Abstract
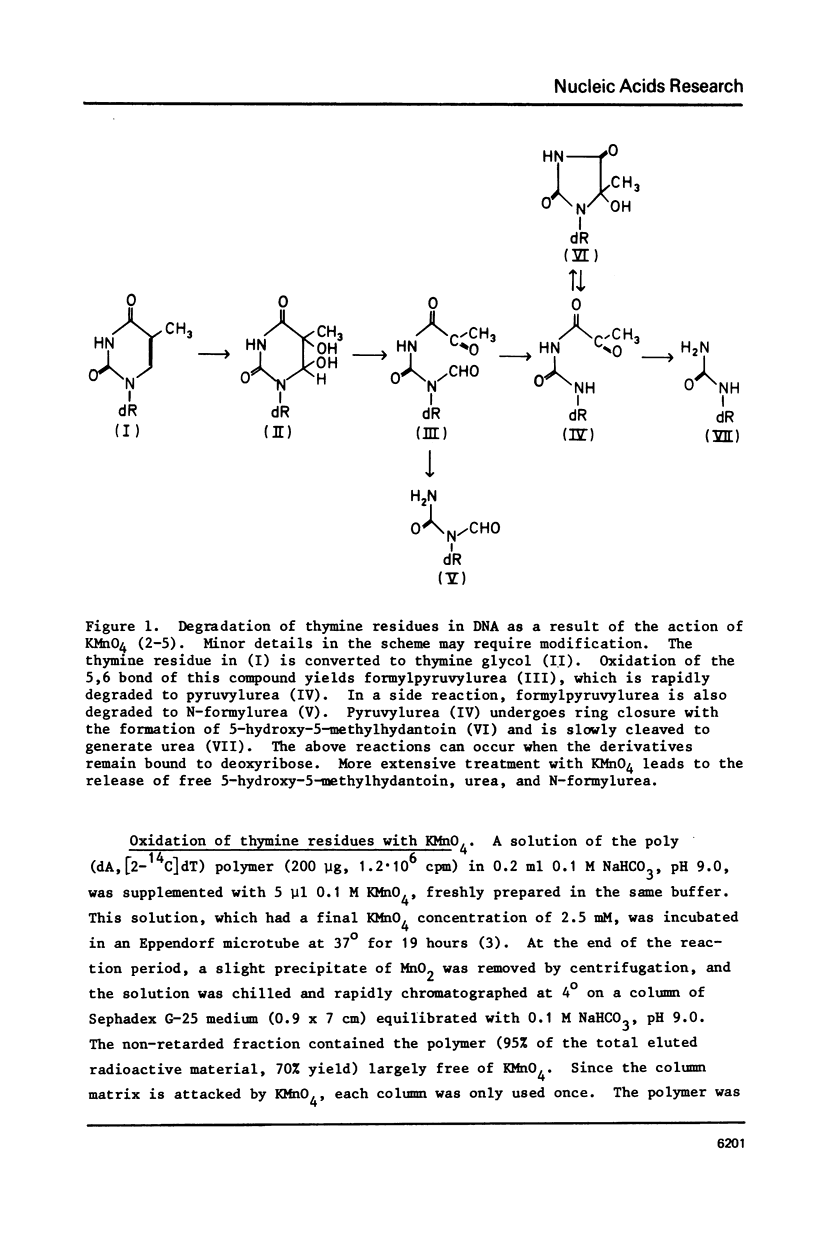
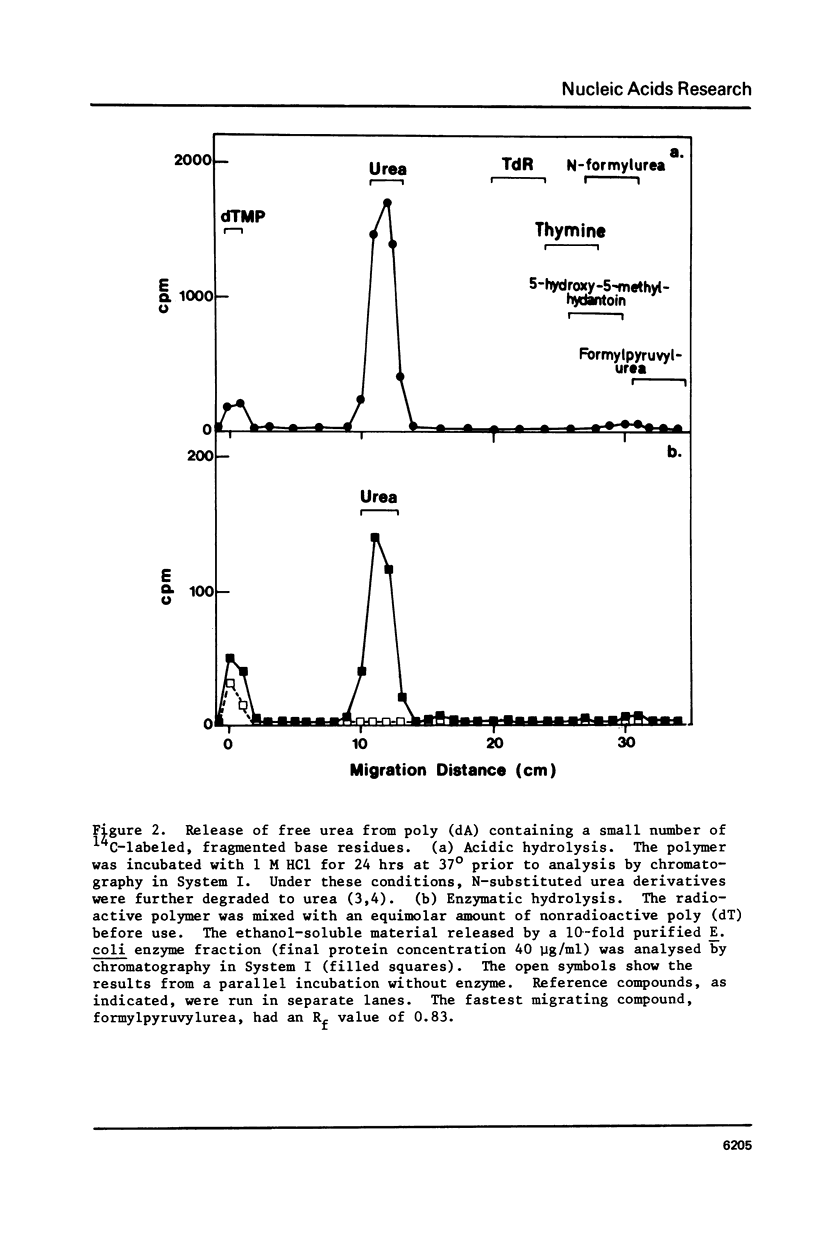
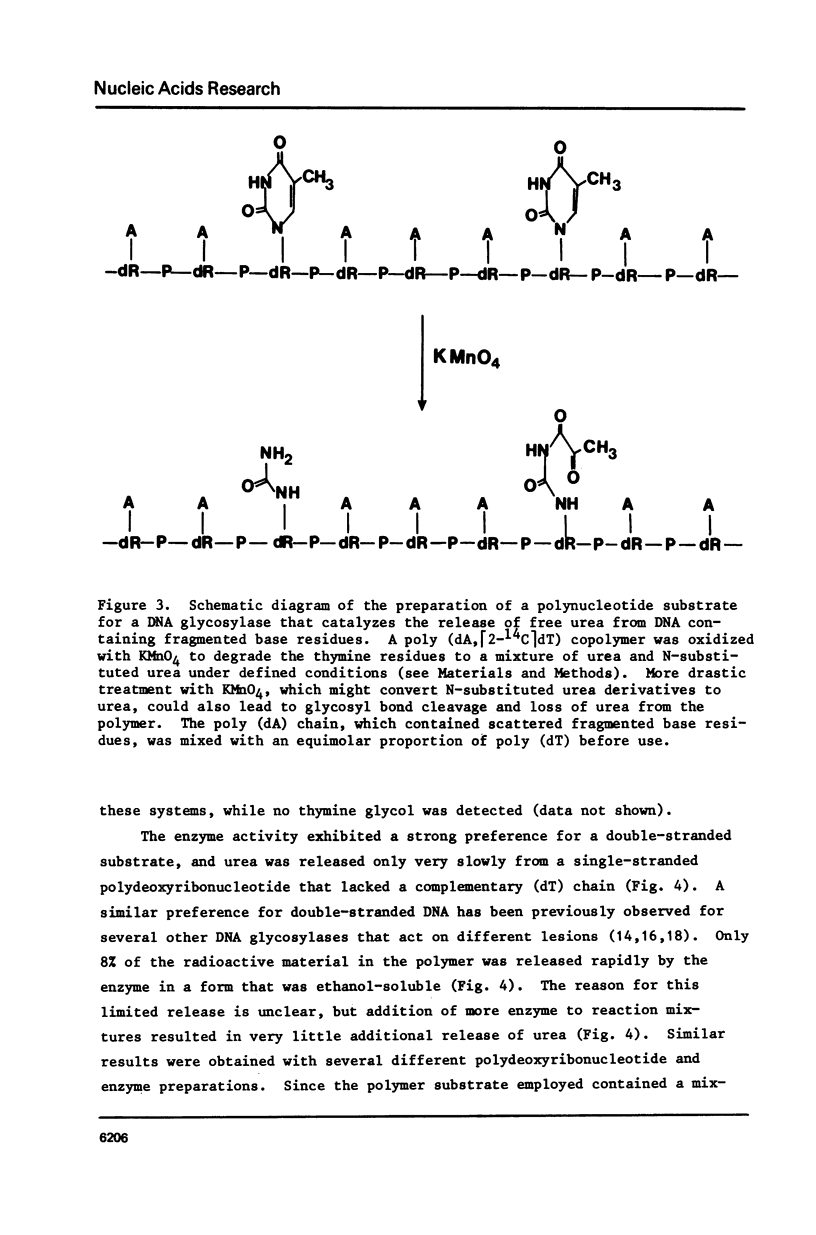
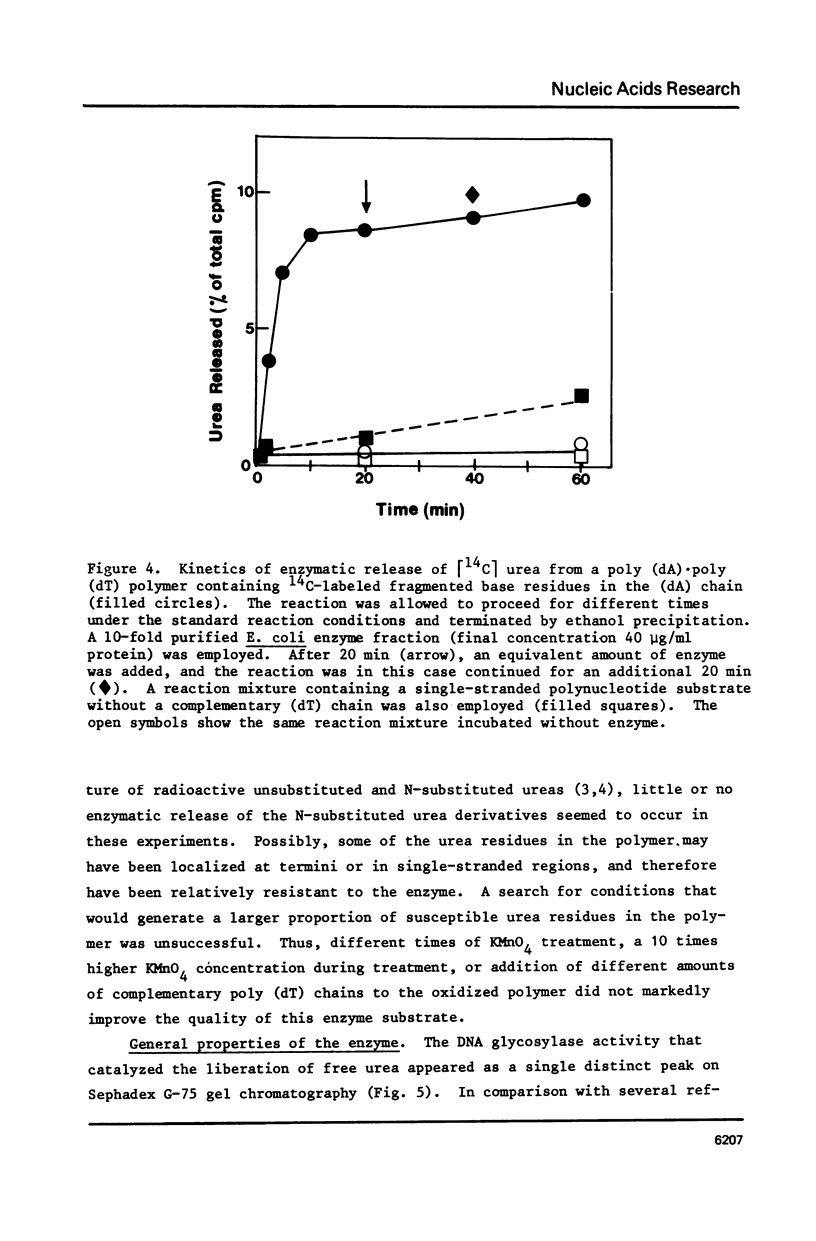
A poly (dA, [2-14C]dT) copolymer has been synthesized using terminal deoxynucleotidyltransferase. Treatment of the polydeoxyribonucleotide with potassium permanganate converts the thymine residues to urea and N-substituted urea derivatives, while the adenine residues are resistant to oxidation. This damaged polymer has been annealed with an equimolar amount of poly (dT) to generate a double-stranded polydeoxyribonucleotide containing scattered fragmented base residues, which are radioactively labeled selectively. On incubation of the latter with crude cell extracts from E. coli, free urea is released by a DNA glycosylase activity. The enzyme has been partly purified, and appears to be different from previously studied DNA glycosylase. It shows a strong preference for a double-stranded substrate, exhibits no cofactor requirement, and has a molecular weight of 20000 - 25000. Since fragmentation of pyrimidine residues is a major type of base lesion introduced in DNA by exposure to ionizing radiation, it seems likely this DNA glycosylase is active in repair of X-ray-induced lesions.