Abstract
Aim
We hypothesized that the anticancer activity of cannabinoids was linked to induction of phosphatases.
Materials and Methods
The effects of cannabidiol (CBD) and the synthetic cannabinoid WIN-55,212 (WIN) on LNCaP (prostate) and SW480 (colon) cancer cell proliferation were determined by cell counting; apoptosis was determined by cleavage of poly(ADP)ribose polymerase (PARP) and caspase-3 (Western blots); and phosphatase mRNAs were determined by real-time PCR. The role of phosphatases and cannabinoid receptors in mediating CBD- and WIN-induced apoptosis was determined by inhibition and receptor knockdown.
Results
CBD and WIN inhibited LNCaP and SW480 cell growth and induced mRNA expression of several phosphatases, and the phosphatase inhibitor sodium orthovanadate significantly inhibited cannabinoid-induced PARP cleavage in both cell lines, whereas only CBD-induced apoptosis was CB1 and CB2 receptor-dependent.
Conclusion
Cannabinoid receptor agonists induce phosphatases and phosphatase-dependent apoptosis in cancer cell lines; however, the role of the CB receptor in mediating this response is ligand-dependent.
Keywords: Cannabinoids, apoptosis, protein tyrosine phosphatases, dual-specificity phosphatases
The anticancer activities of phytochemicals and their synthetic derivatives is complex and dependent on tumor type; however, several studies have shown that an important component of their activity is associated with induction of specific phosphatases which in turn inhibit kinase signaling pathways that are overexpressed in many tumor types. For example, several phytochemical anticancer agents, including betulinic acid and other polycyclic terpenoids, inhibit signal transducer and activator of transcription 3 (STAT3) activation and this has been linked to induction of the protein tyrosine phosphatase, non-receptor type 6 (PTPN6) (1–7). Curcumin, resveratrol and calcitriol (vitamin D analog) also induce expression of the dual specificity phosphatase 10 (DUSP10) mRNA in prostate cancer cell lines (8, 9) and this response has been linked to inhibition of p38 stress kinase activity.
Cannabinoids have long been used for ameliorating the debilitating effects of cytotoxic anticancer drugs; however, these compounds also exhibit antitumorigenic activity against multiple tumor types (9–13) and are already in clinical trials for treatment of brain tumors (e.g. gliomas). The mechanisms of action of cannabinoids are complex and dependent on ligand structure and cell context, and the effects are both receptor (CB1 and/or CB2) dependent and independent. Like many other phytochemical anticancer agents, cannabinoids induce growth inhibitory, proapoptotic and antimetastatic responses which are accompanied by modulation of several kinase activities (14–25). We hypothesized that cannabinoids may also induce phosphatases and the first objective of this study was to investigate the effects of the cannabinoids WIN 55,512-22 (WIN) and cannabidiol (CBD) on apoptosis in colon and prostate cancer cells. The second objective was to investigate the induction of phosphatases and the role of the cannabinoid (CB) receptors (CB1 and CB2) and the phosphatase inhibitor sodium orthovanadate (SOV) on cannabinoid-induced apoptosis.
Materials and Methods
Chemicals, antibodies, and reagents
WIN was purchased from Tocris Bioscience (Ellisville, MO, USA). Cannabidiol was kindly provided by Dr. Norbert. E. Kaminski (Michigan State University, East Lansing, MI, USA). Tyrosine phosphatase inhibitor, SOV, was purchased from Calbiochem (La Jolla, CA, USA). Cleaved poly (ADP-ribose) polymerase (PARP), phospho-c-jun N-terminal kinase (pJNK) and JNK antibodies were obtained from Cell Signaling (Danvers, MA, USA). β-Actin antibody was purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies for phospho-AKT, AKT, CB1 and CB2 receptors, phospho-extracellular signal-regulated kinase (pERK), ERK, phospho-ERBB-2, ErbB-2, phospho-P38, P38, caspase-3 and phospho-signal transducer and activator of transcription 3 (STAT3) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Chemiluminescence reagents for Western blot imaging were purchased from Millipore (Billerica, MA, USA). RPMI medium and Dulbecco’s Modified Eagle Medium (DMEM) were purchased from Sigma Aldrich.
Cell Culture
Human LNCaP prostate carcinoma cells were obtained from the American Type Culture Collection (Manassas, VA, USA). Human SW480 colon carcinoma cell lines were provided by Dr. Stanley Hamilton (M.D. Anderson Cancer Center, Houston, TX, USA). LNCaP cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 100× antibiotic-antimycotic solution (Sigma-Aldrich, St. Louis, MO, USA). SW480 cells were maintained in DMEM/F-12 supplemented with 5% fetal bovine serum and 100× antibiotic-antimycotic solution. Cells were maintained at 37°C in the presence of 5% CO2.
Cell Proliferation Assay
Prostate and colon cancer cells (3×104 per well) were plated in 12-well plates and allowed to attach for 24 h. The medium was then changed to DMEM/Ham’s F-12 medium without phenol red containing 2.5% charcoal-stripped FBS, and either vehicle dimethylsulfoxide (DMSO) or different concentrations of compounds were added. Cells were then trypsinized and counted after 24, 48 and 72 h using a Coulter Z1 cell counter (Sykesville, MD, USA). Each experiment was carried out in triplicate, and results are expressed as means ± SE for each set of experiments.
