission (Chaston and Goodrich-Blair, 2010). In terms of evolution, it was suggested that episymbiosis represents a more primitive stage than endosymbiosis (Dubilier et al., 2008). The internalization of symbionts would then represent the final step of the association. Nevertheless, a recent study based on 16S rRNA analyses demonstrated that bathymodiolin epibionts were not ancestral to bathymodiolin endosymbionts (Duperron et al., 2009a, 2009b). These authors suggested that the location of symbionts was not always a conserved trait and that both the host and the symbiotic bacteria were more versatile in their ability to establish associations than assumed previously.
It should be noted that only three gravid females were used for phylogenetic studies (Supplementary Table S2). More specimens of the first stages of the shrimp life cycle (notably free larvae at each developmental stage) are necessary for complementary analyses.
The methanotrophic metabolism hypothesis
Methanotrophic symbionts use methane as both an electron donor and a carbon source, with oxygen as the final electron acceptor. These symbionts have been described in deep-sea hydrothermal vents and cold seeps, where methane co-occurs with oxygen (Petersen and Dubilier, 2009). In situ observations showed that R. exoculata lives in the mixing zone between reduced hydrothermal fluid (containing methane at Rainbow and Logachev) and oxidized ambient seawater. Methane oxidation metabolism was suspected previously (Zbinden et al., 2008). To our knowledge, all 16S rRNA sequences of methanotrophic symbionts from marine invertebrates belong to a single monophyletic lineage within the Gammaproteobacteria phyla related to type-I methanotrophs. These bacteria are coccoid and have a concentric stacking of intracytoplasmic membranes where the methanotrophic enzymes are located (Hanson and Hanson, 1996). These membranes probably push back the cellular material (including ribosomes) to the cell periphery, explaining the characteristic circular FISH hybridization signal (Figure 2c). In this study, we have shown using molecular and microscopic approaches the presence of active type-I methanotrophic bacteria occurring in the cephalothorax of the Rainbow specimens (Figures 2c and 3c, cluster-2) and located at the base of the filamentous bacteria (Supplementary Figure S1). This result was congruent with the fluid composition of this site, which is highly enriched in methane (Charlou et al., 2002), and confirmed the result of a previous study (Zbinden et al., 2008). Regarding eggs, TEM observations revealed methanotroph-shaped bacteria associated with their membrane (Figure 1g), and sequences affiliated to the methanotrophic cluster were retrieved (Figure 3c, cluster-2). According to their small size, these cells might then be dormant, which could explain the absence of a FISH signal. Logachev, like Rainbow, is enriched in methane (Schmidt et al., 2007) (Supplementary Table S2). Therefore, methanotrophy might also occur at this site, but at a lower activity level. No methanotrophic-related sequence has been retrieved in the Rainbow seawater sample. This could be due to PCR bias; the low number of sequences treated; or could indicate that they were poorly present as free-living forms. Taken altogether, our results indicated the presence of methanotrophic bacteria associated with R. exoculata (eggs and adults) in two sites, reinforcing the symbiosis hypothesis. This could therefore be the first description of an epibiotic association between methanotrophic bacteria and hydrothermal vent crustaceans.
Conclusion
By describing the young larva just after hatching (Figure 4), we improved the knowledge of the R. exoculata life cycle. Nevertheless, the dispersion and recruitment of R. exoculata along the MAR vent sites still unknown (Figure 4). Like larval dispersion, symbiont transmission is obviously an integral factor influencing colonization efficiency (Teixeira et al., 2011). Our results indicated a possible horizontal transmission for the gill chamber epibionts of R. exoculata, which could explain colonization along the MAR.
