Cilia are evolutionarily conserved hair-like structures with key roles in cell locomotion, fluid movement, and sexual reproduction. Recent studies have shown that cilia also sense the extracellular environment and are important signaling organelles, which direct embryonic development and organ function. Abnormal ciliary axonemal structure and function have been linked to the growing class of genetic disorders collectively known as ciliopathies.
The prototypical ciliopathy, primary ciliary dyskinesia (PCD), was the first human disorder linked to ciliary dysfunction, described over a century ago, and since then, our understanding of the genetic and molecular abnormalities of this disease have greatly advanced [1-3]. The importance of cilia in other human diseases are just beginning to be elucidated, and defects have been associated with a growing number of pediatric conditions, including obesity, renal disease, hepatic fibrosis, skeletal dysplasias, endocrinopathies, neurodevelopmental defects, central nervous system (CNS) anomalies, laterality defects, and congenital heart disease (CHD) [2-5]. Research into ciliopathies has rapidly expanded, involving multidisciplinary efforts to define the complex genetics and functional phenotypes of cilia. In this review, we will describe ciliary ultrastructure and function, genetics, clinical characteristics, and overlapping features of these diverse diseases.
CILIA STRUCTURE AND FUNCTION
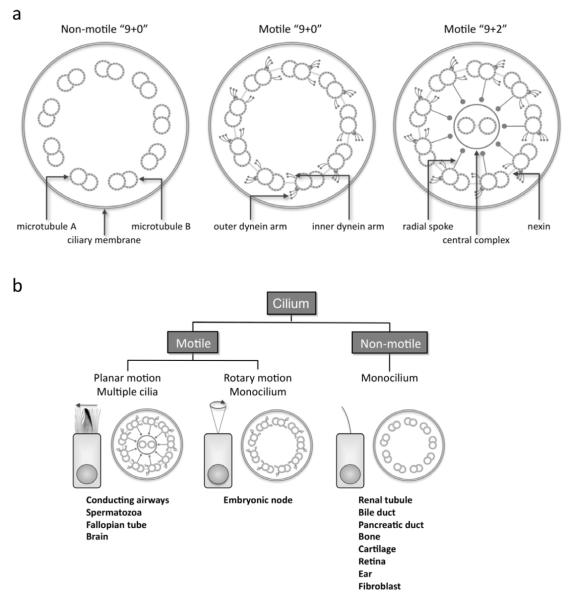
Cilia are found on the surface of most cells. These hair-like appendages, anchored by a basal body to the apical cytoplasm and extending from the cell surface into the extracellular space, consist of hundreds of proteins organized around a microtubular scaffold, which makes up the basic axonemal structure. Based on the arrangement of these longitudinal microtubules, cilia have historically been segregated into motile or primary (or sensory) cilia. The terminology can be confusing, but currently, there are 3 broad classes of cilia: motile “9+2”, motile “9+0”, and non-motile “9+0” (Figure) [1-6]. In simpler organisms, such as unicellular flagellated alga Chlamydomonas reinhardtii, there is considerable overlap between motile and sensory functions, so such classifications may be simplistic. Motor and sensory functions have been considered distinct in mammalian cells, though evidence is mounting that motile cilia are chemosensory and can detect substances in the external environment [7,8].
Figure.
A, Schematic diagram showing the structural elements of motile “9+2”, motile “9+0”, and non-motile “9+0” ciliary axonemes. B, Classification and sites of different cilia, showing the coordinated synchronous motion of motile “9+2” cilia, the rotary motion of motile “9+0” nodal monocilium, and non-motile “9+0” primary monocilium (modified from Leigh, et al [1]).
The basic structure of cilia and their structural counterparts, flagella, is highly conserved across species, and numerous cell and animal models exist. The basal body is a specialized centriole found at the base of a cilium (or flagellum) that organizes ciliogenesis and anchors the cilia in a specific orientation [9]. Synthesis of structural and functional elements of cilia occurs in the cytoplasm, and intraflagellar transport (IFT) is responsible for anterograde and retrograde movement of essential proteins. This process is essential for construction and maintenance of cilia. Ciliary proteins dock at the basal body while awaiting transport into the cilia by microtubule-associated kinesin and dynein [10]. Both motile and sensory cilia consist of organized arrays of microtubules, composed of α - and β-monomers of tubulin arranged into helical protofilaments.
