Abstract
To provide a stable environmental barrier, the epidermis requires an integrated network of cytoskeletal elements and cellular junctions. Nevertheless, the epidermis ranks among the body’s most dynamic tissues, continually regenerating itself and responding to cutaneous insults. As keratinocytes journey from the basal compartment towards the cornified layers, they completely reorganize their adhesive junctions and cytoskeleton. These architectural components are more than just rivets and scaffolds — they are active participants in epidermal morphogenesis that regulate epidermal polarization, signalling and barrier formation.
The epidermis is a highly specialized epithelium that has evolved to perform multiple essential protective functions: it prevents water loss, excludes toxins, resists mechanical stress and participates in immune responses. To establish a barrier between the organism and its environment, keratinocytes, the main cells of the epidermis, form an adhesive network organized into multiple layers, or ‘strata’ (REFS 1,2) (FIG. 1). This presents us with a paradox: although the tissue exhibits incredible stability that shields underlying organs from external insults, its cellular components must remain dynamic to allow tissue regeneration and response to cutaneous insults.
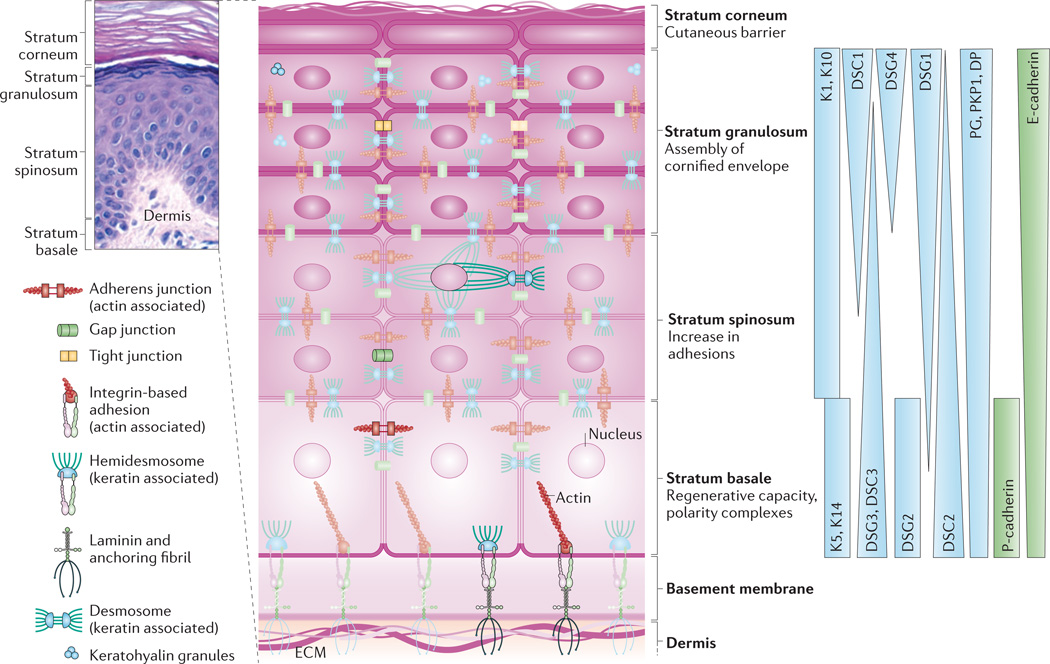
Figure 1. Epidermal architecture.
The epidermis is composed of stratified cell layers, which undergo programmed differentiation to allow for constant renewal of the skin. Four main layers are illustrated by a hematoxylin- and eosin-stained human skin sample and an accompanying schematic of the stratum basale, stratum spinosum, stratum granulosum and stratum corneum. The basal, proliferating cell layer of the epidermis remains in contact with the dermis through hemidesmosomes and integrin-based adhesions, both of which provide connections to the underlying extracellular matrix (ECM). During keratinocyte differentiation, a unique cytoarchitecture is elaborated in each of the four layers that comprises specific cytoskeleton and cell junction types, including adherens junctions, tight junctions, desmosomes and gap junctions. The differentiation-dependent changes in the composition and organization of epidermal cytoarchitecture help to drive tissue morphogenesis while supporting the specific functions of each layer, from the regenerative capacity of the stratum basale to the assembly of the cornified envelope and the sloughing of terminally differentiated cells from the stratum corneum. The graded distribution of specific cytoskeletal and junction components, including specific keratins (Ks), desmogleins (DSGs) and cadherins, is crucial for driving morphogenesis. Image in top left courtesy of R. Lavker, Northwestern University, USA. DP, desmoplakin; DSC, desmocollin; E-cadherin, epithelial cadherin; P-cadherin, placental cadherin; PG, plakoglobin; PKP1, plakophilin 1.
Keratinocytes undergo a dramatic transformation as they differentiate and migrate outwards to replace cells that are shed from the body surface1. While basal cells of the ‘stratum basale’ remain attached to an underlying matrix and proliferate, some of their daughter keratinocytes enter the spinous layer (or ‘stratum spinosum’) through asymmetric mitoses, where they exit the cell cycle, grow larger and establish robust intercellular connections. Cells in the granular layer (the ‘stratum granulosum’) flatten and assemble a water-impermeable cornified envelope underlying the plasma membrane. Finally, corneal layer (or ‘stratum corneum’) keratinocytes release lysosomal enzymes to degrade major organelles, become completely squamous and are tightly crosslinked together to complete the cutaneous barrier2. Thus, the mature epidermis exhibits tissue-level polarization with asymmetric distribution of signalling activity, protein expression and cytoarchitectural organization that reflects the unique functions of its multiple layers.
The embryonic epidermis begins as a single layer of ectodermal cells that then undergo stratification to construct a multilayered epithelium in response to specific transcription factors3. Once constructed, the epidermis is maintained by continual stratification and differentiation of keratinocytes throughout adult life. A number of outstanding articles have reviewed the factors that mediate epidermal development and homeostasis1,4. These factors include cytoskeletal building blocks and their associated intercellular junctions, which have classically been thought to serve as the scaffold and rivets that impart structural integrity to the epidermis. However, these elements of cell architecture are themselves increasingly being viewed as active participants in epidermal morphogenesis.
Various junctional and cytoskeletal proteins control the regenerative capacity of the epidermis by affecting maintenance of the stem cell population that resides primarily in the hair follicles and the stratum basale (reviewed in REF. 5). In this Review, we chronicle the journey of the interfollicular keratinocyte through the epidermal layers. Along the way, we highlight how the cytoplasmic and cortical cytoskeleton and associated adhesion molecules provide instructions for epidermal morphogenesis. We review their contributions to the establishment of polarity and to the alterations in cell signalling, morphology and protein expression that drive the keratinocyte from the basal compartment to the cornified layers. Finally, we discuss how these advances at the cellular and molecular level have improved our understanding of human disease.
Adhesion at the basement membrane
The boundary between the epidermis and the underlying dermis is established by deposition of a specialized layer of extracellular matrix (ECM) called the basement membrane6. Keratinocytes of the basal layer are inherently polarized as their lower surface is anchored to the basement membrane by integrins (FIG. 2). These heterodimeric transmembrane receptors, consisting of α- and β-integrin subunits, bind specific ECM components via their extracellular domains7. At least 11 integrin dimers have been described in epidermal keratinocytes8. On the intracellular side, most integrin tails associate with actin via adaptor proteins. However, α6β4 integrin has a unique role in organizing large integrin complexes called hemidesmosomes, which are tethered to intermediate filaments by the cytolinker proteins plectin and bullous pemphigoid antigen 1e (BPAG1e; also known as BP230 and dystonin)6. A range of human disorders result from integrin-based adhesion being compromised by mutation or by autoantibodies that target specific integrins, integrin-associated proteins or matrix components; such disorders are characterized by varying degrees of epidermal fragility and blistering (TABLE 1 and Supplementary information S1 (table)).
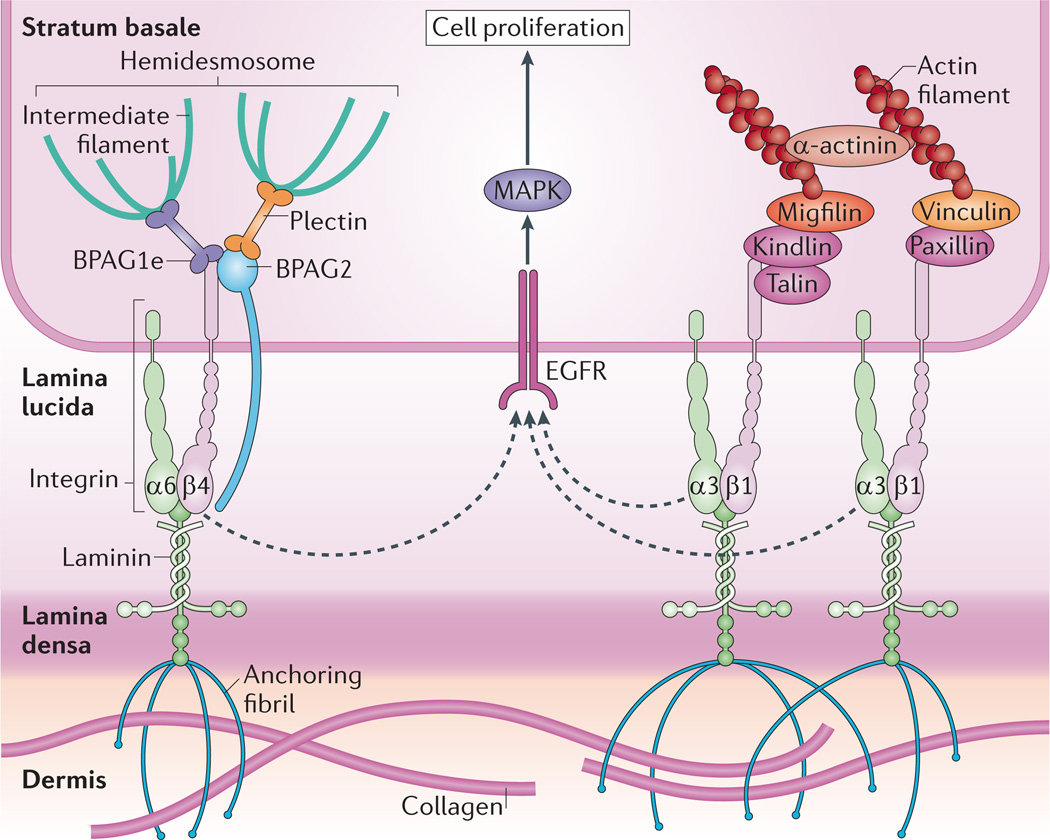
Figure 2. Integrins crosstalk with growth factor receptors to regulate proliferation in the basal layer.
The stratum basale has a high density of hemidesmosomes and integrin-based adhesions that maintain cell attachment to the underlying basement membrane, which is composed of the lamina lucida and lamina densa. Hemidesmosomes connect to the basement membrane through α6β4 integrins and the transmembrane protein bullous pemphigoid antigen 2 (BPAG2; also known as collagen XVII), and are tethered to intermediate filaments by the plakin family members plectin and BPAG1e6. α3β1 integrins provide transmembrane connections to the intracellular actin network through recruitment of several factors to their cytoplasmic tails. Integrins are thought to crosstalk with receptors such as EGFR (epidermal growth factor receptor) to induce proliferation of cells in the stratum basale via mitogen-activated protein kinase (MAPK) signalling. As cells stratify, decreased integrin density allows cell cycle exit and differentiation.
Table 1.
