Abstract
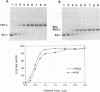
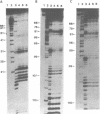
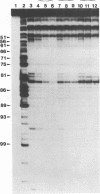
Immature Xenopus laevis oocytes contain large quantities of a 7S ribonucleoprotein particle containing transcription factor IIIA (TFIIIA) and 5S RNA in a 1:1 molar ratio. We have reconstituted RNPs containing 5S RNA and either intact TFIIIA or proteolytic fragments that represent progressive C-terminal deletions of the protein. A partial trypsin digestion fragment encompassing the amino terminal seven zinc fingers of TFIIIA rebinds 5S RNA with nearly the same affinity as intact TFIIIA. We have compared the RNase protection patterns resulting from binding of intact and deleted forms of TFIIIA. RNAse protection assays using cobra venom nuclease were performed on complexes reconstituted with 5' and 3' end-labeled 5S RNA. Similar experiments with 3' end-labeled 5S RNA were performed with nuclease alpha-sarcin. With both nucleases, nucleotides in helix V of 5S RNA show more complete protection from nuclease cleavage when the RNA is bound to intact TFIIIA than when it is bound to a 20 kDa tryptic fragment of TFIIIA lacking the C-terminal portion of the protein. These results suggest that fingers 8 and 9 of TFIIIA interact with the distal portion of helix V in the 5S RNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J., Delihas N. Characterization of RNA-protein interactions in 7 S ribonucleoprotein particles from Xenopus laevis oocytes. J Biol Chem. 1986 Feb 25;261(6):2912–2917. [PubMed] [Google Scholar]
- Andersen J., Delihas N., Hanas J. S., Wu C. W. 5S RNA structure and interaction with transcription factor A. 1. Ribonuclease probe of the structure of 5S RNA from Xenopus laevis oocytes. Biochemistry. 1984 Nov 20;23(24):5752–5759. doi: 10.1021/bi00319a013. [DOI] [PubMed] [Google Scholar]
- Andersen J., Delihas N., Hanas J. S., Wu C. W. 5S RNA structure and interaction with transcription factor A. 2. Ribonuclease probe of the 7S particle from Xenopus laevis immature oocytes and RNA exchange properties of the 7S particle. Biochemistry. 1984 Nov 20;23(24):5759–5766. doi: 10.1021/bi00319a014. [DOI] [PubMed] [Google Scholar]
- Baudin F., Romby P., Romaniuk P. J., Ehresmann B., Ehresmann C. Crosslinking of transcription factor TFIIIA to ribosomal 5S RNA from X. laevis by trans-diamminedichloroplatinum (II). Nucleic Acids Res. 1989 Dec 11;17(23):10035–10046. doi: 10.1093/nar/17.23.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J. M. Zinc fingers and other metal-binding domains. Elements for interactions between macromolecules. J Biol Chem. 1990 Apr 25;265(12):6513–6516. [PubMed] [Google Scholar]
