Abstract
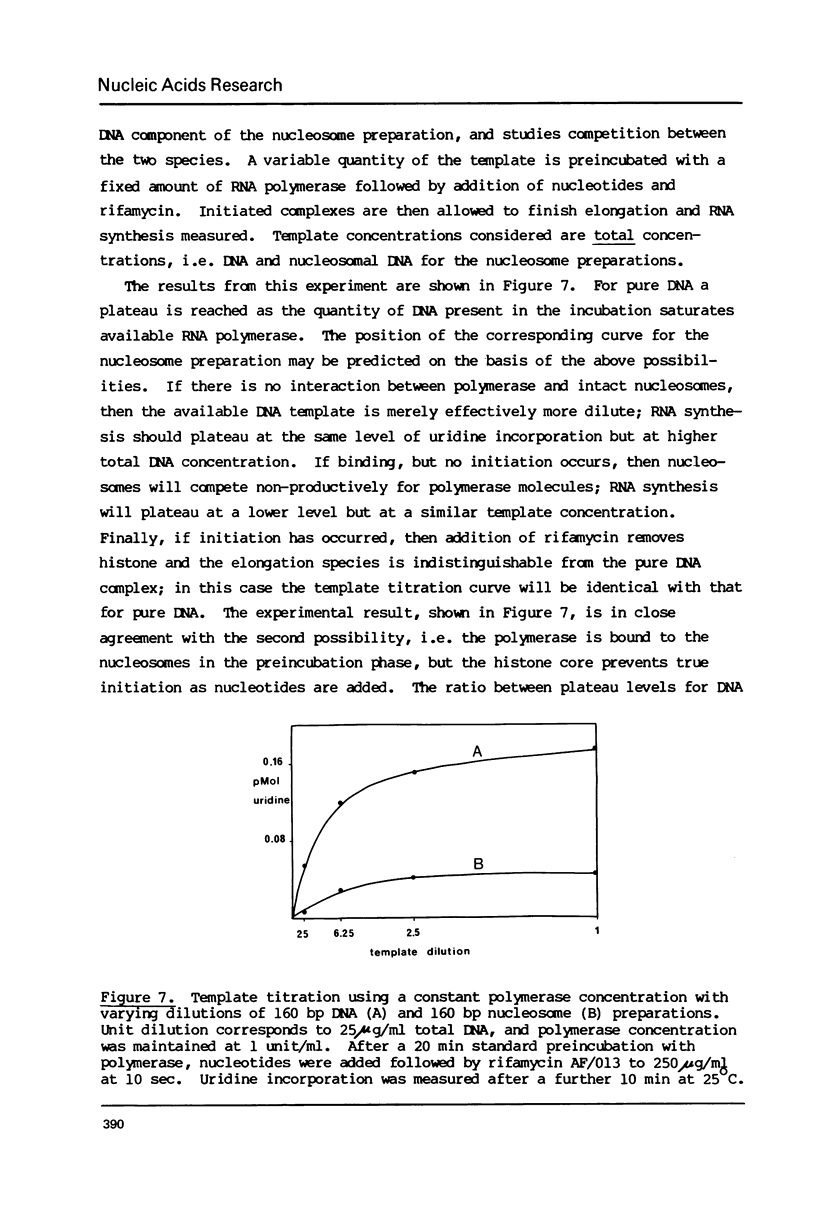
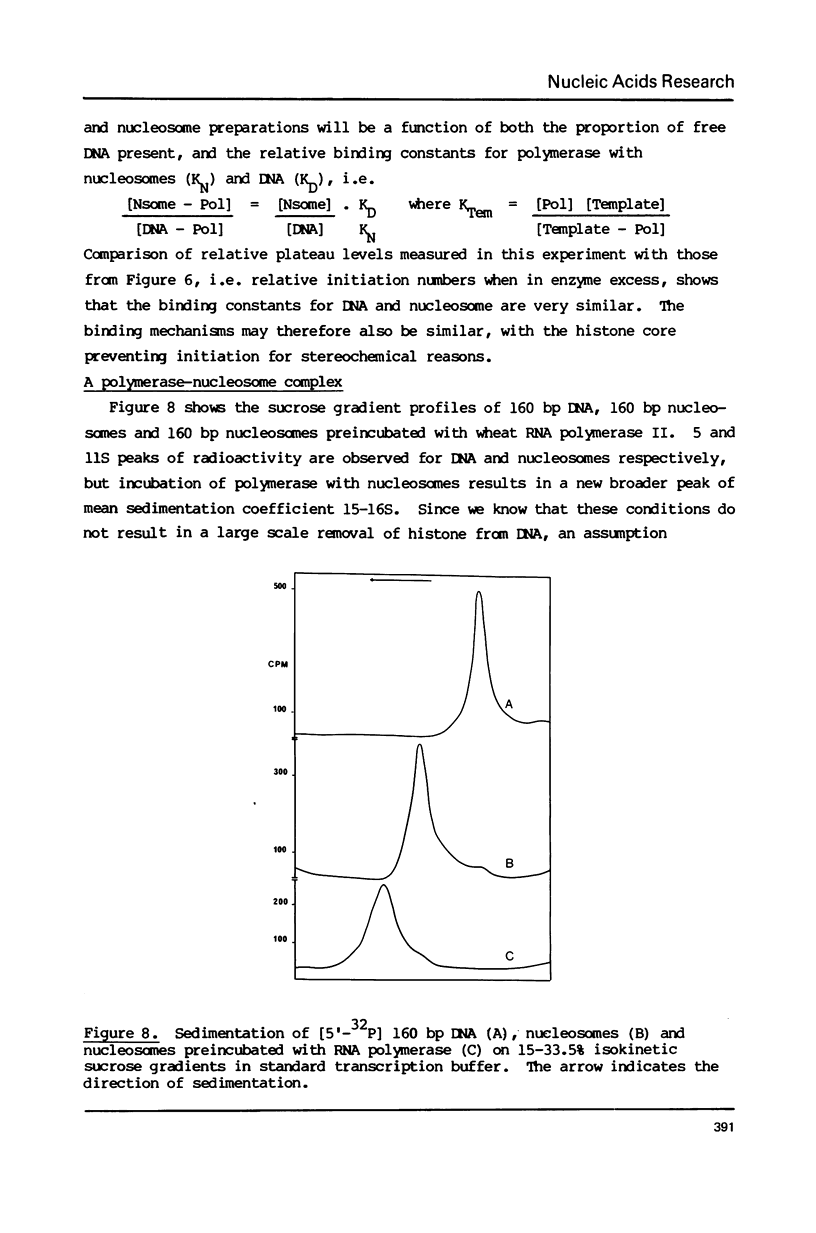
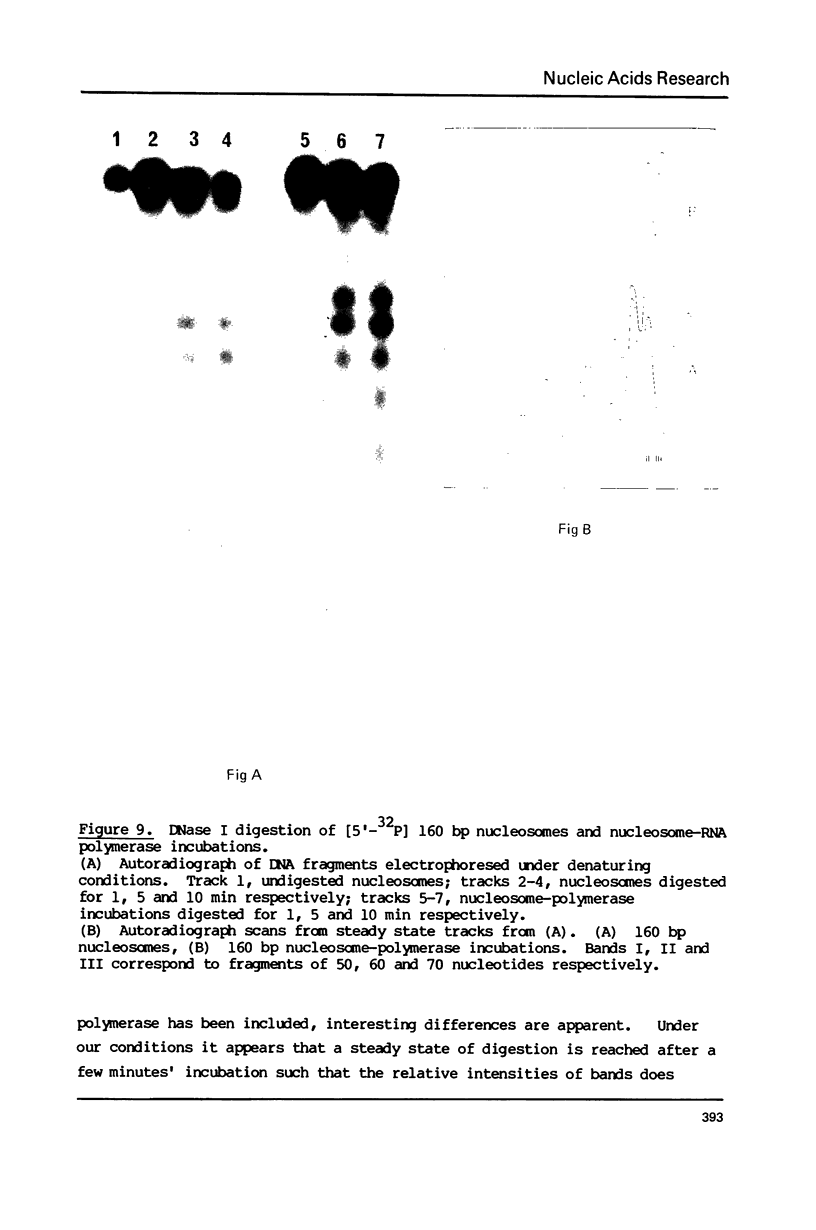
The integrity and stability of nucleosomes under transcription assay conditions has been found to depend on concentration and ionic environment. Rifamycin AF/013, a commonly used inhibitor of initiation, is particularly effective in destabilisation of nucleosomes. Intact nucleosomes are refractory to transcription by wheat RNA polymerase II, the histone core preventing initiation. Template titration suggests that the polymerase can, however, bind to nucleosomes, and a 15--16S complex has been observed on sucrose gradients. DNase I digestion of polymerase-nucleosome incubations indicates that whilst histone is still present in the complex, the nucleosome conformation is altered resulting in enhanced nucleolysis at sites near the DNA centre but reduced overall kinetics of digestion.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bustin M. Binding of E. coli RNA polymerase to chromatin subunits. Nucleic Acids Res. 1978 Mar;5(3):925–932. doi: 10.1093/nar/5.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedar H., Felsenfeld G. Transcription of chromatin in vitro. J Mol Biol. 1973 Jun 25;77(2):237–254. doi: 10.1016/0022-2836(73)90334-3. [DOI] [PubMed] [Google Scholar]
- Chambon P. Summary: the molecular biology of the eukaryotic genome is coming of age. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1209–1234. doi: 10.1101/sqb.1978.042.01.122. [DOI] [PubMed] [Google Scholar]
- Chung S. Y., Hill W. E., Doty P. Characterization of the histone core complex. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1680–1684. doi: 10.1073/pnas.75.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezélée S., Sentenac A., Fromageot P. Role of deoxyribonucleic acid-ribonucleic acid hybrids in eukaryotes. Study of the template requirements of yeast ribonucleic acid polymerases and nature of the ribonucleic acid product. J Biol Chem. 1974 Sep 25;249(18):5971–5977. [PubMed] [Google Scholar]