Motile cilia are found in the apical surface of the upper and lower respiratory tract, ependymal cells lining the ventricles of the central nervous system, the oviducts of the female reproductive system, and as the flagellum of male spermatazoa (Figure). Each motile cilium is organized into nine microtubule pairs (or doublets) surrounding a central pair in a characteristic “9+2” arrangement as shown by cross-sectional views by electron microscopy. The central fibrillar bundle of the cilium, or axoneme, is covered by a membrane continuous with the plasma membrane. Arising from the A microtubule, the dynein arms are complex structures, consisting of several heavy, intermediate, and light chains, contain ATPases that act as molecular motors and slide the peripheral microtubular pairs relative to one another. Nexin links tether and limit the relative motion of adjacent microtubular doublets, and radial spokes control dynein arm activity relaying signals from the central mictrotubular pair to the arms. During a beat, the dynein arms undergo an attachment, retraction, and release cycle with the adjacent doublet, which results in a sliding motion of the microtubule doublets relative to each other. The basal body anchors the microtubules, and the nexin links, radial spokes, and possibly the ciliary membrane restrict the degree of sliding between microtubules, thereby, converting the slide to a bend, characteristic of ciliary waveform. The “9+2” motile cilia beat in a synchronous waveform having a forward effective stroke followed by a return stroke. The direction of stroke depends on the orientation of central microtubules. Cilia move in low viscosity, periciliary fluid at the immediate epithelial surface, which permits a rapid beat frequency in the airway, ranging from approximately 8-20 Hz under normal conditions, and mobilizes the mucus that sits atop the cilia. Ciliary beating is coordinated by calcium signaling between epithelial cells through gap junctions [11], and disturbances to the precise, orchestrated movement of any motile cilia can lead to disease [1].
Another type of motile cilia is present only transiently in the ventral node of the gastrula during embryonic development. These nodal cilia have a “9+0” microtubule arrangement (similar to primary cilia), but are motile with a whirling, rotational movement that directs leftward flow of extracellular fluid. The nodal cilia play a vital role in establishing left-right body orientation, and abnormalities can lead to laterality defects (Figure) [12].
Primary cilia occur on nearly all vertebrate cells, appearing as a single cilium that may occur only during interphase. Until recently, primary cilia were considered vestigial -- solitary organelles of little clinical significance. Nonetheless, it has become increasingly clear that primary cilia contain both extracellular receptors, responding to mechanical stimulation, chemosensation, and in specialized cases, to light, temperature, and gravity, as well as a number of signaling pathways that are important in development and tissue homeostasis [3]. Primary cilia lack the central pair of central microtubules, creating a “9+0” arrangement, and lack dynein arms, thus leaving these structures immotile [2-5]. Newer insights into the structure and function of primary cilia have led to greater understanding of their role in a widening number of pediatric diseases.
MOTOR CILIOPATHIES
The respiratory tract is repeatedly exposed to inhaled irritants and pathogens, and innate host defenses are critical in preventing pulmonary injury and infection. Complex, local defenses have evolved to protect the airway, including the mucociliary escalator, which mechanically eliminates bacteria and particulates that deposit at the epithelial surface. Ciliated cells line the nasopharynx, middle ear, paranasal sinuses, and lower respiratory tract from the trachea to bronchioles. Each ciliated cell has approximately 200 cilia projecting from its surface that beat in a coordinated fashion. The regulation and function of cilia that play a role in mucociliary clearance are complex. Ciliary ultrastructure and orientation are critical for efficient clearance of the lower respiratory tract by moving fluids, mucus, and inhaled foreign materials vectorially from distal to more proximal airways. Indeed, to achieve effective mucociliary clearance, the conducting airways closely coordinate ciliary movement, airway surface fluid volume, fluid composition, and macromolecule (e.g., mucin) secretion [13]. Any disruption, whether cilia are immotile, dysmotile or disoriented in their movement, can manifest with sinopulmonary symptoms.
Primary ciliary dyskinesia was the first human disorder associated with ciliary dysfunction [14]. Usually described as an autosomal recessive condition, though rare cases of autosomal dominant and X-linked inheritance have been reported, the frequency of PCD ranges from 1 in 15,000 to 30,000 live births, but these measures likely underestimate the disease in the general population. Theoretically, mutations in any of the 400 proteins that make up the complex structure of the cilium could cause impaired mucociliary clearance, which is consistent with the wide spectrum of clinical presentations. The truncation or absence of dynein arms is the most common ultrastructural abnormality seen in primary ciliary dyskinesia, accounting for 90 percent of cases with defined ultrastructural defects. Other axonemal changes consistent with disease include microtubular transposition, radial spoke and nexin link defects, and ciliary agenesis. Ciliary disorientation has also been described as a form of primary ciliary dyskinesia, but this phenomenon may be the result of airway injury. It is important to note that the presence of normal axonemal ultrastructure does not exclude PCD [1].