We have also described for the first time epibiotic communities associated with eggs and different stages from adults, and highlighted a community switch between Gammaproteobacteria and Epsilonproteobacteria. By coupling molecular biological and microscopic approaches we have demonstrated the occurrence of type-I methanotrophic Gammaproteobacteria, one of three metabolisms (iron, sulfur and methane oxidation) expected to occur in the gill chamber (Zbinden et al., 2008). Our results indicated that the epibiotic community was globally conserved along the MAR. We suggest that the phylotype relative abundance and the activity of the epibionts could vary according to the shrimp life stage and the geochemical environment, reinforcing the symbiotic hypothesis. Future investigations will focus on identification (by PCR and reverse transcription-PCR) of functional genes implied in these different metabolisms. Deeper sequencing using high-throughput sequencing technologies would be useful to exhaust the diversity. Finally, more sampling in the aggregates and in the water column will be necessary to complete the shrimp life cycle, as well as incubation experiments using gravid females.
Acknowledgments
We thank Y Fouquet and F Gaill, respectively, chief scientists of the Serpentine and MoMARDREAM-Naut cruises, as well as the captain and the crew of the Pourquoi pas? and Nautile/Victor teams. We also thank M Perennou and S Romac from the ‘Plateforme Biogenouest' for sequencing work. TEM was undertaken by the Service de Microscopie Electronique, IFR 83 de Biologie Integrative—CNRS/Paris VI. We also thank I Probert and M Segonzac for advice and comments. This work was supported by Ifremer, CNRS, Brest Metropole Oceane, GDR ECCHIS and ANR Deep Oases.
Footnotes
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Amann RI, Krumholz L, Stahl DA. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol. 1990;172:762–770. doi: 10.1128/jb.172.2.762-770.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates AE, Harmer TL, Roeselers G, Cavanaugh CM. Phylogenetic characterization of episymbiotic bacteria hosted by a hydrothermal vent limpet (Lepetodrilidae, Vetigastropoda) Biol Bull. 2011;220:118–127. doi: 10.1086/BBLv220n2p118. [DOI] [PubMed] [Google Scholar]
- Bent SJ, Forney LJ. The tragedy of the uncommon: understanding limitations in the analysis of microbial diversity. ISME J. 2008;2:689–695. doi: 10.1038/ismej.2008.44. [DOI] [PubMed] [Google Scholar]
- Brazelton WJ, Schrenk MO, Kelley DS, Baross JA. Methane- and sulfur-metabolizing microbial communities dominate the Lost City hydrothermal field ecosystem. Appl Environ Microbiol. 2006;72:6257–6270. doi: 10.1128/AEM.00574-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright M, Bulgheresi S. A complex journey: transmission of microbial symbionts. Nat Rev Microbiol. 2010;8:218–230. doi: 10.1038/nrmicro2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova B, Brunet M, Segonzac M. Impact of bacterial epibiosis on functional-morphology of shrimp associated with the Mid-Atlantic hydrothermal conditions. Cah Biol Mar. 1993;34:573–588. [Google Scholar]
- Charlou JL, Donval JP, Fouquet Y, Jean-Baptiste P, Holm N. Geochemistry of high H2 and CH4 vent fluids issuing from ultramafic rocks at the Rainbow hydrothermal field (36°14′N, MAR) Chem Geol. 2002;191:345–359. [Google Scholar]
- Chaston J, Goodrich-Blair H. Common trends in mutualism revealed by model associations between invertebrates and bacteria. FEMS Microbiol Rev. 2010;34:41–58. doi: 10.1111/j.1574-6976.2009.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copley CEA, Tyler PA, Varney MS. Lipid profiles of hydrothermal vent shrimps. Cah Biol Mar. 1998;39:229–231. [Google Scholar]
