Background: Constitutive activity is central to G protein-coupled receptor signaling but the mechanisms underlying it are still unknown.
Results: The ghrelin receptor monomer reconstituted in a lipid disc activates Gq without agonist and recruits arrestin in a ligand-dependent manner.
Conclusion: High constitutive activity is an intrinsic property of the ghrelin receptor.
Significance: This is the first demonstration that the ghrelin receptor has all the determinants for constitutive activity and ligand-regulated internalization.
Keywords: 7-Helix Receptor, Arrestin, Fluorescence, Membrane Proteins, Signal Transduction, Constitutive Activity, Ghrelin, Internalization
Abstract
Despite its central role in signaling and the potential therapeutic applications of inverse agonists, the molecular mechanisms underlying G protein-coupled receptor (GPCR) constitutive activity remain largely to be explored. In this context, ghrelin receptor GHS-R1a is a peculiar receptor in the sense that it displays a strikingly high, physiologically relevant, constitutive activity. To identify the molecular mechanisms responsible for this high constitutive activity, we have reconstituted a purified GHS-R1a monomer in a lipid disc. Using this reconstituted system, we show that the isolated ghrelin receptor per se activates Gq in the absence of agonist, as assessed through guanosine 5′-O-(thiotriphosphate) binding experiments. The measured constitutive activity is similar in its extent to that observed in heterologous systems and in vivo. This is the first direct evidence for the high constitutive activity of the ghrelin receptor being an intrinsic property of the protein rather than the result of influence of its cellular environment. Moreover, we show that the isolated receptor in lipid discs recruits arrestin-2 in an agonist-dependent manner, whereas it interacts with μ-AP2 in the absence of ligand or in the presence of ghrelin. Of importance, these differences are linked to ligand-specific GHS-R1a conformations, as assessed by intrinsic fluorescence measurements. The distinct ligand requirements for the interaction of purified GHS-R1a with arrestin and AP2 provide a new rationale to the differences in basal and agonist-induced internalization observed in cells.
Introduction
The ability for a vast majority of receptors to display spontaneous constitutive activity has extended our insights into G protein-coupled receptor (GPCR)4-mediated signaling and provided new criteria for drug design and therapeutic strategies (1). Along this line, the behavior of very specific modulators of this basal activity shed light on inverse agonism (2, 3). Another major interest of GPCR constitutive activity relies on characterization of the behavior of the receptor itself, based on the conceptual framework of a complex conformational repertoire (4). Assessing mechanisms underlying constitutive signaling of GPCRs is therefore a constant evolving area. It is predictably challenged through discoveries of new inverse agonists for most receptors displaying native basal signaling, but also further addressed through the analysis of conformational changes concealing distinct biological effects (5).
Modulation of basal activity is a frequent issue of the GPCRs natural mutations (6, 7). Subsequent altered physiological functions and diseases have aroused interest for these mutants and helped extend the multistate model for receptor activation (8). A general mechanism has been proposed that would be shared by all GPCRs (9, 10). Specific residues in the vicinity of these mutation points have been proposed to orchestrate finely tuned microswitches critical for the level of activation in the absence of ligand (11–13). Establishing a naturally occurring modulation of constitutive activity suggests that ligand-independent signaling contributes to biological responses. In connection with this concept, few in vivo studies already highlighted the physiological function of spontaneous or mutation-induced constitutive activity of GPCRs (7, 14–18).
Besides in vivo data, many in vitro studies with heterologous systems have been used to shed light on GPCR constitutive activity. However, characterization of basal activity is often hindered due to assay-dependent parameters or to the occurrence of intrinsic modulators of the constitutive activity. High background signals triggered by neighboring constitutively active receptors, high expression levels in heterologous systems, or endogenous ligands in the media may indeed lead to hampered records (19, 20), leaving on hold the search for the intrinsic determinants of the receptor protein for constitutive signaling.
Among very few GPCRs, ghrelin receptor GHS-R1a has been shown to display both the highest basal activation of Gαq (about 50% of its maximal activity) in vitro, and substantial basal signaling for food intake and weight control in vivo (11, 18, 21, 22). The physiological relevance of GHS-R1a basal activity is substantiated by the occurrence of a human mutation in the GHS-R1a gene that suppresses constitutive activity and is associated with a short-stature phenotype (7). Interestingly, a high basal activity was also observed for MC4R and CB1 receptors, both involved in appetite regulation. Besides G protein-mediated signaling, constitutive activity of the ghrelin receptor family was also found to be necessary to control arrestin-independent endocytosis (23).
The structural features of GPCRs in the absence of ligand as well as the conformational transitions occurring during inverse agonism remain questioned. Previous studies with isolated receptors based on ligand-specific states detected by classical or fluorescence lifetime spectroscopies gave insights into the dynamics of these structural changes (24–26). In a few cases, these studies have allowed a direct molecular characterization of basal activity (10, 25). However, the receptors investigated, i.e. essentially the β2-adrenergic and the 5-HT4(a) serotonin receptors, display only to a limited extent natural constitutive activity.
Studies with isolated GPCRs have been in part hampered by the instability of purified receptors in detergents that mimic very imperfectly the membrane environment. For this reason, an alternative medium, nanoscale phospholipid bilayers, has been developed where purified GPCRs can be assembled (27–30). In the present study, we used this membrane bilayer model to reconstitute the purified ghrelin receptor and analyze its basal activity using a combination of biochemical and conventional spectroscopy methods. These approaches allowed us to address the central question of the intrinsic properties of the receptor protein within a controlled model system. In doing so, we directly demonstrate that the basal active state is an intrinsic feature of the monomeric GHS-R1a receptor.
EXPERIMENTAL PROCEDURES
Materials
All chemicals were from Sigma with the exception of DDM that was purchased from Calbiochem and lipids that were from Avanti Polar Lipids. A8–35 was purchased from Anatrace. Ghrelin was from Polypeptide Laboratories, NEOMPS, MK677 from Axon Medchem BV, and substance P analog (SPA) from Bachem. Alexa Fluor 350 carboxylic acid succinimidyl ester and BODIPY FL GTPγS were from Invitrogen. The pET28a vector encoding MSP1E3 was obtained from Addgene (Cambridge, MA). Cephalopod Gαq was purified from dark-adapted retinas of Sepia officinalis (31). Gαi2 and Gβ1γ2 were produced as described in Banères and Parello (32), whereas Gαs was expressed and purified as reported in Pellissier et al. (33).
Peptide Synthesis
The synthesis of the different peptides used throughout this work is extensively described in the supplemental data.
GHS-R1a Expression and Purification
The sequence encoding the human ghrelin receptor GHS-R1a was cloned downstream from that encoding αI and a thrombin cleavage site (34). Receptor expression and purification under denaturing conditions were carried out as described in Arcimesbéhère et al. (34).
A8–35-mediated Folding
Amphipol-mediated folding of GHS-R1a was carried out as described in Dahmane et al. (35). Briefly, A8–35 was added at a ratio of 5 g of A8–35 per g of SDS-unfolded receptor in the presence of asolectin at a 2:1 (w/w) lipid:protein ratio. After 30 min of incubation at room temperature, receptor folding was initiated by precipitating dodecyl sulfate as its potassium salt. After 30 min, the KDS precipitate was removed by two 10-min centrifugation steps at 13,000 × g. The supernatant was dialyzed against a KP buffer (150 mm KCl, 50 mm potassium phosphate, pH 8).
Alexa Fluor-350 Receptor Labeling
N-terminal labeling with Alexa Fluor-350 was carried out as described by Mesnier and Banères (36). Briefly, the ghrelin receptor folded in A8–35 was extensively dialyzed in 50 mm potassium phosphate, pH 7.5. This pH value was determined from a series of labeling reactions carried out at different pH values (ranging from 7.0 to 9) to define the optimal value for labeling only the protein N terminus and not the lysyl residues. pH 7.0 was the minimum used with A8–35-folded proteins because A8–35 precipitates below this value (35). Alexa Fluor-350 carboxylic acid succinimidyl ester was added to the protein solution (dye:protein molar ratio, 10:1), and the reaction was carried out at room temperature for 2 h under constant stirring. The conjugate was separated from unreacted labeling reagent by dialyzing against KP buffer. The relative efficiency of the labeling reaction was determined by measuring the absorbance of the protein at 276 nm and the dye at its absorbance maximum (346 nm). A ratio of 0.9 fluorescent probes per receptor molecule was found.
Amphipol to Detergent Exchange
The GHS-R1a receptor folded in A8–35 was immobilized on a 1-ml HisTrap column (GE Healthcare). After extensive washing with KP buffer, the protein-bound amphipol was removed through an extensive washing step with KP buffer containing 0.1% DDM and 0.02% cholesteryl hemisuccinate. The protein was then recovered from the column using the same buffer containing 100 mm imidazole.
