Abstract
CYP3A7 is the predominant cytochrome P450 (CYP) expressed in human fetal liver, accounting for 30–50% of the total CYP in fetal liver and 87–100% of total fetal hepatic CYP3A content. However, the lack of a rodent model limits the investigation of CYP3A7 regulation and function. Hence, double-transgenic mice expressing human pregnane X receptor (PXR) and CYP3A4/7 (Tg3A4/7-hPXR) were used to investigate the regulation and function of CYP3A7. Expression of CYP3A7 was monitored in mice that ranged in age from 14.5-d-old embryos to 8.5-d-old newborns; expression of CYP3A7 mRNA was increased before birth in the embryos and decreased after birth in the newborns. This is consistent with the observed developmental regulation of CYP3A7 protein levels and CYP3A7-mediated dehydroepiandrosterone 16α-hydroxylase activities. This developmental flux is also in agreement with previous studies that have investigated the expression of CYP3A7 in developing human liver. The regulation of CYP3A7 was further studied using hepatoblasts from the Tg3A4/7-hPXR mice. Glucocorticoids, including dexamethasone, cortisol, corticosterone, and cortisone all induced the expression of CYP3A7 mRNA, whereas rifampicin, an activator of PXR and an inducer of CYP3A4 in adult liver, had no effect on CYP3A7 expression. Cell-based promoter luciferase and chromatin immunoprecipitation assays further confirmed glucocorticoid receptor-mediated control of the CYP3A7 promoter. These findings indicate that CYP3A7 is developmentally regulated in mouse liver primarily by glucocorticoids through the glucocorticoid receptor. The Tg3A4/7-hPXR mouse model could therefore potentially serve as a tool for investigating CYP3A7 regulation and function.
The primary cytochrome P450 (CYP) of the human CYP3A family, CYP3A7, which is expressed in the human fetus, shares nearly 90% nucleotide sequence identity with CYP3A4. Although CYP3A7 is predominantly expressed in fetal liver (1–3), it also exists in some adult livers but at low levels relative to CYP3A4. It can be found in extrahepatic tissue such as intestine, endometrial, placenta, adrenal gland, prostate, and lung in the fetus (4–7). Some reports revealed that a CYP3A7 polymorphism is associated with high expression of CYP3A7 in the adult (6, 8, 9). CYP3A7 accounts for 30–50% of total CYP in fetal liver (2, 10) and up to 87–100% of total fetal hepatic CYP3A content (1). CYP3A7 shows high catalytic activity toward endogenous steroids such as dehydroepiandrosterone (DHEA), estrone, and retinoic acid (1, 11, 12) but lower activity toward metabolism of the majority of CYP3A4 substrates (13). CYP3A7 can specifically carry out DHEA 16α-hydroxylation, whereas DHEA is not a CYP3A4 substrate, due to differences in amino acid residues from leucine210 to glutamic acid279 between the two proteins (14). The metabolism of these endogenous steroids by CYP3A7 could play an important physiological role in fetal development. CYP3A7 could also be involved in xenobiotic metabolism that would be of clinical consequence with drug administration during pregnancy.
The mechanisms of regulation of fetal CYP3A7 compared with adult CYP3A4 is of considerable interest. There have been limited studies on regulation of the CYP3A7 gene. A base pair mismatch in the promoter region was shown to be sufficient for differential enhancer activity between CYP3A4 and CYP3A7 (15). Several transcription factors are involved in the regulation of CYP3A7, such as nuclear factor I (16) and nuclear factor κB (15). Because pregnane X receptor (PXR) is the main regulator of CYP3A4, it is also of interest to determine the role of PXR activators such as rifampicin in regulation of CYP3A7. It is also of interest to determine the role of the glucocorticoid receptor (GR) in the regulation of CYP3A7 by use of agonists such as dexamethasone (Dex). Earlier studies have shown that rifampicin slightly induces CYP3A7 in human adult liver (17), whereas a later report revealed that rifampicin had no effect on CYP3A7; however, Dex was shown to markedly increase expression of CYP3A7 in human fetal liver (18).
To investigate the expression of CYP3A7 in fetal liver, Tg3A4-hPXR mice were studied. These mice were originally generated by introduction of a bacterial artificial chromosome (BAC) clone of human PXR and CYP3A4 to Pxr-null mice; this BAC also contains the complete CYP3A7 gene (19). Hepatoblasts were also developed from these mice to investigate CYP3A7 function and regulation in fetal liver.
Materials and Methods
Chemicals and animals
Rifampicin (Rif), Dex, cortisol, corticosterone, cortisone, RU486, DHEA, DHEA sulfate (DHEA-S), dimethylsulfoxide (DMSO), nicotinamide adenine dinucleotide phosphate (NADPH), and 1,4-dithioerythritol, ammonium iodide (NH4I) were purchased from Sigma-Aldrich (St. Louis, MO). N-Methyl-N-trifluoroacetamide was obtained from Thermo Scientific (Rockford, IL)
Human CYP3A4/CYP3A7 and PXR double-transgenic (Tg3A4/7-hPXR) mice were housed under controlled conditions and fed NIH-31 rodent chow (Zeigler, Gardners, PA) and water ad libitum. All animal experiments were performed under guidelines approved by the National Cancer Institute Animal Care and Use Committee.
Liver samples from Tg3A4/7-hPXR mice were collected at different developmental time points: embryonic d 14.5 (E14.5), E16.5, E18.5, postnatal d 0.5 (P0.5), P2.5, P4.5, P6.5, and P8.5.
Cell culture
The preparation of fetal hepatic cultures taken from E16.5–E18.5 mouse livers was carried out as previously described (20, 21). Briefly, embryonic livers were minced in 4 C PBS buffer and then dissociated with 0.1% collagenase IV in DMEM on a 37 C shaker for 20 min. The resulting cell suspension was poured through 70-μm filter (BD Falcon, Bedford, MA) and centrifuged at 20 × g for 3 min. The cell pellet was resuspended in PBS buffer and centrifuged at 20 × g for 3 min twice. Cells were cultured on collagen I-coated tissue culture dishes in the DMEM supplemented (DMEM-S) with 5% charcoal-stripped fetal bovine serum (Gemini Bioproducts, West Sacramento, CA), 1× insulin-transferrin-selenium (Life Technologies, Inc., Invitrogen, Carlsbad, CA), and 1× antibiotic-antimycotic solution (Life Technologies, Inc., Invitrogen, Carlsbad, CA). Four hours later, the cells were washed with PBS to remove contaminating hematopoietic cells and cell debris and were cultured in DMEM-S.
After culturing the cells for 24 h, the influence of steroid hormones on mRNA and protein expression was examined. The cells were treated with 0.1% DMSO; 10 μm Rif; 10, 100, and 1000 nm Dex; 300 nm cortisol; 600 nm corticosterone; 300 nm cortisone; and 1, 10, 100 μm DHEA and DHEA-S or cotreated with 100 nm RU486, a GR antagonist. Cells were harvested on d 3 for analysis of mRNA. Cells treated with 0.1% DMSO or 10 or 100 nm Dex, or cotreated with 100 nm RU486 were harvested on d 3 for analysis of protein.