Western Blot Analyses
LNCaP prostate and SW480 colon cancer cells were seeded in DMEM/Ham’s F-12 medium. After 24 h, cells were treated with either vehicle (DMSO) or WIN and CBD for 48 and 24 h, respectively, or pretreated with SOV (0.25 mM and 0.5 mM) for 40 min and then treated with WIN and CBD. Cells were lysed with high salt buffer and subjected to sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting for cleaved PARP, CB1, CB2 and cleaved caspase-3 proteins as described previously (26).
Real-time PCR
LNCaP prostate and SW480 colon cancer cells were seeded and after 24 h, were treated with either vehicle (DMSO) or WIN and CBD for 24 h. Total RNA was isolated using the RNeasy Protect Mini kit according to the manufacturer’s protocol. cDNA was prepared and subjected to real-time PCR analysis as described previously (26). Table I lists the symbols, names and synonyms of genes discussed in this study. DUSP1 (forward-5′-CTCCATGCTCCTTGAGAGGAGAAATGC-3′, reverse-5′-GGTAGGTATGTCAAGCATGAAGAG-3′); DUSP4 (forward 5′-TACAAGTGCATCCCAGTGGA-3′, reverse 5′-CCCGTTTCTTCATCATCAGG-3′); cellular ACPP (forward 5′-TCTCAGTGGTGCCGCATCTA-3′, reverse 5′-CAGGGTGTGAGGATGGCAA-3′); serum ACPP (forward 5′-GGCAGATGATGCTTTGAGAACA-3′, reverse 5′-TCATCCAAAGCCCATTTTCC-3′); PTEN (forward 5′-CGAACTGGTGTAATGATATGT-3′, reverse 5′-CATGAACTTGTCTTCCCGT-3′); PTPRJ (forward 5′-TCGTTCGTGACTACATGAAGCA-3′, reverse 5′-CCCCAGCACTGCAATGC-3′); PTPN6 (forward 5′-AATGCGTCCCATACTGGCCCGA-3′, reverse 5′-CCCGCAGTTGGTCACAGAGT-3′); PTPN1 (forward 5′-TCCTACCTGGCTGTGATCGAG-3′, reverse 5′-CCTTCCACTGATCCTGCACTG-3′); PPP2R4 (forward 5′-GTTGGGAGGTGGCAGTGAG-3′, reverse 5′-AAACACTGGCCTCTGGTGTC-3′) primers were acquired from Sigma Aldrich. DUSP10 (forward 5′-GCTCAGGACCTGGACACCAT-3′, reverse 5′-GGAAGATGAGTGGTGACGTTGAT-3′) primer was acquired from IDT (Coralville, IA, USA). Values for each gene were normalized to expression levels of TATA-binding protein.
Table I.
List of the symbols, names and synonyms of genes discussed in this study.
| Symbol | Name | Synonyms |
|---|---|---|
| DUSP1 | dual specificity phosphatase 1 | MKP1 |
| DUSP4 | dual specificity phosphatase 4 | MKP2 |
| DUSP6 | dual specificity phosphatase 6 | MKP3 |
| DUSP10 | dual specificity phosphatase 10 | MKP5 |
| ACPP | prostatic acid phosphatase, cellular and serum | cPAcP, sPAcP |
| ERBB2 | human epidermal growth factor receptor-2 | HER2 |
| PTEN | phosphatase and tensin homolog | PTEN |
| PTPRJ | protein tyrosine phosphatase, receptor type, J | DEP1 |
| PTPN6 | protein tyrosine phosphatase, non-receptor type 6 | SHP1 |
| PTPN1 | protein tyrosine phosphatase, non-receptor type 1 | PTP1B |
| PPP2R4 | protein phosphatase 2A activator, regulatory subunit 4 | PP2A |
| STAT3 | signal transducer and activator of transcription 3 | STAT3 |
Small Inhibitory RNAs (siRNA) Interference Assay
LNCaP and SW480 cells were seeded (1×105 per well) in six-well plates in DMEM/Ham’s F-12 medium supplemented with 2.5% charcoal-stripped FBS without antibiotic and left to attach for 24 h. The CB1 and CB2 knockdown (iCB1 and iCB2) along with iLamin as control was performed using Lipofectamine 2000 reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). siRNAs were purchased from Sigma-Aldrich.
