Abstract
Cell polarization is important for multiple physiological processes. In polarized cells, microtubules (MTs) are organized into a spatially polarized array. Generally, in non-differentiated cells, it is assumed that MTs are symmetrically nucleated exclusively from centrosome (microtubule organizing center, MTOC) and then reorganized into the asymmetric array. We have recently identified the Golgi complex as an additional MTOC that asymmetrically nucleates MTs toward one side of the cell. Methods used for alternative MTOC identification include microtubule re-growth after complete drug-induced depolymerization and tracking of growing microtubules using fluorescence labeled MT +TIP binding proteins in living cells. These approaches can be used for quantification of MT nucleation sites at diverse sub-cellular structures.
Keywords: Microtubules, MTOC, centrosome, Golgi, cell polarity, cell motility
1. Introduction
The asymmetric distribution of MT network contributes to the establishment or maintenance of cell polarity during a number of biological processes such as cell migration, cell division and embryonic development. How could MTs achieve asymmetric distribution pattern? In the canonical model, MTs are exclusively nucleated from the centrosome with the plus ends extending toward the cell periphery creating a radial distribution pattern. As both in vivo (1) and in vitro (2) experiments have demonstrated that MTs nucleated from the centrosome are symmetric, it is assumed that nucleation does not contribute to the asymmetric organization of MTs. Instead other mechanisms are responsible for it, which include the local stabilization of MTs at a specific region (3), relocating the centrosome away from the cell center (4), or severing and releasing MTs from the centrosome (5;6). However, a number of observations have suggested that MTs may be nucleated independently of the centrosome. For example, during cell division, MTs are nucleated from kinetochores (7). In interphase cells, non-centrosome-based MT organization centers (MTOCs) have been identified in several specialized cells (8;9). These observations have raised two questions. First, are non-centrosomal MTs also nucleated in non-differentiated cells? Second, can the non-centrosomal MTs contribute to the asymmetric pattern of MT array?
Technically, it is difficult to directly quantify MT nucleation at MTOC. The high density of the existing MTs around the MTOC makes it difficult to detect the newly nucleated MTs emerging from the MTOC. Thus, MT nucleation from centrosome has been studied by monitoring the fluorescent labeled +TIP proteins such as CLIP170, EB1 etc (10;11). +TIPs are proteins which only localized to the plus end of the growing MTs. In the region around MTOC, +TIPs are associated only with newly nucleated MTs and move away from the MTOC as a MT polymerizes. Thus, the emergence of one +TIP dot from the MTOC indicates one nucleation event.
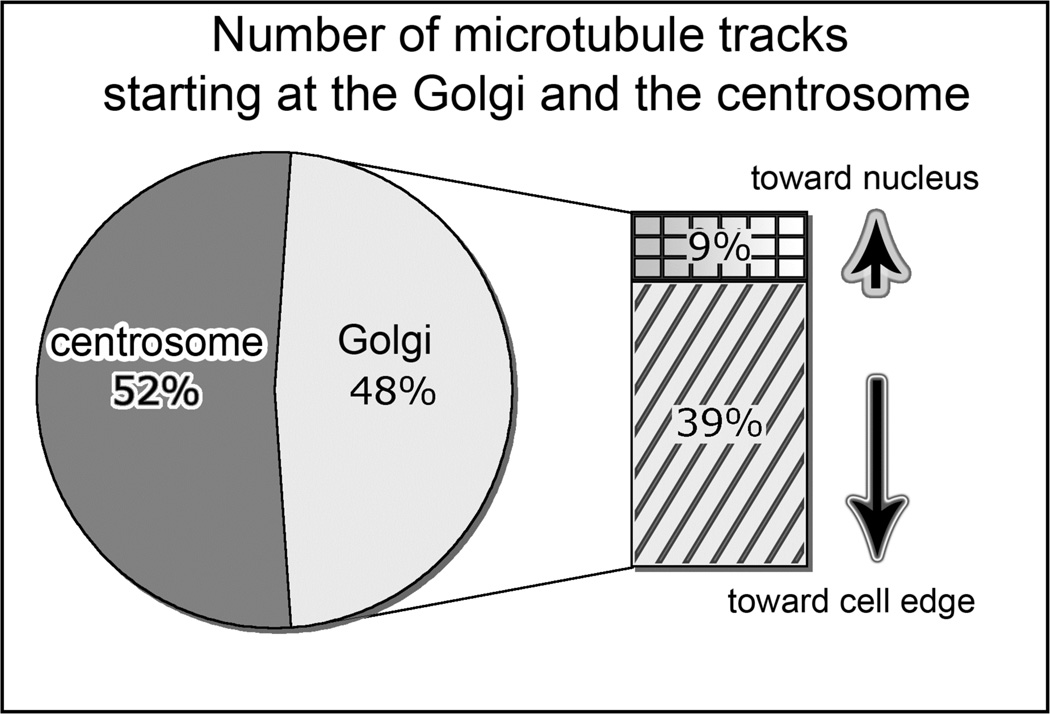
We have adopted this approach to monitor the MT nucleation by tracking the GFP-EB3 in interphase RPE1 cells in the steady state (Fig. 1). A complementary approach involves complete disassembly of the existing MTs by nocodazole and monitoring the initiation of MT nucleation after nocodazole washout. In non-differentiated RPE1 cells, these approaches allow identification of the Golgi apparatus as an alternative MT organizing center (12). MT nucleation at the Golgi is asymmetric, with the majority of MTs oriented toward the leading edge in motile cells (Fig. 2). This observation indicates that MT nucleation can be an alternative way to form the asymmetric MT array.
