Abstract
Proteins without readily available three-dimensional structural data present a difficult problem in the exploration of structure/function relationships. Saturation mutagenesis using contaminated oligonucleotides can identify potentially interesting regions of such a protein. This technique, in which synthesized oligonucleotides contain low-level base substitutions, allows random mutations to be placed throughout a gene sequence. Using double-stranded cassettes, a region of the human interleukin-1 alpha gene has been altered using such mutagenic oligonucleotides. However, instead of contaminating both strands of the gene sequence at the same level, each strand of the insert was contaminated at a different level. Several recombinants were sequenced and the effects of the mutations on the activity of the proteins were examined. Contaminating the two oligonucleotides at different levels produced a significantly different distribution of nucleotide changes from that seen if both strands were contaminated at the same level. The observed distribution followed the average of the distributions for each of the two contamination levels. This resulted in roughly equal frequencies of 1 to 5 nucleotide changes per clone with very few clones containing the wild-type nucleotide sequence. This helped overcome the redundancy in the genetic code, resulting in a high frequency of amino acid changes, and allowed changes at every amino acid to be sampled in a small number of mutants. This procedure can allow a gene sequence to be screened rapidly by removing most wild-type sequences from analysis while making sure that there are many amino acid changes in the resultant mutants.
Full text
PDF

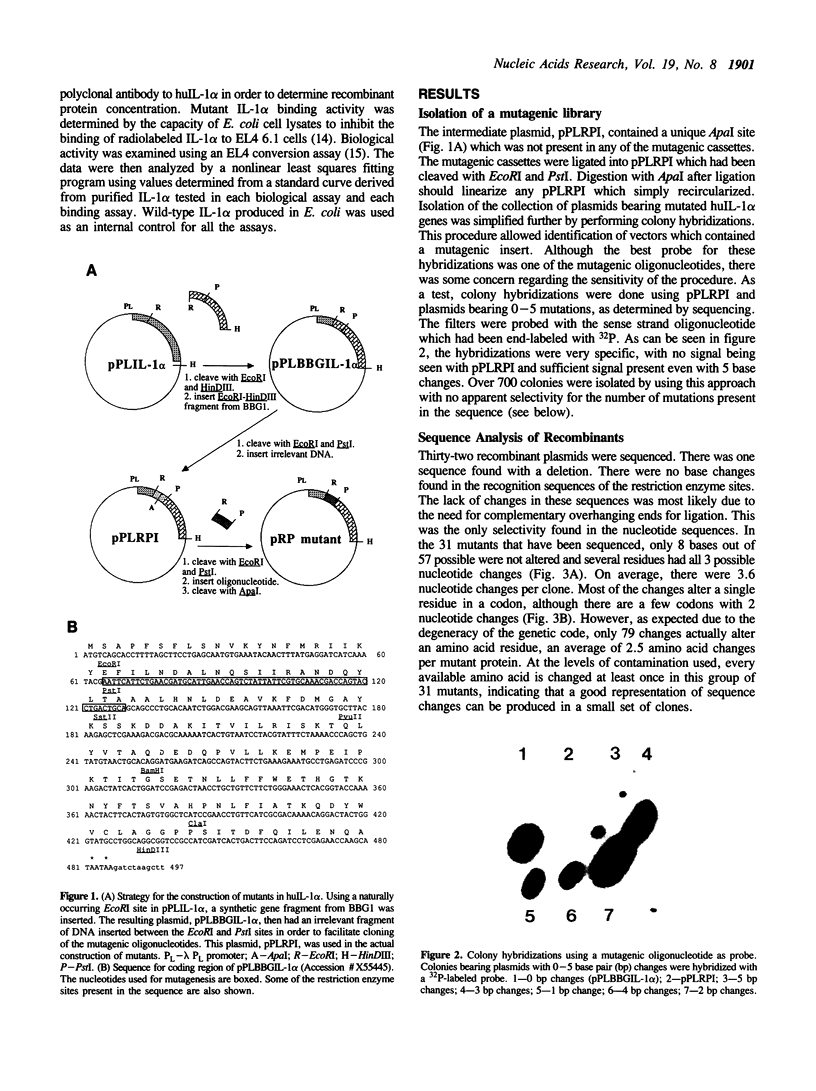
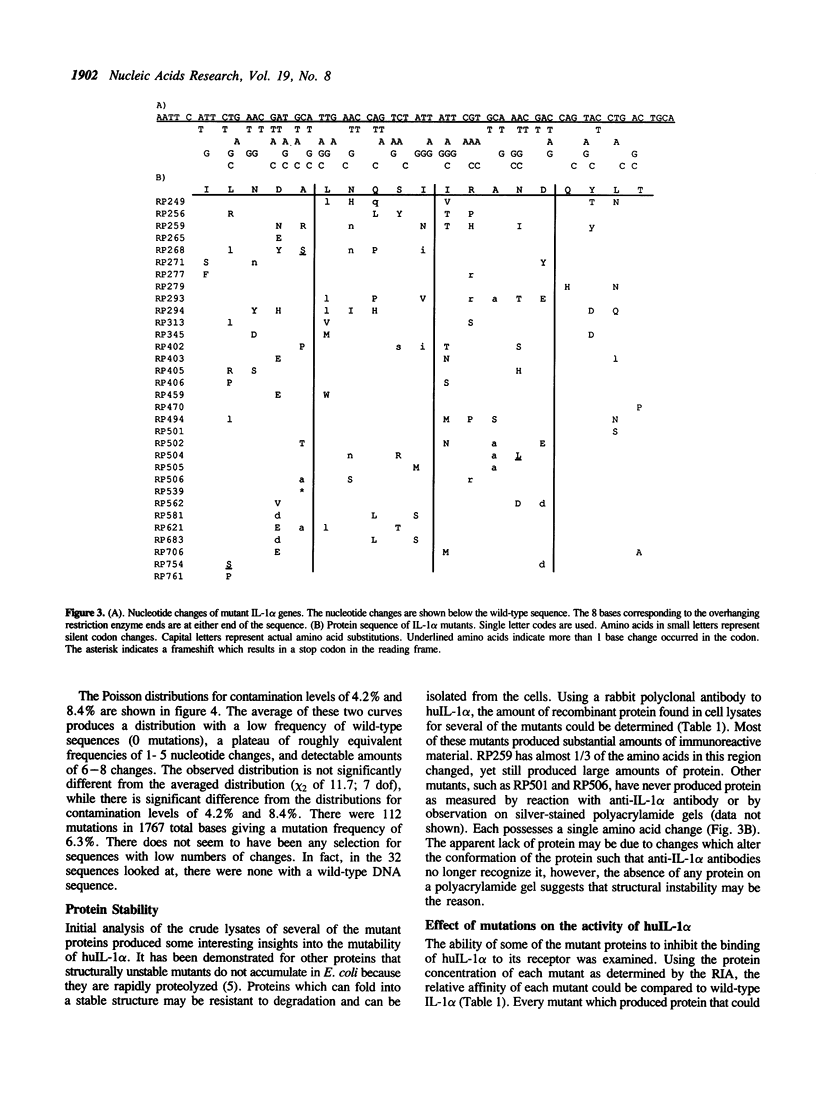


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard H. U., Helinski D. R. Use of the lambda phage promoter PL to promote gene expression in hybrid plasmid cloning vehicles. Methods Enzymol. 1979;68:482–492. doi: 10.1016/0076-6879(79)68037-0. [DOI] [PubMed] [Google Scholar]
- Blundell T. L., Sibanda B. L., Sternberg M. J., Thornton J. M. Knowledge-based prediction of protein structures and the design of novel molecules. 1987 Mar 26-Apr 1Nature. 326(6111):347–352. doi: 10.1038/326347a0. [DOI] [PubMed] [Google Scholar]
- Bowie J. U., Sauer R. T. Identifying determinants of folding and activity for a protein of unknown structure. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2152–2156. doi: 10.1073/pnas.86.7.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. III. Additional correlation of mutational sites with specific amino acid residues. J Mol Biol. 1977 Dec 15;117(3):525–567. doi: 10.1016/0022-2836(77)90056-0. [DOI] [PubMed] [Google Scholar]
