Abstract
It has been shown that some opium derivatives promote cell death via apoptosis. This study was designed to examine the influence of opium addiction on brain and liver cells apoptosis in male and female diabetic and non-diabetic Wistar rats. This experimental study was performed on normal, opium-addicted, diabetic and diabetic opium-addicted male and female rats. Apoptosis was evaluated by TUNEL and DNA fragmentation assays. Results of this study showed that apoptosis in opium-addicted and diabetic opium-addicted brain and liver cells were significantly higher than the both normal and diabetic rats. In addition, we found that apoptosis in brain cells of opium-addicted and diabetic opium-addicted male rats were significantly higher than opium-addicted and diabetic opium-addicted female, whereas apoptosis in liver cells of opium-addicted and diabetic opium-addicted female rats were significantly higher than opium-addicted and diabetic opium-addicted male. Overall, these results indicate that opium probably plays an important role in brain and liver cells apoptosis, therefore, leading neurotoxicity and hepatotoxicity. These findings also in away possibly means that male brain cells are more susceptible than female and interestingly liver of females are more sensitive than males in induction of apoptosis by opium.
Keywords: Addiction, Apoptosis, Brain cells, Diabetes, Liver cells
INTRODUCTION
Apoptosis or programmed cell death is known as a normal process in the development of the nervous system and aging. Apoptosis play a role in neurodegenerative diseases, including Alzheimer's and Parkinson's diseases [1]. Apoptosis is also a pathologic feature of the brain injury, certain inflammatory diseases of the brain and central nervous system infection, such as human immunodeficiency virus (HIV)-associated dementia [2]. Apoptotic effects of opioids such as morphine [3-7], heroines [8], codeine [9], in neurons has been demonstrated.
Opium is a narcotic analgesic drug which is originally obtained from the unripe seed pods of the opium poppy. Opium is used as the raw material for the synthesis of some medications such as morphine, noscapine, papaverine and codeine which contains 8~17%, 1~10%, 0.5~1.5% and 0.7~5% of opium, respectively [10].
Effects of opium on some biochemical parameters [11,12] and TGF-β [13] have been reported. Due to the lack of literature on the effects of opium we aimed this project to examine its effects on brain and liver cells apoptosis. Apoptotic activity of morphine [3-5] noscapine [14-17], codeine [9] and papaverine [18] has been shown in the in vitro and in vivo experimental systems. Morphine promotes Jurkat cells apoptosis [3] Morphine enhances the expression of both Fas and Fas ligand (FasL), and induces macrophage apoptosis [6]. Fas (CD95)-induced hepatocyte apoptosis and cytotoxic activities of infiltrating neutrophils in the injured liver are two major events leading hepatitis [7]. Noscapine and papaverine are other important derivatives of opium [10]. Noscapine is commonly used as an antitussive agent in many countries. It binds stoichiometrically to tubulin, causes microtubule assembly and arrests mammalian cells in mitosis phase, and finally induces apoptosis [14,15]. Noscapine increases sensitivity of HCT116 colon carcinoma cells to apoptotic stimuli [17].
Papaverine is a vasodilator which is commonly used for the treatment of vasospasmic diseases such as cerebral spasm associated with subarachnoid hemorrhage, and in the prevention of spasm of coronary artery bypasses graft by intraluminal and/or extraluminal administration. Papaverine could damage endothelial and smooth muscle cells by inducing changes which are associated with events leading to apoptosis [18].
There are however, more than 20 alkaloids [19] and more than 70 components [20] in opium, thus, its effect on apoptosis and cell functions could hence be different from pure morphine, noscapine, codeine and papaverine.
Our previous findings on the effects of opium on biochemical parameters and TGF-β and also the influence of opium components on apoptosis encouraged us to investigate the effects of opium on apoptosis of brain and liver in male and female rats following repeated daily opium administration at a maximum tolerance dose (150 mg/kg). Due to the fact that, some people around the world believe that opium posses therapeutic properties on many disorders, particularly diabetes mellitus [11,12] in this study we designed a diabetic animal model to challenge this public believe on opium properties in an experimental manner.