Quantitative real-time PCR (qPCR) analysis
cDNA was synthesized from 1 μg total mRNA using SuperScript II reverse transcriptase kit (Invitrogen, Carlsbad, CA). qPCR was performed on the Applied Biosystems (Foster City, CA) Prism 7900HT sequence detection system. Reactions were conducted in a 10-μl volume of 25 ng cDNA, 300 nm of each primer, and 5 μl of SYBR Green PCR Master Mix (Applied Biosystems) at 95 C for 10 min, 95 C for 3 sec and 60 C for 30 sec for 40 cycles. The primer sets are listed in Table 1. The levels of cDNA were quantitated by the comparative threshold cycle method using β-actin or 18S as an internal standard.
Table 1.
Primer sequences
| Primer list for qPCR | Sequence (5′–3′) |
|---|---|
| CYP3A7 | |
| Forward | AAG TCT GGG GTA TTT ATG ACT |
| Reverse | CGC TGG TGA ATG TTG GAG AC |
| CYP3A4 | |
| Forward | GCC TGG TGC TCC TCT ATC TA |
| Reverse | GGC TGT TGA CCA TCA TAA AAG |
| Cyp3a11 | |
| Forward | AGC AGG GAT GGA CCT GG |
| Reverse | CGG TAG AGG AGC ACC AA |
| Cyp3a13 | |
| Forward | TCC CAG AAT TAC AAA ACC AAA GA |
| Reverse | TGT GGT CTC ATA GCC AGC AA |
| Gr | |
| Forward | GGC AAA GGC GAT ACC AGG AT |
| Reverse | TCC AAC CCA GGG CAA ATG |
| mPxr | |
| Forward | AGA AGC AGA CTC TGC CTT G |
| Reverse | ATC TCG CAG GTT CCC TTG CGG A |
| hPXR | |
| Forward | GGC CAC TGG CTA TCA CTT CAA |
| Reverse | TTC ATG GCC CTC CTG AAA A |
| 18S | |
| Forward | ATT GGA GCT GGA ATT ACC GC |
| Reverse | CGG CTA CCA CAT CCA AGG AA |
| β-Actin | |
| Forward | TAT TGG CAA CGA GCG GTT CC |
| Reverse | GGC ATA GAG GTC TTT ACG GAT GTC |
| Primer list for plasmid constructs | |
| p3A7/+103b reverse | AAAAATTAATTAACACTACTTTCCTTCCTTATCTCTCTCCTCTGAGTC |
| p3A7-9.8kb forward | AAAAAGGCGCGCCTCAGGATGATGCAGGCCTCAGAAA |
| p3A7-7.8kb forward | AAAAAGGCGCGCCTCCTCTTGGAACTTCATGCCCGAT |
| p3A7-5.4kb forward | AAAAAGGCGCGCCTGGCTTCATGGCTTAGTCACGTCT |
| p3A7-3.2kb | AAAAAGGCGCGCCTCACTTGGCCACTGGAAGTCAGAA |
| GRE-6.2kb forward | AAAAAGGCGCGCCAGAACAGCTTGTGTGGACAAATGGAGGTCAGTGAGTGGTGTGT |
| GRE-4.9kb forward | AAAAAGGCGCGCCTGTCCCAGCATATGGGACAAATGGAGGTCAGTGAGTGGTGTGT |
| GRE-3.8kb forward | AAAAAGGCGCGCCAGTACATGTCCAAATGGAGGTCAGTGAGTGGTGTGT |
| GRE-1.4kb forward | AAAAAGGCGCGCCTGAACAAGCAAAAATGGAGGTCAGTGAGTGGTGTGT |
| pGL4.10-M | |
| Forward | CTGGCGCGCCGAGCTCACGCGTGCTAGCTTAATTAAC |
| Reverse | TCGAGTTAATTAAGCTAGCACGCGTGAGCTCGGCGCGCCAGGTAC |
Preparation protein samples and Western blot analysis
One milliliter of liver homogenate from whole liver tissue was prepared in 4 C buffer, 50 mm Tris-HCl, 150 mm KCl, and 1 mm EDTA with protease inhibitor (Thermo Scientific). The samples were centrifuged at 9000 × g for 20 min at 4 C, and the supernatant removed and centrifuged at 100,000 × g for 60 min at 4 C. The pellet was suspended in 4 C buffer with 20% glycerol. Membrane protein from cell samples was extracted using a subcellular protein fractionation kit (Thermo Scientific).
Protein concentrations were determined using a BCA protein assay kit (Thermo Scientific). To assess the amount of target protein required, protein was subjected to electrophoresis on a 4–15% SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The target protein was specifically bound to the primary antibodies of CYP3A7-specific polyclonal antibody (9) or antirat CYP3A1/2 monoclonal antibody (22) and detected with a chemiluminescent agent (Thermo Scientific) after incubating with the corresponding secondary antibodies (Cell Signaling, Danvers, MA). Comassie Brilliant Blue staining was applied to confirm same loading amount (1 pmol) of recombinant CYP3A7 and CYP3A4 (BD Biosciences, Bedford, MA). Calnexin was used as a loading control for Western blot.
Detection of CYP3A7 activity at different developmental stages of Tg3A4/7-hPXR mice
CYP3A7 is known to catalyze the 16α-hydroxylation of DHEA. DHEA metabolism was initiated by addition of 20 μl 20 mm NADPH to the reaction mixture and the reaction carried out for 10 min at 37 C. The reaction mixture was preincubated for 5 min in a 10-ml glass tube containing 30 μm DHEA and 50 μg liver microsomes in 500 μl Tris buffer. Hepatic microsomes were prepared from Tg3A4/7-hPXR mice at E14.5, E16.5, E18.5, P0.5, P2.5, P4.5, P6.5, and P8.5 or wild-type mice at P2.5. The organic supernatant was dried, and the residue was dissolved in 40 μl N-methyl-N-trifluoroacetamide/1,4-dithioerythritol/NH4I derivative reagent (1000 μl/4 mg/2 mg). It was then incubated for 35 min at 80 C for gas chromatography (GC)/mass spectrometry (MS) analysis.
For GC/MS analysis, samples (1 μl) were injected in the splitless mode from an Agilent 7683 automatic liquid sampler into an Agilent 5890N network GC system coupled to an Agilent 5973 network mass selective detector and a G1701 DA enhanced ChemStation (Agilent, Palo Alto, CA). Chromatography was performed on a 0.25-μm film thickness HP-5MS capillary column (28 m × 0.25 mm inner diameter). Carrier gas was helium (linear velocity 39 cm/sec) with a temperature program of 10 min at 120 C, then 10 C/min to 250 C, then 20 C/min to 300 C, and then held at 300 C for 3 min (total run time 28.5 min). Injector and interface temperatures were 230 and 280 C, respectively. The mass spectrometer was operated in the EI mode (69.9 eV). Peaks were identified from the retention times and mass spectra of authentic standards.
