Abstract
Development of strategies capable of specifically curbing pathogenic autoimmune responses in a disease- and organ-specific manner without impairing foreign or tumor antigen-specific immune responses represents a long sought-after goal in autoimmune disease research. Unfortunately, our current understanding of the intricate details of the different autoimmune diseases that affect mankind, including type 1 diabetes, is rudimentary. As a result, progress in the development of the so-called “antigen-specific” therapies for autoimmunity has been slow and fraught with limitations that interfere with bench-to-bedside translation. Absent or incomplete understanding of mechanisms of action and lack of adequate immunological biomarkers, for example, preclude the rational design of effective drug development programs. Here, we provide an overview of antigen-specific approaches that have been tested in preclinical models of T1D and, in some cases, human subjects. The evidence suggests that effective translation of these approaches through clinical trials and into patients will continue to meet with failure unless detailed mechanisms of action at the level of the organism are defined.
Progress in the development of antigen-specific therapies for type I diabetes has been slow. Permanent insulin independence may not be the best benchmark for determining the clinical utility of an intervention.
Current approaches toward a potential cure for Type I diabetes (T1D) have focused on three main targets: (1) ablation of the β-cell-specific autoimmune response; (2) β-cell replacement therapy using islet transplantation; and (3) potentiation of β-cell mass and function using pharmacologic agents capable of promoting β-cell proliferation, regeneration and/or repair.
Pancreas and islet-transplantation in the context of systemic immunosuppression are the only approaches that have afforded patients complete independence from exogenous insulin. However, they have also highlighted the fact that, in the absence of immunosuppression, transplantation invariably meets with failure. For example, although 70% of islet-grafted patients remain insulin independent 1 year after transplantation, a significant fraction of them revert to insulin-dependency within 5 years, albeit with significantly lower insulin needs (Shapiro et al. 2000, 2006; Merani and Shapiro 2006; Robertson 2010). Because disease recurrence is typically associated with an anamnestic autoimmune response against the grafted tissue (Laughlin et al. 2008; Monti et al. 2008; Velthuis et al. 2009), all approaches that aim at improving β-cell mass and function must be accompanied by some sort of immunosuppression.
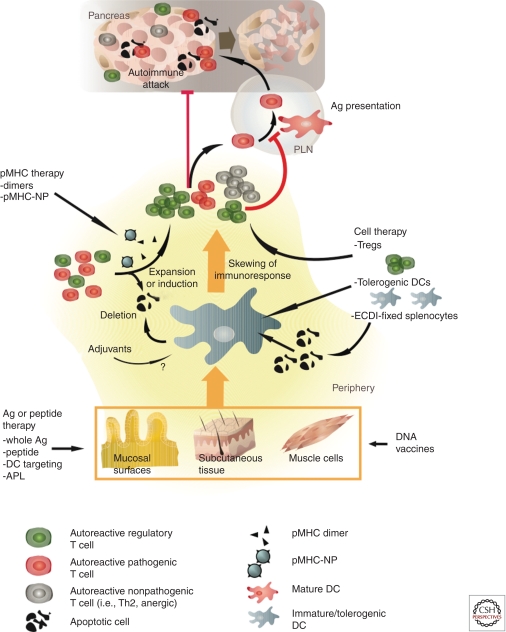
This article provides an overview of antigen-specific strategies for the prevention and/or treatment of T1D, from their conception and testing in preclinical models of T1D (Table 1) to their translation into clinical trials (Table 2). We will discuss these approaches in order of increasing complexity, from peptide-based therapies, with or without adjuvants, through more exotic approaches such as administration of dendritic cell (DC)-targeted compounds, or DNA and peptide–major histocompatibility complex (pMHC)-based vaccines, to more elaborate antigen-specific cell transfer strategies (Fig. 1).
Table 1.
Summary of preclinical studies addressing the remission of hyperglycemic state
| Immunotherapy | Antigen | Criteria for treatment | Protocol | Route1 | Results | Reference | Clinical trial? |
|---|---|---|---|---|---|---|---|
| Peptide | hsp60437–460 (p277) | 15- to 17-wk-old female NOD (50%–80% diabetic mice >11.1 mM) | 50 µg | s.c. | ∼100% survival at 40 wk. Mild hyperglycemia very few reverted to euglycemia | Elias et al. 1994 | YES |
| Ig-peptide fusion | GAD65206–220 | NOD females with BG between 8.9 mM and 13.9 mM for 2 wk were treated. Age of onset 14–30 wk | 300 µg/d for 5 d, then 1 × 300 µg/wk until 52–56 wk | i.p. | Complete reversal of hyperglycemia with continuous weekly boosting. 60% remain diabetes free at 24 wk after treatment after withdrawal of boosting | Jain et al. 2008 | NO |
| DNA vaccination | GAD65 and BAX | 8-wk-old or older NOD females with fasting blood glucoce >7.8 mM | 50 µg/wk for 8 wk | s.c. | Mice considered cured if free of glycosuria and FBG <16.7 mM. 48% diabetes-free at 40 wk of age; 8% diabetes-free in control group. Treatment -induced GAD-specific CD4+ Tregs | Li et al. 2006 | NO |
| Peptide/IFA | InsB9–23 | >10-wk-old NOD females with blood glucose 10–13.9 mM | 100 µg 1× at diagnosis | s.c. | 52% diabetes-free at 35 wk. Induction of Tregs (not Ag-specific) that can transfer protection. Neutralization of either IL-10 or IFNγ abrogated protection | Fousteri et al. 2010 | NO |
| Peptide/IFA | InsB9–23 | >10-wk-old NOD females with blood glucose 13.9–19.4 mM | 100 µg 1× at diagnosis | s.c. | No remission | Fousteri et al. 2010 | NO |
| DNA vaccination | PPIns or PIns | NOD females with first BG 10.5–13.9 mM, and second BG of 9.4–16.7 mM | 50 µg/wk or biweekly or monthly | i.m. | Weekly injection of PIns II-encoding high expression plasmid resulted in reversal of hyperglycemia in 70% of animals, while the original PIns II plasmid afforded 40% hyperglycemia reversal. PPIns I plasmids injection afforded marginal protection | Solvason et al. 2008 | Phase I/II in progress |
| Peptide: MHC dimer | HA110–120 | RIP-HA/TCR-HA mice >11 mM | 2 µg every 5 d up to age of 5 mo | i.v. | 50%–60% reversal of hyperglycemia. IL-10- mediated suppression | Casares et al. 2002 | NO |
| Peptide: MHC dimer | GAD65217–230 | NOD females >13.9–19.4 mM | 1× or 4× weekly 5 µg | i.v. | 20% and 80% reversal in 1× and 4× groups, respectively | Lin et al. 2010 | NO |
| Peptide: MHC dimer | BDC2.5 mimetope | NOD females >13.9 mM | 10 µg | Retro-orbital | No protection | Masteller et al. 2003 | NO |
| Peptide: MHC coated nanoparticles | NRP-V7, MimA2, IGRP265–273, InsB10–18 | NOD or HLA-A2 NOD females (11–18 mM) | 7.5 µg of Fe | i.v. | 75% reversal of hyperglycemia and clearance of insulitis, through IFNγ- and IDO-dependent mechanism and down-regulation/killing of APC | Tsai et al. 2010 | NO |
| Ex vivo expanded Foxp3+ Tregs | BDC2.5 | NOD females >16.6 mM | 107 Tregs | i.v. | 60% reversal of hyperglycemia | Tang et al. 2004 | NO |
| ECDI-fixed splenocytes | Insulin, GAD65206–220, GAD217–236, GAD524–536 NRP-V7 | NOD females >13.9 mM | 50 × 106 splenocytes with 0.5 mg/mL antigen | i.v. | 50% reversal of hyperglycemia in insulin ECDI-treated mice, but not other antigens | Fife et al. 2006 | Planned |
1Abbreviations: s.c. subcutaneous; i.p. intraperitoneal; i.m. intramuscular; i.v. intravenous.
Table 2.
Summary of clinical studies
| Immunotherapy | Antigen | Criteria for treatment | Protocol | Route | Results | Reference | Tested in hyperglycemic NOD mice? |
|---|---|---|---|---|---|---|---|
| Whole Ag | Insulin | Recent onset diabetic patients <4 mo after diagnosis | 5 mg/d for 12 mo | Oral | No effect in preventing c-peptide loss. In patients <15 yr c-peptide levels were lower in the treatment group at 9 and 12 mo. No differences in insulin requirement | Pozzilli et al. 2000 | NO |
| Whole Ag | Insulin | Recent onset diabetic patients <2 wk after diagnosis | 2.5 mg/d or 7.5 mg/d for 12 mo | Oral | No effect in preventing c-peptide loss. No differences in insulin requirement between treated and placebo | Chaillous et al. 2000 | NO |
| Ag/IFA | Insulin B chain | Recent onset diabetic patients <3 mo after diagnosis | 2 mg single injection | i.m. | No difference in stimulated c-peptide over 2 yr | Orban et al. 2010 | NO |
| Ag/alum | GAD65 | Recent onset diabetic patients <18 mo after diagnosis | 20 µg on d1 and 30 | s.c. | No effect in patients treated >6 mo after diagnosis. Decline in fasting and stimulated c-peptide is significantly slower in GAD-alum-treated group at 30 mo follow-up | Ludvigsson et al. 2008 | NO |
| Ag/alum | GAD65 | Recent onset diabetic patients <3 mo after diagnosis | 20 µg on d1 and 30, or 20 µg on d1, 30, 90, and 270 | s.c. | No effect in preserving stimulated c-peptide at 15 mo follow-up | Dyamid Medical, Press Release, June 1, 2011 | NO |
| Peptide | hsp60437–460 (p277) | Recent onset diabetic patients <6 mo after diagnosis | 1 mg at 0, 1, and 6 mo; extension to 12 mo | s.c. | Mantains c-peptide production. Lower insulin requirements. At 18 mo c-peptide is maintained but same requirements of insulin as controls | Raz et al. 2001, 2007 | YES |
| Peptide | hsp60437–460 (p277) | Diabetic patients. <42 mo after diagnosis | 0.2, 1, 2.5 mg at 0, 1, 6, and 12 mo | s.c. | Mantains c-peptide in first 12 mo at 2.5 mg. No changes in insulin requirements | Huurman et al. 2007, 2008 | YES |
| Peptide | hsp60437–460 (p277) | Adult diabetic patients per group. <3 mo after diagnosis | 0.2, 1, 2.5 mg at 0, 1, 6, and 12 mo | s.c. | At 18 mo, 0.1 and 1 mg groups had stable c-peptide. Stable insulin requirements | Schloot et al. 2007 | YES |
| Peptide | hsp60437–460 (p277) | Pediatric diabetic patients. <3 mo after diagnosis | 0.2, 1, and 2.5 mg at 0, 1, 6, and 12 mo | s.c. | At 7 and 18 mo decrease in c-peptide and increase in insulin requirement | Schloot et al. 2007 | YES |
| Peptide | hsp60437–460 (p277) | Pediatric diabetic patients. <6 mo after diagnosis | 1 mg at 0, 1, 6, and 12 mo | s.c. | At 6 mo and onward decrease in c-peptide and increase in insulin requirement | Lazar et al. 2007 | YES |
| APL | InsB9–23 | Teen and 16 adults diabetic patients. <6 mo after diagnosis | 0.1, 1, or 5 mg at 0, 2, 4, 6, and 8 wk | s.c. | During 25- wk follow-up, reduction of IFNγ responses against the APL or the natural peptide in the 5 mg group. Sporadic increase of IL-5 in the other doses | Alleva et al. 2006 | NO |
| APL | InsB9–23 | Adolescent and adult diabetic patients. <6 mo after diagnosis | 0.1, 0.5, and 1 mg at 0, 2, 4, and 23 more monthly injections | s.c. | After 24 months of follow-up, no differences in c-peptide, HbAc1, or insulin requirements could be identified between treated groups and placebo | Walter et al. 2009 | NO |
| DNA vaccine | PIns | Adult patients. <5 yr of diagnosis | 1 mg weekly for 12 injections | i.m. | Preliminary at 6 mo of follow-up: prevention of the decline of C-peptide. Reduction of IAA and GAD autoantibodies | Gottlieb et al. 2008 | YES |
Figure 1.
Putative mechanisms of action of antigen-specific therapies. Diverse mechanisms have been proposed to underlie the suppression of autoimmunity by antigen-specific approaches. This cartoon provides an overview of these mechanisms with the caveat that only a few of them have been documented formally. Capture of exogenous-derived autoantigens (i.e., naturally occurring peptides, APLs, proteins, ECDI-fixed splenocytes, or antigens encoded in DNA vaccines) in peripheral compartments by immature DCs can lead to the deletion of pathogenic effectors and/or induction of nonpathogenic/regulatory T cells. This tolerogenic effect can be enhanced with the use of apoptotic signals (ECDI) or adjuvants (IFA, alum). Strategies based on pMHC administration appear to function by activating autoreactive Treg subsets, which then go on to suppress the activation of pathogenic effectors through a number of mechanisms.