- Dieterich A. E., Axel R., Cantor C. R. Salt-induced structural changes of nucleosome core particles. J Mol Biol. 1979 Apr 25;129(4):587–602. doi: 10.1016/0022-2836(79)90470-4. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Noll M. Nucleosome arcs and helices. Science. 1978 Oct 20;202(4365):280–286. doi: 10.1126/science.694532. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Lutter L. C., Rhodes D., Brown R. S., Rushton B., Levitt M., Klug A. Structure of nucleosome core particles of chromatin. Nature. 1977 Sep 1;269(5623):29–36. doi: 10.1038/269029a0. [DOI] [PubMed] [Google Scholar]
- Foe V. E., Wilkinson L. E., Laird C. D. Comparative organization of active transcription units in Oncopeltus fasciatus. Cell. 1976 Sep;9(1):131–146. doi: 10.1016/0092-8674(76)90059-3. [DOI] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel A., Zolan M., Axel R. Genes transcribed at diverse rates have a similar conformation in chromatin. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4867–4871. doi: 10.1073/pnas.74.11.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariglio P., Buss J., Green M. H. Sarkosyl activation of RNA polymerase activity in mitotic mouse cells. FEBS Lett. 1974 Aug 30;44(3):330–333. doi: 10.1016/0014-5793(74)81170-1. [DOI] [PubMed] [Google Scholar]
- Gariglio P., Llopis R., Oudet P., Chambon P. The template of the isolated native simian virus 40 transcriptional complexes is a minichromosome. J Mol Biol. 1979 Jun 15;131(1):75–105. doi: 10.1016/0022-2836(79)90302-4. [DOI] [PubMed] [Google Scholar]
- Hossenlopp P., Oudet P., Chambon P. Animal DNA-dependent RNA polymerases. Studies on the binding of mammalian RNA polymerases AI and B to Simian virus 40 DNA. Eur J Biochem. 1974 Jan 16;41(2):397–411. doi: 10.1111/j.1432-1033.1974.tb03281.x. [DOI] [PubMed] [Google Scholar]
- Jendrisak J. J., Burgess R. R. A new method for the large-scale purification of wheat germ DNA-dependent RNA polymerase II. Biochemistry. 1975 Oct 21;14(21):4639–4645. doi: 10.1021/bi00692a012. [DOI] [PubMed] [Google Scholar]
