Background: Glycogen Synthase Kinase-3 (GSK-3) is a key regulator of multiple signaling pathways.
Results: Adenomatous Polyposis Coli (APC) positively regulates GSK-3 activity in both Wnt- and Akt-dependent pathways.
Conclusion: Canonical Wnt signaling disrupts APC-Axin interaction and reduces GSK-3 activity.
Significance: APC regulation of GSK-3 provides a novel mechanism for signaling through GSK-3 by Wnt and non-Wnt pathways.
Keywords: β-Catenin, Colon Cancer, Glycogen Synthase Kinase 3, mTOR, Wnt Signaling, Adenomatous Polyposis Coli (APC), Axin, GSK-3, LRP
Abstract
Glycogen synthase kinase-3 (GSK-3) is essential for many signaling pathways and cellular processes. As Adenomatous Polyposis Coli (APC) functions in many of the same processes, we investigated a role for APC in the regulation of GSK-3-dependent signaling. We find that APC directly enhances GSK-3 activity. Furthermore, knockdown of APC mimics inhibition of GSK-3 by reducing phosphorylation of glycogen synthase and by activating mTOR, revealing novel roles for APC in the regulation of these enzymes. Wnt signaling inhibits GSK-3 through an unknown mechanism, and this results in both stabilization of β-catenin and activation of mTOR. We therefore hypothesized that Wnts may regulate GSK-3 by disrupting the interaction between APC and the Axin-GSK-3 complex. We find that Wnts rapidly induce APC dissociation from Axin, correlating with β-catenin stabilization. Furthermore, Axin interaction with the Wnt co-receptor LRP6 causes APC dissociation from Axin. We propose that APC regulates multiple signaling pathways by enhancing GSK-3 activity, and that Wnts induce APC dissociation from Axin to reduce GSK-3 activity and activate downstream signaling. APC regulation of GSK-3 also provides a novel mechanism for Wnt regulation of multiple downstream effectors, including β-catenin and mTOR.
Introduction
Glycogen synthase kinase-3 (GSK-3)3 plays critical roles in a wide variety of essential biological processes during development and throughout adulthood, including tissue patterning, glucose metabolism, apoptosis, stem cell homeostasis, and cell cycle regulation (1). GSK-3 has over 40 known direct substrates, and regulates many signaling pathways including the Wnt, MAPK/ERK, BMP, mTOR, and insulin pathways (1–5). Misregulation of GSK-3 has been proposed to play important roles in human disorders such as bipolar disorder, schizophrenia, Alzheimer disease, and cancer (6–8). Therefore understanding the mechanisms that control GSK-3 activity is essential to understanding many biological processes as well as human disorders.
GSK-3 is a constitutively active kinase that typically inhibits downstream signaling events; thus extracellular signals in general inhibit GSK-3 to induce intracellular signaling events. For example, insulin promotes N-terminal phosphorylation of GSK-3 (Ser-9/21), creating a pseudosubstrate motif that inhibits GSK-3 and allows activation of glycogen synthase (9–11). Wnt signaling also inhibits GSK-3 activity, but through a mechanism that is independent of Ser-9/21 phosphorylation (10, 12, 13). The mechanism of GSK-3 inhibition by Wnts remains poorly understood.
The Wnt signaling pathway is essential for patterning and cell fate specification during embryonic development, and plays critical roles in stem cell homeostasis and tissue regeneration throughout adulthood (14, 15). Ectopic activation of the Wnt pathway causes many human cancers, including breast, prostate, liver, and most notably colorectal cancers (16, 17). In the absence of Wnt ligands, GSK-3, the transcription factor β-catenin, and the tumor suppressor Adenomatous Polyposis Coli (APC) bind directly to the scaffolding protein Axin in a robust complex (18) that facilitates destabilizing phosphorylation of β-catenin by GSK-3. Canonical Wnt ligands bind to their receptor Frizzled and induce phosphorylation of the co-receptor LRP5/6, promoting direct interaction between LRP5/6 and Axin. This reduces GSK-3 activity, allowing stabilization of β-catenin, which then enters the nucleus to activate transcription (14, 15). Several mechanisms have been proposed to explain Wnt-induced inhibition of GSK-3, including Axin degradation (19, 20) and sequestration of GSK-3 within multivesicular endosomes (21). However, these events occur several hours after stimulation, whereas reduced GSK-3 activity and β-catenin stabilization are detected within minutes (12, 13). In addition, the LRP6 intracellular domain can directly inhibit GSK-3, suggesting an alternative and plausible mechanism for inhibition of GSK-3 once it is recruited to the receptor/co-receptor complex (22–24). However, micromolar concentrations of the LRP6 intracellular domain peptide are required to inhibit GSK-3, and the peptide itself is a GSK-3 substrate and thus may simply compete with the substrates that were assayed for phosphorylation by GSK-3.
Similar to GSK-3, APC also negatively regulates the Wnt pathway by promoting β-catenin phosphorylation and degradation (25–27). APC interaction with Axin requires Ser-Ala-Met-Pro (SAMP) repeats on APC (28). Mutations in APC that delete the SAMP repeats and eliminate Axin binding are strongly linked to colorectal cancers, and cause constitutively elevated β-catenin levels and constitutive activation of Wnt target genes (29). These effects can be rescued by reintroduction of wild type APC or fragments of APC that bind Axin through SAMP repeats, but not by SAMP mutant fragments that do not bind Axin (29–31). Therefore APC interaction with Axin is critical for down-regulating β-catenin and maintaining the Wnt pathway in an inactive state, and thus parallels the function of GSK-3 in Wnt signaling.
In addition to their parallel roles in Wnt signaling, APC and GSK-3 have similar functions in processes such as cell cycle regulation and apoptosis (32, 33). For example, APC and GSK-3 are both required for proper spindle formation during mitosis (32, 34, 35). As APC promotes phosphorylation of β-catenin, a direct GSK-3 substrate, and is involved in many of the same processes as GSK-3, we hypothesized that APC regulates additional GSK-3 substrates and GSK-3-dependent signaling pathways. We find that APC directly regulates GSK-3 activity. Thus APC promotes phosphorylation of the direct GSK-3 substrate glycogen synthase independently of Wnt signaling. We also find that APC is required for GSK-3-dependent negative regulation of mTOR. This led us to hypothesize that a key function of APC in the Axin complex is to enhance GSK-3 activity and that Wnts reduce GSK-3 activity by causing dissociation of APC from the Axin complex. In support of this hypothesis, we find that Wnts rapidly disrupt the endogenous APC-Axin interaction. We propose that APC regulates multiple signaling pathways by enhancing GSK-3 activity, and that Wnts cause APC to dissociate from the Axin complex to reduce GSK-3 activity and activate downstream signaling.