Linkage analyses have demonstrated substantial locus heterogeneity, which has made correlations between specific ciliary defects and the underlying mutations problematic. Investigations into the genetic basis of PCD have focused on dynein arm proteins, and to date, mutations have been identified in 11 genes that are known to cause PCD (Table). DNAI1 was the first gene to be linked to PCD based on the candidate approach. Mutations in DNAI1 have been found in patients with PCD with outer dynein arm defects and functional ciliary abnormalities and have been estimated to occur in 10% of patients with PCD [15]. Another gene, DNAH5 has also been identified as a causative gene for PCD using homozygosity mapping. A recent study has indicated that 28% of all and 53% of those patients with PCD with known outer dynein arm defects had mutations in DNAH5 clustered in five exons, making this a promising target for genetic screening [16]. Most recently, another dynein gene, DNAH11 has been linked to PCD with normal ultrastructure [17]. Although the genetic basis of this disease is far from being completely understood, the identification of disease-causing mutations could lead to better testing that should overcome many of the current diagnostic limitations. Commercial laboratories offer testing for specific DNAI1 and DNAH5 defects, but such testing does not currently provide diagnostic advantages over electron microscopy because the mutations surveyed are only associated with outer dynein arm defects. The other PCD genes listed in the Table have only been reported in a small number of patients, and their relative prevalence has not been defined.
Table.
Childhood diseases and syndromes associated with motile and sensory ciliopathies.
| Pediatric ciliopathy | Clinical manifestations | Gene(s) |
|---|---|---|
| Motor | ||
| Primary ciliary dyskinesia | Chronic bronchitis, rhinosinusitis, otitis media, laterality defects, infertility, CHD |
DNAI1, DNAH5, DNAH11, DNAI2, KTU, TXNDC3, LRRC50, RSPH9, RSPH4A, CCDC40, CCDC39 |
| Sensory | ||
| Autosomal recessive polycystic kidney disease |
RFD, CHF | PKHD1 |
| Nephronophthisis | RFD, interstitial nephritis, CHF, RP | NPHP1-8, ALMS1, CEP290 |
| Bardet-Biedl syndrome | Obesity, polydactyly, ID, RP, renal anomalies, anosmia, CHD |
BBS1-12, MKS1, MKS3, CEP290 |
| Meckel-Gruber syndrome | RFD, polydactyly, ID, CNS anomalies, CHD, cleft lip, cleft palate |
MKS1-6, CC2D2A, CEP290, ,
TMEM216 |
| Joubert syndrome | CNS anomalies, ID, ataxia, RP, polydactyly, cleft lip, cleft palate |
NPHP1, JBTS1, JBTS3, JBTS4, CORS2, AHI1, CEP290, TMEM216 |
| Alstrom syndrome | Obesity, RP, DM, hypothyroidism, hypogonadism, skeletal dysplasia, cardiomyopathy, pulmonary fibrosis |
ALMS1 |
| Orofaciodigital syndrome type 1 |
Polydactyly, syndactyly, cleft lip, cleft palate, CNS anomalies, ID, RFD |
OFD1 |
| Ellis van Creveld syndrome | Chondrodystrophy, polydactyly, ectodermal dysplasia, CHD |
EVC, EVC2 |
| Jeune asphyxiating thoracic dystrophy |
Narrow thorax, RFD, RP, dwarfism, polydactyly |
IFT80 |
| Sensenbrenner syndrome | Dolichocephaly, ectodermal dysplasia, dental dysplasia, narrow thorax, RFD, CHD |
IFT122, IFT43, WDR35 |
| Short rib-polydactyly syndromes |
Narrow thorax, short limb dwarfism, polydactyly, renal dysplasia |
WDR35, DYNC2H1, NEK1 |
CHD, congenital heart disease; CHF, congenital hepatic fibrosis; CNS, central nervous system; DM, diabetes mellitus; ID, intellectual disabilities; RFD, renal fibrocystic disease; RP, retinitis pigmentosa.