METHODS
Materials
Opium was dedicated by anti-drug section of Kerman Police (Iran). Based on their information the origin of opium was Helmand in Afghanistan. Streptozocin (STZ) were purchased from Pfizer Company (AG, Zurich, Switzerland). Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-X nick end labeling (TUNEL) kit was purchased from Roche Diagnostic, Manheim, Germany. Proteinase K was from Roche, Germany [20µg/ml in phosphate-buffered saline (PBS)]. DNA extraction kit was from Cinnagen, Iran. Glucose oxidase kit was also prepared from Pars azemoon company (Tehran, Iran). All of other materials were of analytical grade and obtained from standard sources.
Methods
In this experimental study, 70 male and 70 female Wistar rats (weighting 250~300 gr) were entered into the study. Animals were kept on a 12 hours light-dark cycle and had freely accessed to food and water. All animals' procedures were in accordance to "guide for the care and use of laboratory animals (NIH US publication no. 85-23 revised 1985)". 20 of each group were used as control and 50 male and 50 female rats were used for inducing diabetes by injection of streptozocin (dissolved in sodium citrate buffer, pH 4.4) with a single dose of 60 mg/kg of the body weight intravenously into the tail vein [21]. After 3 days, 0.5 ml blood was collected from orbit cavity by a thin heparinized tube. Plasma glucose was measured using glucose oxidase method and the animals with glucose level more than 250 mg/dl were regarded as diabetic [22]. The success rate of inducing diabetes was approximately 90%. 25 of the above animals along with 10 of the control (non-diabetic) animals were treated with a daily double dose (8 AM and 8 PM, ip) of opium for 8 consecutive days (Opium was dissolved in fresh saline). The protocol of opium treatment was as follow: Days one to five, 30, 60, 90, 120 and 150 mg/kg respectively and continued on 150 mg/kg for the consecutive three days (150 mg/kg was the maximum tolerable dose for animals). At day 9 after weight measurement, a single dose of 150 mg/kg opium was injected and after 3 hours animals were humanly killed by decapitation under ether anesthesia. The control groups received normal saline. All control animals survived during 14 days of experiment period. Overall 40% of STZ-diabetic rats survived during this period and there were no significant difference between males and females in this regard. Finally the liver and brain of 7 animals of each group were used for apoptosis assessment. The withdrawal signs in opium-dependent rats were observed from the 5th day of opium injection. These signs which were sometime observed for a short time before next injection were wet-dog shakes as the first sign, and then hyperactivity, irritability, head shakes, ptosis, and writhing.
Tissue preparation for histological studies
The rat brains and liver were rapidly removed and fixed at room temperature in phosphate-buffered saline (PBS; 0.1 M Sodium phosphate, 0.14 M NaCl, pH 7.4) containing 3.7% paraformaldehyde for 30 minutes. The tissues transferred to fresh 3.7% paraformaldehyde and incubated for 7.5 hours. Brains transferred to 75% ethanol and wash for 1 hour and repeated the wash using fresh 75% ethanol. This step was repeated by using of 95% ethanol and washing process was followed by 100% ethanol. The tissues transferred to xylenes and washed twice for 1 hour. The tissues embedded in molten paraffin wax (58℃) for 1 hour using new paraffin wax.
Apoptosis studies
The apoptotic nuclei DNA fragmentation (the final result of the apoptotic process), was evaluated by Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-X nick end labeling (TUNEL) technique using the in situ cell death detection kit as described by manufacturer. Briefly, paraffin-embedded tissue sections (5µm), were treated with proteinase K (2µg/ml in PBS) at room temperature for 10 min and then soaked in PBS for 5 min. Sections were then incubated with 50µl terminal deoxynucleotidyl transferase and nucleotide mixture (TUNEL Label Mix) in a humidified chamber at 37℃ for 1 hour in the dark. The sections were immersed in PBS. Slides were washed in PBS, and visualized with a fluorescent microscope (Micros Austria) using an excitation wavelength of 450~500 nm. Quantitative evaluation of apoptosis index was performed by manual counting of positively stained nuclei in 30 microscopic fields at ×100 magnifications.