Human diseases of the epidermis*
| Molecular target‡ | Disease | Phenotype |
|---|---|---|
| Gap junctions | ||
| CX26 | Keratitis ichthyosis deafness syndrome and hystrix-like ichthyosis deafness syndrome | Vascularizing keratitis; progressive erythrokeratoderma; sensorineural hearing loss |
| Vohwinkel’s syndrome (keratoderma hereditaria mutilans) | Keratoderma of palmoplantar surfaces; circumferential hyperkeratosis of digits leading to autoamputation; moderate sensorineural hearing loss | |
| Bart–Pumphrey syndrome | Hyperkeratosis of knuckle pads; sensorineural hearing loss | |
| CX30 | Clouston syndrome (hidrotic ectodermal dysplasia) | Palmoplantar hyperkeratosis; hair defects (partial to total alopecia); nail deformities |
| CX30.3, CX31 | Erythrokeratodermia variabilis | Local or diffuse hyperkeratosis; migratory erythematous patches |
| Adherens junctions | ||
| P-cadherin (encoded by CDH3) | Hypotrichosis with juvenile macular dystrophy | Hair loss; progressive macular degeneration and early blindness |
| Ectodermal dysplasia, ectrodactyly, macular degeneration syndrome | Hypotrichosis with partial adontia; absence deformities and syndactyly; atrophy of retinal pigment epithelium | |
| Keratin intermediate filaments | ||
| K4, K13 | White sponge nevus | Spongy white plaques, often on buccal mucosa |
| K9 | Epidermolytic palmoplantar keratoderma | Epidermolysis and hyperkeratosis of palms and soles |
| K6, K16, K17 | Pachyonychia congenita | Painful blisters on hands and feet; thickened nails; hyperkeratosis of hair follicles; leukokeratosis of oral mucosa |
| K2e | Ichthyosis bullosa of Siemens | Bullous ichthyosis without erythroderma; epidermolysis limited to upper suprabasal layers |
| K1, K10 | Bullous congenital ichthyosis erythroderma (epidermolytic hyperkeratosis) | Generalized erythema; erosions and blisters owing to fragility of suprabasal keratinocytes |
| K5, K14 | Epidermolysis bullosa simplex | Skin blistering from fragility of basal keratinocytes |
| Desmosomes | ||
| DSG1 | Bullous impetigo | Epidermal blisters at the granular layer caused by a bacterial protease |
| Staphylococcal scalded skin syndrome | ||
| Pemphigus foliaceus | Epidermal blisters at the granular layer caused by autoantibodies | |
| DSG3 | Pemphigus vulgaris | Epidermal blisters at the basal–suprabasal cell interface caused by autoantibodies |
| DSG4 | Hypotrichosis | Sparse, fragile hair with abnormal hair follicles; epidermal hyperproliferation |
| DSC3 | Hypotrichosis with skin vesicles | Sparse, fragile hair with normal hair follicles; recurrent skin vesicles |
| DSC2 | Arrhythmogenic right ventricular cardiomyopathy with mild palmoplantar keratoderma and woolly hair | Ventricular arrhythmias with fibro-fatty replacement of heart tissue; thickening of palms and soles; tightly coiled hair |
| DP, DSG1 | Striate palmoplantar keratoderma | Linear and focal hyperkeratosis of palms and soles |
| DP | Carvajal syndrome | Epidermolytic palmoplantar keratoderma with woolly hair and dilated cardiomyopathy |
| Lethal acantholytic epidermolysis bullosa | Acantholysis and shedding of skin at birth, leading to early death | |
| PKP1 | Ectodermal dysplasia-skin fragility syndrome | Skin fragility; plantar keratoderma; nail dystrophy; alopecia |
| PG | Naxos disease | Woolly hair; palmoplantar keratoderma; arrhythmogenic right ventricular cardiomyopathy |
| Lethal congenital epidermolysis bullosa | Lethal epidermal blistering at birth | |
| Hemidesmosomes | ||
| Laminins, integrins, BPAG2 | Junctional epidermolysis bullosa | Generalized blistering at dermal–epidermal junction due to either congenital mutation or acquired autoantibodies |
| BPAG1e | Epidermolysis bullosa simplex | Trauma-induced epidermal blisters and episodic limb numbness |
| BPAG2 | Bullous pemphigoid | Fluid-filled subepidermal blisters with deroofing of epidermis caused by autoantibodies |
| Stratum corneum | ||
| ABCA12 (lipid transport) | Harlequin ichthyosis | Hard, thickened skin with fissures |
| Filaggrin | Ichthyosis vulgaris | Dry skin; mild hyperkeratosis |
| CDSN | Hypotrichosis simplex of scalp | Childhood-onset loss of hair |
| Generalized peeling skin syndrome | Peeling of the skin and itching | |
| SPINK5 (also known as LEKT1; serine protease inhibitor) | Netherton syndrome | Exfoliative erythroderma; hair abnormalities; atopic manifestations |
| TGase 1 | Lamellar ichthyosis | Newborns covered with colloid membrane that is later shed; erythema with white scales; hyperkeratosis of palms and soles |
| Loricrin | Vohwinkel’s syndrome (keratoderma hereditaria mutilans) | Keratoderma of palmoplantar surfaces; circumferential hyperkeratosis of digits leading to autoamputation; moderate sensorineural hearing loss |
| Progressive symmetric erythrokeratodermia | Hyperpigmented, hyperkeratotic plaques with symmetrical growth | |
| Other | ||
| ATP2C1 (calcium pump) | Hailey–Hailey disease | Outbreaks of rashes and blisters, usually in skin folds |
| ATP2A2 (calcium pump) | Darier’s disease | Acantholysis; abnormal keratinization; greasy, hyperkeratotic papules |
| Collagen VII | Dystrophic epidermolysis bullosa | Severe blistering with atrophic scarring |
| Kindlin 1 | Kindler syndrome | Skin atrophy with blistering at dermal–epidermal junction |
ABCA12, ATP-binding cassette, sub-family A, member 12; ATP2A2, sarcoplasmic/endoplasmic reticulum calcium ATPase 2; ATP2C1, calcium-transporting ATPase type 2C member 1; BPAG, bullous pemphigoid antigen; CDH3, cadherin 3; CDSN, corneodesmosin; CX, connexin; DP, desmoplakin; DSC, desmocollin; DSG, desmoglein; K, keratin; PG, plakoglobin; PKP1, plakophilin 1; TGase 1, transglutaminase 1.
A version of this table including references is available in Supplementary information S1 (table).
Listed molecular targets are affected by gene mutation in these diseases unless otherwise specified.
Intriguingly, integrin complexes also contain signalling proteins and are well positioned to transduce external cues into intracellular signals. Studies are beginning to suggest a broader role for integrins and their associated proteins in regulating epidermal homeostasis and basement membrane organization. However, the extent to which the functions of integrins in tissue integrity can truly be separated from their roles in morphogenesis and homeostasis remains controversial8.
Integrins in epidermal homeostasis
Homeostasis of the epidermis requires precise regulation of basal cell proliferation to offset keratinocyte loss through sloughing of the cornified layer4. Early in vitro experiments indicated that releasing keratinocytes from an underlying matrix induced cell cycle withdrawal and expression of differentiation markers9,10. In intact epidermis, keratinocytes cease proliferating as they leave the basal layer, which coincides with loss of contact with the basement membrane and suppression of integrin expression. Thus, it has been hypothesized that stratification-associated downregulation of integrins dampens proliferation in the suprabasal epidermal layers7,8. Under normal circumstances, mitogenic signalling from epidermal growth factor receptor (EGFR) via the mitogen-activated protein kinase (MAPK) pathway is limited to basal keratinocytes, which express abundant integrins11,12 (FIG. 2). Integrins can crosstalk with receptor tyrosine kinases, including EGFR, providing a possible mechanism for how they may regulate proliferation8,13,14. In fact, integrin ligation by certain ECM molecules leads to MAPK activation in vitro12,15.
In mice, deletion of β4 integrin prohibits formation of hemidesmosomes, resulting in severe blistering16,17. Loss of β4 integrin also appeared to induce apoptosis of basal cells16, whereas deleting the cytoplasmic tail of β4 impairs proliferation18, together suggesting that hemidesmosomes regulate tissue homeostasis. Conversely, tamoxifen-induced deletion of α6 integrin, the partner of β4 in hemidesmosomes, increases proliferation and skin inflammation19. Nevertheless, all of these effects could simply be secondary to defective basement membrane adhesion. Consistent with this, mice lacking α6 and α3 integrin in the skin show normal morphogenesis and differentiation in all non-blistered epidermis20. Similarly, mosaic epidermal deletion of β4 integrin disrupted proliferation only in areas of basement membrane detachment21.
Integrin expression is seen beyond the basal layer in hyperproliferative skin in humans, such as in psoriasis and carcinomas14,22,23. Accordingly, ectopic integrin expression in mice increases proliferation and impairs differentiation. For example, ectopic expression of β1 integrin in suprabasal cells leads to a phenotype resembling psoriasis24, and expression of α6β4 or α5β1 integrin in the suprabasal compartment increases susceptibility to epidermal carcinogenesis25,26. Conversely, deletion of β1 integrin in skin produces a hypoproliferative epidermis with more differentiating keratinocytes than in normal epidermis27,28, although it also results in extensive epidermal blistering. Inducible deletion of β1 integrin in adult epidermis results in hyperproliferation in targeted areas of the skin29. So although integrin deletion or mis-expression may alter proliferation, it remains difficult to dissect the potential homeostatic function of integrins from their role in maintaining skin integrity.
Forming the basement membrane
Keratinocytes can secrete ECM components, including collagen IV and laminin 5, to help establish and organize the matrix, an ability that depends on integrins themselves6,30. Total loss of α3 integrin results in neonatal lethality and subepidermal blistering owing to a discontinuous basement membrane between hemidesmosomes31. Epidermal-specific deletion of α3 integrin results in duplicated areas of the basement membrane and microblisters between the stratum basale and the dermis (the dermal– epidermal junction)32. Although these animals differentiate and proliferate normally, loss of α3 integrin actually accelerated wound sealing, suggesting that defective basement membrane adhesion may allow more efficient migration of keratinocytes into wound beds. In addition to causing thinning of the epidermis and blistering, deletion of β1 integrin in mice results in a defective basement membrane, with large areas of the skin lacking a subepidermal matrix27. Mice deficient in integrin-linked kinase (ILK), a cytoplasmic pseudokinase associated with integrins, have similar abnormalities33. In addition to epidermal hyperplasia and impaired differentiation, ILK-deficient mice display a discontinuous basement membrane. Thus, integrin complexes are crucial for proper morphogenesis of the basement membrane.
Kindlin 1 regulates epidermal integrity and homeostasis
A novel epidermal function has been uncovered for kindlins, which are β-integrin-binding proteins that are thought to cooperate with talin to regulate integrin-based adhesion34 (FIG. 2). Kindlin 1 (also known as FERMT1), which is highly expressed in the epidermis35, is targeted in Kindler syndrome, an autosomal recessive disease characterized by transient perinatal blistering at the dermal–epidermal junction followed by longer term skin atrophy36. In patients with Kindler syndrome, the basement membrane is grossly disorganized with both aberrant thickening and substantial gaps in the matrix (or lamina densa) between the basal keratinocytes and the dermis37. Thus, kindlin 1 seems to be essential for organizing the epidermal ECM. Although it has no intrinsic enzymatic activity, kindlin 1 probably functions as a scaffold in integrin complexes and may reorganize microfilaments through its association with α-actinin and migfilin, two actin-binding proteins38,39.
Genetic ablation of kindlin 1 in mice results in skin atrophy with reduced epidermal proliferation and thickness40. Keratinocytes isolated from patients with Kindler syndrome have cell autonomous defects in proliferation and apoptosis, suggesting that kindlin 1 may directly affect signalling41. Patients with Kindler syndrome are also more prone to epidermal carcinogenesis later in life, implying that kindlin 1 may affect long-term skin homeostasis34. Surprisingly, mice lacking kindlin 1 do not develop epidermal blisters, although keratinocytes from these mice show reduced substrate adhesion in vitro40. Instead, the animals die from sloughing of the intestinal epithelium owing to faulty substratum adhesion; thus, kindlin 1 may have an additional role in maintaining the integrity of the gut, another highly regenerative organ. Interestingly, kindlin 2 (also known as FERMT2), which is also expressed in the epidermis, colocalizes with epithelial cadherin (E-cadherin; also known as cadherin 1) at intercellular contacts35 and is required for motility and intercellular adhesion in keratinocytes42. The potential function of kindlin 2 in epidermal integrity and morphogenesis remains unexplored.
Serum response factor promotes differentiation
During stratification, basal keratinocytes produce suprabasal cells that no longer have cell–matrix adhesions, a process that completely alters cell polarity and cytoskeletal architecture7,43. In isolated keratinocytes, actin associates with integrin-based adhesions, but then assembles into a robust cortical actin ring during stratification in vitro44–46. Moreover, manipulating the area or shape of keratinocyte adhesion on micropatterned substrates coated with ECM molecules affects their differentiation47, and this effect is not altered by the ECM component used.
Serum response factor (SRF) signalling has been proposed to provide a potential mechanotransduction mechanism that may explain this shape-dependent effect47. SRF drives transcription with its cofactor MAL (also known as MRTF), which can be bound and sequestered by globular actin (G-actin)48. When substratum area is restricted, G-actin levels decrease, allowing SRF–MAL to activate transcription and initiate differentiation47. This provides a key example of the inverse relationship between substrate adhesion and differentiation, and should spark further investigation of how mechanical signalling and stratification-associated cell shape changes directly regulate differentiation.
In vivo, targeted ablation of SRF causes epidermal hyperproliferation accompanied by defects in actin organization, reduced adhesion through desmosomes and hemidesmosomes, and disrupted compaction of the epidermal layers49. Others have found that deletion of SRF in epidermis correlates with transcriptional downregulation of both actin and its regulators and blocks cortical enrichment of actin and myosin IIA during keratinocyte mitosis50. These cortical cytoskeletal defects disrupt the apical polarity complex in basal cells, causing aberrant orientation of mitotic spindles and disorganized epidermal architecture. Together, these studies suggest an intriguing model in which substratum adhesion and actin can directly influence SRF signalling to regulate epidermal morphogenesis. This signalling network may offer a new treatment avenue for cutaneous diseases characterized by aberrant morphology and differentiation. For example, levels of SRF and its target gene, JUNB, are reduced in psoriasis51,52, and this pathway may be a relevant therapeutic target.
Adherens junctions, actin and polarity
Moving upwards from the basement membrane, cad-herin-based junctions, including adherens junctions and desmosomes, are first assembled in the lateral region between basal keratinocytes (FIG. 1). Gap junctions in the epidermis allow direct cytoplasmic communication between keratinocytes and are implicated in epidermal morphogenesis and disease (BOX 1, TABLE 1 and Supplementary information S1 (table); for excellent review articles, see REFS 53,54). Differences in the type or levels of cadherins are crucial for epithelial morphogenesis because they allow cells to be sorted into discrete epithelial layers55,56. Epidermal adherens junctions are composed of the classic cadherins — E-cadherin and placental cadherin (P-cadherin; also known as cadherin 3) — both of which are important for skin morphogenesis: targeted ablation of E-cadherin causes hyperproliferation, defective differentiation and impaired barrier formation with loosening of tight junctions57–59, whereas depletion of both E- and P-cadherin results in lethal blistering60. E-cadherin loss can disrupt morphogenesis without overt loss of integrity, suggesting additional roles beyond adhesion. In fact, the cytoplasmic domains of classic cadherins interact with various signalling-competent partners, including p120 catenin and β-catenin, the latter of which associates with α-catenin (also known as αE-catenin), an actin-binding protein61 (BOX 1). However, adherens junction components affect not only adhesion but also epidermal polarity, stratification and inflammatory signalling.
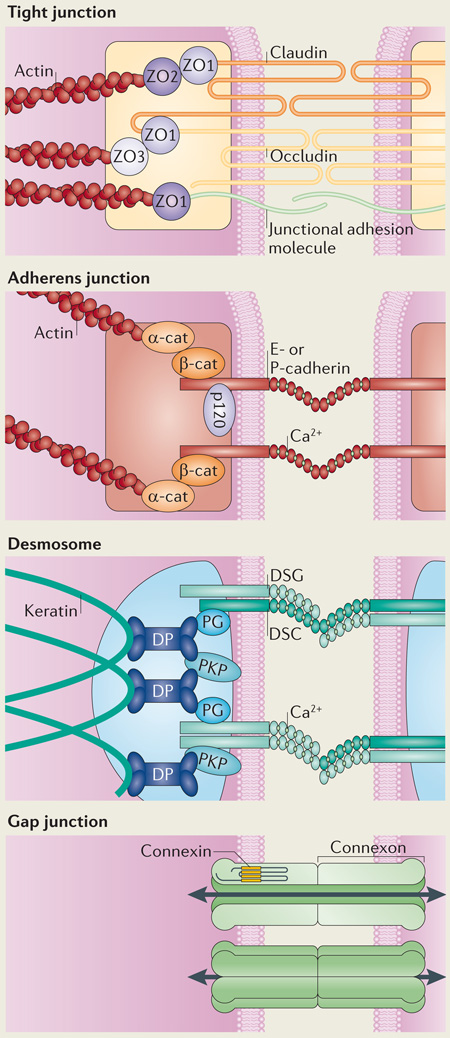
Intercellular junctions of the epidermis.