Most patients with PCD present with persistent hypoxemia or even acute respiratory failure during the immediate newborn period. Despite this early clinical feature, the association of newborn respiratory distress with PCD has been underappreciated. The upper respiratory tract is almost universally involved in PCD, frequently present since early infancy. Inadequate innate mucus clearance commonly manifests as chronic sinusitis, and some patients develop nasal polyposis. Middle ear disease is described in virtually all cases of PCD with varying degrees of conductive hearing loss. Impaired mucociliary clearance of the lower respiratory tract leads to recurrent episodes of pneumonia or bronchitis with nontypeable Haemophilus influenzae, Staphylococcus aureus, and Streptococcus pneumoniae. Pseudomonas aeruginosa infection has also been reported, typically found in older individuals with advanced disease. Chronic lung infection and inflammation result in persistent atelectasis and bronchiectasis in many individuals, even very young children, typically involving the right (or left) middle lobe. Pulmonary function testing shows progressive intrathoracic airway obstruction [18,19]. Despite its early, unambiguous pulmonary manifestations, the mean age of diagnosis in most studies is approximately 4 years of age [19].
Left-right laterality is a cilia-dependent mechanism, and one-half of the patients have situs inversus totalis (SI) with complete reversal of the thoracic and abdominal organs. Asymmetry is determined by the embryonic node, a dish-like structure containing motile cilia that is transiently present at the distal tip of the embryonic notochord before development of the left-right axis. Recent studies have indicated that both non-motile primary cilia and specialized motile cilia populate the embryonic node. Whirling, motile cilia generate the leftward flow of extra-embryonic fluid, thereby establishing left-right sidedness, while a second array of non-motile cilia senses the flow. Without functional nodal cilia in the embryonic period, thoracoabdominal orientation is random [12]. Other forms of left-right asymmetry have been reported in association with PCD. Laterality defects may involve some but not all organs in the thorax and abdomen, situs ambiguus, or be associated with heterotaxy, or abnormal organ positioning, with associated CHD, asplenia, or polysplenia [20]. It is curious that some ciliary ultrastructural defects, such as transposition defects or central microtubular agenesis, have not been associated with SI [21]. This phenomenon has been attributed to the fact that cilia with these defects have a circular beat pattern similar to that of nodal cilia. It has been postulated that movement of nodal cilia would not be affected and flow across the node would be in the usual direction.
Males with PCD are typically infertile as a result of impaired spermatozoa motility secondary to defective sperm flagella, although male infertility is not a universal finding in this disease. In fact, males with PCD can have some spermatozoa motility suggesting sperm tails retain some function or could actually be under different genetic control than cilia. Fertility issues in women have also been reported, possibly due to ciliary dysfunction in Fallopian tubes.
Other clinical manifestations of PCD are rare and less well understood. Motile cilia line the cerebral ventricles and aqueduct. In contrast to the airway, ependymal cells have fewer and longer cilia that beat at twice the frequency. Their orientation and distribution suggest that they are involved in cerebral spinal fluid flow, and ependymal ciliary defects can lead to hydrocephalus in model systems. Nevertheless, though nearly universal in mouse models, hydrocephalus is extremely rare in patients with primary ciliary dyskinesia. Slightly enlarged brain ventricles have been noted in prenatal ultrasound examinations of embryos with SI, but progression of hydrocephalus is unusual [22].
SENSORY CILIOPATHIES
Cilia-basal body-centrosome complexes are critical organelles, central to several basic biological processes. In some tissues, primary cilia serve as chemoreceptors and mechanoreceptors of the extracellular environment [3,23,24]. Centrioles and the surrounding centrosomes are necessary for cell division, serving as poles of the mitotic spindle, and appear to regulate the critical balance between cell proliferation and apoptosis [25]. So in retrospect, it should not be surprising that primary ciliary defects can lead to broad categories of disease. In addition, though sensory ciliopathies were first thought to be organ-specific (e.g., kidney), it has become increasingly clear that these conditions involve many different organs and share clinical features.
Autosomal dominant polycystic kidney disease (ADPKD), a common cause of chronic renal failure in adults, was one of the first diseases linked to sensory cilia dysfunction, due to mutations in PKD1 and PKD2 genes, which encode polycystin 1 or polycystin 2 respectively [24-26]. Primary cilia in epithelial cells lining renal tubules detect flow by bending and transmitting a calcium-mediated signal to the cell. The scope of renal fibrocystic diseases caused by defective primary cilia has widened since this discovery, including a number of genetically heterogeneous pediatric diseases. Autosomal recessive polycystic kidney disease (ARPKD) is the most common childhood-onset ciliopathy, characterized by dilated renal collecting ducts resulting in progressive cystic degeneration of the kidneys and congenital hepatic fibrosis. A significant number of patients with ARPKD die shortly after birth and the majority of those who survive develop renal failure during childhood or young adulthood [27]. ARPKD is caused by mutations in PKHD1 that encodes polyductin, a protein involved in differentiation of cells lining the collecting ducts [24,28]. Another ciliopathy, nephronophthisis, is an autosomal-recessive cystic renal disease of childhood, due to mutations in nine different genes encoding nephrocystins (NPHP1-8 and ALMS1) that localize to cilia, basal bodies, centrosomes, adherens junctions, and focal adhesions [24,29]. Collectively, the various forms of nephronophthisis are the most common cause of end-stage renal failure in children. Patients with nephronophthisis can also have extrarenal manifestations, including retinal degeneration, congenital hepatic fibrosis, laterality defects, and skeletal anomalies .