Detection of brain cell apoptosis by gel electrophoresis
Gel electrophoresis is a simple method for detection of apoptosis. DNA extraction was performed according to the manufacturer's protocol. Briefly, a volume of 100µl protease was added to 50 mg brain or liver tissue and was vigorously vortexed, and followed by addition of 5µl of protease and then was incubated at 55℃ for 2 hours. At the end of incubation time 400µl of lyses solution was added, vortexed and homogenized for 15~20 minutes and followed by addition of 300µl of precipitation solution and vortexing for 3~5 seconds and then centrifuged for 10 minutes at 1,200 g. The supernatant was discarded and 1 ml of washing buffer was added to the resultant pellet, 3~5 seconds vigorously vortexed and centrifuged for 5 minutes at 1,200 g. The washing step was repeated twice and at the end of second step washing buffer was discarded and the pellet was dried at 65℃ for 5 minutes. Finally 50µl of solvent buffer was added, gently vortexed and incubated at 65℃ for 5 minutes, centrifuged for 30 seconds at 1,200 g. The resultant product is a biphasic solution which the upper layer is DNA product and the lower is the other materials. DNA was run on a 1.8% agarose gel electrophoresis at 5 V/cm in 0.5×TE buffer (Tris 10 mM; EDTA 1mM, pH 8.0) containing 10µg/ml ethidum bromide.
Statistical analysis
All analysis was performed by SPSS (version 18; SPSS Inc.). For comparison of mean values between two groups, the student's t-test was used. To compare values between multiple groups, analysis of variance (ANOVA) was applied. All values are means±SEM. p<0.05 was considered statistically significant.
RESULTS
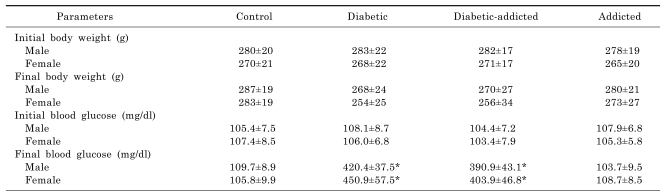
The changes in blood glucose level and body weights are shown in Table 1. There is no significant difference between weights of control, opium-addicted (non-diabetic) and opium-addicted diabetic rats.
Table 1.
Blood glucose and weights of the study groups. Values are mean±SEM of 7 rats in each group
*p<0.001 versus control.
Our results showed that there was a significant difference in apoptosis as follow:
Brain
1. In male rats
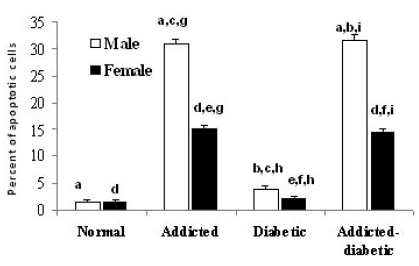
The difference between normal (1.50%±0.373) and opium-addicted diabetic (31.80%±0.774) and opium-addicted non-diabetic (31.00%±0.830) was statically significant (p<0.001 and p<0.001 respectively), while there was not significant difference between normal (1.50%±0.373) and non-addicted diabetic (3.90%±0.526) groups (p= 0.62). There was also a significant difference between non-addicted diabetic (3.90%±0.526) and opium-addicted diabetic (31.80%±0.774) groups (p<0.001). Comparison of our data indicated that a significant difference between opium-addicted non-diabetic (31.00%±0.830) and non-addicted diabetic (3.90%±0.526) groups was also observed (p<0.001) (Fig. 1).
Fig. 1.
Comparison of brain cells apoptosis in normal, opium addicted, diabetic and opium diabetic-addicted Wistar rats. Data are from 7 animals is presented in each group. In male rats: aSignificant difference between normal, opium-addicted diabetic and opium-addicted non-diabetic (p<0.001 and p<0.001 respectively). bSignificant difference between non-addicted diabetic and opium-addicted diabetic groups (p<0.001). cSignificant difference between opium-addicted non-diabetic and non-addicted diabetic groups (p<0.001). In female rats: dSignificant differences between normal, opium-addicted diabetic and opium-addicted non-diabetic groups (p<0.001 and p<0.001 respectively). eSignificant difference between opium-addicted and non-addicted diabetic groups (p <0.001). fSignificant difference between non-addicted diabetic and opium-addicted diabetic groups (p<0.001). Sex associated: gSignificant difference between opium-addicted male and female (p< 0.001). hSignificant difference between non-addict diabetics male and non-addict diabetics female (p=0.006). iSignificant difference between opium-addicted diabetic males and opium-addicted diabetic females (p<0.001).
2. In female rats
We observed significant differences in female normal (1.40%±0.0.267) and opium-addicted diabetic (14.36%±0.256) and opium-addicted non-diabetic (15.20%±0.696) groups (p<0.001 and p<0.001 respectively). The difference is clearly significant in opium-addicted (15.20%±0.696) and non-addicted diabetic (2.00%±0.05) groups when they were compared (p<0.001). Interestingly, the non-addicted diabetic (2.00%±0.05) and opium-addicted diabetic (14.36%±0.256) groups had also differences when they were compared (p<0.001) (Fig. 1).