- Jendrisak J. J., Burgess R. R. Studies of the subunit structure of wheat germ ribonucleic acid polymerase II. Biochemistry. 1977 May 3;16(9):1959–1964. doi: 10.1021/bi00628a032. [DOI] [PubMed] [Google Scholar]
- Karagyozov L. K., Valkanov M. A., Hadjiolov A. A. Transcription of DNA-histone complexes by yeast RNA polymerase B. Nucleic Acids Res. 1978 Jun;5(6):1907–1917. doi: 10.1093/nar/5.6.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedinger C., Gissinger F., Chambon P. Animal DNA-dependent RNA polymerases. Molecular structures and immunological properties of calf-thymus enzyme AI and of calf-thymus and rat-liver enzymes B. Eur J Biochem. 1974 May 15;44(2):421–436. doi: 10.1111/j.1432-1033.1974.tb03500.x. [DOI] [PubMed] [Google Scholar]
- Lacy E., Axel R. Analysis of DNA of isolated chromatin subunits. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3978–3982. doi: 10.1073/pnas.72.10.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley D. M. Active chromatin structure. Cell Biol Int Rep. 1978 Jan;2(1):1–10. doi: 10.1016/0309-1651(78)90078-4. [DOI] [PubMed] [Google Scholar]
- Lilley D. M., Houghton M. The interaction of RNA polymerase II from wheat with supercoiled and linear plasmid templates. Nucleic Acids Res. 1979 Feb;6(2):507–523. doi: 10.1093/nar/6.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter L. C. Kinetic analysis of deoxyribonuclease I cleavages in the nucleosome core: evidence for a DNA superhelix. J Mol Biol. 1978 Sep 15;124(2):391–420. doi: 10.1016/0022-2836(78)90306-6. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Martinson H. G., True R. J., Burch J. B. Specific histone-histone contacts are ruptured when nucleosomes unfold at low ionic strength. Biochemistry. 1979 Mar 20;18(6):1082–1089. doi: 10.1021/bi00573a023. [DOI] [PubMed] [Google Scholar]
- Meilhac M., Tysper Z., Chambon P. Animal DNA-dependent RNA polymerases. 4. Studies on inhibition by rifamycin derivatives. Eur J Biochem. 1972 Jul 13;28(2):291–300. doi: 10.1111/j.1432-1033.1972.tb01913.x. [DOI] [PubMed] [Google Scholar]