X-linked retinitis pigmentosa (RP) is caused by defective XRP2 and X-linked retinitis pigmentosa GTPase regulator (RPGR) proteins, which are localized to photoreceptor connecting cilia [30]. Retinal photoreceptor cells rely on IFT to move proteins between inner and outer segments of the connecting cilium, and defects in intersegmental transport can lead to retinal degeneration. Proteins defective in syndromic ciliopathies, such as Senior-Løken syndrome, also lead to retinal degeneration.
Genetically heterogeneous primary ciliary defects have been shown to cause several, overlapping syndromes (Table). For instance, Bardet-Biedl syndrome (BBS) is a rare, genetically heterogeneous, autosomal recessive disorder with varied phenotypes, including RP, polycystic kidneys, truncal obesity, polydactyly, hypogonadism, intellectual disabilities (ID), diabetes mellitus (DM), and CHD. Approximately one-third of patients with BBS will have anosmia, due to defective non-motile sensory “9+2” cilia, present on olfactory neurons. There are at least twelve different BBS genes, found only in ciliated cells and localized to the basal body and ciliary axoneme, and expressed proteins are involved in microtubule anchoring and coordination of the cell cycle [31-33].
Meckel-Gruber syndrome (MGS) is a perinatally fatal ciliopathy, characterized by cystic renal disease, polydactyly, posterior encephalocele, and other central nervous system abnormalities. It is caused by abnormalities of various proteins located in the cilia-basal body-centrosome complex, including MKS1 and MKS3 [34,35]. Joubert syndrome and related disorders (JSRD) are characterized by a specific form of cerebellar vermis hypoplasia, hypotonia, neurodevelopmental delay, ataxia, and irregular breathing patterns. Other less common features include retinal dystrophy, fibrocystic renal disease, congenital hepatic fibrosis, occipital encephalocele, and polydactyly. These disorders have genetic heterogeneity with at least 8 different genes, including NPHP1, JBTS1, JBTS3, JBTS4, and CORS2, all encoding proteins that localize to the primary cilium [36]. Interestingly, some genotype-phenotype correlations have emerged, and causative genes for some of these syndromes have been implicated in other disorders, illustrating the genetic overlap and shared pathologies of sensory ciliopathies. For instance, mutations in a single gene CEP290 (centrosomal protein 290kDa) have been linked to Joubert syndrome, BBS, MGS, nephronophthisis, and Leber congenital amaurosis, a severe form of retinal degeneration [37].
Alstrom syndrome is an autosomal recessive disorder caused by mutations in ALMS1, which are localized to centrisomes and basal bodies of primary cilia. Its disruption leads to rod-cone retinal dystrophy, sensorineural hearing loss, obesity, and insulin resistant DM and cardiomyopathy. Other features include hypothyroidism, hypogonadism, pulmonary fibrosis, and progressive hepatic and renal failure [38]. Orofaciodigital syndrome type 1 is an X-linked dominant disorder, lethal in males. Defects in the OFD1 gene, which encodes an essential protein that regulates the length and organization of centrosomes [39], lead to defective primary cilia causing characteristic oral, dental, facial and digital anomalies in girls. Similar to other sensory ciliopathies, affected individuals can also have renal cysts and the CNS abnormalities [40].
Two other ciliopathies, Ellis van Creveld syndrome, an autosomal-recessive condition typically characterized by chondrodystrophy with associated dwarfism, polydactyly, ectodermal dysplasia, and CHD, and Weyers acrodental dysostosis, an autosomal-dominant syndrome that results in polydactyly, nail dysplasia, and orofacial abnormalities, are caused by loss-of-function mutations in Evc and Evc2 proteins, which localize to the basal bodies of primary cilia and are involved in hedgehog signaling [41].