Tight junctions form a belt at the apical side of keratinocytes of the stratum granulosum, providing an additional barrier beneath the stratum corneum that controls fluid loss and protects against pathogens. They consist of the transmembrane proteins claudins, occludins and junctional adhesion molecules and are scaffolded by the cytoplasmic zonula occludens (ZO) proteins ZO1, ZO2 and ZO3. These ZO proteins also link these junctions with the actin cytoskeleton134.
Adherens junctions are intercellular connections that coordinate the assembly and organization of the cortical actin cytoskeleton throughout the epidermis. Classic cadherins (such as epithelial (E)-cadherin and placental (P)-cadherin) make up the adhesive core of the junctions through homophilic interactions mediated by characteristic extracellular homology domains, the conformation of which is regulated by calcium. Their cytoplasmic tails recruit p120 catenin and β-catenin (β-cat), which bind directly to cadherins via armadillo repeat domains, and α-catenin, which is linked to the cadherin complex by β-catenin. α-catenin (α-cat) associates with actin and coordinates the activity of actin nucleating proteins at the cell cortex to regulate assembly of the adherens junction plaque61. The plaque is reinforced by additional actin binding partners such as vinculin and α-actinin.
Desmosomes are a third class of intercellular adhesions; in contrast to adherens junctions, desmosomes link to the intracellular network of keratin intermediate filaments. In a similar way to adherens junctions, desmosomes from neighbouring cells are connected by members of the cadherin family, called desmogleins (DSGs) and desmocollins (DSCs). In contrast to the classic cadherins of adherens junctions, DSGs have unique extended cytoplasmic tails, the functions of which are yet to be fully elucidated. Plakoglobin (PG) and plakophilins (PKPs) are homologous to β-catenin and p120 catenin, respectively, and bind to desmosomal cadherins via armadillo repeat domains and amino-terminal head domains, respectively. PG and PKPs also bind desmoplakin (DP), a plakin family protein that tethers keratin intermediate filaments to the desmosomal plaque. The exact nature of DSG and DSC complexes has yet to be defined in situ; however, both homophilic and heterophilic interactions have been reported188–190.
Gap junctions are unique in their ability to provide a direct connection between neighbouring cells. Gap junctions are formed by connexons, which are composed of oligomers of six connexins (CXs); in the skin, these include CX26, CX43, CX30, CX30.3 and CX31 (also known as GJB2, GJA1, GJB6, GJB4 and GJB3, respectively)53. These connexons can join heterotypically or homotypically to form an open channel, allowing for the transport of ions and other small molecules that aid in signal transmission between cells.
Cell adhesion is essential for keratinocyte polarity
Although the embryonic epidermis begins as a single layer, around embryonic day 12.5 keratinocytes undergo stratification to establish the multiple layers needed for an environmental barrier3,62. This vertical expansion has been suggested to occur through reorientation of mitotic spindles in the basal layer63. While a horizontal axis of division (parallel to the basement membrane) produces two basal cells, vertical mitotic spindles allow stratification by production of one daughter cell that occupies the suprabasal compartment (FIG. 3a). A complex of the polarity protein partitioning defective 3 (PAR3), mouse inscuteable (MINSC) and LGN is recruited to the apical region of basal cells undergoing mitosis63 (FIG. 3b). The PAR3–MINSC–LGN complex also associates with nuclear mitotic apparatus protein 1 (NUMA1), a regulator of the mitotic spindle, and dynactin, a microtubule motor-associated protein, suggesting a mechanism by which this polarity complex could directly facilitate spindle reorientation to promote asymmetric division64,65. In fact, LGN, NUMA1 and dynactin are all essential for proper spindle positioning and asymmetric cell division during epidermal stratification64. Moreover, compromising these apical polarity complex components disrupts Notch signalling, which is a critical driver of differentiation in the suprabasal compartment64.
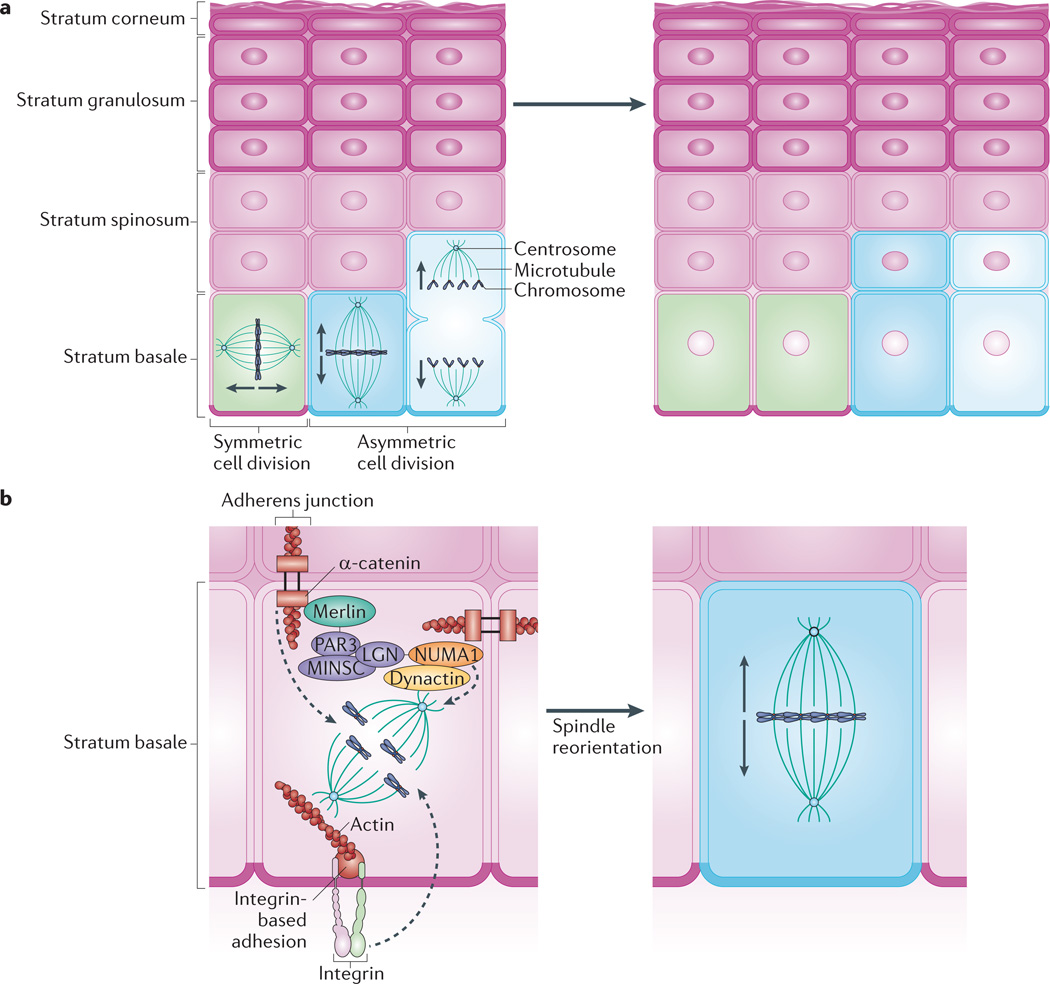
Figure 3. Mitotic spindle orientation directs stratification.
Keratinocytes of the stratum basale provide the regenerative capacity of the epidermis. a | Basal cells use symmetric cell division to promote lateral expansion of the epidermis and undergo asymmetric cell division to enable vertical expansion through the production of differentiated keratinocytes. The direction of expansion is thought to depend on the orientation of the mitotic spindle: spindles that lie parallel to the basement membrane favour symmetric division, whereas spindles perpendicular to the basement membrane promote asymmetric division. b | Asymmetric cell division depends on apical polarity factors, including the partitioning defective 3 (PAR3)–mouse inscuteable (MINSC)–LGN complex, which interacts with nuclear mitotic apparatus protein 1 (NUMA1) and dynactin to regulate spindle orientation63,65. The adherens junction component α-catenin is thought to recruit this complex via merlin, which links α-catenin and PAR3 (REF. 75). Through knockout studies, p120 catenin and β1 integrin have also been shown to have a role in mitotic spindle orientation63,70. The roles of these factors in regulating mitotic spindle pole orientation and subsequent division, including the precise location of complexes within basal cells, are yet to be fully elucidated.
Cellular junctions have a polarized organization in basal keratinocytes and contribute to mitotic spindle orientation during stratification66 (FIG. 3b). Mice lacking β1 integrin fail to recruit the polarity complex to the apex of dividing keratinocytes and have randomized mitotic axes63. Likewise, in epidermis lacking α-catenin, spindle poles are randomly aligned, and this correlates with loss of polarity, the presence of suprabasal mitoses and a propensity to develop carcinomas67,68. Surprisingly, epidermal deletion of another adherens junction component, p120 catenin, does not affect adhesion69. However, loss of p120 catenin impairs cytokinesis and produces binucleate cells in the suprabasal layers, probably owing to impaired mitosis70. p120 catenin regulates RHO signalling, which directly affects actin organization44,71,72, and this may explain how p120 catenin might mechanically orient mitotic spindles.
How do epithelial cells integrate the formation of adhesive junctions and the establishment of cellular polarity? Depletion of the adherens junction component E-cadherin disrupts localization of atypical protein kinase C (aPKC), which is a crucial regulator of cell polarity73, in the upper epidermis59; this suggests that E-cadherin might recruit aPKC to adhesive complexes in the suprabasal layers. Although asymmetric divisions in the basal layer depend on α-catenin63, exactly how adherens junctions associate with the PAR3–MINSC–LGN polarity complex had been unclear. The FERM (4.1 protein, ezrin, radixin, moesin) domain protein merlin, which localizes to adherens junctions and promotes their formation74, may have such a role75. In mitotic basal keratinocytes, merlin binds both PAR3 and α-catenin and is required for their association75. Accordingly, epidermal loss of merlin results in impaired basal cell polarity, randomized mitotic spindle orientation and a disrupted epidermal barrier owing to faulty tight junctions. Thus, through its association with adherens junctions, merlin promotes assembly of the apical polarity complex to orientate cell division during epidermal morphogenesis.
Although apical–basal polarity is essential for stratification of the interfollicular epidermis, keratinocytes also exhibit planar cell polarity, which allows coordinated movement of cells within an epithelial sheet76. Signalling proteins in the planar cell polarity pathway, including the frizzled 6 (FZD6) receptor and its effectors VANGL2 and CELSR1 are required for cell patterning across the surface of the skin, which drives the orientation of hair follicles and allows the repair of epidermal wounds in mice77,78. Whether junctional or cytoskeletal components in basal cells affect signalling via planar cell polarity proteins to regulate epidermal wound healing or morphogenesis remains to be determined.
Actin dynamics during keratinocyte stratification
Whereas actin filaments are highly associated with cell–matrix junctions in undifferentiated keratinocytes, actin in differentiating keratinocytes becomes coupled to intercellular junctions44,46,79. During keratinocyte stratification in vitro, cadherin–catenin complexes at adherens junctions are linked into a cortical ring of bundled microfilaments, which effectively couples the actin cytoskeletons of neighbouring cells80. Through association with motor proteins such as myosin, these actin-linked adhesions are thought to generate the tension required for a keratinocyte to move over a neighbouring cell44,46. Other in vitro studies have revealed that the RHO actin modulators and their effectors, RHO-associated protein kinase 1 (ROCK1) and ROCK2, are required for keratinocyte stratification and differentiation46,81. RHOE, in particular, directly promotes keratinocyte stratification82. Moreover, ROCK1 and ROCK2 have opposing roles in balancing the trade-off between matrix adhesion and differentiation83. Given the identification of SRF as an actin-regulated transcription factor that is crucial for skin morphogenesis47,49,50, it will be important to identify how these actin regulatory proteins might couple the mechanical signalling pathways driving acto-myosin contraction during stratification to the nuclear transcriptional events that initiate differentiation.
Catenin control of epidermal inflammation
In addition to their traditional roles in adhesion, adherens junction components regulate inflammatory signalling in the epidermis84. Epidermal deletion of α-catenin produces hyperproliferation in mouse skin grafts, and this depends on elevated mitogenic MAPK signalling68,85. Moreover, α-catenin deficiency also disrupts keratinocyte polarity and promotes epidermal tumour formation through p53-mediated apoptotic signalling68,86. Although impaired adhesion might have been assumed to be a primary trigger for carcinogenesis, nuclear factor-κB (NF-κB) signalling is markedly dysregulated in α-catenin-null tumours. Elevated NF-κB activity is also observed in keratinocytes lacking α-catenin that are cultured in low calcium (without robust intercellular adhesion), suggesting that loss of α-catenin causes an intrinsic signalling defect that not secondary to tissue infiltration by immune cells or loss of adhesion. p120 catenin similarly affects inflammatory signalling in the epidermis69. Epidermal deletion of p120 catenin results in increased RHOA activity, but also aberrantly upregulates NF-κB signalling. As a result, p120 catenin loss in the skin results in hyperproliferation and increased MAPK signalling with prominent dermal inflammation. p120 catenin loss also leads to increased epidermal tumour formation70. Interestingly, the ability of p120 catenin to regulate NF-κB signalling is independent of its ability to bind and stabilize E-cadherin, again implying a supra-adhesive function. Together, these studies show that rather than simply ensuring epidermal integrity, α-catenin and p120 catenin have unappreciated roles in suppressing an inflammatory cascade that promotes tumorigenesis.