EXPERIMENTAL PROCEDURES
Cell Culture, Conditioned Medium, and Transfections
HEK293 cells (ATCC #CRL-1573), HEK293T OT-Luciferase cells (36), L cells (ATCC #CRL-2648), L-Wnt3a cells (ATCC #CRL-2647), and SW480 cells (ATCC #CCL-228) were cultured in Dulbecco's modified Eagle's medium (GIBCO #11965) supplemented with 10% fetal bovine serum (Hyclone #SH30071.03) and 1% penicillin/streptomycin (GIBCO #15140). Insulin was purchased from Sigma (#I9278). Control and Wnt3a conditioned media were prepared from L and l-Wnt3a cells, respectively, according to ATCC instructions. Cells were transfected 24 h after plating using Lipofectamine 2000 (Invitrogen #11668) for plasmids or Lipofectamine RNAiMax (Invitrogen #13778) for siRNAs according to manufacturer's instructions. Transfections were done in Opti-MEM Reduced Serum Medium (GIBCO #31985).
Plasmids and siRNAs
Myc-SAMP (APC 1211–2075) in pEF-BOS vector was provided by Akira Kikuchi (27). VSV-G-LRP6ΔN1 (constitutively active LRP6) and VSV-G-LRP6ΔN1ab (nonfunctional LRP6) both in pCS2+ vector were provided by Xi He (37). Axin in pCS2+MT vector was described previously (38). APC in pCMV vector was provided by Bert Vogelstein and Kenneth Kinzler (39). Myc-tagged stabilized β-catenin (T41A/S45A) in pCS2+MT vector was provided by Ed Morrisey (40). Axin and GFP shRNAs expressed from pSUPER vector were provided by Xiao-Fan Wang (41). APC siRNA expressed from pSUPER vector (pH1iAPC729) was provided by Jean Schneikert (42). The following siRNAs were purchased from Applied Biosystems and transfected at a final concentration of 75 nm; Silencer Negative Control #1 (Catalogue #AM4635), APC (siRNA ID #42812), APC (siRNA ID #s1435).
Western Blotting, Immunoprecipitations, and Luciferase Assays
Cells were lysed in buffer containing 20 mm Tris pH 7.5, 140 mm NaCl, 1 mm EDTA, 10% glycerol, 1% Triton X-100, 1 mm DTT, 50 mm NaF, and protease inhibitor mixture (Sigma P8340), phosphatase inhibitor mixture #1 (Sigma P2850) and #2 (Sigma P5726) or #2 and #3 (Sigma P0044) used 1:100 each. Lysis buffer used in Fig. 4 contained 20 mm Tris, pH 8.0 instead of pH 7.5. Supernatants were collected after centrifugation at 13,000 rpm for 5 min at 4 °C. For immunoprecipitation, lysates were incubated with antibodies to Axin (43), APC (Santa Cruz Biotechnology #sc-896), VSV-G (Sigma #V4888), or control IgG (Thermo Scientific #31235) for 2 h, and then protein G-agarose beads (Invitrogen #15920) were added for an additional 2 h. Antibody-bound beads were washed with lysis buffer three times for 5 min each and resuspended in standard 2× Laemmli Sample Buffer. All steps were done at 4 °C. Samples were heated for 5 min at 95 °C before SDS-PAGE and Western blot analysis using the following antibodies from Cell Signaling: phosphoglycogen synthase (#3891), glycogen synthase (#3893), phospho-Akt (#4058), phospho-GSK-3 (#9331), Axin (#3323), phospho-S6 (#4858), total S6 (#2317), phospho-β-catenin (#9561), GAPDH (#2118), or antibodies to GSK-3 (Calbiochem #368662), APC (Santa Cruz Biotechnology #sc-896 or Abcam #ab58), Myc (Sigma #C3956), β-catenin (BD Transduction Laboratories #610153), VSV-G (Sigma #V4888), Axin (43), phospho-Tau (PHF-1 antibody provided by Peter Davies (44)), or total Tau (T14/46 antibodies provided by Virginia Lee). For luciferase assay, cells were lysed in 1X Promega Passive Lysis Buffer (Promega E194A) and luciferase assay was performed using the Promega Dual Luciferase Reporter Assay System (Promega #E1910) according to the manufacturer's instructions.
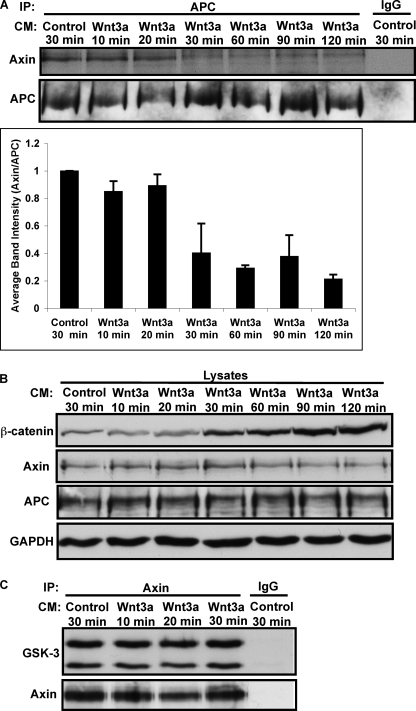
FIGURE 4.
Wnt signaling disrupts the interaction between APC and Axin. L cells were treated with Control or Wnt3a conditioned medium (CM) for indicated times. A, APC was immunoprecipitated from control and Wnt3a-treated cells, and samples were immunoblotted for Axin or APC. For image quantification, Axin was normalized to APC and then all lanes were normalized to control (lane 1). Error bars represent S.E. of four independent experiments. B, lysates from panel A were immunoblotted for β-catenin, Axin, APC, and GAPDH. β-catenin accumulation in response to Wnt activation correlates with Axin/APC dissociation in panel A. C, Wnt3a does not affect GSK-3 association with Axin.
In Vitro Kinase Reactions and Image Quantification
Purified recombinant proteins were incubated in kinase reaction buffer (100 mm Tris pH 7.5, 5 mm DTT, 10 mm MgCl2, 100 μm ATP) for 10 min at 30 °C. Reactions were stopped by adding standard 2× Laemmli Sample Buffer and incubating at 95 °C for 5 min. The following protein concentrations were used; 80 nm GST-SAMP, 100 nm His-β-catenin, 100 nm His-Axin, 100 nm Tau, 80 nm BSA (Sigma #A9647), 2 units/μl GSK-3 (New England Biolabs #P6040), 4 units/μl CKI (New England Biolabs #P6030). Western blot images were quantified using ImageJ software. For supplemental Fig. S7, cells were lysed in standard RIPA buffer, immunoprecipitated with anti-GSK-3 antibody, and GSK-3 kinase activity assay was performed as previously described (45).