PROTEIN/PEPTIDE-BASED IMMUNOTHERAPIES
GAD
Glutamic acid decarboxylase (GAD) is the rate-limiting enzyme that converts glutamic acid into γ aminobutyric acid (GABA). Human and/or mouse pancreatic β cells express up to two isoforms of GAD (Christgau et al. 1991; Erlander et al. 1991; Bu et al. 1992); whereas human β cells only express the 64 kDa isoform (GAD65) (Karlsen et al. 1992), their murine counterparts predominantly express the 67 kDa isoform (GAD67) (Faulkner-Jones et al. 1993).
Early studies suggested that GAD might be an important autoantigen in T1D. Anti-GAD antibodies (Baekkeskov et al. 1982, 1987; Christie et al. 1992; De Aizpurua et al. 1992; Thivolet et al. 1992; Atkinson et al. 1993; Seissler et al. 1993; Tuomilehto et al. 1994; Verge et al. 1996) as well as GAD-reactive T cells (Honeyman et al. 1993; Panina-Bordignon et al. 1995; Rudy et al. 1995; Endl et al. 1997) were found in patients with preclinical or recent-onset T1D. In NOD mice, GAD autoreactivity was found at as early as 4 weeks of age (Kaufman et al. 1993; Tisch et al. 1993; Elliott et al. 1994). Notwithstanding the overwhelming evidence supporting a role for GAD autoreactivity in T1D, the role of GAD in diabetogenesis remains unclear. For example, whereas some GAD-reactive CD4+ T-cell clones are diabetogenic, others afford T1D protection (Zekzer et al. 1998; You et al. 2004). Likewise, although suppression of β-cell-specific GAD65/67 expression via a GAD antisense RNA approach prevented T1D in NOD mice (Yoon et al. 1999), induction of GAD-specific tolerance by transgenic overexpression of GAD did not (Bridgett et al. 1998; Geng et al. 1998; Jaeckel et al. 2003; Tian et al. 2009).
The identification of GAD as a prevalent autoantigen in T1D prompted several groups to attempt to inhibit the progression of disease by tolerizing GAD-reactive T cells. Young NOD mice treated intravenously with recombinant GAD65 (Kaufman et al. 1993; Ramiya et al. 1997; Tisch et al. 1999) or GAD67 (Elliott et al. 1994) were significantly protected from T1D, an effect that was ascribed to induction of GAD-specific T-cell tolerance. Administration of GAD through other routes, including intrathymic (Tisch et al. 1993; Cetkovic-Cvrlje et al. 1997), intraperitoneal (Pleau et al. 1995), intranasal (Tian et al. 1996), or oral routes (in the form of GAD67- [Ma et al. 1997] or GAD65/IL-4- transgenic plant tissues [Ma et al. 2004]) also had antidiabetogenic effects. Rather than inducing classical forms of T-cell tolerance, some of these approaches induced GAD-specific Th2 responses, elevating serum IgG1 levels while decreasing GAD-specific IFNγ production (Tian et al. 1996; Ma et al. 1997; Carter et al. 2006). Whether these antigen-specific Th2 responses were directly responsible for disease protection, however, was not formally established.
Other groups attempted to induce GAD-specific T-cell tolerance using GAD-derived peptides. Intraperitoneal injection of GAD65217–236, GAD65247–265, GAD65290–309, and GAD65524–543 peptides emulsified in incomplete Freund’s adjuvant (IFA) afforded disease protection when given at the onset of insulitis, but were largely ineffective when given to prediabetic mice (Tisch et al. 1999). Intraperitoneal delivery of GAD206–220 and GAD221–235 in IFA elicited peptide-specific IFNγ/IL-10-secreting Tr1-like T cells capable of inhibiting disease transfer by NOD splenic T cells into NOD.scid hosts (Chen et al. 2003). Nasal or oral delivery of GAD524–543 followed by injection of complete Freund's adjuvant (CFA) in the footpad resulted in the generation of Th2-like T cells that secreted IL-4, IL-10, and TGFβ and could suppress diabetes transfer by NOD splenic T cells (Maron et al. 1999). Likewise, intranasal administration of GAD247–266, GAD509–527, and GAD524–543 delayed islet graft rejection in a syngeneic model of islet transplantation in NOD mice, in association with enhanced IL-10 and decreased IFNγ production in GAD antigen-induced recall responses (Ravanan et al. 2007).
Alum-formulated GAD has been tested in human clinical trials for safety and efficacy as an anti-T1D therapy. Preclinical studies and a phase I clinical trial sponsored by Diamyd Therapeutics found that administration of recombinant human GAD with or without adjuvants did not induce adverse side effects or exacerbate T1D in man and mice (Plesner et al. 1998; Uibo and Lernmark 2008). A subsequent phase II trial in LADA (Latent Autoimmune Diabetes in Adults) patients receiving placebo or two doses of alum-formulated GAD (Diamyd) injected subcutaneously at weeks 1 and 4, confirmed the safety of this approach. The 20 µg dose was found to preserve insulin secretion/C-peptide production over the 24 week study period, and patients receiving Diamyd at this dose had increased CD4+ CD25+/CD4+ CD25− ratios in peripheral blood (Agardh et al. 2005). A subsequent phase IIb study tested the therapeutic effects of two doses of 20 µg of Diamyd given 4 weeks apart to 10–18 year-old new onset diabetic patients (Ludvigsson et al. 2008). At 30 (but not 15) weeks after the initiation of treatment, fasting and stimulated C-peptide levels in the Diamyd-treated group were significantly higher than in the placebo-treated cohort. GAD treatment increased serum GAD autoantibody levels, Foxp3 and TGFβ gene transcription, as well as IFNγ, IL-5, IL-10, IL-13, IL-17, and TNFα expression in PBMCs (Ludvigsson et al. 2008). Further analysis of the collected samples revealed an early (one month after treatment) and sustained (9 months after treatment) increase in GAD-specific IL-5 and IL-13 responses (Axelsson et al. 2010). GAD-alum treatment also led to increases in IgG3/IgG4 and decreases in IgG1 (Cheramy et al. 2010), as well as increases in GAD65-specific CD4+ CD25hi Foxp3+ T cells (Hjorth et al. 2011). Patients who had been treated with GAD-alum within 6 months of diagnosis had better preservation of fasting C-peptide levels than those that were treated later (Ludvigsson et al. 2011), although these beneficial effects did not translate into reduced insulin requirement.
Casting a shadow on these apparently encouraging results are two recent Phase II/III studies (performed independently by Diamyd and TrialNet), which failed to meet their primary efficacy endpoint in preserving insulin production (Wherrett et al. 2011). To the best of our knowledge, however, alum-formulated GAD has not been reported to reverse hyperglycaemia in NOD mice (Table 1). Thus, in the absence of adequate preclinical work in diabetic mice, it is not possible to rationalize whether lack of efficacy in the human trials was related to the choice of route of administration, antigen formulation, dosing or other factors.
Insulin
Insulin was the first β-cell autoantigen described in T1D patients (Palmer et al. 1983). The biologically active form of insulin is processed from its precursor, preproinsulin (PPIns), by sequential enzymatic cleavages that release the leader peptide (to make proinsulin; PIns) and the C-peptide. The role of insulin as a T1D autoantigen in both humans and NOD mice has been extensively reviewed elsewhere (Zhang et al. 2008). In humans, autoantibodies specific for both insulin and PIns are present in recent-onset diabetic patients (Kuglin et al. 1988; Yu et al. 2000), and T1D-associated T cells target multiple epitopes within insulin and its precursors (Lieberman and DiLorenzo 2003). In NOD mice, presence of insulin autoantibodies at 8 weeks of age is associated with high risk of early T1D onset (Yu et al. 2000), and prevalent intra-islet insulin-specific CD4+ T-cell responses have been documented in prediabetic NOD mice (Wegmann et al. 1994a,b). Importantly, there is substantial evidence pointing to insulin as an initiating autoantigen in T1D (Nakayama et al. 2005).
Administration of insulin through the oral (Zhang et al. 1991; Sai et al. 1996), parenteral (Atkinson et al. 1990), nasal (Harrison et al. 1996; Aspord and Thivolet 2002) and intravenous (Hutchings and Cooke 1995) routes has shown efficacy in preventing T1D in NOD mice. In some instances, conjugation of insulin to an adjuvant such as cholera toxin enhanced the therapeutic efficacy of insulin administration (Harrison et al. 1996; Bergerot et al. 1997; Aspord and Thivolet 2002). Subcutaneous immunization with insulin B chain in IFA at 4, 12, 16, and 22 weeks of age also afforded significant protection, although immunization with insulin A chain did not (Muir et al. 1995; Coon et al. 1999).
The efficacy of insulin-based vaccination using peptides, as opposed to full-length PPIns, PIns or insulin, has proven to be epitope-dependent and highly sensitive to the timing and route of administration. Certain combinations of routes and epitopes favor tolerance whereas others accelerate disease (Hutchings and Cooke 1998; Chen et al. 2001). For example, whereas intraperitoneal vaccination with PIns B24-C33 at 18 days of age reduced the incidence and delayed the onset of T1D, subcutaneous immunization with B24-C33 promoted disease acceleration (Chen et al. 2001). Intranasal delivery of insulin and PIns peptides has largely had antidiabetogenic effects, as shown by studies using B24-C33, B24-C32 and B9–23 (Martinez et al. 2003; Daniel and Wegmann 1996a,b). Furthermore, intranasal delivery of PIns B24-C33 (CTL-epitope mutated at C34) synergized with anti-CD3 mAb therapy to expand antigen-specific IL-10- and TGFβ-producing CD4+ Foxp3+ Tregs and reverse hyperglycemia in recent-onset diabetic animals (Bresson et al. 2006). Subcutaneous delivery of insulin peptides has yielded mixed results. For example, whereas immunization with the insulin-1 B9–23 peptide starting at 4 weeks of age accelerated T1D, immunization with insulin-2 B9–23 peptide afforded T1D protection (Devendra et al. 2004). In one study, subcutaneous injection of insulin B9–23 or B13–23 generated high titres of autoantibodies and caused fatal anaphylaxis in NOD mice after 6 weeks of treatment (Liu et al. 2002). Immunization with insulin B9–23 emulsified in IFA delayed the onset and lowered the incidence of T1D in preinsulitic or prediabetic NOD females (Muir et al. 1995; Daniel and Wegmann 1996a; Hutchings and Cooke 1998; Fousteri et al. 2010) but failed to reverse hyperglycaemia in recent onset diabetic mice (Fousteri et al. 2010). This treatment induced CD4+ CD25+ Tregs and relied on IL-10 and IFNγ for its protective function (Fousteri et al. 2010).
The human clinical trials conducted to date using insulin as a therapeutic or prophylactic immunotherapy have yielded disappointing results. In a safety-assessment trial, individuals at risk of developing T1D were treated with 1.6 mg of aerosolized insulin daily for 10 consecutive days, followed by 2 days/week of 1.6 mg for 6 months. Treatment did not generate adverse side effects or accelerate β-cell loss, but decreased insulin-specific T-cell responses (Harrison et al. 2004). A subsequent study in recent-onset diabetic individuals showed that intranasal insulin decreased serum insulin autoantibodies (IAAs) and, in a subset of patients, reduced insulin-specific T-cell (IFNγ) responses. The treatment, however, did not delay β-cell destruction (Fourlanos et al. 2011). In the Finnish T1D Prediction and Prevention Study (DIPP), intranasal insulin (1 unit/kg daily) did not delay or prevent T1D when given to infants carrying high risk HLA haplotypes or to siblings positive for two or more T1D-associated autoantibodies (Nanto-Salonen et al. 2008). As part of the T1D Prevention Trial (DPT-1), a study was conducted to test the efficacy of ultralente insulin (0.25 unit/Kg/d subcutaneously and one annual 4 day continuous intravenous infusion) as a prophylactic vaccine in at risk individuals presenting with a 5-year projected risk of >50%. The results showed that this protocol did not prevent or delay T1D development over a median 3.7 year follow-up period (Diabetes Prevention Trial—Type 1 Diabetes Study Group 2002). In another DPT-1 trial, first- or second-degree relatives with a 5-yr projected risk of 26%–50% (determined by metabolic, immunological, and genetic staging) received oral insulin (7.5 mg/d) or placebo. The median follow-up was 4.3 years. Overall, the treatment did not prevent or delay T1D (Skyler et al. 2005). However, subgroup analyses revealed that oral insulin had beneficial effects in patients with high IAA levels. Based on these results, TrialNet is conducting an Oral Insulin Prevention Trial enrolling subjects with similar characteristics to the subgroup mentioned above—relatives with normal glucose tolerance carrying at least two autoantibody specificities in serum, one of which must be anti-insulin, and presenting with 35% risk of T1D within 5 years (Skyler et al. 2008). In the immunotherapy T1D (IMDIAB) trial, oral insulin (5 mg/d) was given in conjunction with intensive subcutaneous insulin therapy to recent-onset diabetic patients within 4 weeks of diagnosis for 12 months. The results suggested that oral insulin failed to preserve C-peptide levels and reduce insulin requirement at 12 months of follow-up (Pozzilli et al. 2000). Furthermore, there was evidence of accelerated β-cell loss in patients younger than 15 years. In the T1D Insuline Orale study, daily oral insulin administration (2.5 mg or 7.5 mg/d) also failed to blunt established T1D in recent-onset diabetic patients (Chaillous et al. 2000). Lastly, a recent phase I clinical trial with 12 recent-onset diabetic subjects (within 3 months of diagnosis) undergoing treatment with a single intramuscular injection of insulin B chain/IFA showed that this approach elicits robust antibody and T-cell responses against insulin without causing adverse events (Orban et al. 2010). However, no significant differences in C-peptide level between the two groups could be detected. Clearly, unless the protocols that are tested in humans are previously tested in rodents at similar stages of disease progression, and the mechanisms of action explored fully, testing of similar immunotherapeutic approaches in clinical trials will be less likely to be successful.