Results
WIN and CBD induce apoptosis and inhibit proliferation of LNCaP and SW480 cells
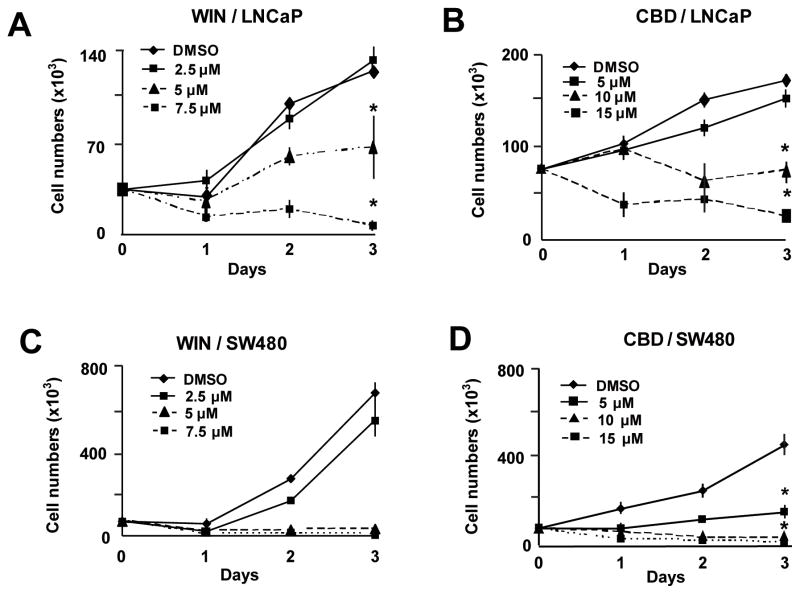
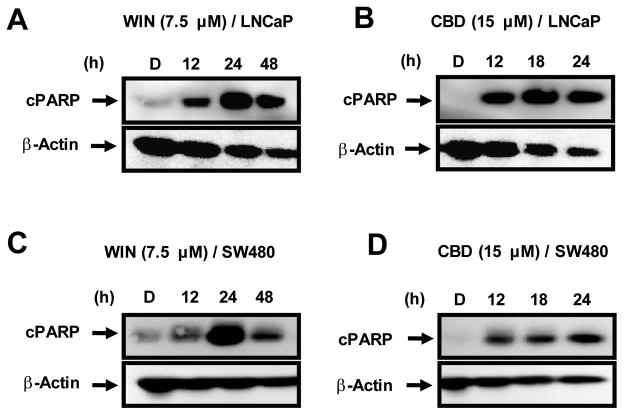
Results summarized in Figures 1A and 1B show that WIN and CBD inhibited LNCaP cell growth, and half-maximal concentrations for growth inhibition (IC50)after 2 days were 5.1 and 10.0 μM and, after 3 days, were 4.59 and 9.43 μM, respectively. Similar results were observed for SW480 cells (Figures 1C and 1D); IC50 values (for days 2 and 3) were 3.3 and 3.5 μM for WIN and 5.95 and 5.06 μM for CBD. The concentration-dependent effects of CBD on cell proliferation were observed within 24 h after treatment, whereas the growth-inhibitory effects of WIN were somewhat delayed in both cell lines. WIN and CBD also induced a time-dependent increase in PARP cleavage, a marker of apoptosis, in LNCaP and SW480 (Figure 2) cells. Maximal induction by CBD was observed within 12 h after treatment in both cell lines, whereas WIN-induced PARP cleavage was maximal after 24 h.
Figure 1.
Concentration-dependent effects of WIN and CBD on LNCaP (A, B) and SW480 (C, D) cell proliferation. Cells were treated with DMSO and 2.5–7.5 μM of the synthetic cannabinoid WIN-55,212 (WIN) and 5–15 μM of cannabidiol (CBD) for 72 hr. Cells were counted as described in the Materials and Methods. Results are expressed as mean ± S.E. for three replicate determinations for each treatment group, and significantly (p<0.05) reduced proliferation is indicated (*). Proliferation of cells treated with DMSO (solvent control) was set at 100%. β-Actin served as a loading control for all Western blots.
Figure 2.
Induction of apoptosis by the synthetic cannabinoid WIN-55,212 (WIN) and cannabidiol (CBD). Time-dependent effects of WIN and CBD on cleaved poly(ADP)ribose polymerase (PARP) expression in LNCaP (A, B) and SW480 (C, D) cells. LNCaP and SW480 cells were treated with dimethylsulfoxide (DMSO), 7.5 μM WIN (12, 24 and 48 h) and 15 μM CBD (12, 18, and 24 h), and cPARP expression was determined by Western blot analysis of whole-cell lysates as described in the Materials and Methods.
WIN and CBD induce phosphatase mRNA expression in LNCaP and SW480 cells
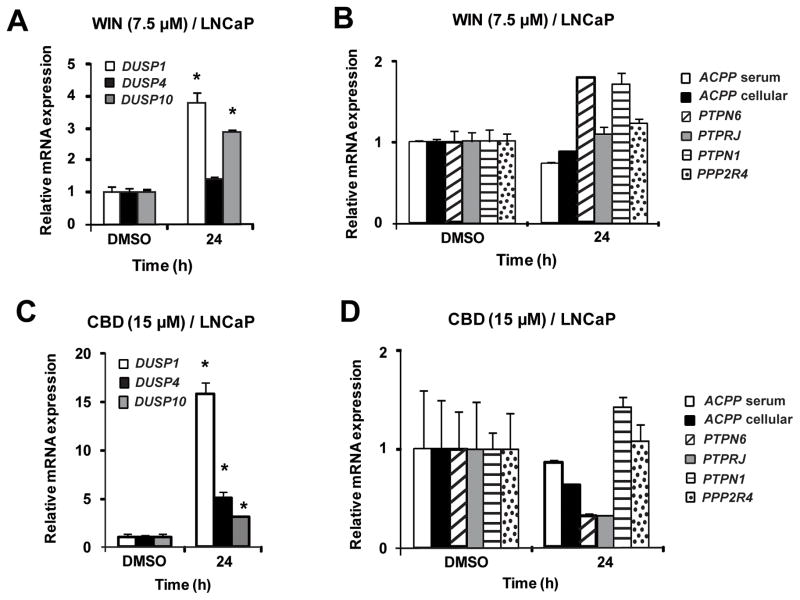
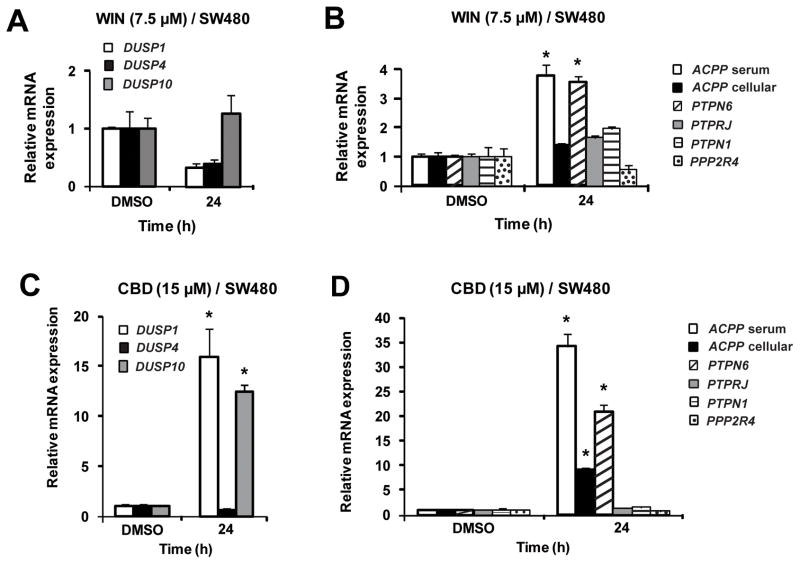
Figure 3 summarizes the effects of WIN and CBD on induction of phosphatase mRNA levels in LNCaP cells. WIN induced DUSP1 and DUSP10 and CBD induced DUSP1, DUSP4 and DUSP10 mRNA expression after 24 hr. Similar results were observed at earlier time points, and WIN also induced mRNA expression of DUSP4 after 48 h (data not shown). In contrast, none of the selected protein tyrosine phosphatases were induced by WIN or CBD in LNCaP cells. In SW480 cells, WIN did not induce the dual specificity phosphatases but induced serum ACPP and PTPN6 after treatment for 24 h (Figure 4); however, after 48 h, induction of cellular ACPP, DUSP1 and