RESULTS
APC Regulates Glycogen Synthase through GSK-3
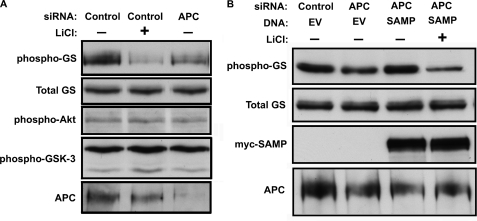
APC promotes phosphorylation of the direct GSK-3 substrate β-catenin, and APC and GSK-3 function in many of the same cellular processes (1, 32); we therefore asked whether APC promotes phosphorylation of additional GSK-3 substrates. Glycogen synthase (GS), the eponymous substrate for GSK-3, is expressed in 293T cells and is constitutively phosphorylated by GSK-3 to inhibit its activity. Knocking down APC with siRNA reduced site-specific phosphorylation of GS, similar to treating with the GSK-3 inhibitor LiCl (Fig. 1A) or alternative small molecule GSK-3 inhibitors (supplemental Fig. S1). These results were confirmed using an additional non-overlapping APC siRNA (supplemental Fig. S2). Insulin and other growth factors reduce GS phosphorylation through phosphorylation of Akt and GSK-3 (9, 10, 46). Interestingly, knocking down APC did not affect phosphorylation of Akt or GSK-3, indicating APC does not regulate GS by activating Insulin signaling upstream of GSK-3 (Fig. 1A). GS phosphorylation was rescued after APC knockdown by expressing a large fragment of APC (SAMP fragment) that also rescues β-catenin levels in colorectal carcinoma cells that lack wild type APC (Fig. 1B). Importantly, LiCl blocked the rescue of GS phosphorylation by APC-SAMP, showing that the rescue is dependent on GSK-3 activity (Fig. 1B). Knocking down APC in these cells also activated Wnt signaling, as assessed by activity of a β-catenin responsive reporter (supplemental Fig. S3A), consistent with the known role for APC as a negative regulator of the Wnt pathway (14, 15). To rule out the possibility that GS phosphorylation was indirectly regulated by Wnt signaling downstream of the APC/Axin degradation complex, we expressed a stabilized and hence constitutively active form of β-catenin. Although stabilized β-catenin potently activated the Wnt reporter, it had no effect on phospho-GS (supplemental Fig. S3B), demonstrating that the reduction in GS phosphorylation in response to APC knockdown is not due to downstream activation of the Wnt pathway. These results suggest a novel role for APC in the regulation of GSK-3 activity.
FIGURE 1.
APC regulates glycogen synthase through GSK-3. A, APC was knocked down by siRNA in 293T cells and phosphorylation of GS at an established GSK-3 target site (GS-Ser641) was assessed by immunoblotting with a phosphospecific antibody. The same samples were immunoblotted for total GS (loading control), phospho-Akt (Ser-473), phospho-GSK-3 (Ser-9/21), and APC. Cells in lane 2 were also treated with 15 mm LiCl. Cells were also transfected with a scrambled siRNA (control). APC knockdown reduced APC protein levels and endogenous GS phosphorylation without affecting total GS or phosphorylation of Akt or GSK-3. B, to rescue GS phosphorylation in APC knockdown cells, the Myc-tagged SAMP fragment of APC was co-transfected with scrambled (control) or APC siRNAs. EV indicates empty vector control. The SAMP fragment of APC rescues phosphorylation of GS in APC knockdowns, and this rescue is blocked by LiCl.
We also asked whether APC knockdown affects GS regulation by insulin. We generated an HEK293 cell line that stably expresses an siRNA targeting APC (42). Stable knockdown of APC reduces GS phosphorylation (supplemental Fig. S4), similar to Fig. 1. Insulin also reduces GS phosphorylation in the parental HEK293 line (46), but combined APC knockdown and insulin treatment do not further reduce GS phosphorylation (supplemental Fig. S4). Insulin did not induce phosphorylation of GSK-3 stably associated with the endogenous APC/Axin complex (supplemental Fig. S5), consistent with previous observations that the Axin-associated/Wnt-regulated subcellular pool of GSK-3 is distinct from insulin-regulated GSK-3 (10, 12). Stable association of APC and GSK-3 independent of the Axin complex has not been demonstrated to our knowledge.
APC Regulates mTOR through GSK-3
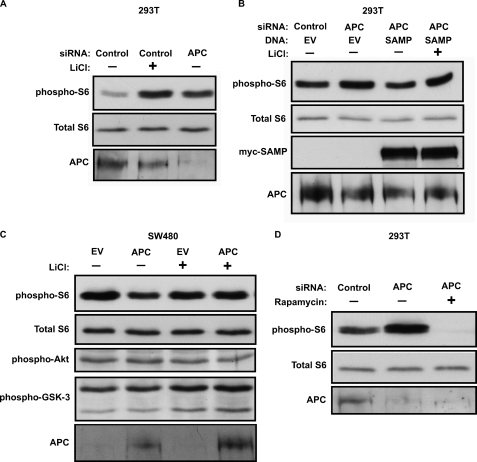
GSK-3 negatively regulates mammalian Target Of Rapamycin (mTOR), and either pharmacological or genetic inhibition of GSK-3 activates mTOR in vivo (2, 47). If APC promotes phosphorylation of other substrates by GSK-3, in addition to GS and β-catenin, then knockdown of APC may also activate mTOR. In support of this, knockdown of APC activated mTOR, as assessed by increased phosphorylation of ribosomal protein S6, similar to inhibiting GSK-3 with LiCl (Fig. 2A), other small molecule GSK-3 inhibitors (supplemental Fig. S1), or knocking down Gsk3 expression (2, 47). This was also confirmed using a non-overlapping APC siRNA (supplemental Fig. S2), and rescued by expressing the SAMP fragment of APC. This rescue was also blocked by LiCl (Fig. 2B). The effect of APC knockdown on S6 phosphorylation was blocked by the mTOR inhibitor rapamycin (Fig. 2D), further supporting our conclusion that APC functions upstream of mTOR. As above, expression of stabilized β-catenin had no effect on S6 phosphorylation (supplemental Fig. S3), indicating that mTOR activation resulting from APC knockdown is not due to activation of Wnt signaling downstream of β-catenin.
FIGURE 2.
APC regulates mTOR through GSK-3. A, APC was knocked down by siRNA, as in Fig. 1, and mTOR activity was assessed as phosphorylation of ribosomal protein S6 (Ser-235/236, upper panel). Inhibition of GSK-3 with LiCl also activated mTOR. Total S6 (middle panel) is shown as a loading control. The cell lysates in panels A and B are the same as those used in Fig. 1. B, APC knockdown increased S6 phosphorylation and this effect was rescued by the SAMP fragment of APC. C, expression of full-length APC reduces S6 phosphorylation in SW480 colorectal carcinoma cells, which only express a truncated APC that lacks the SAMP repeats. Transfection efficiency was estimated at 30% based on expression of YFP (data not shown). LiCl increased S6 phosphorylation in cells expressing wild type APC. D, rapamycin (10 nm) blocks S6 phosphorylation after APC knockdown.