Diverse roles for desmosomes and keratins
Although desmosomes are present in basal keratinocytes, stratification induces a marked increase in the concentration of these adhesive structures87 and also reorganizes their associated cytoskeletal elements88. Desmosomes are built from clustered transmembrane cadherins called desmogleins (DSGs) and desmocollins (DSCs). These bind to plakoglobin (PG) and plakophilins (PKPs), which are members of the intracellular armadillo protein family. In turn, this binding recruits the cytolinker desmoplakin (DP), which binds keratin intermediate filaments89 (BOX 1). Through this chain of interactions, desmosomal components directly link intercellular junctions and the intermediate filament cytoskeleton that fills the cytoplasm and surrounds the nucleus. Epidermal differentiation induces dramatic changes in desmosomal cadherin expression and the partitioning of PKPs between the nucleus and cell junctions. Suprabasal keratinocytes also change their complement of keratins88, which have roles in the regulation of cellular growth and metabolism addition to supporting the cytoarchitecture of the epidermis90,91. Desmosomal proteins also have supra-adhesive effects in the epidermis, including effects on microtubule reorganization during differentiation.
Desmosomal cadherins regulate differentiation
Desmosomes in vivo are tightly adherent and exhibit property called hyperadhesion, reflecting an insensitivity to extracellular calcium92. However, these intercellular rivets can also revert to a more plastic state during wound healing through PKC activity93. In fact, PKC may govern desmosome stability by regulating interactions with keratin, as mutation of a PKC consensus site in DP increases affinity for intermediate filaments and promotes hyperadhesion in vitro191. The complement of desmosomal components is extensively modified as keratinocytes their dynamic nature89,94 (FIG. 1). For example, DSG2 is normally restricted to low expression in the proliferative basal layer, but becomes upregulated in epidermal carcinomas95,96. Increased PG, PKP1 and DP in the suprabasal layers is accompanied by upregulation of specific cadherins, beginning with DSG1 expressed as cells emerge from the basal layer, along with DSC1 in the spinous layers and DSG4 in the granular layers96–98.
In addition to maintaining epidermal integrity, the regulated expression of these desmosomal components is crucial for altering keratinocyte morphology and signalling to drive epidermal morphogenesis89,99,100. In particular, desmosomal cadherins modulate intracellular signalling and control differentiation101–105. Ectopic expression of DSG2 in the mouse suprabasal epidermis augments various signalling pathways downstream of EGFR, resulting in suppressed differentiation, impaired apoptosis and pre-malignant papillomas106. In contrast to DSG2, DSG1 expression increases as EGFR activity is downregulated (FIG. 1). DSG1 is essential for suppressing EGFR signalling to MAPKs to allow differentiation in organotypic epidermis107. DSG1 can promote differentiation independently of a functional adhesive ectodomain, implying an adhesion-independent effect. Together, these data suggest that desmosomal cadherins might exert opposing roles in keratinocyte signalling, perhaps promoting the switch from proliferation to differentiation upon stratification. This is further supported by other in vivo models that show altered epidermal morphogenesis and homeostasis upon deletion or aberrant expression of desmosomal cadherins89,99.
The expanding role of epidermal keratins
The epidermis provides an excellent demonstration of the diverse functions of various keratin isoforms, the expression of which is highly regulated in this tissue. Although keratins are crucial in hair follicles108, here we focus on their importance in the interfollicular epidermis, which has been underscored by mutations that result in diseases ranging from hyperthickened palms and soles (mutations in keratin 1 (K1), K2e, K6, K9 or K10) to lethal blistering (K5 or K14 mutation)109,110. Other epidermal roles of keratins include regulation of signalling, growth and apoptosis90,91.
In epidermal keratinocytes, intermediate filaments fill the cytoplasm and mechanically connect hemidesmosomes at the basement membrane and desmosomes at the lateral membrane to the cell nucleus. The intermediate-filament-associated protein plectin binds α6β4 integrin at hemidesmosomes but also associates with nesprin 3, a protein embedded in the outer nuclear membrane111 (FIG. 2). Nesprin 2 regulates tissue thickness and nuclear morphology in the epidermis112. Thus, a desmosome–nesprin connection could provide a unique mechanosensory apparatus in keratinocytes, allowing mechanical forces perceived at the periphery to directly influence nuclear activity during epidermal morphogenesis. It has also been hypothesized that the regulation of cytoplasmic viscosity by specific keratin expression might regulate keratinocyte mobility, for example during stratification or wound healing90,113. Supporting this model, K6 and K16 are upregulated in basal cells as they seal cutaneous wounds, and they probably contribute to the migration of these cells114.
Keratinocytes flatten as they move upwards in the epithelium, and keratins are thought to contribute to the morphological transformation of these cells through their intrinsic bundling properties, their association with filaggrin and their crosslinking to desmosomes and cornified envelopes2,115. In the suprabasal compartment, expression of K5 and K14 is replaced by expression of K1 and K10, along with K9 in the palms and soles88,110. Mice lacking K10 exhibit larger-than-normal suprabasal keratinocytes and defective flattening116. Loss of K10 in the epidermis also triggers hyperproliferation with aberrant activation of MAPKs and the signalling scaffold protein 14-3-3σ117.
Keratins may directly control cell size through the regulation of metabolic signalling. Deletion of K17, which is normally expressed only in activated epidermis (for example, during wounding or in psoriasis), reduces keratinocyte size118. Loss of K17 also diminishes total protein synthesis through AKT and mammalian target of rapamycin (mTOR) signalling; this function of K17 depends on its association with cytosolic 14-3-3σ to inhibit its nuclear translocation. Keratins can also interact with the protein translation apparatus, including ribosomes, which suggests that the regulated expression of keratins may modulate global protein synthesis to allow proper evolution of cell morphology in complex tissues such as the epidermis90. Further support for keratins regulating cellular metabolism is found in mice lacking all keratin genes, in which lethal growth defects are associated with mislocalization of glucose transporters, resulting in AMP kinase activation and downregulation of mTOR targets119. In addition, drug treatment that mimics oxidative stress modulates expression of K16 and K17, suggesting that keratin expression can be dynamically regulated in response to environmental stimuli120.
An immunomodulatory role for specific keratins is supported by the recent demonstration of increased recruitment of Langerhans cells to the epidermis of mice and human patients harbouring mutations in K5 but not K14 (REF. 121). K17-null cells also show increased sensitivity to tumour necrosis factor (TNF; also known as TNFα) in vitro, suggesting that it affects susceptibility to apoptosis122. Moreover, K17 potentiates chronic proinflammatory signalling in the epidermis, which leads to basal cell carcinoma formation and may also contribute to psoriasis123. Furthermore, the keratin cytoskeleton collapses in epidermal blistering diseases such as pemphigus, which is induced by auto-antibodies against desmosomal cadherins124. One intriguing possibility is that this collapse contributes to the aberrant signalling that occurs in response to pathogenic antibodies in pemphigus125, but this effect might also arise from altered mechanics of the cytoplasm, allowing increased nuclear import. Moreover, the phenotypic manifestations of skin diseases targeting desmosome components and keratins, which can be limited to areas of high stress, such as palms and soles126, could perhaps be explained by altered mechanotransduction between desmosomes, keratin and the nucleus. Accordingly, the mutations in K5 and K14 that cause severe skin blistering also downregulate transcription of cell junction components both in cells from patients and in normal keratinocytes transfected with mutant keratins127.
Desmosomal crosstalk with microtubules and actin
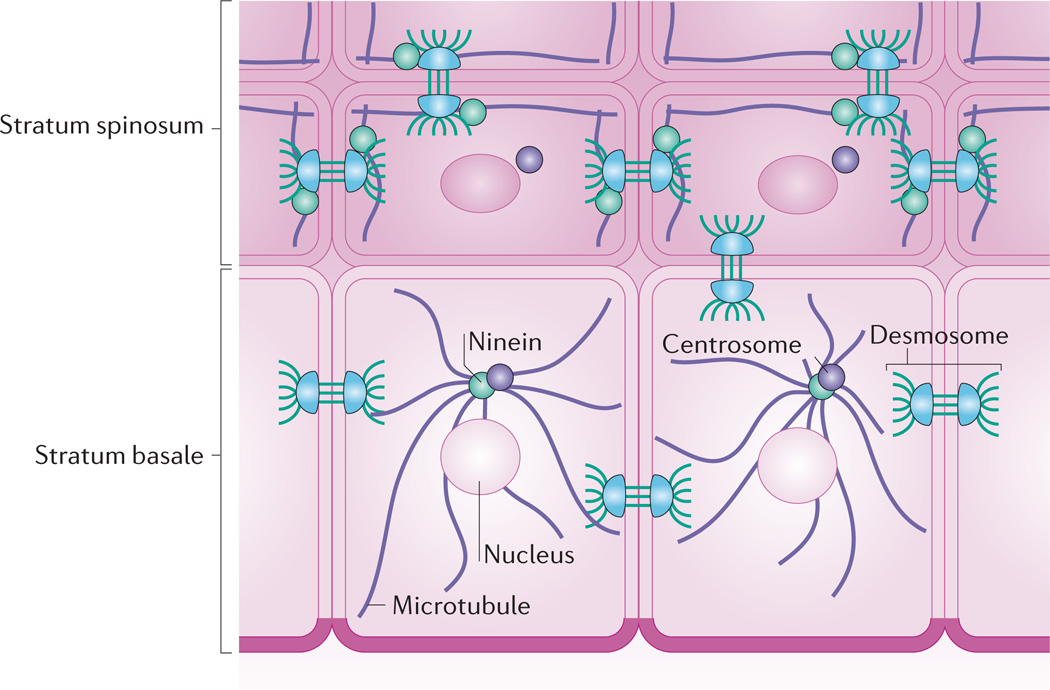
During epidermal differentiation, microtubules are substantially restructured128. Although there is a normal astral array of tubulin filaments emanating from a perinuclear centrosome in basal cells, microtubules concentrate at the cortical region of suprabasal cells and colocalize with intercellular junctions (FIG. 4). A connection between adherens junctions and microtubule dynamics has been demonstrated129. More recently, the desmosome protein DP has been found to be required for microtubule reorganization in suprabasal mouse keratinocytes128. Here, DP colocalizes at intercellular junctions with ninein (a microtubule-anchoring protein that is normally found in the centrosome130), which may recruit and/or anchor microtubules to desmosomes. Interestingly, microtubule organization by DP does not require its interaction with intermediate filaments128.
Figure 4. Desmoplakin regulates microtubule reorganization in the stratified epidermis.
Whereas keratinocytes of the stratum basale have an astral array of microtubules emanating from perinuclear centrosomes, microtubules in suprabasal keratinocytes reorganize to lie parallel with cell borders. This reorganization depends on ninein, a protein that localizes to centrosomes but is recruited to desmosomes in suprabasal keratinocytes through association with desmoplakin128.
Desmosomal components are also functionally linked to the actin cytoskeleton. In fact, the p120-related desmosomal protein PKP1 associates with actin and can induce filopodia formation131. In addition, PKP2 regulates RHO activity and actin organization during intercellular junction assembly in keratinocytes132. Actin reorganization and reduced activity of RHOA have been suggested to underlie the pathogenesis of pemphigus133, which may imply that links between desmosomal adhesion and the actin cytoskeleton are essential for supporting epidermal integrity in vivo.
These studies have revealed that although desmosomes have been traditionally associated with intermediate filaments, they more broadly regulate actin and microtubules in epidermal keratinocytes.
Tight junctions in the epidermis
As keratinocytes move into the granular layers, tight junctions help to promote an environmental barrier134. Tight junctions seal multicellular sheets by forming belt-like adhesion between cells, allowing the passage of only small molecules and ions135. Several families of transmembrane proteins contribute to tight junctions, including claudins, junctional adhesion molecules, occludins and tricellulin, an occludin-like protein found at tricellular intersections134 (BOX 1). Intracellular tight junction components include scaffolding proteins of the zonula occludens (ZO) family, which cluster transmembrane proteins and allow coupling to actin. In simple epithelial layers, tight junctions reside at the apical region of polarized cells. However, in the epidermis, although claudins are expressed in lower cell layers, functional tight junctions only form in the granular layers. This appears to be driven by differentiation-dependent expression of ZO proteins136 and relies on E-cadherin, which is needed for the localization of claudins and ZO1 in the granular layers59. Here, we discuss how tight junctions allow the simultaneous functioning of the epidermis as both an environmental shield and as a site where external antigens are ‘sampled’ by immune cells.
Tight junctions provide an epidermal barrier
In the epidermis, the regulated expression of multiple claudin genes is thought to ‘tailor’ barrier selectivity134,137. The first in vivo evidence demonstrating that tight junctions are essential for epidermal barrier function came from mice lacking claudin 1 (REF. 138), which die shortly after birth owing to profound trans-epidermal water loss (TEWL). A human gastrointestinal syndrome associated with epidermal scaling (ichthyosis) was subsequently linked to mutations in claudin 1 (REF. 139). In other animal models, ectopic expression of claudin 6 in the suprabasal compartment results in a dose-dependent defect in epidermal barrier function, suggesting that perturbing the ratio claudins in the epidermis can disrupt epidermal morphogenesis140. It has been suggested that the unique roles different claudins (for example, in intracellular signalling) may result from their divergent cytoplasmic tails. support of this, ectopic expression of claudin 6 mutants lacking a full cytoplasmic tail resulted in epidermal hyperproliferation and lethal skin barrier dysfunction age-related dermatitis and increased TEWL, depending on the extent of the deletion141,142.
Dynamic tight junctions permit immune sampling
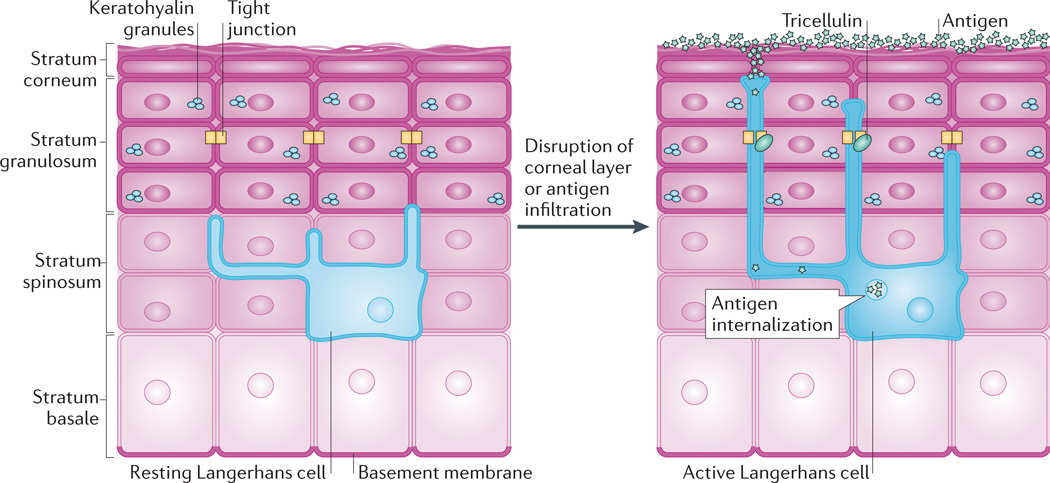
The epidermis is also a site of active immune surveillance abundant antigen-presenting cells143. Langerhans cells, particular, reside within the lower epidermis, but their cytoplasmic projections form an extensive network that permeates the upper layers despite the presence of tight junctions (FIG. 5).
