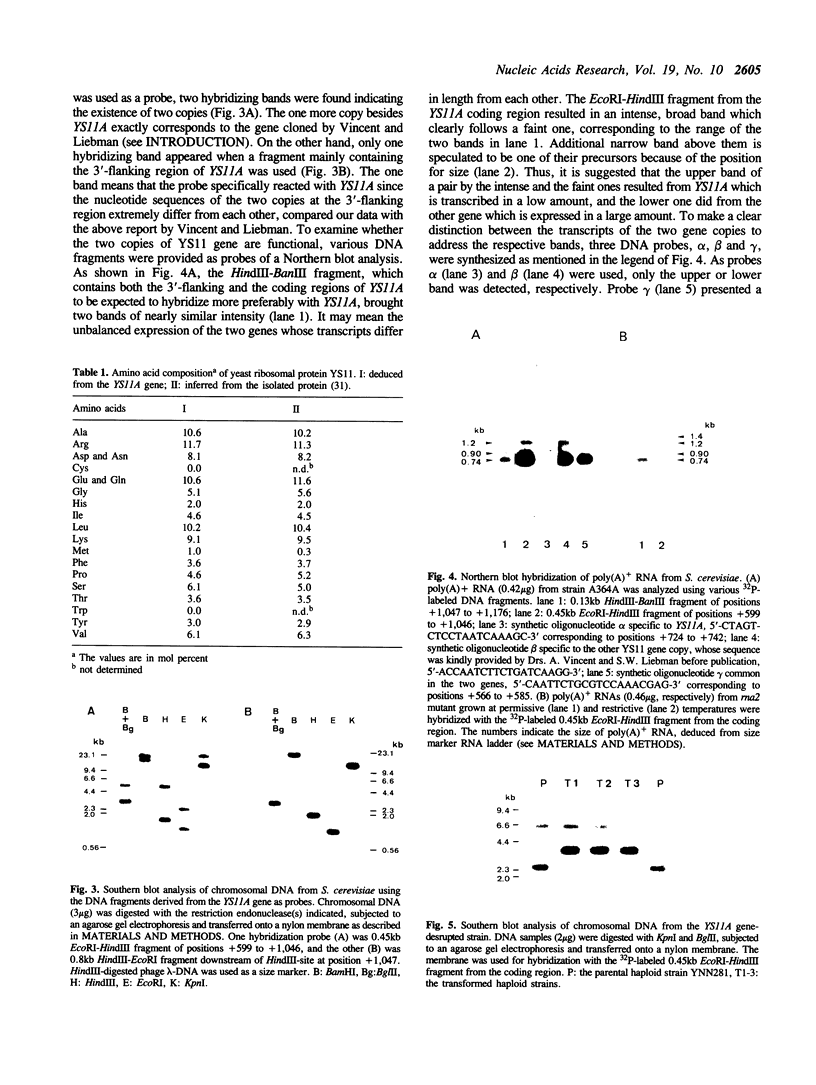
Abstract
We isolated and sequenced a gene, YS11A, encoding ribosomal protein YS11 of Saccharomyces cerevisiae. YS11A is one of two functional copies of the YS11 gene, located on chromosome XVI and transcribed in a lower amount than the other copy which is located on chromosome II. The disruption of YS11A has no effect on the growth of yeast. The 5'-flanking region contains a similar sequence to consensus UASrpg and the T-rich region. The open reading frame is interrupted with an intron located near the 5'-end. The predicted amino acid sequence reveals that yeast YS11 is a homologue to E. coli S4, one of the ram proteins, three chloroplast S4s and others out of the ribosomal protein sequences currently available.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abovich N., Rosbash M. Two genes for ribosomal protein 51 of Saccharomyces cerevisiae complement and contribute to the ribosomes. Mol Cell Biol. 1984 Sep;4(9):1871–1879. doi: 10.1128/mcb.4.9.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- All-Robyn J. A., Brown N., Otaka E., Liebman S. W. Sequence and functional similarity between a yeast ribosomal protein and the Escherichia coli S5 ram protein. Mol Cell Biol. 1990 Dec;10(12):6544–6553. doi: 10.1128/mcb.10.12.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedwell D., Davis G., Gosink M., Post L., Nomura M., Kestler H., Zengel J. M., Lindahl L. Nucleotide sequence of the alpha ribosomal protein operon of Escherichia coli. Nucleic Acids Res. 1985 Jun 11;13(11):3891–3903. doi: 10.1093/nar/13.11.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley S., Hereford L., Rosbash M. Further evidence that the rna2 mutation of Saccharomyces cerevisiae affects mRNA processing. Mol Cell Biol. 1982 Oct;2(10):1205–1211. doi: 10.1128/mcb.2.10.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Higo K., Otaka E. Isolation and characterization of fourteen ribosomal proteins from small subunits of yeast. Biochemistry. 1979 Sep 18;18(19):4191–4196. doi: 10.1021/bi00586a024. [DOI] [PubMed] [Google Scholar]
- Hori H., Osawa S. Origin and evolution of organisms as deduced from 5S ribosomal RNA sequences. Mol Biol Evol. 1987 Sep;4(5):445–472. doi: 10.1093/oxfordjournals.molbev.a040455. [DOI] [PubMed] [Google Scholar]
- Ishiguro J., Ono B. I., Masurekar M., McLaughlin C. S., Sherman F. Altered ribosomal protein S11 from the SUP46 suppressor of yeast. J Mol Biol. 1981 Apr 15;147(3):391–397. doi: 10.1016/0022-2836(81)90491-5. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Otaka E., Matsui K. A. Primary structures of ribosomal protein YS25 from Saccharomyces cerevisiae and its counterparts from Schizosaccharomyces pombe and rat liver. Biochemistry. 1985 Dec 3;24(25):7418–7423. doi: 10.1021/bi00346a058. [DOI] [PubMed] [Google Scholar]