Liver
1. In male rats
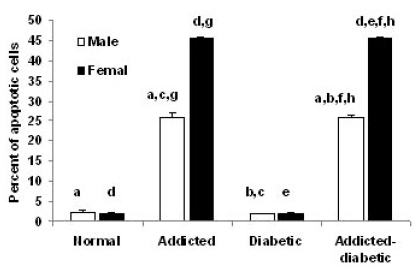
The difference between normal (2.30%±0.448), opium-addicted diabetics (25.85±0.558) and opium-addicted non-diabetics (26.20%±0.800) was statistically significant (p<0.001 and p<0.001 respectively), but a significant difference was not observed between normal (2.30%±0.448) and non-addicted diabetic (2.02%±0.064) groups (p=0.649). There was also a significant difference between non-addicted diabetics (2.02%±0.064) and opium-addicted diabetics (25.85%±0.558) (p<0.001).Our data analysis indicated a significant difference between opium-addicted non-diabetic (26.20%±0.800) and non-addicted diabetic (2.02%±0.064) groups (p<0.001) (Fig. 2).
Fig. 2.
Comparison of liver cells apoptosis in normal, opium addicted, diabetic and opium diabetic-addicted rats. Data are from 7 animals is presented in each group. In male rats: aSignificant difference between normal, opium-addicted diabetics and opiumaddicted non-diabetics (p<0.001 and p<0.001 respectively). bSignificant difference between non-addicted diabetics and opium-addicted diabetics (p<0.001). cSignificant difference between opium-addicted non-diabetic and non-addicted diabetic groups (p<0.001). In female rats: dSignificant difference between normal, opium-addicted diabetics and opium-addicted non-diabetics (p<0.001 and p <0.001 respectively). eSignificant difference between opium-addicted and non-addicted diabetic groups (p<0.001). fSignificant difference between non-addicted diabetics and opium-addicted diabetics (p<0.001). Sex associated: gSignificant difference between opiumaddicted male and female rats (p<0.001). hSignificant difference between opium-addicted diabetic females and addicted diabetic males (p<0.001).
2. In female rats
We found significant differences in female normal (1.90±0.314), opium-addicted diabetics (45.50±0.522) and opium-addicted non-diabetics (45.00±0.715) (p<0.001 and p<0.001 respectively). There is an obvious significant difference between opium-addicted (45.00±0.715) and non-addicted diabetic (2.09±0.08) groups (p<0.001). Obviously, the non-addicted diabetics (2.09±0.08) and opium-addicted diabetics (45.50±0.522) also showed significant difference when they compared (p<0.001) (Fig. 2).
Sex associated apoptosis
1. Brain
Assessment of the effects of sex on apoptosis in our study showed that there was a significant difference between opium-addicted male (31.00±0.830) and female (15.20±0.696) (p<0.001). There was also a significant difference between non-addict diabetics male (3.90%±0.526) and non-addict diabetics female rats (2.00%±0.05) (p= 0.006). We also observed that opium-addicted diabetic males (31.80±0.774) have been significantly more affected by apoptosis than addicted diabetic females (14.36±0.256) (p<0.001) (Fig. 1).
2. Liver
In this study, we showed a gender dependent pattern of the impact of opium on liver cells apoptosis. Evaluation of the gender related effects of opium on apoptosis showed that there was a significant difference between opium-addicted male (26.20±0.800) and female rats (45.00±0.715) (p<0.001). We also showed that opium-addicted diabetic females (45.50±0.522) were more significantly affected by apoptosis than addicted diabetic males (25.85±0.558) (p<0.001) (Fig. 2).
Based on our findings apoptosis is following a differential pattern in male and female rats, so that in male rats more apoptotic brain cells but in females more apoptotic liver cells were observed.
DISCUSSION
In the present study we found that apoptosis is induced by opium addiction in diabetic and non-diabetic rat's brain and liver. In addition brain cells of male rats are more susceptible to induction of apoptosis than female and interestingly liver of females are more sensitive than males in induction of apoptosis by opium. Some people in communities believe that opium has positive therapeutic effects on some disorders [12] including diabetes mellitus [11]. This is why opium is prescribed by ordinary people and it makes an excuse for consumers for explaining of its application. Afghanistan, the eastern neighbor of Iran is the largest opium producer and along the road of poppy transit to Europe. Based on a WHO report, more than 2.8% of the adult population of Iran is opium-addicted [23].