Promoter-luciferase analysis
The human CYP3A7 5′-flanking regions and deletion constructs were cloned into the modified pGL4.10 basic vector (Promega, San Luis Obispo, CA) (pGL4.10-M), which contains AscI and PacI restriction enzyme sites in the multicloning sites, using PCR amplicons from the BAC clone RP11-757A13 (Resgen/Invitrogen Corp., Huntsville, AL). All PCR amplicons were obtained using the KOD Hot Start DNA polymerase kit (Novagen, San Diego, CA) by the following PCR: polymerase activation step at 95 C for 2 min, denaturing step at 95 C for 20 sec, annealing step at 60 C for 20 sec, extension step at 70 C for 25 sec/kb, repeated for 27 cycles, and a final extension step at 70 C for 10 min. Each plasmid construct for the luciferase assay was named as follows: p3A7-9852/+103 (p3A7-9.8kb), p3A7-7852/+103 (p3A7-7.8kb), p3A7-5432/+103 (p3A7-5.4kb), p3A7-3219kb/+103 (p3A7-3.2kb), p3A7-GRE-6265/-6247&-161/+103 (GRE-6.2kb), p3A7-GRE-4922/-4904&-161/+103 (GRE-4.9kb), p3A7-GRE-3837/-3826&-161/+103 (GRE-3.8kb), p3A7-GRE-1469/-1457&-161/+103 (GRE-1.4kb). All primers for the cloning are listed in Table 1. pSG5-hGR, an expression vector encoding human GR, was provided by Alan Anderson (Centre de Recherche, L'Hôtel-Dieu de Québec, Québec, Canada)
Fetal hepatoblasts were chosen as the platform to investigate the regulation of CYP3A7. After preparing and culturing the hepatoblasts for 24 h, the cells were cultured in Opti-MEM I reduced serum medium (Life Technologies) and cotransfected with 100 ng human GR, 300 ng of p3A7-9.8kb, p3A7-7.8kb, p3A7-5.4kb, p3A7-3.2kb, GRE-6.2kb, GRE-4.9kb, GRE-3.8kb, GRE-1.4kb plasmid or pGL4.10-M basic plasmid, and 30 ng internal control plasmid phRL-SV40 (Promega, CA). At 6 h after transfection, the cells were treated with 0.1% DMSO, 10 nm Dex, or 100 nm Dex. After 24 h treatment, cells were harvested and the dual luciferase assay was performed according to Promega's protocol on the Veritas microplate luminometer (Turner Biosystems, Sunnyvale, CA).
Chromatin immunoprecipitation (ChIP) assays
Freshly minced liver samples from E14.5 and P2.5 Tg3A4/7-hPXR mice were fixed with 1% formaldehyde for 10 min in DMEM containing 0.5% fetal bovine serum at room temperature. To stop cross-linking, glycine (1.25 m) was added to the medium for 5 min and the samples washed briefly with cold PBS. The cell pellet aliquots were frozen until use. Twenty micrograms of each frozen sample were lysed with 400 μl M-PER buffer (Thermo Scientific) containing 0.2% Triton X-100 and protease inhibitors for 30 min. The samples were sonicated using a Bioruptor system (Cosmo Bio, Tokyo, Japan) twice for 15 min each (30 sec on/30 sec off mode) on ice and centrifuged at 12,000 rpm for 15 min at 4 C. Each 100-μl aliquot was mixed with 400 μl 1× ChIP buffer (Cell Signaling) and incubated with rabbit IgG or GR antibody cocktail [ABR PA1-511A (2.5 μl), ABR MA1-510 (5 μl), and SC-1004 (1 μl)] overnight at 4 C. To pull down the DNA-protein complex, 30 μl ChIP-grade protein G magnetic beads (Cell Signaling) were added and incubated for 1 h at 4 C with rotation. After incubation, the samples were washed with low-salt and high-salt washing buffer (Cell Signaling). Reverse-cross-linked DNA was purified using a QIAGEN (Valencia, CA) PCR purification kit. PCR was performed with 1 μl ChIP DNA samples, using the following primers for four different regions of the CYP3A7 promoter (for the 6.2-kb region, 5′-ATG ATG CCC TGG TTG ACT CCT GAA-3′ and 5′-TGT GTT CGT GTC TCC TTG TGT CCA-3′; for the 4.9-kb region, 5′-CCA GTC CTC ACT GAC AAG TTT GCT-3′ and 5′-TGC CCA AGG ACA CAC AGA GTT AGT-3′; for the 3.8-kb region, 5′-AGT GGC ATC GAA GTG AGA CAC CTA-3′ and 5′-CAA TGC TTT CGG GCC TCA TGC TTA-3′; and for the 1.4-kb region, 5′-TGC AGG AGT GCT GAA CCT GAG AAA-3′ and 5′-TCT GTG TCT GCT TGG CCT GAG AAA-3′). The ChIP assay was repeated twice and the bands quantitated by both qPCR and resolution of the PCR bands on gels.
Statistical analysis
Results are expressed as means ± sd. Differences between different groups were examined using Student's t test.
Results
Expression of CYP3A7 mRNA in liver of Tg3A4/7-hPXR mice
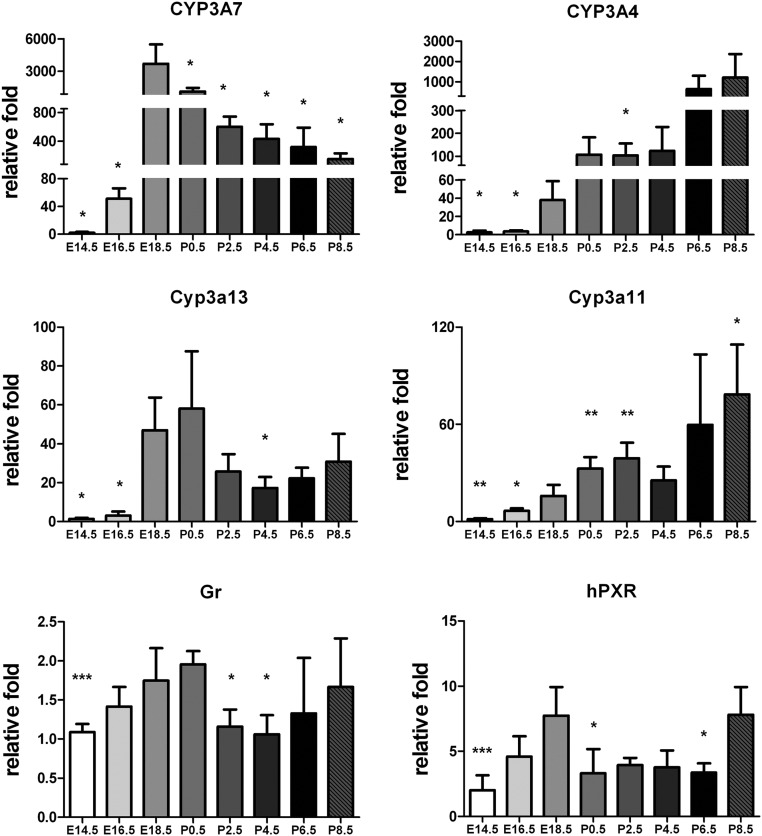
The mRNA encoding CYP3A7, CYP3A4, Cyp3a11, Cyp3a13, human PXR (hPXR), and mouse GR were measured by qPCR (Fig. 1). Levels of CYP3A7 mRNA were detected at E16.5, reached maximal levels at E18.5, and then progressively decreased to P8.5. The expression pattern of mouse Cyp3a13 mRNA, the closest mouse homolog of human CYP3A7, was similar with that of CYP3A7 with maximal expression noted at E18.5 and P0.5. Expression of CYP3A4 mRNA was found at E18.5 and increased to P8.5, comparable to that of the endogenous mouse Cyp3a11 mRNA; the most similar mouse homolog of human CYP3A4. Mouse GR and hPXR did not show evidence for developmental changes in mRNA expression.