- Jensen R., Sprague G. F., Jr, Herskowitz I. Regulation of yeast mating-type interconversion: feedback control of HO gene expression by the mating-type locus. Proc Natl Acad Sci U S A. 1983 May;80(10):3035–3039. doi: 10.1073/pnas.80.10.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y., Nishikawa K., Takahashi S., Ooi T. Correspondence of homologies in amino acid sequence and tertiary structure of protein molecules. Biochim Biophys Acta. 1982 Feb 18;701(2):242–252. doi: 10.1016/0167-4838(82)90120-0. [DOI] [PubMed] [Google Scholar]
- Leer R. J., van Raamsdonk-Duin M. M., Molenaar C. M., Witsenboer H. M., Mager W. H., Planta R. J. Yeast contains two functional genes coding for ribosomal protein S10. Nucleic Acids Res. 1985 Jul 25;13(14):5027–5039. doi: 10.1093/nar/13.14.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masurekar M., Palmer E., Ono B. I., Wilhelm J. M., Sherman F. Misreading of the ribosomal suppressor SUP46 due to an altered 40 S subunit in yeast. J Mol Biol. 1981 Apr 15;147(3):381–390. doi: 10.1016/0022-2836(81)90490-3. [DOI] [PubMed] [Google Scholar]
- Ono B. I., Stewart J. W., Sherman F. Serine insertion caused by the ribosomal suppressor SUP46 in yeast. J Mol Biol. 1981 Apr 15;147(3):373–379. doi: 10.1016/0022-2836(81)90489-7. [DOI] [PubMed] [Google Scholar]
- Otaka E., Higo K., Itoh T. Yeast ribosomal proteins: VII. Cytoplasmic ribosomal proteins from Schizosaccharomyces pombe. Mol Gen Genet. 1983;191(3):519–524. doi: 10.1007/BF00425772. [DOI] [PubMed] [Google Scholar]
- Otaka E., Higo K., Osawa S. Isolation of seventeen proteins and amino-terminal amino acid sequences of eight proteins from cytoplasmic ribosomes of yeast. Biochemistry. 1982 Sep 14;21(19):4545–4550. doi: 10.1021/bi00262a005. [DOI] [PubMed] [Google Scholar]
- Otaka E., Ooi T., Itoh T., Kumazaki T. Examination of protein sequence homologies: III. Ribosomal protein YS25 from Saccharomyces cerevisiae and its counterparts from Schizosaccharomyces pombe, rat liver, and Escherichia coli. J Mol Evol. 1986;23(4):337–342. doi: 10.1007/BF02100643. [DOI] [PubMed] [Google Scholar]
- Otaka E., Ooi T., Kumazaki T., Itoh T. Examination of protein sequence homologies: I. Eleven Escherichia coli L7/L12-type ribosomal "A" protein sequences from eubacteria and chloroplast. J Mol Evol. 1984;21(4):339–345. doi: 10.1007/BF02115652. [DOI] [PubMed] [Google Scholar]
- Otaka E., Ooi T., Suzuki K. Examination of protein sequence homologies. VI. The evolution of Escherichia coli L7/L12 equivalent ribosomal proteins ('A' proteins), and the tertiary structure. Protein Seq Data Anal. 1989 Aug;2(5):395–402. [PubMed] [Google Scholar]
- Otaka E., Suzuki K., Hashimoto T. Examination of protein sequence homologies. VII. The complementary molecular coevolution of ribosomal proteins equivalent to Escherichia coli L7/L12 and L10. Protein Seq Data Anal. 1990 Mar;3(1):11–19. [PubMed] [Google Scholar]