Finally, Jeune asphyxiating thoracic dystrophy, an autosomal recessive disorder that causes thoracic cage deformity, leading to respiratory insufficiency, fibrocystic renal disease, retinal degeneration, short-limbed dwarfism, and polydactyly, is one of the first human diseases linked to intraflagellar transport gene mutations (IFT80) [42]. Two other conditions associated with thoracic cage defects, Sensenbrenner syndrome and short rib-polydactyly syndromes, have also been linked to defective sensory cilia [43,44].
OVERLAPPING SYNDROMES
As noted previously, sensory and motor ciliopathies can overlap. For instance, a large consanguineous family with an X-linked recessive syndrome of intellectual disabilities and macrocephaly, caused by an OFD1 frameshift mutation, also had respiratory features consistent with PCD [45]. A few patients with PCD and left-right laterality defects have had features that indicate defective sensory cilia, including RP [46]. Conversely, several adults with ADPKD were found to have bronchiectasis, a classic finding in PCD [47], suggesting impaired motor cilia function.
DISCUSSION
Ciliopathies are a group of genetically heterogeneous disorders of motor or sensory ciliary dysfunction. Our understanding of the genetics, molecular abnormalities, and clinical manifestations in children has mushroomed during the past decade. It is a rapidly expanding field of research, involving multiple disciplines, which will improve diagnostic testing and identify new therapeutic targets.
Acknowledgments
Supported by National Institutes of Health awards (HL096458 to T.F. and M.L. and HL082657 to TF.) and the Children’s Discovery Institute (to T.F.).
ABBREVIATIONS
- ADPKD
autosomal dominant polycystic kidney disease
- ARPKD
Autosomal recessive polycystic kidney disease
- BBS
Bardet-Biedl syndrome
- CHD
congenital heart disease
- CNS
central nervous system
- DM
diabetes mellitus
- MGS
Meckel-Gruber syndrome
- ID
intellectual disabilities
- PCD
primary ciliary dyskinesia
- RP
retinitis pigmentosa
- SI
situs inversus totalis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- 1.Leigh MW, Pittman JE, Carson JL, Ferkol TW, Dell SD, Davis SD, Knowles MR, Zariwala MA. Clinical and genetic aspects of primary ciliary dyskinesia and Kartagener syndrome. Genet Med. 2009;11:473–87. doi: 10.1097/GIM.0b013e3181a53562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardenas-Rodriguez M, Badano JL. Ciliary biology: understanding the cellular and genetic basis of human ciliopathies. Am J Med Genet C Semin Med Genet. 2009;151:263–80. doi: 10.1002/ajmg.c.30227. [DOI] [PubMed] [Google Scholar]
- 3.Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci. 2010;123:499–503. doi: 10.1242/jcs.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Ann Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 5.Tobin JL, Beales PL. The nonmotile ciliopathies. Genet Med. 2009;11:386–402. doi: 10.1097/GIM.0b013e3181a02882. [DOI] [PubMed] [Google Scholar]
- 6.Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Ann Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- 7.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–4. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloodgood RA. Sensory reception is an attribute of both primary cilia and motile cilia. J Cell Sci. 2010;123:505–9. doi: 10.1242/jcs.066308. [DOI] [PubMed] [Google Scholar]
- 9.Hoyer-Fender S. Centriole maturation and transformation to basal body. Semin Cell Dev Biol. 2010;21:142–7. doi: 10.1016/j.semcdb.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr Top Dev Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- 11.Sanderson MJ, Chow I, Dirksen ER. Intercellular communication between ciliated cells in culture. Am J Physiol. 1988;254:C63–74. doi: 10.1152/ajpcell.1988.254.1.C63. [DOI] [PubMed] [Google Scholar]
- 12.Basu B, Brueckner M. Cilia multifunctional organelles at the center of vertebrate left-right asymmetry. Curr Top Dev Biol. 2008;85:151–74. doi: 10.1016/S0070-2153(08)00806-5. [DOI] [PubMed] [Google Scholar]