DUSP10 by WIN were also observed (data not shown). CBD induced expression of DUSP1, DUSP10, serum ACPP, cellular ACPP and PTPN6 in SW480 cells after treatment for 24 h. These results demonstrate that like other anticancer agents, cannabinoids induce mRNA expression of several dual specificity phosphatases and protein tyrosine phosphatases, and current studies are focused on the induction of gene expression and activation of individual phosphatase proteins and their functions in cancer cells.
Figure 3.
Effects of the synthetic cannabinoid WIN-55,212 (WIN) and cannabidiol (CBD) on phosphatase mRNA levels in LNCaP cells. Induction by WIN (A, B) and CBD (C, D). LNCaP cells were treated with dimethylsulfoxide (DMSO), 7.5 μM WIN and 15 μM CBD for 24 h, and dual specificity phosphatase 1 (DUSP1), dual specificity phosphatase 4 (DUSP4), dual specificity phosphatase 10 (DUSP10), serum prostatic acid phosphate (sACPP), cellular prostatic acid phosphate (cACPP), protein tyrosine phosphatase, non-receptor type 1 (PTPN1), protein phosphatase 2A activator, regulatory subunit 4 (PPP2R4), protein tyrosine phosphatase, receptor type, J (PTPRJ) and protein tyrosine phosphatase, non-receptor type 6 (PTPN6) mRNA levels were determined by real-time PCR as described in the Materials and Methods. Results are expressed as mean ± S.E. for 3 replicate experiments and significant (p<0.05) increases are indicated (*).
Figure 4.
Effects of the synthetic cannabinoid WIN-55,212 (WIN) and cannabidiol (CBD) on phosphatase mRNA levels in SW480 cells. Induction by WIN (A, B) and CBD (C, D). SW480 cells were treated with dimethylsulfoxide (DMSO), 7.5 μM WIN and 15 μM CBD for 24 h, and dual specificity phosphatase 1 (DUSP1), dual specificity phosphatase 4 (DUSP4), dual specificity phosphatase 10 (DUSP10), serum prostatic acid phosphate (sACPP), cellular prostatic acid phosphate (cACPP), protein tyrosine phosphatase, non-receptor type 1 (PTPN1), protein phosphatase 2A activator, regulatory subunit 4 (PPP2R4), protein tyrosine phosphatase, receptor type, J (PTPRJ) and protein tyrosine phosphatase, non-receptor type 6 (PTPN6) mRNA levels were determined by real-time PCR as described in the Materials and Methods. Results are expressed as mean ± S.E. for 3 replicate experiments and significant (p<0.05) increases are indicated (*).
The effects of WIN- and CBD-induced phosphatases on dephosphorylation of p42/44 MAPK, pAKT, pSTAT3, pJNK, pERBB2, and pP38 MAPK were investigated in LNCaP and SW480 cells and, with few exceptions, the data were difficult to interpret since both phospho-and total kinase protein levels were reduced simultaneously (data not shown). This suggested that cannabinoid-induced phosphatases reduced multiple kinase activities but also activated other pathways that affect expression of their corresponding proteins (total). The mechanisms associated with this reduction are currently being investigated.