Wnts regulate the GSK-3/APC/Axin complex to inhibit GSK-3 and activate β-catenin and mTOR (2, 14). As APC promotes glycogen synthase phosphorylation by GSK-3, we asked whether Wnts affect glycogen synthase phosphorylation. L cells were treated with control or Wnt3a conditioned medium for up to 2 h, but no change in GS phosphorylation was detected (supplemental Fig. S6A). Furthermore, Axin knockdown activates mTOR as observed previously (2), but does not affect GS phosphorylation (supplemental Fig. S6B). Taken together, these data suggest Wnts and Axin regulate mTOR but not GS.
The SW480 human colorectal carcinoma cell line lacks wild-type APC, and instead expresses a truncated APC that fails to interact with Axin or to down-regulate β-catenin (29, 48). Phosphorylation and degradation of β-catenin can be restored in these cells by reintroduction of wild type APC (or the SAMP fragment) (25–27, 29). Interestingly, S6 phosphorylation readily detected in untreated SW480 cells was not further increased by LiCl, in contrast to the effects observed in 293T cells, which express wild type APC (Fig. 2C, compare lanes 1 and 3). We hypothesized that mTOR is aberrantly active in these cells due to the truncating APC mutations. Consistent with this idea, reintroduction of wild type APC reduces S6 phosphorylation, suggesting a novel role for APC as a negative regulator of mTOR. Furthermore, when wild-type APC was introduced into SW480 cells, LiCl activated mTOR (Fig. 2C, compare lanes 2 and 4), indicating that restoring wild-type APC restores GSK-3 activity and mTOR sensitivity to LiCl. Restoring wild type APC in SW480 cells did not affect Akt or GSK-3 phosphorylation. These data support a signaling pathway in which APC, acting through GSK-3, suppresses mTOR activity.
APC Directly Enhances GSK-3 Activity
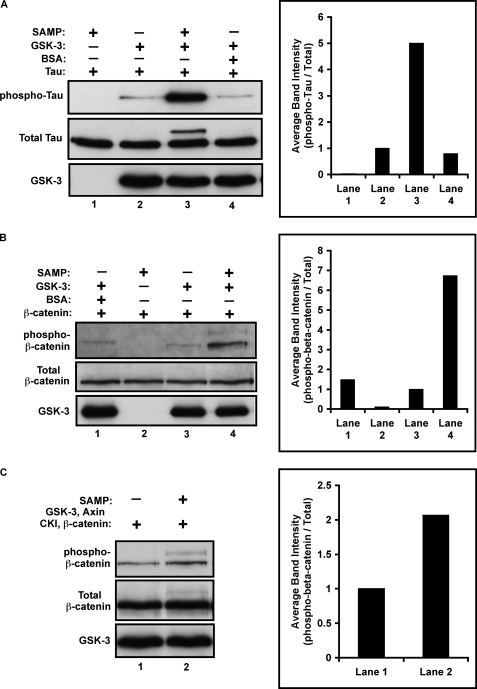
Knocking down APC reduced phosphorylation of GS, a direct GSK-3 substrate, without affecting known signaling mechanisms upstream of GSK-3. To test whether APC regulates GSK-3 activity directly, we performed in vitro kinase reactions using purified recombinant proteins. Surprisingly, addition of the SAMP fragment of APC increases GSK-3-dependent phosphorylation of both Tau protein (Fig. 3A, lanes 2 and 3) and β-catenin (Fig. 3B, lanes 3 and 4) by ∼5–7-fold. GSK-3 dependent phosphorylation of β-catenin normally occurs within the Axin complex, and requires a priming phosphorylation of β-catenin by casein kinase Iα (CKIα) (49). Thus, we added Axin and casein kinase I (CKI) to the in vitro kinase reaction and again observed that SAMP further enhanced β-catenin phosphorylation by GSK-3. Thus, APC directly enhances GSK-3 activity toward multiple substrates both in the presence and absence of the Axin complex.
FIGURE 3.
APC directly enhances GSK-3 activity. In vitro kinase reactions were carried out using purified recombinant proteins. A, addition of the SAMP fragment (80 nm) of APC, but not BSA, directly enhances Tau phosphorylation (Ser-396/404) by GSK-3 by ∼5 fold. For image quantification, phospho-Tau was normalized to total Tau and then all lanes were normalized to lane 2. B, SAMP, but not BSA, directly enhances β-catenin phosphorylation (Ser-33/37/41) by GSK-3 by ∼7-fold. For image quantification, phospho-β-catenin was normalized to total β-catenin and then all lanes were normalized to lane 3. C, SAMP enhances β-catenin phosphorylation by GSK-3 in the presence of Axin and CKI by ∼2-fold. For image quantification, phospho-β-catenin was normalized to total β-catenin and then lane 2 was normalized to lane 1.
We also asked whether restoring wild type APC in APC-deficient colorectal carcinoma cells could enhance GSK-3 activity. We used an inducible APC cell line derived from HT29 cells, human colorectal carcinoma cells that express a truncated APC similar to SW480 cells (50). HT29-APC cells express full length APC under control of the zinc-inducible metallothionein promoter and control HT29-β-gal cells express β-galactosidase (supplemental Fig. 7C). We immunoprecipitated endogenous GSK-3 from untreated or zinc-treated cells and measured phosphorylation of a peptide derived from glycogen synthase (GS-2) by incorporation of 32P (supplemental Fig. S7). APC induction enhances GS-2 phosphorylation, whereas zinc treatment in HT29-β-gal cells has no effect. The GSK-3 inhibitor SB216763 blocks GS-2 phosphorylation in all groups indicating that GS-2 is phosphorylated primarily by GSK-3 in this assay. These data confirm that wild type APC enhances the activity of GSK-3 recovered from APC-deficient colorectal carcinoma cells.
Wnt3a Induces Axin/APC Dissociation
In the canonical Wnt pathway, β-catenin is constitutively phosphorylated by GSK-3 within the Axin complex and targeted for degradation. APC interaction with Axin is required for β-catenin phosphorylation and destabilization. Binding of Wnts to the surface receptor complex leads to inhibition of GSK-3 activity through an as yet poorly characterized mechanism (14, 15). As our data show that APC enhances GSK-3 activity in general and can specifically enhance GSK-3 phosphorylation of β-catenin within the Axin complex (Fig. 3), we hypothesized that Wnt signaling may reduce GSK-3 activity by inducing dissociation of APC from the Axin complex. To test this, we treated L cells with control or Wnt3a conditioned medium for up to 2 h, immunoprecipitated endogenous APC, and detected endogenous APC-associated Axin. The amount of Axin associated with APC was dramatically reduced by 30 min, and interaction was not restored even after 2 h (Fig. 4A). Similar results were obtained when Axin was immunoprecipitated, when purified recombinant Wnt3a was used instead of Wnt3a-conditioned medium, and when 293T cells were treated with Wnt3a (data not shown). Importantly, observation of Wnt-induced Axin/APC dissociation was sensitive to lysis and IP conditions, especially the pH of the lysis buffer (see “Experimental Procedures”). The amount of GSK-3 associated with Axin did not change under these conditions (Fig. 4C). Interestingly, the onset of β-catenin accumulation correlated with APC/Axin dissociation (Fig. 4B). Taken together, these results show that activation of the Wnt pathway causes APC/Axin dissociation, and this may contribute to reduced GSK-3 activity and subsequent β-catenin stabilization.