Having chronic exposure to opiates lead to impairment of impairs learning and memory [24] and opiate tolerance and dependency have been suggested to induce a pathological form of learning and memory [25] There are many reports regarding induction of apoptosis by major components of opium including; morphine, codeine, noscapine and papaverine.
Chronic treatment with high doses of morphine induces cell death via apoptosis in both neurons and glial cells [4]. Chronic, but not acute, intrathecal administration of morphine to rats produced neuronal apoptosis in the dorsal horn spinal cord [26]. In addition, chronic intraperitoneal treatment with morphine or heroin and heroin withdrawal induce apoptosis in the cerebral cortex of rats [5,27]. It has been shown that acute morphine (single dose) did not induced apoptosis but chronic administration of large morphine doses induced apoptosis in brain [4]. The morphological studies showed that chronic morphine treatment leading substantial injuries of brain. For example, chronic morphine treatment causes structural defects in cerebral cortex, hippocampus, and huge reduction in dendritic complexity and decreases denderitic growth in the cerebral cortex and hippocampus [28,29].
Life threatening effects of morphine are generally expected to happen at high doses, and this was our reason for selecting of maximum tolerance dose of opium (150 mg/kg) in the current study. Morphine enhances apoptosis of human umbilical vein endothelial cells (HUVECs) via enhancement of intracellular reactive oxygen species (ROS), leading reduced mitochondrial membrane potentials (MMPs) and also release of NO and activated NF-kappa B [30].
Buprenorphine which is a semi-synthetic opioid derivative commonly is used for treatment of heroin addiction can induce liver and kidney failure in consumers, possibly through direct mitochondrial toxicity [31]. Liver plays a major role in body defense against xenobiotics hence this organ was chosen to investigate in opium addicted situation. It has been reported that systemic disease, most notably liver disease, is common among fatal opioid toxicity cases [23]. Morphine-induced apoptosis is reported in SH-SY5Y cells through activation of JNK mitochondrial death pathway, and ROS signaling exerts its positive feedback regulation on JNK activity [32].
Papaverine is a vasodilator which indicated to induce apoptosis in vascular endothelial and smooth muscle cells [18].
Noscapine which serves as a tubulin-binding agent has also been demonstrated to facilitate apoptosis of a colorectal carcinoma cells [17].
Although there are huge amounts of reports regarding the effects of opiate derivatives on brain cells apoptosis in database, to our knowledge this is the first study on the effects of opium on brain cells apoptosis.
Although, gender differences our results showed that apoptosis in brain cells of addicted male rats were significantly higher than addicted female, but in liver cells of addicted female rats were significantly higher than addicted male. This finding is consistent with our previous studies on biochemical parameters [11,12], TGF-β [13] and many other reports on brain apoptosis and brain injuries [33,34], liver apoptosis [35] or injury [36]. It has been shown that females are more protected from cerebrovascular diseases than males. The underlying mechanisms which are involved in these sex differences remained unclear but exposure to gonadal hormones, particularly estrogen, has been through to play a major role [33,37] Apoptosis is believed to be triggered by intracellular signals and or extracellular by extrinsic death activators [1]. The responsible agents of activation of a specific triggering event in each pathway have very variated results in male or female animals or tissue, suggesting that intrinsic differences are mostly based on sex [33]. One of the differences regarding gender depending effects of opioid could probably be due to the variation in the individual tissues and also possibly differential interruption of sex hormone functions in male and female. It is also probable that the level of apoptosis such as inhibitory apoptosis protein (IAP) or products of bcl-gene groups are different in various gender and tissues; therefore, it is valuable to examine the level of these proteins in different genders. On the other hand, the expression of these regulators of apoptosis may have defects at the level of translation, transcription or even degrade due to the opium consumption.
Diabetes demonstrated that induce apoptosis in central nervous system and also in neuronal related tissues, including peripheral nervous system, hippocampus, and ganglions [22,38,39].