Figure 5. Tight junction dynamics during antigen sampling.
Tight junctions contribute to the barrier between the superficial epidermis and underlying stratum spinosum but must also be dynamic to allow antigen sampling. In the resting state, Langerhans cells reside among keratinocytes in the stratum spinosum and extend dendrites through suprabasal layers. When activated, for instance by disruption of the stratum corneum or by infiltration of antigens, the dendrites of Langerhans cells dock with tight junctions and gain the ability to extend into the stratum corneum. The transmembrane protein tricellulin localizes to these tricellular tight junctions formed between keratinocytes and Langerhans cells. This dynamic adjustment of tight junctions allows Langerhans cells to internalize antigens, which can then be presented to the host immune system144.
Advances in three-dimensional imaging have revealed the mechanism by which the epidermis allows antigen sampling but preserves the cutaneous barrier144: Langerhans cells form tight junctions with keratinocytes in the epidermis, and tight junctions undergo an intricate reorganization to permit epidermal antigen surveillance. This local rearrangement allows dendritic processes Langerhans cells to sample for antigens beyond the interkeratinocyte tight junction, but the overall epidermal barrier is maintained by unique tricellular tight junctions between two adjacent keratinocytes and a penetrating Langerhans cell. Indeed, Langerhans cells express claudin 1 and ZO1 at their cell membranes, which permits the formation of tight junctions between their dendrites and granular layer keratinocytes.
The upper tips of the Langerhans cell projections also act as endocytic sites for antigen uptake into Birbeck granules, which allows the processing and presentation of external antigens to the host immune system144. Thus, this study has revealed the dynamic nature of tight junctions and the essential role that their reorganization has in immune surveillance. The signalling pathways that drive this reorganization remain unknown but might underlie cutaneous pathologies, such as atopic dermatitis, that are caused by impaired barrier function and enhanced antigen uptake145. Intriguingly, tight junction organization is altered early in the development of psoriatic epidermal lesions146. Further investigation is likely to improve our understanding of the role that tight junctions have in other skin diseases caused by, or resulting in, defective barrier function and chronic inflammation.
Filaggrin in barrier function
In addition to tight junctions, another key morphological feature of the granular layer is the keratohyalin granule, a multiprotein complex that is mostly composed of profilaggrin147. Profilaggrin consists of 10 to 12 repeated units and is cleaved to generate mature filaggrin, which binds to and promotes the aggregation of intermediate filaments in the upper layers. Thus, filaggrin helps to stabilize the keratin network, which serves as a crucial scaffold for other components of the cornified envelope2. In vitro studies suggest that filaggrin may also promote collapse of the intermediate filament cytoskeleton during later stages of differentiation, which might facilitate cell compaction in the upper epidermis148. This structural protein also helps to maintain the cutaneous barrier. Genetic studies have confirmed that mutation of the filaggrin gene underlies the scaly skin phenotype seen in ichthyosis vulgaris and is a major risk factor for atopic dermatitis (eczema), a prevalent condition characterized by cutaneous inflammation149–151. Mice harbouring a mutation in the filaggrin gene have increased immunological response to topical antigens, indicating a defective cutaneous barrier152. Furthermore, filaggrin processing affects skin hydration: mice lacking a protease that cleaves profilaggrin have a dehydrated stratum corneum153. Moreover, mutations in this protease are found in certain patients with atopic dermatitis, which may explain the chronic dry skin typical of this disease. These studies have provided crucial advances in our understanding of the pathogenesis of atopic dermatitis and may have further implications for the treatment of common related diseases such as asthma, hay fever and even food allergies147,154,155.
Cornified envelopes and corneodesmosomes
As keratinocytes progress towards their ultimate destination in the upper epidermis, they undergo a unique process of cell death termed cornification, which is distinct from apoptosis2. Cornification involves biochemical crosslinking of various keratinocyte proteins such as loricrin and involucrin by transglutaminases (TGases), but it eventually terminates with nuclei and organelles being broken down by intracellular and secreted proteases, including a specialized caspase156,157. However, before dying, these keratinocytes produce specialized proteins and lipids to construct the cornified envelope, which is a heavily crosslinked submembranous sheath that provides structure to the upper epidermis and acts as a water-impermeable barrier2. In this barrier, desmosomal components are crosslinked to the cornified envelope to form corneodesmosomes, which bind cornified cells together and are essential for barrier formation158. However, these linkages must be reversed to allow shedding of cornified cells (desquamation) during normal epidermal turnover159,160.
The cornified envelope promotes barrier function
Crosslinking of involucrin and loricrin by TGase 1 promotes their incorporation into a submembranous matrix of tightly knit proteins formed by keratin filaments and filaggrin in the granular layer2. TGase 1 is upregulated in the upper layers and is essential for epidermal barrier function in vivo161,162. In addition to these components, specialized plakin proteins, called envoplakin and periplakin, and the secreted desmosomal protein corneodesmosin (CDSN) are incorporated into the cornified envelope2,163,164. Envoplakin and periplakin are intermediate- filament-binding proteins that localize to desmosomes in the upper layers, where they are thought to integrate the keratin cytoskeletons of the stratum corneum165,166.
Surprisingly, deletion of individual components of the cornified envelope, such as loricrin, involucrin, periplakin and envoplakin, produce minor phenotypes, suggesting that these constituents can compensate for one another in barrier function167–170. In a more aggressive approach, a triple-knockout mouse lacking periplakin, envoplakin and involucrin shows barrier dysfunction and impaired desquamation of the cornified layers, probably owing to the faulty processing and aberrant retention of corneodesmosomes in addition to defective degradation of DSG1 during desquamation171. Mutations in cornified envelope components may contribute to genetic susceptibility to chronic barrier defects, as in atopic dermatitis172,173.
Corneodesmosomes in barrier formation and desquamation
Corneodesmosomes contain the cadherins DSG1 and DSC1, which must be cleaved by serine proteases to allow proper shedding of the outer epidermis160. In fact, deficiency of SPINK5 (also known as LEKT1), a serine protease inhibitor, results in weakened adhesion in the cornified layers, premature desquamation and defective barrier function in mice160. SPINK5 mutations have also been linked to Netherton syndrome, a human disease of similar phenotype174.
CDSN is an essential component of corneodesmosomes that is secreted in the upper granular layers during the final stages of differentiation164. It is thought to function as intercellular ‘glue’ that helps to solidify the linkage of desmosomal cadherin ectodomains between keratinocytes in the stratum corneum, but it may have inherent adhesive properties175. CDSN is also thought to be essential for desquamation176. Its targeted ablation in the epidermis results in death shortly after birth owing to sloughing of the outer epidermal layers, accompanied the appearance of split corneodesmosomes177. In humans, mutations in CDSN cause chronic skin inflammation and peeling owing to defective epidermal desquamation and cutaneous barrier dysfunction178 and can also associated with congenital hair loss179.
Thus, the structural linkages that form in cornified keratinocytes are dynamic and must undergo a finely tuned cycle of aggregation and desquamation in precise balance with basal cell proliferation to simultaneously preserve epidermal homeostasis and provide environmental barrier.
Conclusions
In this Review we have traced the journey of the keratinocyte as it progresses outwards through the epidermal layers, a process that is paralleled by key alterations adhesive junctions and their associated cytoskeletal elements. These cytoarchitectural elements are far from passive scaffolds — they actively cooperate with transcriptional and translational pathways to establish cell and tissue polarity, guide differentiation and regulate cutaneous responses to environmental insults and pathogens. For instance, stratification-associated alterations integrin- and cadherin-based adhesions are important for balancing proliferation and differentiation. Although tight junctions are crucial for preventing water loss, their remodelling also has an active role in antigen sampling. The proper construction of corneodesmosomes makes essential contribution to the skin barrier, but their timely breakdown is necessary for normal epidermal turnover. Our increased understanding of these processes has turn provided insights into the dysfunction of adhesive and cytoskeletal proteins that leads to epidermal pathology in humans and has suggested potential therapeutic avenues for cutaneous disease.
However, many questions remain about the detailed mechanisms by which cytoskeletal and adhesive proteins directly contribute to epidermal morphogenesis and disease. These mechanisms are likely to hinge on interconnections between keratinocytes and the cytoskeletal scaffolding that connects the cell surface with the nucleus. This scaffold could contribute to various functions ranging from mechanosensing to regulation of the diffusion or nuclear import of signalling proteins180. Differentiation-dependent remodelling of this scaffold could coordinate changes in morphology with altered signalling and transcription. Moreover, the ability of adhesive junctions to interconnect the cells of the epidermis may regulate communication among layers and allow coordinated responses to pathogenic insults or mechanical strain. It has been suggested that the meshwork of interlaced cytoskeletons that fill the cytoplasm may globally regulate cell plasticity and signalling during dynamic processes such as tissue stratification or cell migration90,113,181. However, we still have much to learn about the specific contributions of epidermal cytoskeletal elements. For instance, the significance of microtubule redistribution during stratification128 is completely unknown. In addition, junction proteins themselves, including catenins, PKPs and even cadherin fragments, can enter the nucleus and affect transcription182,183. This dual localization at junctions and in transcriptional complexes places these versatile proteins in a prime position to orchestrate alterations in adhesion with changes in signalling during epidermal morphogenesis. In fact, PKP1 can control cell size and proliferation through the regulation of protein translation184.
How are the mechanical cues borne by epidermal cytoskeletal scaffolds translated into chemical information? One can envision that signal transmission occurs through a series of molecular sensors and actuators present in cell junctions and cortical cytoskeletal attachments, and that information is communicated through alterations in protein conformation and protein–protein interactions. To illuminate exactly how these cell elements translate environmental cues, strategies will be needed that directly couple the delivery and measurement of mechanical forces with molecular probes that report how information is perceived and processed. The development of molecular fluorescent sensors is still in its infancy, but one example is a biosensor that assesses tension in the actin-binding protein vinculin, which is found in both focal contacts and adherens junctions185. This sensor has provided insights into the relationship between force transmission and junction assembly and size. Combining the use of these probes with super-resolution microscopy, intravital imaging techniques and physiologically relevant models of epidermal morphogenesis will be needed to directly assess how endogenous contractile forces or environmentally applied stress is transmitted through the epidermal cell architecture. Applications of these strategies could be used, for instance, to understand how genetic deficiencies impair the coupling of contractile forces with the differentiation programme that normally directs stratification.
Although cell biologists have for years promoted the idea that cytoarchitecture governs cell fate186,187, we still have a lot to learn about how this occurs at a molecular level. The next decade holds much promise for grappling with these complex questions. We are now in a new era of interdisciplinary cooperation, with teams bringing to bear their combined expertise in bioengineering and materials science, quantitative biology, optical imaging, cell and molecular biology. Advances resulting from these interdisciplinary teams promise to illuminate how the hard-wiring of cells transforms their behaviour and fate.
Supplementary Material
Acknowledgements
The authors would like to thank M. Amagai, J. Jones, T. Lechler and T. Magin for critical reading of the manuscript and/or advice on figures. We also apologize to our colleagues whose work we were unable to include owing to space limitations. The authors are supported by US National Institutes of Health grants AR043380, AR041836 and CA122151, the Leducq Foundation and the J.L. Mayberry Endowment to K.J.G.
Glossary
- Organotypic
An in vitro reconstituted model of a tissue grown from cultured cellular elements
- Filopodia
Thin, transient actin protrusions that extend out from the cell surface and are formed by the elongation of bundled actin filaments in their cores
- Endocytic sites
Sites of endocytosis, which is the internalization and transport of extracellular material and plasma membrane proteins from the cell surface to intracellular organelles known as endosomes
- Transglutaminases
(TGases). A family of enzymes that can catalyse covalent bond formation between a glutamine on a peptide and a free amine group
Footnotes
Competing interests statement
The authors declare no competing financial interests.