Hsp60
In 1990, Cohen’s group identified antibody and T-cell reactivity against the micobacterial heat shock protein 6 (hsp60) as well as its human and murine homologs during the prediabetic stage (Elias et al. 1990, 1991; Birk et al. 1996). Hsp60-reactive T-cell clones were found to have diabetogenic properties (Elias et al. 1990, 1995). In humans, 87% of T1D patients showed a strong but transient T-cell reactivity toward multiple epitopes of hsp60 (Abulafia-Lapid et al. 1999, 2003).
Administration of hsp60 or an immunodominant hsp60 peptide spanning residues 437–460 (p277) in IFA or mineral oil prevented (Elias et al. 1991, 1997) and delayed (but did not reverse) the progression of hyperglycemia in NOD mice (Elias and Cohen 1994). This was associated with the induction of an antigen-specific shift from a Th1 (IFNγ) to Th2 (IL-5 and IL-10) response (Elias et al. 1991; Ablamunits et al. 1998) and a form of cell-mediated, transferrable tolerance (Elias and Cohen 1995). Because hsp60 and p277 also bind to TLR2, promoting a series of anti-inflammatory events, such as up-regulation of SOCS3 leading to a reduction of T-cell chemotaxis and enhancement of Treg function (Zanin-Zhorov et al. 2005, 2006; Nussbaum et al. 2006), the mechanisms by which hsp60 might afford protection remain unclear.
Peptor and DeveloGen, currently known as Andromeda Biotech, performed several clinical trials to determine the tolerability and therapeutic efficacy of a variant of p277 in which the two cysteines (6 and 11) were mutated to valines to increase stability (referred to as Diapep277). These trials have been extensively reviewed elsewhere and are not discussed here in detail (Fischer et al. 2010). In initial phase II clinical trials, C-peptide production was maintained after several months of follow-up (Raz et al. 2001; Huurman et al. 2007, 2008), but the therapeutic effects were marginal overall. Another trial involving adult patients also showed a marginal trend toward improved maintenance of β-cell function for some of the doses that were tested (Schloot et al. 2007). None of the two trials involving pediatric patients was able to identify differences in C-peptide loss between groups (Lazar et al. 2007; Schloot et al. 2007). Patients with p277-specific responses prior to treatment (baseline responders) changed their cytokine responses toward IL-10 or showed a reduction in p277-specific responses. Both effects correlated with better preservation of β-cell function. Two additional clinical trials were subsequently performed in LADA patients with inconclusive results (Fischer et al. 2010). The first phase III trial started in 2005, and the follow-up was for 24 months with an extended 21-month study (Fischer et al. 2010).
DC-targeted Peptide Therapy
In the steady state (i.e., absence of danger signals) DCs display an immature phenotype characterized by low expression of MHC and costimulatory molecules. These so-called immature DCs present antigens but do not generate an immunogenic response (Steinman et al. 2003). Rather, engagement of cognate pMHC by T-cells on immature DCs results in tolerogenic responses, including the deletion of cognate T-cells (Bonifaz et al. 2002) and/or the induction/expansion of regulatory T cells (Mahnke et al. 2003).
These observations predicted that targeting antigens to immature DCs should promote tolerance induction. One of the most widely used approaches to target antigens to DCs is to conjugate the antigen (a protein or peptide) to an antibody against DEC-205 (CD205), a C-type lectin that functions as an endocytosis receptor (Jiang et al. 1995). Antigen-coupled anti-DEC-205 antibodies are efficiently captured and processed by immature DCs. In 2005, Bruder et al. tested the ability of this approach to blunt diabetogenesis in a transgenic mouse model of T1D expressing the influenza A/PR/8/34 virus hemagglutinin (HA) under the control of the rat insulin promoter on β cells and the HA110–120-specific 14.3.d TCR (RIP-HA/TCR-HA) on T cells (Bruder et al. 2005). Treatment of newborn mice with HA linked to anti-DEC-205 prevented the development of T1D, and this was associated with increased recruitment of T cells expressing Foxp3, CTLA-4, and IL-10. Subsequent application of this approach in a polyclonal system led to mixed results, with treatment outcome being a function of the autoantigen used (Petzold et al. 2011). For example, in mice treated with anti-DEC-205 mAb conjugated with a mimotope of the β-cell autoreactive BDC2.5 CD4+ T-cell clone, there was an increase in the number of cognate CD4+ T-cells expressing Foxp3 but this had no effect on disease progression. In contrast, treatment with PIns-conjugated anti-DEC-205 mAb afforded significant protection from T1D, presumably because this antigen plays a dominant role in disease initiation. In another study, anti-DEC-205 mAb conjugated with a mimotope of the T1D autoantigen dystrophia myotonica kinase (MimA2) induced the deletion and anergy of cognate TCR-transgenic CD8+ T-cells (Mukhopadhaya et al. 2008). Whether this approach can blunt diabetogenesis in nontransgenic NOD mice has not been determined.
A similar approach involves incorporating autoantigenic peptide sequences within the CDR3 region of an immunoglobulin molecule (Gregg et al. 2004; Jain et al. 2008; Tartar et al. 2010). In this case, binding of the autoantigen-encoding Ig molecule to FcRγ on APCs enables the uptake, processing and presentation of the autoantigen by immature DCs (Brumeanu et al. 1993; Zaghouani et al. 1993). Intraperitoneal injections of aggregated but not soluble Ig-GAD65524–543 to preinsulitic NOD mice expanded cognate Foxp3+ Tregs and afforded T1D protection. However, treatment was ineffective when initiated at the prediabetic or diabetic stages (Gregg et al. 2004). In another study, intraperitoneal injections of soluble Ig-GAD65206–220 failed to prevent T1D in 4–6 week-old NOD mice, but blunted disease progression in prediabetic and new-onset diabetic animals, in an IFNγ-dependent manner (Jain et al. 2008). These results suggest that the therapeutic efficacy of this approach is both epitope- and disease stage-dependent.
Altered Peptide Ligands (APLs)
APLs (Evavold and Allen 1991; Evavold et al. 1993; Sloan-Lancaster and Allen 1996) can be classified according to the type of T-cell response that they induce. Antagonists block T-cell activation by cognate agonists; partial agonists induce an incomplete or alternative activation state in responder T cells; and superagonists trigger stronger activation responses than the natural agonists. The potential ability of APLs to compete with agonist peptides for MHC binding, to trigger immune deviation, or to induce anergy or deletion of cognate T cells suggested that APLs might be useful tools as autoimmune disease therapeutic agents. Induction of disease exacerbation in MS clinical trials (Bielekova et al. 2000; Kappos et al. 2000), however, exposed the potential dangers of this approach. The clonal complexity (and TCR diversity) of the individual antigenic T-cell specificities targeted by specific APLs cannot guarantee a tolerogenic outcome; some clones may see the APL as a superagonist (Anderton et al. 1998).
In one of the first reports using T1D-relevant APLs, APLs of Imogen 38 were shown to block antigen-induced activation of a cognate human T-cell clone, albeit through unclear mechanisms (Geluk et al. 1998). The APL experience in the NOD model of T1D has been largely disappointing. In 2000, we reported that treatment of prediabetic NOD mice with agonistic mimotopes of IGRP206–214-specific CD8+ T cells (NRP and NRP-A7) could effectively inhibit disease progression by blunting the avidity maturation of this T-cell subset (Amrani et al. 2000). We subsequently discovered that these mimotopes did not afford disease protection by selectively deleting high-avidity clonotypes, as we had proposed, but rather indirectly, by fostering the recruitment of their low-avidity counterparts (Han et al. 2005). We have recently shown that these low avidity clonotypes are a source of a negative feedback regulatory loop (memory-like autoregulatory CD8+ T cells) that aims to counter disease progression effected by high avidity clonotypes (Tsai et al. 2010). In fact, complete deletion of the IGRP206–214-specific CD8+ T-cell pool (including low-avidity clonotypes) by high doses of a superagonistic mimotope (NRP-V7) failed to blunt disease progression in prediabetic NOD mice. Furthermore, nondeletional doses of partial (NRP-I4) or full agonists (NRP-A7) could also afford disease protection, but this effect was only seen over very narrow, APL-specific dose ranges in which treatment fostered the recruitment of low-avidity clonotypes. Thus, near complete deletion of a single antigenic-specificity in a complex autoimmune disease is unlikely to have therapeutic value, unless such antigenic specificity(ies) play(s) a critical role in disease initiation and treatment is initiated before secondary specificities become involved.
Neurocrine Biosciences, Inc. developed an APL of the dominant epitope of insulin B chain (residues 9–23) referred to as NBI-6024 (16,19 → A) (Alleva et al. 2002). In mice, this APL induced APL-specific Th2 cells that did not crossreact with insulin B9–23. NBI-6024 delayed T1D onset in preinsulitic mice and, when combined with IFA, in prediabetic mice (Alleva et al. 2002). When given to recently diagnosed T1D patients, NBI-6024 had no therapeutic effects (Walter et al. 2009).
DNA “VACCINES”
Plasmids encoding autoantigens have also been used as a mean to induce tolerance against specific autoantigens in autoimmune diseases. After cellular uptake (by immature DCs), the plasmids are maintained as episomes (and hence do not integrate into the host genome), although their expression efficiency is generally low. Protocols have been established to enhance gene transfer, including local electroporation (Prud’homme et al. 2007; Frelin et al. 2010), pretreatment with bupivicane, cardiotoxin or other myotoxic drugs (Wolff et al. 1990; Danko et al. 1994), or delivery via a gene gun (Chen et al. 2002). Plasmids encoding several different β-cell antigens, including insulin and its precursors, GAD65, Hsp60, and the 2.5mi mimotope have been tested in the context of T1D through various routes, including intradermal, intramuscular, oral, and intranasal.
Insulin DNA Vaccination
Vaccination with PPIns, PIns or insulin-encoding plasmids produced mixed results depending on the type of antigen used, the route of administration, the expression efficiency, and the dosing regimen. Intramuscular vaccination with Insulin B chain- or Insulin B9–23-encoding DNA afforded T1D protection in NOD and LCMV-NP models (Coon et al. 1999; Bot et al. 2001; Urbanek-Ruiz et al. 2001), whereas injections of plasmids encoding Insulin A7–21 had no effects (Urbanek-Ruiz et al. 2001).
In one study, intramuscular delivery of PPIns II-encoding DNA was found to promote, rather than suppress, T1D development when given to prediabetic NOD mice (Karges et al. 2002). More recently, Solvason et al. tested the ability of PPIns I/II-, or PIns I/II-encoding plasmids to prevent disease in prediabetic mice or to restore normoglycemia in newly diagnosed diabetic animals (Solvason et al. 2008). Vaccination of hyperglycemic mice with a high-expression version of a PIns II-encoding plasmid blunted disease progression in a significant fraction of mice (Solvason et al. 2008). Although long-term PIns II vaccination induced IL-10- and IFNγ-producing T cells, the mechanism of action of this approach remains unresolved.
