Abstract
Background
The identification of proteins by mass spectrometry is a standard method in biopharmaceutical quality control and biochemical research. Prior to identification by mass spectrometry, proteins are usually pre-separated by electrophoresis. However, current protein staining and de-staining protocols are tedious and time consuming, and therefore prolong the sample preparation time for mass spectrometry.
Methodology and Principal Findings
We developed a 1-minute covalent pre-gel staining protocol for proteins, which does not require de-staining before the mass spectrometry analysis. We investigated the electrophoretic properties of derivatized proteins and peptides and studied their behavior in mass spectrometry. Further, we elucidated the preferred reaction of proteins with Uniblue A and demonstrate the integration of the peptide derivatization into typical informatics tools.
Conclusions and Significance
The Uniblue A staining method drastically speeds up the sample preparation for the mass spectrometry based identification of proteins. The application of this chemo-proteomic strategy will be advantageous for routine quality control of proteins and for time-critical tasks in protein analysis.
Introduction
Proofing the identity of compounds during the manufacturing chain is a basic obligation in the pharmaceutical industries. Adequate quality control procedures are therefore mandatory and strictly supervised by regulatory bodies [1]. Additionally, fake drugs threaten the health of patients [2]–[4]. This makes additional quality controls necessary for products, which are already in circulation or imported. But also in biological research and development, the confirmation of the identity of molecules is crucial. This is especially true for laboratories working with proteins, since those are usually purified from complex mixtures and difficult to distinguish by their biochemical properties only.
Nowadays, proteins in most of the cases are identified based on mass spectrometry (MS) data, since current MS methods offer high sensitivity, speed and accuracy and hence permit reliable conclusions on the nature of a protein in reasonable time. Moreover, MS methods are applicable to virtually any protein and are not limited to the N-terminal sequence such as Edman sequencing.
Usually proteins need to be pre-separated, before they can be subjected to MS analysis. This is efficiently done by gel electrophoresis, which has the additional advantage to remove low molecular weight contaminants such as salts. For extremely complex samples, such as entire cell disintegrates with several thousand proteins, a two-dimensional gel electrophoresis (2D-GE) is necessary, which separates the proteins first by their isoelectric point and subsequently by their molecular weight [5]. However, 2D-GE is time and labor intense.
For analyzing partially purified or pure proteins, a one-dimensional gel electrophoresis (1D-GE) [6] is sufficient and provides the advantage, that several samples can be run in parallel on a single gel. Considering the much higher possible through-put compared to two-dimensional gel electrophoresis, we focus in our study on one-dimensional gel electrophoreses.
Independently, if 1D-GE or 2D-GE is chosen, the proteins need to be stained or labeled in order to be visible. In some cases, a selective stain might be applicable [7]. But for most cases, a general protein dye needs to be applied. The most sensitive protein stain, which is visible at natural light, is the silver staining. However, it is cumbersome and troubling in mass spectrometry analyses [8]. Therefore, the less sensitive Coomassie stain became the current de facto standard for protein staining [9]. Several protocols for Coomassie staining are given in the literature, which are either optimized for sensitivity, speed or mass spectrometry compatibility [10], [11]. Out of those, the staining with colloidal Coomassie is currently the method of choice, if the samples are intended for later analysis by mass spectrometry. However, considering the quickest protocols, three hours are necessary for colloidal Coomassie staining [11], and another four hours for preparing selected gel pieces for MS [12]–[15]. A significant part of this time is consumed by de-staining steps.
In comparison, the 1D-GE and the MS analysis take only about one to two hours each.
Besides the time issue, many tedious manual steps are necessary for the processing of Coomassie stained gel pieces, which increases the risk of sample contamination, for example by human keratin. Automation of the sample processing is possible, but its costs are considerable and the reliability and flexibility of robots is sometimes not satisfactory.
Altogether, we identified a vast potential for optimization in the sample preparation for mass spectrometry; especially in the protein staining/de-staining procedures. We sought after a protein staining method, which reduces the sample preparation effort before mass spectrometry to a minimum and consequently permits faster protein identification results. The requirements for such a staining method would be: Rapidity, visibility of stained proteins at natural light, compatibility with gel electrophoresis, compatibility with mass spectrometry and current data processing work-flows as well as simple adoption to existing laboratory procedures.
In the following study we demonstrate how those conditions can be met by covalent pre-gel staining of the proteins with Uniblue A.
Results
Covalent staining procedure and electrophoretic properties of derivatized proteins
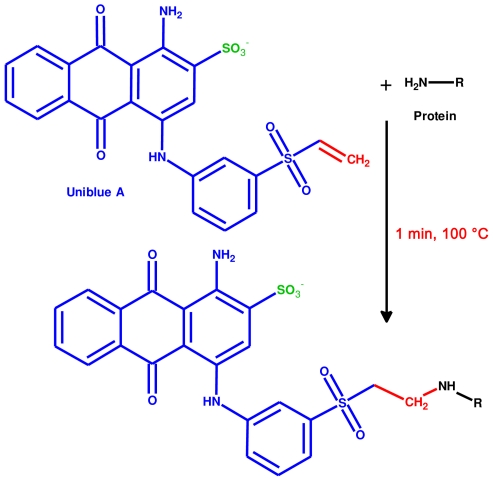
After some theoretical considerations and initial testing of several reactive protein dyes, Uniblue A seemed to be the most promising candidate, due to its solubility in water, commercial availability with adequate purity and low price. Additionally, its blue color aids in achieving a sufficient optical contrast. Uniblue A exhibits broad and intense absorption in the visible range with a maximum at 593.5 nm (Supplemental Fig. S1, λmax = 596 nm according to Sigma Aldrich). This is practically the same absorption maximum as for Coomassie with a λmax of 595 nm [16]. Therefore the same settings for the scanning of gels can be used for obtaining the best contrast. Since we suspected a reaction with amines (Fig. 1) at basic pH, we performed the staining reaction in an amine-free NaHCO3 buffer at a pH of 8–9. We discovered that staining can be obtained at different temperatures, ranging from 37°C up to 100°C. But whereas the reaction requires about 1 hour at 60°C, sufficient covalent pre-gel staining of the protein with Uniblue A can be obtained within only one minute at 100°C (Fig. 2A). Prolonged incubation at this temperature results in thermal protein degradation.
Figure 1. Covalent staining of proteins by nucleophilic addition of Uniblue A.
The vinyl sulfone group (red) reacts with primary amines, preferably on lysine residues. The sulfate group (green) supports the solubility of the dye and affects the ionization properties of the labeled peptide during mass spectrometry measurements.
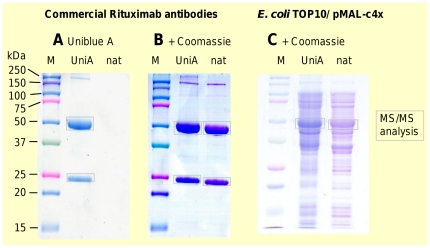
Figure 2. Electrophoretic properties of Uniblue A stained proteins.