Fig. 1.
qPCR analysis of mRNA in the livers of Tg3A4/7-hPXR mice at different developmental days: E14.5, E16.5, E18.5, P0.5, P2.5, P4.5, P6.5, and P8.5. The profiles are shown of PXR typical target genes, CYP3A and nuclear receptors hPXR and murine GR, at different developmental days. The mRNA expression levels were quantitated by the ΔΔCt method using β-actin as an internal control. The relative fold of expression level at different developmental stages is expressed as mean ± sd; n = 5. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with mice born at E18.5.
Expression of CYP3A7 protein in liver
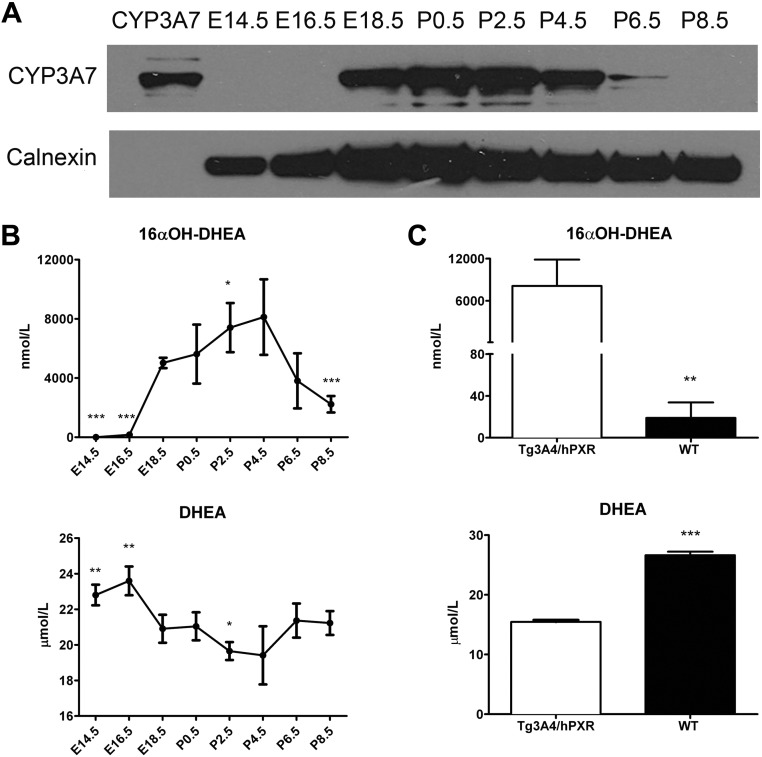
Western blot analysis of CYP3A7 protein levels demonstrated a pattern of expression similar to the mRNA analysis; protein was detected from E18.5 to P4.5 (Fig. 2A). CYP3A7 protein at E14.5, E16.5, and P8.5 was undetectable even though mRNA could be measured at E16.5 and P8.5. The reason for this discrepancy could be related to the exact timing of harvesting the fetus and suggests increased expression at around E16.5 to E18.5. Enzymatic activity using DHEA, a CYP3A7 substrate, correlated with the Western blot data (Fig. 2B). High DHEA 16α-hydroxylation activity occurred from E18.5 to P4.5, whereas a low conversion of DHEA to 16α-OH DHEA was observed at E14.5, E16.5, P6.5, and P8.5. In addition, a decrease in DHEA concentration paralleled the increasing levels of 16α-OH DHEA. Because rodent Cyp3a may also be involved in the metabolism of DHEA to 16α-OH DHEA (23, 24), a comparison of metabolic activities of microsomes from Tg3A4/7-hPXR and wild-type mice was determined. Tg3A4/7-hPXR microsomes had much higher activity than wild-type liver microsomes, thus indicating that the contribution of conversion of DHEA to 16α-OH DHEA was mostly due to CYP3A7 (Fig. 2C).
Fig. 2.
CYP3A7 protein level and its activity at different developmental days. A, Western blot analysis of pooled hepatic microsomes prepared from Tg3A4/7-hPXR mice detected using polyclonal antibody specific to human CYP3A7. B, CYP3A7 enzymatic activity as measured by the increase of 16α-OH DHEA and indicated by the decrease of DHEA, a relatively specific substrate of CYP3A7. Results are shown as the means ± sd of individual liver microsomes; n = 5. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with mice born at E18.5. C, CYP3A7 enzymatic activity in Tg3A4/7-hPXR mice and wild-type mice at P2.5. Data are shown as mean ± sd of individual liver microsomes; n = 4. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with Tg3A4/7-hPXR mice.
Induction of gene expression in hepatoblasts from Tg3A4/7-hPXR mice
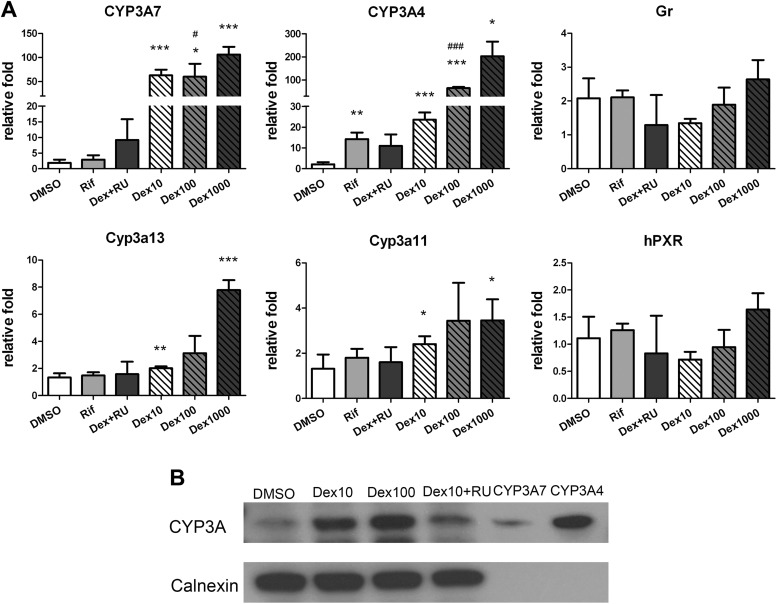
Because of the similar expression of CYP3A7 protein in the Tg3A4/7-hPXR mouse embryos compared with human fetus (1), the induction of CYP3A and the expression of nuclear receptors, hPXR and GR involved in CYP3A control, were consistent with previous studies (17, 18). Induction of both CYP3A4 and CYP3A7 mRNA by Dex was reversed by the GR antagonist RU486. The PXR agonist Rif induced only CYP3A4 mRNA expression, not CYP3A7 (Fig. 3A). Total CYP3A protein levels paralleled mRNA expression after treatment with Dex and RU486 (Fig. 3B). Moreover, there was no evident dose-dependent induction of mouse Cyp3a13 and Cyp3a11 by Dex, but a more than 4-fold induction of Cyp3a13 was seen with the high dose of Dex. RU486 did not inhibit their expression either (Fig. 3A). In addition, Dex had no effect on the levels of expression of mRNA encoding nuclear receptors, hPXR and GR.