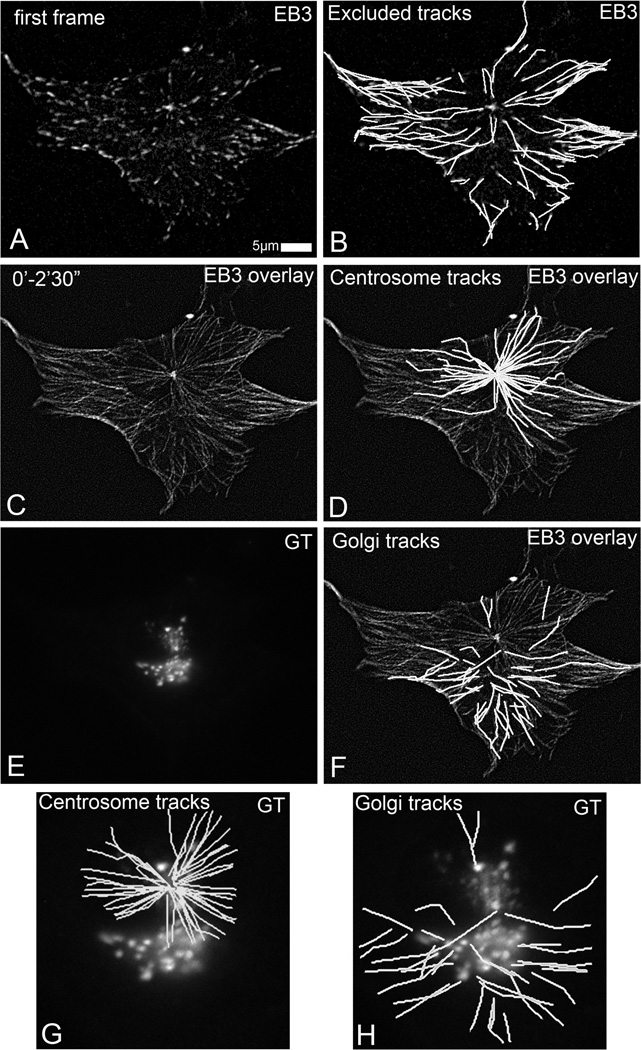
Fig 1.
Detection of Golgi-originated MTs in time-lapse recording of GFP-EB3- and mCherry-GT-expressing RPE1 cells (5 s/frame). (A) GFP-EB3 in the first frame of the video. Currently growing MTs are detected as EB3 dots. (B) MT tracks starting at EB3 dot at the first frame. These tracks are excluded from the analysis. (C) Overlaid GFP-EB3 images within 2.5 min mark all MT growth paths. (D) MT tracks starting from a single site are identified as centrosomal tracks. (E) mCherry-GT identifies Golgi complex localization. (F) noncentrosomal MT tracks (G) Centrosomal MT tracks and their relation to the Golgi position. (H) noncentrosomal (Golgi-derived) MT tracks in the cell center and their relation to the Golgi position. Modified for grayscale from [Efimov et al, 2007] with permission from [Elsevier Limited].
Fig 2.
