Abstract
Island environments typically share characteristics such as impoverished biotas and less-seasonal climates, which should be conducive to specific adaptations by organisms. However, with the exception of morphological studies, broad-scale tests of patterns of adaptation on islands are rare. Here, I examine reproductive patterns in island birds worldwide. Reproductive life histories are influenced by latitude, which could affect the response to insularity; therefore, I additionally test this hypothesis. Island colonizers showed mostly bi-parental care, but there was a significant increase in cooperative breeding on islands. Additionally, I found support for previous suggestions of reduced fecundity, longer developmental periods and increased investment in young on islands. However, clutch size increased with latitude at a rate nearly five times faster on the mainland than on the islands revealing a substantially stronger effect of insularity at higher latitudes. Latitude and insularity may also interact to determine egg volume and incubation periods, but these effects were less clear. Analyses of reproductive success did not support an effect of reduced nest predation as a driver of reproductive change, but this requires further study. The effect of latitude detected here suggests that the evolutionary changes associated with insularity relate to environmental stability and improved adult survival.
Keywords: developmental periods, fecundity, insularity, life-history evolution, parental care
1. Introduction
Islands offer unique opportunities to study the ecology and evolution of organisms. They are characterized by specific features, such as lower species diversity, fewer predators, milder climates and a lower number of habitats available [1–3]. The colonization of islands by organisms therefore presupposes the ability to adapt to this unique environment, which is expected to result in a series of convergent adaptations often called ‘island syndrome’ [1,3–7].
In terms of reproduction, island animals are believed to exhibit a shift towards ‘slower’ (or ‘K-selected’) life-history strategies [2,4,6,8]. This shift is believed to arise from improved survival on islands resulting from milder climates, and less predators and parasites (but see [9]). According to life-history studies, improved survival favours reduced fecundity as part of a strategy to allocate more resources into self-maintenance, as maximizing survival is key to maximizing lifetime reproductive success [10–13]. In addition, islands usually hold higher population densities [2,14–17], which has been suggested to favour reduced fecundity through competition for food [7,18,19]. Conversely, if juvenile survival is also high, there should be increased investment per young in order to improve offspring quality or survival [2,8], which could translate in larger eggs (see [20–22]). High densities and higher mate fidelity on islands [23] also could promote increased investment in young if this increases quality and competitiveness [4,8]. In addition, developmental periods of long-lived species could increase as a result of parental or life-history strategies to maximize lifetime fitness [24–26] or in response to environmental factors such as food availability and predator and parasite pressures (reviewed by Dmitriew [27]; see also [28–30]). Finally, mating and parental care strategies may change following island colonization. A previous study has produced evidence that extra-pair paternity on islands is lower [23]. This higher certainty of paternity may increase reproductive investment by males, leading to an increased incidence of bi-parental care (see [31]). On the other hand, island environments may experience a frequency in species that breed cooperatively, which has been suggested to arise from higher population densities that limit the opportunities of independent breeding for younger individual [32].
This reproductive shift in island species has been supported by studies on birds and other vertebrates that found reduced fecundity on islands [6,8,14,33–36]. Other reproductive traits are less well-studied; studies on tits (Parus spp.) found longer developmental periods on islands [4,37], while variation in egg size or in mating and parental care strategies of island birds is poorly documented.
Importantly, no broad-scale test of these changes on islands has ever been performed [4,6]. Whether an ‘island syndrome’ does exist or not can only be established by demonstrating that a set of traits has arisen independently several times in unrelated taxa and geographically distinct areas [1,4,6]. In addition, avian life histories vary markedly with latitude [24,35,38,39], which needs to be accounted. Specifically, temperate islands usually experience milder climates than mainland regions at the same latitude [3], while tropical mainland areas already experience mild, relatively aseasonal climates and hence would not be expected to differ as much from island areas [8]. In this study, I compare pairs of island species and their close mainland relatives to test the hypothesis that bird species change their reproductive strategy after becoming isolated on islands and examine some of the hypotheses put forward to explain these changes. Specifically, I address the following questions: (i) Do reproductive traits (clutch size, egg size and developmental periods) of island birds generally follow the ‘island syndrome’? (ii) Is life-history change on islands influenced by latitude? (iii) Is island evolution associated with changes in social mating system and parental behaviour? Finally, I discuss the results obtained in light of recent developments in life-history studies and identify several gaps in our current knowledge regarding life-history evolution on islands.