Fig. 3.
qPCR analysis of mRNA in the hepatoblast isolated from E16.5 TgCYP3A4/7-hPXR. Expression CYP3A, hPXR, and murine GR in the hepatoblast was detected after 3 d of treatment. A, Treatment with 0.1% DMSO; 10 μm Rif; and 1, 10, and 100 nm Dex or cotreated with 100 nm Dex and a GR antagonist, RU486 (100 nm). *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with cells treated with 0.1% DMSO; #, P < 0.05; ###, P < 0.001 compared with cells treated with 100 nm RU486. B, Western blot analysis of pooled membrane protein from E18.5 Tg3A4/7-hPXR hepatoblasts treated with 0.1% DMSO, 10 μm Rif, and 10 and 100 nm Dex or cotreated with 10 nm Dex and 100 nm RU486. CYP3A protein was detected using antirat CYP3A1/2 monoclonal antibody on the third day. The same loading amounts of recombinant CYP3A7 and CYP3A4 (1 pmol) was detected with Coomassie blue staining (51.5 kDa).
During gestation, there are increased levels of circulating hormones, so the effect of three other endogenous glucocorticoids, cortisol, cortisone, and corticosterone, was evaluated in relation to CYP3A expression (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). CYP3A7, CYP3A4, Cyp3a11, and Cyp3a13 mRNA were all induced by these three glucocorticoids, and their induction was inhibited by RU486. Although there was significant induction of GR by cortisol and corticosterone and hPXR by corticosterone, the induction was less than 1.5-fold; therefore, the glucocorticoids had no significant effect on the expression of GR and hPXR.
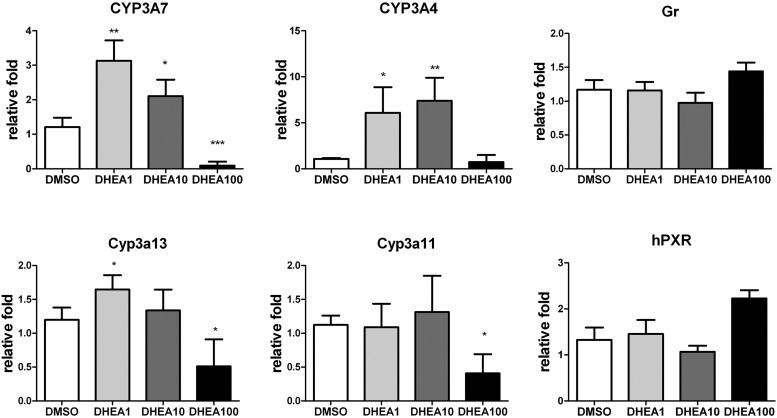
During fetal development, DHEA and DHEA-S concentrations are high. These steroids play important roles in the synthesis of downstream androgen and estrogen hormones and act directly on neurological, immunological, and cardiovascular systems (25). There are specific substrates of CYP3A7, and their effect on the expression levels of CYP3A7 and other CYP3A was investigated. At physiological levels (24), DHEA significantly induced the expression of CYP3A7 and CYP3A4 at the lower doses of 1 and 10 μm but inhibited CYP3A7 expression at the high dose of 100 μm (Fig. 4). DHEA had no influence on the expression of Cyp3a13, Cyp3a11, GR, and hPXR at the physiological concentration range. DHEA-S, which serves as a reservoir for DHEA, did not induce expression of CYP3A7 at low concentrations. At 100 μm DHEA-S, expression was slightly increased, less than 2-fold (Supplemental Fig. 1A). Although there was an indication that DHEA-S may increase CYP3A4 and Cyp3a11 expression, it was not statistically significant. DHEA-S had no effect on the expression level of Cyp3a13, GR, and hPXR.
Fig. 4.
qPCR analysis of mRNA in the hepatoblast isolated from E16.5 TgCYP3A4/7-hPXR. Expression of CYP3A, hPXR, and murine GR in hepatoblast was determined after 3 d of treatment with 0.1% DMSO and 1, 10, and 100 μm DHEA. Cells were harvested on the third day for analysis of mRNA by the ΔΔCt method, using 18S as an internal control. The relative fold expression level at different developmental stages was expressed as mean ± sd; n = 4. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with cell treated with 0.1% DMSO.
GR mediates the transcription activation of CYP3A7 through the distal promoter
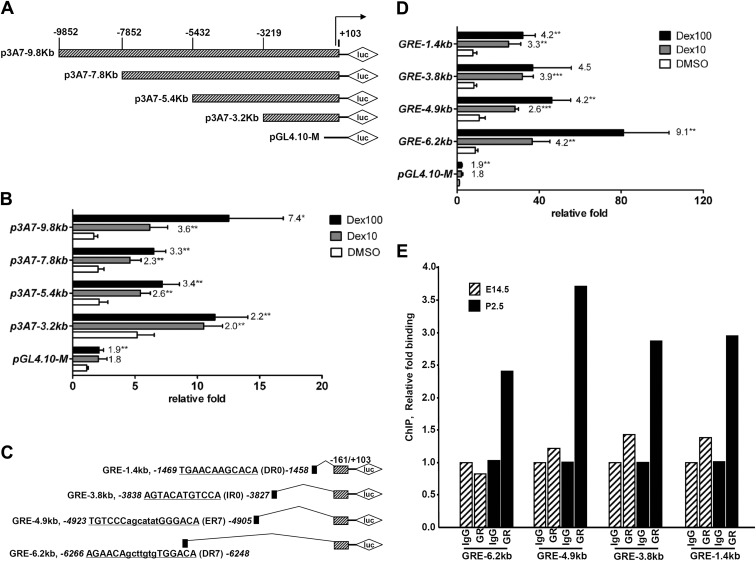
To determine whether the induction of CYP3A7 mRNA by Dex was through activation of transcription, reporter assays were performed in hepatoblasts. Constructs including different lengths of the CYP3A7 5′-flanking region and basic plasmid pGL4.10-M were cotransfected with a human GR expression plasmid (Fig. 5A). Although there was less than a 2-fold increase of luciferase activity for the basic construct, transactivation of the other four CYP3A7 promoter constructs were higher than the basic plasmid to various extents (Fig. 5B). When treated with Dex, a dose-dependent increase in luciferase activity was observed for all four CYP3A7 promoter reporter constructs. The most potent activation occurred with the longest promoter reporter, p3A7-9.8kb, where the response to Dex was 7.4- and 3.6-fold with 10 and 100 nm Dex, respectively. In the absence of Dex, the proximal promoter reporter, p3A7-3.2kb, showed the highest responsiveness to Dex-independent transactivation among all the constructs. Thus, the distal promoter may play a critical role in GR-dependent transcription, whereas the proximal promoter possibly has a response element for the GR-independent transcription.
Fig. 5.
Induction of CYP3A7 via the far upstream region mediated by the GR. A–D, Luciferase constructs with various lengths of CYP3A7 5′-flanking region (A) were subjected to luciferase reporter assays (B) or isolated GRE located in the upstream region of CYP3A7 (C) were transiently transfected into hepatoblasts from E18.5 Tg3A4/7-hPXR (D). After culture of hepatoblasts for 24 h, cells were cotransfected with 100 ng human GR, 300 ng pGL4.10-M basic plasmid or various luciferase constructs, and 30 ng internal control plasmid phRL-SV40. At 6 h after transfection, the cells were treated with 0.1% DMSO, 10 nm Dex, or 100 nm Dex. After 24 h treatment, the cells were harvested for the dual luciferase assay. E, Age-related GR binding in predicted GRE binding sites determined by ChIP assays with E14.5 and P2.5 mouse livers; shown is the qPCR analysis of the immunoprecipitated DNA. Histograms of the PCR products resolved on a gel, done in a separate experiment, are shown in Supplemental Fig. 2. The relative fold increases of luciferase activity are expressed as mean ± sd; n = 4. *, P < 0.05; **, P < 0.01 compared with DMSO-treated cells. Numbers on the top of columns denote the fold differences between the Dex-treated group and DMSO-treated group in each construct.