- Bieker J. J., Roeder R. G. Physical properties and DNA-binding stoichiometry of a 5 S gene-specific transcription factor. J Biol Chem. 1984 May 25;259(10):6158–6164. [PubMed] [Google Scholar]
- Bogenhagen D. F., Sakonju S., Brown D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: II. The 3' border of the region. Cell. 1980 Jan;19(1):27–35. doi: 10.1016/0092-8674(80)90385-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Christiansen J., Brown R. S., Sproat B. S., Garrett R. A. Xenopus transcription factor IIIA binds primarily at junctions between double helical stems and internal loops in oocyte 5S RNA. EMBO J. 1987 Feb;6(2):453–460. doi: 10.1002/j.1460-2075.1987.tb04775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
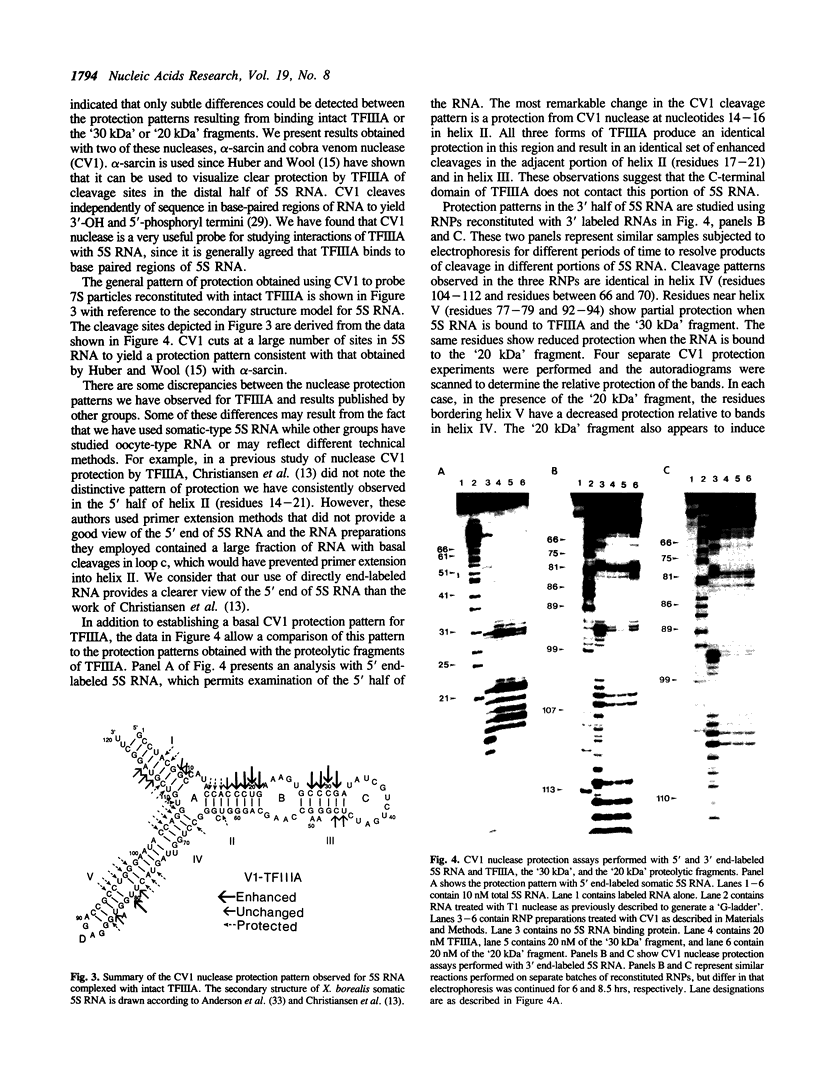
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelke D. R., Ng S. Y., Shastry B. S., Roeder R. G. Specific interaction of a purified transcription factor with an internal control region of 5S RNA genes. Cell. 1980 Mar;19(3):717–728. doi: 10.1016/s0092-8674(80)80048-1. [DOI] [PubMed] [Google Scholar]
- Hanas J. S., Bogenhagen D. F., Wu C. W. Cooperative model for the binding of Xenopus transcription factor A to the 5S RNA gene. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2142–2145. doi: 10.1073/pnas.80.8.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanas J. S., Hazuda D. J., Bogenhagen D. F., Wu F. Y., Wu C. W. Xenopus transcription factor A requires zinc for binding to the 5 S RNA gene. J Biol Chem. 1983 Dec 10;258(23):14120–14125. [PubMed] [Google Scholar]
- Honda B. M., Roeder R. G. Association of a 5S gene transcription factor with 5S RNA and altered levels of the factor during cell differentiation. Cell. 1980 Nov;22(1 Pt 1):119–126. doi: 10.1016/0092-8674(80)90160-9. [DOI] [PubMed] [Google Scholar]
- Huber P. W., Wool I. G. Identification of the binding site on 5S rRNA for the transcription factor IIIA: proposed structure of a common binding site on 5S rRNA and on the gene. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1593–1597. doi: 10.1073/pnas.83.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockard R. E., Kumar A. Mapping tRNA structure in solution using double-strand-specific ribonuclease V1 from cobra venom. Nucleic Acids Res. 1981 Oct 10;9(19):5125–5140. doi: 10.1093/nar/9.19.