- 13.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–7. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afzelius BA. A human syndrome caused by immotile cilia. Science. 1976;193:317–319. doi: 10.1126/science.1084576. [DOI] [PubMed] [Google Scholar]
- 15.Zariwala MA, Leigh MW, Ceppa F, Kennedy MP, Noone PG, Carson JL, et al. Mutations of DNAI1 in primary ciliary dyskinesia: evidence of founder effect in a common mutation. Am J Respir Crit Care Med. 2006;174:858–66. doi: 10.1164/rccm.200603-370OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hornef N, Olbrich H, Horvath J, Zariwala MA, Fliegauf M, Loges NT, et al. DNAH5 mutations are a common cause of primary ciliary dyskinesia with outer dynein arm defects. Am J Respir Crit Care Med. 2006;174:120–6. doi: 10.1164/rccm.200601-084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwabe GC, Hoffmann K, Loges NT, Birker D, Rossier C, de Santi MM, et al. Primary ciliary dyskinesia associated with normal axoneme ultrastructure is caused by DNAH11 mutations. Hum Mutat. 2008;29:289–98. doi: 10.1002/humu.20656. [DOI] [PubMed] [Google Scholar]
- 18.Noone PG, Leigh MW, Sannuti A, Minnix SL, Carson JL, Hazucha M, Zariwala MA, Knowles MR. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med. 2004;169:459–67. doi: 10.1164/rccm.200303-365OC. [DOI] [PubMed] [Google Scholar]
- 19.Coren ME, Meeks M, Morrison I, Buchdahl RM, Bush A. Primary ciliary dyskinesia: age at diagnosis and symptom history. Acta Paediatr. 2002;91:667–9. doi: 10.1080/080352502760069089. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy MP, Omran H, Leigh MW, Dell S, Morgan L, Molina PL, et al. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation. 2007;115:2814–21. doi: 10.1161/CIRCULATIONAHA.106.649038. [DOI] [PubMed] [Google Scholar]
- 21.Ferkol T, Mitchison H, O’Callaghan C, Leigh M, Carson J, Lie H, et al. Current issues in the basic mechanisms, pathophysiology, diagnosis, and management of primary ciliary dyskinesia. Eur Respir Monogr. 2006;37:291–313. [Google Scholar]
- 22.Wessels MW, den Hollander NS, Willems PJ. Mild fetal cerebral ventriculomegaly as a prenatal sonographic marker for Kartagener syndrome. Prenat Diagn. 2003;23:239–42. doi: 10.1002/pd.551. [DOI] [PubMed] [Google Scholar]
- 23.Gerdes GM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:132–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hildebrandt F, Otto E. Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat Rev Genet. 2005;6:928–40. doi: 10.1038/nrg1727. [DOI] [PubMed] [Google Scholar]
- 25.Gunay-Aygun M. Liver and kidney disease in ciliopathies. Am J Med Genet C Semin Med Genet. 2009;151:296–306. doi: 10.1002/ajmg.c.30225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoder BK. Role of primary cilia in the pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2007;18:1381–8. doi: 10.1681/ASN.2006111215. [DOI] [PubMed] [Google Scholar]
- 27.Gunay-Aygun M, Font-Montgomery E, Lukose L, Tuchman M, Graf J, Bryant JC, Kleta R, Garcia A, Edwards H, Piwnica-Worms K, Adams D, Bernardini I, Fischer RE, Krasnewich D, Oden N, Ling A, Quezado Z, Zak C, Daryanani K, Turkbey B, Choyke P, Guay-Woodford L, Gahl W. Correlation of kidney function, volume and imaging findings, and PKHD1 mutations in 73 patients with autosomal recessive polycystic kidney disease. Clin J Am Soc Nephrol. 2010;5:972–8. doi: 10.2215/CJN.07141009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S, Luo Y, Wilson PD, Witman GB, Zhou J. The autosomal recessive polycystic kidney disease protein is localized to primary cilia, with concentration in the basal body area. J Am Soc Nephrol. 2004;15:592–602. doi: 10.1097/01.asn.0000113793.12558.1d. [DOI] [PubMed] [Google Scholar]