- Corbari L, Zbinden M, Cambon-Bonavita MA, Gaill F, Compère P. Bacterial symbionts and mineral deposits in the branchial chamber of the hydrothermal vent shrimp Rimicaris exoculata: relationship to moult cycle. Aquat Biol. 2008;1:225–238. [Google Scholar]
- Costello AM, Lidstrom ME. Molecular characterization of functional and phylogenetic genes from natural populations of methanotrophs in lake sediments. Appl Environ Microbiol. 1999;65:5066–5074. doi: 10.1128/aem.65.11.5066-5074.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JM, Viney C. Water–mucin phases: conditions for mucus liquid crystallinity. Thermochim Acta. 1998;315:39–49. [Google Scholar]
- Desbruyères D, Biscoito M, Caprais JC, Colaço A, Comtet T, Crassous P, et al. Variations in deep-sea hydrothermal vent communities on the Mid-Atlantic Ridge near the Azores plateau. Deep Sea Res I. 2001;48:1325–1346. [Google Scholar]
- Douville E, Charlou JL, Oelkers EH, Bienvenu P, Colon CFJ, Donval JP, et al. The Rainbow vent fluids (36°14′N, MAR): the influence of ultramafic rocks and phase separation on trace metal content in Mid-Atlantic Ridge hydrothermal fluids. Chem Geol. 2002;184:37–48. [Google Scholar]
- Dubilier N, Bergin C, Lott C. Symbiotic diversity in marine animals: the art of harnessing chemosynthesis. Nat Rev Microbiol. 2008;6:725–740. doi: 10.1038/nrmicro1992. [DOI] [PubMed] [Google Scholar]
- Duperron S, De Beer D, Zbinden M, Boetius A, Schipani V, Kahil N, et al. Molecular characterization of bacteria associated with the trophosome and the tube of Lamellibrachia sp., a siboglinid annelid fromcold seeps in the eastern Mediterranean. FEMS Microbiol Ecol. 2009a;69:395–409. doi: 10.1111/j.1574-6941.2009.00724.x. [DOI] [PubMed] [Google Scholar]
- Duperron S, Lorion J, Samadi S, Gros O, Gaill F. Symbioses between deep-sea mussels (Mytilidae: Bathymodiolinae) and chemosynthetic bacteria: diversity, function and evolution. C R Biol. 2009b;332:298–310. doi: 10.1016/j.crvi.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Duperron S, Nadalig T, Caprais JC, Sibuet M, Fiala-Medioni A, Amann R, et al. Dual symbiosis in a Bathymodiolus sp mussel from a methane seep on the Gabon continental margin (southeast Atlantic): 16S rRNA phylogeny and distribution of the symbionts in gills. Appl Environ Microbiol. 2005;71:1694–1700. doi: 10.1128/AEM.71.4.1694-1700.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand L, Zbinden M, Cueff-Gauchard V, Duperron S, Roussel EG, Shillito B, et al. Microbial diversity associated with the hydrothermal shrimp Rimicaris exoculata gut and occurrence of a resident microbial community. FEMS Microbiol Ecol. 2010;71:291–303. doi: 10.1111/j.1574-6941.2009.00806.x. [DOI] [PubMed] [Google Scholar]
- Fabri MC, Bargain A, Briand P, Gebruk A, Fouquet Y, Morineaux M, et al. The hydrothermal vent community of a new deep-sea field, Ashadze-1, 12°58′N on the Mid-Atlantic Ridge. J Mar Biol Assoc UK. 2011;91:1–13. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Galtier N, Gouy M, Gautier C. SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput Appl Biosci. 1996;12:543–548. doi: 10.1093/bioinformatics/12.6.543. [DOI] [PubMed] [Google Scholar]
- Gebruk AV, Pimenov NV, Savvichev AS. Feeding specialization of bresilid shrimps in the TAG site hydrothermal community. Mar Ecol Prog Ser. 1993;98:247–253. [Google Scholar]
- Gilturnes MS, Fenical W. Embryos of Homarius americanus are protected by epibiotic bacteria. Biol BuII. 1992;182:105–108. doi: 10.2307/1542184. [DOI] [PubMed] [Google Scholar]