Reconstitution in Lipid Discs and Functional Purification
MSP1E3(−) was expressed and purified as described by Denisov et al. (49). MSP1E3(−) was mixed at a 1:120 molar ratio with purified lipids (POPC/POPG/POPS; 3:1:1 molar ratio) dissolved at a 24 mm concentration in a 20 mm HEPES, 100 mm NaCl, 1 mm EDTA, 48 mm sodium cholate, pH 7.5, buffer (28). Sodium cholate concentration was kept constant at 25 mm. The mixture was incubated for 15 min on ice. The purified receptor in the KP buffer containing 0.1% DDM and 0.02% cholesteryl hemisuccinate was then added to the MSP/lipid mixture at 0.1:1 receptor:MSP molar ratio and further incubated for 30 min on ice. Detergents were removed through an extensive dialysis step against 20 mm Tris-HCl, 150 mm NaCl, 0.5 mm EDTA, pH 7.4. Empty lipid discs as well as discs containing unfolded receptor were removed through an affinity purification step. To this end, ghrelin-derived peptide JMV 4947 (see supplemental Data S1) was immobilized on a streptavidin column (Pierce) following the manufacturer's instructions. The protein mixture after lipid disc reconstitution was directly loaded on the column. After washing with 20 mm Tris-HCl, 150 mm NaCl, 0.5 mm EDTA, pH 7.4, the bound proteins were recovered by washing the column with the 20 mm Tris-HCl, 150 mm NaCl, 1 mm EDTA, pH 7.4, buffer containing 0.1 mm JMV 4101, a low affinity antagonist (in the 0.1 μm range) derived from JMV 3002 (37). The antagonist was then removed through extensive dialysis against a 20 mm Tris-HCl, 150 mm NaCl, 0.5 mm EDTA, pH 7.4, buffer. Finally, GHS-R1a containing discs were separated from protein aggregates through a size-exclusion chromatography step: the discs recovered from the affinity column were loaded on a S200-HR column (10 × 400 mm; GE Healthcare) and elution was carried out at a 0.2 ml/min flow rate with the 20 mm Tris-HCl, 150 mm NaCl, 0.5 mm EDTA, pH 7.4, buffer. 0.5-ml fractions were recovered.
Receptor:MSP Stoichiometry
The oligomeric state of the GHS-R1a receptor in lipid discs was determined using blue native electrophoresis performed as described by Schägger et al. (38). Receptor:MSP ratios were also independently determined from Alexa Fluor-350-labeled GHS-R1a lipid disc preparations using the known extinction coefficients for Alexa Fluor-350 (19,000 m−1 cm−1 at λ = 346 nm) and MSP1E3(−) (26,600 m−1 cm−1 at λ = 280 nm) and the receptor:Alexa Fluor-350 molar ratio measured before reconstitution into lipid discs (0.9; see above).
FRET-monitored Ligand Binding and GTPγS Binding Assays
Direct ligand binding experiments and competition assays were performed using fluorescence energy transfer with the purified receptor labeled with Alexa Fluor-350 and a ghrelin peptide labeled with FITC (JMV 4946; see supplemental Data S1). Titration experiments were carried out with protein concentrations in the 10 nm protein concentration range and increasing ligand concentrations. Competition experiments were carried out by adding increasing concentrations of the competing compound to a receptor/JMV 4946 mixture (100 nm concentration range). Fluorescence emission spectra were recorded at 20 °C between 400 and 600 nm on a Cary Eclipse spectrofluorimeter (Varian) with excitation at 346 or 488 nm. Buffer contributions were systematically subtracted. The FRET ratio corresponds to the ratio of the acceptor emitted fluorescence at 520 nm from excitation at two different wavelengths, 346 and 488 nm (39). All binding data were analyzed using GraphPad Prism software (version 4.0). GTPγS binding experiments were carried out for 5 min at 20 °C using the fluorescent BODIPY FL GTPγS analog as described by McEwen et al. (40). Ligand concentrations of 1 μm and 1:10 receptor:G protein molar ratios were used in all cases.
Arrestin Expression, Purification, and Labeling
The arrestin-2 mutant L68C,R169E was produced in Escherichia coli and purified as described in Gurevich and Benovic (41). The purified protein was then labeled on C68 with the thiol alkylating fluorescent compound monobromobimane as described in Sommer et al. (42).
μ-AP2 Expression, Purification, and Labeling
Residues 160–435 of μ-AP2 were expressed in E. coli as an NH2-terminal hexahistidine fusion protein, as described by Owen and Evans (43). For monobromobimane labeling, the protein and monobromobimane were incubated at a 1:10 protein:dye ratio for 16 h at 4 °C. Dye excess was removed by desalting on a PD10 column (Bio-Rad). The relative efficiency of the labeling reaction was determined by measuring the absorbance of the protein at 276 nm and the dye at its absorbance maximum (392 nm). A ratio of 0.8 fluorescent probes per receptor molecule was found. No tentative identification of the labeled cysteine was carried out at this stage of analysis. Monobromobimane labeling did not affect the way AP2 interacts with the purified receptor, as assessed by similar behavior of the labeled and unlabeled proteins when complex formation was analyzed by size exclusion chromatography (supplemental Data S2).
Arrestin and μ-AP2 Recruitment Assay
For the arrestin and the μ-AP2 recruitment assays, the partner proteins were first added to the ghrelin receptor in lipid discs at 1:2 receptor:signaling protein molar ratio with protein concentrations in the 0.5 μm range. A 20 mm Tris-HCl, 250 mm NaCl, 0.5 mm EDTA, pH 7.4, buffer was used in all experiments. For the experiments in the presence of both AP2 and arrestin, an AP2:arrestin 1:1 molar ratio was used. Incubation was carried out for 45 min at 4 °C. The different ligands were then added at a 1 μm concentration and incubated for an addition 45 min at 4 °C. Fluorescence emission was recorded at 20 °C between 400 and 600 nm on a Cary Eclipse spectrofluorimeter (Varian) with excitation at 380 nm. All data were normalized to the fluorescence measured under the same conditions with empty discs.
Tryptophan Emission Measurements
All tryptophan fluorescence emission spectra were recorded at 20 °C between 305 and 405 nm using a Cary Eclipse spectrofluorimeter (Varian) with an excitation wavelength at 295 nm. Buffer and ligand contributions were systematically subtracted. A protein concentration of 0.2 μm was used in all measurements. Trp emission spectra were systematically recorded for GHS-R1a containing discs and empty lipid discs under the same experimental conditions.
Cell Culture and Transfection
HEK293T cells were maintained in DMEM/Glutamax (Invitrogen) supplemented with antibiotics (penicillin, 50 units/ml, streptomycin, 50 μg/ml), HEPES 2 mm, 1% nonessential amino acids, and 10% fetal calf serum. Transfections were performed in 96-well plates using a cell density of 50,000 cells per well. Prior to cell plating, wells were pre-coated with poly-l-ornithine (50 μl of 10 mg/ml) for 30 min at 37 °C. Transfection mixtures were prepared using 100 ng of GHS-R1a constructs, 0.25 μl of Lipofectamine 2000 (Invitrogen), respectively, and 50 μl of Opti-MEM culture medium per well. Prior to their addition in plates, transfection mixtures were preincubated for 20 min at room temperature. Then 100 μl of HEK293T cells at a density of 500,000 cells/ml were plated in each well and incubated at 37 °C under 5% CO2 for 48 h.
Inositol Phosphate Accumulation Assay
The inositol phosphate accumulation assay was carried out 48 h after transfection on adherent cells in a 96-well black plate (Greiner Bio One) at a density of 50,000 cells/well. As a control, inositol phosphate accumulation corresponding to basal activity of the cells that do not express GHS-R1a was measured on cells previously transfected with the pcDNA3 empty vector. IP1 accumulation was measured using the IP-One HTRF kit (Cisbio Bioassays) according to the manufacturer's instructions as described (44). Briefly, cells were stimulated for 45 min at 37 °C with the ligand to be tested in 70 μl of IP stimulation buffer. An anti-IP1 antibody labeled with Lumi4-Tb (15 μl) and an IP1-d2 derivative (15 μl) were added to the cells. The medium was incubated for 1 h at room temperature. Signals at 665 and 620 nm were detected using a RUBYstar (BMG Labtech) fluorescence reader. Values are expressed as ΔF. ΔF corresponds to: (ratio 665 nm/620 nm of the assay − ratio 665 nm/620 nm of the negative control)/ratio 665 nm/620 nm of the negative control.
The negative control corresponded to the Lumi4-Tb blank and was used as an internal assay control. Inositol phosphate accumulation was expressed as the percentage of the maximal ghrelin response using the formula: (ΔF mock cells − ΔF receptor transfected cells)/(ΔF mock cells − ΔF maximal ghrelin stimulation for receptor transfected cells).
Arrestin Recruitement Assay
Interaction between GHS-R1a-YFP and Rluc-Arrestin-3 was measured in HEK293T by BRET1 in 96-well white plates (Greiner Bio One). Briefly, cells were transfected by Lipofectamine with 100 ng of GHS-R1a-YFP and 5 ng of Rluc-arrestin. 48 h after transfection the cells were washed with PBS and then incubated for 45 min at 37 °C with 50 μl of ligand in DMEM, 0.1% BSA. After stimulation, cells were washed with 100 μl of PBS and then 50 μl of 0.5 mm coelenterazine H (Interchim) solution in PBS was added to the cells, and reading was performed in a Mithras LB 940 plate reader (Berthold Biotechnologies) that allows sequential integration of the luminescence signal (5 cycles of 0.05 s) with two filter settings (Rluc filter, 485 ± 20 nm, and YFP filter, 530 ± 25 nm). The BRET ratio was defined as the difference of the ratio 530/485 nm of the cotransfected Rluc and YFP proteins and the ratio of the Rluc protein alone. Results are expressed in mBRET corresponding to a ratio of (530 nm/485 nm) × 1000.