A Phase I/II study by Bayhill Therapeutics assessed the safety of 12 weekly doses of a human PIns-encoding plasmid (BHT-3201) in recent-onset diabetic patients (Gottlieb et al. 2008). Preliminary data from the vaccinated patients showed that BHT-3021 is well tolerated and may be associated with preservation of pancreatic β-cell function.
GAD65 DNA Vaccination
Intramuscular vaccination with DNA encoding GAD also yielded mixed results across different laboratories. Vaccination with rat GAD65 and GAD67 encoded in a plasmid under the control of the human cytomegalovirus promoter did not affect T1D incidence in NOD mice (Wiest-Ladenburger et al. 1998). Likewise, intramuscular vaccination with constructs encoding the full-length intracellular or truncated soluble forms of human GAD65 (Lieberman and DiLorenzo 2003), or a GAD-IgG Fc fusion protein (Tisch et al. 2001) enhanced Th1 responses and did not afford protection. In another study, a GAD-encoding DNA vaccine induced Th2 responses and promoted T1D (Gauvrit et al. 2004).
Administration of GAD-encoding DNA through the oral or intradermal routes had incremental protective effects (Lieberman and DiLorenzo 2003). In some cases, injection of a control plasmid encoding an irrelevant protein also resulted in protection (Filippova et al. 2001). More promising results were obtained in a recent study in which a gene gun biolistic approach was used to deliver a GAD-IgG Fc-encoding plasmid through the skin. Gene gun delivery of this construct, as opposed to intramuscular delivery, induced IL-4-producing CD4+ T cells and delayed T1D onset in NOD mice (Goudy et al. 2008).
DNA Vaccination with Other Autoantigens
DNA vaccination of prediabetic NOD mice with a hsp60-encoding plasmid down-regulated hsp60-specific T-cell responses, up-regulated hsp60-specifc autoantibodies of the IgG2b subclass, and inhibited diabetogenesis (Quintana et al. 2000). In another study, vaccination with a plasmid encoding a lysosome-targeted 2.5mi mimotope afforded T1D protection in a BDC2.5 TCR-transgenic CD4+ T-cell transfer model. Targeting the 2.5mi mimotope to the APC lysosomal compartment in which MHC class II loading takes place presumably enhances the efficiency of antigen presentation, hence the induction of tolerance (Rivas et al. 2011).
Codelivery of Antigen-Encoding DNA with Other Immunomodulatory Genes or Molecules
An advantage of DNA vaccination as an immunotherapeutic approach is the ease with which additional immunomodulatory genes and sequences can be codelivered with the target autoantigen. Plasmids encoding IL-4, IL-10, or a CTLA-4-binding ligand have been codelivered with insulin- or GAD-encoding plasmids to obtain synergistic effects (Tisch et al. 2001; Prud’homme et al. 2002; Chang et al. 2005; Glinka et al. 2006; Pop et al. 2007).
DNA vaccination has also been tested in combination with immunomodulatory monoclonal antibodies. Anti-CD3 mAb treatment enhanced the protective effect of vaccination with GAD-encoding DNA in the RIP-LCMV-GP (C57BL/6) model of T1D. However, this effect could not be reproduced in NOD mice, suggesting that the genetic background conditions the therapeutic efficacy of this approach (Bresson et al. 2010). Intranasal delivery of a PIns II-encoding plasmid in mice cotreated with a blocking anti-CD40L mAb elicited antidiabetogenic CD4+ Tregs. In the absence of anti-CD40L blocking Abs, however, the treatment likely also primes pathogenic T cells, therefore rendering it ineffective in preventing T1D (Every et al. 2006).
PEPTIDE–MHC-BASED THERAPIES
Administration of autoantigenic pMHC class I or II complexes has also been explored as an approach to induce tolerance in autoimmune diseases. Different outcomes and mechanisms of action have been reported, depending on the structure that was used for therapy (monomers versus dimers or multimers coupled to nanoparticles) (reviewed in Clemente-Casares et al. 2011). Whereas pMHC class I monomers cannot activate T cells efficiently, higher-order structures can do so in a manner that is proportional to the valency, hence avidity, of the pMHC–TCR interaction (Herrmann and Mescher 1986; McCluskey et al. 1989; Abastado et al. 1995). Here, we briefly summarize the outcome of studies using dimers and nanoparticle-conjugated multimeric structures.
Dimers
Administration of dimeric pMHC have proved effective in the prevention of diabetes in spontaneous and transfer models (Masteller et al. 2003; Li et al. 2009; Lin et al. 2010) and reversal of hyperglycemia (Casares et al. 2002; Lin et al. 2010). These outcomes were attributed to the generation of anergic IL-10-producing CD4+ T cells. Although alternative mechanisms were not formally excluded, this interpretation of the data is consistent with the observation that in vitro stimulation of PBMCs from T1D patients and healthy controls with GAD65271–285 or PIns73–90/DR4-IgG1 dimers elicits IL-10-producing T cells (Preda et al. 2005).
pMHC-Coated Nanoparticles
We have recently shown that iron oxide nanoparticles (NPs) coated with mono-specific, T1D-relevant pMHC class I monomers afford disease protection to prediabetic NOD mice and effectively restore normoglycemia to newly diagnosed diabetic animals (Tsai et al. 2010). We have shown that this approach functions by boosting a naturally occurring negative feedback regulatory loop that aims to blunt the progression of diabetogenic autoimmunity. This negative feedback loop consists of memory-like autoregulatory CD8+ T cells that arise (during disease progression, prior to pMHC-NP therapy) from low-avidity autoreactive T-cell clones in response to chronic autoantigenic stimulation. We have proposed that autoreactive T-cell memory in diabetogenesis primarily (albeit not exclusively) arises from low-avidity clones and thus is regulatory in nature. Because memory T cells are costimulation-independent, cross-linking of cognate TCRs on these memory-like autoregulatory T cells by the pMHC complexes coated onto the NPs induces autoregulatory T-cell expansion. In turn, these pMHC-NP-expanded mono-specific autoregulatory T cells blunt disease progression by suppressing the presentation of both cognate and noncognate autoantigens by autoantigen-loaded professional APCs to all other naïve autoreactive T cells partaking in the disease process, through a number of mechanisms that include APC-killing. Nonautoantigen-loaded APCs are spared. A more in-depth discussion of the significance of this new immunological paradigm and therapeutic approach can be found in the work of Clemente-Casares et al. (2011).
ANTIGEN-SPECIFIC CELL THERAPIES
DC-Based Therapies
Studies in the early nineties showed that transfer of DCs isolated from the pancreatic lymph nodes of NOD mice (autoantigen-loaded) could prevent the onset of T1D by inducing regulatory T cells (Clare-Salzler et al. 1992). Similar results were obtained with immature bone marrow-derived DCs (BMDCs) generated in the presence of GM-CSF and IL-4 (Feili-Hariri et al. 1999; Morel et al. 1999), which presumably act by skewing the autoreactive T-cell response toward Th2 on autoantigen capture in vivo (Feili-Hariri et al. 2002). Another study reported that adoptive transfer of BMDCs generated in the presence of GM-CSF and IL-10 could prevent T1D almost completely (Tai et al. 2011). DCs generated in the presence of IL-10 express much lower levels of costimulatory molecules, produce high amounts of IL-10, and foster the expansion of memory and Foxp3+ CD4+ T cells. In humans, DCs generated from monocytes in the presence of GM-CSF, IL-4, IL10, and TGFβ were found to tolerize insulin-reactive CD4+ T cells in an antigen-specific manner (Torres-Aguilar et al. 2010). The tolerized cells produced low levels of IFNγ and IL-2 and significantly higher levels of IL-10 than CD4+ T cells challenged with control DCs. DCs genetically modified to express IL-4 (Feili-Hariri et al. 2003; Creusot et al. 2008; Ruffner and Robbins 2010), galactin-1 (Perone et al. 2006), PD-L1 (He et al. 2008) or treated with antisense oligonucleotides against costimulatory molecules (Machen et al. 2004) or NF-kB (Ma et al. 2003) have also been shown to have antidiabetogenic properties.
ECDI-Fixed Splenocytes
Another version of APC-based therapy is the administration of antigen-pulsed, ethylene carbodiimide-fixed splenocytes (ECDI-SP). It has been suggested that capture and processing of apoptotic ECDI-fixed splenocytes by endogenous DCs (Turley and Miller 2007) renders them tolerogenic (Xia et al. 2007; Marin-Gallen et al. 2010), in part by inducing the up-regulation of suppressive molecules such as PD-L1 (Fife et al. 2006, 2009; Turley and Miller 2007). Insulin- but not GAD or IGRP epitope-coupled ECDI-fixed splenocytes could reverse hyperglycemia in a fraction of newly diagnosed diabetic NOD mice (Fife et al. 2006). ECDI-fixed splenocytes coupled with CD8+ T-cell-associated epitopes of insulin and IGRP inhibited T1D development in a humanized NOD model (Niens et al. 2011). Curiously, when the splenocytes were pulsed with one or more epitopes from a single autoantigen, only those coupled with IGRP, but not insulin epitopes prevented T1D, presumably by tolerizing cognate autoreactive T cells (Niens et al. 2011).
CONCLUDING REMARKS
Insulin independence is the ultimate goal of any therapeutic approach in T1D. However, from a pragmatic standpoint, the goal of treating T1D is to prevent the development of acute metabolic instability such as diabetic ketoacidosis and severe hypoglycemia, so as to enable normal growth and development in children, and to prevent the development of long-term complications without significantly impairing normal activity of living or quality of life. In this regard, insulin independence is not an absolute prerequisite for success. This concept is in part exemplified by the long-term outcome of islet transplantation. Transplanted patients with detectable endogenous insulin production maintained significantly lower plasma HbA1c levels than those who experienced graft failure (Shapiro et al. 2000, 2006; Merani and Shapiro 2006). In addition, patients who received islet transplants had a significant reduction in the incidence of hypoglycemic coma and reported an improvement in their quality of life (Merani and Shapiro 2006; Speight et al. 2010). Because a reduction in HbA1c levels is highly predictive of a reduction in T1D-related complication rates (The Diabetes Control and Complications Trial Research Group [1993]), any strategy that results in a simplified insulin regimen and a lower HbA1c may have clinical benefits. Indeed, restoration of β-cell mass to “normal” levels may not be necessary, as maintenance of modest β-cell activity has been shown to improve clinical outcome (Steffes et al. 2003). More importantly, the body appears to have “metabolic memory.” Results from the T1D Control and Complications Trial (DCCT) clearly showed that during a mean of 4–17 years of follow-up, patients who were originally assigned to the intense treatment group (who also had lower HbA1c levels) had a significant reduction in the progression of retinopathy and nephropathy (The Diabetes Control and Complications Trial Research Group [2000]), in the risk of cardiovascular disease, including the risk of death (Nathan et al. 2005), and in the progression of intimal-thickness (Nathan et al. 2003). These results suggest that improvement of metabolic control for only a few years (i.e., 6.5 years during the DCCT) is sufficient to provide long-term protection to the patients, despite worsening hyperglycemia later on.
The limited success of the clinical trials reviewed in this article shall not discourage us but rather heighten the hope that, with proper optimization of current and new therapies, and the development of well-designed clinical trials, clear beneficial effects in the diabetic population can be achieved. The aforementioned results of the DCCT study suggest that permanent insulin independence, although obviously the ideal outcome, should not be the only benchmark for deciding on the clinical utility of an intervention. Any intervention that can result in lower HbA1c levels, even if only for a short term, can potentially have a positive impact on the disease course and quality of life of the patients. These approaches, however, should be carefully developed and judiciously offered to our patients, to ensure that any associated side effects do not out-weigh the benefits derived from improved glycemic control and better quality of life.
ACKNOWLEDGMENTS
The work described here was funded by grants from the Canadian Institutes of Health Research (CIHR), the Natural Sciences and Engineering Research Council of Canada (NSERC), the Juvenile Diabetes Research Foundation (JDRF), the Diabetes Association (Foothills) and the Canadian Diabetes Association. S.T. and X.C.C. are supported by studentships from Alberta Innovates—Health Solutions (AIHS, formerly AHFMR) and the AXA Research Fund, respectively. P.S. is a Scientist of the AIHS and a JDRF Scholar. The JMDRC is supported by the Diabetes Association (Foothills).