Axin Binding to LRP6 Causes APC Dissociation
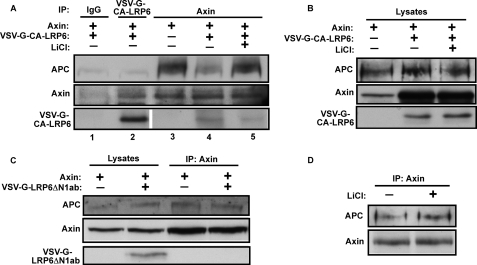
The Wnt co-receptor LRP6 is phosphorylated in response to canonical Wnt ligands, and LRP6 phosphorylation promotes direct interaction with Axin (37, 51, 52). As Wnt3a causes dissociation of APC from Axin, we hypothesized that the recruitment of Axin to phosphorylated LRP6 may displace APC from the Axin complex. Deletion of the N-terminal extracellular domain of LRP6 results in a constitutively active LRP6 fragment (CA-LRP6) that is phosphorylated by GSK-3, interacts with Axin, and results in β-catenin stabilization and activation of Wnt target genes (37, 51), (supplemental Fig. S8). We found that co-expression of CA-LRP6 and Axin markedly reduces the relative amount of APC associated with Axin (Fig. 5A, compare lanes 3 and 4). In addition, inhibition of GSK-3 with LiCl reduced the Axin-CA-LRP6 interaction and restored APC-Axin interaction (Fig. 5A, lanes 3–5), consistent with the known role for GSK-3 in phosphorylation and activation of LRP6. Conversely, LiCl had no effect on Axin-APC association in the absence of the CA-LRP6 fragment (Fig. 5D). Importantly, the nonfunctional LRP6 mutant LRP6ΔN1Ab did not recruit Axin or activate Wnt signaling, as shown previously (37, 51), (supplemental Fig. S8), and had no effect on Axin-APC interaction (Fig. 5C). Importantly, there was no detectable APC association with CA-LRP6 despite significant Axin association with CA-LRP6 (Fig. 5A, lane 2). These results show that recruitment of Axin to the activated form of LRP6 displaces APC from the Axin complex, and suggest that Wnt induced Axin-LRP6 interaction causes APC dissociation.
FIGURE 5.
LRP6 binding to Axin causes APC dissociation. A, a constitutively active form of LRP6 (VSV-G-CA-LRP6) was expressed in 293T cells with Axin, and cell lysates were immunoprecipitated with antibodies to VSV-G, Axin, or control antibody, and then immunoblotted for APC, Axin, or VSV-G-CA-LRP6. Axin IP samples were adjusted for equal loading of Axin. Constitutively active LRP6 reduces APC association with Axin (compare lanes 3 and 4). LiCl (15 mm) reduces LRP6/Axin association and restores APC/Axin association (lane 5). APC association with CA-LRP6 was not detected (lane 2). B, Western blot analysis of APC, Axin, and CA-LRP6 in lysates used for immunoprecipitation in panel A. Co-expression of Axin and CA-LRP6, but not LRP6ΔN1ab, increased Axin levels for unclear reasons. C, unphosphorylatable VSV-G-LRP6ΔN1ab does not associate with Axin and does not affect Axin/APC interaction. D, 15 mm LiCl alone does not affect Axin/APC interaction.
DISCUSSION
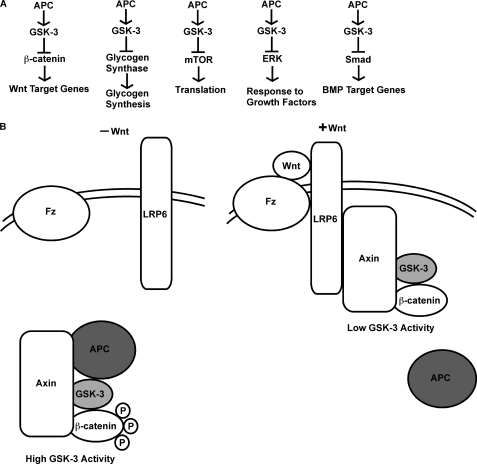
In this study, we defined a role for APC in the regulation of GSK-3 and GSK-3-dependent pathways, including glycogen synthase, mTOR, and Wnt signaling (Fig. 6A).
FIGURE 6.
Model. A, APC negatively regulates multiple signaling pathways, including the Wnt, glycogen synthase, mTOR, ERK, and BMP pathways by enhancing GSK-3 activity. B, Wnts disrupt the Axin/APC interaction to reduce GSK-3 activity and thus activate downstream signaling.
APC mutations associated with colorectal cancer strongly activate Wnt signaling, and here we show that these mutations also activate mTOR, a metabolic sensor that is aberrantly activated in many cancers (53). As GSK-3 suppresses both Wnt and mTOR pathways (2, 14), our demonstration that APC enhances GSK-3 activity provides a mechanistic link between APC loss of function and activation of these two downstream signaling pathways. In support of this mechanism, tumor formation in mice with similar APC mutations is blocked by mTOR inhibitors (54, 55). Furthermore, bladder-specific β-catenin stabilization synergizes with PTEN deletion, which activates mTOR, to promote bladder cancer in mice (56). Taken together, these data support the hypothesis that mTOR activation is required for APC-mediated tumor formation and suggest mTOR as a potential therapeutic target for treatment of cancers resulting from APC mutations.
We also show that APC promotes phosphorylation of glycogen synthase by GSK-3, implicating APC in glucose metabolism and glycogen synthesis for the first time. Interestingly, addition of Wnt ligands or knockdown of Axin activates β-catenin and mTOR but does not affect GS phosphorylation. These data suggest APC and GSK-3 regulation of GS is independent of the Axin complex, unlike regulation of β-catenin and mTOR. Axin knockdown in Drosophila S2 cells increases glycogen levels but this may be through regulation of the Drosophila c-Cbl-associated protein (DCAP) rather than GSK-3 and GS (57).