RESULTS
From GHS-R1a Expression in Inclusion Bodies to Assembly into Lipid Discs
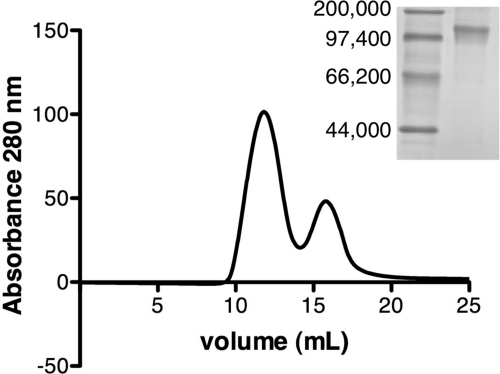
To achieve a high-level production of the functional ghrelin receptor in lipid discs, we adopted a strategy that consists of accumulating the protein in E. coli inclusion bodies, folding it back to its native state, and then assembling it into the lipid particles. To get the receptor in high yields, GHS-R1a was first produced as a fusion protein with the integrin αI fragment previously described (34, 45). Because direct reconstitution of the SDS-unfolded protein into the lipid disc occurred with very low yields, we then devised a new strategy that consists of using an amphipol-folded state as a starting point for disc assembly. We previously established that amphipols are a particularly relevant medium to fold GPCRs back to their native state (35). Amphipol-trapped proteins can hardly be inserted directly into a bilayer such as the lipid disc without significant protein destabilization (37, 46), but they can be easily transferred to a detergent environment simply by exchanging A8–35 with the corresponding detergent (47). The receptor after the amphipol to detergent exchange can then be used for disc assembly. The ghrelin receptor was thus folded in the presence of A8–35 amphipol and lipids (35). We then exchanged A8–35 for a DDM/cholesteryl hemisuccinate mixture. The latter was selected based on studies showing that it provides a stabilizing environment for different GPCRs such as the β2-adrenergic (48) or the μ-opioid (30) receptors. A8–35 to DDM exchange was carried out with the receptor immobilized on a nickel column through its C-terminal His6 tag to ensure full removal of amphipol. The receptor in DDM/cholesteryl hemisuccinate was then directly used for reconstitution in lipid discs using MSP1E3, as previously described for other GPCRs such as rhodopsin (27). To remove empty discs as well as discs containing unfolded protein, we used an affinity chromatographic step with an immobilized ghrelin peptide. Disc particles containing GHS-R1a were finally resolved with size exclusion chromatography. Two distinct populations were observed on the size exclusion chromatography profile (Fig. 1). The first one eluted in the void volume of the column. This fraction was not analyzed further but likely corresponds to large aggregates, presumably of liposome-like structures, containing functional receptors because they were specifically adsorbed on the ghrelin-affinity column. The second peak corresponds to a homogeneous fraction with an apparent Stokes diameter in the 12-nm range, in agreement with the expected value for a lipid disc formed with MSP1E3 (49). The fractions in this peak were pooled and used throughout this work.
FIGURE 1.
Reconstitution of the purified GHS-R1a in lipid discs. The affinity-purified GHS-R1a after reconstitution in lipid discs was loaded onto a Superdex S200HR column (10 × 400 mm) and elution was carried out as described under “Experimental Procedures”; inset, blue native-PAGE followed by Coomassie Blue staining of the particles in peak 2. Lane 1, molecular weight markers, lane 2, GHS-R1a -containing discs (peak 2 pooled fractions). The molecular mass of the markers is indicated in Da.
To determine the number of GHS-R1a receptors incorporated per disc, the complex eluted from the size exclusion chromatography column was analyzed by blue native PAGE (Fig. 1, inset). The purified complex migrates predominantly as a single band of a mass compatible with that of a complex composed of one receptor and two MSP proteins. If one considers that a lipid disc is formed from 2 MSP proteins (49), then such a composition is indicative of a particle composed of a single GHS-R1a protein per lipid disc. The stoichiometry was also independently estimated by labeling the purified receptor in A8–35 before insertion in the lipid disc with Alexa Fluor-350 and then quantitating MSP1E3 and receptor contributions after reconstitution through the absorbance at 280 and 346 nm, respectively (see ”Experimental Procedures“). A GHS-R1a:MSP ratio of 0.47 (i.e. about one receptor for two MSP proteins) was obtained using this procedure, in full agreement with the blue native PAGE analysis. Altogether, these data strongly indicate that reconstitution of the purified GHS-R1a under our conditions predominantly yields a single receptor incorporated in a disc structure.
The Isolated GHS-R1a Displays Normal Ligand Binding Properties
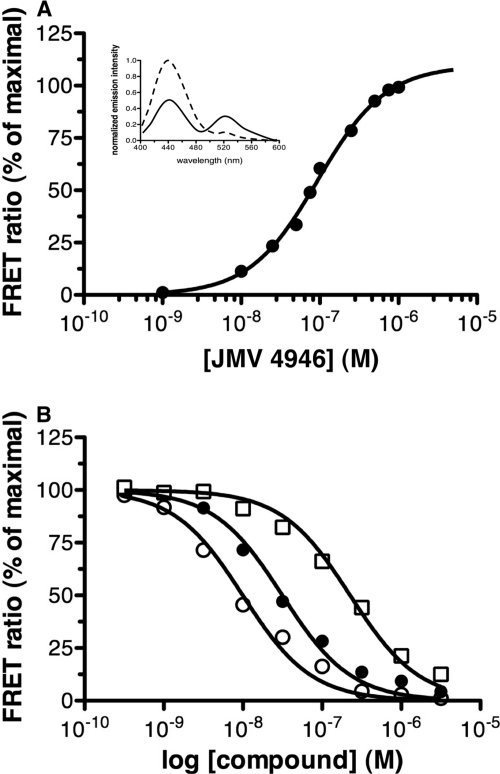
Homogeneity of the purified receptor in lipid discs was demonstrated through a FRET-based ligand-binding assay. To this end, the purified receptor was labeled at its N terminus with Alexa Fluor-350. Under such conditions, a significant FRET signal was observed upon binding a ghrelin peptide labeled with FITC (JMV 4946; see supplemental Data S1), as shown in the inset to Fig. 2A. Plotting the variations in FRET efficiency as a function of peptide concentration resulted in a classical dose-response profile (Fig. 2A) that revealed the occurrence of a single population of binding sites with a Kd value of 99.9 nm. Subsequent FRET-monitored competition experiments were carried out with two synthetic GHS-R1a antagonists, JMV 3002 and JMV 3018, as well as with the inverse agonist SPA. The competition profiles in Fig. 2B were obtained by plotting the changes in the Alexa Fluor-350:FITC FRET signal as a function of the compound concentration. The Ki values inferred from such competition experiments, i.e. 9.3, 18.3, and 253.7 nm for JMV 3002, JMV 3018, and SPA, respectively, are within the same range as those inferred through radioactive and TagLite-based measurements from HEK293 cells transiently expressing GHS-R1a (44). This strongly suggests that reconstitution of the ghrelin receptor in lipid discs allows the receptor to adopt a conformation closely related to that of the receptor in the plasma membrane, at least in terms of ligand binding.
FIGURE 2.
Purified GHS-R1a binds its ligands. A, FRET-monitored binding of an FITC-labeled ghrelin peptide (JMV 4946) to Alexa Fluor 350-labeled GHS-R1a assembled in lipid discs. The binding data are presented as variations in FRET ratio as a function of JMV 4946 concentration. Inset, fluorescence emission spectrum obtained for Alexa Fluor 350-labeled GHS-R1a in lipid discs in the presence of saturating concentrations in JMV 4946 (solid line) and in the presence of JMV 4946 and an excess of the JMV3002 antagonist (dotted line). B, FRET-monitored competition experiments. JMV 4946 was displaced from its binding site in the presence of increasing concentrations in the JMV3002 antagonist (open circles), the JMV3018 antagonist (closed circles), or the SPA inverse agonist (open squares). Data are presented as the mean ± S.D. from three independent experiments.
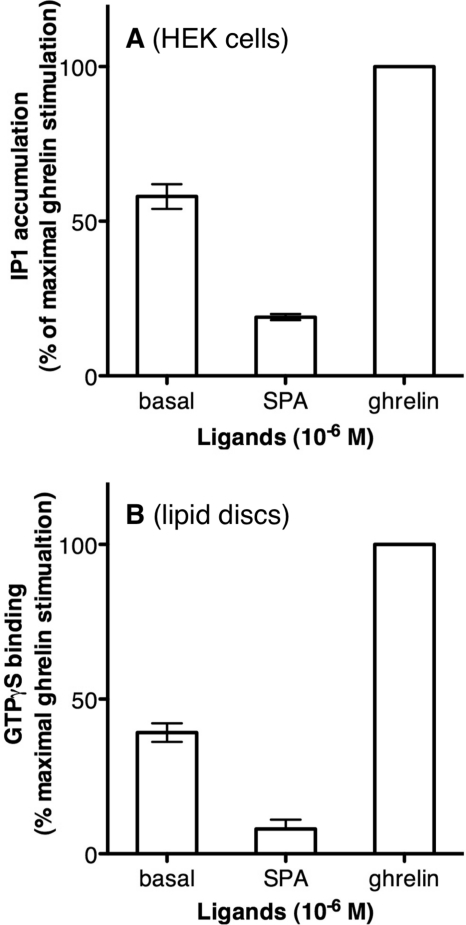
GHS-R1a Displays Constitutive Activity per se
In agreement with previous reports in the literature (11), we observed that the GHS-R1a receptor displayed a particularly high level of Gαq-directed constitutive activity when expressed in HEK cells (Fig. 3A). To assess whether this is an intrinsic property of the receptor or not, we investigated if the purified monomeric GHS-R1a receptor in lipid discs displayed a similar behavior at activating its cognate G protein partner. To this end, we monitored receptor-catalyzed GTPγS binding using a fluorescent analog (BODIPY FL GTPγS) (40) and purified Gαq in the presence of Gβγ. Gαq is the primary cognate G protein partner of the ghrelin receptor. As shown in Fig. 3B, a significant increase in GTPγS binding to Gαq was observed in the presence of the purified ligand-free GHS-R1a receptor, as compared with the binding measured in the absence of the receptor. Such an increase was specific of Gαq, because no significant change in GTPγS was observed with the two other Gα subtypes, i.e. Gαi and Gαs (supplemental Data S3).