SUPPLEMENTARY INFORMATION
See online article: S1 (table)
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nature Rev. Genet. 2002;3:199–209. doi: 10.1038/nrg758. [DOI] [PubMed] [Google Scholar]
- 2.Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nature Rev. Mol. Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 3.Mack JA, Anand S, Maytin EV. Proliferation and cornification during development of the mammalian epidermis. Birth Defects Res. C Embryo Today. 2005;75:314–329. doi: 10.1002/bdrc.20055. [DOI] [PubMed] [Google Scholar]
- 4.Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nature Rev. Mol. Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu YC, Pasolli HA, Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011;144:92–105. doi: 10.1016/j.cell.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuruta D, Hashimoto T, Hamill KJ, Jones JC. Hemidesmosomes and focal contact proteins: functions and cross-talk in keratinocytes, bullous diseases and wound healing. J. Dermatol. Sci. 2011;62:1–7. doi: 10.1016/j.jdermsci.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watt FM. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 2002;21:3919–3926. doi: 10.1093/emboj/cdf399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Margadant C, Charafeddine RA, Sonnenberg A. Unique and redundant functions of integrins in the epidermis. FASEB J. 2010;24:4133–4152. doi: 10.1096/fj.09-151449. [DOI] [PubMed] [Google Scholar]
- 9.Watt F. In: Keratinocyte Methods. Leigh I, Watt F, editors. Cambridge: Cambridge Univ. Press; 1994. p. 113. [Google Scholar]
- 10.Green H. Terminal differentiation of cultured human epidermal cells. Cell. 1977;11:405–416. doi: 10.1016/0092-8674(77)90058-7. [DOI] [PubMed] [Google Scholar]
- 11.De Potter IY, Poumay Y, Squillace KA, Pittelkow MR. Human EGF receptor (HER) family and heregulin members are differentially expressed in epidermal keratinocytes and modulate differentiation. Exp. Cell Res. 2001;271:315–328. doi: 10.1006/excr.2001.5390. [DOI] [PubMed] [Google Scholar]
- 12.Aplin AE, Juliano RL. Integrin and cytoskeletal regulation of growth factor signaling to the MAP kinase pathway. J. Cell Sci. 1999;112:695–706. doi: 10.1242/jcs.112.5.695. [DOI] [PubMed] [Google Scholar]
- 13.Muller EJ, Williamson L, Kolly C, Suter MM. Outside-in signaling through integrins and cadherins: a central mechanism to control epidermal growth and differentiation? J. Invest. Dermatol. 2008;128:501–516. doi: 10.1038/sj.jid.5701248. [DOI] [PubMed] [Google Scholar]
- 14.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nature Rev. Mol. Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 15.Aplin AE, Stewart SA, Assoian RK, Juliano RL. Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1. J. Cell Biol. 2001;153:273–282. doi: 10.1083/jcb.153.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dowling J, Yu QC, Fuchs E. β4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J. Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin β4 null mice. Nature Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- 18.Murgia C, et al. Cell cycle and adhesion defects in mice carrying a targeted deletion of the integrin β4 cytoplasmic domain. EMBO J. 1998;17:3940–3951. doi: 10.1093/emboj/17.14.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niculescu C, et al. Conditional ablation of integrin α6 in mouse epidermis leads to skin fragility and inflammation. Eur. J. Cell Biol. 2011;90:270–277. doi: 10.1016/j.ejcb.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 20.DiPersio CM, et al. α3β1 and α6β4 integrin receptors for laminin-5 are not essential for epidermal morphogenesis and homeostasis during skin development. J. Cell Sci. 2000;113:3051–3062. doi: 10.1242/jcs.113.17.3051. [DOI] [PubMed] [Google Scholar]
- 21.Raymond K, Kreft M, Janssen H, Calafat J, Sonnenberg A. Keratinocytes display normal proliferation, survival and differentiation in conditional β4-integrin knockout mice. J. Cell Sci. 2005;118:1045–1060. doi: 10.1242/jcs.01689. [DOI] [PubMed] [Google Scholar]
- 22.Hertle MD, Kubler MD, Leigh IM, Watt FM. Aberrant integrin expression during epidermal wound healing and in psoriatic epidermis. J. Clin. Invest. 1992;89:1892–1901. doi: 10.1172/JCI115794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haase I, Hobbs RM, Romero MR, Broad S, Watt FM. A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis. J. Clin. Invest. 2001;108:527–536. doi: 10.1172/JCI12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carroll JM, Romero MR, Watt FM. Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell. 1995;83:957–968. doi: 10.1016/0092-8674(95)90211-2. [DOI] [PubMed] [Google Scholar]
- 25.Owens DM, Romero MR, Gardner C, Watt FM. Suprabasal α6β4 integrin expression in epidermis results in enhanced tumourigenesis and disruption of TGFβ signalling. J. Cell Sci. 2003;116:3783–3791. doi: 10.1242/jcs.00725. [DOI] [PubMed] [Google Scholar]
- 26.Owens DM, Broad S, Yan X, Benitah SA, Watt FM. Suprabasal α5β1 integrin expression stimulates formation of epidermal squamous cell carcinomas without disrupting TGFβ signaling or inducing spindle cell tumors. Mol. Carcinog. 2005;44:60–66. doi: 10.1002/mc.20118. [DOI] [PubMed] [Google Scholar]
- 27.Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of β1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J. Cell Biol. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brakebusch C, et al. Skin and hair follicle integrity is crucially dependent on β1 integrin expression on keratinocytes. EMBO J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez-Rovira T, Silva-Vargas V, Watt FM. Different consequences of β1 integrin deletion in neonatal and adult mouse epidermis reveal a context-dependent role of integrins in regulating proliferation, differentiation, and intercellular communication. J. Invest. Dermatol. 2005;125:1215–1227. doi: 10.1111/j.0022-202X.2005.23956.x. [DOI] [PubMed] [Google Scholar]
- 30.McMillan JR, Akiyama M, Shimizu H. Epidermal basement membrane zone components: ultrastructural distribution and molecular interactions. J. Dermatol. Sci. 2003;31:169–177. doi: 10.1016/s0923-1811(03)00045-8. [DOI] [PubMed] [Google Scholar]
- 31.DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO. α3β1 Integrin is required for normal development of the epidermal basement membrane. J. Cell Biol. 1997;137:729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Margadant C, et al. Integrin α3β1 inhibits directional migration and wound re-epithelialization in the skin. J. Cell Sci. 2009;122:278–288. doi: 10.1242/jcs.029108. [DOI] [PubMed] [Google Scholar]
- 33.Lorenz K, et al. Integrin-linked kinase is required for epidermal and hair follicle morphogenesis. J. Cell Biol. 2007;177:501–513. doi: 10.1083/jcb.200608125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai-Cheong JE, Parsons M, McGrath JA. The role of kindlins in cell biology and relevance to human disease. Int. J. Biochem. Cell Biol. 2010;42:595–603. doi: 10.1016/j.biocel.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 35.Ussar S, Wang HV, Linder S, Fassler R, Moser M. The Kindlins: subcellular localization and expression during murine development. Exp. Cell Res. 2006;312:3142–3151. doi: 10.1016/j.yexcr.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 36.Siegel DH, et al. Loss of kindlin-1, a human homolog of the Caenorhabditis elegans actin–extracellular-matrix linker protein UNC-112, causes Kindler syndrome. Am. J. Hum. Genet. 2003;73:174–187. doi: 10.1086/376609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimizu H, et al. Immunohistochemical, ultrastructural, and molecular features of Kindler syndrome distinguish it from dystrophic epidermolysis bullosa. Arch. Dermatol. 1997;133:1111–1117. [PubMed] [Google Scholar]
- 38.Lai-Cheong JE, Ussar S, Arita K, Hart IR, McGrath JA. Colocalization of kindlin-1, kindlin-2, and migfilin at keratinocyte focal adhesion and relevance to the pathophysiology of Kindler syndrome. J. Invest. Dermatol. 2008;128:2156–2165. doi: 10.1038/jid.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Has C, et al. Kindlin-1 is required for RhoGTPase-mediated lamellipodia formation in keratinocytes. Am. J. Pathol. 2009;175:1442–1452. doi: 10.2353/ajpath.2009.090203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ussar S, et al. Loss of Kindlin-1 causes skin atrophy and lethal neonatal intestinal epithelial dysfunction. PLoS Genet. 2008;4:e1000289. doi: 10.1371/journal.pgen.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Herz C, et al. Kindlin-1 is a phosphoprotein involved in regulation of polarity, proliferation, and motility of epidermal keratinocytes. J. Biol. Chem. 2006;281:36082–36090. doi: 10.1074/jbc.M606259200. Uses a mouse model to show that loss of kindlin 1 function, which causes a skin disorder called Kindler syndrome, is also a direct cause of ulcerative colitis-like symptoms, which occur as a result of impaired integrin inactivation and delamination of the intestinal epithelia in response to force.
- 42.He Y, Esser P, Heinemann A, Bruckner-Tuderman L, Has C. Kindlin-1 and -2 have overlapping functions in epithelial cells implications for phenotype modification. Am. J. Pathol. 2011;178:975–982. doi: 10.1016/j.ajpath.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raghavan S, Vaezi A, Fuchs E. A role for αβ1 integrins in focal adhesion function and polarized cytoskeletal dynamics. Dev. Cell. 2003;5:415–427. doi: 10.1016/s1534-5807(03)00261-2. [DOI] [PubMed] [Google Scholar]
- 44.Perez-Moreno M, Jamora C, Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–548. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 45.Vasioukhin V, Fuchs E. Actin dynamics and cell-cell adhesion in epithelia. Curr. Opin. Cell Biol. 2001;13:76–84. doi: 10.1016/s0955-0674(00)00177-0. [DOI] [PubMed] [Google Scholar]
- 46.Vaezi A, Bauer C, Vasioukhin V, Fuchs E. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev. Cell. 2002;3:367–381. doi: 10.1016/s1534-5807(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 47. Connelly JT, et al. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nature Cell Biol. 2010;12:711–718. doi: 10.1038/ncb2074. Using a micropatterning strategy, the authors reveal a novel mechanism by which cell shape drives keratinocyte differentiation through SRF signalling, irrespective of substrate area or composition.
- 48.Miano JM, Long X, Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am. J. Physiol. Cell Physiol. 2007;292:C70–C81. doi: 10.1152/ajpcell.00386.2006. [DOI] [PubMed] [Google Scholar]
- 49. Koegel H, et al. Loss of serum response factor in keratinocytes results in hyperproliferative skin disease in mice. J. Clin. Invest. 2009;119:899–910. doi: 10.1172/JCI37771. Reports the first keratinocyte-specific deletion of mouse SRF, revealing that postnatal loss of SRF is associated with development of psoriasis-like skin lesions characterized by inflammation, hyperproliferation, abnormal keratinocyte differentiation and disruption of the actin cytoskeleton.
- 50. Luxenburg C, Amalia Pasolli H, Williams SE, Fuchs E. Developmental roles for Srf, cortical cytoskeleton and cell shape in epidermal spindle orientation. Nature Cell Biol. 2011;13:203–214. doi: 10.1038/ncb2163. In contrast to reference 49, this study reports that SRF-deficient keratinocytes fail to undergo cortical actin-dependent mitotic shape changes, which are required for localizing proteins that are important for directing spindle orientation, stratification and differentiation.
- 51.Mehic D, Bakiri L, Ghannadan M, Wagner EF, Tschachler E. Fos and jun proteins are specifically expressed during differentiation of human keratinocytes. J. Invest. Dermatol. 2005;124:212–220. doi: 10.1111/j.0022-202X.2004.23558.x. [DOI] [PubMed] [Google Scholar]
- 52.Zenz R, et al. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature. 2005;437:369–375. doi: 10.1038/nature03963. [DOI] [PubMed] [Google Scholar]
- 53.Mese G, Richard G, White TW. Gap junctions: basic structure and function. J. Invest. Dermatol. 2007;127:2516–2524. doi: 10.1038/sj.jid.5700770. [DOI] [PubMed] [Google Scholar]
- 54.Lai-Cheong JE, Arita K, McGrath JA. Genetic diseases of junctions. J. Invest. Dermatol. 2007;127:2713–2725. doi: 10.1038/sj.jid.5700727. [DOI] [PubMed] [Google Scholar]
- 55.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nature Rev. Mol. Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 56.Runswick SK, O’Hare MJ, Jones L, Streuli CH, Garrod DR. Desmosomal adhesion regulates epithelial morphogenesis and cell positioning. Nature Cell Biol. 2001;3:823–830. doi: 10.1038/ncb0901-823. [DOI] [PubMed] [Google Scholar]
- 57.Tinkle CL, Lechler T, Pasolli HA, Fuchs E. Conditional targeting of E-cadherin in skin: insights into hyperproliferative and degenerative responses. Proc. Natl Acad. Sci. USA. 2004;101:552–557. doi: 10.1073/pnas.0307437100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young P, et al. E-cadherin controls adherens junctions in the epidermis and the renewal of hair follicles. EMBO J. 2003;22:5723–5733. doi: 10.1093/emboj/cdg560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tunggal JA, et al. E-cadherin is essential for in vivo epidermal barrier function by regulating tight junctions. EMBO J. 2005;24:1146–1156. doi: 10.1038/sj.emboj.7600605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tinkle CL, Pasolli HA, Stokes N, Fuchs E. New insights into cadherin function in epidermal sheet formation and maintenance of tissue integrity. Proc. Natl Acad. Sci. USA. 2008;105:15405–15410. doi: 10.1073/pnas.0807374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niessen CM, Gottardi CJ. Molecular components of the adherens junction. Biochim. Biophys. Acta. 2008;1778:562–571. doi: 10.1016/j.bbamem.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koster MI, Roop DR. Mechanisms regulating epithelial stratification. Annu. Rev. Cell Dev. Biol. 2007;23:93–113. doi: 10.1146/annurev.cellbio.23.090506.123357. [DOI] [PubMed] [Google Scholar]
- 63. Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. The first paper to show that basal cells in the mammalian epidermis undergo asymmetric divisions to drive stratification during epidermal morphogenesis. It also identifies the cytoarchitectural elements regulating this process.
- 64.Williams SE, Beronja S, Pasolli HA, Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353–358. doi: 10.1038/nature09793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poulson ND, Lechler T. Robust control of mitotic spindle orientation in the developing epidermis. J. Cell Biol. 2010;191:915–922. doi: 10.1083/jcb.201008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nieben MT, Niessen CM. Regulation of cell and tissue polarity: implications for skin homeostasis and disease. Expert Rev. Dermatol. 2010;5:671–687. [Google Scholar]
- 67.Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of α-catenin in skin. Cell. 2001;104:605–617. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 68.Kobielak A, Fuchs E. Links between α-catenin, NF-κB, and squamous cell carcinoma in skin. Proc. Natl Acad. Sci. USA. 2006;103:2322–2327. doi: 10.1073/pnas.0510422103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perez-Moreno M, et al. p120-catenin mediates inflammatory responses in the skin. Cell. 2006;124:631–644. doi: 10.1016/j.cell.2005.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Perez-Moreno M, Song W, Pasolli HA, Williams SE, Fuchs E. Loss of p120 catenin and links to mitotic alterations, inflammation, and skin cancer. Proc. Natl Acad. Sci. USA. 2008;105:15399–15404. doi: 10.1073/pnas.0807301105. References 69 and 70 report that, rather than resulting in overt defects in adherens junctions, conditional ablation of p120 catenin in mouse skin leads to chronic inflammatory responses through NF-κB activation, as well as mitotic defects through elevation of RHOA GTPase activity, leading to skin neoplasias.