2. Material and methods
(a). Data
This study was based on pairs of island species worldwide and their mainland counterparts. I used different handbooks and monographs (see electronic supplementary material, 1) to gather data for a total of 306 bird species (i.e. 153 pairs) comprising 192 passerines and 114 non-passerines (electronic supplementary material, 2). However, the number of pairs used in each analysis varied owing to differences in data available for each trait, as the paired analyses required data for both species in a pair. Pairs were established by identifying an island endemic for which information was available and then searching for the nearest relative on the nearest mainland for which corresponding data were available. In some cases, I included populations differentiated at the sub-specific level (a total of 16 pairs or 10.5%). Although different sub-species are less differentiated than full species, this should make the results more conservative. Different genera in the same family were sometimes also used (13 pairs, or 8.5%) in order to allow including data available for some genera that were endemic to islands and that would otherwise be excluded from comparisons. Only data from wild populations were used. Both oceanic and continental islands were used (27 islands, or 17.6%, were continental islands), but islands had to be smaller than ca 12 km2 to be included here. Again, differentiation may be stronger on oceanic islands, but including species from both types of island is expected to be conservative.
Data were collected for the following life-history and behavioural traits (electronic supplementary material, 2): (i) average clutch size, (ii) egg volume (calculated from length and width after Hoyt [40]), (iii) incubation period, (iv) incubating sex (female or bi-parental), (v) nestling period, (vi) nestling care (bi-parental or cooperative), (vii) post-fledging period (number of days the offspring remain with the parents after fledging) and (viii) mating system (monogamous, polyandrous, polygynous). Data obtained from the literature are variable in quality and sample size. To account for this, I assumed that whenever an average was given, it was based on an acceptable sample. However, I also used data when a trait appeared invariable (e.g. ‘fixed’ clutch-size). For developmental periods, I also used measures given as a range (e.g. if incubation was said to vary between 16 and 18 days, I entered 17 days as the incubation period).
(b). Statistical methods
Comparative analyses have to account for the phylogenetic structure of the data, as the species used are not independent phylogenetically and closely related species share more characteristics than more distantly related ones [41,42]. I therefore conducted comparisons between pairs of species matched for phylogeny [43]. However, simple matched-pair comparisons do not allow including other variables that may be of interest or that may covary with the traits studied and need to be controlled for. Hence, I analysed the data through general or generalized linear mixed models (GLMMs) with body mass and latitude as covariates, and island–mainland as a dichotomous factor. To account for phylogenetic dependency and for the paired structure of the analyses, I included in all models a random term consisting of ‘pair’ nested in family nested in order. The inclusion of ‘pair’ as a nested random term takes into account the correlation between the two closely related species that form the pair. The phylogenetic structure was decided after performing a nested analyses with different phylogenetic structures included as random terms (for example ‘family’ nested in ‘order’ or ‘genus’ nested in ‘family’) and selecting the one that best-explained the variation observed [42]. GLMMs were run assuming normal or binomial distribution of error terms. All analyses were carried out in R v. 2.11.1 [44] using packages nlme [45] and lme4 [46]. I used backward deletion and, for model selection, used both the Akaike information criteria (AIC) and F-tests with associated p-values. Both criteria usually produced the same results, unless indicated; otherwise, for simplicity, I give only the results obtained with the F-tests, p-values and parameter estimates.
3. Results
(a). Mating system and parental care
The species studied here were overwhelmingly socially monogamous, both on the mainland and islands (89%, n = 72 species or 36 pairs). The large majority of mainland birds (90%, n = 79) had bi-parental care. There were no significant changes in the incubating sex following colonization: 54 per cent of species were female-only incubators and the remaining were bi-parental incubators (n = 35 pairs). However, there were differences in nestling care, with a significant increase in the frequency of cooperative breeders from 7 per cent on the mainland to 33 per cent on the islands (z = −1.96, p = 0.05; estimate ± s.e. of the binomial model: mainland −3.39 ± 0.95, islands −1.54 ± 0.54; n = 32 pairs). The remaining species had bi-parental care.