- Meneguzzi G., Pignatti P. F., Barbanti-Brodano G., Milanesi G. Minichromosome from BK virus as a template for transcription in vitro. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1126–1130. doi: 10.1073/pnas.75.3.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll M. DNA folding in the nucleosome. J Mol Biol. 1977 Oct 15;116(1):49–71. doi: 10.1016/0022-2836(77)90118-8. [DOI] [PubMed] [Google Scholar]
- Onishi T., Muramatsu M. Inhibition by derivatives of rifamycin of soluble ribonucleic acid polymerase from rat liver. Biochem J. 1972 Aug;128(5):1361–1364. doi: 10.1042/bj1281361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudet P., Germond J. E., Bellard M., Spadafora C., Chambon P. Nucleosome structure. Philos Trans R Soc Lond B Biol Sci. 1978 May 11;283(997):241–258. doi: 10.1098/rstb.1978.0021. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Mulvihill E. R., McKnight G. S., Senear A. W. Regulation of gene expression in the chick oviduct by steroid hormones. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):639–647. doi: 10.1101/sqb.1978.042.01.066. [DOI] [PubMed] [Google Scholar]
- Pardon J. F., Worcester D. L., Wooley J. C., Cotter R. I., Lilley D. M., Richards R. M. The structure of the chromatin core particle in solution. Nucleic Acids Res. 1977 Sep;4(9):3199–3214. doi: 10.1093/nar/4.9.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards B. M., Pardon J. F., Lilley D. M., Cotter R. I., Wooley J. C., Worcester D. L. Nucleosome sub-structure during transcription and replication. Philos Trans R Soc Lond B Biol Sci. 1978 May 11;283(997):287–289. doi: 10.1098/rstb.1978.0025. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P. A., Sahasrabuddhe C. G., Hodo H. G., 3rd, Saunders G. F. Transcription of nucleosomes from human chromatin. Nucleic Acids Res. 1978 Aug;5(8):2999–3012. doi: 10.1093/nar/5.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. T., Whitlock J. P. Mapping DNAase l-susceptible sites in nucleosomes labeled at the 5' ends. Cell. 1976 Oct;9(2):347–353. doi: 10.1016/0092-8674(76)90124-0. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Felsenfeld G. Pancreatic DNAase cleavage sites in nuclei. Cell. 1977 Mar;10(3):537–547. doi: 10.1016/0092-8674(77)90040-x. [DOI] [PubMed] [Google Scholar]
