Abstract
Photophysical studies have been undertaken to characterize the binding interactions of enantiomers of Ru(phen)3(2+), Ru(DIP)3(2+), and racemic Ru(bpy)2dppz2+ (where phen = 1,10-phenanthroline, DIP = 4,7-diphenylphenanthroline, and dppz = dipyridophenazine) with Z-form poly d(GC). Parallel enhancements in steady state luminescent intensity and a lengthening of luminescent lifetimes are seen for ruthenium enantiomers with Z-DNA as for B-DNA but with enantioselectivities reversed. Greater enhancements are seen for delta-isomers with the right-handed helix but for lambda-isomers with the left-handed helix. Ru(bpy)2dppz2+, an avid intercalator in B-DNA, displays no luminescence free in aqueous solution, but luminesces brightly bound to either B- or Z-poly d(GC). Stern-Volmer quenching studies also support the enantioselective preference in binding to B-DNA by delta-isomers and a reversal with binding to Z-DNA preferentially by the lambda-isomers. Steady state polarization studies indicate a rigid association of the complexes with both B- and Z-DNA on the time-scale of their emission and again with symmetrical enantioselectivities for the left and right-handed helices. Given the well characterized intercalative association of the complexes with B-DNA, the parallel results seen here with Z-DNA point strongly to a comparable intercalative association with the Z-form helix. That molecules may interact with Z-DNA through intercalation has not been demonstrated previously and now requires consideration in describing the range of interactions of small molecules and proteins with Z-DNA.
Full text
PDF

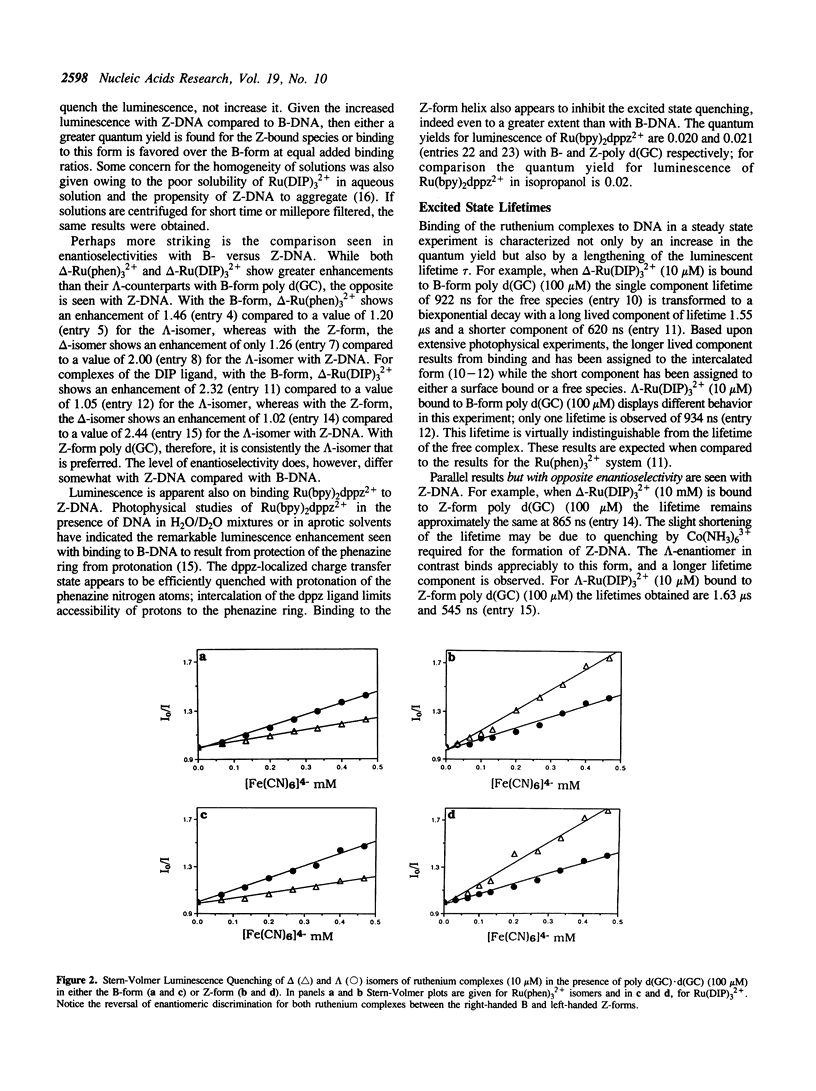
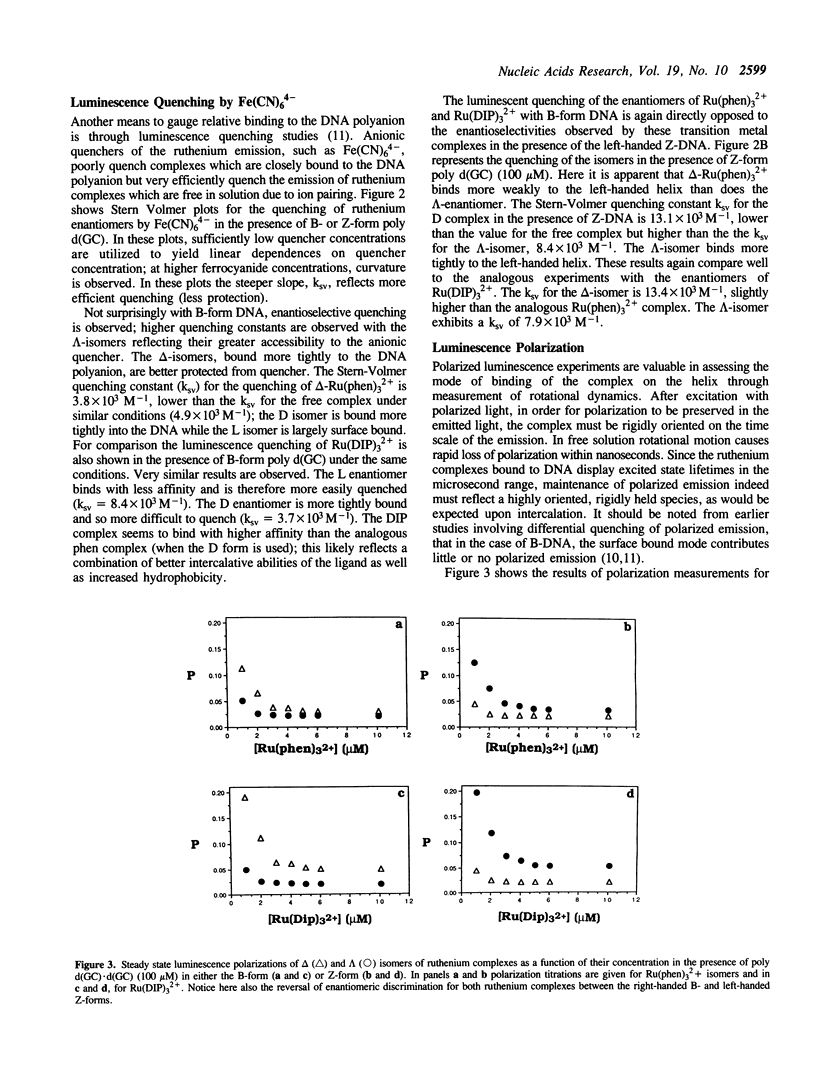