- 29.Hildebrandt F, Attanasio M, Otto E. Nephronophthisis: disease mechanisms of a ciliopathy. J Am Soc Nephrol. 2009;20:23–35. doi: 10.1681/ASN.2008050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Q, Zhang Q, Pierce E. Photoreceptor sensory cilia and inherited retinal degeneration. Adv Exp Med Biol. 2010;664:223–32. doi: 10.1007/978-1-4419-1399-9_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nigg EA, Raff JW. Centrioles, centrosomes, and cilia in health and disease. Cell. 2009;139:663–78. doi: 10.1016/j.cell.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 32.Tobin JL, Beales PL. Bardet-Biedl syndrome: beyond the cilium. Pediatr Nephrol. 2007;22:926–36. doi: 10.1007/s00467-007-0435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaghloul NA, Katsanis N. Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. J Clin Invest. 2009;119:428–437. doi: 10.1172/JCI37041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JE, Gleeson JG. Cilia in the nervous system: linking cilia function and neurodevelopmental disorders. Curr Opin Neurol. 2011;24:98–105. doi: 10.1097/WCO.0b013e3283444d05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dawe HR, Smith UM, Cullinane AR, Gerrelli D, Cox P, Badano JL, et al. The Meckel-Gruber Syndrome proteins MKS1 and meckelin interact and are required for primary cilium formation. Hum Mol Genet. 2007;16:173–86. doi: 10.1093/hmg/ddl459. [DOI] [PubMed] [Google Scholar]
- 36.Doherty D. Joubert syndrome: insights into brain development, cilium biology, and complex disease. Semin Pediatr Neurol. 2009;16:143–54. doi: 10.1016/j.spen.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coppieters F, Lefever S, Leroy BP, De Baere E. CEP290, a gene with many faces: mutation overview and presentation of CEP290 base. Hum Mutat. 2010;31:1097–108. doi: 10.1002/humu.21337. [DOI] [PubMed] [Google Scholar]
- 38.Hearn T, Spalluto C, Phillips VJ, Renforth GL, Copin N, Hanley NA, Wilson DI. Subcellular localization of ALMS1 supports involvement of centrosome and basal body dysfunction in the pathogenesis of obesity, insulin resistance, and type 2 diabetes. Diabetes. 2005;54:1581–7. doi: 10.2337/diabetes.54.5.1581. [DOI] [PubMed] [Google Scholar]
- 39.Singla V, Romaguera-Ros M, Garcia-Verdugo JM, Reiter JF. Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev Cell. 2010;18:410–24. doi: 10.1016/j.devcel.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferrante MI, Zullo A, Barra A, Bimonte S, Messaddeq N, Studer M, Dollé P, Franco B. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nature Genet. 2006;38:112–17. doi: 10.1038/ng1684. [DOI] [PubMed] [Google Scholar]
- 41.Ruiz-Perez VL, Goodship JA. Ellis-van Creveld syndrome and Weyers acrodental dysostosis are caused by cilia-mediated diminished response to hedgehog ligands. Am J Med Genet C Semin Med Genet. 2009;151:341–51. doi: 10.1002/ajmg.c.30226. [DOI] [PubMed] [Google Scholar]
- 42.Beales PL, Bland E, Tobin JL, Bacchelli C, Tuysuz B, Hill J, Rix S, Pearson CG, Kai M, Hartley J, Johnson C, Irving M, Elcioglu N, Winey M, Tada M, Scambler PJ. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet. 2007;39:727–9. doi: 10.1038/ng2038. [DOI] [PubMed] [Google Scholar]
- 43.Konstantinidou AE, Fryssira H, Sifakis S, Karadimas C, Kaminopetros P, Agrogiannis G, Velonis S, Nikkels PG, Patsouris E. Cranioectodermal dysplasia: a probable ciliopathy. Am J Med Genet. 2009;149A:2206–11. doi: 10.1002/ajmg.a.33013. [DOI] [PubMed] [Google Scholar]
- 44.Mill P, Lockhart PJ, Fitzpatrick E, Mountford HS, Hall EA, Reijns MA, Keighren M, Bahlo M, Bromhead CJ, Budd P, Aftimos S, Delatycki MB, Savarirayan R, Jackson IJ, Amor DJ. Human and mouse mutations in WDR35 cause short-rib polydactyly syndromes due to abnormal ciliogenesis. Am J Hum Genet. 2011;88:508–15. doi: 10.1016/j.ajhg.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Budny B, Chen W, Omran H, Fliegauf M, Tzschach A, Wisniewska M, et al. A novel X-linked recessive mental retardation syndrome comprising macrocephaly and ciliary dysfunction is allelic to oral-facial-digital type I syndrome. Hum Genet. 2006;120:171–8. doi: 10.1007/s00439-006-0210-5. [DOI] [PubMed] [Google Scholar]
- 46.Moore A, Escudier E, Roger G, Tamalet A, Pelosse B, Marlin S, et al. RPGR is mutated in patients with a complex X linked phenotype combining primary ciliary dyskinesia and retinitis pigmentosa. J Med Genet. 2006;43:326–33. doi: 10.1136/jmg.2005.034868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Driscoll JA, Bhalla S, Liapis H, Ibricevic A, Brody SL. Autosomal dominant polycystic kidney disease is associated with an increased prevalence of radiographic bronchiectasis. Chest. 2008;133:1181–8. doi: 10.1378/chest.07-2147. [DOI] [PubMed] [Google Scholar]