WIN- and CBD-induced apoptosis is phosphatase dependent: the role of CB receptors is ligand dependent
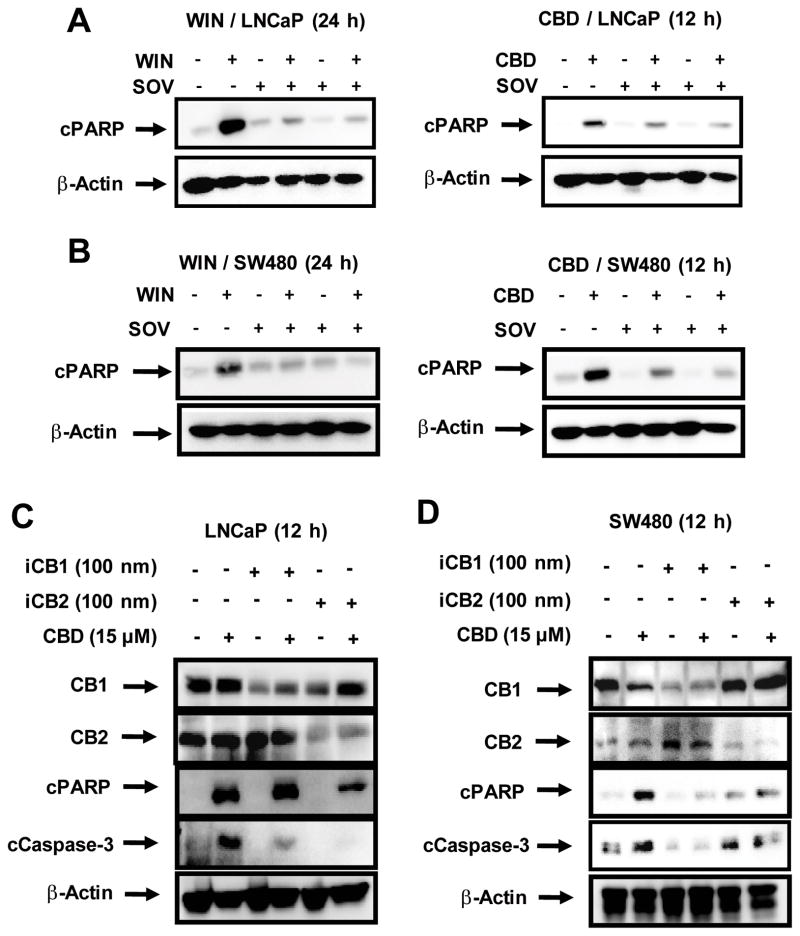
Preliminary studies on the effects of phosphatase inhibitor cocktails and their components on CBD- and WIN-induced growth inhibition and apoptosis showed that SOV was the most effective inhibitor and this was consistent with CBD- and WIN-dependent induction of dual-specificity and protein tyrosine phosphatases which are inhibited by SOV. WIN and CBD induced PARP cleavage as early as 12 h after treatment, and PARP cleavage induced by WIN and CBD persisted for 48 and 24 h, respectively (Figure 2). Since near maximal induction of PARP cleavage by WIN and CBD was observed after treatment of SW480 and LNCaP cells for 24 or 12 h, respectively, these time points were used for investigating the effects of SOV on WIN- and CBD-induced PARP cleavage. Cells were treated with the cannabinoids alone and in combination with SOV. The phosphatase inhibitor SOV significantly reduced WIN- and CBD-induced PARP cleavage in LNCaP (Figure 5A) and SW480 (Figure 5B) cells, demonstrating that the CBD- and WIN-induced phosphatase activities play an important role in the pro-apoptotic activity of these compounds.
Figure 5.
Effects of phosphatase inhibitor sodium orthovanadate (SOV) on cannabinoid-induced PARP cleavage in LNCaP (A) and SW480 (B) cells. Cells were pre-treated with SOV followed by treatment with the synthetic cannabinoid WIN-55,212 (WIN) and cannabidiol (CBD) for 24 and 12 h, respectively, and whole-cell lysates were analyzed by Western blot analysis as described in the Materials and Methods. β-Actin served as a loading control for all Western blots. Effects of cannabinoids and CB1 and CB2 receptor knockdown by RNA interference in LNCaP (C) and SW480 (D) cells. Cells were transfected with siRNAs against CB1 and CB2 receptors followed by treatment with CBD for 12 h and whole-cell lysates were examined for expression of cleaved poly(ADP)ribose polymerase (cPARP), cleaved caspase-3, and CB1 and CB2 receptor proteins determined by Western blot analysis as indicated in the Materials and Methods. β-Actin served as a loading control for all Western blot analyses.
Receptor-dependent and -independent induction of apoptosis by WIN and CBD was investigated in LNCaP and SW480 cells treated with the cannabinoids alone or after transfection with siRNAs targeting CB1 and CB2 receptors. CBD-induced PARP and caspase-3 cleavage in LNCaP cells was inhibited after knockdown of the CB2 receptor (Figure 5C), whereas after CB1 receptor knockdown, CBD-induced caspase activation was partially inhibited but PARP cleavage was not affected. In SW480 cells, both CB1 and CB2 receptor knockdown inhibited CBD-induced PARP cleavage and caspase-3 activation (cleavage) (Figure 5D). In contrast, WIN-induced apoptosis and caspase-3 cleavage was unaffected by knockdown of CB1 or CB2 receptors in LNCaP or SW480 cells (data not shown). Thus, the proapoptotic activities of WIN and CBD were phosphatase dependent in both cell lines but cannibinoid receptor independent and dependent, respectively, in LNCaP and SW480 cells.
Discussion
Cannabinoids have emerged as an important new class of anticancer drugs that bind cannabinoid receptors and activate several downstream pathways leading to inhibition of cancer cell proliferation, and induction of apoptosis (10–13). Their mechanisms of action are complex and dependent on cell context and ligand structure and there are examples of cannabinoid-mediated activities that are receptor dependent and independent (14–24). Several studies show that cannabinoids such as WIN, Δ9-tetrahydrocannabinol (ΔTHC) and CBD modulate kinase activities in cancer cell lines and this includes inhibition of membrane-bound and intracellular kinases, resulting in their dephosphorylation. The mechanisms associated with cannabinoid-induced inactivation of kinases is unknown; however, several different classes of anticancer drugs induce dual specificity and protein tyrosine phosphatases which selectively inactivate phosphokinases that play a role in cancer cell growth and survival (1–8, 27–33).