A) SDS-PAGE gel showing pre-stained molecular weight marker and Uniblue A (Uni A) derivatized Rituximab antibody chains. The staining was achieved within 1 minute. The third lane contains the equal concentration of un-derivatized Rituximab sample (nat). B) The same gel after subsequent staining with Coomassie, now also revealing the un-derivatized Rituximab sample. Staining intensity and protein profiles are comparable. C) E. coli TOP10, transformed with pMAL-c4x and auto-induced. Uniblue A (Uni A) derivatized and un-derivatized (nat) disintegration sample display comparable protein profiles after Coomassie staining. The assumed recombinant protein band was cut and subjected to nanoLC-MS/MS analysis, confirming the identity with >80% MS/MS based sequence coverage in both samples.
Further steps of the protocol include quenching of excess Uniblue A, reduction and alkylation. Altogether, the sample preparation for the SDS-PAGE can be completed in less than ten minutes. Additionally to the blue protein bands, also the reaction products with Tris buffer are visible and serve as running front indicator. Those low-molecular compounds disappear rapidly during the fixing of the gel.
For recombinant cystatin we determined a quantitative sensitivity of about 1 µg protein. This is less sensitive than current Coomassie staining protocols. However, the Uniblue A derivatization is fully compatible with subsequent Coomassie staining. Therefore, the intensity of protein bands gels can be increased by double-staining, if required (Fig. 2B, C and S3). Clearly, several of the advantages of the Uniblue A protocol would be lost after sequential staining, in particular the saving of analysis time. However, even assuming insufficient staining by Uniblue A for some samples, only a few minutes are required for the sample preparation, which is very little in comparison to several hours, which generally can be saved. As an analytical strategy, several lanes of the same sample, with and without prior Uniblue A derivatization, can be run in the same gel. In this approach, one lane with Uniblue A derivatized proteins could be used as internal standard for the progress of electrophoresis and for rapid identification by MS, whereas the other lanes of derivatized or un-derivatized protein could be subsequently stained, in order to evaluate purity.
The apparent molecular weights of pre-stained and un-labeled Coomassie stained proteins are in agreement (see Fig. 2B, C and S2). Hence, the electrophoretic mobility of the proteins is not changed significantly by their covalent staining, which is in congruence with previous studies employing dabsyl chloride [17] or Remazol dyes [18]. Presumably, these small appendices do not contribute to the binding of SDS.
On the other side, the negatively charged Uniblue A does strongly influences the isoelectric point of the derivatized proteins. Modified proteins are shifted towards the basic region of a 2D gel. Even prolonged isoelectric focusing does not result in defined spots (Fig. S4). The 2D analysis also reveals that increased derivatization leads to more diffuse spots, although the apparent molecular weight is not affected significantly.
Sensitivity and resolution are reduced for pre-stained proteins, but protein patterns of pre-stained and un-labeled Coomassie stained proteins are comparable, as demonstrated for the Escherichia coli disintegrate (Fig. 2C).
For SDS-PAGE gels intended for subsequent mass spectrometric analyses, the staining intensity and the resolution are perfectly adequate.
Shortened work-up for mass spectrometry and peptide tracking
De-staining is not required for the work-up of gel pieces. Also reduction and alkylation can be skipped, since those steps are already integrated into the SDS-PAGE sample preparation. In comparison to the current best-in-class methods, the staining time could be reduced from three hours to less than ten minutes, and the sample work-up time from four hours to about two hours. In total, the required sample processing time was condensed to less than a third, and the manual handling steps could be significantly reduced, which reduces the risk of contamination. No stain particles are present, which reduces the chance of blockages which occasionally occur in the NanoLC analysis of Coomassie stained samples.
Tagged proteins and peptides display color in the visible spectrum and their fate can be tracked visually. This allows for the direct monitoring of sample processing steps, such as extraction and re-dissolution of peptides. This feature facilitates optimization and validation of sample preparation methods.
The additional sulfate group increases the solubility of derivatized proteins and peptides, which supports their extraction, especially in cases of very hydrophobic species.
Elucidation of amino acid serving as reaction partner for Uniblue A
Uniblue A contains a single vinyl sulfone group that may react with primary amines via nucleophilic addition (Fig. 1). Covalently modified residues will have a defined monoisotopic mass shift of 484.0399 Da.
However, also other potential reaction partners such as sulfhydryl groups or hydroxyl groups might be possible. The actual reaction needed to be evaluated by mass spectrometry data. Therefore the data sets were run allowing for potential Uniblue A modifications on lysine, cysteine, asparagine, glutamine, threonine, arginine, and tyrosine. Surprisingly, it turned out, that under the given conditions only lysine residues were derivatized, but neither other amine containing residues such as asparagine and glutamine nor alternative functional groups. Therefore it can be suggested that the ε-amino group of lysine is the preferred reaction partner for a nucleophilic addition of Uniblue A. Moreover, only a fraction of the lysines was derivatized. Based on assigned peptide spectra, up to 17 Uniblue A modifications were found for BSA, a lysine rich protein (Tab. 1). Surprisingly, only few or no Uniblue A modifications were found for other samples, although the protein was successfully stained, as evaluated visually from the SDS-PAGE gels. This indicates that the sensitivity is reduced for Uniblue A derivatized peptides. But it also has to be kept in mind, that this quantification method for judging the ratio of modified peptides, the so-called “spectral counting”, is limited and might show huge variations, especially when it comes to low abundance peptides [19]. On the other side, when detected, Uniblue A modified peptides exhibit a different, chemically assisted, fragmentation behavior, which supports their evaluation with high significance, as discussed below.
Table 1. NanoLC-MS/MS identification results for gel bands of proteins.
| Bovine serum albumin | Rituximab, heavy chain | Rituximab, light chain | lacZ-á from E.coli | ||||||||
| Uni A | Uni A+ | Coom | Uni A | Uni A+ | Coom | Uni A | Uni A+ | Coom | Uni A | Coom | |
| Coom | Coom | Coom | |||||||||
| MS/MS spectra | 2898 | 2851 | 2778 | 2905 | 2818 | 2852 | 2887 | 2818 | 2869 | 2980 | 2838 |
| ProteinProphet Probability | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| MS/MS Sequence coverage | 32.5% | 52.1% | 40.2% | 27.7% | 26.0% | 41.6% | 33.8% | 58.1% | 62.9% | 82.3% | 83.8% |
| Total ident. peptides | 43 | 90 | 93 | 19 | 14 | 102 | 20 | 55 | 125 | 111 | 120 |
| Uniblue A deriv. | 0 | 17 | N.A. | 0 | 0 | N.A. | 0 | 0 | N.A. | 6 | N.A. |
| PRIDE accession # | 12567 | 12565 | 12564 | 12571 | 12569 | 12568 | 12575 | 12573 | 12572 | 13516 | 13515 |
Proteins were either covalently labeled with Uniblue A (Uni A) before electrophoresis or stained with Coomassie (Coom) after electrophoresis. Also sequential staining with both methods was applied (Uni A+Coom). The bands of interest were cut, tryptically digested and subjected to nanoLC-MS/MS identification (see Methods).
These data are crucial for the design of efficient database searches, since only one potential modification site, namely +484.0399 Da at lysine, has to be considered.
The successful staining of cysteine-free recombinant cystatin adds biochemical proof that the staining does not depend on the presence of cysteine (Fig. S2, S3, S4).