Footnotes
Editors: Jeffrey A. Bluestone, Mark A. Atkinson, and Peter R. Arvan
Additional Perspectives on Type 1 Diabetes available at www.perspectivesinmedicine.org
REFERENCES
- Abastado JP, Lone YC, Casrouge A, Boulot G, Kourilsky P 1995. Dimerization of soluble major histocompatibility complex-peptide complexes is sufficient for activation of T cell hybridoma and induction of unresponsiveness. J Exp Med 182: 439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ablamunits V, Elias D, Reshef T, Cohen IR 1998. Islet T cells secreting IFN-γ in NOD mouse diabetes: Arrest by p277 peptide treatment. J Autoimmun 11: 73–81 [DOI] [PubMed] [Google Scholar]
- Abulafia-Lapid R, Elias D, Raz I, Keren-Zur Y, Atlan H, Cohen IR 1999. T cell proliferative responses of type 1 diabetes patients and healthy individuals to human hsp60 and its peptides. J Autoimmun 12: 121–129 [DOI] [PubMed] [Google Scholar]
- Abulafia-Lapid R, Gillis D, Yosef O, Atlan H, Cohen IR 2003. T cells and autoantibodies to human HSP70 in type 1 diabetes in children. J Autoimmun 20: 313–321 [DOI] [PubMed] [Google Scholar]
- Agardh CD, Cilio CM, Lethagen A, Lynch K, Leslie RD, Palmer M, Harris RA, Robertson JA, Lernmark A 2005. Clinical evidence for the safety of GAD65 immunomodulation in adult-onset autoimmune diabetes. J Diabetes Complications 19: 238–246 [DOI] [PubMed] [Google Scholar]
- Alleva DG, Gaur A, Jin L, Wegmann D, Gottlieb PA, Pahuja A, Johnson EB, Motheral T, Putnam A, Crowe PD, et al. 2002. Immunological characterization and therapeutic activity of an altered-peptide ligand, NBI-6024, based on the immunodominant type 1 diabetes autoantigen insulin B-chain (9-23) peptide. Diabetes 51: 2126–2134 [DOI] [PubMed] [Google Scholar]
- Alleva DG, Maki RA, Putnam AL, Robinson JM, Kipnes MS, Dandona P, Marks JB, Simmons DL, Greenbaum CJ, Jimenez RG, et al. 2006. Immunomodulation in type 1 diabetes by NBI-6024, an altered peptide ligand of the insulin B epitope. Scand J Immunol 63: 59–69 [DOI] [PubMed] [Google Scholar]
- Amrani A, Verdaguer J, Serra P, Tafuro S, Tan R, Santamaria P 2000. Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature 406: 739–742 [DOI] [PubMed] [Google Scholar]
- Anderton SM, Manickasingham SP, Burkhart C, Luckcuck TA, Holland SJ, Lamont AG, Wraith DC 1998. Fine specificity of the myelin-reactive T cell repertoire: Implications for TCR antagonism in autoimmunity. J Immunol 161: 3357–3364 [PubMed] [Google Scholar]
- Aspord C, Thivolet C 2002. Nasal administration of CTB-insulin induces active tolerance against autoimmune diabetes in non-obese diabetic (NOD) mice. Clin Exp Immunol 130: 204–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson MA, Maclaren NK, Luchetta R 1990. Insulitis and diabetes in NOD mice reduced by prophylactic insulin therapy. Diabetes 39: 933–937 [DOI] [PubMed] [Google Scholar]
- Atkinson MA, Kaufman DL, Newman D, Tobin AJ, Maclaren NK 1993. Islet cell cytoplasmic autoantibody reactivity to glutamate decarboxylase in insulin-dependent diabetes. J Clin Invest 91: 350–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson S, Hjorth M, Akerman L, Ludvigsson J, Casas R 2010. Early induction of GAD(65)-reactive Th2 response in type 1 diabetic children treated with alum-formulated GAD(65). Diabetes Metab Res Rev 26: 559–568 [DOI] [PubMed] [Google Scholar]
- Baekkeskov S, Landin M, Kristensen JK, Srikanta S, Bruining GJ, Mandrup-Poulsen T, de Beaufort C, Soeldner JS, Eisenbarth G, Lindgren F, et al. 1987. Antibodies to a 64,000 Mr human islet cell antigen precede the clinical onset of insulin-dependent diabetes. J Clin Invest 79: 926–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekkeskov S, Nielsen JH, Marner B, Bilde T, Ludvigsson J, Lernmark A 1982. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature 298: 167–169 [DOI] [PubMed] [Google Scholar]
- Bergerot I, Ploix C, Petersen J, Moulin V, Rask C, Fabien N, Lindblad M, Mayer A, Czerkinsky C, Holmgren J, et al. 1997. A cholera toxoid-insulin conjugate as an oral vaccine against spontaneous autoimmune diabetes. Proc Natl Acad Sci 94: 4610–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielekova B, Goodwin B, Richert N, Cortese I, Kondo T, Afshar G, Gran B, Eaton J, Antel J, Frank JA, et al. 2000. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83-99) in multiple sclerosis: Results of a phase II clinical trial with an altered peptide ligand. Nat Med 6: 1167–1175 [DOI] [PubMed] [Google Scholar]
- Birk OS, Elias D, Weiss AS, Rosen A, van-der Zee R, Walker MD, Cohen IR 1996. NOD mouse diabetes: The ubiquitous mouse hsp60 is a β-cell target antigen of autoimmune T cells. J Autoimmun 9: 159–166 [DOI] [PubMed] [Google Scholar]
- Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM 2002. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med 196: 1627–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bot A, Smith D, Bot S, Hughes A, Wolfe T, Wang L, Woods C, von Herrath M 2001. Plasmid vaccination with insulin B chain prevents autoimmune diabetes in nonobese diabetic mice. J Immunol 167: 2950–2955 [DOI] [PubMed] [Google Scholar]
- Bresson D, Togher L, Rodrigo E, Chen Y, Bluestone JA, Herold KC, von Herrath M 2006. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J Clin Invest 116: 1371–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresson D, Fradkin M, Manenkova Y, Rottembourg D, von Herrath M 2010. Genetic-induced variations in the GAD65 T-cell repertoire governs efficacy of anti-CD3/GAD65 combination therapy in new-onset type 1 diabetes. Mol Ther 18: 307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgett M, Cetkovic-Cvrlje M, O’Rourke R, Shi Y, Narayanswami S, Lambert J, Ramiya V, Baekkeskov S, Leiter EH 1998. Differential protection in two transgenic lines of NOD/Lt mice hyperexpressing the autoantigen GAD65 in pancreatic β-cells. Diabetes 47: 1848–1856 [DOI] [PubMed] [Google Scholar]
- Bruder D, Westendorf AM, Hansen W, Prettin S, Gruber AD, Qian Y, von Boehmer H, Mahnke K, Buer J 2005. On the edge of autoimmunity: T-cell stimulation by steady-state dendritic cells prevents autoimmune diabetes. Diabetes 54: 3395–3401 [DOI] [PubMed] [Google Scholar]
- Brumeanu TD, Swiggard WJ, Steinman RM, Bona CA, Zaghouani H 1993. Efficient loading of identical viral peptide onto class II molecules by antigenized immunoglobulin and influenza virus. J Exp Med 178: 1795–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu DF, Erlander MG, Hitz BC, Tillakaratne NJ, Kaufman DL, Wagner-McPherson CB, Evans GA, Tobin AJ 1992. Two human glutamate decarboxylases, 65-kDa GAD and 67-kDa GAD, are each encoded by a single gene. Proc Natl Acad Sci 89: 2115–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JE 3rd, Yu J, Choi NW, Hough J, Henderson D, He D, Langridge WH 2006. Bacterial and plant enterotoxin B subunit-autoantigen fusion proteins suppress diabetes insulitis. Mol Biotechnol 32: 1–15 [DOI] [PubMed] [Google Scholar]
- Casares S, Hurtado A, McEvoy RC, Sarukhan A, von Boehmer H, Brumeanu TD 2002. Down-regulation of diabetogenic CD4+ T cells by a soluble dimeric peptide-MHC class II chimera. Nat Immunol 3: 383–391 [DOI] [PubMed] [Google Scholar]
- Cetkovic-Cvrlje M, Gerling IC, Muir A, Atkinson MA, Elliott JF, Leiter EH 1997. Retardation or acceleration of diabetes in NOD/Lt mice mediated by intrathymic administration of candidate β-cell antigens. Diabetes 46: 1975–1982 [DOI] [PubMed] [Google Scholar]
- Chaillous L, Lefevre H, Thivolet C, Boitard C, Lahlou N, Atlan-Gepner C, Bouhanick B, Mogenet A, Nicolino M, Carel JC, et al. 2000. Oral insulin administration and residual β-cell function in recent-onset type 1 diabetes: A multicentre randomised controlled trial. Diabete Insuline Orale group. Lancet 356: 545–549 [DOI] [PubMed] [Google Scholar]
- Chang Y, Yap S, Ge X, Piganelli J, Bertera S, Giannokakis N, Mathews C, Prud’homme G, Trucco M 2005. DNA vaccination with an insulin construct and a chimeric protein binding to both CTLA4 and CD40 ameliorates type 1 diabetes in NOD mice. Gene Ther 12: 1679–1685 [DOI] [PubMed] [Google Scholar]
- Chen W, Bergerot I, Elliott JF, Harrison LC, Abiru N, Eisenbarth GS, Delovitch TL 2001. Evidence that a peptide spanning the B-C junction of proinsulin is an early autoantigen epitope in the pathogenesis of type 1 diabetes. J Immunol 167: 4926–4935 [DOI] [PubMed] [Google Scholar]
- Chen D, Maa YF, Haynes JR 2002. Needle-free epidermal powder immunization. Expert Rev Vaccines 1: 265–276 [DOI] [PubMed] [Google Scholar]
- Chen C, Lee WH, Yun P, Snow P, Liu CP 2003. Induction of autoantigen-specific Th2 and Tr1 regulatory T cells and modulation of autoimmune diabetes. J Immunol 171: 733–744 [DOI] [PubMed] [Google Scholar]
- Cheramy M, Skoglund C, Johansson I, Ludvigsson J, Hampe CS, Casas R 2010. GAD-alum treatment in patients with type 1 diabetes and the subsequent effect on GADA IgG subclass distribution, GAD65 enzyme activity and humoral response. Clin Immunol 137: 31–40 [DOI] [PubMed] [Google Scholar]
- Christgau S, Schierbeck H, Aanstoot HJ, Aagaard L, Begley K, Kofod H, Hejnaes K, Baekkeskov S 1991. Pancreatic β cells express two autoantigenic forms of glutamic acid decarboxylase, a 65-kDa hydrophilic form and a 64-kDa amphiphilic form which can be both membrane-bound and soluble. J Biol Chem 266: 23516. [PubMed] [Google Scholar]
- Christie MR, Tun RY, Lo SS, Cassidy D, Brown TJ, Hollands J, Shattock M, Bottazzo GF, Leslie RD 1992. Antibodies to GAD and tryptic fragments of islet 64K antigen as distinct markers for development of IDDM. Studies with identical twins. Diabetes 41: 782–787 [DOI] [PubMed] [Google Scholar]
- Clare-Salzler MJ, Brooks J, Chai A, Van Herle K, Anderson C 1992. Prevention of diabetes in nonobese diabetic mice by dendritic cell transfer. J Clin Invest 90: 741–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente-Casares X, Tsai S, Yang Y, Santamaria P 2011. Peptide-MHC-based nanovaccines for the treatment of autoimmunity: A “one size fits all” approach? J Mol Med 89: 733–742 [DOI] [PubMed] [Google Scholar]
- Coon B, An LL, Whitton JL, von Herrath MG 1999. DNA immunization to prevent autoimmune diabetes. J Clin Invest 104: 189–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creusot RJ, Yaghoubi SS, Kodama K, Dang DN, Dang VH, Breckpot K, Thielemans K, Gambhir SS, Fathman CG 2008. Tissue-targeted therapy of autoimmune diabetes using dendritic cells transduced to express IL-4 in NOD mice. Clin Immunol 127: 176–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel D, Wegmann DR 1996a. Intranasal administration of insulin peptide B: 9–23 protects NOD mice from diabetes. Ann NY Acad Sci 778: 371–372 [DOI] [PubMed] [Google Scholar]
- Daniel D, Wegmann DR 1996b. Protection of nonobese diabetic mice from diabetes by intranasal or subcutaneous administration of insulin peptide B-(9-23). Proc Natl Acad Sci 93: 956–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danko I, Fritz JD, Jiao S, Hogan K, Latendresse JS, Wolff JA 1994. Pharmacological enhancement of in vivo foreign gene expression in muscle. Gene Ther 1: 114–121 [PubMed] [Google Scholar]
- De Aizpurua HJ, Wilson YM, Harrison LC 1992. Glutamic acid decarboxylase autoantibodies in preclinical insulin-dependent diabetes. Proc Natl Acad Sci 89: 9841–9845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devendra D, Paronen J, Moriyama H, Miao D, Eisenbarth GS, Liu E 2004. Differential immune response to B:9-23 insulin 1 and insulin 2 peptides in animal models of type 1 diabetes. J Autoimmun 23: 17–26 [DOI] [PubMed] [Google Scholar]
- Diabetes Prevention Trial—Type 1 Diabetes Study Group 2002. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 346: 1685–1691 [DOI] [PubMed] [Google Scholar]