Increased mitochondrial ROS, has been reported in hyperglycemic liver cells, which in turn activates apoptosis signal-regulating kinase 1 (ASK1) and c-jun NH2-terminal kinases (JNK), increases serine phosphorylation of IRS-1, and decreases insulin-stimulated tyrosine phosphorylation of IRS-1 [40]. Based on vulgar believe, some diabetics individuals consume opium for reduction of diabetic defects [11]. These believe encouraged us to investigate the effects of opium on brain and liver apoptosis in diabetic rats. Although differential expression of genes in liver in diabetes were shown [41], but as it is clear from Fig. 1 there is no difference between diabetic and non-diabetic groups in this regard. Pathogenesis of hyperglycemia needs probably longer time from that we had in this study. The difference between groups may be attributed to the effect of withdrawal on apoptosis, however this is unlikely because the withdrawal time in this study was short as opium injection was repeated each 12 hours (see method section).
In summary, our results showed that opium addiction could possibly impose damage in brain and liver cells by inducing apoptosis. In addition herein we showed that brain cells of male rats are more susceptible to opium induced apoptosis than female, but liver cells of female rats are more susceptible to induction of apoptosis by opium than male. It is worth to note that some patients, in particular diabetics' individuals often consume opium at high doses for a long period of time [11] that could be deleterious for the patients' liver and nervous system.
ACKNOWLEDGEMENTS
The authors would like to thank Farzan Institute for Research and Technology for technical assistance. We also thank Prof. Hamid Najafipur for his helpful scientific advice.
Source of Support: This work was supported by grants from Rafsanjan University of Medical Sciences, Iran. The authors thankfully acknowledge for support of the dissertation grant.
ABBREVIATIONS
- HIV
human immunodeficiency virus
- TGF-β
transforming growth factor beta
- FasL
Fas ligand
- STZ
streptozocin
- PBS
phosphate-buffered saline
- TUNEL
terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-X nick end labeling
- ANOVA
analysis of variance
- EDTA
ethylene diamine tetra acetic acid
- WHO
world health organization
- HUVECs
human umbilical vein endothelial cells
- ROS
reactive oxygen species
- MMPs
mitochondrial membrane potentials
- SPSS
statistical package for the social sciences
- NIH
national institutes of health
- NO
nitric oxide
- NF-κB
nuclear factor kappa B
- TE
tris-EDTA
- IAP
inhibitory apoptosis protein
- ASK1
apoptosis signal-regulating kinase 1
- JNK
c-jun NH2-terminal kinases
References
- 1.Sastry PS, Rao KS. Apoptosis and the nervous system. J Neurochem. 2000;74:1–20. doi: 10.1046/j.1471-4159.2000.0740001.x. [DOI] [PubMed] [Google Scholar]
- 2.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 3.Yin D, Woodruff M, Zhang Y, Whaley S, Miao J, Ferslew K, Zhao J, Stuart C. Morphine promotes Jurkat cell apoptosis through pro-apoptotic FADD/P53 and anti-apoptotic PI3K/Akt/NF-kappaB pathways. J Neuroimmunol. 2006;174:101–107. doi: 10.1016/j.jneuroim.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Emeterio EP, Tramullas M, Hurlé MA. Modulation of apoptosis in the mouse brain after morphine treatments and morphine withdrawal. J Neurosci Res. 2006;83:1352–1361. doi: 10.1002/jnr.20812. [DOI] [PubMed] [Google Scholar]
- 5.Boronat MA, García-Fuster MJ, García-Sevilla JA. Chronic morphine induces up-regulation of the pro-apoptotic Fas receptor and down-regulation of the anti-apoptotic Bcl-2 oncoprotein in rat brain. Br J Pharmacol. 2001;134:1263–1270. doi: 10.1038/sj.bjp.0704364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singhal P, Kapasi A, Reddy K, Franki N. Opiates promote T cell apoptosis through JNK and caspase pathway. Adv Exp Med Biol. 2001;493:127–135. doi: 10.1007/0-306-47611-8_15. [DOI] [PubMed] [Google Scholar]