Centrosome-nucleated MTs are symmetric and Golgi-nucleated MTs are asymmetric. Percentage and directionality of Golgi-associated tracks (583 tracks in ten cells, analyzed as in Fig 1. Modified for grayscale from [Efimov et al, 2007] with permission from [Elsevier Limited].
2. Materials
2.1 cell culture and transfection
Dulbecco's Modification of Eagle's Medium/Ham's F-12 50/50 Mix (DMEM/F12K) from mediatech, supplemented with 1.5 mM NaHCO3 solution (Thermo Scientific HyClone),10% fetal bovine serum(Maverick Biosciences), and 1 mM L-glutamine (Mediatech).
Trypsin: 0.25% Trypsin solution with EDTA, without sodium bicarbonate, calcium and magnesium from mediatech.
Fugene 6 transfection reagent from Roche.
Plasmid: +TIP marked with EGFP-EB3 (kind gift of Dr. J.V. Small, Vienna); and the centrosome marked with GFP-centrin (kind gift of Dr. M. Bornens, Paris); Golgi apparatus marked with mCherry-GT (modified from Clontech).
2.2 Live cell imaging and Immunofluorescence
-
1.
Cell culture dishes for live cell imaging: 35 mm glass bottom dish with No. 1.5 coverslips from MatTek coorperation. No. 1.5 microscope coverslips and microscope slides for immunofluorescence from Fisher Scientific.
-
2.
Human Fibronectin (BD Biosciences) is dissolved 1M urea up to 2.27 µM (1 mg/ml) stock concentration.
-
3.
Phosphate-Buffered Saline (PBS): Prepare 10× stock solution contain 1.37M NaCl, 27 mM KCl, 80 mM Na2HPO4, and 20 mM KH2PO4 is autoclaved. The stock solution is diluted into 1×working solution before use.
-
4.
Fixative: Methanol from EMD Chemicals Inc.
-
6.
Nocodazole (Sigma) is dissolve into 16.6 mM stock with DMSO and stored at −20 °C. Taxol is dissolved into 5 µM stock with DMSO stored at −20 °C
-
7.
Extracting buffer: 60mM PIPES, 25mM HEPES, 10mM EGTA, 2mM MgCl2, 0.1% Saponin adjust pH to 6.9. Store in 4°C. Immediate before use, supplemented with 0.25 nM nocodazole and 0.25nM taxol (13).
-
8.
Blocking and antibody dilution buffer: 1% BSA (Bovine Serum Albumin)/ PBS supplemented with 5% DHS (Donor Horse Serum).
-
9.
Primary antibody: monoclonal anti-alpha tubulin antibody (1:500 dilution) from Sigma. Guinea pig anti-GCC 185 antibody (1:800) were developed by Covance Inc.
-
10.
Secondary antibody: Alexa Fluor 568 anti-guinea pig IgG, highly cross absorbed Alexa Fluor 568 anti-mouse IgG from Invitrogen.
-
11.
Mounting medium: ProLong Gold antifade reagent from Invitrogen. Stored at −20 °C.
2.3 Confocal microscope
Microscope: Nikon TE2000E microscope with a built-in Z motor is equipped with a heated stage (Warner Instruments), and a PerfectFocus automated focusing device.
Optics: a Yokogawa QLC-100/CSU-10 spinning disk head (Visitec assembled by Vashaw) is attached to the microscope. Samples is illuminted by a krypton-argon laser (75 mW 488/568; Melles Griot) with AOTF and visualized with CFI PLAN APO VC 100× oil lens, double dichroic mirror and filters (Chroma) in a filter wheel (Ludl) were used for emission light.
Camera: back illuminated EM-CCD camera Cascade 512B (Photometrics) is driven by IPLab software (Scanalytics).
Software packages such as IPLab and NIH ImageJ can be used for image analysis.
3. Methods
Here, we suggest three experiments allowing identification of non-centrosomal nucleation sites, with the Golgi as an example.
First experiment involves transfection of cells with a +TIP marker and a Golgi marker with subsequent live cell imaging of MT tip movement in steady state (Fig. 1). This method allows identification of nucleation sites in cells with unchanged MT organization.
However, monitoring MTs nucleated at the Golgi as compared to the centrosome may be challenging due to the fact that Golgi apparatus is localized adjacent to the centrosome. This can be avoided by monitoring those cells where Golgi and centrosome are physically separated will help the analysis.