To elucidate the location of the CYP3A7 promoter region containing the GR response element (GRE), four putative sites were selected according to the analysis from the NUBIScan website when setting the raw score as 0.8. In the presence of Dex, all four putative GRE reporter constructs (Fig. 5C) had higher activation folds than the basal activation of the basic plasmid. The GRE-6.2kb construct showed the most potent responsiveness, 9.1- and 4.2-fold induction of luciferase activity, when treated with 10 and 100 nm Dex, respectively, whereas the other three GRE reporter constructs had a similar response to Dex-mediated transactivation (Fig. 5D). In addition, GR binding sites in E14.5 and P2.5 mouse livers were determined by ChIP assays. These results revealed a 2- to 3-fold increase of GR binding to CYP3A7 in livers of P2.5 mice compared with that of E14.5 mice, which correlate with the higher expression of CYP3A7 in livers of P2.5 mice compared with low expression of CYP3A7 in livers of E14.5 mice (Fig. 5E and Supplemental Fig. 2).
Discussion
CYP3A7 is generally considered a fetal-specific gene mainly expressed in human fetal or newborn liver. However, the deficit of available liver samples and the sensitive nature of obtaining fetal liver limits the scope of investigations of CYP3A7 studies in human fetal liver. Thus, it is necessary to develop a new model to elucidate the regulation and function of CYP3A7. Although HepG2 cells expressing low levels of CYP3A7 have been applied as a tool to study this CYP, they are not suitable to determine the role of CYP3A7 in mammals. Although HepG2 cells are dedifferentiated and more closely resemble adult than human fetal liver, CYP expression is diminished over time in cell culture. In addition, PXR expression is also different in HepG2 cells; the receptor is expressed at a functional level, whereas human fetal liver lacks measurable expression of PXR (26).
A double-transgenic mouse model expressing hPXR and CYP3A4/CYP3A7 has been generated for investigation of CYP3A4 regulation and function (19). The Tg3A4/7-hPXR mouse model was shown in the current study to demonstrate high expression of CYP3A7 in the developmental stage of the mouse from E18.5 to P4.5 and low expression from E14.5 to E16.5 and from P6.5 to P8.5. Comparatively, in humans, CYP3A7 was shown to decline in the fetus from the 13th week of pregnancy (1). Conversely, it was also reported to increase throughout pregnancy, peaking at the 2-wk postnatal age and declining thereafter to levels more characteristic of adult liver (27). However, these data were extrapolated from 16α-hydroxylation of DHEA-mediated enzymatic activity of human fetal liver microsomes, because conversion of DHEA to 16α-OH DHEA is carried out by CYP3A7. DHEA-S, which serves as a reservoir to maintain the equilibrium of circulating DHEA, through SULT2A1 (28) and steroid sulfatase C (29), is considered as a relatively specific substrate of CYP3A7 that carries out 16α-hydroxylation of this metabolite (30). DHEA and DHEA-S, the most abundant circulating steroid hormones in humans, have important roles in the transformation of other androgen or estrogen hormones (31). The serum level of DHEA-S in the human fetus increases from 28–40 wk and decreases from 40–42 wk (32) followed by a decline after birth and through the first 5 yr of life (24). DHEA levels undergo a peak of 10 μm before 30 yr of age, but the levels fall with aging (33). Thus, an attempt was made to measure the levels of DHEA and DHEA-S by ELISA in the fetal Tg3A4/7-hPXR mice. However, there was some concern about the level of specificity for these steroids. Liquid chromatography/MS and GC-MS were also used to determine DHEA and DHEA-S levels, but these measurements were unsuccessful because both steroids were below the limits of detection. Thus, it could not be concluded whether the ELISA signal is specific for DHEA/DHEA-S or a related steroid or that the DHEA or DHEA-S are at levels below those found in humans. The expression of CYP3A7 does not affect the concentration of DHEA or DHEA-S in mice; they were, however, at lower concentrations than those seen in humans. In some mice, they were completely absent. In any case, a similar developmental pattern of CYP3A7 in the Tg3A4/7-hPXR mouse with that in humans would render it an appealing model for studies on CYP3A7 physiologically.
Hepatoblasts derived from fetal Tg3A4/7-hPXR mice were used to investigate the regulation of CYP3A7. Although the CYP3A7 and CYP3A4 genes share around 90% identity in the 5′-flanking region, the gradual shift in expression of CYP3A7 to CYP3A4 after birth and the different catalytic capacity of CYP3A7 and CYP3A4 for endogenous and exogenous chemicals (1, 11–13, 34) suggest the existence of different regulatory mechanisms for these two CYP. Human PXR is very important in the regulation of CYP3A4, but it remains to be determined whether hPXR also induces CYP3A7 expression. CYP3A7 expression could be induced by Rif in human adult hepatocytes, HepG2 cells, or HuH7 cells, as seen for CYP3A4 in humans (17, 35), but three mutations in the CYP3A7 ER6 sequence resulted in no activation of the CYP3A7 gene by PXR (36, 37). The BAC clone containing CYP3A4/CYP3A7 used in the generation of Tg3A4/7-hPXR mouse contains this ER6 sequence, AGGTCA. However, it should be noted that studies showing the induction of CYP3A7 by Rif were conducted in a nonfetal liver platform. A recent study did show that PXR was not involved in the Rif-mediated induction of CYP3A7, but GR was in fact active in human fetal hepatocytes, consistent with the results of the present study. The lack of expression of hPXR during embryogenesis might explain these conflicting data (26). Evidence for up-regulation of mRNA through Dex and the other three glucocorticoids, cortisol, corticosterone, and cortisone, by cell-based luciferase assays and ChIP all supported the regulation of CYP3A7 by GR. Therefore, GR binding to the distal element of the 5′-flanking region of CYP3A7 could be responsible for the transactivation of CYP3A7, whereas another mechanism may be involved in PXR-mediated induction of CYP3A7 expression. CYP3A4 expression was also inducible by glucocorticoids, suggesting that transactivation of GR was not the critical mechanism for the shift of CYP3A7 to CYP3A4 expression after birth.
DHEA/DHEA-S is the most abundant circulating steroid hormone in humans and its metabolism to an inactive derivative during the embryonic to postnatal stage is mainly mediated by CYP3A7. Thus, the question arises whether DHEA/DHEA-S may be involved in up-regulation of CYP3A4 after birth. Indeed, DHEA also induced the expression of CYP3A4 at physiological concentrations but inhibited expression at the higher concentration of 100 μm; DHEA-S had no effect on expression of CYP3A4 and CYP3A7 mRNA at low concentrations. It was reported that the activities of DHEA/DHEA-S were related to other nuclear receptors other than GR such as the estrogen receptor, androgen receptor, peroxisome proliferator-activated receptor α, and PXR yet not directly or through their downstream metabolites (38). Among these nuclear receptors, it is unlikely that DHEA/DHEA-S activates PXR considering the high concentrations used in vitro (37, 39) and low inhibition efficiency by SR12813 (40). Although the mechanism for the shift of CYP3A7 to CYP3A4 expression after birth is still not clear, the alteration of endogenous and exogenous chemicals after birth may initiate the increasing expression of CYP3A4 (3).