FIGURE 3.
Isolated GHS-R1a triggers Gq protein activation. A, IP1 production induced by the GHS-R1a receptor expressed in HEK293T cells in the absence of ligand, in the presence of the inverse agonist SPA, or in the presence of the full agonist ghrelin. IP1 accumulation is expressed as the percentage of the maximal ghrelin response and 0 represents the IP1 accumulation in cells transfected with an empty vector. The figure is representative of one experiment of three. B, BODIPY FL GTPγS binding to Gαq protein induced by GHS-R1a in the absence of ligand, in the presence of SPA, or in the presence of ghrelin. Data are presented as the percentage of maximum BODIPY FL fluorescence change measured in the presence of ghrelin and represent the mean ± S.D. from three independent experiments.
We then monitored the effects of two different kind of ligands, i.e. a full agonist (ghrelin) and an inverse agonist (SPA), on receptor-catalyzed GTPγS binding. As shown in Fig. 3B, an additional increase in receptor-catalyzed GTPγS binding was observed after addition of the full agonist ghrelin. Such an increase is the direct consequence of ligand binding, as shown by the agonist concentration-dependent changes in GTPγS binding (supplemental Data S4). Comparing the amount of GTPγS bound in the absence and presence of the full agonist indicates that basal binding corresponds to about 40% of the maximal effect triggered by ghrelin. The extent in basal activity observed with the purified receptor closely approximates the 50% basal activity in Gαq-dependent pathways reported for GHS-R1a expressed in heterologous systems (Fig. 3A) (50) and in vivo (18). These data are direct evidence that the purified receptor displays per se a constitutive activity comparable with that it displays in a more complex cellular environment. If the basal receptor-catalyzed GTPγS binding observed with the purified receptor is a specific property of the protein, then it should be reverted in the presence of the inverse agonist SPA. The latter has been reported as the only and most potent inverse agonist for GHS-R1a (50). As shown in Fig. 3B, a significant reduction of GTPγS binding was observed in the presence of SPA. As in the case of ghrelin, this is likely a direct consequence of SPA binding, as shown by the dose-dependent reversal of GHS-R1a constitutive activity (supplemental Data S4). All these data indicate that (i) the basal activity observed in the absence of ligand is an intrinsic property of the purified receptor and (ii) this basal activity can be up- and down-regulated in vitro upon binding of a full and an inverse agonist, respectively.
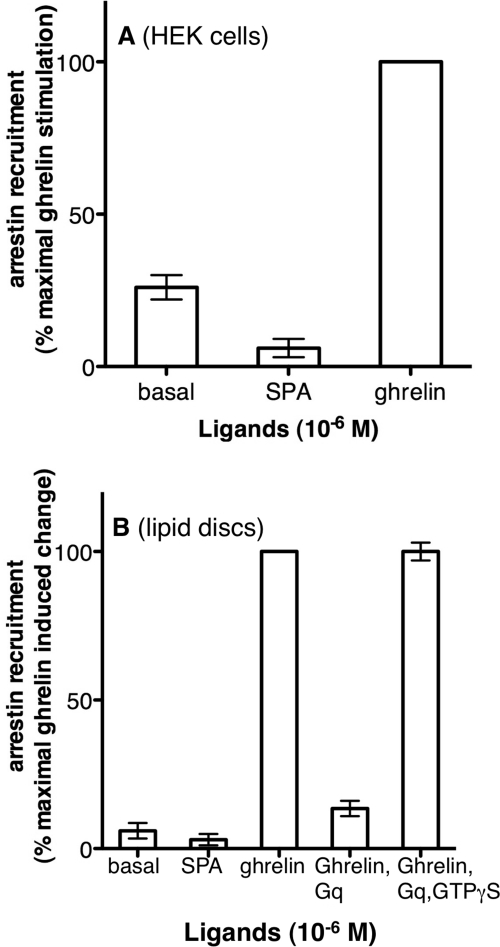
The Isolated GHS-R1a Recruits Arrestin in an Agonist-dependent Manner
It has been shown that basal and agonist-activated states of the ghrelin receptor differ in arrestin requirements (23). Indeed, GHS-R1a recruits arrestins essentially in an agonist-dependent manner as previously reported (23), and as also shown here with the GHS-R1a receptor expressed in HEK cells (Fig. 4A). To assess whether agonist-dependent arrestin recruitment is a specific property of the GHS-R1a protein, as is the case for Gαq activation, we subsequently analyzed the ability of the purified receptor to recapitulate the behavior of GHS-R1a with regard to agonist-induced arrestin recruitment. To this end, we monitored the interaction between the purified receptor in lipid discs and a monobromobimane-labeled arrestin-2. Bimane is a small size fluorophore with high sensitivity to the polarity of its molecular environment that can be used as a sensor to detect interactions between arrestin and its protein partners, as previously reported for visual arrestin and rhodopsin (42). We used here the phosphorylation-independent mutant of arrestin-2 because the receptor is produced in bacteria and is therefore not phosphorylated. As shown in the supplemental Data, similar effects were observed with a phosphorylation-independent mutant of arrestin-3, although with lower signal-to-noise ratios.
FIGURE 4.
Purified GHS-R1a recruits arrestin in an agonist-dependent manner. A, ligand-dependent arrestin recruitment by GHS-R1a in HEK293T cells. BRET signal (ratio 530 nm/485 nm) is expressed as a percentage of the maximal ghrelin response and 0 represents BRET signal of mock cells transfected with an empty vector. The figure is one experiment representative of three. B, changes in emission intensity of bimane-labeled arrestin induced by GHS-R1a in the absence of ligand, in the presence of ghrelin, or in the presence of SPA, ghrelin, Gαβγq, and Gαβγq and GTPγS. Data are presented as the percentage of maximum bimane fluorescence change measured in the presence of ghrelin and represent the mean ± S.D. from three independent experiments.
As shown in Fig. 4B, incubation of arrestin-2 with the purified ghrelin receptor affected bimane emission properties only to a very limited extent, suggesting that only a very slight constitutive interaction between the receptor and its signaling partner occurs in the absence of ligands, in accordance with what is observed with this receptor expressed in HEK cells (Fig. 4A). This limited constitutive recruitment was, at least to some extent, reversed by the inverse agonist SPA, both in HEK cells and in vitro (Fig. 4). In the presence of the full agonist ghrelin, a very significant change in the emission properties of bimane was observed, with an increase in the emission intensity of about 30%. This is likely the direct consequence of ghrelin binding to the receptor because it occurs in a ligand concentration-dependent manner (supplemental Data S5). Of importance, a decreased extent in arrestin recruitment was observed in the presence of the G protein and in the absence of GTP (Fig. 4B), indicating that both processes, G protein and arrestin recruitment, are mutually exclusive, with the G protein recruitment step occurring preferentially. It was only upon dissociating the receptor·G protein complex in the presence of GTPγS that the interaction with arrestin was restored (Fig. 4B). This clearly shows that the purified monomeric ghrelin receptor in lipid discs interacts with arrestins only in its agonist-activated state. As is the case for G protein activation, these data are direct evidence for the fact that the differential requirements in arrestin for basal and agonist-induced internalization are again an intrinsic property of the receptor protein rather than the consequence of any influence of the cellular environment. Additionally, our data with a purified monomeric receptor indicate that, as previously reported for rhodopsin (53, 54), a monomeric GPCR is totally able to interact with arrestin. All these data establish that the monomeric GHS-R1a reconstituted in lipid discs is functional toward its two main partners, Gαq and arrestin.
The Isolated GHS-R1a Binds μ-AP2 in the Absence and Presence of Agonist
In an heterologous expression system, no arrestin recruitment by GHS-R1a in the absence of agonist is observed while a basal internalization occurs. It has been suggested that the latter may be related to interaction of the receptor in its basal state with another protein involved in GPCR internalization, namely the μ subunit of AP2 (23). However, no direct evidence of such an interaction has yet been reported. To assess if such an interaction indeed occurs, we analyzed whether the purified GHS-R1a in lipid discs could bind AP2. To this end, we expressed in E. coli the signal-binding domain of μ-AP2 (residues 160 to 435) (43). This fragment has been shown to directly bind isolated GPCR fragments such as the C-terminal tails of the α1b receptor (55) and PAR1 (56). The AP2 fragment was labeled with monobromobimane to monitor its possible recruitment by the purified receptor, as described above for arrestin.