Another way to solve this difficulty is by monitoring MT re-polymerization after nocodazole washout. When MTs are completely dissembled by nocodazole, Golgi is also dissembled into ministacks distributed all over the cell and imaging cells shortly after nocodazole treatment can clearly identify those MTs which are nucleated from the Golgi. Thus, the second experiment is live cell imaging of cells expressing a +TIP marker and a Golgi marker in the course of nocodazole washout. This experiment is conclusive but time consuming. For the purpose of statistical evaluation of MT number organized at the Golgi, we suggest the third experiment that involves nocodazole washout approach in non-transfected cells with fixation at specific time points and immunostaining to visualize MTs and the Golgi. Below, detailed techniques required for these three experiments are described.
3.1. Live cell imaging of MT nucleation in steady state
-
1.
Cell maintenance and preparation for transfection: RPE1 cells are maintained in 5 ml tissue culture flask and passaged when reaching 80% confluence. To passage, cells were treated with trypsin for 1–2min at room temperature and split into a new 5 ml flask for maintainance at 1:4 or 1:8 dilution. For transfection, cells are plating at 1:16 dilution in 35 mm tissue culture dishes. 2 hours incubation before transfection is necessary for cells to attach to the culture dish and achieve 40%-60% confluence (see Notes 1).
-
2.
Transfection: The cells are transfected with 0.5 µg of PEGFP-EB3 using Fugene 6 according to manufacturer's instructions. Briefly, in a 1.5 ml tube, 3µl Fugene 6 is added into100 µl plain DMEM/F12k medium, then 0.5 µg GFP-EB3 is added to form the transfection mixture. After incubated at room temperature for 30 min, 1.4 ml complete medium is added to stop the reaction. The final mixture is added to cells in the 35 mm culture dish. Cells are then incubated with the transfection mixture overnight (see Notes 2).
-
3.
Glass coating: fibronectin stock solution is diluted with 1×PBS at 1:100 dilution. 150 µl diluted fibronectin is added onto the center of the Glass coverslip in MatTek dish and then incubated at 37 °C for 30 min or room temperature for one hour.
-
5.
Plating transfected cells on glass: At least 4 hours prior to live cell imaging, fibronectin is removed from the glass surface by aspiration. Transfected cells are plated on the glass bottom culture and incubated to allow cells attach to the glass surface.
-
6.
Before placing the cells on the stage, microscope stage is warmed up to 37 °C.
-
7.
For imaging, cell culture medium are overlaid with a layer of mineral oil to avoid evaporation of medium and then placed on the microscope stage.
-
8.
In order to monitor all the MTs nucleation toward all directions, Z-stack images of the entire cells with 0.2 µm Z-interval are captured at 5 sec/frame. All the images are captured with laser power 100–150 mw, and 200 ms-500 ms exposure time.
3. 2. Live cell imaging of MT nucleation after nocodazole washout
Cells are maintained and transfected as the above procedure (see step 1–5 in Subheading 3.1.).
Nocodazole treatment: To dissemble MT cytoskeleton, nocodazole stock is diluted with complete medium at 1:2000 dilution. Then the solution is added to the cells and incubated at 37 °C for 2 hours
Nocodazole washout: cells incubated with nocodazole were directly placed on the microscope stage and imaged, then washed with cold medium at the stage. Image recording is resumed immediately after washing.
To monitor MT nucleation after nocodazole washout, images are captured at 5 sec/frame with laser power 100–150 mw, and 200 ms-500 ms exposure time. Three Z-slices with 0.2 µm interval is taken at each time point to capture the Golgi mini-stack (see Notes 3).
3.3 Immunofluorescence analysis of MT nucleation after nocodazole washout
Cells are maintained as the above procedure (see step 1 in Subheading 3.1.).
Preparation of coverslip: Place a pack of coverlips in glass Petri dish and autoclave; the sterilized coverslips can be kept in the tissue culture hood.
Glass coating: use the forceps to lift the coverslip from the Petri dish and place inside the 35 mm plastic culture dish. 1 mg/ml fibronectin stock solution is then diluted with 1×PBS at 1:100 dilution. 60 µl-100 µl diluted fibronectin is added onto the center of the coverslip and then incubated at 37 °C for 30 min or room temperature for one hour.
Plating transfected cells on glass: At least 4 hours prior to nocodazole treatment, fibronectin is removed from the glass surface by aspiration. Cells are plated on 35 mm tissue culture dish containing coated coverslips and incubated to allow cells attach to the glass surface.
Nocodazole treatment: To dissemble MT cytoskeleton, nocodazole stock is diluted with complete medium at 1:2000 dilution. Then solution is added to the cells and incubated at 37 °C for 2 hours.