(b). Clutch size
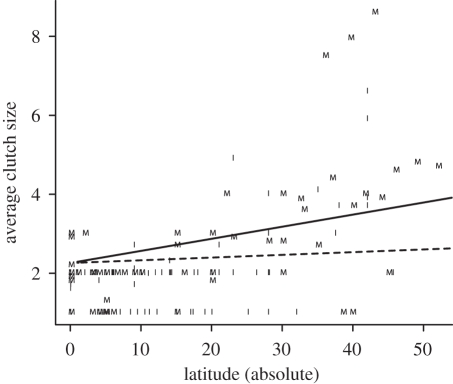
Clutch size response to insularity was influenced by an interesting interaction with latitude (F1,71 = 15.303, p = 0.0002), indicating that, on the mainland, clutch size increased with latitude at a pace that was ca 4.5 times faster than the latitudinal increase observed on the islands (slope estimate ± s.e. mainland 0.031 ± 0.006; islands 0.007 ± 0.006; figure 1). Hence, the response to the island environment was dependent on latitude.
Figure 1.
Clutch size increased with latitude at a pace that was ca 4.5 times higher on the mainland (solid line) than on the islands (dashed line). The letters ‘M’ and ‘I’ correspond to the data points for the mainland and island species used (n = 148 species). The effect of the interaction remains significant if the three outliers (and their island counterparts) are removed from the analysis (F1,68 = 6.42, p = 0.014).
(c). Egg volume
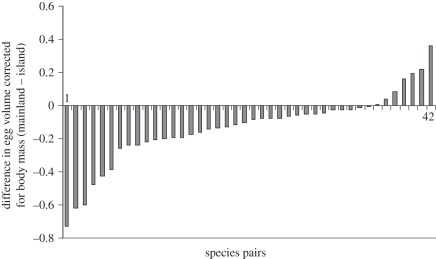
Using a log–log allometric relationship for egg volume and body mass, I found that eggs were significantly larger on islands overall (F1,40 = 15.841, p < 0.0001; estimate ± s.e. for log-transformed data: mainland 5.633 ± 0.034, islands 5.767 ± 0.174; figure 2). The interaction between the island effect and latitude was not supported by the F-test, but could not be rejected based on the AIC criteria. The ΔAIC was 1.25, suggesting that the two models were not significantly different. The parameter estimates indicated a slight tendency for egg volume on the islands to decrease with latitude, while on the mainland areas there was an increase. However, the standard errors overlapped these estimates, indicating that this trend is not very robust (estimate ± s.e. for log-transformed data: mainland 0.0012 ± 0.002; islands −0.0007 ± 0.002). There was no main effect of latitude.
Figure 2.
The egg volume of islands species was generally larger than that of the mainland counterparts. This graph represents the difference between the mainland value and the island value for the pairs used in the analyses (hence negative values correspond to a larger egg volume of the insular species). To account for body size, the figure was based on the residuals of a regression of egg volume against body mass (but not the analyses—see text).