- Staynov D. Z., Spencer M., Allan J., Gould H. J. A reversible conformational transition in chromatin. Nature. 1979 May 17;279(5710):263–265. doi: 10.1038/279263a0. [DOI] [PubMed] [Google Scholar]
- Tien Kuo M., Sahasrabuddhe C. G., Saunders G. F. Presence of messenger specifying sequences in the DNA of chromatin subunits. Proc Natl Acad Sci U S A. 1976 May;73(5):1572–1575. doi: 10.1073/pnas.73.5.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Thevenin G., Oudet P., Chambon P. Transcription of in vitro assembled chromatin by Escherichia coli RNA polymerase. J Mol Biol. 1979 Mar 5;128(3):411–440. doi: 10.1016/0022-2836(79)90095-0. [DOI] [PubMed] [Google Scholar]
- Weihe A., von Mickwitz C. U., Grade K., Lindigkeit R. Complexes of DNA with arginine-rich and slightly lysine-rich histones. Transcription and electron microscopy. Biochim Biophys Acta. 1978 Mar 29;518(1):172–176. doi: 10.1016/0005-2787(78)90126-0. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Worcel A., Alberts B. A model for chromatin based upon two symmetrically paired half-nucleosomes. Cell. 1976 Nov;9(3):409–417. doi: 10.1016/0092-8674(76)90085-4. [DOI] [PubMed] [Google Scholar]
- Williamson P., Felsenfeld G. Transcription of histone-covered T7 DNA by Escherichia coli RNA polymerase. Biochemistry. 1978 Dec 26;17(26):5695–5705. doi: 10.1021/bi00619a015. [DOI] [PubMed] [Google Scholar]
- Zama M., Bryan P. N., Harrington R. E., Olins A. L., Olins D. E. Conformational states of chromatin. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):31–41. doi: 10.1101/sqb.1978.042.01.006. [DOI] [PubMed] [Google Scholar]
- de Pomerai D. I., Chesterton C. J., Butterworth P. H. The effect of heparin on the structure and template properties of chromatin. FEBS Lett. 1974 Jun 1;42(2):149–153. doi: 10.1016/0014-5793(74)80773-8. [DOI] [PubMed] [Google Scholar]