As shown in Fig. 5A, incubation of bimane-labeled μ-AP2 with ligand-free GHS-R1a leads to a significant quenching of bimane emission intensity, indicating a recruitment of AP2 to the receptor. The formation of such a receptor·AP2 complex was confirmed by size exclusion chromatography (supplemental Data S2). A similar change in bimane emission intensity was measured in the presence of ghrelin, suggesting that agonist-induced activation of the receptor does not modify its association to AP2. In contrast, in the presence of the inverse agonist SPA, bimane emission intensity was similar to that observed with empty discs, suggesting that AP2 is not recruited to the ground state of the receptor. Dissociation of the GHS-R1a·AP2 complex directly results from SPA binding, as shown by the typical dose-response profile (supplemental Data S6). This could be a direct explanation to the observation that SPA significantly reduces constitutive internalization (23). All these data directly show that, in our model system, the basal- and agonist-activated states of GHS-R1a are both able to interact μ-AP2, in contrast to the ground state stabilized by an inverse agonist.
FIGURE 5.
Isolated GHS-R1a binds μ-AP2. A, changes in emission intensity of bimane-labeled μ-AP2 induced by GHS-R1a in the absence of ligand, with 1 μm SPA, or 1 μm ghrelin. Data are presented as the percentage of maximum bimane fluorescence change measured in the presence of ghrelin and represent the mean ± S.D. from three independent experiments. B, changes in emission intensity of bimane-labeled μ-AP2 induced by GHS-R1a in the presence of purified arrestin-2 and in the absence of ligand, in the presence of SPA, or in the presence ghrelin. Data are presented as the percentage of maximum bimane fluorescence change measured in the presence of ghrelin and in the absence of arrestin-2 and represent the mean ± S.D. from three independent experiments.
Ghrelin-induced Arrestin Recruitment Dissociates GHS-R1a·AP2 Complex
Ghrelin-induced activation triggers arrestin recruitment to the purified GHS-R1a receptor (see above). To assess whether such an agonist-induced arrestin recruitment affects the way the receptor interacts with μ-AP2, we monitored the changes in emission of bimane-labeled μ-AP2 in the presence of unlabeled arrestin-2. As shown in Fig. 5B, no effect of arrestin was observed on AP2 bimane emission in the absence of ligand, in agreement with the observation that the basal state of the receptor does not recruit arrestin. In contrast, a nearly full reversal of the changes in AP2-bimane emission intensity was observed when ghrelin was added in the presence of arrestin (Fig. 5B). This is a strong indication that ghrelin-induced arrestin binding triggers dissociation of the GHS-R1a·AP2 complex. As expected because neither μ-AP2 nor arrestin bind to the ground state of the receptor, no change in AP2 bimane emission intensity was observed in the presence of the inverse agonist SPA (Fig. 5B).
Ligand-binding Triggers Specific GHS-R1a Conformational Changes
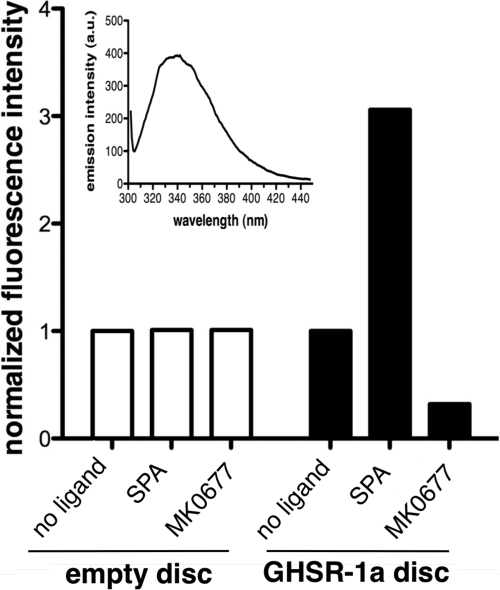
All ligand-induced changes in G protein activation or arrestin- and μ-AP2 recruitment are likely to be associated to ligand-specific receptor conformations. To assess if this is indeed the case, we have used tryptophan intrinsic fluorescence spectroscopy to detect conformational changes of the GHS-R1a receptor in a lipid disc that would occur upon ligand binding. Because the MSP1E3(−) protein also contains Trp residues, we systematically compared the effects of ligands on the emission intensity of ghrelin-containing discs to that of empty discs (Fig. 6). As expected, no change in MSP1E3(−) Trp emission was observed with any of the ligands considered. In contrast, addition of the SPA inverse agonist or the MK677 full agonist induced a change in emission intensity with no significant change in the maximum emission wavelength. MK677 was used here as a full agonist instead of ghrelin because the latter contains tryptophan residues that could contribute to changes in Trp emission intensity. MK677 triggers the same effects as ghrelin on the purified GHS-R1a with regard to G protein activation or arrestin and AP2 recruitment. Of interest, the full and inverse agonists triggered opposite effects on the GHS-R1a emission spectrum; whereas an approximatel 60% decrease inthe GHS-R1a tryptophan emission intensity was observed with MK677, addition of SPA induced an approximately 200% increase in emission intensity. Although it must be kept in mind that Trp intrinsic fluorescence only gives information on global conformational changes in the receptor, these data nevertheless, indicate that ligands with different efficacies with regard to G protein activation and arrestin- and μ-AP2 recruitment are likely to trigger and/or stabilize distinct receptor conformations.
FIGURE 6.
Ligand binding affects GHS-R1a conformation. Intrinsic fluorescence spectra of GHS-R1a in lipid discs in the absence and presence of ligands. Empty discs or monomeric GHS-R1a in lipid discs were incubated with or without the drug to be tested (MK0677, SPA; 1 μm). Data are presented as the intensity of tryptophan emission at its maximum normalized to that in the absence of the ligand. Inset, fluorescence emission spectrum of the ligand-free receptor in lipid discs. Data are presented as the mean ± S.D. from three independent experiments.
DISCUSSION
To investigate the molecular mechanisms of ghrelin receptor functioning, we produced and reconstituted GHS-R1a in a lipid disc. To assess what really is an intrinsic property of the receptor protein per se, we assembled lipid particles with the receptor in its monomeric state. Using this purified monomer receptor model system, we clearly established that the isolated receptor displays constitutive activity toward Gαq similar in its extent to that of the same receptor in living cells. To our knowledge, this is the first direct demonstration that such a high constitutive activity is an intrinsic feature of the ghrelin receptor protein and not the consequence of an influence of the cellular environment. Furthermore, we showed that the purified receptor recruits arrestin-2 in an agonist-dependent manner, whereas μ-AP2 binding occurs both in the absence of ligand and in the presence of the full agonist ghrelin. This provides the first direct experimental proof to the model of basal- and agonist-dependent GHS-R1a internalization proposed by Holliday et al. (23). Additionally, all our data with the purified monomeric GHS-R1a isolated in a lipid disc provides further demonstration that a GPCR monomer can both activate its G protein partner and recruit arrestins (27, 28, 34, 53, 54, 57).
Manipulation of solubilized membrane proteins is a prerequisite to understanding their structure and function. However, because most membrane proteins, and in particular GPCRs, are unstable when exposed to detergents, alternate membrane-mimicking environments have been developed during the last years. In this context, lipid discs appear as a particularly well suited system for in vitro biophysical studies in a membrane-like environment (58). Lipid discs have been used to characterize a very few GPCRs, essentially the β2-adrenergic receptor (10, 28), the μ-opioid receptor (30), and rhodopsin (27, 29, 54). We have used here this lipid disc-based approach to assess the functional features of the isolated ghrelin receptor GHS-R1a. To this end, we developed an original approach for inserting a GPCR produced in E. coli into the disc. This approach relies on the expression of the GPCR in bacterial inclusion bodies, followed by amphipol-mediated in vitro refolding and then assembly into the disc after amphipol-to-detergent exchange. Based on our data with the GHS-R1a receptor, it appears that the amphipol-folded state could be a particularly relevant intermediate state for insertion of unfolded membrane proteins produced in E. coli into lipid discs.
As stated under the Introduction, constitutive activity of GPCRs is the focus of intensive research due to its possible role in the control of GPCR-mediated signaling and the potential use of the inverse agonist in therapeutic applications. This is particularly true for the ghrelin receptor that displays a strikingly high constitutive activity, in the 50% range, both in heterologous systems (50) and in vivo (18). This constitutive activity is physiologically relevant as it has been proposed to play a role in food intake (59) or to be involved in the control of apoptotic mechanisms (60). In accordance with such a physiological role, a human mutation in the GHS-R1a gene that suppresses constitutive activity without affecting stimulation by ghrelin has been shown to be associated with a short-stature phenotype (7). It is therefore crucial to shed light on such a process. However, as recently pointed out by Stoddart and Milligan (20) in the case of GPR40, it can be particularly difficult to assess to what extent constitutive activity is really relevant of the receptor itself and what is related to either the assay or the cellular environment. In this context, isolated receptors in lipid discs provide an unprecedented system to analyze the molecular mechanisms of constitutive activity and functioning of GPCRs in a cell-free environment. Using such a system, we unambiguously establish here that the monomeric GHS-R1a displays a high constitutive activity toward its cognate G protein partner, Gαq (Fig. 7). The extent in constitutive activity measured through GTPγS binding assays amounts to about 40% of the maximal effect observed in the presence of the full agonist ghrelin. This is a first direct demonstration that the high constitutive activity of the ghrelin receptor is an intrinsic feature of the receptor protein rather than the consequence of the influence of the cellular environment. This does not rule out, however, that this environment cannot modulate the intrinsic activity of GHS-R1a. Besides, our model system composed of the isolated ghrelin receptor in the lipid disc provides us with a unique system to further investigate the molecular mechanisms associated with this constitutive activity and its regulation by agonists and inverse agonists. The latter have a potential therapeutic application, for instance, in the regulation of food intake (59).