- 71.Hatzfeld M. The p120 family of cell adhesion molecules. Eur. J. Cell Biol. 2005;84:205–214. doi: 10.1016/j.ejcb.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 72.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 73.Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev. Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lallemand D, Curto M, Saotome I, Giovannini M, McClatchey AI. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev. 2003;17:1090–1100. doi: 10.1101/gad.1054603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gladden AB, Hebert AM, Schneeberger EE, McClatchey AI. The NF2 tumor suppressor, Merlin, regulates epidermal development through the establishment of a junctional polarity complex. Dev. Cell. 2010;19:727–739. doi: 10.1016/j.devcel.2010.10.008. Provides insight into the mechanism by which the FERM protein merlin promotes epidermal polarity and morphogenesis in vivo by coupling adherens junctions and the PAR3 polarity complex.
- 76.Bayly R, Axelrod JD. Pointing in the right direction: new developments in the field of planar cell polarity. Nature Rev. Genet. 2011;12:385–391. doi: 10.1038/nrg2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caddy J, et al. Epidermal wound repair is regulated by the planar cell polarity signaling pathway. Dev. Cell. 2010;19:138–147. doi: 10.1016/j.devcel.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nature Cell Biol. 2008;10:1257–1268. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kubler MD, Jordan PW, O’Neill CH, Watt FM. Changes in the abundance and distribution of actin and associated proteins during terminal differentiation of human epidermal keratinocytes. J. Cell Sci. 1991;100:153–165. doi: 10.1242/jcs.100.1.153. [DOI] [PubMed] [Google Scholar]
- 80.Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 81.McMullan R, et al. Keratinocyte differentiation is regulated by the Rho and ROCK signaling pathway. Curr. Biol. 2003;13:2185–2189. doi: 10.1016/j.cub.2003.11.050. [DOI] [PubMed] [Google Scholar]
- 82.Liebig T, et al. RhoE is required for keratinocyte differentiation and stratification. Mol. Biol. Cell. 2009;20:452–463. doi: 10.1091/mbc.E07-11-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lock FE, Hotchin NA. Distinct roles for ROCK1 and ROCK2 in the regulation of keratinocyte differentiation. PLoS ONE. 2009;4:e8190. doi: 10.1371/journal.pone.0008190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev. Cell. 2006;11:601–612. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beronja S, Livshits G, Williams S, Fuchs E. Rapid functional dissection of genetic networks via tissue-specific transduction and RNAi in mouse embryos. Nature Med. 2010;16:821–827. doi: 10.1038/nm.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Silvis MR, et al. α-Catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci. Signal. 2011;4:ra33. doi: 10.1126/scisignal.2001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.White FH, Gohari K. Desmosomes in hamster cheek pouch epithelium: their quantitative characterization during epithelial differentiation. J. Cell Sci. 1984;66:411–429. doi: 10.1242/jcs.66.1.411. [DOI] [PubMed] [Google Scholar]
- 88.Fuchs E. Epidermal differentiation and keratin gene expression. J. Cell Sci. 1993;17:197–208. doi: 10.1242/jcs.1993.supplement_17.28. [DOI] [PubMed] [Google Scholar]
- 89.Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J. Invest. Dermatol. 2007;127:2499–2515. doi: 10.1038/sj.jid.5701015. [DOI] [PubMed] [Google Scholar]
- 90.Gu LH, Coulombe PA. Keratin function in skin epithelia: a broadening palette with surprising shades. Curr. Opin. Cell Biol. 2007;19:13–23. doi: 10.1016/j.ceb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 91.Magin TM, Vijayaraj P, Leube RE. Structural and regulatory functions of keratins. Exp. Cell Res. 2007;313:2021–2032. doi: 10.1016/j.yexcr.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 92.Garrod D, Kimura TE. Hyper-adhesion: a new concept in cell–cell adhesion. Biochem. Soc. Trans. 2008;36:195–201. doi: 10.1042/BST0360195. [DOI] [PubMed] [Google Scholar]
- 93.Kimura TE, Merritt AJ, Garrod DR. Calcium-independent desmosomes of keratinocytes are hyper-adhesive. J. Invest. Dermatol. 2007;127:775–781. doi: 10.1038/sj.jid.5700643. [DOI] [PubMed] [Google Scholar]
- 94.Getsios S, Huen AC, Green KJ. Working out the strength and flexibility of desmosomes. Nature Rev. Mol. Cell Biol. 2004;5:271–281. doi: 10.1038/nrm1356. [DOI] [PubMed] [Google Scholar]
- 95.Brennan D, Mahoney MG. Increased expression of Dsg2 in malignant skin carcinomas: a tissue-microarray based study. Cell Adh. Migr. 2009;3:148–154. doi: 10.4161/cam.3.2.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mahoney MG, et al. Delineation of diversified desmoglein distribution in stratified squamous epithelia: implications in diseases. Exp. Dermatol. 2006;15:101–109. doi: 10.1111/j.1600-0625.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 97.Arnemann J, Sullivan KH, Magee AI, King IA, Buxton RS. Stratification-related expression of isoforms of the desmosomal cadherins in human epidermis. J. Cell Sci. 1993;104:741–750. doi: 10.1242/jcs.104.3.741. [DOI] [PubMed] [Google Scholar]
- 98.King IA, et al. Hierarchical expression of desmosomal cadherins during stratified epithelial morphogenesis in the mouse. Differentiation. 1997;62:83–96. doi: 10.1046/j.1432-0436.1997.6220083.x. [DOI] [PubMed] [Google Scholar]
- 99.Cheng X, Koch PJ. In vivo function of desmosomes. J. Dermatol. 2004;31:171–187. doi: 10.1111/j.1346-8138.2004.tb00654.x. [DOI] [PubMed] [Google Scholar]
- 100.Garrod D, Chidgey M. Desmosome structure, composition and function. Biochim. Biophys. Acta. 2008;1778:572–587. doi: 10.1016/j.bbamem.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 101.Merritt AJ, et al. Suprabasal desmoglein 3 expression in the epidermis of transgenic mice results in hyperproliferation and abnormal differentiation. Mol. Cell. Biol. 2002;22:5846–5858. doi: 10.1128/MCB.22.16.5846-5858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hardman MJ, et al. Desmosomal cadherin misexpression alters β-catenin stability and epidermal differentiation. Mol. Cell. Biol. 2005;25:969–978. doi: 10.1128/MCB.25.3.969-978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Elias PM, et al. Desmoglein isoform distribution affects stratum corneum structure and function. J. Cell Biol. 2001;153:243–249. doi: 10.1083/jcb.153.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chidgey M, et al. Mice lacking desmocollin 1 show epidermal fragility accompanied by barrier defects and abnormal differentiation. J. Cell Biol. 2001;155:821–832. doi: 10.1083/jcb.200105009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kljuic A, et al. Desmoglein 4 in hair follicle differentiation and epidermal adhesion. Evidence from inherited hypotrichosis and acquired pemphigus vulgaris. Cell. 2003;113:249–260. doi: 10.1016/s0092-8674(03)00273-3. [DOI] [PubMed] [Google Scholar]
- 106.Brennan D, et al. Suprabasal Dsg2 expression in transgenic mouse skin confers a hyperproliferative and apoptosis-resistant phenotype to keratinocytes. J. Cell Sci. 2007;120:758–771. doi: 10.1242/jcs.03392. [DOI] [PubMed] [Google Scholar]
- 107. Getsios S, et al. Desmoglein 1-dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. J. Cell Biol. 2009;185:1243–1258. doi: 10.1083/jcb.200809044. Demonstrates that DSG1, which was previously well-established as being crucial for adhesion in the epidermal suprabasal layers, also regulates epidermal differentiation through its attenuation of MAPK signalling; this function does not involve DSG1’s adhesive ectodomain or binding to the associated armadillo protein PG.
- 108.Schweizer J, Langbein L, Rogers MA, Winter H. Hair follicle-specific keratins and their diseases. Exp. Cell Res. 2007;313:2010–2020. doi: 10.1016/j.yexcr.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 109.Omary MB, Coulombe PA, McLean WH. Intermediate filament proteins and their associated diseases. N. Engl. J. Med. 2004;351:2087–2100. doi: 10.1056/NEJMra040319. [DOI] [PubMed] [Google Scholar]
- 110.Szeverenyi I, et al. The Human Intermediate Filament Database: comprehensive information on a gene family involved in many human diseases. Hum. Mutat. 2008;29:351–360. doi: 10.1002/humu.20652. [DOI] [PubMed] [Google Scholar]
- 111.Wilhelmsen K, et al. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J. Cell Biol. 2005;171:799–810. doi: 10.1083/jcb.200506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Luke Y, et al. Nesprin-2 Giant (NUANCE) maintains nuclear envelope architecture and composition in skin. J. Cell Sci. 2008;121:1887–1898. doi: 10.1242/jcs.019075. [DOI] [PubMed] [Google Scholar]
- 113.Beil M, et al. Sphingosylphosphorylcholine regulates keratin network architecture and visco-elastic properties of human cancer cells. Nature Cell Biol. 2003;5:803–811. doi: 10.1038/ncb1037. [DOI] [PubMed] [Google Scholar]
- 114.Coulombe PA. Wound epithelialization: accelerating the pace of discovery. J. Invest. Dermatol. 2003;121:219–230. doi: 10.1046/j.1523-1747.2003.12387.x. [DOI] [PubMed] [Google Scholar]
- 115.Lee CH, Coulombe PA. Self-organization of keratin intermediate filaments into cross-linked networks. J. Cell Biol. 2009;186:409–421. doi: 10.1083/jcb.200810196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reichelt J, Magin TM. Hyperproliferation, induction of c-Myc and 14-3-3σ, but no cell fragility in keratin-10-null mice. J. Cell Sci. 2002;115:2639–2650. doi: 10.1242/jcs.115.13.2639. [DOI] [PubMed] [Google Scholar]
- 117.Reichelt J, Furstenberger G, Magin TM. Loss of keratin 10 leads to mitogen-activated protein kinase (MAPK) activation, increased keratinocyte turnover, and decreased tumor formation in mice. J. Invest. Dermatol. 2004;123:973–981. doi: 10.1111/j.0022-202X.2004.23426.x. [DOI] [PubMed] [Google Scholar]
- 118. Kim S, Wong P, Coulombe PA. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature. 2006;441:362–365. doi: 10.1038/nature04659. Shows that the wound-induced intermediate filament protein K17 regulates cell growth by associating with the adaptor 14-3-3σ to govern its localization in the cytoplasm, where it stimulates AKT–mTOR signalling and protein synthesis.
- 119. Vijayaraj P, et al. Keratins regulate protein biosynthesis through localization of GLUT1 and -3 upstream of AMP kinase and Raptor. J. Cell Biol. 2009;187:175–184. doi: 10.1083/jcb.200906094. A targeted gene deletion strategy is used to generate transgenic mice that completely lack all keratin intermediate family members, resulting in severe growth retardation and embryonic lethality, which occurs owing to mislocalization of membrane-associated glucose transporter type 1 (GLUT1) and GLUT3 and consequent activation of an energy sensing cascade that represses protein synthesis.
- 120.Kerns M, DePianto D, Yamamoto M, Coulombe PA. Differential modulation of keratin expression by sulforaphane occurs via Nrf2-dependent and -independent pathways in skin epithelia. Mol. Biol. Cell. 2010;21:4068–4075. doi: 10.1091/mbc.E10-02-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Roth W, Reuter U, Wohlenberg C, Bruckner-Tuderman L, Magin TM. Cytokines as genetic modifiers in K5−/− mice and in human epidermolysis bullosa simplex. Hum. Mutat. 2009;30:832–841. doi: 10.1002/humu.20981. [DOI] [PubMed] [Google Scholar]
- 122.Tong X, Coulombe PA. Keratin 17 modulates hair follicle cycling in a TNFα-dependent fashion. Genes Dev. 2006;20:1353–1364. doi: 10.1101/gad.1387406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Depianto D, Kerns ML, Dlugosz AA, Coulombe PA. Keratin 17 promotes epithelial proliferation and tumor growth by polarizing the immune response in skin. Nature Genet. 2010;42:910–914. doi: 10.1038/ng.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Diercks GF, Pas HH, Jonkman MF. The ultrastructure of acantholysis in pemphigus vulgaris. Br. J. Dermatol. 2009;160:460–461. doi: 10.1111/j.1365-2133.2008.08971.x. [DOI] [PubMed] [Google Scholar]
- 125.Sharma P, Mao X, Payne AS. Beyond steric hindrance: the role of adhesion signaling pathways in the pathogenesis of pemphigus. J. Dermatol. Sci. 2007;48:1–14. doi: 10.1016/j.jdermsci.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 126.Braun-Falco M. Hereditary palmoplantar keratodermas. J. Dtsch Dermatol. Ges. 2009;7:971–984. doi: 10.1111/j.1610-0387.2009.07058.x. [DOI] [PubMed] [Google Scholar]
- 127.Liovic M, et al. Severe keratin 5 and 14 mutations induce down-regulation of junction proteins in keratinocytes. Exp. Cell. Res. 2009;315:2995–3003. doi: 10.1016/j.yexcr.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 128. Lechler T, Fuchs E. Desmoplakin: an unexpected regulator of microtubule organization in the epidermis. J. Cell Biol. 2007;176:147–154. doi: 10.1083/jcb.200609109. Makes the surprising discovery that the desmosomal plaque protein DP not only anchors the intermediate filament cytoskeleton at the plasma membrane, but is also required for a switch in epidermal microtubule organization from centrosome-associated organization (in the basal proliferating layers) to cortical, intercellular junction-associated organization (in the suprabasal layers).