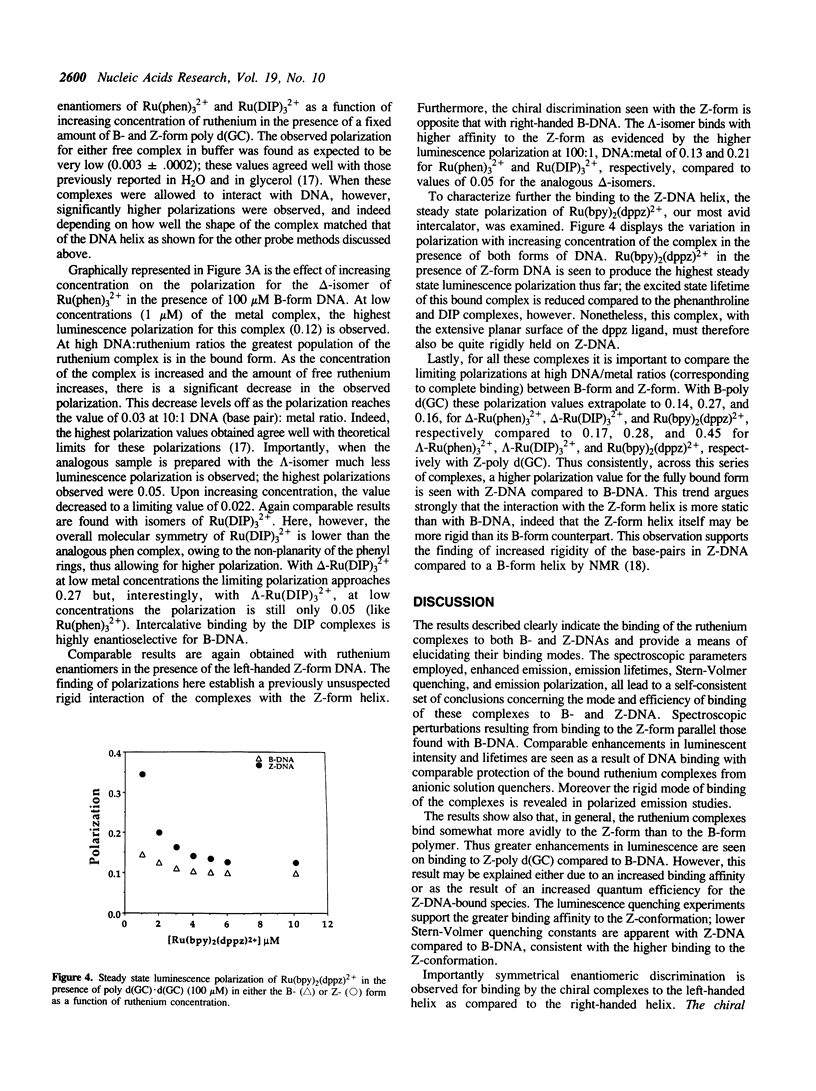


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton J. K., Basile L. A., Danishefsky A., Alexandrescu A. Chiral probes for the handedness of DNA helices: enantiomers of tris(4,7-diphenylphenanthroline)ruthenium(II). Proc Natl Acad Sci U S A. 1984 Apr;81(7):1961–1965. doi: 10.1073/pnas.81.7.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton J. K., Raphael A. L. Site-specific cleavage of left-handed DNA in pBR322 by lambda-tris(diphenylphenanthroline)cobalt(III). Proc Natl Acad Sci U S A. 1985 Oct;82(19):6460–6464. doi: 10.1073/pnas.82.19.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. C., Aggarwal A. K. DNA recognition by proteins with the helix-turn-helix motif. Annu Rev Biochem. 1990;59:933–969. doi: 10.1146/annurev.bi.59.070190.004441. [DOI] [PubMed] [Google Scholar]
- Jain S. C., Tsai C. C., Sobell H. M. Visualization of drug-nucleic acid interactions at atomic resolution. II. Structure of an ethidium/dinucleoside monophosphate crystalline complex, ethidium:5-iodocytidylyl (3'-5') guanosine. J Mol Biol. 1977 Aug 15;114(3):317–331. doi: 10.1016/0022-2836(77)90253-4. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum M. R., Tribolet R., Barton J. K. Rh(DIP)3(3+): a shape-selective metal complex which targets cruciforms. Nucleic Acids Res. 1988 Aug 25;16(16):7943–7960. doi: 10.1093/nar/16.16.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamos M. L., Walker G. T., Krugh T. R., Turner D. H. Fluorescence-detected circular dichroism of ethidium bound to poly(dG-dC) and poly(dG-m5dC) under B- and Z-form conditions. Biochemistry. 1986 Feb 11;25(3):687–691. doi: 10.1021/bi00351a027. [DOI] [PubMed] [Google Scholar]