Based on the results relating to CYP3A7 expression, endogenous metabolic activities on DHEA and DHEA-S, and regulation of CYP3A7 by GR, the Tg3A4/7-hPXR mouse model may provide an optimal platform for CYP3A7 characterization. It could be used as an in vivo platform to study potential drug-drug interactions caused by CYP3A7-mediated metabolism of major prescription or over-the-counter drugs; the decreasing catalytic capacity of midazolam from preneonate to adult (41) indicates a potential risk of overdosing drugs metabolized by CYP3A4 during the fetal or postnatal stage. In this study, the Tg3A4/7-hPXR mouse model demonstrated a good specificity for the CYP3A7 substrate DHEA based on the high DHEA 16α-hydroxylase activity of Tg3A4/7-hPXR mouse liver at P2.5 compared with wild-type mice. Thus, the Tg3A4/7-hPXR mouse model could be a useful tool for CYP3A7 functional studies.
Supplementary Material
Acknowledgments
This work was supported by the National Cancer Institute Intramural Research Program. X.-Y.P. was partially supported by a fellowship from China Scholarship Council. We thank Caroline H. Johnson for careful review of the manuscript, and John Buckley and Linda G Byrd for assistance with the mouse studies.
Present address for X.-Y.P.: Department of Pharmacognosy, School of Traditional Chinese Pharmacy, China Pharmaceutical University, Nanjing 210009, Jiangsu Province, People's Republic of China and Shanghai Research Center for Modernization of Traditional Chinese Medicine, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Zhangjiang, Shanghai, People's Republic of China.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BAC
- Bacterial artificial chromosome
- ChIP
- chromatin immunoprecipitation
- CYP
- cytochrome P450
- Dex
- dexamethasone
- DHEA
- dehydroepiandrosterone
- DHEA-S
- DHEA sulfate
- DMEM-S
- DMEM supplemented
- DMSO
- dimethylsulfoxide
- E14.5
- embryonic d 14.5
- GC
- gas chromatography
- GR
- glucocorticoid receptor
- GRE
- GR response element
- hPXR
- human PXR
- MS
- mass spectrometry
- NADPH
- nicotinamide adenine dinucleotide phosphate
- P0.5
- postnatal d 0.5
- PXR
- pregnane X receptor
- qPCR
- quantitative real-time PCR
- Rif
- rifampicin.
References
- 1. Stevens JC, Hines RN, Gu C, Koukouritaki SB, Manro JR, Tandler PJ, Zaya MJ. 2003. Developmental expression of the major human hepatic CYP3A enzymes. J Pharmacol Exp Ther 307:573–582 [DOI] [PubMed] [Google Scholar]
- 2. Shimada T, Yamazaki H, Mimura M, Wakamiya N, Ueng YF, Guengerich FP, Inui Y. 1996. Characterization of microsomal cytochrome P450 enzymes involved in the oxidation of xenobiotic chemicals in human fetal liver and adult lungs. Drug Metab Dispos 24:515–522 [PubMed] [Google Scholar]
- 3. Stevens JC. 2006. New perspectives on the impact of cytochrome P450 3A expression for pediatric pharmacology. Drug Discov Today 11:440–445 [DOI] [PubMed] [Google Scholar]
- 4. Schuetz JD, Beach DL, Guzelian PS. 1994. Selective expression of cytochrome P450 CYP3A mRNA in embryonic and adult human liver. Pharmacogenetics 4:11–20 [DOI] [PubMed] [Google Scholar]
- 5. Schuetz JD, Kauma S, Guzelian PS. 1993. Identification of the fetal liver cytochrome CYP3A7 in human endometrium and placenta. J Clin Invest 92:1018–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burk O, Tegude H, Koch I, Hustert E, Wolbold R, Glaeser H, Klein K, Fromm MF, Nuessler AK, Neuhaus P, Zanger UM, Eichelbaum M, Wojnowski L. 2002. Molecular mechanisms of polymorphic CYP3A7 expression in adult human liver and intestine. J Biol Chem 277:24280–24288 [DOI] [PubMed] [Google Scholar]
- 7. Koch I, Weil R, Wolbold R, Brockmöller J, Hustert E, Burk O, Nuessler A, Neuhaus P, Eichelbaum M, Zanger U, Wojnowski L. 2002. Interindividual variability and tissue-specificity in the expression of cytochrome P450 3A mRNA. Drug Metab Dispos 30:1108–1114 [DOI] [PubMed] [Google Scholar]
- 8. Smit P, van Schaik RH, van der Werf M, van den Beld AW, Koper JW, Lindemans J, Pols HA, Brinkmann AO, de Jong FH, Lamberts SW. 2005. A common polymorphism in the CYP3A7 gene is associated with a nearly 50% reduction in serum dehydroepiandrosterone sulfate levels. J Clin Endocrinol Metab 90:5313–5316 [DOI] [PubMed] [Google Scholar]
- 9. Sim SC, Edwards RJ, Boobis AR, Ingelman-Sundberg M. 2005. CYP3A7 protein expression is high in a fraction of adult human livers and partially associated with the CYP3A7*1C allele. Pharmacogenet Genomics 15:625–631 [DOI] [PubMed] [Google Scholar]
- 10. Wrighton SA, Molowa DT, Guzelian PS. 1988. Identification of a cytochrome P-450 in human fetal liver related to glucocorticoid-inducible cytochrome P-450HLp in the adult. Biochem Pharmacol 37:3053–3055 [DOI] [PubMed] [Google Scholar]
- 11. Lee AJ, Conney AH, Zhu BT. 2003. Human cytochrome P450 3A7 has a distinct high catalytic activity for the 16α-hydroxylation of estrone but not 17β-estradiol. Cancer Res 63:6532–6536 [PubMed] [Google Scholar]
- 12. Chen H, Fantel AG, Juchau MR. 2000. Catalysis of the 4-hydroxylation of retinoic acids by cyp3a7 in human fetal hepatic tissues. Drug Metab Dispos 28:1051–1057 [PubMed] [Google Scholar]
- 13. Williams JA, Ring BJ, Cantrell VE, Jones DR, Eckstein J, Ruterbories K, Hamman MA, Hall SD, Wrighton SA. 2002. Comparative metabolic capabilities of CYP3A4, CYP3A5, and CYP3A7. Drug Metab Dispos 30:883–891 [DOI] [PubMed] [Google Scholar]
- 14. Torimoto N, Ishii I, Toyama K, Hata M, Tanaka K, Shimomura H, Nakamura H, Ariyoshi N, Ohmori S, Kitada M. 2007. Helices F-G are important for the substrate specificities of CYP3A7. Drug Metab Dispos 35:484–492 [DOI] [PubMed] [Google Scholar]
- 15. Saito T, Takahashi Y, Hashimoto H, Kamataki T. 2001. Novel transcriptional regulation of the human CYP3A7 gene by Sp1 and Sp3 through nuclear factor kappa B-like element. J Biol Chem 276:38010–38022 [DOI] [PubMed] [Google Scholar]