In addition to regulating Wnt, glycogen synthase, and mTOR signaling, APC and GSK-3 both promote mitotic spindle formation, negatively regulate MAPK/ERK and BMP signaling (Fig. 6A), and regulate apoptosis (3–5, 32, 33, 58, 59). Taken together, these observations suggest a widespread role for APC in the regulation of GSK-3-dependent signaling. Future studies are needed to determine whether APC regulates additional GSK-3-dependent pathways such as the Notch, Hedgehog, and NF-κB pathways.
The finding that APC enhances GSK-3 activity prompted us to ask whether APC is also regulated by Wnt signaling to reduce GSK-3 activity. Canonical Wnts reduce GSK-3 activity through unknown mechanisms, and this is critical for β-catenin stabilization (12, 13). We find that Wnts rapidly induce APC dissociation from Axin, in close temporal correlation with β-catenin accumulation. Furthermore, Axin interaction with activated LRP6, the Wnt co-receptor, causes APC dissociation. Therefore we propose that Wnt-induced Axin recruitment to LRP6 displaces APC, reducing GSK-3 activity and activating downstream signaling (Fig. 6B). Consistently, Wnt-1 induces membrane localization of Axin and GSK-3β, but not APC (60).
Previous work showed APC is required for β-catenin phosphorylation and degradation, and this was attributed to APC recruiting β-catenin to the Axin complex (25, 27). While this work is compatible with our findings, we also find that APC enhances GSK-3 activity toward multiple substrates, including β-catenin. Consistently, deletion of the RGS domain of Axin, which binds APC, reduces GSK-3 activity and activates Wnt signaling (38). Interestingly, APC also enhances activity of topoisomerase IIα (61), possibly suggesting a novel widespread function for APC in regulating enzymatic activity.
APC also blocks dephosphorylation of β-catenin by protein phosphatase-2A (PP2A) (26). Therefore Wnt induced APC-Axin dissociation may stabilize β-catenin by both reducing GSK-3 activity and allowing PP2A mediated β-catenin dephosphorylation. Interestingly, this mechanism parallels glycogen synthase activation by Insulin which induces both inhibition of GSK-3 and dephosphorylation of glycogen synthase by protein phosphatase-1 (62).
Recent work has shown that Wnt signaling induces sequestration of the GSK-3 into multivesicular bodies, and this was suggested as a mechanism for attenuating phosphorylation and degradation of β-catenin (21). However, that work examined GSK-3 several hours after stimulation with Wnts, whereas we observe dissociation of APC from Axin within 30 min, closely correlated with accumulation of β-catenin protein. Because of the different time scales, the work of Taelman et al. is not incompatible with our findings, and may represent a later consequence of Wnt signaling.
GSK-3 negatively regulates mTOR by phosphorylating Tuberous Sclerosis Complex 2 (TSC2). TSC2 associates with the Axin complex, and canonical Wnts activate mTOR by inhibiting GSK-3 and reducing TSC2 phosphorylation (2). As we show APC enhances GSK-3 activity and negatively regulates mTOR, Wnt-induced APC dissociation from Axin also provides a mechanism for how Wnts activate mTOR.
Because APC regulates multiple GSK-3 dependent processes, and is regulated by Wnts, additional studies are needed to determine whether APC can be regulated to reduce GSK-3 activity in other contexts. For example, during hippocampal development, axonal-dendritic polarity is established by selection of one of several minor neurites to grow into the mature axon (63). This process involves localized inhibition of GSK-3 through unknown mechanisms (independent of S9/21 phosphorylation), and concomitant spatial relocation of APC (64–66). Thus, APC sequestration might reduce GSK-3 activity to promote hippocampal neurite outgrowth.
In conclusion, we demonstrate novel roles for APC as a negative regulator of both glycogen synthase and mTOR, like GSK-3, and uncover a novel ability of APC to directly enhance GSK-3 activity. We also show that Wnts induce APC-Axin dissociation, suggesting a new mechanism for how Wnts decrease GSK-3 activity. Further studies are needed to investigate a role for APC in additional GSK-3-dependent processes, and to determine whether APC is regulated in other contexts to reduce GSK-3 activity.
Supplementary Material
Acknowledgments
We thank all members of the Klein Laboratory for discussions and comments on the manuscript, Dr. Shelby Blythe for helpful comments and technical assistance, and Michael O'Donnell and Arpine Arzoumanian for technical assistance. We also thank Drs. Jon Epstein, Virginia Lee, Peter Davies for antibodies and Drs. Xi He, Jean Schneikert, Akira Kikuchi, Ed Morrisey, Bert Vogelstein, Kenneth Kinzler, and Xiao-Fan Wang for plasmids.
This work was supported, in whole or in part, by Grant 1R01MH58324 from the NIMH and 1R01HL110806-01 from the NHLBI, National Institutes of Health.

This article contains supplemental Figs. S1–S8.
- GSK
- glycogen synthase kinase
- APC
- adenomatous polyposis coli
- mTOR
- mammalian target of rapamycin
- CKI
- casein kinase I.