(d). Developmental periods
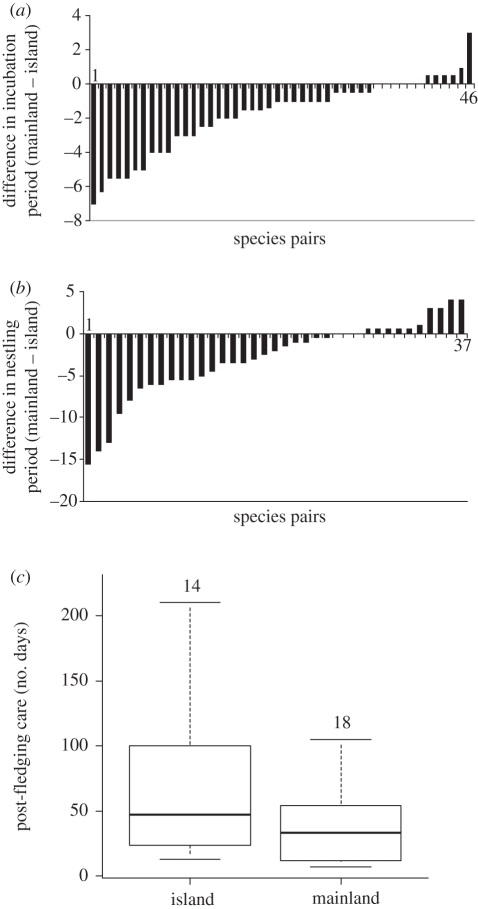
Incubation was significantly longer on islands (F1,44 = 47.384, p < 0.0001; estimate ± s.e.: mainland 12.85 ± 2.09; islands 14.45 ± 2.41; figure 3a). There was also a positive body mass effect (F1,44 = 26.61, p < 0.0001; estimate ± s.e.: 1.71 ± 0.33). As above, there was indication that the relationship between the incubation period and latitude was different on the mainland and on the islands (F1,42 = 3.6, p = 0.065), with both decreasing with latitude, but a sharper decrease for the islands (estimate ± s.e.: mainland −0.003 ± 0.028; islands −0.057 ± 0.028). However, there were only 16 temperate species (i.e. eight pairs) with known incubation data, which probably impaired the capacity to test the interaction (and this uncertainty is again reflected in the large, overlapping standard errors, particularly for the mainland).
Figure 3.
Developmental periods were generally longer on insular species compared with their mainland counterparts. (a,b) Difference between the mainland value and the island value for the pairs used in the analyses for incubation and nestling periods, respectively (negative values correspond to a longer period on the island). (c) The analyses of post-fledging care periods were conducted on unpaired data. Sample minimum, lower quartile, median, upper quartile, and sample maximum for islands and mainland species. The numbers above the boxes are the sample sizes.
Nestling periods were also longer on islands globally (F1,35 = 10.65, p = 0.0025; estimate ± s.e.: mainland 12.34 ± 6.36, islands 14.92 ± 5.6; figure 3b). There was a positive body mass effect (F1,35 = 33.98, p < 0.0001; estimate ± s.e.: 5.22 ± 0.89) and no indication of an interaction between latitude and insularity.
I analysed the duration of post-fledging care through unpaired analyses because there were not enough data for both species in a sufficient number of pairs. Based on 32 species, I found a significantly longer (and more variable) post-fledging care on islands (F1,11 = 6.95, p = 0.023; estimate ± s.e.: mainland −30.9 ± 14.4, islands 6.96 ± 19.97; figure 3c). There was also a positive effect of body mass (F1,11 = 14.02, p = 0.003; estimate ± s.e.: 16.8 ± 4.5) and no effect of latitude or of the interaction insularity × latitude.
(e). Nesting success
As above, the analyses were based on unpaired data. Nesting success was entered as the percentage of nests reported in the literature as successful. In most cases, there was no information on sample sizes and the data were not corrected for observation periods [47]. This means that the data available were crude estimates of nesting success. Based on this data, I found no effects of insularity, latitude or body size.
4. Discussion
This study confirms the frequently cited but seldom tested expectation that island birds generally have lower fecundity, greater reproductive investment and extended developmental periods when compared with their mainland counterparts. However, for some traits, this effect was more pronounced with increasing latitude. Clutch size increased with latitude at a rate nearly five times faster on the mainland than on the islands revealing a stronger insularity effect at higher latitudes. The effect of insularity on egg volume and incubation period also showed a tendency to be influenced by latitude, but this trend was not statistically significant. Finally, these results suggest that island colonizers are usually species with bi-parental care (90% of the mainland species analysed here compared with an estimated 81% for birds in general; [48]), but following colonization, there is a significant increase in the frequency of cooperative breeding on islands (see also [32]). To my knowledge, this is the first study to test global patterns of breeding adaptations in island birds and to reveal a latitudinal effect in response to insularity.