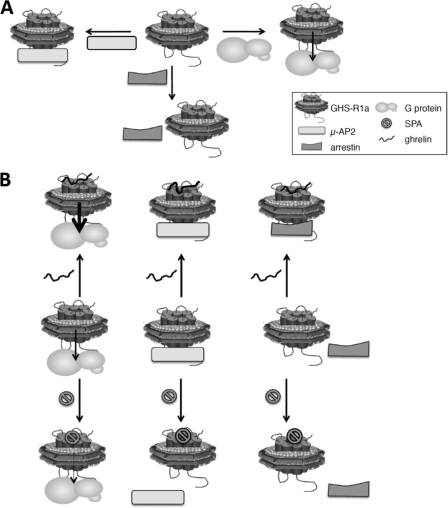
FIGURE 7.
Schematic representation of the functional behavior of the purified GHS-R1a. A, in the absence of ligand, the purified receptor activates Gαq and recruits μ-AP2, whereas no significant basal recruitment of arrestin occurs. B, in the presence of the ghrelin agonist, Gαq is further activated, arrestin-2 is recruited, and μ-AP2 still interacts with the receptor; in contrast, binding of the inverse agonist SPA significantly reduces the receptor constitutive activity and dissociates the complex with μ-AP2. The width of the arrow in the receptor·Gq complexes represents the extent in G protein activation.
The ghrelin receptor displays differences in ligand requirements with respect to G protein activation but also to internalization (23). Whereas basal internalization occurs in an arrestin-independent manner, ghrelin-stimulated GHS-R1a recruits arrestins. In full agreement with these data, we provide direct evidence that the agonist-free receptor, although it activates its G protein partner, does not recruit arrestin-2 in a significant manner (Fig. 7). In contrast, upon ghrelin activation, arrestin binding occurs (supplemental Data S7). Importantly, arrestin and G protein compete for binding to the ghrelin-activated receptor, with a preferential association to the G protein. Upon GTPγS binding to Gα, arrestin recruitment was restored, indicating that dissociation of the receptor·G protein complex that occurs as a consequence of G protein activation enables a subsequent interaction of arrestin with the receptor, as is the case in cellular systems. This again provides strong evidence that the purified receptor in lipid discs fully recapitulates the behavior the protein displays in more integrated systems. Besides their role in GPCR trafficking, arrestins are involved in ERK/MAP kinase scaffolding; the differences observed here in arrestin recruitment depending upon whether the GHS-R1a receptor is activated by ghrelin or not may therefore impact selective basal- and agonist-induced signaling besides trafficking.
Although such an interaction has not been demonstrated so far, it has been proposed that basal internalization of the ghrelin receptor could involve direct μ-AP2 binding to its putative binding motif in the C-tail of the receptor (23). AP2 is a plasma membrane-localized clathrin adaptor composed of α, β2, μ2, and σ2 adaptin subunits (61). The μ2 subunit binds directly to tyrosine-based sorting signals within the cytoplasmic regions of transmembrane proteins to facilitate internalization through clathrin-coated pits. Our data with the purified receptor provide evidence that the ghrelin receptor can, per se, interact with AP2 in the absence of agonist, indicating that μ-AP2 could indeed be involved in basal regulation of GHS-R1a trafficking. Such a direct interaction is reminiscent of the interaction reported between μ-AP2 and the α1b (55) or PAR1 (56) C-tails. Interestingly, the constitutively active human viral chemokine US28 GPCR has also been reported to internalize through a clathrin-dependent pathway involving AP2 and not arrestins (62). This suggests that both highly constitutive active receptors, US28 and GHS-R1a, could share similar mechanisms of ligand-independent internalization. However, direct binding of AP2 to the YXXØ motif in the C-tail region of US28 remains to be assessed.
Strikingly, no difference between the basal state and the agonist-loaded states was observed when AP2 binding was considered. Only the inverse agonist SPA reversed the interaction observed between the receptor and its internalization partner, in agreement with the observation that this inverse agonist reduced basal internalization (23). However, AP2 is removed from its binding site on the receptor in lipid discs when arrestin is recruited upon binding of ghrelin. This could be the consequence of steric hindrance preventing both proteins to interact with the receptor monomer at sites that are likely to be close in space; indeed, the C-tail has been described as a potential interaction site for both AP2 and arrestin. Another possibility would be that arrestin stabilizes the receptor in a conformation that is no more able to make a stable complex with AP2. Whatever the explanation is, our data with the purified GHS-R1a provides the first direct experimental evidence for the mechanism proposed to account for the differences in basal and agonist-dependent ghrelin receptor internalization (23).
The differences observed in terms of G protein activation or arrestin and AP2 recruitment reported above are likely to be associated to a conformational adaptability of the receptor that would conceal distinct and specific biological responses. In accordance with this conformational selectivity model, we observed striking differences in Trp emission of the purified ghrelin receptor in the lipid disc in the absence of ligand, in the presence of the full agonist MK677, or in the presence of the inverse agonist SPA. This clearly indicates that these different states differ in their conformational features. These data with the isolated ghrelin receptor are in accordance with what we previously reported (25) with the purified monomeric serotonin receptor; the latter indeed displays a different conformation whether one considers the basal state in the absence of ligands, the ground state stabilized by an inverse agonist, or the activated states stabilized by agonists. Similar differences have been reported for other receptors, in particular, the β2-adrenergic receptor (4, 5). Finally, the wealth of GPCR crystal structures reported so far also point to differences in receptor conformation depending upon whether the receptor is complexed with an antagonist, an agonist, or an agonist and a G protein-mimicking nanobody (for a review, see Ref. 63). It is to be noted here, however, that the fluorescence intensity observed in the absence of ligand is intermediate between that measured for the agonist-activated state and that of the inverse agonist-loaded state. Because Trp fluorescence is a global method we cannot, at this stage of analysis, assess whether the ligand-free state displays a well defined, individualized conformation, as previously demonstrated for 5-HT4(a) (25) or whether it corresponds to a mixture of ground and agonist-active conformations.
Interestingly, it has been proposed that the ghrelin receptor activation relies, at least in part, on a toggle switch mechanism involving the highly conserved Trp residue in the sixth transmembrane domain (13, 64). Such changes in Trp rotamers occurring upon activation could be associated to the changes in Trp emission observed, even if the ghrelin receptor includes several other Trp residues besides that in TM6 that could also contribute to changes in emission fluorescence. It is to be noted, however, that we previously showed with a similar class A GPCR, the leukotriene B4 receptor BLT1, which changes in Trp emission observed during agonist binding, were exclusively due to the contribution of the similarly conserved Trp residue in TM6 (65). This is also the case for the purified serotonin receptor.5
There is increasing evidence that dimerization and/or oligomerization is a common process in the GPCR superfamily (66). Whether or not GPCR dimerization is required for G protein activation has been the matter of intense debate during these last years (67, 68). In this context, it has been reported that several monomeric GPCRs such as rhodopsin (27), the β2-adrenergic receptor (28), the neurotensin NTS1 receptor (57), the μ-opioid receptor (30), and the leukotriene B4 receptor BLT2 (34) efficiently activate their cognate G proteins. More recently, it has also been reported that monomeric rhodopsin binds visual arrestin (53) and is phosphorylated by GRKs (54). Our data with the monomeric ghrelin receptor embedded in lipid discs provides further evidence that a GPCR monomer has, per se, all the molecular determinants that allow it to interact with proteins involved in its signaling and trafficking. This does not rule out that dimerization can have a regulatory effect on all these processes, as reported for G protein activation (33, 34, 57). In this context, the lipid disc system used here may provide an invaluable tool to investigate the effects of dimerization on protein function by allowing direct comparison of well defined particles with one or two receptor molecules. The use of such a well controlled model system could, for instance, help have a clear view on the way the ghrelin receptor dimer functions, something that has been the matter of debate (52, 69).
Supplementary Material
Acknowledgments
We thank D. A. Sahlender and M. Robinson (University of Cambridge, Cambridge Institute for Medical Research, Wellcome Trust, MRC building, Hills Road, UK) for the gift of the μ-AP2 pIRES neo vector that was used as a template for our μ-AP2 expressing construct.
This work was supported by Universities of Montpellier 1 and Montpellier 2, CNRS, and Agence Nationale pour la Recherche Contract PCV08_323163.

This article contains supplemental Figs. S1–S7.
J. L. Banères, unpublished data.
- GPCR
- G protein-coupled receptor
- BRET
- bioluminescence resonance energy transfer
- DDM
- dodecyl β-d-maltoside
- GTPγS
- guanosine 5′-O-(thiotriphosphate)
- α5I
- α5 integrin
- IP1
- inositol phosphate 1
- SPA
- substance P analog.