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Brown D. D. A specific transcription factor that can bind either the 5S RNA gene or 5S RNA. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4170–4174. doi: 10.1073/pnas.77.7.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard B., Wegnez M. Isolation of a 7S particle from Xenopus laevis oocytes: a 5S RNA-protein complex. Proc Natl Acad Sci U S A. 1979 Jan;76(1):241–245. doi: 10.1073/pnas.76.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieler T., Erdmann V. A. Isolation and characterization of a 7 S RNP particle from mature Xenopus laevis oocytes. FEBS Lett. 1983 Jul 4;157(2):283–287. doi: 10.1016/0014-5793(83)80562-6. [DOI] [PubMed] [Google Scholar]
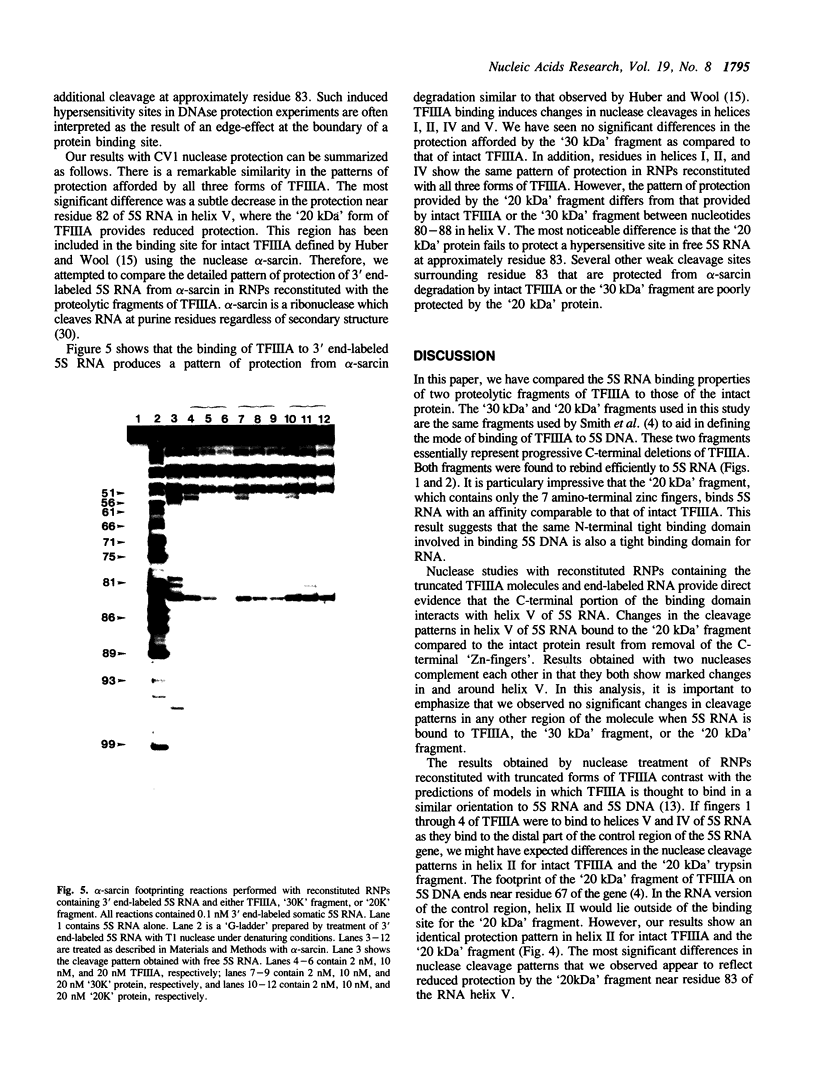
- Pieler T., Guddat U., Oei S. L., Erdmann V. A. Analysis of the RNA structural elements involved in the binding of the transcription factor III A from Xenopus laevis. Nucleic Acids Res. 1986 Aug 11;14(15):6313–6326. doi: 10.1093/nar/14.15.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniuk P. J. Characterization of the RNA binding properties of transcription factor IIIA of Xenopus laevis oocytes. Nucleic Acids Res. 1985 Jul 25;13(14):5369–5387. doi: 10.1093/nar/13.14.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniuk P. J., de Stevenson I. L., Wong H. H. Defining the binding site of Xenopus transcription factor IIIA on 5S RNA using truncated and chimeric 5S RNA molecules. Nucleic Acids Res. 1987 Mar 25;15(6):2737–2755. doi: 10.1093/nar/15.6.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakonju S., Brown D. D. Contact points between a positive transcription factor and the Xenopus 5S RNA gene. Cell. 1982 Dec;31(2 Pt 1):395–405. doi: 10.1016/0092-8674(82)90133-7. [DOI] [PubMed] [Google Scholar]
- Sands M. S., Bogenhagen D. F. TFIIIA binds to different domains of 5S RNA and the Xenopus borealis 5S RNA gene. Mol Cell Biol. 1987 Nov;7(11):3985–3993. doi: 10.1128/mcb.7.11.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrana K. E., Churchill M. E., Tullius T. D., Brown D. D. Mapping functional regions of transcription factor TFIIIA. Mol Cell Biol. 1988 Apr;8(4):1684–1696. doi: 10.1128/mcb.8.4.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhof E., Romby P., Romaniuk P. J., Ebel J. P., Ehresmann C., Ehresmann B. Computer modeling from solution data of spinach chloroplast and of Xenopus laevis somatic and oocyte 5 S rRNAs. J Mol Biol. 1989 May 20;207(2):417–431. doi: 10.1016/0022-2836(89)90264-7. [DOI] [PubMed] [Google Scholar]