Automated and manual MS/MS data evaluation and integration of modification into standard bioinformatic work-flows
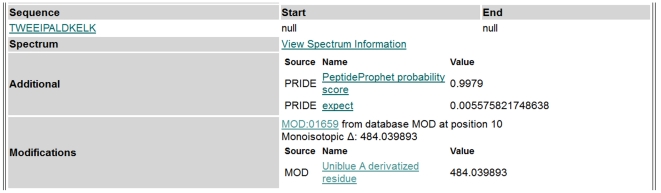
Raw MS/MS data were converted into mzXML and evaluated automatically (see Materials and Methods). In short, the search was performed against a concatenated target-decoy database [20] using the Open Mass Spectrometry Search Algorithm [21] (OMSSA). The peptide hits were validated by PeptideProphet [22] and ProteinProphet [23]. After this automatic processing, the raw data and identification results could be easily converted into valid PRoteomics IDEntifications database [24] (PRIDE) XML, using the PRIDE converter tool [25], and uploaded to the repository. Covalent derivatization with Uniblue A has been added by the PRIDE team as a protein modification (PSI-MOD) for the ontology lookup service (OLS) with the comma separated value (CSV) term MOD: 01659. Figure 3 shows the entry of an identified peptide with Uniblue A modification as deposited in the PRIDE database. This peptide was assigned with high significance, expressed by an X!Tandem E-value of 0.0056 and a PeptideProphet probability score of 0.9979.
Figure 3. Annotation of a Uniblue A derivatization in the PRIDE repository.
The peptide TWEEIPALDKELK was modified at position 10 (MOD:01659) and belongs to the identified recombinant protein MALELZ|MALELZ_LACZA|MALE-lacZ of experiment # 13516.
Trypsin requires a basic arginine or lysine side chain for substrate recognition [26]. Even small chemical modifications that remove the charge, such as methylation or acetic anhydride acetylation result in inhibition of the trypsin activity [27], [28]. Consequently, at positions with Uniblue A derivatized lysines no proteolytic cleavage could be found.
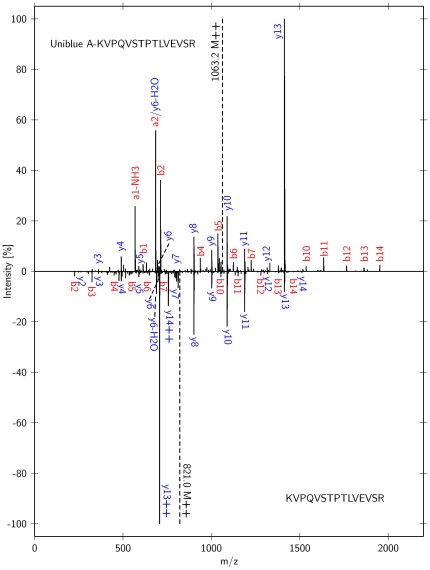
During mass spectrometric analysis, tagged and un-tagged peptides exhibited slightly different behavior. In general, the Uniblue A modification has a tendency to reduce the charge state of the molecules in positive ionization mode due to its negative sulfate group. Figure 4 compares the fragmentation spectrum of a doubly charged Uniblue A derivatized peptide with the fragmentation spectrum of a triply charged untagged peptide of the same sequence. Both spectra were found in the same sample (BSA_dry, PRIDE accession #11793, scans 820 and 1547). In this example, the N-terminal lysine of the tryptic peptide is derivatized. The mass shift allows the clear assignment of the N-terminal fragment ions a1-NH3 and b1, which otherwise would be outside the mass range. Whereas the position of the C-terminal y-ions was not affected, all N-terminal a/b-series ions were shifted, which facilitates the assignment of the peaks b10 to b14. Additionally, the signal-to-noise ratio of N-terminal ions was significantly improved. Altogether more fragment ions can be assigned automatically for the Uniblue A derivatized peptide.
Figure 4. MS/MS fragmentation of an Uniblue A derivatized peptide.
The direct comparison of MS/MS fragmentation spectra of Uniblue A derivatized (top) versus un-labeled (bottom) peptide KVPQVSTPTLVEVSR displays significantly increased signal intensities for the derivatized N-terminal ions (a- and b- series, shown in red). The defined mass shift of 484.0399 m/z for modified residues allows the detection of fragments, which otherwise would be outside the measuring range (fragments a1-NH3 and b1).
This finding was further investigated by comparing MS/MS spectra of native and Uniblue A derivatized peptides in the same sample, to exclude other influences such as different concentrations or run-to-run variability. Three MS/MS spectra pairs are provided as Supplemental Spectra S1 . Especially for short peptides the derivatized peptide spectra provide more evaluable and assignable signals. The E-value of individual peptide identifications was typically improved by at least one order of magnitude for the derivatized version of the peptide compared to the native one.
Comparison of protein identification results after Uniblue A staining and Coomassie staining
To examine the overall performance of this rapid covalent derivatization protocol in comparison to the standard Coomassie based strategy, we compared the identification results after PeptideProphet/ProteinProphet validation (Tab. 1). Both methods yield identification results which comply with strict acceptance criteria. All proteins were identified with a ProteinProphet probability of 1.0000. At least 6 unique peptides were proven and the MS/MS based sequence coverage was at least 26% in all procedures.
This is remarkable, since analytically challenging proteins have been employed for this study. Bovine serum albumin undergoes post-translational proteolytical processing and contains 17 disulfide bonds. Further, three phosphorylation sites and a copper binding site are probable [29]. Its high lysine content might support the staining, but increase at the same time the data complexity due to partial cleavage events. The Rituximab mouse-human chimeric antibody on the other side, consists of 2×2 subunits, which are connected by disulfide bonds [30]. Additionally, glycosylations might be present. The successful identification of those real-life samples underlines the practical usability of our method.
The reduced number of identified peptides when using only Uniblue A is probably caused by matrix suppression effects during the mass spectrometry, since the samples are washed for less time compared to the Coomassie protocol. This was confirmed by the analysis of samples which were first derivatized with Uniblue A and after electrophoresis stained with Coomassie. For two of the three samples, the double staining led to a dramatically increased number of validated peptides, whereas in only one case the number remained about the same. This demonstrates that Uniblue A derivatization is in principle compatible with mass spectrometry based protein identification. Optimized protocols that address sample-to-sample variation and matrix suppression might further improve possible sequence coverage results.
Application of Uniblue A derivatization method to complex samples
To prove the suitability of the method for complex samples, we applied the covalent derivatization to disintegrates of Escherichia coli cells producing a recombinant protein. Uniblue A derivatized and Coomassie stained samples exhibit the sample protein profile (see Fig. 2C), underlining the suitability of the method e.g. for expression clone screening. The supposed recombinant protein at approximately 50 kDa (theoretical molecular weight from sequence: 50,871 Da) was cut and subjected to NanoLC-MS/MS, yielding an excellent MS/MS based sequence coverage above 80% in both cases. Since for some parts of the sequence the data are complementary, the combined MS/MS sequence coverage reaches 92.0% (Fig. S5). Further, the overall detection of proteins in the putative MalE-lacZα bands was compared, to investigate the sensitivity for low abundance proteins (Fig. 5). Applying a ProteinProphet threshold of 0.9, 12 proteins were identified with both staining strategies. Another 13 proteins were found only in the Uniblue A pre-stained band, compared to 8 proteins, which only were detected in the Coomassie stained band. Altogether it can be concluded that both staining strategies exhibit comparable sensitivity for low abundance proteins, and that the staining methods complement each other.