REFERENCES
- 1. Kockeritz L., Doble B., Patel S., Woodgett J. R. (2006) Glycogen synthase kinase-3–an overview of an over-achieving protein kinase. Current Drug Targets 7, 1377–1388 [DOI] [PubMed] [Google Scholar]
- 2. Inoki K., Ouyang H., Zhu T., Lindvall C., Wang Y., Zhang X., Yang Q., Bennett C., Harada Y., Stankunas K., Wang C. Y., He X., MacDougald O. A., You M., Williams B. O., Guan K. L. (2006) TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126, 955–968 [DOI] [PubMed] [Google Scholar]
- 3. Wang Q., Zhou Y., Wang X., Evers B. M. (2006) Glycogen synthase kinase-3 is a negative regulator of extracellular signal-regulated kinase. Oncogene 25, 43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fuentealba L. C., Eivers E., Ikeda A., Hurtado C., Kuroda H., Pera E. M., De Robertis E. M. (2007) Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell 131, 980–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fukuda T., Kokabu S., Ohte S., Sasanuma H., Kanomata K., Yoneyama K., Kato H., Akita M., Oda H., Katagiri T. (2010) Canonical Wnts and BMPs cooperatively induce osteoblastic differentiation through a GSK3β-dependent and β-catenin-independent mechanism. Differentiation 80, 46–52 [DOI] [PubMed] [Google Scholar]
- 6. Jope R. S., Roh M. S. (2006) Glycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions. Curr. Drug Targets 7, 1421–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hernández F., de Barreda E. G., Fuster-Matanzo A., Goñi-Oliver P., Lucas J. J., Avila J. (2009) The role of GSK3 in Alzheimer disease. Brain Research Bulletin 80, 248–250 [DOI] [PubMed] [Google Scholar]
- 8. Luo J. (2009) Glycogen synthase kinase 3β (GSK3β) in tumorigenesis and cancer chemotherapy. Cancer Letters 273, 194–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 [DOI] [PubMed] [Google Scholar]
- 10. McManus E. J., Sakamoto K., Armit L. J., Ronaldson L., Shpiro N., Marquez R., Alessi D. R. (2005) Role that phosphorylation of GSK3 plays in insulin and Wnt signaling defined by knockin analysis. EMBO J. 24, 1571–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dajani R., Fraser E., Roe S. M., Young N., Good V., Dale T. C., Pearl L. H. (2001) Crystal structure of glycogen synthase kinase 3β: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell 105, 721–732 [DOI] [PubMed] [Google Scholar]
- 12. Ding V. W., Chen R. H., McCormick F. (2000) Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signaling. J. Biol. Chem. 275, 32475–32481 [DOI] [PubMed] [Google Scholar]
- 13. Cook D., Fry M. J., Hughes K., Sumathipala R., Woodgett J. R., Dale T. C. (1996) Wingless inactivates glycogen synthase kinase-3 via an intracellular signaling pathway which involves a protein kinase C. EMBO J. 15, 4526–4536 [PMC free article] [PubMed] [Google Scholar]
- 14. MacDonald B. T., Tamai K., He X. (2009) Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clevers H. (2006) Wnt/β-catenin signaling in development and disease. Cell 127, 469–480 [DOI] [PubMed] [Google Scholar]
- 16. Salahshor S., Woodgett J. (2005) The links between axin and carcinogenesis. J. Clin. Pathol. 58, 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paul S., Dey A. (2008) Wnt signaling and cancer development: therapeutic implication. Neoplasma 55, 165–176 [PubMed] [Google Scholar]
- 18. Peterson-Nedry W., Erdeniz N., Kremer S., Yu J., Baig-Lewis S., Wehrli M. (2008) Unexpectedly robust assembly of the Axin destruction complex regulates Wnt/Wg signaling in Drosophila as revealed by analysis in vivo. Dev. Biol. 320, 226–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tolwinski N. S., Wehrli M., Rives A., Erdeniz N., DiNardo S., Wieschaus E. (2003) Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3β activity. Dev. Cell 4, 407–418 [DOI] [PubMed] [Google Scholar]
- 20. Lee E., Salic A., Kruger R., Heinrich R., Kirschner M. (2003) The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 1, 116–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taelman V. F., Dobrowolski R., Plouhinec J. L., Fuentealba L. C., Vorwald P. P., Gumper I., Sabatini D. D., De Robertis E. M. (2010) Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 143, 1136–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu G., Huang H., Garcia Abreu J., He X. (2009) Inhibition of GSK3 phosphorylation of β-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS ONE 4, e4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cselenyi C. S., Jernigan K. K., Tahinci E., Thorne C. A., Lee L. A., Lee E. (2008) LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3's phosphorylation of β-catenin. Proc. Natl. Acad. Sci. U.S.A. 105, 8032–8037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Piao S., Lee S. H., Kim H., Yum S., Stamos J. L., Xu Y., Lee S. J., Lee J., Oh S., Han J. K., Park B. J., Weis W. I., Ha N. C. (2008) Direct inhibition of GSK3β by the phosphorylated cytoplasmic domain of LRP6 in Wnt/β-catenin signaling. PLoS ONE 3, e4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang J., Zhang W., Evans P. M., Chen X., He X., Liu C. (2006) Adenomatous polyposis coli (APC) differentially regulates β-catenin phosphorylation and ubiquitination in colon cancer cells. J. Biol. Chem. 281, 17751–17757 [DOI] [PubMed] [Google Scholar]
- 26. Su Y., Fu C., Ishikawa S., Stella A., Kojima M., Shitoh K., Schreiber E. M., Day B. W., Liu B. (2008) APC is essential for targeting phosphorylated β-catenin to the SCFβ-TrCP ubiquitin ligase. Mol. Cell 32, 652–661 [DOI] [PubMed] [Google Scholar]
- 27. Hinoi T., Yamamoto H., Kishida M., Takada S., Kishida S., Kikuchi A. (2000) Complex formation of adenomatous polyposis coli gene product and axin facilitates glycogen synthase kinase-3β-dependent phosphorylation of β-catenin and down-regulates β-catenin. J. Biol. Chem. 275, 34399–34406 [DOI] [PubMed] [Google Scholar]
- 28. Behrens J., Jerchow B. A., Würtele M., Grimm J., Asbrand C., Wirtz R., Kühl M., Wedlich D., Birchmeier W. (1998) Functional interaction of an axin homolog, conductin, with β-catenin, APC, and GSK3β. Science 280, 596–599 [DOI] [PubMed] [Google Scholar]
- 29. Munemitsu S., Albert I., Souza B., Rubinfeld B., Polakis P. (1995) Regulation of intracellular β-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc. Natl. Acad. Sci. U.S.A. 92, 3046–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kawahara K., Morishita T., Nakamura T., Hamada F., Toyoshima K., Akiyama T. (2000) Down-regulation of β-catenin by the colorectal tumor suppressor APC requires association with Axin and β-catenin. J. Biol. Chem. 275, 8369–8374 [DOI] [PubMed] [Google Scholar]
- 31. von Kries J. P., Winbeck G., Asbrand C., Schwarz-Romond T., Sochnikova N., Dell'Oro A., Behrens J., Birchmeier W. (2000) Hot spots in β-catenin for interactions with LEF-1, conductin and APC. Nat. Struct. Biol. 7, 800–807 [DOI] [PubMed] [Google Scholar]
- 32. Hanson C. A., Miller J. R. (2005) Non-traditional roles for the Adenomatous Polyposis Coli (APC) tumor suppressor protein. Gene 361, 1–12 [DOI] [PubMed] [Google Scholar]