- Gehrke L., Jobling S. A., Paik L. S., McDonald B., Rosenwasser L. J., Auron P. E. A point mutation uncouples human interleukin-1 beta biological activity and receptor binding. J Biol Chem. 1990 Apr 15;265(11):5922–5925. [PubMed] [Google Scholar]
- Maliszewski C. R., Baker P. E., Schoenborn M. A., Davis B. S., Cosman D., Gillis S., Cerretti D. P. Cloning, sequence and expression of bovine interleukin 1 alpha and interleukin 1 beta complementary DNAs. Mol Immunol. 1988 May;25(5):429–437. doi: 10.1016/0161-5890(88)90162-9. [DOI] [PubMed] [Google Scholar]
- McNeil J. B., Smith M. Saccharomyces cerevisiae CYC1 mRNA 5'-end positioning: analysis by in vitro mutagenesis, using synthetic duplexes with random mismatch base pairs. Mol Cell Biol. 1985 Dec;5(12):3545–3551. doi: 10.1128/mcb.5.12.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley B., Dower S. K., Gillis S., Cosman D. Determination of the minimum polypeptide lengths of the functionally active sites of human interleukins 1 alpha and 1 beta. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4572–4576. doi: 10.1073/pnas.84.13.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley B., Urdal D. L., Prickett K. S., Larsen A., Cosman D., Conlon P. J., Gillis S., Dower S. K. The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. J Biol Chem. 1987 Mar 5;262(7):2941–2944. [PubMed] [Google Scholar]
- Murray R., Pederson K., Prosser H., Muller D., Hutchison C. A., 3rd, Frelinger J. A. Random oligonucleotide mutagenesis: application to a large protein coding sequence of a major histocompatibility complex class I gene, H-2DP. Nucleic Acids Res. 1988 Oct 25;16(20):9761–9773. doi: 10.1093/nar/16.20.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D., Botstein D. Directed mutagenesis with sodium bisulfite. Methods Enzymol. 1983;100:457–468. doi: 10.1016/0076-6879(83)00073-7. [DOI] [PubMed] [Google Scholar]
- Yamayoshi M., Ohue M., Kawashima H., Kotani H., IIda M., Kawata S., Yamada M. A human IL-1 alpha derivative which lacks prostaglandin E2 inducing activity and inhibits the activity of IL-1 through receptor competition. Lymphokine Res. 1990 Fall;9(3):405–413. [PubMed] [Google Scholar]
- van Gunsteren W. F. The role of computer simulation techniques in protein engineering. Protein Eng. 1988 Apr;2(1):5–13. doi: 10.1093/protein/2.1.5. [DOI] [PubMed] [Google Scholar]