- 7.Jaume M, Jacquet S, Cavaillès P, Macè G, Stephan L, Blanpied C, Demur C, Brousset P, Dietrich G. Opioid receptor blockade reduces Fas-induced hepatitis in mice. Hepatology. 2004;40:1136–1143. doi: 10.1002/hep.20428. [DOI] [PubMed] [Google Scholar]
- 8.Cunha-Oliveira T, Rego AC, Garrido J, Borges F, Macedo T, Oliveira CR. Street heroin induces mitochondrial dysfunction and apoptosis in rat cortical neurons. J Neurochem. 2007;101:543–554. doi: 10.1111/j.1471-4159.2006.04406.x. [DOI] [PubMed] [Google Scholar]
- 9.Hitosugi N, Hatsukari I, Ohno R, Hashimoto K, Mihara S, Mizukami S, Nakamura S, Sakagami H, Nagasaka H, Matsumoto I, Kawase M. Comparative analysis of apoptosisinducing activity of codeine and codeinone. Anesthesiology. 2003;98:643–650. doi: 10.1097/00000542-200303000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Schiff PL. Opium and its alkaloids. Am J Pharm Educ. 2002;66:186–194. [Google Scholar]
- 11.Karam GA, Reisi M, Kaseb AA, Khaksari M, Mohammadi A, Mahmoodi M. Effects of opium addiction on some serum factors in addicts with non-insulin-dependent diabetes mellitus. Addict Biol. 2004;9:53–58. doi: 10.1080/13556210410001674095. [DOI] [PubMed] [Google Scholar]
- 12.Karam GA, Rashidinejad HR, Aghaee MM, Ahmadi J, Rahmani MR, Mahmoodi M, Azin H, Mirzaee MR, Khaksari M. Opium can differently alter blood glucose, sodium and potassium in male and female rats. Pak J Pharm Sci. 2008;21:180–184. [PubMed] [Google Scholar]
- 13.Asadikaram G, Asiabanha M, Sayadi A, Jafarzadeh A, Hassanshahi G. Impact of opium on the serum levels of TGF-β in diabetic, addicted and addicted-diabetic rats. Iran J Immunol. 2010;7:186–192. [PubMed] [Google Scholar]
- 14.Ye K, Ke Y, Keshava N, Shanks J, Kapp JA, Tekmal RR, Petros J, Joshi HC. Opium alkaloid noscapine is an antitumor agent that arrests metaphase and induces apoptosis in dividing cells. Proc Natl Acad Sci USA. 1998;95:1601–1606. doi: 10.1073/pnas.95.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahmoudian M, Mojaverian N. Efffect of noscapine, the antitussive opioid alkaloid, on bradykinin-induced smooth muscle contraction in the isolated ileum of the guinea-pig. Acta Physiol Hung. 2001;88:231–237. doi: 10.1556/APhysiol.88.2001.3-4.5. [DOI] [PubMed] [Google Scholar]
- 16.Newcomb EW, Lukyanov Y, Smirnova I, Schnee T, Zagzag D. Noscapine induces apoptosis in human glioma cells by an apoptosis-inducing factor-dependent pathway. Anticancer Drugs. 2008;19:553–563. doi: 10.1097/CAD.0b013e3282ffd68d. [DOI] [PubMed] [Google Scholar]
- 17.Aneja R, Ghaleb AM, Zhou J, Yang VW, Joshi HC. p53 and p21 determine the sensitivity of noscapine-induced apoptosis in colon cancer cells. Cancer Res. 2007;67:3862–3870. doi: 10.1158/0008-5472.CAN-06-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao YJ, Stead S, Lee RM. Papaverine induces apoptosis in vascular endothelial and smooth muscle cells. Life Sci. 2002;70:2675–2685. doi: 10.1016/s0024-3205(02)01525-4. [DOI] [PubMed] [Google Scholar]
- 19.Venturella VS. Natural Product. In: Gennard AR, editor. The Science and Practice of Pharmacy. 19th ed. Remington: Mack Publishing Company; 1995. pp. 400–402. [Google Scholar]
- 20.Buchbauer G, Nikiforov A, Remberg B. Headspace constituents of opium. Planta Med. 1994;60:181–183. doi: 10.1055/s-2006-959447. [DOI] [PubMed] [Google Scholar]
- 21.Akbarzadeh A, Norouzian D, Mehrabi MR, Jamshidi Sh, Farhangi A, Allah Verdi A, Mofidian1 SMA, Lame Rad B. Induction of diabetes by streptozocin in rats. Indian J Clin Biochem. 2007;22:60–64. doi: 10.1007/BF02913315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jafari Anarkooli I, Sankian M, Ahmadpour S, Varasteh AR, Haghir H. Evaluation of Bcl-2 family gene expression and Caspase-3 activity in hippocampus STZ-induced diabetic rats. Exp Diabetes Res. 2008;2008:638467. doi: 10.1155/2008/638467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chawla S, Korenblik A, Kunnen S. Annual prevalence of drug abuse. Austria: Wold drug report, United Nations Publication, United Nations Office on Drugs and Crime Vienna; 2005. pp. 363–366. [Google Scholar]