- Elias D, Cohen IR 1994. Peptide therapy for diabetes in NOD mice. Lancet 343: 704–706 [DOI] [PubMed] [Google Scholar]
- Elias D, Cohen IR 1995. Treatment of autoimmune diabetes and insulitis in NOD mice with heat shock protein 60 peptide 277. Diabetes 44: 1132–1138 [DOI] [PubMed] [Google Scholar]
- Elias D, Markovits D, Reshef T, van der Zee R, Cohen IR 1990. Induction and therapy of autoimmune diabetes in the non-obese diabetic (NOD/Lt) mouse by a 65-kDa heat shock protein. Proc Natl Acad Sci 87: 1576–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias D, Reshef T, Birk OS, van der Zee R, Walker MD, Cohen IR 1991. Vaccination against autoimmune mouse diabetes with a T-cell epitope of the human 65-kDa heat shock protein. Proc Natl Acad Sci 88: 3088–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias D, Marcus H, Reshef T, Ablamunits V, Cohen IR 1995. Induction of diabetes in standard mice by immunization with the p277 peptide of a 60-kDa heat shock protein. Eur J Immunol 25: 2851–2857 [DOI] [PubMed] [Google Scholar]
- Elias D, Meilin A, Ablamunits V, Birk OS, Carmi P, Konen-Waisman S, Cohen IR 1997. Hsp60 peptide therapy of NOD mouse diabetes induces a Th2 cytokine burst and downregulates autoimmunity to various β-cell antigens. Diabetes 46: 758–764 [DOI] [PubMed] [Google Scholar]
- Elliott JF, Qin HY, Bhatti S, Smith DK, Singh RK, Dillon T, Lauzon J, Singh B 1994. Immunization with the larger isoform of mouse glutamic acid decarboxylase (GAD67) prevents autoimmune diabetes in NOD mice. Diabetes 43: 1494–1499 [DOI] [PubMed] [Google Scholar]
- Endl J, Otto H, Jung G, Dreisbusch B, Donie F, Stahl P, Elbracht R, Schmitz G, Meinl E, Hummel M, et al. 1997. Identification of naturally processed T cell epitopes from glutamic acid decarboxylase presented in the context of HLA-DR alleles by T lymphocytes of recent onset IDDM patients. J Clin Invest 99: 2405–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ 1991. Two genes encode distinct glutamate decarboxylases. Neuron 7: 91–100 [DOI] [PubMed] [Google Scholar]
- Evavold BD, Allen PM 1991. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science 252: 1308–1310 [DOI] [PubMed] [Google Scholar]
- Evavold BD, Sloan-Lancaster J, Allen PM 1993. Tickling the TCR: Selective T-cell functions stimulated by altered peptide ligands. Immunol Today 14: 602–609 [DOI] [PubMed] [Google Scholar]
- Every AL, Kramer DR, Mannering SI, Lew AM, Harrison LC 2006. Intranasal vaccination with proinsulin DNA induces regulatory CD4+ T cells that prevent experimental autoimmune diabetes. J Immunol 176: 4608–4615 [DOI] [PubMed] [Google Scholar]
- Faulkner-Jones BE, Cram DS, Kun J, Harrison LC 1993. Localization and quantitation of expression of two glutamate decarboxylase genes in pancreatic β-cells and other peripheral tissues of mouse and rat. Endocrinology 133: 2962–2972 [DOI] [PubMed] [Google Scholar]
- Feili-Hariri M, Dong X, Alber SM, Watkins SC, Salter RD, Morel PA 1999. Immunotherapy of NOD mice with bone marrow-derived dendritic cells. Diabetes 48: 2300–2308 [DOI] [PubMed] [Google Scholar]
- Feili-Hariri M, Falkner DH, Gambotto A, Papworth GD, Watkins SC, Robbins PD, Morel PA 2003. Dendritic cells transduced to express interleukin-4 prevent diabetes in nonobese diabetic mice with advanced insulitis. Hum Gene Ther 14: 13–23 [DOI] [PubMed] [Google Scholar]
- Feili-Hariri M, Falkner DH, Morel PA 2002. Regulatory Th2 response induced following adoptive transfer of dendritic cells in prediabetic NOD mice. Eur J Immunol 32: 2021–2030 [DOI] [PubMed] [Google Scholar]
- Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, Yagita H, Azuma M, Sayegh MH, Bluestone JA 2006. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med 203: 2737–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA 2009. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol 10: 1185–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippova M, Liu J, Escher A 2001. Effects of plasmid DNA injection on cyclophosphamide-accelerated diabetes in NOD mice. DNA Cell Biol 20: 175–181 [DOI] [PubMed] [Google Scholar]
- Fischer B, Elias D, Bretzel RG, Linn T 2010. Immunomodulation with heat shock protein DiaPep277 to preserve β cell function in type 1 diabetes—an update. Expert Opin Biol Ther 10: 265–272 [DOI] [PubMed] [Google Scholar]
- Fourlanos S, Perry C, Gellert SA, Martinuzzi E, Mallone R, Butler J, Colman PG, Harrison LC 2011. Evidence that nasal insulin induces immune tolerance to insulin in adults with autoimmune diabetes. Diabetes 60: 1237–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fousteri G, Dave A, Bot A, Juntti T, Omid S, von Herrath M 2010. Subcutaneous insulin B:9-23/IFA immunisation induces Tregs that control late-stage prediabetes in NOD mice through IL-10 and IFNγ. Diabetologia 53: 1958–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelin L, Brass A, Ahlen G, Brenndorfer ED, Chen M, Sallberg M 2010. Electroporation: A promising method for the nonviral delivery of DNA vaccines in humans? Drug News Perspect 23: 647–653 [DOI] [PubMed] [Google Scholar]
- Gauvrit A, Debailleul M, Vu AT, Sai P, Bach JM 2004. DNA vaccination encoding glutamic acid decarboxylase can enhance insulitis and diabetes in correlation with a specific Th2/3 CD4 T cell response in non-obese diabetic mice. Clin Exp Immunol 137: 253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geluk A, van Meijgaarden KE, Roep BO, Ottenhoff TH 1998. Altered peptide ligands of islet autoantigen Imogen 38 inhibit antigen specific T cell reactivity in human type-1 diabetes. J Autoimmun 11: 353–361 [DOI] [PubMed] [Google Scholar]
- Geng L, Solimena M, Flavell RA, Sherwin RS, Hayday AC 1998. Widespread expression of an autoantigen-GAD65 transgene does not tolerize non-obese diabetic mice and can exacerbate disease. Proc Natl Acad Sci 95: 10055–10060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka Y, Chang Y, Prud’homme GJ 2006. Protective regulatory T cell generation in autoimmune diabetes by DNA covaccination with islet antigens and a selective CTLA-4 ligand. Mol Ther 14: 578–587 [DOI] [PubMed] [Google Scholar]
- Gottlieb P, Eisenbarth G, Kipnes M, Ratner R, Rockell J, Wagner R, Solvason N, Tersini K, Valone F, Woody K, et al. 2008. Interim results of a phase I/II clinical trial of a DNA plasmid vaccine (BHT-3021) for type 1 diabetes. Diabetologia 51 (Abstr): 574 [Google Scholar]
- Goudy KS, Wang B, Tisch R 2008. Gene gun-mediated DNA vaccination enhances antigen-specific immunotherapy at a late preclinical stage of type 1 diabetes in nonobese diabetic mice. Clin Immunol 129: 49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RK, Jain R, Schoenleber SJ, Divekar R, Bell JJ, Lee HH, Yu P, Zaghouani H 2004. A sudden decline in active membrane-bound TGF-β impairs both T regulatory cell function and protection against autoimmune diabetes. J Immunol 173: 7308–7316 [DOI] [PubMed] [Google Scholar]
- Han B, Serra P, Amrani A, Yamanouchi J, Maree AF, Edelstein-Keshet L, Santamaria P 2005. Prevention of diabetes by manipulation of anti-IGRP autoimmunity: High efficiency of a low-affinity peptide. Nat Med 11: 645–652 [DOI] [PubMed] [Google Scholar]
- Harrison LC, Dempsey-Collier M, Kramer DR, Takahashi K 1996. Aerosol insulin induces regulatory CD8 γ δ T cells that prevent murine insulin-dependent diabetes. J Exp Med 184: 2167–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison LC, Honeyman MC, Steele CE, Stone NL, Sarugeri E, Bonifacio E, Couper JJ, Colman PG 2004. Pancreatic β-cell function and immune responses to insulin after administration of intranasal insulin to humans at risk for type 1 diabetes. Diabetes Care 27: 2348–2355 [DOI] [PubMed] [Google Scholar]
- He FR, Zhu HF, Huang H, Dai YD, Shen X, Wang M, Li L, Xing W, Shen GX 2008. Programmed death-1 ligands-transfected dendritic cells loaded with glutamic acid decarboxylase 65 (GAD65) inhibit both the alloresponse and the GAD65-reactive lymphocyte response. Clin Exp Immunol 151: 86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann SH, Mescher MF 1986. The requirements for antigen multivalency in class I antigen recognition and triggering of primed precursor cytolytic T lymphocytes. J Immunol 136: 2816–2825 [PubMed] [Google Scholar]
- Hjorth M, Axelsson S, Ryden A, Faresjo M, Ludvigsson J, Casas R 2011. GAD-alum treatment induces GAD65-specific CD4+CD25highFOXP3+ cells in type 1 diabetic patients. Clin Immunol 138: 117–126 [DOI] [PubMed] [Google Scholar]
- Honeyman MC, Cram DS, Harrison LC 1993. Glutamic acid decarboxylase 67-reactive T cells: A marker of insulin-dependent diabetes. J Exp Med 177: 535–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings PR, Cooke A 1995. Comparative study of the protective effect afforded by intravenous administration of bovine or ovine insulin to young NOD mice. Diabetes 44: 906–910 [DOI] [PubMed] [Google Scholar]
- Hutchings P, Cooke A 1998. Protection from insulin dependent diabetes mellitus afforded by insulin antigens in incomplete Freund’s adjuvant depends on route of administration. J Autoimmun 11: 127–130 [DOI] [PubMed] [Google Scholar]
- Huurman VA, Decochez K, Mathieu C, Cohen IR, Roep BO 2007. Therapy with the hsp60 peptide DiaPep277 in C-peptide positive type 1 diabetes patients. Diabetes Metab Res Rev 23: 269–275 [DOI] [PubMed] [Google Scholar]
- Huurman VA, van der Meide PE, Duinkerken G, Willemen S, Cohen IR, Elias D, Roep BO 2008. Immunological efficacy of heat shock protein 60 peptide DiaPep277 therapy in clinical type I diabetes. Clin Exp Immunol 152: 488–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeckel E, Klein L, Martin-Orozco N, von Boehmer H 2003. Normal incidence of diabetes in NOD mice tolerant to glutamic acid decarboxylase. J Exp Med 197: 1635–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R, Tartar DM, Gregg RK, Divekar RD, Bell JJ, Lee HH, Yu P, Ellis JS, Hoeman CM, Franklin CL, et al. 2008. Innocuous IFNγ induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J Exp Med 205: 207–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, Nussenzweig MC 1995. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature 375: 151–155 [DOI] [PubMed] [Google Scholar]
- Kappos L, Comi G, Panitch H, Oger J, Antel J, Conlon P, Steinman L 2000. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. The Altered Peptide Ligand in Relapsing MS Study Group. Nat Med 6: 1176–1182 [DOI] [PubMed] [Google Scholar]
- Karges W, Pechhold K, Al Dahouk S, Riegger I, Rief M, Wissmann A, Schirmbeck R, Barth C, Boehm BO 2002. Induction of autoimmune diabetes through insulin (but not GAD65) DNA vaccination in nonobese diabetic and in RIP-B7.1 mice. Diabetes 51: 3237–3244 [DOI] [PubMed] [Google Scholar]
- Karlsen AE, Hagopian WA, Petersen JS, Boel E, Dyrberg T, Grubin CE, Michelsen BK, Madsen OD, Lernmark A 1992. Recombinant glutamic acid decarboxylase (representing the single isoform expressed in human islets) detects IDDM-associated 64,000-M(r) autoantibodies. Diabetes 41: 1355–1359 [DOI] [PubMed] [Google Scholar]
- Kaufman DL, Clare-Salzler M, Tian J, Forsthuber T, Ting GS, Robinson P, Atkinson MA, Sercarz EE, Tobin AJ, Lehmann PV 1993. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature 366: 69–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuglin B, Gries FA, Kolb H 1988. Evidence of IgG autoantibodies against human proinsulin in patients with IDDM before insulin treatment. Diabetes 37: 130–132 [DOI] [PubMed] [Google Scholar]