I used datasets of different sizes for the different traits analysed and hence my power to detect effects was not the same for all traits. This might have consequences in testing the interactions with latitude, as the large majority of the world's islands are tropical, and hence the data available for temperate islands are always fewer than for tropical islands. In addition, similar to any comparative analyses, I used data from varied sources with varied quality, and used data from different taxonomic levels and from oceanic and continental islands; all this might make patterns more difficult to detect. Nonetheless, the results obtained here provide a clear indication of a general pattern of adaptation to the island environment on what concerns reproductive traits. It is less clear, however, which factors underlie the evolution of the patterns of adaptation shown here.
Reduced fecundity has long been assumed as one of the main life-history changes on islands, and this was supported by previous studies [8,33–36]. However, the effect of latitude on the response to the island conditions had not been revealed previously. This result was nonetheless anticipated, as one of the main factors believed to trigger life-history change and, specifically, reduced fecundity on islands is improved adult survival resulting from reduced seasonality of resources and a benign climate [2,4,8,18,35]. This mechanism is likely to have a stronger effect on temperate islands, where climatic fluctuation is reduced when compared with the mainland [2,3]. Tropical mainland regions, on the other hand, already experience fairly benign climates and less-fluctuating resource levels, which should promote survival. Survival on tropical islands might nevertheless be higher than on mainland areas since there should be less predators and parasites, but data to support this expectation are still patchy. Simultaneously, intra-specific competition on islands may be higher owing to inflated population densities, also favouring reduced clutch size [4,16–18,49]. However, mainland tropical species already exhibit small clutches making it difficult, in some instances, for selection to reduce clutch size further.
Egg size is normally negatively related to clutch size, which is seen as an energetic trade-off [50]. However, I found generally higher egg volume on islands, but no clear evidence of an interaction between insularity and latitude as it was found for clutch size. To test this trade-off hypothesis, I conducted a post hoc regression of the egg size increase on islands in relation to the decrease in clutch size and, as expected, I found a significant negative relationship between the two (F1,9 = 5.7, p = 0.04; estimate ± s.e.: −0.3 ± 0.12; n = 21 species). This indicates a change in reproductive strategy on islands, with investment per young being favoured in relation to number of young produced, confirming previous suggestions [2,8]. Increased investment per young could be important if it improves offspring quality or survival [21,22] particularly in competitive island environments [51]. The pattern of larger eggs could be related also to lower nest predation [52], which is believed to be an important trait of island environments. The analyses of nesting success conducted here, however, produced no indication that nest predation is lower on islands. Still, these results should be interpreted with caution given that they were not based on matched-pair analyses and were based on raw data and not estimates corrected for nest exposure [47]. In addition, these results probably reflect conditions experienced currently on islands and not those under which island forms evolved, as most islands nowadays have introduced predators (e.g. rats, Rattus spp.). Finally, egg size has been linked to bi-parental incubation [20,52], but I found no differences in incubation behaviour between island and mainland species, suggesting that this explanation is not particularly important for island birds.
This study found support for the expectation of longer developmental periods on islands, confirming previous suggestions [4,37]. Extended incubation periods may be required to incubate larger eggs, and there was indication that the incubation period on islands was affected by latitude, which could reflect the similar tendency found for egg size. However, with only 11 species with data for both egg size and incubation period, the power for analysis was limited and this relationship remains untested. Developmental periods in animals often occur at rates lower than the physiological maximum, which implies that slower growth might be beneficial, but some environmental factors favour a faster growth rate (reviewed by Dmitriew [27]). Empirical studies of incubation and nestling periods in birds have found that these stages are affected by nest predation pressure (either directly or by constraining foraging activity; [28,29,53–55]), parental longevity [24], food limitation [56,57], and parasite levels and immunity [28–30,58]. The generally impoverished predator faunas on islands may release island birds from predation pressure (but see above), resulting in the longer developmental periods found here. However, the factors indicated above are expected to interact, especially at a broad geographical level [25,28,29], and thus detailed data from different regions are needed to understand the developmental patterns on islands.