- Goffredi SK. Indigenous ectosymbiotic bacteria associated with diverse hydrothermal vent invertebrates. Environ Microbiol Rep. 2010;2:479–488. doi: 10.1111/j.1758-2229.2010.00136.x. [DOI] [PubMed] [Google Scholar]
- Goffredi SK, Waren A, Orphan VJ, Van Dover CL, Vrijenhoek RC. Novel forms of structural integration between microbes and a hydrothermal vent gastropod from the Indian Ocean. Appl Environ Microbiol. 2004;70:3082–3090. doi: 10.1128/AEM.70.5.3082-3090.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good IJ. The population frequencies of species and the estimation of population parameters. Biometrika. 1953;40:237–264. [Google Scholar]
- Grabovich MY, Muntyan MS, Lebedeva VY, Ustiyan VS, Dubinina GA. Lithoheterotrophic growth and electron transfer chain components of the filamentous gliding bacterium Leucothrix mucor DSM 2157 during oxidation of sulfur compounds. FEMS Microbiol Lett. 1999;178:155–161. [Google Scholar]
- Hanson RS, Hanson TE. Methanotrophic bacteria. Microbiol Rev. 1996;60:439–471. doi: 10.1128/mr.60.2.439-471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hügler M, Petersen JM, Dubilier N, Imhoff JF, Sievert SM. Pathways of carbon and energy metabolism of the epibiotic community associated with the deep-sea hydrothermal vent shrimp Rimicaris exoculata. PLoS Biol. 2011;6:e16018. doi: 10.1371/journal.pone.0016018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki F, Takai K, Nealson KH, Horikoshi K. Sulfurovum lithotrophicum gen. nov., sp. nov., a novel sulfur-oxidizing chemolithoautotroph within the e-Proteobacteria isolated from Okinawa Trough hydrothermal sediments. Int J Syst Evol Microbiol. 2004;54:1477–1482. doi: 10.1099/ijs.0.03042-0. [DOI] [PubMed] [Google Scholar]
- Komai T, Segonzac M. Taxonomic review of the hydrothermal vent shrimp genera Rimicaris Williams & Rona and Chorocaris Martin & Hessler (Crustacea: Decapoda: Caridea: Alvinocarididae) J Shellfish Res. 2008;27:21–41. [Google Scholar]
- Lane D. 16S/23S rRNA sequencing. Nucleic Acid Techn Bact Syst. 1991;1:115–176. [Google Scholar]
- Lin X, Wakeham SG, Putnam IF, Astor YM, Scranton MI, Chistoserdov AY, et al. Comparison of vertical distributions of prokaryotic assemblages in the anoxic Cariaco Basin and Black Sea by use of fluorescence in situ hybridization. Appl Environ Microbiol. 2006;72:2679–2690. doi: 10.1128/AEM.72.4.2679-2690.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llodra ER, Tyler PA, Copley JTP. Reproductive biology of three caridean shrimp, Rimicaris exoculata, Chorocaris chacei and Mirocaris fortunata (Carudea: Decapoda), from hydrothermal vents. J Mar Biol Assoc UK. 2000;80:473–484. [Google Scholar]
- Lopez-Garcia P, Duperron S, Philippot P, Foriel J, Susini J, Moreira D. Bacterial diversity in hydrothermal sediment and epsilon proteobacterial dominance in experimental microcolonizers at the Mid-Atlantic Ridge. Environ Microbiol. 2003;5:961–976. doi: 10.1046/j.1462-2920.2003.00495.x. [DOI] [PubMed] [Google Scholar]
- Loy A, Lehner A, Lee N, Adamczyk J, Meier H, Ernst J, et al. Oligonucleotide microarray for 16S rRNA gene-based detection of all recognized lineages of sulfate-reducing prokaryotes in the environment. Appl Environ Microbiol. 2002;68:5064–5081. doi: 10.1128/AEM.68.10.5064-5081.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz W, Amann R, Ludwig W, Vancanneyt M, Schleifer KH. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum cytophaga-flavobacter-bacteroides in the natural environment. Microbiology. 1996;142:1097–1106. doi: 10.1099/13500872-142-5-1097. [DOI] [PubMed] [Google Scholar]