- 129.Stehbens SJ, Akhmanova A, Yap AS. Microtubules and cadherins: a neglected partnership. Front. Biosci. 2009;14:3159–3167. doi: 10.2741/3442. [DOI] [PubMed] [Google Scholar]
- 130.Mogensen MM, Malik A, Piel M, Bouckson-Castaing V, Bornens M. Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. J. Cell Sci. 2000;113:3013–3023. doi: 10.1242/jcs.113.17.3013. [DOI] [PubMed] [Google Scholar]
- 131.Hatzfeld M, Haffner C, Schulze K, Vinzens U. The function of plakophilin 1 in desmosome assembly and actin filament organization. J. Cell Biol. 2000;149:209–222. doi: 10.1083/jcb.149.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Godsel LM, et al. Plakophilin 2 couples actomyosin remodeling to desmosomal plaque assembly via RhoA. Mol. Biol. Cell. 2010;21:2844–2859. doi: 10.1091/mbc.E10-02-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Waschke J, et al. Inhibition of Rho A activity causes pemphigus skin blistering. J. Cell Biol. 2006;175:721–727. doi: 10.1083/jcb.200605125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Niessen CM. Tight junctions/adherens junctions: basic structure and function. J. Invest. Dermatol. 2007;127:2525–2532. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- 135.Morita K, Miyachi Y. Tight junctions in the skin. J. Dermatol. Sci. 2003;31:81–89. doi: 10.1016/s0923-1811(03)00038-0. [DOI] [PubMed] [Google Scholar]
- 136.Langbein L, et al. Tight junctions and compositionally related junctional structures in mammalian stratified epithelia and cell cultures derived therefrom. Eur. J. Cell Biol. 2002;81:419–435. doi: 10.1078/0171-9335-00270. [DOI] [PubMed] [Google Scholar]
- 137.Kirschner N, Bohner C, Rachow S, Brandner JM. Tight junctions: is there a role in dermatology? Arch. Dermatol. Res. 2010;302:483–493. doi: 10.1007/s00403-010-1058-z. [DOI] [PubMed] [Google Scholar]
- 138.Furuse M, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J. Cell Biol. 2002;156:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hadj-Rabia S, et al. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: a tight junction disease. Gastroenterology. 2004;127:1386–1390. doi: 10.1053/j.gastro.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 140.Turksen K, Troy TC. Permeability barrier dysfunction in transgenic mice overexpressing claudin 6. Development. 2002;129:1775–1784. doi: 10.1242/dev.129.7.1775. [DOI] [PubMed] [Google Scholar]
- 141.Troy TC, Arabzadeh A, Lariviere NM, Enikanolaiye A, Turksen K. Dermatitis and aging-related barrier dysfunction in transgenic mice overexpressing an epidermal-targeted claudin 6 tail deletion mutant. PLoS ONE. 2009;4:e7814. doi: 10.1371/journal.pone.0007814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Arabzadeh A, Troy TC, Turksen K. Role of the Cldn6 cytoplasmic tail domain in membrane targeting and epidermal differentiation in vivo. Mol. Cell. Biol. 2006;26:5876–5887. doi: 10.1128/MCB.02342-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Clausen BE, Kel JM. Langerhans cells: critical regulators of skin immunity? Immunol. Cell Biol. 2010;88:351–360. doi: 10.1038/icb.2010.40. [DOI] [PubMed] [Google Scholar]
- 144. Kubo A, Nagao K, Yokouchi M, Sasaki H, Amagai M. External antigen uptake by Langerhans cells with reorganization of epidermal tight junction barriers. J. Exp. Med. 2009;206:2937–2946. doi: 10.1084/jem.20091527. Beautiful imaging performed in this study shows how Langerhans cells, which are dendritic cells in the skin, dock with and extend through tight junctions when activated by antigen infiltration of the overlying stratum corneum.
- 145.De Benedetto A, et al. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2011;127:773–786. doi: 10.1016/j.jaci.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kirschner N, et al. Alteration of tight junction proteins is an early event in psoriasis: putative involvement of proinflammatory cytokines. Am. J. Pathol. 2009;175:1095–1106. doi: 10.2353/ajpath.2009.080973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McGrath JA, Uitto J. The filaggrin story: novel insights into skin-barrier function and disease. Trends Mol. Med. 2008;14:20–27. doi: 10.1016/j.molmed.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 148.Dale BA, Presland RB, Lewis SP, Underwood RA, Fleckman P. Transient expression of epidermal filaggrin in cultured cells causes collapse of intermediate filament networks with alteration of cell shape and nuclear integrity. J. Invest. Dermatol. 1997;108:179–187. doi: 10.1111/1523-1747.ep12334205. [DOI] [PubMed] [Google Scholar]
- 149. Palmer CN, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nature Genet. 2006;38:441–446. doi: 10.1038/ng1767. This ground-breaking work identifies loss of function mutations in filaggrin as a major contributor to the development of atopic dermatitis, supporting the idea that this common disorder is primarily caused by disturbed barrier function, and that the associated inflammation is an indirect effect of infiltrating environmental toxins, allergens and pathogens.
- 150.Sandilands A, Sutherland C, Irvine AD, McLean WH. Filaggrin in the frontline: role in skin barrier function and disease. J. Cell Sci. 2009;122:1285–1294. doi: 10.1242/jcs.033969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Smith FJ, et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nature Genet. 2006;38:337–342. doi: 10.1038/ng1743. [DOI] [PubMed] [Google Scholar]
- 152.Fallon PG, et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nature Genet. 2009;41:602–608. doi: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Matsui T, et al. SASPase regulates stratum corneum hydration through profilaggrin-to-filaggrin processing. EMBO Mol. Med. 2011;3:320–333. doi: 10.1002/emmm.201100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.O’Regan GM, Irvine AD. The role of filaggrin in the atopic diathesis. Clin. Exp. Allergy. 2010;40:965–972. doi: 10.1111/j.1365-2222.2010.03522.x. [DOI] [PubMed] [Google Scholar]
- 155.Brown SJ, et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J. Allergy Clin. Immunol. 2011;127:661–667. doi: 10.1016/j.jaci.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Eckhart L, et al. Terminal differentiation of human keratinocytes and stratum corneum formation is associated with caspase-14 activation. J. Invest. Dermatol. 2000;115:1148–1151. doi: 10.1046/j.1523-1747.2000.00205.x. [DOI] [PubMed] [Google Scholar]
- 157.Denecker G, Ovaere P, Vandenabeele P, Declercq W. Caspase-14 reveals its secrets. J. Cell Biol. 2008;180:451–458. doi: 10.1083/jcb.200709098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ishida-Yamamoto A, Igawa S, Kishibe M. Order and disorder in corneocyte adhesion. J. Dermatol. 2011;38:645–654. doi: 10.1111/j.1346-8138.2011.01227.x. [DOI] [PubMed] [Google Scholar]
- 159.Caubet C, et al. Degradation of corneodesmosome proteins by two serine proteases of the kallikrein family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J. Invest. Dermatol. 2004;122:1235–1244. doi: 10.1111/j.0022-202X.2004.22512.x. [DOI] [PubMed] [Google Scholar]
- 160.Descargues P, et al. Spink5-deficient mice mimic Netherton syndrome through degradation of desmoglein 1 by epidermal protease hyperactivity. Nature Genet. 2005;37:56–65. doi: 10.1038/ng1493. [DOI] [PubMed] [Google Scholar]
- 161.Matsuki M, et al. Defective stratum corneum and early neonatal death in mice lacking the gene for transglutaminase 1 (keratinocyte transglutaminase) Proc. Natl Acad. Sci. USA. 1998;95:1044–1049. doi: 10.1073/pnas.95.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Huber M, et al. Mutations of keratinocyte transglutaminase in lamellar ichthyosis. Science. 1995;267:525–528. doi: 10.1126/science.7824952. [DOI] [PubMed] [Google Scholar]
- 163.DiColandrea T, Karashima T, Maatta A, Watt FM. Subcellular distribution of envoplakin and periplakin: insights into their role as precursors of the epidermal cornified envelope. J. Cell Biol. 2000;151:573–586. doi: 10.1083/jcb.151.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lundstrom A, Serre G, Haftek M, Egelrud T. Evidence for a role of corneodesmosin, a protein which may serve to modify desmosomes during cornification, in stratum corneum cell cohesion and desquamation. Arch. Derm. Res. 1994;286:369–375. doi: 10.1007/BF00371795. [DOI] [PubMed] [Google Scholar]
- 165.Karashima T, Watt FM. Interaction of periplakin and envoplakin with intermediate filaments. J. Cell Sci. 2002;115:5027–5037. doi: 10.1242/jcs.00191. [DOI] [PubMed] [Google Scholar]
- 166.Kalinin AE, et al. Co-assembly of envoplakin and periplakin into oligomers and Ca2+-dependent vesicle binding: implications for cornified cell envelope formation in stratified squamous epithelia. J. Biol. Chem. 2004;279:22773–22780. doi: 10.1074/jbc.M313660200. [DOI] [PubMed] [Google Scholar]
- 167.Aho S, et al. Periplakin gene targeting reveals a constituent of the cornified cell envelope dispensable for normal mouse development. Mol. Cell. Biol. 2004;24:6410–6418. doi: 10.1128/MCB.24.14.6410-6418.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Maatta A, DiColandrea T, Groot K, Watt FM. Gene targeting of envoplakin, a cytoskeletal linker protein and precursor of the epidermal cornified envelope. Mol. Cell. Biol. 2001;21:7047–7053. doi: 10.1128/MCB.21.20.7047-7053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Koch PJ, et al. Lessons from loricrin-deficient mice: compensatory mechanisms maintaining skin barrier function in the absence of a major cornified envelope protein. J. Cell Biol. 2000;151:389–400. doi: 10.1083/jcb.151.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Djian P, Easley K, Green H. Targeted ablation of the murine involucrin gene. J. Cell Biol. 2000;151:381–388. doi: 10.1083/jcb.151.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sevilla LM, et al. Mice deficient in involucrin, envoplakin, and periplakin have a defective epidermal barrier. J. Cell Biol. 2007;179:1599–1612. doi: 10.1083/jcb.200706187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sugiura H, et al. Large-scale DNA microarray analysis of atopic skin lesions shows overexpression of an epidermal differentiation gene cluster in the alternative pathway and lack of protective gene expression in the cornified envelope. Br. J. Dermatol. 2005;152:146–149. doi: 10.1111/j.1365-2133.2005.06352.x. [DOI] [PubMed] [Google Scholar]
- 173.Guttman-Yassky E, et al. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J. Allergy Clin. Immunol. 2009;124:1235–1244. doi: 10.1016/j.jaci.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 174.Chavanas S, et al. Mutations in SPINK5, encoding a serine protease inhibitor, cause Netherton syndrome. Nature Genet. 2000;25:141–142. doi: 10.1038/75977. [DOI] [PubMed] [Google Scholar]
- 175.Jonca N, et al. Corneodesmosin, a component of epidermal corneocyte desmosomes, displays homophilic adhesive properties. J. Biol. Chem. 2002;277:5024–5029. doi: 10.1074/jbc.M108438200. [DOI] [PubMed] [Google Scholar]
- 176.Simon M, et al. Refined characterization of corneodesmosin proteolysis during terminal differentiation of human epidermis and its relationship to desquamation. J. Biol. Chem. 2001;276:20292–20299. doi: 10.1074/jbc.M100201200. [DOI] [PubMed] [Google Scholar]
- 177.Leclerc EA, et al. Corneodesmosin gene ablation induces lethal skin-barrier disruption and hair-follicle degeneration related to desmosome dysfunction. J. Cell Sci. 2009;122:2699–2709. doi: 10.1242/jcs.050302. [DOI] [PubMed] [Google Scholar]
- 178.Oji V, et al. Loss of corneodesmosin leads to severe skin barrier defect, pruritus, and atopy: unraveling the peeling skin disease. Am. J. Hum. Genet. 2010;87:274–281. doi: 10.1016/j.ajhg.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Levy-Nissenbaum E, et al. Hypotrichosis simplex of the scalp is associated with nonsense mutations in CDSN encoding corneodesmosin. Nature Genet. 2003;34:151–153. doi: 10.1038/ng1163. [DOI] [PubMed] [Google Scholar]
- 180.Janmey PA, McCulloch CA. Cell mechanics: integrating cell responses to mechanical stimuli. Annu. Rev. Biomed. Eng. 2007;9:1–34. doi: 10.1146/annurev.bioeng.9.060906.151927. [DOI] [PubMed] [Google Scholar]
- 181.Ingber DE, Tensegrity II. How structural networks influence cellular information processing networks. J. Cell Sci. 2003;116:1397–1408. doi: 10.1242/jcs.00360. [DOI] [PubMed] [Google Scholar]
- 182.Cavallaro U, Dejana E. Adhesion molecule signalling: not always a sticky business. Nature Rev. Mol. Cell. Biol. 2011;12:189–197. doi: 10.1038/nrm3068. [DOI] [PubMed] [Google Scholar]
- 183.Bass-Zubek AE, Godsel LM, Delmar M, Green KJ. Plakophilins: multifunctional scaffolds for adhesion and signaling. Curr. Opin. Cell Biol. 2009;21:708–716. doi: 10.1016/j.ceb.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Wolf A, et al. Plakophilin 1 stimulates translation by promoting eIF4A1 activity. J. Cell Biol. 2010;188:463–471. doi: 10.1083/jcb.200908135. Highlights the emerging importance of adhesionin-dependent roles for the ‘junctional’ armadillo proteins, in this case showing that the desmosomal protein PKP1 binds to eukaryotic initiation factor 4A (eIF4A) to increase translational activity of the eIF4A complex.
- 185.Grashoff C, et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–266. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bissell MJ, Barcellos-Hoff MH. The influence of extracellular matrix on gene expression: is structure the message? J. Cell Sci. 1987;8:327–343. doi: 10.1242/jcs.1987.supplement_8.18. [DOI] [PubMed] [Google Scholar]
- 187.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 188.Nie Z, Merritt A, Rouhi-Parkouhi M, Tabernero L, Garrod D. Membrane-impermeable cross-linking provides evidence for homophilic, isoform-specific binding of desmosomal cadherins in epithelial cells. J. Biol. Chem. 2011;286:2143–2154. doi: 10.1074/jbc.M110.192245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chitaev NA, Troyanovsky SM. Direct Ca2+-dependent heterophilic interaction between desmosomal cadherins, desmoglein and desmocollin, contributes to cell-cell adhesion. J. Cell Biol. 1997;138:193–201. doi: 10.1083/jcb.138.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Spindler V, et al. Desmocollin 3-mediated binding is crucial for keratinocyte cohesion and is impaired in pemphigus. J. Biol. Chem. 2009;284:30556–30564. doi: 10.1074/jbc.M109.024810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hobbs RP, Green KJ. Desmoplakin regulates desmosome hyperadhesion. J. Invest. Dermatol. doi: 10.1038/jid.2011.318. (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.