Preparation of fixative: During the Nocodazole incubation, 100% methanol is added into 12 well plate with 1–2 ml/well and chilled at −20 °C.
Nocodazole washout for immunofluorescence: Place two 60 mm tissue culture dishes containing 5 ml complete medium and extraction buffer, respectively, on a floating raft in the 37 °C water bath. Directly remove the 35mm culture dish containing nocodazole treated cells from the incubator and place it on ice. Wash the cells with ice-cold plain medium 5 times on ice (see Notes 4), then quickly lift the coverslip and immerse it into the complete medium in the water bath, gently swirl the dish for 45 sec to allow the re-growth of the MT (see Notes 5). Then immediately remove the coverslip from the medium and immerse it into the extraction buffer and gently swirl the dish for 40 sec to extract the free tubulin from the cytoplasm. Cells were then subjected to fixation.
Fixation: Carefully pick up the coverslip from the culture dish with the forceps and quickly submerge the coverslip into methanol prechilled in step 6 with the cell side facing up.
Cells are incubated with methonal at −20 °C for 5 min. Then washed with PBS and kept in PBS for at least one hour to rehydrate.
Cut a piece of parafilm and place it into a plastic box. Attach a piece of wet Kimwipe inside the cover of the box to keep the humidity.
Add 18 µl drops of blocking solution onto the parafilm and carefully place each coverslip onto a solution drop with the cell side facing the solution. Let the coverslip rest on the solution so that the cell side is in contact with the solution uniformly. Do not press the coverslip toward parafilm! Incubate at room temperature for 1 hour.
In a separate plastic box, prepare a similar humidity chamber as in step (4). Place 18 µl drops of primary antibody solution on parafilm. Gently lift the coverslip from the blocking solution by forceps, and place the coverslip on primary antibody drops by the same procedure described above. Incubate at room temperature for 1 hour.
Immerse the coverslip into PBS in 12 well plate with the cell side up. Wash with 3 changes of PBS each for 5 min.
Repeat steps (6) and (7) for secondary antibody.
Add a drop of mounting medium on the microscopic slide and invert the coverslip on to it with the cell side facing down. Then keep the slide at room temperature overnight or 37 °C for 1hour to dry. Seal the edge of the coverslip with the nail polish.
Once the nail polish has dried, carefully clean the surface of the coverslip with ethanol to remove the precipitates from PBS, and samples and samples are ready for microscopic examination. For long-term storage, samples should be kept at −20°C.
3.4 Image processing for plus-tip tracking
IPLab, ImageJ, Metamorph or any other appropriate software package can be used for processing. To subtract background, time-lapse images are processed by rolling ball background correction. Brightness and contrast, gamma settings were then adjusted to make both the major structure and the minor structures visible.
Z-stack images at each time point and fluorescent channel is projected by Maxi-Projection algorithm individually. In order to clearly follow MT tip dislocation, image sequence is processed to create a rolling average sequence over 4 frames.
All MT plus tip tracks are determined by a tracking algorithm such as “Manual Tracking” plugin of ImageJ software. As all the plus tip signals existing in the first frame of the sequence present already existing MTs nucleated before the observation, those MTs tracks have to be excluded from the further analysis. All the other tracks initiated during the imaging have to be tracked back to the sites of origin and classified according to the origin (Fig. 1).
Identify centrosomal MT. As +TIP binding proteins bind to centrosome, and MT tracks continually emanating form centrosome. Thus the position of the centrosome can be identified as a single spot with high fluorescence intensity. This spot normally is less than 1µm in diameter. All the MT tracks emanating from this common spot can be considered centrosomal MT.
Identify Golgi-derived MTs. To indentfiy Golgi-derived MTs, sites of MT origin have to be colocalized with the Golgi. Similarly, specific markers can be used for any sub-cellular structure under consideration.
Footnotes
Transfection can also be performed directly with the cells growing on the glass surface. As it is easy to image when cells are very well spread and flat, a dense culture should be avoided. We find that it is easy to adjust the cell confluence when transfecting cells in 35 mm plastic dishes and then splitting onto the glass surface on the day before imaging.