Previous findings on the effects of cannabinoids on activated phosphokinases are highly variable in different cancer cell lines and dependent on the structure of the cannabinoid and cell context. WIN inhibited growth and induced apoptosis in LNCaP cells and this was due in part to sustained phosphorylation of p42/44 MAPK and lower p-AKT expression (22). The phytochemical ΔTHC inhibited growth and induced apoptosis in SW480 cells and this was accompanied by reduced expression of phospho-p42/p44 MAPK and phospho-AKT (19).
Although the reported effects of cannabinoids on phosphokinases were highly variable among different cell lines, we observed that WIN and CBD induced mRNA expression of several dual specificity and protein tyrosine phosphatases in LNCaP and SW480 cells (Figures 3 and 4). Selection of the phosphatases was based on their putative anticarcinogenic activities and on previous reports showing that dual specificity and protein tyrosine phosphatases were induced by several phytochemical anticancer agents including curcumin, resveratrol, ursolic acid, betulinic acid, guaiane sesquiterpenoids, guggulsterone, epigallocatechin-3-gallate and retinoic acid (1–8, 27–33). Previous studies with these compounds primarily focused on induction of a single phosphatase gene, whereas the results of this study showed that WIN and CBD induced mRNA expression of multiple and overlapping phosphatases (Figures 3 and 4). WIN and CBD induced DUSP1 and DUSP10 after treatment for 24 h in LNCaP cells, whereas DUSP4 induction by CBD and WIN was time dependent (24 and 48 h, respectively) (Figure 3). In SW480 cells, the pattern of phosphatase induction by WIN and CBD differed after treatment for 24 h primarily due to induction of DUSP1 by CBD and not WIN (Figure 4); however, after 48 h, WIN also induced DUSP1 (data not shown). Since both CB receptor antagonists alone induced expression of phosphatase mRNAs (data not shown), the role of the CB receptor in mediating induction of individual phosphatase mRNAs was not further investigated. In addition, the effects of CBD-and WIN-dependent induction of gene expression of dual specificity and protein tyrosine phosphatases on dephosphorylation of phosphokinase proteins was confounded by the parallel decrease of most phosphokinase and total kinase proteins (data not shown). It was apparent in SW480 cells that WIN dramatically reduced AKT phosphorylation without reducing total AKT protein, and this was comparable to the effects reported for ΔTHC in SW480 cells (19). The only other consistent observation was that WIN and CBD enhanced phosphorylation of the p38 stress protein kinase in both cell lines (data not shown). These results demonstrate that WIN-/CBD-induced phosphatases may play a role in kinase inactivation in SW480 cells; however, these results were confounded by the parallel decrease in kinase proteins, and the mechanisms of the latter response are currently being investigated.
CBD and WIN also inhibited LNCaP and SW480 cell growth (Figure 1) and induced apoptosis (Figure 2) in both cell lines, and induction of apoptosis by cannabinoids is an important element of their anticancer activity (6–9). Induction of caspase-dependent PARP cleavage was used as an indicator of apoptosis and it was clear that both WIN and CBD induced PARP cleavage in LNCaP and SW480 cells (Figure 2) and this is consistent with cannabinoid-induced apoptosis in many other cancer cell lines (10–24). Moreover, we also observed that WIN-/CBD-induced PARP cleavage dramatically decreased in cells co-treated with the phosphatase inhibitor SOV (Figure 5A and B), indicating that cannabinoid-induced phosphatases played a role in the induction of SW480 and LNCaP cell death. It should be noted that although induction of PARP cleavage by WIN was maximal after 24 h, there was significant induction within 12 h in LNCaP and SW480 cells (Figure 2). In contrast, induction of phosphatase mRNA levels after treatment with WIN for 12 h was relatively low in SW480 cells, whereas modest but significant induction was observed in LNCaP cells (data not shown). These results suggest that other ‘proapoptotic’ phosphatases may also be induced or activated by WIN and CBD and these are currently being investigated. CB1 and CB2 receptor knockdown by siRNA was used to determine the role of the CB receptors in mediating CB- and WIN-induced proapoptotic activity (cleavage of PARP and caspase-3) and the results demonstrate that the effects of WIN were CB receptor independent, whereas CBD-induced apoptosis was CB receptor dependent (Figure 5).
In summary, this study shows for the first time that cannabinoids induced mRNA expression of several phosphatases in LNCaP and SW480 cells and this was consistent with induction of phosphatases by other phytochemical anticancer drugs (1–8, 27–33). The direct effects of phosphatase induction on reduced phosphokinase protein expression was confounded by a parallel decrease in most (total) kinase proteins. This response may also be important for the anticancer activities of WIN and CBD and is currently being investigated. Previous studies have reported induction of DUSP1 and DUSP6 by cannabinoids in microglial cells and this resulted in inactivation of MAPK; however, effects on apoptosis were not determined (34, 35). The results obtained using the phosphatase inhibitor SOV demonstrate that induction of apoptosis by WIN and CBD was significantly blocked by the phosphatase inhibitor SOV, and this represents a novel proapoptotic pathway induced by cannabinoids. Current studies are focused on identification of specific proapoptotic phosphatases and their mechanisms of induction or activation by cannabinoids.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health (R01-CA136571) and Texas AgriLife.