- Ramirez C., Shimmin L. C., Newton C. H., Matheson A. T., Dennis P. P. Structure and evolution of the L11, L1, L10, and L12 equivalent ribosomal proteins in eubacteria, archaebacteria, and eucaryotes. Can J Microbiol. 1989 Jan;35(1):234–244. doi: 10.1139/m89-036. [DOI] [PubMed] [Google Scholar]
- Rotenberg M. O., Moritz M., Woolford J. L., Jr Depletion of Saccharomyces cerevisiae ribosomal protein L16 causes a decrease in 60S ribosomal subunits and formation of half-mer polyribosomes. Genes Dev. 1988 Feb;2(2):160–172. doi: 10.1101/gad.2.2.160. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Shimmin L. C., Newton C. H., Ramirez C., Yee J., Downing W. L., Louie A., Matheson A. T., Dennis P. P. Organization of genes encoding the L11, L1, L10, and L12 equivalent ribosomal proteins in eubacteria, archaebacteria, and eucaryotes. Can J Microbiol. 1989 Jan;35(1):164–170. doi: 10.1139/m89-025. [DOI] [PubMed] [Google Scholar]
- Song J. M., Picologlou S., Grant C. M., Firoozan M., Tuite M. F., Liebman S. Elongation factor EF-1 alpha gene dosage alters translational fidelity in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Oct;9(10):4571–4575. doi: 10.1128/mcb.9.10.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel L. F., Smyth A., Jacobson A. Nucleotide sequence and characterization of the transcript of a Dictyostelium ribosomal protein gene. Nucleic Acids Res. 1987 Dec 23;15(24):10285–10298. doi: 10.1093/nar/15.24.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A. R., Steinmetz A., Bogorad L. Maize chloroplast DNA encodes a protein sequence homologous to the bacterial ribosome assembly protein S4. Nucleic Acids Res. 1983 Aug 11;11(15):5277–5286. doi: 10.1093/nar/11.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Hashimoto T., Otaka E. Yeast ribosomal proteins: XI. Molecular analysis of two genes encoding YL41, an extremely small and basic ribosomal protein, from Saccharomyces cerevisiae. Curr Genet. 1990 Mar;17(3):185–190. doi: 10.1007/BF00312608. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Otaka E. Cloning and nucleotide sequence of the gene encoding yeast ribosomal protein YS25. Nucleic Acids Res. 1988 Jul 11;16(13):6223–6223. doi: 10.1093/nar/16.13.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K., Inokuchi H., Shiki Y., Takeuchi M., Chang Z., Fukuzawa H., Kohchi T., Shirai H., Ohyama K., Ozeki H. Structure and organization of Marchantia polymorpha chloroplast genome. II. Gene organization of the large single copy region from rps'12 to atpB. J Mol Biol. 1988 Sep 20;203(2):299–331. doi: 10.1016/0022-2836(88)90002-2. [DOI] [PubMed] [Google Scholar]
- Vincent A., Petes T. D. Mitotic and meiotic gene conversion of Ty elements and other insertions in Saccharomyces cerevisiae. Genetics. 1989 Aug;122(4):759–772. doi: 10.1093/genetics/122.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. R. Synthesis of ribosomes in Saccharomyces cerevisiae. Microbiol Rev. 1989 Jun;53(2):256–271. doi: 10.1128/mr.53.2.256-271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]