REFERENCES
- 1. Arvanitakis L., Geras-Raaka E., Gershengorn M. C. (1998) Constitutively signaling G protein-coupled receptors and human disease. Trends Endocrinol. Metab. 9, 27–31 [DOI] [PubMed] [Google Scholar]
- 2. Kenakin T. (2001) Inverse, protean, and ligand-selective agonism. Matters of receptor conformation. FASEB J. 15, 598–611 [DOI] [PubMed] [Google Scholar]
- 3. Milligan G. (2003) Constitutive activity and inverse agonists of G protein-coupled receptors. A current perspective. Mol. Pharmacol. 64, 1271–1276 [DOI] [PubMed] [Google Scholar]
- 4. Deupi X., Kobilka B. K. (2010) Energy landscapes as a tool to integrate GPCR structure, dynamics, and function. Physiology 25, 293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kobilka B. K. (2007) G protein-coupled receptor structure and activation. Biochim. Biophys. Acta 1768, 794–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bond R. A., Ijzerman A. P. (2006) Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol. Sci. 27, 92–96 [DOI] [PubMed] [Google Scholar]
- 7. Pantel J., Legendre M., Cabrol S., Hilal L., Hajaji Y., Morisset S., Nivot S., Vie-Luton M. P., Grouselle D., de Kerdanet M., Kadiri A., Epelbaum J., Le Bouc Y., Amselem S. (2006) Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature. J. Clin. Invest. 116, 760–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Samama P., Cotecchia S., Costa T., Lefkowitz R. J. (1993) A mutation-induced activated state of the β2-adrenergic receptor. Extending the ternary complex model. J. Biol. Chem. 268, 4625–4636 [PubMed] [Google Scholar]
- 9. Ratnala V. R., Kobilka B. (2009) Understanding the ligand-receptor-G protein ternary complex for GPCR drug discovery. Methods Mol. Biol. 552, 67–77 [DOI] [PubMed] [Google Scholar]
- 10. Yao X. J., Vélez Ruiz G., Whorton M. R., Rasmussen S. G., DeVree B. T., Deupi X., Sunahara R. K., Kobilka B. (2009) The effect of ligand efficacy on the formation and stability of a GPCR-G protein complex. Proc. Natl. Acad. Sci. U.S.A. 106, 9501–9506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holst B., Holliday N. D., Bach A., Elling C. E., Cox H. M., Schwartz T. W. (2004) Common structural basis for constitutive activity of the ghrelin receptor family. J. Biol. Chem. 279, 53806–53817 [DOI] [PubMed] [Google Scholar]
- 12. Morisset S., Rouleau A., Ligneau X., Gbahou F., Tardivel-Lacombe J., Stark H., Schunack W., Ganellin C. R., Schwartz J. C., Arrang J. M. (2000) High constitutive activity of native H3 receptors regulates histamine neurons in brain. Nature 408, 860–864 [DOI] [PubMed] [Google Scholar]
- 13. Holst B., Nygaard R., Valentin-Hansen L., Bach A., Engelstoft M. S., Petersen P. S., Frimurer T. M., Schwartz T. W. (2010) A conserved aromatic lock for the tryptophan rotameric switch in TM-VI of seven-transmembrane receptors. J. Biol. Chem. 285, 3973–3985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Milano C. A., Dolber P. C., Rockman H. A., Bond R. A., Venable M. E., Allen L. F., Lefkowitz R. J. (1994) Myocardial expression of a constitutively active α1B-adrenergic receptor in transgenic mice induces cardiac hypertrophy. Proc. Natl. Acad. Sci. U.S.A. 91, 10109–10113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vassart G., Costagliola S. (2011) Nat. Rev. Endocrinol. 7, 362–372 [DOI] [PubMed] [Google Scholar]
- 16. Holst P. J., Rosenkilde M. M., Manfra D., Chen S. C., Wiekowski M. T., Holst B., Cifire F., Lipp M., Schwartz T. W., Lira S. A. (2001) Tumorigenesis induced by the HHV8-encoded chemokine receptor requires ligand modulation of high constitutive activity. J. Clin. Invest. 108, 1789–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tolle V., Low M. J. (2008) In vivo evidence for inverse agonism of Agouti-related peptide in the central nervous system of proopiomelanocortin-deficient mice. Diabetes 57, 86–94 [DOI] [PubMed] [Google Scholar]
- 18. Petersen P. S., Woldbye D. P., Madsen A. N., Egerod K. L., Jin C., Lang M., Rasmussen M., Beck-Sickinger A. G., Holst B. (2009) In vivo characterization of high basal signaling from the ghrelin receptor. Endocrinology 150, 4920–4930 [DOI] [PubMed] [Google Scholar]
- 19. Schneider E. H., Seifert R. (2010) Coexpression systems as models for the analysis of constitutive GPCR activity. Methods Enzymol. 485, 527–557 [DOI] [PubMed] [Google Scholar]
- 20. Stoddart L. A., Milligan G. (2010) Constitutive activity of GPR40/FFA1 intrinsic or assay dependent? Methods Enzymol. 484, 569–590 [DOI] [PubMed] [Google Scholar]
- 21. Els S., Beck-Sickinger A. G., Chollet C. (2010) Ghrelin receptor. High constitutive activity and methods for developing inverse agonists. Methods Enzymol. 485, 103–121 [DOI] [PubMed] [Google Scholar]
- 22. Mokrosiński J., Holst B. (2010) Modulation of the constitutive activity of the ghrelin receptor by use of pharmacological tools and mutagenesis. Methods Enzymol. 484, 53–73 [DOI] [PubMed] [Google Scholar]
- 23. Holliday N. D., Holst B., Rodionova E. A., Schwartz T. W., Cox H. M. (2007) Importance of constitutive activity and arrestin-independent mechanisms for intracellular trafficking of the ghrelin receptor. Mol. Endocrinol. 21, 3100–3112 [DOI] [PubMed] [Google Scholar]
- 24. Ghanouni P., Gryczynski Z., Steenhuis J. J., Lee T. W., Farrens D. L., Lakowicz J. R., Kobilka B. K. (2001) Functionally different agonists induce distinct conformations in the G protein coupling domain of the β2-adrenergic receptor. J. Biol. Chem. 276, 24433–24436 [DOI] [PubMed] [Google Scholar]
- 25. Banères J. L., Mesnier D., Martin A., Joubert L., Dumuis A., Bockaert J. (2005) Molecular characterization of a purified 5-HT4 receptor. A structural basis for drug efficacy. J. Biol. Chem. 280, 20253–20260 [DOI] [PubMed] [Google Scholar]
- 26. Granier S., Kim S., Shafer A. M., Ratnala V. R., Fung J. J., Zare R. N., Kobilka B. (2007) Structure and conformational changes in the C-terminal domain of the β2-adrenoceptor. Insights from fluorescence resonance energy transfer studies. J. Biol. Chem. 282, 13895–13905 [DOI] [PubMed] [Google Scholar]
- 27. Bayburt T. H., Leitz A. J., Xie G., Oprian D. D., Sligar S. G. (2007) Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J. Biol. Chem. 282, 14875–14881 [DOI] [PubMed] [Google Scholar]
- 28. Whorton M. R., Bokoch M. P., Rasmussen S. G., Huang B., Zare R. N., Kobilka B., Sunahara R. K. (2007) A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc. Natl. Acad. Sci. U.S.A. 104, 7682–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Banerjee S., Huber T., Sakmar T. P. (2008) Rapid incorporation of functional rhodopsin into nanoscale apolipoprotein bound bilayer (NABB) particles. J. Mol. Biol. 377, 1067–1081 [DOI] [PubMed] [Google Scholar]
- 30. Kuszak A. J., Pitchiaya S., Anand J. P., Mosberg H. I., Walter N. G., Sunahara R. K. (2009) Purification and functional reconstitution of monomeric μ-opioid receptors. Allosteric modulation of agonist binding by Gi2. J. Biol. Chem. 284, 26732–26741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hartman J. L., IV, Northup J. K. (1996) Functional reconstitution in situ of 5-hydroxytryptamine 2c (5HT2c) receptors with αq and inverse agonism of 5HT2c receptor antagonists. J. Biol. Chem. 271, 22591–22597 [DOI] [PubMed] [Google Scholar]
- 32. Banères J. L., Parello J. (2003) Structure-based analysis of GPCR function. Evidence for a novel pentameric assembly between the dimeric leukotriene B4 receptor BLT1 and the G-protein. J. Mol. Biol. 329, 815–829 [DOI] [PubMed] [Google Scholar]
- 33. Pellissier L. P., Barthet G., Gaven F., Cassier E., Trinquet E., Pin J. P., Marin P., Dumuis A., Bockaert J., Banères J. L., Claeysen S. (2011) G protein activation by serotonin type 4 receptor dimers. Evidence that turning on two protomers is more efficient. J. Biol. Chem. 286, 9985–9997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arcemisbéhère L., Sen T., Boudier L., Balestre M. N., Gaibelet G., Detouillon E., Orcel H., Mendre C., Rahmeh R., Granier S., Vivès C., Fieschi F., Damian M., Durroux T., Banères J. L., Mouillac B. (2010) Leukotriene BLT2 receptor monomers activate the G(i2) GTP-binding protein more efficiently than dimers. J. Biol. Chem. 285, 6337–6347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dahmane T., Damian M., Mary S., Popot J. L., Banères J. L. (2009) Amphipol-assisted in vitro folding of G protein-coupled receptors. Biochemistry 48, 6516–6521 [DOI] [PubMed] [Google Scholar]