The pattern of post-fledging care on islands found here was not based on matched-pair analyses and hence should be seen as preliminary. Nonetheless, this is an interesting result that provides additional support to the trend of greater investment in offspring on islands. Prolonged investment in offspring is possible when parents have predictable access to resources and the additional investment significantly benefits the offspring [59,60]. This might be the case in stable island environments, in spite of—or because of—enhanced competition. This result is also relevant given the increase in the frequency of cooperative breeding on islands (this study; [32]). A prolonged association with parents and delayed onset of reproduction are usually prerequisites for cooperative breeding. Cockburn [32] suggested that the higher incidence of cooperative breeding on islands provided good support for the ‘ecological constraints’ hypothesis [61], according to which individuals cooperate because they live in saturated habitats, where they have less possibilities of breeding independently. However, the present study suggests that the prolonged association between parents and offspring may start before the breeding season and hence might involve more than simply constraints on reproduction.
In summary, this study confirmed the existence of a global pattern of convergent adaptations in reproductive traits of island birds worldwide and revealed that some differences between island and mainland birds are accentuated by latitude. This effect of latitude suggests that milder, less-seasonal climates are important agents of life-history change, possibly through their effects on adult survival. This provides support for suggestions of early island biogeography theory [2,8] and concurs with more recent life-history work [11,24,35,38,62]. Reduced seasonality and predation and increased competition for food on islands may also lead to reduced metabolic rates, which may enhance (or mediate) the effects of these environmental variables on life-history traits [36]. However, data on the key factors potentially affecting life-history evolution on islands remain scarce. Namely, data on adult mortality, predation rates, food levels, metabolic rates, parasite levels and immunity are needed. Currently, the factors underlying the evolution of the reproductive traits reported here for island birds remain largely unknown.
Acknowledgements
I am most thankful to P. Jones for kindly giving me full access to his comprehensive ornithological library and for helpful advice. I also thank M. Koopman at the Niven Library (Percy FitzPatrick Institute, University of Cape Town) for assistance. O. Gimenez, C. Spottiswoode and P.-Y. Henry provided helpful statistical advice. M. Melo, S. Anderson, R. Bowie, R. Lopes, R. Heleno and P. Rodrigues gave me access to unpublished data. The manuscript was improved by comments from T. Arnold, C. Doutrelant, P. Jones, M. Melo and an anonymous reviewer. P. Tarroso helped in preparing figure 1. I was funded by a Marie Curie Fellowship (EU) and the Portuguese Science and Technology Foundation (FCT) during the preparation of this paper.
References
- 1.Losos J. B., Ricklefs R. E. 2009. Adaptation and diversification on islands. Nature 457, 830–836 10.1038/nature07893 (doi:10.1038/nature07893) [DOI] [PubMed] [Google Scholar]
- 2.MacArthur R. H., Wilson E. O. 1967. The theory of island biogeography. Princeton, NJ: Princeton University Press [Google Scholar]
- 3.Whittaker R. J., Fernández-Palacios J. M. 2007. Island biogeography ecology, evolution, and conservation. Oxford, UK: Oxford University Press [Google Scholar]
- 4.Blondel J. 2000. Evolution and ecology of birds on islands: trends and prospects. Vie et Milieu 50, 205–220 [Google Scholar]