- Mei H. Y., Barton J. K. Tris(tetramethylphenanthroline)ruthenium(II): a chiral probe that cleaves A-DNA conformations. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1339–1343. doi: 10.1073/pnas.85.5.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirau P. A., Behling R. W., Kearns D. R. Internal motions in B- and Z-form poly(dG-dC).poly(dG-dC): 1H NMR relaxation studies. Biochemistry. 1985 Oct 22;24(22):6200–6211. doi: 10.1021/bi00343a026. [DOI] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Rehmann J. P., Barton J. K. 1H NMR studies of tris(phenanthroline) metal complexes bound to oligonucleotides: characterization of binding modes. Biochemistry. 1990 Feb 20;29(7):1701–1709. doi: 10.1021/bi00459a006. [DOI] [PubMed] [Google Scholar]
- Rehmann J. P., Barton J. K. 1H NMR studies of tris(phenanthroline) metal complexes bound to oligonucleotides: structural characterizations via selective paramagnetic relaxation. Biochemistry. 1990 Feb 20;29(7):1710–1717. doi: 10.1021/bi00459a007. [DOI] [PubMed] [Google Scholar]
- Schevitz R. W., Otwinowski Z., Joachimiak A., Lawson C. L., Sigler P. B. The three-dimensional structure of trp repressor. 1985 Oct 31-Nov 6Nature. 317(6040):782–786. doi: 10.1038/317782a0. [DOI] [PubMed] [Google Scholar]
- Shafer R. H., Roques B. P., LePecq J. B., Delepierre M. Chromomycin A3 binds to left-handed poly(dG-m5dC). Eur J Biochem. 1988 Apr 15;173(2):377–382. doi: 10.1111/j.1432-1033.1988.tb14009.x. [DOI] [PubMed] [Google Scholar]
- Sobell H. M., Tsai C. C., Jain S. C., Gilbert S. G. Visualization of drug-nucleic acid interactions at atomic resolution. III. Unifying structural concepts in understanding drug-DNA interactions and their broader implications in understanding protein-DNA interactions. J Mol Biol. 1977 Aug 15;114(3):333–365. doi: 10.1016/0022-2836(77)90254-6. [DOI] [PubMed] [Google Scholar]