- 24.Pu L, Bao GB, Xu NJ, Ma L, Pei G. Hippocampal long-term potentiation is reduced by chronic opiate treatment and can be restored by re-exposure to opiates. J Neurosci. 2002;22:1914–1921. doi: 10.1523/JNEUROSCI.22-05-01914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- 26.Mao J, Sung B, Ji RR, Lim G. Neuronal apoptosis associated with morphine tolerance: evidence for an opioid-induced neurotoxic mechanism. J Neurosci. 2002;22:7650–7661. doi: 10.1523/JNEUROSCI.22-17-07650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Fuster MJ, Ferrer-Alcón M, Miralles A, García-Sevilla JA. Modulation of Fas receptor proteins and dynamin during opiate addiction and induction of opiate withdrawal in rat brain. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:421–431. doi: 10.1007/s00210-003-0801-9. [DOI] [PubMed] [Google Scholar]
- 28.Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci USA. 1996;93:11202–11207. doi: 10.1073/pnas.93.20.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson TE, Gorny G, Savage VR, Kolb B. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse. 2002;46:271–279. doi: 10.1002/syn.10146. [DOI] [PubMed] [Google Scholar]
- 30.Hsiao PN, Chang MC, Cheng WF, Chen CA, Lin HW, Hsieh CY, Sun WZ. Morphine induces apoptosis of human endothelial cells through nitric oxide and reactive oxygen species pathways. Toxicology. 2009;256:83–91. doi: 10.1016/j.tox.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Faulkner L, Altmann DM, Ellmerich S, Huhtaniemi I, Stamp G, Sriskandan S. Sexual dimorphism in superantigen shock involves elevated TNF-alpha and TNF-alpha induced hepatic apoptosis. Am J Respir Crit Care Med. 2007;176:473–482. doi: 10.1164/rccm.200611-1712OC. [DOI] [PubMed] [Google Scholar]
- 32.Fuggetta MP, Di Francesco P, Falchetti R, Cottarelli A, Rossi L, Tricarico M, Lanzilli G. Effect of morphine on cell-mediated immune responses of human lymphocytes against allogeneic malignant cells. J Exp Clin Cancer Res. 2005;24:255–263. [PubMed] [Google Scholar]
- 33.Lang JT, McCullough LD. Pathways to ischemic neuronal cell death: are sex differences relevant? J Transl Med. 2008;6:33. doi: 10.1186/1479-5876-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vagnerova K, Koerner IP, Hurn PD. Gender and the injured brain. Anesth Analg. 2008;107:201–214. doi: 10.1213/ane.0b013e31817326a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sueoka E, Sueoka N, Kai Y, Okabe S, Suganuma M, Kanematsu K, Yamamoto T, Fujiki H. Anticancer activity of morphine and its synthetic derivative, KT-90, mediated through apoptosis and inhibition of NF-kappaB activation. Biochem Biophys Res Commun. 1998;252:566–570. doi: 10.1006/bbrc.1998.9695. [DOI] [PubMed] [Google Scholar]
- 36.Maneckjee R, Minna JD. Opioids induce while nicotine suppresses apoptosis in human lung cancer cells. Cell Growth Differ. 1994;5:1033–1040. [PubMed] [Google Scholar]
- 37.Liu M, Dziennis S, Hurn PD, Alkayed NJ. Mechanisms of gender-linked ischemic brain injury. Restor Neurol Neurosci. 2009;27:163–179. doi: 10.3233/RNN-2009-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sas K, Robotka H, Toldi J, Vécsei L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J Neurol Sci. 2007;257:221–239. doi: 10.1016/j.jns.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 39.Sima AA, Zhang W, Li ZG, Kamiya H. The effects of C-peptide on type 1 diabetic polyneuropathies and encephalopathy in the BB/Wor-rat. Exp Diabetes Res. 2008;2008:230458. doi: 10.1155/2008/230458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishikawa T, Araki E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal. 2007;9:343–353. doi: 10.1089/ars.2006.1458. [DOI] [PubMed] [Google Scholar]
- 41.Seo E, Park EJ, Park MK, Kim DK, Lee HJ, Hong SH. Differential expression of metabolism-related genes in liver of diabetic obese rats. Korean J Physiol Pharmacol. 2010;14:99–103. doi: 10.4196/kjpp.2010.14.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]