- 5.Clegg S. M., Owens I. P. F. 2002. The 'island rule' in birds: medium body size and its ecological explanation. Proc. R. Soc. Lond. B 269, 1359–1365 10.1098/rspb.2002.2024 (doi:10.1098/rspb.2002.2024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant P. R. 1998. Patterns on islands and microevolution. In Evolution on islands (ed. Grant P. R.), pp. 1–17 Oxford, UK: Oxford University Press [Google Scholar]
- 7.McNab B. K. 1994. Resource use and the survival of land and freshwater vertebrates on oceanic islands. Am. Nat. 144, 643–660 10.1086/285698 (doi:10.1086/285698) [DOI] [Google Scholar]
- 8.Cody M. L. 1966. A general theory of clutch size. Evolution 20, 174–184 10.2307/2406571 (doi:10.2307/2406571) [DOI] [PubMed] [Google Scholar]
- 9.Blondel J., Pradel R., Lebreton D. 1992. Low fecundity insular blue tits do not survive better as adults than high fecundity mainland ones. J. Anim. Ecol. 61, 205–213 10.2307/5523 (doi:10.2307/5523) [DOI] [Google Scholar]
- 10.Barbraud C., Weimerskirch H. 2001. Emperor penguins and climate change. Nature 411, 183–186 10.1038/35075554 (doi:10.1038/35075554) [DOI] [PubMed] [Google Scholar]
- 11.Charlesworth B. 1994. Evolution in age-structured populations Cambridge, UK: Cambridge University Press [Google Scholar]
- 12.Clutton-Brock T. H. 1988. Reproductive success. Chicago, IL: University of Chicago Press [Google Scholar]
- 13.Goodman D. 1974. Natural selection and a cost ceiling on reproductive effort. Am. Nat. 108, 247–268 10.1086/282906 (doi:10.1086/282906) [DOI] [Google Scholar]
- 14.Buckley L. B., Walter Jetz W. 2007. Insularity and the determinants of lizard population density. Ecol. Lett. 10, 481–489 10.1111/j.1461-0248.2007.01042.x (doi:10.1111/j.1461-0248.2007.01042.x) [DOI] [PubMed] [Google Scholar]
- 15.Crowell K. L. 1962. Reduced interspecific competition among the birds of Bermuda. Ecology 43, 75–88 10.2307/1932042 (doi:10.2307/1932042) [DOI] [Google Scholar]
- 16.MacArthur R. H., Diamond J. M., Karr J. R. 1972. Density compensation in island faunas. Ecology 53, 330–342 10.2307/1934090 (doi:10.2307/1934090) [DOI] [Google Scholar]
- 17.Blondel J., Chessel D., Frochot B. 1988. Bird species impoverishment, niche expansion and density inflation in Mediterranean island habitats. Ecology 69, 1899–1917 10.2307/1941167 (doi:10.2307/1941167) [DOI] [Google Scholar]
- 18.Ashmole N. P. 1963. The regulation of numbers of tropical oceanic birds. Ibis 103, 458–473 [Google Scholar]
- 19.Ricklefs R. E. 1980. Geographical variation in clutch size among passerine birds: Ashmole's hypothesis. Auk 97, 38–49 [Google Scholar]
- 20.Martin T. E., Bassar R. D., Bassar S. K., Fontaine J. J., Lloyd P., Mathewson H. A., Niklison A. M., Chalfoun A. 2006. Life history and ecological correlates of geographic variation in egg and clutch mass among passerine species. Evolution 60, 390–398 [PubMed] [Google Scholar]
- 21.Styrsky J. D., Dobbs R. C., Thompson C. F. 2000. Food-supplementation does not override the effect of egg mass on fitness-related traits of nestling house wrens. J. Anim. Ecol. 69, 690–702 10.1046/j.1365-2656.2000.00427.x (doi:10.1046/j.1365-2656.2000.00427.x) [DOI] [Google Scholar]
- 22.Christians J. K. 2002. Avian egg size: variation within species and inflexibility within individuals. Biol. Rev. 77, 1–26 10.1017/S1464793101005784 (doi:10.1017/S1464793101005784) [DOI] [PubMed] [Google Scholar]
- 23.Griffith S. C. 2000. High fidelity on islands: a comparative study of extra-pair paternity in passerine birds. Behav. Ecol. 11, 265–273 10.1093/beheco/11.3.265 (doi:10.1093/beheco/11.3.265) [DOI] [Google Scholar]