- 33. Forde J. E., Dale T. C. (2007) Glycogen synthase kinase 3: a key regulator of cellular fate. Cell. Mol. Life Sci. 64, 1930–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Caldwell C. M., Kaplan K. B. (2009) The role of APC in mitosis and in chromosome instability. Adv. Exp. Med. Biol. 656, 51–64 [DOI] [PubMed] [Google Scholar]
- 35. Wakefield J. G., Stephens D. J., Tavare J. M. (2003) A role for glycogen synthase kinase-3 in mitotic spindle dynamics and chromosome alignment. J. Cell Science 116, 637–646 [DOI] [PubMed] [Google Scholar]
- 36. Phiel C. J., Zhang F., Huang E. Y., Guenther M. G., Lazar M. A., Klein P. S. (2001) Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 276, 36734–36741 [DOI] [PubMed] [Google Scholar]
- 37. Tamai K., Zeng X., Liu C., Zhang X., Harada Y., Chang Z., He X. (2004) A mechanism for Wnt coreceptor activation. Molecular Cell 13, 149–156 [DOI] [PubMed] [Google Scholar]
- 38. Hedgepeth C. M., Deardorff M. A., Rankin K., Klein P. S. (1999) Regulation of glycogen synthase kinase 3β and downstream Wnt signaling by axin. Mol. Cell. Biol. 19, 7147–7157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smith K. J., Levy D. B., Maupin P., Pollard T. D., Vogelstein B., Kinzler K. W. (1994) Wild-type but not mutant APC associates with the microtubule cytoskeleton. Cancer Res. 54, 3672–3675 [PubMed] [Google Scholar]
- 40. Xu L., Corcoran R. B., Welsh J. W., Pennica D., Levine A. J. (2000) WISP-1 is a Wnt-1- and β-catenin-responsive oncogene. Genes Dev. 14, 585–595 [PMC free article] [PubMed] [Google Scholar]
- 41. Guo X., Ramirez A., Waddell D. S., Li Z., Liu X., Wang X. F. (2008) Axin and GSK3- control Smad3 protein stability and modulate TGF signaling. Genes Dev. 22, 106–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schneikert J., Behrens J. (2006) Truncated APC is required for cell proliferation and DNA replication. Int. J. Cancer 119, 74–79 [DOI] [PubMed] [Google Scholar]
- 43. Kofron M., Klein P., Zhang F., Houston D. W., Schaible K., Wylie C., Heasman J. (2001) The role of maternal axin in patterning the Xenopus embryo. Dev. Biol. 237, 183–201 [DOI] [PubMed] [Google Scholar]
- 44. Greenberg S. G., Davies P., Schein J. D., Binder L. I. (1992) Hydrofluoric acid-treated tau PHF proteins display the same biochemical properties as normal tau. J. Biol. Chem. 267, 564–569 [PubMed] [Google Scholar]
- 45. Klein P. S., Melton D. A. (1996) A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. U.S.A. 93, 8455–8459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alessi D. R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B. A. (1996) Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15, 6541–6551 [PMC free article] [PubMed] [Google Scholar]
- 47. Huang J., Zhang Y., Bersenev A., O'Brien W. T., Tong W., Emerson S. G., Klein P. S. (2009) Pivotal role for glycogen synthase kinase-3 in hematopoietic stem cell homeostasis in mice. J. Clin. Invest. 119, 3519–3529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kishida S., Yamamoto H., Ikeda S., Kishida M., Sakamoto I., Koyama S., Kikuchi A. (1998) Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of β-catenin. J. Biol. Chem. 273, 10823–10826 [DOI] [PubMed] [Google Scholar]
- 49. Liu C., Li Y., Semenov M., Han C., Baeg G. H., Tan Y., Zhang Z., Lin X., He X. (2002) Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108, 837–847 [DOI] [PubMed] [Google Scholar]
- 50. Morin P. J. (1999) β-Catenin signaling and cancer. Bioessays 21, 1021–1030 [DOI] [PubMed] [Google Scholar]
- 51. Zeng X., Tamai K., Doble B., Li S., Huang H., Habas R., Okamura H., Woodgett J., He X. (2005) A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 438, 873–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mao J., Wang J., Liu B., Pan W., Farr G. H., 3rd, Flynn C., Yuan H., Takada S., Kimelman D., Li L., Wu D. (2001) Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol. Cell 7, 801–809 [DOI] [PubMed] [Google Scholar]
- 53. Zoncu R., Efeyan A., Sabatini D. M. (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Koehl G. E., Spitzner M., Ousingsawat J., Schreiber R., Geissler E. K., Kunzelmann K. (2010) Rapamycin inhibits oncogenic intestinal ion channels and neoplasia in APC(Min/+) mice. Oncogene 29, 1553–1560 [DOI] [PubMed] [Google Scholar]
- 55. Fujishita T., Aoki K., Lane H. A., Aoki M., Taketo M. M. (2008) Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in ApcDelta716 mice. Proc. Natl. Acad. Sci. U.S.A. 105, 13544–13549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ahmad I., Morton J. P., Singh L. B., Radulescu S. M., Ridgway R. A., Patel S., Woodgett J., Winton D. J., Taketo M. M., Wu X. R., Leung H. Y., Sansom O. J. (2011) β-Catenin activation synergizes with PTEN loss to cause bladder cancer formation. Oncogene 30, 178–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yamazaki H., Yanagawa S. (2003) Axin and the Axin/Arrow-binding protein DCAP mediate glucose-glycogen metabolism. Biochem. Biophys. Res. Commun. 304, 229–235 [DOI] [PubMed] [Google Scholar]
- 58. Park K. S., Jeon S. H., Kim S. E., Bahk Y. Y., Holmen S. L., Williams B. O., Chung K. C., Surh Y. J., Choi K. Y. (2006) APC inhibits ERK pathway activation and cellular proliferation induced by RAS. J. Cell Science 119, 819–827 [DOI] [PubMed] [Google Scholar]
- 59. Miclea R. L., van der Horst G., Robanus-Maandag E. C., Löwik C. W., Oostdijk W., Wit J. M., Karperien M. (2011) Apc bridges Wnt/β-catenin and BMP signaling during osteoblast differentiation of KS483 cells. Exp. Cell Res. 317, 1411–1421 [DOI] [PubMed] [Google Scholar]
- 60. Chen H. J., Lin C. M., Lin C. S., Perez-Olle R., Leung C. L., Liem R. K. (2006) The role of microtubule actin cross-linking factor 1 (MACF1) in the Wnt signaling pathway. Genes Dev. 20, 1933–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang Y., Azuma Y., Moore D., Osheroff N., Neufeld K. L. (2008) Interaction between tumor suppressor adenomatous polyposis coli and topoisomerase IIα: implication for the G2/M transition. Mol. Biol. Cell 19, 4076–4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brady M. J., Saltiel A. R. (2001) The role of protein phosphatase-1 in insulin action. Rec. Progr. Horm. Res. 56, 157–173 [DOI] [PubMed] [Google Scholar]
- 63. Dotti C. G., Sullivan C. A., Banker G. A. (1988) The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 8, 1454–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jiang H., Guo W., Liang X., Rao Y. (2005) Both the establishment and the maintenance of neuronal polarity require active mechanisms: critical roles of GSK-3β and its upstream regulators. Cell 120, 123–135 [DOI] [PubMed] [Google Scholar]
- 65. Gärtner A., Huang X., Hall A. (2006) Neuronal polarity is regulated by glycogen synthase kinase-3 (GSK-3β) independently of Akt/PKB serine phosphorylation. J. Cell Sci. 119, 3927–3934 [DOI] [PubMed] [Google Scholar]
- 66. Shi S. H., Cheng T., Jan L. Y., Jan Y. N. (2004) APC and GSK-3β are involved in mPar3 targeting to the nascent axon and establishment of neuronal polarity. Curr. Biol. 14, 2025–2032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.