Figure 5. Comparison of identified proteins in the putative E. coli MalE-lacZα band.
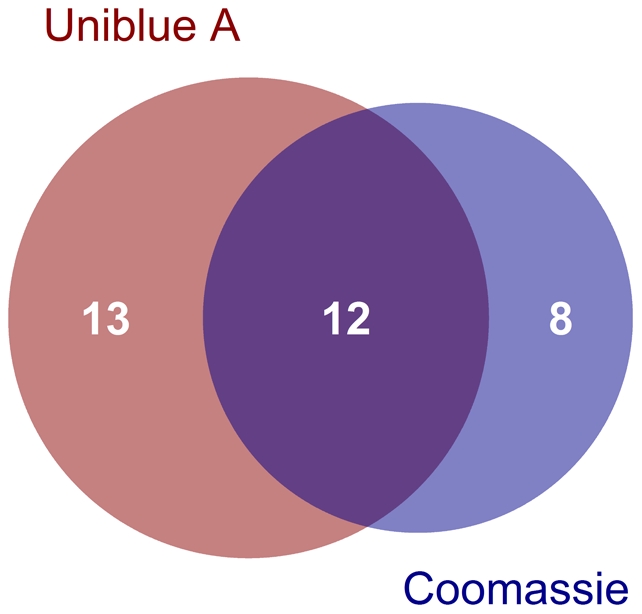
Different proteins were identified from the Uniblue A and the Coomassie stained band of the over-expressed protein. Applying a ProteinProphet score of 0.9 as threshold, 12 proteins were found with both staining methods. 13 proteins were exclusively detected with the Uniblue A staining method, 8 proteins only with the Coomassie staining.
Discussion
In our study we present a protocol for the rapid staining of proteins and their subsequent analysis by SDS-PAGE and mass spectrometry. The method is applicable for pure proteins as well as for complex mixtures. The SDS-PAGE based protein profiles of derivatized samples are comparable with those of un-derivatized samples in one-dimensional gel electrophoresis, indicating that the electrophoretic mobility in SDS-PAGE is not significantly affected. This was already demonstrated for other covalent protein dyes, namely dabsyl chloride [17] or Remazol dyes [18]. Uniblue A derivatization is not compatible with two-dimensional gel electrophoresis, since the isoelectric properties of the proteins are compromised. The resolution of Uniblue A derivatized proteins is slightly reduced for the 1D-GE, and the staining is less intense compared to standard Commassie staining protocols. However, for the localization of abundant proteins in a 1D-GE gel for subsequent mass spectrometry analysis, those restrictions are not critical. The rapidity of the protocol allows for the “at-line” analysis of proteins. This can be applied for example in the monitoring of the enzymatic cleavage of recombinant fusion proteins.
The required time for sample work-up prior to mass spectrometric analyses can be reduced drastically. Additionally, the visibility of the modified proteins and peptides enables their direct tracking and facilitates the optimization and validation of protocols.
By allowing for different potential derivatization sites in the database search, we could prove that the nucleophilic addition with Uniblue A reaction was highly selective for lysine residues. Integrating the defined mass shift +484.0399 Da at lysine into proteomics software tools enabled automated data evaluation of data sets generated after Uniblue A derivatization. The EBI RESID database for protein modifications does not contain any modification, which would result in a monoisotopic weight correction of 480 to 490 Da [http://www.ebi.ac.uk/RESID/; RESID-CWeightp#480:490]. Therefore the false annotation of a Uniblue A modification is impossible.
Previous studies have demonstrated that sulfonation at the N-term of peptides supports the generation of fragmentation spectra with improved y-series and therefore facilitates peptide identification and de-novo sequencing by MALDI-PSD-MS and ESI-MS [31], [32]. The same phenomenon can be observed after derivatization of peptides with Uniblue A, which also contains a sulfone side group, although the derivatization could take place at various positions of a peptide, depending on the location of the lysine. Consequently, Uniblue A tagging is beneficial for automatic or manual peptide identification.
The inhibition of tryptic cleavage at derivatized lysines can be employed to generate longer tryptic peptide fragments. Some peptides, such as YENGKYDIK and KFEKDTGIK in PRIDE Experiment #13516 (MalE-lacZα) only were identified as Uniblue A derivatized peptide. Covalent lysine derivatization by using propionic anhydride, resulting in the neutralization of the charge and thus blocking trypsin cleavages, is employed in the study of histone modifications [33]. Combining tryptic digestion MS/MS data sets of Coomassie stained and Uniblue A derivatized samples improved the total MS/MS based sequence coverage of a recombinant protein. Further, different proteins of lower abundance could be detected. Both findings indicate that those two different staining methods are complementary.
The “peptidic diversity” of tryptic digestions of Uniblue A derivatized proteins is increased by partial derivatization, inhibition of derivatized lysine cleavage sites, different chromatographic properties between un-derivatized and labeled peptides, defined mass shifts during mass spectrometry and different ionization behavior. In theory, this reduces on one side the sensitivity for the mass spectrometric analysis, but on the other side supports improved LC-MS based sequence coverages. The sensitivity of current mass spectrometry instrumentation is already extraordinary high and should not represent a bottleneck. From a practical point of view, we did not experience technical problems in the detection and identification of peptides. The employed algorithms deal well with the potential modifications and the identifications were executed by the automated scripts in about 20 minutes using standard computers.
Further, the enhanced solubility of derivatized proteins and peptides might be used for the analysis and recovery of highly hydrophobic proteins.
Our proposed method is primarily thought to provide a novel and rapid interface between gel electrophoresis and mass spectrometry. This should be especially interesting for the pharmaceutical quality control, but also for speeding up the protein analysis in research and development. Further, the covalent staining has a number of implications concerning the biophysical properties of derivatized proteins and peptides, which can be exploited for defined analytical questions.
Additionally, we invite to think about novel strategies in the development of really mass spectrometry compatible stains and provide a suitable chemical strategy.
Materials and Methods
Cultivation of Escherichia coli TOP10/pMAL-c4x and production of recombinant protein
E. coli TOP10/pMAL-c4x (transformed by electroporation) was grown on Overnight Express™ Instant TB Medium (Novagen Inc.). 50 mL of sterile Overnight Express medium containing 50 µg/ml carbenicillin were given into a 100 mL Erlenmeyer flask and inoculated with 100 µL of a 50% glycerol stock solution of recombinant E. coli TOP10/pMAL-c4x (stored at −80°C). The cells were grown for 16 hours at 37°C and 250 rpm. The production of the gene product of the vector, a MalE-lacZα fusion protein was auto-induced after consumption of glucose and the following growth on lactose. The cultivation broth was harvested by centrifugation at 4°C. The pellets were stored at −20°C.