- Laughlin E, Burke G, Pugliese A, Falk B, Nepom G 2008. Recurrence of autoreactive antigen-specific CD4+ T cells in autoimmune diabetes after pancreas transplantation. Clin Immunol 128: 23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar L, Ofan R, Weintrob N, Avron A, Tamir M, Elias D, Phillip M, Josefsberg Z 2007. Heat-shock protein peptide DiaPep277 treatment in children with newly diagnosed type 1 diabetes: A randomised, double-blind phase II study. Diabetes Metab Res Rev 23: 286–291 [DOI] [PubMed] [Google Scholar]
- Li A, Ojogho O, Franco E, Baron P, Iwaki Y, Escher A 2006. Pro-apoptotic DNA vaccination ameliorates new onset of autoimmune diabetes in NOD mice and induces foxp3+ regulatory T cells in vitro. Vaccine 24: 5036–5046 [DOI] [PubMed] [Google Scholar]
- Li L, Yi Z, Wang B, Tisch R 2009. Suppression of ongoing T cell-mediated autoimmunity by peptide-MHC class II dimer vaccination. J Immunol 183: 4809–4816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman SM, DiLorenzo TP 2003. A comprehensive guide to antibody and T-cell responses in type 1 diabetes. Tissue Antigens 62: 359–377 [DOI] [PubMed] [Google Scholar]
- Lin M, Stoica-Nazarov C, Surls J, Kehl M, Bona C, Olsen C, Brumeanu TD, Casares S 2010. Reversal of type 1 diabetes by a new MHC II-peptide chimera: “Single-epitope-mediated suppression” to stabilize a polyclonal autoimmune T-cell process. Eur J Immunol 40: 2277–2288 [DOI] [PubMed] [Google Scholar]
- Liu E, Moriyama H, Abiru N, Miao D, Yu L, Taylor RM, Finkelman FD, Eisenbarth GS 2002. Anti-peptide autoantibodies and fatal anaphylaxis in NOD mice in response to insulin self-peptides B:9-23 and B:13-23. J Clin Invest 110: 1021–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsson J, Faresjo M, Hjorth M, Axelsson S, Cheramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C, et al. 2008. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 359: 1909–1920 [DOI] [PubMed] [Google Scholar]
- Ludvigsson J, Hjorth M, Cheramy M, Axelsson S, Pihl M, Forsander G, Nilsson NO, Samuelsson BO, Wood T, Aman J, et al. 2011. Extended evaluation of the safety and efficacy of GAD treatment of children and adolescents with recent-onset type 1 diabetes: A randomised controlled trial. Diabetologia 54: 634–640 [DOI] [PubMed] [Google Scholar]
- Ma SW, Zhao DL, Yin ZQ, Mukherjee R, Singh B, Qin HY, Stiller CR, Jevnikar AM 1997. Transgenic plants expressing autoantigens fed to mice to induce oral immune tolerance. Nat Med 3: 793–796 [DOI] [PubMed] [Google Scholar]
- Ma L, Qian S, Liang X, Wang L, Woodward JE, Giannoukakis N, Robbins PD, Bertera S, Trucco M, Fung JJ, et al. 2003. Prevention of diabetes in NOD mice by administration of dendritic cells deficient in nuclear transcription factor-κB activity. Diabetes 52: 1976–1985 [DOI] [PubMed] [Google Scholar]
- Ma S, Huang Y, Yin Z, Menassa R, Brandle JE, Jevnikar AM 2004. Induction of oral tolerance to prevent diabetes with transgenic plants requires glutamic acid decarboxylase (GAD) and IL-4. Proc Natl Acad Sci 101: 5680–5685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machen J, Harnaha J, Lakomy R, Styche A, Trucco M, Giannoukakis N 2004. Antisense oligonucleotides down-regulating costimulation confer diabetes-preventive properties to nonobese diabetic mouse dendritic cells. J Immunol 173: 4331–4341 [DOI] [PubMed] [Google Scholar]
- Mahnke K, Qian Y, Knop J, Enk AH 2003. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood 101: 4862–4869 [DOI] [PubMed] [Google Scholar]
- Marin-Gallen S, Clemente-Casares X, Planas R, Pujol-Autonell I, Carrascal J, Carrillo J, Ampudia R, Verdaguer J, Pujol-Borrell R, Borras FE, et al. 2010. Dendritic cells pulsed with antigen-specific apoptotic bodies prevent experimental type 1 diabetes. Clin Exp Immunol 160: 207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron R, Melican NS, Weiner HL 1999. Regulatory Th2-type T cell lines against insulin and GAD peptides derived from orally- and nasally-treated NOD mice suppress diabetes. J Autoimmun 12: 251–258 [DOI] [PubMed] [Google Scholar]
- Martinez NR, Augstein P, Moustakas AK, Papadopoulos GK, Gregori S, Adorini L, Jackson DC, Harrison LC 2003. Disabling an integral CTL epitope allows suppression of autoimmune diabetes by intranasal proinsulin peptide. J Clin Invest 111: 1365–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masteller EL, Warner MR, Ferlin W, Judkowski V, Wilson D, Glaichenhaus N, Bluestone JA 2003. Peptide-MHC class II dimers as therapeutics to modulate antigen-specific T cell responses in autoimmune diabetes. J Immunol 171: 5587–5595 [DOI] [PubMed] [Google Scholar]
- McCluskey J, Blok R, Kjer-Nielsen L 1989. The role of major histocompatibility molecules in antigen-specific immune responses. Transplant Proc 21: 591–594 [PubMed] [Google Scholar]
- Merani S, Shapiro AM 2006. Current status of pancreatic islet transplantation. Clin Sci (Lond) 110: 611–625 [DOI] [PubMed] [Google Scholar]
- Monti P, Scirpoli M, Maffi P, Ghidoli N, De Taddeo F, Bertuzzi F, Piemonti L, Falcone M, Secchi A, Bonifacio E 2008. Islet transplantation in patients with autoimmune diabetes induces homeostatic cytokines that expand autoreactive memory T cells. J Clin Invest 118: 1806–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel PA, Vasquez AC, Feili-Hariri M 1999. Immunobiology of DC in NOD mice. J Leukoc Biol 66: 276–280 [DOI] [PubMed] [Google Scholar]
- Muir A, Peck A, Clare-Salzler M, Song YH, Cornelius J, Luchetta R, Krischer J, Maclaren N 1995. Insulin immunization of nonobese diabetic mice induces a protective insulitis characterized by diminished intraislet interferon-γ transcription. J Clin Invest 95: 628–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhaya A, Hanafusa T, Jarchum I, Chen YG, Iwai Y, Serreze DV, Steinman RM, Tarbell KV, DiLorenzo TP 2008. Selective delivery of β cell antigen to dendritic cells in vivo leads to deletion and tolerance of autoreactive CD8+ T cells in NOD mice. Proc Natl Acad Sci 105: 6374–6379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, et al. 2005. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 435: 220–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanto-Salonen K, Kupila A, Simell S, Siljander H, Salonsaari T, Hekkala A, Korhonen S, Erkkola R, Sipila JI, Haavisto L, et al. 2008. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: A double-blind, randomised controlled trial. Lancet 372: 1746–1755 [DOI] [PubMed] [Google Scholar]
- Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, Backlund JY, O’Leary DH, Genuth S 2003. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 348: 2294–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B 2005. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353: 2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niens M, Grier AE, Marron M, Kay TW, Greiner DL, Serreze DV 2011. Prevention of “humanized” diabetogenic CD8 T-cell responses in HLA-transgenic NOD mice by a multipeptide coupled-cell approach. Diabetes 60: 1229–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum G, Zanin-Zhorov A, Quintana F, Lider O, Cohen IR 2006. Peptide p277 of HSP60 signals T cells: Inhibition of inflammatory chemotaxis. Int Immunol 18: 1413–1419 [DOI] [PubMed] [Google Scholar]
- Orban T, Farkas K, Jalahej H, Kis J, Treszl A, Falk B, Reijonen H, Wolfsdorf J, Ricker A, Matthews JB, et al. 2010. Autoantigen-specific regulatory T cells induced in patients with type 1 diabetes mellitus by insulin B-chain immunotherapy. J Autoimmun 34: 408–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JP, Asplin CM, Clemons P, Lyen K, Tatpati O, Raghu PK, Paquette TL 1983. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science 222: 1337–1339 [DOI] [PubMed] [Google Scholar]
- Panina-Bordignon P, Lang R, van Endert PM, Benazzi E, Felix AM, Pastore RM, Spinas GA, Sinigaglia F 1995. Cytotoxic T cells specific for glutamic acid decarboxylase in autoimmune diabetes. J Exp Med 181: 1923–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perone MJ, Bertera S, Tawadrous ZS, Shufesky WJ, Piganelli JD, Baum LG, Trucco M, Morelli AE 2006. Dendritic cells expressing transgenic galectin-1 delay onset of autoimmune diabetes in mice. J Immunol 177: 5278–5289 [DOI] [PubMed] [Google Scholar]
- Petzold C, Riewaldt J, Koenig T, Schallenberg S, Kretschmer K 2011. Dendritic cell-targeted pancreatic β-cell antigen leads to conversion of self-reactive CD4+ T cells into regulatory T cells and promotes immunotolerance in NOD mice. Rev Diabet Stud 7: 47–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleau JM, Fernandez-Saravia F, Esling A, Homo-Delarche F, Dardenne M 1995. Prevention of autoimmune diabetes in nonobese diabetic female mice by treatment with recombinant glutamic acid decarboxylase (GAD 65). Clin Immunol Immunopathol 76: 90–95 [DOI] [PubMed] [Google Scholar]
- Plesner A, Worsaae A, Dyrberg T, Gotfredsen C, Michelsen BK, Petersen JS 1998. Immunization of diabetes-prone or non-diabetes-prone mice with GAD65 does not induce diabetes or islet cell pathology. J Autoimmun 11: 335–341 [DOI] [PubMed] [Google Scholar]
- Pop SM, Wong CP, He Q, Wang Y, Wallet MA, Goudy KS, Tisch R 2007. The type and frequency of immunoregulatory CD4+ T-cells govern the efficacy of antigen-specific immunotherapy in nonobese diabetic mice. Diabetes 56: 1395–1402 [DOI] [PubMed] [Google Scholar]
- Pozzilli P, Pitocco D, Visalli N, Cavallo MG, Buzzetti R, Crino A, Spera S, Suraci C, Multari G, Cervoni M, et al. 2000. No effect of oral insulin on residual β-cell function in recent-onset type I diabetes (the IMDIAB VII). IMDIAB Group. Diabetologia 43: 1000–1004 [DOI] [PubMed] [Google Scholar]
- Preda I, McEvoy RC, Lin M, Bona CA, Rapaport R, Brumeanu TD, Casares S 2005. Soluble, dimeric HLA DR4-peptide chimeras: An approach for detection and immunoregulation of human type-1 diabetes. Eur J Immunol 35: 2762–2775 [DOI] [PubMed] [Google Scholar]
- Prud’homme GJ, Chang Y, Li X 2002. Immunoinhibitory DNA vaccine protects against autoimmune diabetes through cDNA encoding a selective CTLA-4 (CD152) ligand. Hum Gene Ther 13: 395–406 [DOI] [PubMed] [Google Scholar]
- Prud’homme GJ, Draghia-Akli R, Wang Q 2007. Plasmid-based gene therapy of diabetes mellitus. Gene Ther 14: 553–564 [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Rotem A, Carmi P, Cohen IR 2000. Vaccination with empty plasmid DNA or CpG oligonucleotide inhibits diabetes in nonobese diabetic mice: Modulation of spontaneous 60-kDa heat shock protein autoimmunity. J Immunol 165: 6148–6155 [DOI] [PubMed] [Google Scholar]
- Ramiya VK, Shang XZ, Wasserfall CH, Maclaren NK 1997. Effect of oral and intravenous insulin and glutamic acid decarboxylase in NOD mice. Autoimmunity 26: 139–151 [DOI] [PubMed] [Google Scholar]
- Ravanan R, Wong SF, Morgan NG, Mathieson PW, Smith RM 2007. Inhalation of glutamic acid decarboxylase 65-derived peptides can protect against recurrent autoimmune but not alloimmune responses in the non-obese diabetic mouse. Clin Exp Immunol 148: 368–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR 2001. β-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): A randomised, double-blind, phase II trial. Lancet 358: 1749–1753 [DOI] [PubMed] [Google Scholar]
- Raz I, Avron A, Tamir M, Metzger M, Symer L, Eldor R, Cohen IR, Elias D 2007. Treatment of new-onset type 1 diabetes with peptide DiaPep277 is safe and associated with preserved beta-cell function: Extension of a randomized, double-blind, phase II trial. Diabetes Metab Res Rev 23: 292–298 [DOI] [PubMed] [Google Scholar]
- Rivas EI, Driver JP, Garabatos N, Presa M, Mora C, Rodriguez F, Serreze DV, Stratmann T 2011. Targeting of a T cell agonist peptide to lysosomes by DNA vaccination induces tolerance in the nonobese diabetic mouse. J Immunol 186: 4078–4087 [DOI] [PubMed] [Google Scholar]