Footnotes
Disclosures/Conflicts of Interest: None
References
- 1.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–30. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 2.Pathak AK, Bhutani M, Nair AS, Ahn KS, Chakraborty A, Kadara H, Guha S, Sethi G, Aggarwal BB. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol Cancer Res. 2007;5:943–55. doi: 10.1158/1541-7786.MCR-06-0348. [DOI] [PubMed] [Google Scholar]
- 3.Ahn KS, Sethi G, Sung B, Goel A, Ralhan R, Aggarwal BB. Guggulsterone, a farnesoid × receptor antagonist, inhibits constitutive and inducible STAT3 activation through induction of a protein tyrosine phosphatase SHP-1. Cancer Res. 2008;68:4406–15. doi: 10.1158/0008-5472.CAN-07-6696. [DOI] [PubMed] [Google Scholar]
- 4.Lee J, Hahm ER, Singh SV. Withaferin A inhibits activation of signal transducer and activator of transcription 3 in human breast cancer cells. Carcinogenesis. 2010;31:1991–8. doi: 10.1093/carcin/bgq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandey MK, Sung B, Aggarwal BB. Betulinic acid suppresses STAT3 activation pathway through induction of protein tyrosine phosphatase SHP-1 in human multiple myeloma cells. Int J Cancer. 2010;127:282–92. doi: 10.1002/ijc.25059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu LJ, Wu ML, Li H, Chen XY, Wang Q, Sun Y, Kong QY, Liu J. Inhibition of STAT3 expression and signaling in resveratrol-differentiated medulloblastoma cells. Neoplasia. 2008;10:736–44. doi: 10.1593/neo.08304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glienke W, Maute L, Wicht J, Bergmann L. Curcumin inhibits constitutive STAT3 phosphorylation in human pancreatic cancer cell lines and down-regulation of survivin/BIRC5 gene expression. Cancer Invest. 2010;28:166–71. doi: 10.3109/07357900903287006. [DOI] [PubMed] [Google Scholar]
- 8.Nonn L, Duong D, Peehl DM. Chemopreventive anti-inflammatory activities of curcumin and other phytochemicals mediated by MAP kinase phosphatase-5 in prostate cells. Carcinogenesis. 2007;28:1188–96. doi: 10.1093/carcin/bgl241. [DOI] [PubMed] [Google Scholar]
- 9.Krishnan AV, Moreno J, Nonn L, Malloy P, Swami S, Peng L, Peehl DM, Feldman D. Novel pathways that contribute to the antiproliferative and chemopreventive activities of calcitriol in prostate cancer. J Steroid Biochem Mol Biol. 2007;103:694–702. doi: 10.1016/j.jsbmb.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 10.Guzman M. Cannabinoids: potential anticancer agents. Nat Rev Cancer. 2003;3:745–55. doi: 10.1038/nrc1188. [DOI] [PubMed] [Google Scholar]
- 11.Sarfaraz S, Adhami VM, Syed DN, Afaq F, Mukhtar H. Cannabinoids for cancer treatment: progress and promise. Cancer Res. 2008;68:339–42. doi: 10.1158/0008-5472.CAN-07-2785. [DOI] [PubMed] [Google Scholar]
- 12.Bernadette H. Cannabinoid therapeutics: high hopes for the future. Drug Discov Today. 2005;10 :459–62. doi: 10.1016/S1359-6446(05)03417-3. [DOI] [PubMed] [Google Scholar]
- 13.Hall W, Christie M, Currow D. Cannabinoids and cancer: causation, remediation, and palliation. Lancet Oncol. 2005;6:35–42. doi: 10.1016/S1470-2045(04)01711-5. [DOI] [PubMed] [Google Scholar]
- 14.Carracedo A, Lorente M, Egia A, Blazquez C, Garcia S, Giroux V, Malicet C, Villuendas R, Gironella M, Gonzalez-Feria L, Piris MA, Iovanna JL, Guzman M, Velasco G. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell. 2006;9:301–12. doi: 10.1016/j.ccr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Blazquez C, Salazar M, Carracedo A, Lorente M, Egia A, Gonzalez-Feria L, Haro A, Velasco G, Guzman M. Cannabinoids inhibit glioma cell invasion by down-regulating matrix metalloproteinase-2 expression. Cancer Res. 2008;68:1945–52. doi: 10.1158/0008-5472.CAN-07-5176. [DOI] [PubMed] [Google Scholar]
- 16.Sarfaraz S, Afaq F, Adhami VM, Mukhtar H. Cannabinoid receptor as a novel target for the treatment of prostate cancer. Cancer Res. 2005;65:1635–41. doi: 10.1158/0008-5472.CAN-04-3410. [DOI] [PubMed] [Google Scholar]
- 17.Sarfaraz S, Afaq F, Adhami VM, Malik A, Mukhtar H. Cannabinoid receptor agonist-induced apoptosis of human prostate cancer cells LNCaP proceeds through sustained activation of ERK1/2 leading to G1 cell cycle arrest. J Biol Chem. 2006;281:39480–91. doi: 10.1074/jbc.M603495200. [DOI] [PubMed] [Google Scholar]
- 18.Caffarel MM, Sarrio D, Palacios J, Guzman M, Sanchez C. Delta9-tetrahydrocannabinol inhibits cell cycle progression in human breast cancer cells through CDC2 regulation. Cancer Res. 2006;66:6615–21. doi: 10.1158/0008-5472.CAN-05-4566. [DOI] [PubMed] [Google Scholar]