- Steitz T. A. Structural studies of protein-nucleic acid interaction: the sources of sequence-specific binding. Q Rev Biophys. 1990 Aug;23(3):205–280. doi: 10.1017/s0033583500005552. [DOI] [PubMed] [Google Scholar]
- Walker G. T., Stone M. P., Krugh T. R. Ethidium binding to left-handed (Z) DNAs results in regions of right-handed DNA at the intercalation site. Biochemistry. 1985 Dec 3;24(25):7462–7471. doi: 10.1021/bi00346a065. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Nathans J., van der Marel G., van Boom J. H., Rich A. Molecular structure of a double helical DNA fragment intercalator complex between deoxy CpG and a terpyridine platinum compound. Nature. 1978 Nov 30;276(5687):471–474. doi: 10.1038/276471a0. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Zein N., Sinha A. M., McGahren W. J., Ellestad G. A. Calicheamicin gamma 1I: an antitumor antibiotic that cleaves double-stranded DNA site specifically. Science. 1988 May 27;240(4856):1198–1201. doi: 10.1126/science.3240341. [DOI] [PubMed] [Google Scholar]
- Zhang R. G., Joachimiak A., Lawson C. L., Schevitz R. W., Otwinowski Z., Sigler P. B. The crystal structure of trp aporepressor at 1.8 A shows how binding tryptophan enhances DNA affinity. Nature. 1987 Jun 18;327(6123):591–597. doi: 10.1038/327591a0. [DOI] [PubMed] [Google Scholar]
- van de Sande J. H., Jovin T. M. Z* DNA, the left-handed helical form of poly[d(G-C)] in MgCl2-ethanol, is biologically active. EMBO J. 1982;1(1):115–120. doi: 10.1002/j.1460-2075.1982.tb01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