- Robertson RP 2010. Islet transplantation a decade later and strategies for filling a half-full glass. Diabetes 59: 1285–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy G, Stone N, Harrison LC, Colman PG, McNair P, Brusic V, French MB, Honeyman MC, Tait B, Lew AM 1995. Similar peptides from two β cell autoantigens, proinsulin and glutamic acid decarboxylase, stimulate T cells of individuals at risk for insulin-dependent diabetes. Mol Med 1: 625–633 [PMC free article] [PubMed] [Google Scholar]
- Ruffner MA, Robbins PD 2010. Dendritic cells transduced to express interleukin 4 reduce diabetes onset in both normoglycemic and prediabetic nonobese diabetic mice. PLoS One 5: e11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai P, Damage C, Rivereau AS, Hoeltzel A, Gouin E 1996. Prophylactic oral administration of metabolically active insulin entrapped in isobutylcyanoacrylate nanocapsules reduces the incidence of diabetes in nonobese diabetic mice. J Autoimmun 9: 713–722 [DOI] [PubMed] [Google Scholar]
- Schloot NC, Meierhoff G, Lengyel C, Vandorfi G, Takacs J, Panczel P, Barkai L, Madacsy L, Oroszlan T, Kovacs P, et al. 2007. Effect of heat shock protein peptide DiaPep277 on β-cell function in paediatric and adult patients with recent-onset diabetes mellitus type 1: Two prospective, randomized, double-blind phase II trials. Diabetes Metab Res Rev 23: 276–285 [DOI] [PubMed] [Google Scholar]
- Seissler J, Amann J, Mauch L, Haubruck H, Wolfahrt S, Bieg S, Richter W, Holl R, Heinze E, Northemann W, et al. 1993. Prevalence of autoantibodies to the 65- and 67-kD isoforms of glutamate decarboxylase in insulin-dependent diabetes mellitus. J Clin Invest 92: 1394–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV 2000. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 343: 230–238 [DOI] [PubMed] [Google Scholar]
- Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, et al. 2006. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 355: 1318–1330 [DOI] [PubMed] [Google Scholar]
- Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LE, Chase HP, Leschek E 2005. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial–Type 1. Diabetes Care 28: 1068–1076 [DOI] [PubMed] [Google Scholar]
- Skyler JS, Greenbaum CJ, Lachin JM, Leschek E, Rafkin-Mervis L, Savage P, Spain L 2008. Type 1 Diabetes TrialNet—an international collaborative clinical trials network. Ann NY Acad Sci 1150: 14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan-Lancaster J, Allen PM 1996. Altered peptide ligand-induced partial T cell activation: Molecular mechanisms and role in T cell biology. Annu Rev Immunol 14: 1–27 [DOI] [PubMed] [Google Scholar]
- Solvason N, Lou YP, Peters W, Evans E, Martinez J, Ramirez U, Ocampo A, Yun R, Ahmad S, Liu E, et al. 2008. Improved efficacy of a tolerizing DNA vaccine for reversal of hyperglycemia through enhancement of gene expression and localization to intracellular sites. J Immunol 181: 8298–8307 [DOI] [PubMed] [Google Scholar]
- Speight J, Reaney MD, Woodcock AJ, Smith RM, Shaw JA 2010. Patient-reported outcomes following islet cell or pancreas transplantation (alone or after kidney) in Type 1 diabetes: A systematic review. Diabet Med 27: 812–822 [DOI] [PubMed] [Google Scholar]
- Steffes MW, Sibley S, Jackson M, Thomas W 2003. β-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 26: 832–836 [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC 2003. Tolerogenic dendritic cells. Annu Rev Immunol 21: 685–711 [DOI] [PubMed] [Google Scholar]
- Tai N, Yasuda H, Xiang Y, Zhang L, Rodriguez-Pinto D, Yokono K, Sherwin R, Wong FS, Nagata M, Wen L 2011. IL-10-conditioned dendritic cells prevent autoimmune diabetes in NOD and humanized HLA-DQ8/RIP-B7.1 mice. Clin Immunol 139: 336–349 [DOI] [PubMed] [Google Scholar]
- Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA 2004. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med 199: 1455–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartar DM, VanMorlan AM, Wan X, Guloglu FB, Jain R, Haymaker CL, Ellis JS, Hoeman CM, Cascio JA, Dhakal M, et al. 2010. FoxP3+RORγt+ T helper intermediates display suppressive function against autoimmune diabetes. J Immunol 184: 3377–3385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group 1993. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329: 977–986 [DOI] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group 2000. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med 342: 381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thivolet CH, Tappaz M, Durand A, Petersen J, Stefanutti A, Chatelain P, Vialettes B, Scherbaum W, Orgiazzi J 1992. Glutamic acid decarboxylase (GAD) autoantibodies are additional predictive markers of type 1 (insulin-dependent) diabetes mellitus in high risk individuals. Diabetologia 35: 570–576 [DOI] [PubMed] [Google Scholar]
- Tian J, Atkinson MA, Clare-Salzler M, Herschenfeld A, Forsthuber T, Lehmann PV, Kaufman DL 1996. Nasal administration of glutamate decarboxylase (GAD65) peptides induces Th2 responses and prevents murine insulin-dependent diabetes. J Exp Med 183: 1561–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Dang H, von Boehmer H, Jaeckel E, Kaufman DL 2009. Transgenically induced GAD tolerance curtails the development of early β-cell autoreactivities but causes the subsequent development of supernormal autoreactivities to other β-cell antigens. Diabetes 58: 2843–2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisch R, Yang XD, Singer SM, Liblau RS, Fugger L, McDevitt HO 1993. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature 366: 72–75 [DOI] [PubMed] [Google Scholar]
- Tisch R, Wang B, Serreze DV 1999. Induction of glutamic acid decarboxylase 65-specific Th2 cells and suppression of autoimmune diabetes at late stages of disease is epitope dependent. J Immunol 163: 1178–1187 [PubMed] [Google Scholar]
- Tisch R, Wang B, Weaver DJ, Liu B, Bui T, Arthos J, Serreze DV 2001. Antigen-specific mediated suppression of β cell autoimmunity by plasmid DNA vaccination. J Immunol 166: 2122–2132 [DOI] [PubMed] [Google Scholar]
- Torres-Aguilar H, Sanchez-Torres C, Jara LJ, Blank M, Shoenfeld Y 2010. IL-10/TGF-β-treated dendritic cells, pulsed with insulin, specifically reduce the response to insulin of CD4+ effector/memory T cells from type 1 diabetic individuals. J Clin Immunol 30: 659–668 [DOI] [PubMed] [Google Scholar]
- Tsai S, Shameli A, Yamanouchi J, Clemente-Casares X, Wang J, Serra P, Yang Y, Medarova Z, Moore A, Santamaria P 2010. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity 32: 568–580 [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Zimmet P, Mackay IR, Koskela P, Vidgren G, Toivanen L, Tuomilehto-Wolf E, Kohtamaki K, Stengard J, Rowley MJ 1994. Antibodies to glutamic acid decarboxylase as predictors of insulin-dependent diabetes mellitus before clinical onset of disease. Lancet 343: 1383–1385 [DOI] [PubMed] [Google Scholar]
- Turley DM, Miller SD 2007. Peripheral tolerance induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J Immunol 178: 2212–2220 [DOI] [PubMed] [Google Scholar]
- Uibo R, Lernmark A 2008. GAD65 autoimmunity-clinical studies. Adv Immunol 100: 39–78 [DOI] [PubMed] [Google Scholar]
- Urbanek-Ruiz I, Ruiz PJ, Paragas V, Garren H, Steinman L, Fathman CG 2001. Immunization with DNA encoding an immunodominant peptide of insulin prevents diabetes in NOD mice. Clin Immunol 100: 164–171 [DOI] [PubMed] [Google Scholar]
- Velthuis JH, Unger WW, van der Slik AR, Duinkerken G, Engelse M, Schaapherder AF, Ringers J, van Kooten C, de Koning EJ, Roep BO 2009. Accumulation of autoreactive effector T cells and allo-specific regulatory T cells in the pancreas allograft of a type 1 diabetic recipient. Diabetologia 52: 494–503 [DOI] [PubMed] [Google Scholar]
- Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase HP, Eisenbarth GS 1996. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes 45: 926–933 [DOI] [PubMed] [Google Scholar]
- Walter M, Philotheou A, Bonnici F, Ziegler AG, Jimenez R 2009. No effect of the altered peptide ligand NBI-6024 on β-cell residual function and insulin needs in new-onset type 1 diabetes. Diabetes Care 32: 2036–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann DR, Gill RG, Norbury-Glaser M, Schloot N, Daniel D 1994a. Analysis of the spontaneous T cell response to insulin in NOD mice. J Autoimmun 7: 833–843 [DOI] [PubMed] [Google Scholar]
- Wegmann DR, Norbury-Glaser M, Daniel D 1994b. Insulin-specific T cells are a predominant component of islet infiltrates in pre-diabetic NOD mice. Eur J Immunol 24: 1853–1857 [DOI] [PubMed] [Google Scholar]
- Wherrett DK, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Herold KC, Marks JB, et al. 2011. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: A randomised double-blind trial. Lancet 378: 319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest-Ladenburger U, Fortnagel A, Richter W, Reimann J, Boehm BO 1998. DNA vaccination with glutamic acid decarboxylase (GAD) generates a strong humoral immune response in BALB/c, C57BL/6, and in diabetes-prone NOD mice. Horm Metab Res 30: 605–609 [DOI] [PubMed] [Google Scholar]
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL 1990. Direct gene transfer into mouse muscle in vivo. Science 247: 1465–1468 [DOI] [PubMed] [Google Scholar]
- Xia CQ, Peng R, Qiu Y, Annamalai M, Gordon D, Clare-Salzler MJ 2007. Transfusion of apoptotic β-cells induces immune tolerance to β-cell antigens and prevents type 1 diabetes in NOD mice. Diabetes 56: 2116–2123 [DOI] [PubMed] [Google Scholar]
- Yoon JW, Yoon CS, Lim HW, Huang QQ, Kang Y, Pyun KH, Hirasawa K, Sherwin RS, Jun HS 1999. Control of autoimmune diabetes in NOD mice by GAD expression or suppression in β cells. Science 284: 1183–1187 [DOI] [PubMed] [Google Scholar]
- You S, Chen C, Lee WH, Brusko T, Atkinson M, Liu CP 2004. Presence of diabetes-inhibiting, glutamic acid decarboxylase-specific, IL-10-dependent, regulatory T cells in naive nonobese diabetic mice. J Immunol 173: 6777–6785 [DOI] [PubMed] [Google Scholar]
- Yu L, Robles DT, Abiru N, Kaur P, Rewers M, Kelemen K, Eisenbarth GS 2000. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci 97: 1701–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghouani H, Steinman R, Nonacs R, Shah H, Gerhard W, Bona C 1993. Presentation of a viral T cell epitope expressed in the CDR3 region of a self immunoglobulin molecule. Science 259: 224–227 [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A, Tal G, Shivtiel S, Cohen M, Lapidot T, Nussbaum G, Margalit R, Cohen IR, Lider O 2005. Heat shock protein 60 activates cytokine-associated negative regulator suppressor of cytokine signaling 3 in T cells: Effects on signaling, chemotaxis, and inflammation. J Immunol 175: 276–285 [DOI] [PubMed] [Google Scholar]
- Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR 2006. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest 116: 2022–2032 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zekzer D, Wong FS, Ayalon O, Millet I, Altieri M, Shintani S, Solimena M, Sherwin RS 1998. GAD-reactive CD4+ Th1 cells induce diabetes in NOD/SCID mice. J Clin Invest 101: 68–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZJ, Davidson L, Eisenbarth G, Weiner HL 1991. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc Natl Acad Sci 88: 10252–10256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Nakayama M, Eisenbarth GS 2008. Insulin as an autoantigen in NOD/human diabetes. Curr Opin Immunol 20: 111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]