Disintegration of E. coli and TCA/acetone precipitation of intracellular proteins
The E. coli pellet was re-suspended in 1 mL of 100 mM carbonate buffer (derivatization buffer solution), transferred into a 1.5 mL Eppendorf tube and sonicated for 5 min. The suspension was clarified by centrifugation at 4°C. Subsequently, 0.1 mL of ice-cooled TCA/acetone solution (1 g/mL TCA in acetone) was added to 0.9 mL of supernatant. This mixture was kept at 4°C for 2 h and centrifuged for 10 min in a pre-cooled micro-centrifuge. The supernatant was discarded and the pellet was washed three times with 1 mL of 90% acetone. Excess of acetone was eliminated by drying and the pellet was re-suspended in 100 µL of 100 mM carbonate buffer (derivatization buffer solution).
Buffer exchange by ultrafiltration: Preparation of Rituximab antibodies
Commercial MabThera® Rituximab (Hoffmann- La Roche, S.A.) was pre-treated by ultrafiltration. 250 µL of Rituximab (500 mg/50 ml) and 250 µL of 100 mM carbonate buffer (derivatization buffer solution) were placed into a 0.5 ml 3,000 MWCO Amicon® Ultra centrifugal filter unit (Millipore™). After 30 min centrifugation at 14,000× g, the permeate was discharged and new carbonate buffer was added to the retentate. This procedure was repeated at least five times. Finally, the Rituximab retentate was resuspended in 125 µL of 100 mM carbonate buffer. The final concentration of Rituximab was 20 mg/mL.
Production and purification of recombinant amaranth cystatin
Recombinant amaranth cystatin (see Supplemental Sequence S1) was produced and purified as previously described [34]. Briefly, E. coli M15 (pREP 4) cells transformed with the pQE-2 vector, containing the amaranth cystatin coding sequence, were grown under agitation at 37°C in Super Broth medium, containing 100 µgmL-1 of carbenicillin and 25 µgmL−1 of kanamycin, until they reached an OD600 of 0.5. Cystatin expression was induced by the addition of 0.1 mM IPTG and the cells were harvested after 5 h by centrifugation. The amaranth cystatin was purified from cell lysates using an affinity nickel resin column that was previously equilibrated with 50 mM NaH2PO4 buffer containing 300 mM NaCl and 10 mM imidazole (pH 8.0). The cystatin was eluted by 250 mM imidazole, dissolved in the same buffer. The purified cystatin was exhaustively dialyzed against water in a microdialysis system (BRL Life technologies, Inc.) with a molecular weight cut-off of 1,000 Da and concentrated in a Savant speedVac vacuum. The protein concentration of the purified cystatin was determined by the BioRad microassay, using serum albumin as standard. Theoretical properties of the recombinant cystatin were calculated using ProtParam [35].
Uniblue A stain for visualization of proteins
10 µL of 200 mM Uniblue A (Sigma-Aldrich, #298409) solution in derivatization buffer, consisting of 100 mM NaHCO3 and 10% SDS, pH 8–9, were added to 90 µL protein solution. Following the sample were heated at 100°C for one minute to perform the staining. Subsequently 100 µL of reducing solution composed of 10% glycerol and 20 mM dithiotreitol (DTT) in 200 mM Tris buffer with pH 6.8, were added in order to reduce cysteins and to adjust the pH for the electrophoresis. Excess Uniblue A reacts with Tris, resulting in a blue compound, which serves as running front indicator in electrophoresis. The sample was heated another minute at 100°C in order to achieve efficient reduction and allowed to cool to room temperature. Subsequently, 20 µL alkylation solution containing 550 mM iodoacetamide (IAA) was added. After 5 minutes incubation time the samples were subjected to SDS-PAGE.
Dry protein samples or samples in compatible buffers (i.e. free of amines) can be diluted directly with the derivatization buffer solution to a protein concentration of 5 mg/mL. In other cases, a prior trichloroacetic acid (TCA)/acetone precipitation or buffer exchange by ultrafiltration is recommended (see below).
As positive control, bovine serum albumin (BSA) in derivatization buffer was used at a concentration of 10 mg/mL.
1D-GE, SDS-PAGE
SDS-PAGE was carried out according to the methods of Laemmli [6] and Sambrook [36]. Different concentrations of cystatin and the Uniblue A stained cystatin (10–0.1 µg) were analyzed in SDS–PAGE 12.5%. The protein in the gel was fixed in 40% (v/v) ethanol and 10% (v/v) acetic acid for 20 min and stained with PhastGel Blue R-350 (Amersham, BioScience) following the supplier's instructions. The Uniblue A stained cystatin was detected directly in the gel.
Two-dimensional (2D) gel electrophoresis
Two-dimensional gel electrophoresis was performed according to the method of Bjellqvist et al. [37]. Dry IPG strips (7 cm long, pH 3–10 linear) were rehydrated at 20 0C for 14 h in 125 µl of isoelectric focusing buffer (7 M urea, 2 M thiourea, 20 mM DTT, 4% CHAPS, 0.5% ampholite 3–10, 0.001% bromophenol blue), containing 1 µg of protein sample. IEF was conducted with an Ettan IPGphor II system (Amersham Biosciences). Focusing was carried out in four steps: 250 V for 1 h, 500 V 0.5 h followed by 1000 V 0.5 h, and finally 8000 V for 2.5 h. After focusing, the gels were equilibrated twice for 15 min in equilibration solution. The first equilibration was performed in a solution containing 6 M urea, 30% w/v glycerol, 2% w/v SDS, 0.001% bromophenol blue, 50 mM Tris-HCl buffer, pH 8.8 and 1% w/v DTT. The second equilibration solution was modified by the replacement of DTT by 2.5% w/v iodoacetamide. For the second dimension, the proteins were separated on 12.5% SDS polyacrylamide gels. Protein spots were visualized using PhastGel Blue R-350 (Amersham Biosciences).
In-gel digestion for protein bands
For in-gel digestion of protein bands, the protocols of Shevchenko [14], [38] have been adopted with few modifications. In-gel reduction/alkylation is not required since this step is already included in the SDS-PAGE sample preparation.
After the SDS-PAGE, the Uniblue A stained bands could be cut directly from the gel and chopped into cubes with about 1 mm of edge length. The cubes were transferred to vials and covered by acetonitrile. Typically the cubes became whitish and shrunk after about 5 minutes. If not, the acetonitrile solution was exchanged one or two times. The shrunk gel pieces were dried in a vacuum centrifuge. The dry gel pieces were re-hydrated in 10 ng/µL trypsin solution (Promega V511A in 10 mM ammonium bicarbonate) and incubated for 30 min at 60°C. Previous studies had shown that the reductive methylation of Promega trypsin shifts its catalytic optimum to 50–60°C. Therefore, similar peptide yields can be obtained after 30 min digestion at elevated temperatures, compared to overnight digestions at 37°C [39]. After tryptic digestion, the peptides could be extracted by addition of an acetonitrile: 5% trifluoroacetic acid mixture (2∶1) and incubation for 15 min at 60°C.
The extraction solution was collected into a new tube and dried in a vacuum centrifuge. Prior to LC-MS/MS analysis, the peptides were dissolved in 20 µL of 0.1% (v/v) formic acid.
Gels were washed and fixed, if they were going to be scanned or stored. First, the gel was shaken for 5 min in a solution containing 40% methanol and 10% acetic acid, following for 20 min in a solution containing 10% methanol and 7.5% acetic acid. Finally, the gel was washed with the first solution for at least 3 h, until the excess of colorant was eliminated.