- Manz W, Amann R, Ludwig W, Wagner M, Schleifer KH. Phylogenetic oligodeoxynucleotide probes for the major subclasses of Proteobacteria: problems and solutions. Syst Appl Microbiol. 1992;15:593–600. [Google Scholar]
- Mira A, Moran NA. Estimating population size and transmission bottlenecks in maternally transmitted endosymbiotic bacteria. Microbial Ecol. 2002;44:137–143. doi: 10.1007/s00248-002-0012-9. [DOI] [PubMed] [Google Scholar]
- Muyzer G, Teske A, Wirsen CO, Jannasch HW. Phylogenetic relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturing gradient gel electrophoresis of 16S rDNA fragments. Arch Microbiol. 1995;164:165–172. doi: 10.1007/BF02529967. [DOI] [PubMed] [Google Scholar]
- Nussbaumer AD, Bright M, Baranyi C, Beisser CJ, Ott JA. Attachment mechanism in a highly specific association between ectosymbiotic bacteria and marine nematodes. Aquat Microb Ecol. 2004;34:239–246. [Google Scholar]
- Petersen JM, Dubilier N. Methanotrophic symbioses in marine invertebrates. Environ Microbiol. 2009;1:319–335. doi: 10.1111/j.1758-2229.2009.00081.x. [DOI] [PubMed] [Google Scholar]
- Petersen JM, Ramette A, Lott C, Cambon-Bonavita MA, Zbinden M, Dubilier N. Dual symbiosis of the vent shrimp Rimicaris exoculata with filamentous gamma- and epsilonproteobacteria at four Mid-Atlantic Ridge hydrothermal vent fields. Environ Microbiol. 2009;12:2204–2218. doi: 10.1111/j.1462-2920.2009.02129.x. [DOI] [PubMed] [Google Scholar]
- Polz MF, Cavanaugh CM. Dominance of one bacterial phylotype at a Mid-Atlantic Ridge hydrothermal vent site. Proc Natl Acad Sci USA. 1995;92:7232–7236. doi: 10.1073/pnas.92.16.7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polz MF, Robinson JJ, Cavanaugh CM, Van Dover CL. Trophic ecology of massive shrimp aggregations at a Mid-Atlantic Ridge hydrothermal vent site. Limnol Oceanogr. 1998;43:1631–1638. [Google Scholar]
- Pond DW, Gebruk A, Southward EC, Southward AJ, Fallick AE, Bell MV, et al. Unusual fatty acid composition of storage lipids in the bresilioid shrimp Rimicaris exoculata couples the photic zone with MAR hydrothermal vent sites. Mar Ecol Prog Ser. 2000;198:171–179. [Google Scholar]
- Pond DW, Segonzac M, Bell MV, Dixon DR, Fallick AE, Sargent JR. Lipid and lipid carbon stable isotope composition of the hydrothermal vent shrimp Mirocaris fortunata: evidence for nutritional dependence on photosynthetically fixed carbon. Mar Ecol Prog Ser. 1997;157:221–231. [Google Scholar]
- Priede IG, Froese R, Bailey DM, Bergstad OA, Collins MA, Dyb JE, et al. The absence of sharks from abyssal regions of the world's oceans. Proc Biol Sci. 2006;273:1435–1441. doi: 10.1098/rspb.2005.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu XY, Wu LY, Huang HS, McDonel PE, Palumbo AV, Tiedje JM, et al. Evaluation of PCR-generated chimeras: mutations, and heteroduplexes with 16S rRNA gene-based cloning. Appl Environ Microbiol. 2001;67:880–887. doi: 10.1128/AEM.67.2.880-887.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravenschlag K, Sahm K, Pernthaler J, Amann R. High bacterial diversity in permanently cold marine sediments. Appl Environ Microbiol. 1999;65:3982–3989. doi: 10.1128/aem.65.9.3982-3989.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieley G, Van Dover CL, Hedrick DB, Eglinton G. Trophic ecology of Rimicaris exoculata: a combined lipid abundance stable isotope approach. Mar Biol. 1999;133:495–499. [Google Scholar]