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burton K., Riley W. T. Selective degradation of thymidine and thymine deoxynucleotides. Biochem J. 1966 Jan;98(1):70–77. doi: 10.1042/bj0980070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetsanga C. J., Lindahl T. Release of 7-methylguanine residues whose imidazole rings have been opened from damaged DNA by a DNA glycosylase from Escherichia coli. Nucleic Acids Res. 1979 Aug 10;6(11):3673–3684. doi: 10.1093/nar/6.11.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby G. K., Jones A. S., Tittensor J. R., Walker R. T. Chemical degradation of DNA oxidized by permanganate. Nature. 1967 Nov 25;216(5117):793–794. doi: 10.1038/216793a0. [DOI] [PubMed] [Google Scholar]
- Demple B., Linn S. DNA N-glycosylases and UV repair. Nature. 1980 Sep 18;287(5779):203–208. doi: 10.1038/287203a0. [DOI] [PubMed] [Google Scholar]
- Karran P., Lindahl T. Enzymatic excision of free hypoxanthine from polydeoxynucleotides and DNA containing deoxyinosine monophosphate residues. J Biol Chem. 1978 Sep 10;253(17):5877–5879. [PubMed] [Google Scholar]
- Karran P., Lindahl T., Ofsteng I., Evensen G. B., Seeberg E. Escherichia coli mutants deficient in 3-methyladenine-DNA glycosylase. J Mol Biol. 1980 Jun 15;140(1):101–127. doi: 10.1016/0022-2836(80)90358-7. [DOI] [PubMed] [Google Scholar]
- Kato K. I., Gonçalves J. M., Houts G. E., Bollum F. J. Deoxynucleotide-polymerizing enzymes of calf thymus gland. II. Properties of the terminal deoxynucleotidyltransferase. J Biol Chem. 1967 Jun 10;242(11):2780–2789. [PubMed] [Google Scholar]
- Lindahl T. DNA glycosylases, endonucleases for apurinic/apyrimidinic sites, and base excision-repair. Prog Nucleic Acid Res Mol Biol. 1979;22:135–192. doi: 10.1016/s0079-6603(08)60800-4. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Ljungquist S., Siegert W., Nyberg B., Sperens B. DNA N-glycosidases: properties of uracil-DNA glycosidase from Escherichia coli. J Biol Chem. 1977 May 25;252(10):3286–3294. [PubMed] [Google Scholar]