- 16. Riffel AK, Schuenemann E, Vyhlidal CA. 2009. Regulation of the CYP3A4 and CYP3A7 promoters by members of the nuclear factor I transcription factor family. Mol Pharmacol 76:1104–1114 [DOI] [PubMed] [Google Scholar]
- 17. Greuet J, Pichard L, Bonfils C, Domergue J, Maurel P. 1996. The fetal specific gene CYP3A7 is inducible by rifampicin in adult human hepatocytes in primary culture. Biochem Biophys Res Commun 225:689–694 [DOI] [PubMed] [Google Scholar]
- 18. Matsunaga T, Maruyama M, Harada E, Katsuyama Y, Sugihara N, Ise H, Negishi N, Ikeda U, Ohmori S. 2004. Expression and induction of CYP3A in human fetal hepatocytes. Biochem Biophys Res Commun 318:428–434 [DOI] [PubMed] [Google Scholar]
- 19. Ma X, Cheung C, Krausz KW, Shah YM, Wang T, Idle JR, Gonzalez FJ. 2008. A double transgenic mouse model expressing human pregnane X receptor and cytochrome P450 3A4. Drug Metab Dispos 36:2506–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kamiya A, Gonzalez FJ. 2004. TNF-α regulates mouse fetal hepatic maturation induced by oncostatin M and extracellular matrices. Hepatology 40:527–536 [DOI] [PubMed] [Google Scholar]
- 21. Curran TR, Jr, Bahner RI, Jr, Oh W, Gruppuso PA. 1993. Mitogen-independent DNA synthesis by fetal rat hepatocytes in primary culture. Exp Cell Res 209:53–57 [DOI] [PubMed] [Google Scholar]
- 22. Goldfarb I, Korzekwa K, Krausz KW, Gonzalez F, Gelboin HV. 1993. Cross-reactivity of thirteen monoclonal antibodies with ten vaccinia cDNA expressed rat, mouse and human cytochrome P450s. Biochem Pharmacol 46:787–790 [DOI] [PubMed] [Google Scholar]
- 23. Fitzpatrick JL, Ripp SL, Smith NB, Pierce WM, Jr, Prough RA. 2001. Metabolism of DHEA by cytochromes P450 in rat and human liver microsomal fractions. Arch Biochem Biophys 389:278–287 [DOI] [PubMed] [Google Scholar]
- 24. Miller KK, Cai J, Ripp SL, Pierce WM, Jr, Rushmore TH, Prough RA. 2004. Stereo- and regioselectivity account for the diversity of dehydroepiandrosterone (DHEA) metabolites produced by liver microsomal cytochromes P450. Drug Metab Dispos 32:305–313 [DOI] [PubMed] [Google Scholar]
- 25. Tchernof A, Labrie F. 2004. Dehydroepiandrosterone, obesity and cardiovascular disease risk: a review of human studies. Eur J Endocrinol 151:1–14 [DOI] [PubMed] [Google Scholar]
- 26. Maruyama M, Matsunaga T, Harada E, Ohmori S. 2007. Comparison of basal gene expression and induction of CYP3A in HepG2 and human fetal liver cells. Biol Pharm Bull 30:2091–2097 [DOI] [PubMed] [Google Scholar]
- 27. Lacroix D, Sonnier M, Moncion A, Cheron G, Cresteil T. 1997. Expression of CYP3A in the human liver–evidence that the shift between CYP3A7 and CYP3A4 occurs immediately after birth. Eur J Biochem 247:625–634 [DOI] [PubMed] [Google Scholar]
- 28. Strott CA. 2002. Sulfonation and molecular action. Endocr Rev 23:703–732 [DOI] [PubMed] [Google Scholar]
- 29. Reed MJ, Purohit A, Woo LW, Newman SP, Potter BV. 2005. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr Rev 26:171–202 [DOI] [PubMed] [Google Scholar]
- 30. Kitada M, Kamataki T, Itahashi K, Rikihisa T, Kanakubo Y. 1987. P-450 HFLa, a form of cytochrome P-450 purified from human fetal livers, is the 16 α-hydroxylase of dehydroepiandrosterone 3-sulfate. J Biol Chem 262:13534–13537 [PubMed] [Google Scholar]
- 31. Labrie F, Luu-The V, Bélanger A, Lin SX, Simard J, Pelletier G, Labrie C. 2005. Is dehydroepiandrosterone a hormone? J Endocrinol 187:169–196 [DOI] [PubMed] [Google Scholar]
- 32. Parker CR., Jr 1999. Dehydroepiandrosterone and dehydroepiandrosterone sulfate production in the human adrenal during development and aging. Steroids 64:640–647 [DOI] [PubMed] [Google Scholar]
- 33. Webb SJ, Geoghegan TE, Prough RA, Michael Miller KK. 2006. The biological actions of dehydroepiandrosterone involves multiple receptors. Drug Metab Rev 38:89–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen H, Howald WN, Juchau MR. 2000. Biosynthesis of all-trans-retinoic acid from all-trans-retinol: catalysis of all-trans-retinol oxidation by human P-450 cytochromes. Drug Metab Dispos 28:315–322 [PubMed] [Google Scholar]
- 35. Pascussi JM, Jounaidi Y, Drocourt L, Domergue J, Balabaud C, Maurel P, Vilarem MJ. 1999. Evidence for the presence of a functional pregnane X receptor response element in the CYP3A7 promoter gene. Biochem Biophys Res Commun 260:377–381 [DOI] [PubMed] [Google Scholar]
- 36. Itoh S, Yanagimoto T, Tagawa S, Hashimoto H, Kitamura R, Nakajima Y, Okochi T, Fujimoto S, Uchino J, Kamataki T. 1992. Genomic organization of human fetal specific P-450IIIA7 (cytochrome P-450HFLa)-related gene(s) and interaction of transcriptional regulatory factor with its DNA element in the 5′ flanking region. Biochim Biophys Acta 1130:133–138 [DOI] [PubMed] [Google Scholar]
- 37. Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter CM, Ong ES, Evans RM. 1998. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev 12:3195–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Widstrom RL, Dillon JS. 2004. Is there a receptor for dehydroepiandrosterone or dehydroepiandrosterone sulfate? Semin Reprod Med 22:289–298 [DOI] [PubMed] [Google Scholar]
- 39. Ripp SL, Fitzpatrick JL, Peters JM, Prough RA. 2002. Induction of CYP3A expression by dehydroepiandrosterone: involvement of the pregnane X receptor. Drug Metab Dispos 30:570–575 [DOI] [PubMed] [Google Scholar]
- 40. Jones SA, Moore LB, Shenk JL, Wisely GB, Hamilton GA, McKee DD, Tomkinson NC, LeCluyse EL, Lambert MH, Willson TM, Kliewer SA, Moore JT. 2000. The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol Endocrinol 14:27–39 [DOI] [PubMed] [Google Scholar]
- 41. Jacqz-Aigrain E, Daoud P, Burtin P, Maherzi S, Beaufils F. 1992. Pharmacokinetics of midazolam during continuous infusion in critically ill neonates. Eur J Clin Pharmacol 42:329–332 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.