- 19.Greenhough A, Patsos HA, Williams AC, Paraskeva C. The cannabinoid delta(9)-tetrahydrocannabinol inhibits RAS-MAPK and PI3K-AKT survival signalling and induces BAD-mediated apoptosis in colorectal cancer cells. Int J Cancer. 2007;121:2172–80. doi: 10.1002/ijc.22917. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Wang H, Ning W, Backlund MG, Dey SK, DuBois RN. Loss of cannabinoid receptor 1 accelerates intestinal tumor growth. Cancer Res. 2008;68:6468–76. doi: 10.1158/0008-5472.CAN-08-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaccani A, Massi P, Colombo A, Rubino T, Parolaro D. Cannabidiol inhibits human glioma cell migration through a cannabinoid receptor-independent mechanism. Br J Pharmacol. 2005;144:1032–6. doi: 10.1038/sj.bjp.0706134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Massi P, Vaccani A, Ceruti S, Colombo A, Abbracchio MP, Parolaro D. Antitumor effects of cannabidiol, a nonpsychoactive cannabinoid, on human glioma cell lines. J Pharmacol Exp Ther. 2004;308:838–45. doi: 10.1124/jpet.103.061002. [DOI] [PubMed] [Google Scholar]
- 23.Ramer R, Merkord J, Rohde H, Hinz B. Cannabidiol inhibits cancer cell invasion via up-regulation of tissue inhibitor of matrix metalloproteinases-1. Biochem Pharmacol. 2010;79:955–66. doi: 10.1016/j.bcp.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 24.McKallip RJ, Jia W, Schlomer J, Warren JW, Nagarkatti PS, Nagarkatti M. Cannabidiol-induced apoptosis in human leukemia cells: A novel role of cannabidiol in the regulation of p22PHOX and NOX4 expression. Mol Pharmacol. 2006;70:897–908. doi: 10.1124/mol.106.023937. [DOI] [PubMed] [Google Scholar]
- 25.Xian XS, Park H, Cho YK, Lee IS, Kim SW, Choi MG, Chung IS, Han KH, Park JM. Effect of a synthetic cannabinoid agonist on the proliferation and invasion of gastric cancer cells. J Cell Biochem. 2010;110:321–32. doi: 10.1002/jcb.22540. [DOI] [PubMed] [Google Scholar]
- 26.Sreevalsan S, Jutooru I, Chadalapaka G, Walker M, Safe S. 1,1-Bis(3′-indolyl)-1-(p-bromophenyl)methane and related compounds repress survivin and decrease gamma-radiation-induced survivin in colon and pancreatic cancer cells. Int J Oncol. 2009;35:1191–9. doi: 10.3892/ijo_00000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King EM, Holden NS, Gong W, Rider CF, Newton R. Inhibition of NF-kappaB-dependent transcription by MKP-1: transcriptional repression by glucocorticoids occurring via p38 MAPK. J Biol Chem. 2009;284:26803–15. doi: 10.1074/jbc.M109.028381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawan A, Al-Harthi S, Cadalbert L, McCluskey AG, Shweash M, Grassia G, Grant A, Boyd M, Currie S, Plevin R. Deletion of the dual specific phosphatase-4 (DUSP-4) gene reveals an essential non-redundant role for MAP kinase phosphatase-2 (MKP-2) in proliferation and cell survival. J Biol Chem. 2011;286:12933–43. doi: 10.1074/jbc.M110.181370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qin J, Chen HG, Yan Q, Deng M, Liu J, Doerge S, Ma W, Dong Z, Li DW. Protein phosphatase-2A is a target of epigallocatechin-3-gallate and modulates p53-Bak apoptotic pathway. Cancer Res. 2008;68:4150–62. doi: 10.1158/0008-5472.CAN-08-0839. [DOI] [PubMed] [Google Scholar]
- 30.Ramirez CJ, Haberbusch JM, Soprano DR, Soprano KJ. Retinoic acid-induced repression of AP-1 activity is mediated by protein phosphatase 2A in ovarian carcinoma cells. J Cell Biochem. 2005;96:170–82. doi: 10.1002/jcb.20520. [DOI] [PubMed] [Google Scholar]
- 31.Chuang TD, Chen SJ, Lin FF, Veeramani S, Kumar S, Batra SK, Tu Y, Lin MF. Human prostatic acid phosphatase, an authentic tyrosine phosphatase, dephosphorylates ERBB-2 and regulates prostate cancer cell growth. J Biol Chem. 2010;285:23598–606. doi: 10.1074/jbc.M109.098301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balavenkatraman KK, Jandt E, Friedrich K, Kautenburger T, Pool-Zobel BL, Ostman A, Bohmer FD. DEP-1 protein tyrosine phosphatase inhibits proliferation and migration of colon carcinoma cells and is upregulated by protective nutrients. Oncogene. 2006;25:6319–24. doi: 10.1038/sj.onc.1209647. [DOI] [PubMed] [Google Scholar]
- 33.Choi JY, Na M, Hyun Hwang I, Ho Lee S, Young Bae E, Yeon Kim B, Seog Ahn J. Isolation of betulinic acid, its methyl ester and guaiane sesquiterpenoids with protein tyrosine phosphatase 1B inhibitory activity from the roots of Saussurea lappa C. B. Clarke. Molecules. 2009;14:266–72. doi: 10.3390/molecules14010266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eljaschewitsch E, Witting A, Mawrin C, Lee T, Schmidt PM, Wolf S, Hoertnagl H, Raine CS, Schneider-Stock R, Nitsch R, Ullrich O. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron. 2006;49:67–79. doi: 10.1016/j.neuron.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 35.Romero-Sandoval EA, Horvath R, Landry RP, DeLeo JA. Cannabinoid receptor type 2 activation induces a microglial anti-inflammatory phenotype and reduces migration via MKP induction and ERK dephosphorylation. Mol Pain. 2009;5:25–39. doi: 10.1186/1744-8069-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.