- Riazuddin S., Lindahl T. Properties of 3-methyladenine-DNA glycosylase from Escherichia coli. Biochemistry. 1978 May 30;17(11):2110–2118. doi: 10.1021/bi00604a014. [DOI] [PubMed] [Google Scholar]
- Teoule R., Bert C., Bonicel A. Thymine fragment damage retained in the DNA polynucleotide chain after gamma irradiation in aerated solutions. II. Radiat Res. 1977 Nov;72(2):190–200. [PubMed] [Google Scholar]
- Teoule R., Bonicel A., Bert C., Cadet J., Polverelli M. Identification of radioproducts resulting from the breakage of thymine moiety by gamma irradiation of E. coli DNA in an aerated aqueous solution. Radiat Res. 1974 Jan;57(1):46–58. [PubMed] [Google Scholar]
- Téoule R., Cadet J. Effet des rayons gamma sur les PYRIMIDINES. Isolement et identification d'une nouvelle substance obtenue par radiolyse de la thymine--le méthyl-5-hydroxy-5-hydantoïne. C R Acad Sci Hebd Seances Acad Sci D. 1969 May 19;268(20):2501–2503. [PubMed] [Google Scholar]
- YONEDA M., BOLLUM F. J. DEOXYNUCLEOTIDE-POLYMERIZING ENZYMES OF CALF THYMUS GLAND. I. LARGE SCALE PURIFICATION OF TERMINAL AND REPLICATIVE DEOXYNUCLEOTIDYL TRANSFERASES. J Biol Chem. 1965 Aug;240:3385–3391. [PubMed] [Google Scholar]