- Ruehland C, Dubilier N. Gamma- and epsilonproteobacterial ectosymbionts of a shallow-water marine worm are related to deep-sea hydrothermal vent ectosymbionts. Environ Microbiol. 2010;12:2312–2326. doi: 10.1111/j.1462-2920.2010.02256.x. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schloss PD, Handelsman J. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol. 2005;71:1501–1506. doi: 10.1128/AEM.71.3.1501-1506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Le Bris N, Gaill F. Interactions of deep-sea vent invertebrates with their environment: the case of Rimicaris exoculata. J Shellfish Res. 2008;27:79–90. [Google Scholar]
- Schmidt K, Koschinsky A, Garbe-Schönberg D, de Carvalho LM, Seifert R. Geochemistry of hydrothermal fluids from the ultramafic-hosted Logatchev hydrothermal field, 15°N on the Mid-Atlantic Ridge: temporal and spatial investigation. Chem Geol. 2007;242:1–21. [Google Scholar]
- Segonzac M. The hydrothermal vent communities of Snake Pit area (Mid-Atlantic Ridge, 23°N, 3480 m)—megafaunal composition and distribution. C R Acad Sci Paris Life Sci. 1992;314:593–600. [Google Scholar]
- Segonzac M, Saint-Laurent M, Casanova B. Enigma of the trophic adaptation of the shrimp Alvinocarididae in hydrothermal areas along the Mid-Atlantic Ridge. Cah Biol Mar. 1993;34:535–571. [Google Scholar]
- Shillito B, Le Bris N, Gaill A, Rees JF, Zal F. First access to live Alvinella. High Pressure Res. 2004;24:169–172. [Google Scholar]
- Stahl D, Amann R.1991Development and application of nucleic acid probesIn: Stackebrandt E, Goodfellow M (eds). Nucleic Acid Techniques in Bacterial Systematics Wiley Inc.: New York; 205–247. [Google Scholar]
- Teixeira S, Cambon-Bonavita MA, Serrão EA, Desbruyéres D, Arnaud-Haond S. Recent population expansion and connectivity in the hydrothermal shrimp Rimicaris exoculata along the Mid-Atlantic Ridge. J Biogeography. 2011;38:564–574. [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. ClustalW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda G, Yamada A, Nakano K, Arita NO, Yamasaki H. Colonization of Sulfurovum sp on the gill surfaces of Alvinocaris longirostris, a deep-sea hydrothermal vent shrimp. Mar Ecol. 2008;29:106–114. [Google Scholar]
- Tyler PA, Young CM. Reproduction and dispersal at vents and cold seeps. J Mar Biol Assoc UK. 1999;79:193–208. [Google Scholar]
- Van Dover CL, Fry B, Grassle JF, Humphris S, Rona PA. Feeding biology of the shrimp Rimicaris exoculata at hydrothermal vents on the Mid-Atlantic Ridge. Mar Biol. 1988;98:209–216. [Google Scholar]
- Vereshchaka AL, Vinogradov GM, Ivanenko VN. General properties of the reproductive biology of some hydrothermal crustacea (shrimp, Amphipods, Copepods) Doklady Biol Sci. 1998;360:269–270. [Google Scholar]
- Williams AB, Rona PA. Two new caridean shrimps (Bresilidae) from a hydrothermal field on the Mid-Atlantic Ridge. J Crust Biol. 1986;6:446–462. [Google Scholar]
- Zbinden M, Cambon-Bonavita MA. Occurrence of Deferribacterales and Entomoplasmatales in the deep-sea Alvinocarid shrimp Rimicarisexoculata gut. FEMS Microbiol Ecol. 2003;46:23–30. doi: 10.1016/S0168-6496(03)00176-4. [DOI] [PubMed] [Google Scholar]
- Zbinden M, Le Bris N, Gaill F, Compère P. Distribution of bacteria and associated minerals in the gill chamber of the vent shrimp Rimicaris exoculata and related biogeochemical processes. Mar Ecol Prog Ser. 2004;284:237–251. [Google Scholar]
- Zbinden M, Shillito B, Le Bris N, Villardi de Montlaur C, Roussel E, Guyot F, et al. New insights on the metabolic diversity among the epibiotic microbial community of the hydrothermal shrimp Rimicaris exoculata. J Exp Mar Biol Ecol. 2008;359:131–140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.