NanoLC-MS/MS measurements
NanoLC-MS/MS analyses were performed on an Agilent 1100 HPLC sytem with nanoLC-ChipCube, coupled with an Agilent LC/MSD Trap XCT Ultra. For instrument control the vendor's programs ChemStation Rev.B.01.03 and TrapControl version 6.1 were used. Solvent A for chromatography was 0.1% formic acid, solvent B 99% acetonitrile in 0.1% formic acid. 8 µL of sample were loaded with a flow of 4 µL/min solvent A on the 40 nL enrichment column of a Agilent G4240-62001 chip column. The flow rate for the analytical chromatography was 0.3 µL/min. After 5 min washing with 3% solvent B, the flow path was changed to the analytical column (43 mm×75 mm, Zorbax 300SB-C18, 5 mm). During the following 27 min the solvent B concentration was increased to 45%. Following the column was cleaned by a 3-minute gradient to 95% solvent B and re-equilibrated 6.5 min with initial conditions. The total time for the chromatography method was 42.5 min. The electron spray ionization was enabled by a capillary voltage of 1,900 V and a nitrogen gas flow of 4 L/min at 325°C. Parent spectra were measured in positive mode, standard-enhanced with an integrated ion current (ICC) smart target setting of 200,000 and a maximal accumulation time of 100 ms. The scan range was from 200 to 1400 m/z. Collision induced dissociation (CID) fragmentation was performed automatically with preference for multiply charged precursor ions. The fragmentation energy was adjusted online by smart parameter setting.
Evaluation of nanoLC-MS/MS data
In order to enable a target-decoy search strategy [20] with estimation of false positive rates (FDR), a decoy database with reverse sequences was generated and merged with the original one. The forward database was constructed by using the entries of the SwissProt database, supplemented by the sequences of the Rituximab chains, as reported in DrugBank [40] (accession number DB00073), and the theoretical amino acid sequence of the pMAL-c4x vector gene product, MalE-lacZα (sequence derived from technical information of New England BioLabs). The final search data base consisted of approx. 1,000,000 entries.
Agilent XCT ultra *.yep data files were converted to *.mzXML files using the CompassXport 3.0.x program, provided by Bruker. Following the mzXML files were automatically processed by a DOS BATCH script OMSSAVALIDATION.BAT (see below), employing OMSSA 2.1.x [21] as database search engine and PeptideProphet [22] and ProteinProphet [23], versions of Trans-Proteomic Pipeline v4.x [41] for hit validation.
To account for the additional potential protein modification on lysine with Uniblue A, an additional entry was defined in the usermods.xml file in the OMSSA directory:
<MSModSpec>
<MSModSpec_mod>
<MSMod value = “usermod3”>121</MSMod>
</MSModSpec_mod>
<MSModSpec_type>
<MSModType value = “modaa”>0</MSModType>
</MSModSpec_type>
<MSModSpec_name>Uniblue A on K</MSModSpec_name>
<MSModSpec_monomass>484.039891</MSModSpec_monomass>
<MSModSpec_averagemass>484.5016</MSModSpec_averagemass>
<MSModSpec_n15mass>0</MSModSpec_n15mass>
<MSModSpec_residues>
<MSModSpec_residues_E>K</MSModSpec_residues_E>
</MSModSpec_residues>
</MSModSpec>
The modification 121 now is known by OMSSA as “Uniblue A on K” and identified by a mass shift of 484.039891 Da monoisotopic mass, or 484.5016 Da average mass, respectively.
For OMSSA searches, two missed cleavages were allowed, “carbamidomethyl on cysteine” was defined as fixed modification and “deamidation on glutamine/asparagine” and “Uniblue A on lysine” as variable modifications. “Oxidation on methionine” could be omitted, since no methionine oxidation was found in exploratory data base searches. This finding can be attributed to the rapid sample work-up. To allow for subsequent validation of hits by PeptideProphet/ProteinProphet, the e-value was set to 1E6. Precursor and fragment mass tolerances were left at the OMSSA default values, i.e. 2.0 Da for the precursor and 0.8 Da for the fragment masses.
For the PeptideProphet/ProteinProphet hit validations, a minimal peptide length of 5 amino acid residues was specified and a non-parametric validation model based on decoy results.
Raw data and identification results were converted into standard-compliant PRIDE XML using the PRIDE converter, v2.x [25], applying a ProteinProphet probability cut-off of 0.9 and a cut-off for peptides of 0.05. This PRIDE XML files were submitted to the PRIDE server (http://www.ebi.ac.uk/pride/), where the data can be found in the project “Rapid pre-gel visualization of proteins with mass spectrometry compatibility”. Covalent derivatization with Uniblue A has been added by the PRIDE team as a protein modification (PSI-MOD) for the ontology lookup service (OLS) with the comma separated value (CSV) term MOD: 01659.
Manual calculations of fragmentation spectra were conducted with mmass 3.7 [42].
DOS BATCH script for automated processing of mzXML files
For automated database search and hit validation a DOS BATCH script called OMSSAVALIDATION.BAT has been written. Provided, that the necessary software is installed, the following code can be pasted into a *.bat file and executed on all *.mzXML files of a directory.
@ECHO OFF
ECHO *** Converting mzXML files to mgf ***
FOR %%I IN (*.mzXML) DO MzXML2Search -mgf -ACID -M2-2 -P5 -I0.01 -B10 -T5000.0%%I
ECHO *** OMSSA search: Decoy database, trypsin, pep.xml output ***
REM decoy entries in database customdb start with decoy_; reverse decoy database generated with FastaTools 0.9 and merged with original database using mergeFasta.pl
FOR %%I IN (*.mgf) DO omssacl -d c:\blastdb\customdb -fm %%I -v 2 -mf 3 -mv 4,121 -he 1e6 -w -op %%I_OMSSA_TPP.pep.xml
ECHO *** Fix linking of MS/MS spectra ***
sed -i “s/\.mgf_OMSSA_TPP\.pep\.xml//ig” *OMSSA_TPP.pep.xml
ECHO *** Validation of OMSSA results with PeptideProphet/ProteinProphet; NOOCAM NOGROUPS ***
FOR %%I IN (*OMSSA_TPP.pep.xml) DO interactparser ppval_%%I %%I -L5 -Etrypsin -C -P
FOR %%I IN (ppval*.pep.xml) DO peptideprophetparser %%I DECOY = decoy MINPROB = 0 NONPARAM
FOR %%I IN (ppval*.pep.xml) DO refreshparser %%I c:\blastdb\customdb
FOR %%I IN (ppval*.pep.xml) DO proteinprophet %%I %%I.prot.xml NOOCCAM NOGROUPS
DEL ppval_ppval*
DEL sed*
ECHO *** Standard OMSSA Search ***
FOR %%I IN (*.mgf) DO omssacl -d c:\blastdb\customdb -fm %%I -v 2 -mf 3 -mv 4,121 -w -ob %%I_OMSSA_DECOY.oms
UV/VIS spectrum of Uniblue A
An aqueous solution of 0.01 mg/mL Uniblue A was measured in a range from 300 to 800 nm against water as blank on a BECKMAN DU 640 spectrometer. The resulting data were converted into ASCII and visualized using a LaTeX typesetting system.