- 36. Mesnier D., Banères J. L. (2004) Cooperative conformational changes in a G protein-coupled receptor dimer, the leukotriene B(4) receptor BLT1. J. Biol. Chem. 279, 49664–49670 [DOI] [PubMed] [Google Scholar]
- 37. Moulin A., Demange L., Ryan J., Mousseaux D., Sanchez P., Bergé G., Gagne D., Perrissoud D., Locatelli V., Torsello A., Galleyrand J. C., Fehrentz J. A., Martinez J. (2008) New trisubstituted 1,2,4-triazole derivatives as potent ghrelin receptor antagonists. 3 Synthesis and pharmacological in vitro and in vivo evaluations. J. Med. Chem. 51, 689–693 [DOI] [PubMed] [Google Scholar]
- 38. Schägger H., Cramer W. A., von Jagow G. (1994) Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal. Biochem. 217, 220–230 [DOI] [PubMed] [Google Scholar]
- 39. Hickerson R., Majumdar Z. K., Baucom A., Clegg R. M., Noller H. F. (2005) Measurement of internal movements within the 30 S ribosomal subunit using Förster resonance energy transfer. J. Mol. Biol. 354, 459–472 [DOI] [PubMed] [Google Scholar]
- 40. McEwen D. P., Gee K. R., Kang H. C., Neubig R. R. (2001) Fluorescent BODIPY-GTP analogs. Real-time measurement of nucleotide binding to G proteins. Anal. Biochem. 291, 109–117 [DOI] [PubMed] [Google Scholar]
- 41. Gurevich V. V., Benovic J. L. (2000) Arrestin, mutagenesis, expression, purification, and functional characterization. Methods Enzymol. 315, 422–437 [DOI] [PubMed] [Google Scholar]
- 42. Sommer M. E., Smith W. C., Farrens D. L. (2005) Dynamics of arrestin-rhodopsin interactions. Arrestin and retinal release are directly linked events. J. Biol. Chem. 280, 6861–6871 [DOI] [PubMed] [Google Scholar]
- 43. Owen D. J., Evans P. R. (1998) A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 282, 1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leyris J. P., Roux T., Trinquet E., Verdié P., Fehrentz J. A., Oueslati N., Douzon S., Bourrier E., Lamarque L., Gagne D., Galleyrand J. C., M'kadmi C., Martinez J., Mary S., Banères J. L., Marie J. (2011) Homogeneous time-resolved fluorescence-based assay to screen for ligands targeting the growth hormone secretagogue receptor type 1a. Anal. Biochem. 408, 253–262 [DOI] [PubMed] [Google Scholar]
- 45. Banères J. L., Popot J. L., Mouillac B. (2011) New advances in production and functional folding of G protein-coupled receptors. Trends Biotechnol. 29, 314–322 [DOI] [PubMed] [Google Scholar]
- 46. Popot J. L., Althoff T., Bagnard D., Banères J. L., Bazzacco P., Billon-Denis E., Catoire L. J., Champeil P., Charvolin D., Cocco M. J., Crémel G., Dahmane T., de la Maza L. M., Ebel C., Gabel F., Giusti F., Gohon Y., Goormaghtigh E., Guittet E., Kleinschmidt J. H., Kühlbrandt W., Le Bon C., Martinez K. L., Picard M., Pucci B., Sachs J. N., Tribet C., van Heijenoort C., Wien F., Zito F., Zoonens M. (2011) Amphipols from A to Z. Annu. Rev. Biophys. 40, 379–408 [DOI] [PubMed] [Google Scholar]
- 47. Zoonens M., Giusti F., Zito F., Popot J. L. (2007) Dynamics of membrane protein/amphipol association studied by Förster resonance energy transfer. Implications for in vitro studies of amphipol-stabilized membrane proteins. Biochemistry 46, 10392–10404 [DOI] [PubMed] [Google Scholar]
- 48. Rosenbaum D. M., Cherezov V., Hanson M. A., Rasmussen S. G., Thian F. S., Kobilka T. S., Choi H. J., Yao X. J., Weis W. I., Stevens R. C., Kobilka B. K. (2007) GPCR engineering yields high-resolution structural insights into β2-adrenergic receptor function. Science 318, 1266–1273 [DOI] [PubMed] [Google Scholar]
- 49. Denisov I. G., Grinkova Y. V., Lazarides A. A., Sligar S. G. (2004) Directed self-assembly of monodisperse phospholipid bilayer nanodiscs with controlled size. J. Am. Chem. Soc. 126, 3477–3487 [DOI] [PubMed] [Google Scholar]
- 50. Holst B., Cygankiewicz A., Jensen T. H., Ankersen M., Schwartz T. W. (2003) High constitutive signaling of the ghrelin receptor. Identification of a potent inverse agonist. Mol. Endocrinol. 17, 2201–2210 [DOI] [PubMed] [Google Scholar]
- 51. Camiña J. P., Lodeiro M., Ischenko O., Martini A. C., Casanueva F. F. (2007) Stimulation by ghrelin of p42/p44 mitogen-activated protein kinase through the GHS-R1a receptor. Role of G-proteins and β-arrestins. J. Cell Physiol. 213, 187–200 [DOI] [PubMed] [Google Scholar]
- 52. Bennett K. A., Langmead C. J., Wise A., Milligan G. (2009) Growth hormone secretagogues and growth hormone releasing peptides act as orthosteric super-agonists but not allosteric regulators for activation of the G protein Gα(o1) by the ghrelin receptor. Mol. Pharmacol. 76, 802–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tsukamoto H., Sinha A., DeWitt M., Farrens D. L. (2010) Monomeric rhodopsin is the minimal functional unit required for arrestin binding. J. Mol. Biol. 399, 501–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bayburt T. H., Vishnivetskiy S. A., McLean M. A., Morizumi T., Huang C. C., Tesmer J. J., Ernst O. P., Sligar S. G., Gurevich V. V. (2011) Monomeric rhodopsin is sufficient for normal rhodopsin kinase (GRK1) phosphorylation and arrestin-1 binding. J. Biol. Chem. 286, 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Diviani D., Lattion A. L., Abuin L., Staub O., Cotecchia S. (2003) The adaptor complex 2 directly interacts with the β1b-adrenergic receptor and plays a role in receptor endocytosis. J. Biol. Chem. 278, 19331–19340 [DOI] [PubMed] [Google Scholar]
- 56. Paing M. M., Johnston C. A., Siderovski D. P., Trejo J. (2006) Clathrin adaptor AP2 regulates thrombin receptor constitutive internalization and endothelial cell resensitization. Mol. Cell. Biol. 26, 3231–3242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. White J. F., Grodnitzky J., Louis J. M., Trinh L. B., Shiloach J., Gutierrez J., Northup J. K., Grisshammer R. (2007) Dimerization of the class A G protein-coupled neurotensin receptor NTS1 alters G protein interaction. Proc. Natl. Acad. Sci. U.S.A. 104, 12199–12204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bayburt T. H., Sligar S. G. (2010) Membrane protein assembly into nanodiscs. FEBS Lett. 584, 1721–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Holst B., Schwartz T. W. (2006) Ghrelin receptor mutations. Too little height and too much hunger. J. Clin. Invest. 116, 637–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lau P. N., Chow K. B., Chan C. B., Cheng C. H., Wise H. (2009) The constitutive activity of the ghrelin receptor attenuates apoptosis via a protein kinase C-dependent pathway. Mol. Cell Endocrinol. 299, 232–239 [DOI] [PubMed] [Google Scholar]
- 61. Bonifacino J. S., Traub L. M. (2003) Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72, 395–447 [DOI] [PubMed] [Google Scholar]
- 62. Fraile-Ramos A., Kohout T. A., Waldhoer M., Marsh M. (2003) Endocytosis of the viral chemokine receptor US28 does not require β-arrestins but is dependent on the clathrin-mediated pathway. Traffic 4, 243–253 [DOI] [PubMed] [Google Scholar]
- 63. Deupi X., Standfuss J. (2011) Structural insights into agonist-induced activation of G-protein-coupled receptors. Curr. Opin. Struct. Biol. 21, 541–551 [DOI] [PubMed] [Google Scholar]
- 64. Floquet N., M'Kadmi C., Perahia D., Gagne D., Bergé G., Marie J., Banères J. L., Galleyrand J. C., Fehrentz J. A., Martinez J. (2010) Activation of the ghrelin receptor is described by a privileged collective motion. A model for constitutive and agonist-induced activation of a subclass A G-protein coupled receptor (GPCR). J. Mol. Biol. 395, 769–784 [DOI] [PubMed] [Google Scholar]
- 65. Baneres J. L., Martin A., Hullot P., Girard J. P., Rossi J. C., Parello J. (2003) Structure-based analysis of GPCR function. Conformational adaptation of both agonist and receptor upon leukotriene B4 binding to recombinant BLT1. J. Mol. Biol. 329, 801–814 [DOI] [PubMed] [Google Scholar]
- 66. Milligan G. (2009) G protein-coupled receptor heterodimerization. Contribution to pharmacology and function. Br. J. Pharmacol. 158, 5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chabre M., le Maire M. (2005) Monomeric G protein-coupled receptor as a functional unit. Biochemistry 44, 9395–9403 [DOI] [PubMed] [Google Scholar]
- 68. Chabre M., Deterre P., Antonny B. (2009) The apparent cooperativity of some GPCRs does not necessarily imply dimerization. Trends Pharmacol. Sci. 30, 182–187 [DOI] [PubMed] [Google Scholar]
- 69. Holst B., Brandt E., Bach A., Heding A., Schwartz T. W. (2005) Nonpeptide and peptide growth hormone secretagogues act both as ghrelin receptor agonist and as positive or negative allosteric modulators of ghrelin signaling. Mol. Endocrinol. 19, 2400–2411 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.