The exact amount of plasmid used and the incubation time after transfection need to be adjusted according to the desired expression level for imaging. Increasing the amount of plasmid and incubation time normally leads to the higher expression level. It is easy to obtain high quality images with the strong fluorescence signal. However, when overexpressed, +TIPs decorate the entire MT network and affects MT dynamics. On the other hand, weak fluorescence signal requires high laser intensity and/or long exposure time to image, which may induce photo damage to the cells. Therefore it is crucial to select cells with appropriate expression levels. Unfortunately, different imaging systems have different sensitivity, so no fixed criteria can be applied to select the appropriate cells. It is necessary to determine the best condition through trial and error. Alternatively, a stable cell line expressing +TIP at the appropriate level can be established first, and then used for imaging.
Both the time interval and Z interval need to be adjusted according to the cell line and the imaging system used. Nyquist sampling rate can be calculated according to the individual image system and used as a starting point to test for Z interval. Apart from photodamage, oversampling is better than undersampling.
It is important to wash on ice to inhibit the MT re-polymerization during the washing step. To completely wash away any trace amount of nocodazole, wash media should fill the whole volume of the cell culture dish. For one 35 mm culture dish, 5 ml medium is necessary per wash.
Short regrowth times should be considered. Because MTs are very dynamic structures, a small variation in time may generate significant difference in MT organization. In general, MT nucleation and growth are rapid. To monitor the nucleation, images should be captured with short interval. Since MT nucleation occurs in 3D, it is also necessary to take Z-stack images at each time point in order to quantify the nucleation events.
References
- 1.Salaycik KJ, Fagerstrom CJ, Murthy K, Tulu US, Wadsworth P. Quantification of microtubule nucleation, growth and dynamics in wound-edge cells. J Cell Sci. 2005;118:4113–4122. doi: 10.1242/jcs.02531. [DOI] [PubMed] [Google Scholar]
- 2.Bergen LG, Kuriyama R, Borisy GG. Polarity of microtubules nucleated by centrosomes and chromosomes of Chinese hamster ovary cells in vitro. J. Cell Biol. 1980;84:151–159. doi: 10.1083/jcb.84.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirschner M, Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- 4.Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3[beta] and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- 5.Keating TJ, Peloquin JG, Rodionov VI, Momcilovic D, Borisy GG. Microtubule release from the ΓÇëcentrosome. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:5078–5083. doi: 10.1073/pnas.94.10.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad FJ, Baas PW. Microtubules released from the neuronal centrosome are transported into the axon. J Cell Sci. 1995;108:2761–2769. doi: 10.1242/jcs.108.8.2761. [DOI] [PubMed] [Google Scholar]
- 7.Khodjakov A, Copenagle L, Gordon MB, Compton DA, Kapoor TM. Minus-end capture of preformed kinetochore fibers contributes to spindle morphogenesis. The Journal of Cell Biology. 2003;160:671–683. doi: 10.1083/jcb.200208143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bugnard E, Zaal KJ, Ralston E. Reorganization of microtubule nucleation during muscle differentiation. Cell Motil Cytoskeleton. 2005;60:1–13. doi: 10.1002/cm.20042. [DOI] [PubMed] [Google Scholar]
- 9.Malikov V, Kashina A, Rodionov V. Cytoplasmic Dynein Nucleates Microtubules to Organize Them into Radial Arrays In Vivo. Mol. Biol. Cell. 2004;15:2742–2749. doi: 10.1091/mbc.E03-10-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komarova YA, Vorobjev IA, Borisy GG. Life cycle of MTs: persistent growth in the cell interior, asymmetric transition frequencies and effects of the cell boundary. J Cell Sci. 2002;115:3527–3539. doi: 10.1242/jcs.115.17.3527. [DOI] [PubMed] [Google Scholar]
- 11.Piehl M, Tulu US, Wadsworth P, Cassimeris L. Centrosome maturation: Measurement of microtubule nucleation throughout the cell cycle by using GFP-tagged EB1. PNAS. 2004;101:1584–1588. doi: 10.1073/pnas.0308205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Efimov A, Kharitonov A, Efimova N, Loncarek J, Miller PM, Andreyeva N, Gleeson P, Galjart N, Maia AR, McLeod IX, Yates JR, Maiato H, Khodjakov A, Akhmanova A, Kaverina I. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev Cell. 2007;12:917–930. doi: 10.1016/j.devcel.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhai Y, Borisy GG. Quantitative determination of the proportion of microtubule polymer present during the mitosis-interphase transition. J Cell Sci. 1994;107:881–890. doi: 10.1242/jcs.107.4.881. [DOI] [PubMed] [Google Scholar]