Venn diagram
The R package VennDiagram [43] was employed for drawing the Venn diagram with R 2.14.0. Proteins of the PRIDE experiments #13515 and #13516 from the analysis of the putative MalE-lacZα fusion protein were included, with a ProteinProphet score of >0.9 as treshold.
Patent Application
For the pre-gel staining strategy with Uniblue A a patent was filed with the number MX/a/2009/013417.
Mass spectrometry data availability
Mass spectrometry raw data and identification results have been deposited at the EBI PRIDE server (http://www.ebi.ac.uk/pride/). The data will be made public after acceptance of the manuscript. For review, the data can be accessed with Username: review00783 and Password: JT-FskaG. PRIDE accession codes for individual samples are given in Table 1 or within the manuscript. Peptides modified with Uniblue A are annotated in the individual protein hit view as follows: MOD: 01659 from database MOD at position XX. Monoisotopic Δ: 484.039893, Uniblue A derivatized residue. An example is given in Figure 3.
Supporting Information
Recombinant amaranth cystatin fasta sequence.
(DOC)
UV/VIS spectrum of Uniblue A (0.01 mg/mL in water). Uniblue A exhibits strong absorption in the visible wavelength region with a maximum λmax at 593.5 nm and a shoulder at about 630 nm.
(DOC)
Comparison of electrophoretic mobility of Uniblue A derivatized recombinant cystatin with the electrophoretic mobility of un-derivatized cystatin (Coomassie staining). There is no significant change of the electrophoretic mobility of cystatin detectable.
(DOC)
Evaluation of the sensitivity of Uniblue A pre-staining using recombinant cystatin. Gel A shows the gel directly after running and fixation, gel B after additional staining with PhastGel Blue R. Uniblue A derivatized cystatin bands can be detected visually down to about 1 µg of loaded protein. Subsequent staining with PhastGel Blue R allows the detection of about 0.1 µg of loaded protein.
(DOC)
Two-dimensional gel electrophoresis of Uniblue A derivatized recombinant cystatin. Uniblue A derivatized recombinant cystatin cannot be focused, even after 18930Vh of isoelectric-focusing. An increasing derivatization degree leads to a shift of the isoelectric point of the protein towards the basic region. Further, the band gets more diffuse, although the apparent molecular weight does not change significantly.
(DOC)
MS/MS based sequence coverage for MalE-lacZα fusion protein from E. coli expression vector pMAL-c4x.
(DOC)
Supplemental MS/MS spectra of native and Uniblue A derivatized peptides.
(DOC)
Acknowledgments
We greatly appreciate the technical assistance by the OMSSA team of the National Center for Biotechnology Information (NCBI) and the team of the European Bioinformatic Institute (EBI). The Rituximab sample was kindly donated from the research chair for bio-pharmaceuticals (grant CAT 122). We also want to thank Mirna Gonzalez for the preparation of glycerol stocks of E. coli TOP10/pMAL-c4x and Luis González de la Vara for his help with UV/VIS measurements.
Footnotes
Competing Interests: The Tecnologico de Monterrey (ITESM, Monterrey, Mexico) filed a patent on the rapid covalent protein staining with mass spectrometry compatibility (Reg. MX/a/2009/013417, filed on 09/DEC/2009). MAMG, MTY, RW are listed in this application as inventors. However, according to ITESM's policy, none of the authors is eligible to receive benefits from potential commercial exploitation. All reagents - including the stain Uniblue A - are available from providers such as Sigm-Aldrich without restrictions. The authors only used software, which is either installed by default on the mass spectrometry devices or which can be obtained for free. For the data evaluation the authors took special care and only used freely available software and open community standards (the references are included in the manuscript). In case of the approval of the patent, the commercial use of invention might become restricted in the territory of Mexico; especially the preparation and commercialization of a respective kit. However, an international patent application was not filed; the deadline for an international application passed December 2010. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This work was supported by the research chair CAT 161 of the Instituto Tecnológico y de Estudios Superiores de Monterrey (Monterrey Institute of Technology and Higher Education, ITESM) and by institutional funding of the Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional (Center for Research and Advanced Studies of the National Polytechnic Institute, CINVESTAV), Unidad Irapuato. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rathore A. Roadmap for implementation of quality by design (QbD) for biotechnology products. Trends in Biotechnology. 2009;27:546–553. doi: 10.1016/j.tibtech.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Lewis K. China's counterfeit medicine trade booming. CMAJ: Canadian Medical Association journal = journal de l'Association medicale canadienne. 2009;181 doi: 10.1503/cmaj.109-3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akunyili D. Fake drugs: lessons for the world. Dora Akunyili interviewed by Meredith Wadman. Nature. 2008;452 doi: 10.1038/452924a. [DOI] [PubMed] [Google Scholar]
- 4.Lancet Fighting fake drugs: the role of WHO and pharma. Lancet. 2011;377 doi: 10.1016/S0140-6736(11)60656-9. [DOI] [PubMed] [Google Scholar]
- 5.Rabilloud T. Two-dimensional gel electrophoresis in proteomics: Old, old fashioned, but it still climbs up the mountains. Proteomics. 2002;2:3–10. [PubMed] [Google Scholar]
- 6.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 7.Winkler R, García-Lara S. Activity-directed identification of maize kernel peroxidases associated with postharvest insect resistance. Mol Biosyst. 2010;6:1810–1812. doi: 10.1039/c003112g. [DOI] [PubMed] [Google Scholar]
- 8.Gharahdaghi F, Weinberg C, Meagher D, Imai B, Mische S. Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: a method for the removal of silver ions to enhance sensitivity. Electrophoresis. 1999;20:601–605. doi: 10.1002/(SICI)1522-2683(19990301)20:3<601::AID-ELPS601>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Sumpton D, Bienvenut W. Coomassie stains: are they really mass spectrometry compatible? Rapid communications in mass spectrometry: RCM. 2009;23:1525–1529. doi: 10.1002/rcm.4029. [DOI] [PubMed] [Google Scholar]
- 10.Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, et al. Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis. 2004;25:1327–1333. doi: 10.1002/elps.200305844. [DOI] [PubMed] [Google Scholar]
- 11.Westermeier R. Sensitive, quantitative, and fast modifications for Coomassie Blue staining of polyacrylamide gels. Proteomics. 2006;6(Suppl 2):61–64. doi: 10.1002/pmic.200690121. [DOI] [PubMed] [Google Scholar]
- 12.Jenö P, Mini T, Moes S, Hintermann E, Horst M. Internal Sequences from Proteins Digested in Polyacrylamide Gels. Analytical Biochemistry. 1995;224:75–82. doi: 10.1006/abio.1995.1010. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld J, Capdevielle J, Guillemot J, Ferrara P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Analytical Biochemistry. 1992;203:173–179. doi: 10.1016/0003-2697(92)90061-b. [DOI] [PubMed] [Google Scholar]
- 14.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Analytical chemistry. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 15.Shevchenko A, Tomas H, Havlis J, Olsen J, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2006;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 16.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 17.Parkinson D, Redshaw J. Visible labeling of proteins for polyacrylamide gel electrophoresis with dabsyl chloride. Analytical Biochemistry. 1984;141:121–126. doi: 10.1016/0003-2697(84)90434-2. [DOI] [PubMed] [Google Scholar]
- 18.Compton M, Lapp S, Pedemonte R. Generation of multicolored, prestained molecular weight markers for gel electrophoresis. Electrophoresis. 2002;23:3262–3265. doi: 10.1002/1522-2683(200210)23:19<3262::AID-ELPS3262>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Usaite R, Wohlschlegel J, Venable J, Park S, Nielsen J, et al. Characterization of Global Yeast Quantitative Proteome Data Generated from the Wild-Type and Glucose Repression Saccharomyces cerevisiae Strains: The Comparison of Two Quantitative Methods. J Proteome Res. 2008;7:266–275. doi: 10.1021/pr700580m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elias J, Gygi S. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nature methods. 2007;4:207–214. doi: 10.1038/nmeth1019. [DOI] [PubMed] [Google Scholar]
- 21.Geer L, Markey S, Kowalak J, Wagner L, Xu M, et al. Open Mass Spectrometry Search Algorithm. Journal of Proteome Research. 2004;3:958–964. doi: 10.1021/pr0499491. [DOI] [PubMed] [Google Scholar]
- 22.Keller A, Nesvizhskii A, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Analytical chemistry. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 23.Nesvizhskii A, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Analytical chemistry. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 24.Vizcaíno JA, Côté R, Reisinger F, Foster J, Mueller M, et al. A guide to the Proteomics Identifications Database proteomics data repository. Proteomics. 2009;9:4276–4283. doi: 10.1002/pmic.200900402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barsnes H, Vizcaino J, Eidhammer I, Martens L. PRIDE Converter: making proteomics data-sharing easy. Nature Biotechnology. 2009;27:598–599. doi: 10.1038/nbt0709-598. [DOI] [PubMed] [Google Scholar]
- 26.Perona J, Craik C. Structural basis of substrate specificity in the serine proteases. Protein Science. 1995;4:337–360. doi: 10.1002/pro.5560040301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice R. Stabilization of bovine trypsin by reductive methylation. Biochimica et Biophysica Acta (BBA) - Protein Structure. 1977;492:316–321. doi: 10.1016/0005-2795(77)90082-4. [DOI] [PubMed] [Google Scholar]
- 28.Labouesse J, Gervais M. Preparation of Chemically Defined εN-Acetylated Trypsin. European Journal of Biochemistry. 1967;2:215–223. doi: 10.1111/j.1432-1033.1967.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 29.Winkler R. ESIprot: a universal tool for charge state determination and molecular weight calculation of proteins from electrospray ionization mass spectrometry data. Rapid communications in mass spectrometry: RCM. 2010;24:285–294. doi: 10.1002/rcm.4384. [DOI] [PubMed] [Google Scholar]
- 30.Smith M. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003;22:7359–7368. doi: 10.1038/sj.onc.1206939. [DOI] [PubMed] [Google Scholar]
- 31.Keough T, Youngquist RS, Lacey MP. A method for high-sensitivity peptide sequencing using postsource decay matrix-assisted laser desorption ionization mass spectrometry. Proceedings of the National Academy of Sciences. 1999;96:7131–7136. doi: 10.1073/pnas.96.13.7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bauer M, Sun Y, Keough T, Lacey M. Sequencing of sulfonic acid derivatized peptides by electrospray mass spectrometry. Rapid Commun. Mass Spectrom. 2000;14:924–929. doi: 10.1002/(SICI)1097-0231(20000530)14:10<924::AID-RCM967>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 33.Garcia B, Mollah S, Ueberheide B, Busby S, Muratore T, et al. Chemical derivatization of histones for facilitated analysis by mass spectrometry. Nat. Protocols. 2007;2:933–938. doi: 10.1038/nprot.2007.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valdes-Rodriguez S, Cedro-Tanda A, Aguilar-Hernandez V, Cortes-Onofre E, Blanco-Labra A, et al. Recombinant amaranth cystatin (AhCPI) inhibits the growth of phytopathogenic fungi. Plant physiology and biochemistry: PPB/Société française de physiologie végétale. 2010;48:469–475. doi: 10.1016/j.plaphy.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, et al. Walker JM, editor. Protein Identification and Analysis Tools on the ExPASy Server. The proteomics protocols handbook. 2005. pp. 571–607.
- 36.Sambrook J, Russell D, Sambrook J. Molecular cloning: a laboratory manual (3-volume set). Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 37.Bjellqvist B, Ek K, Righetti P, Gianazza E, Görg A, et al. Isoelectric focusing in immobilized pH gradients: principle, methodology and some applications. Journal of biochemical and biophysical methods. 1982;6:317–339. doi: 10.1016/0165-022x(82)90013-6. [DOI] [PubMed] [Google Scholar]
- 38.Shevchenko A, Tomas H, Havlis J, Olsen J, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nature Protocols. 2007;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- 39.Havlis J, Thomas H, Sebela M, Shevchenko A. Fast-response proteomics by accelerated in-gel digestion of proteins. Analytical chemistry. 2003;75:1300–1306. doi: 10.1021/ac026136s. [DOI] [PubMed] [Google Scholar]
- 40.Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, et al. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic acids research. 2008;36:D901–906. doi: 10.1093/nar/gkm958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keller A, Eng J, Zhang N, Li X, Aebersold R. A uniform proteomics MS/MS analysis platform utilizing open XML file formats. Molecular Systems Biology. 2005;1:msb4100024-E1–msb4100024-E8. doi: 10.1038/msb4100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strohalm M, Kavan D, Novák P, Volný M, Havlícek V. mMass 3: A Cross-Platform Software Environment for Precise Analysis of Mass Spectrometric Data. Analytical Chemistry. 2010;82:4648–4651. doi: 10.1021/ac100818g. [DOI] [PubMed] [Google Scholar]
- 43.Chen H, Boutros P. VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinformatics. 2011;12:35. doi: 10.1186/1471-2105-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Recombinant amaranth cystatin fasta sequence.
(DOC)
UV/VIS spectrum of Uniblue A (0.01 mg/mL in water). Uniblue A exhibits strong absorption in the visible wavelength region with a maximum λmax at 593.5 nm and a shoulder at about 630 nm.
(DOC)
Comparison of electrophoretic mobility of Uniblue A derivatized recombinant cystatin with the electrophoretic mobility of un-derivatized cystatin (Coomassie staining). There is no significant change of the electrophoretic mobility of cystatin detectable.
(DOC)
Evaluation of the sensitivity of Uniblue A pre-staining using recombinant cystatin. Gel A shows the gel directly after running and fixation, gel B after additional staining with PhastGel Blue R. Uniblue A derivatized cystatin bands can be detected visually down to about 1 µg of loaded protein. Subsequent staining with PhastGel Blue R allows the detection of about 0.1 µg of loaded protein.
(DOC)
Two-dimensional gel electrophoresis of Uniblue A derivatized recombinant cystatin. Uniblue A derivatized recombinant cystatin cannot be focused, even after 18930Vh of isoelectric-focusing. An increasing derivatization degree leads to a shift of the isoelectric point of the protein towards the basic region. Further, the band gets more diffuse, although the apparent molecular weight does not change significantly.
(DOC)
MS/MS based sequence coverage for MalE-lacZα fusion protein from E. coli expression vector pMAL-c4x.
(DOC)
Supplemental MS/MS spectra of native and Uniblue A derivatized peptides.
(DOC)