- 24.Martin T. E. 2002. A new view for avian life history evolution tested on an incubation paradox. Proc. R. Soc. Lond. B 269, 309–316 10.1098/rspb.2001.1879 (doi:10.1098/rspb.2001.1879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin T. E., Auer S. K., Bassar R. D., Niklison A. M., Lloyd P. 2007. Geographic variation in avian incubation periods and parental influences on embryonic temperature. Evolution 61, 2558–2569 10.1111/j.1558-5646.2007.00204.x (doi:10.1111/j.1558-5646.2007.00204.x) [DOI] [PubMed] [Google Scholar]
- 26.Ricklefs R. E. 2006. Embryo development and ageing in birds and mammals. Proc. R. Soc. B 273, 2077–2082 10.1098/rspb.2006.3544 (doi:10.1098/rspb.2006.3544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dmitriew C. M. 2011. The evolution of growth trajectories: what limits growth rate? Biol. Rev. 86, 97–116 10.1111/j.1469-185X.2010.00136.x (doi:10.1111/j.1469-185X.2010.00136.x) [DOI] [PubMed] [Google Scholar]
- 28.Martin T. E., Arriero E., Majewska A. 2011. A trade-off between embryonic development rate and immune function of avian offspring is revealed by considering embryonic temperature. Biol. Lett. 7, 425–428 10.1098/rsbl.2010.1031 (doi:10.1098/rsbl.2010.1031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin T. E., Lloyd P., Bosque C., Barton D. C., Biancucci A. L., Cheng Y.-R., Ton R. 2011. Growth rate variation among passerine species in tropical and temperate sites: an antagonistic interaction between parental food provisioning and nest predation risk. Evolution 65, 1607–1622 10.1111/j.1558-5646.2011.01227.x (doi:10.1111/j.1558-5646.2011.01227.x) [DOI] [PubMed] [Google Scholar]
- 30.Ricklefs R. E. 1992. Embryonic development period and the prevalence of avian blood parasites. Proc. Natl Acad. Sci. USA 89, 4722–4725 10.1073/pnas.89.10.4722 (doi:10.1073/pnas.89.10.4722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennet P. M., Owens I. P. F. 2002. Evolutionary ecology of birds. Oxford series in ecology and evolution. Oxford, UK: Oxford University Press [Google Scholar]
- 32.Cockburn A. 2003. Cooperative breeding in Oscine passerines: does sociality inhibit speciation? Proc. R. Soc. Lond. B 270, 2207–2214 10.1098/rspb.2003.2503 (doi:10.1098/rspb.2003.2503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blondel J. 1985. Breeding strategies of the blue tit and the coal tit (Parus) in Mainland and island Mediterranean habitats: a comparison. J. Anim. Ecol. 54, 531–556 10.2307/4497 (doi:10.2307/4497) [DOI] [Google Scholar]
- 34.Crowell K. L., Rothstein S. I. 1981. Clutch size and breeding strategies among Bermudan and North American passerines. Ibis 123, 42–50 10.1111/j.1474-919X.1981.tb00171.x (doi:10.1111/j.1474-919X.1981.tb00171.x) [DOI] [Google Scholar]
- 35.Jetz W., Sekercioglu C. H., Böhning-Gaese K. 2008. The worldwide variation in avian clutch size across species and space. PLoS Biol. 6, 2650–2657 10.1371/journal.pbio.0060303 (doi:10.1371/journal.pbio.0060303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNab B. K., Ellis H. I. 2006. Flightless rails endemic to islands have lower energy expenditures and clutch sizes than flighted rails on islands and continents. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 145, 295–311 10.1016/j.cbpa.2006.02.025 (doi:10.1016/j.cbpa.2006.02.025) [DOI] [PubMed] [Google Scholar]
- 37.Higuchi H., Momose H. 1981. Deferred independence and prolonged infantile behaviour in young varied tits, Parus varius, of an island population. Anim. Behav. 29, 523–528 10.1016/S0003-3472(81)80114-5 (doi:10.1016/S0003-3472(81)80114-5) [DOI] [Google Scholar]
- 38.Ghalambor C. K., Martin T. E. 2001. Fecundity-survival trade-offs and parental risk-taking in birds. Science 292, 494–497 10.1126/science.1059379 (doi:10.1126/science.1059379) [DOI] [PubMed] [Google Scholar]
- 39.Lack D. 1948. The significance of clutch size. III. Some interspecific comparisons. Ibis 90, 25–45 10.1111/j.1474-919X.1948.tb01399.x (doi:10.1111/j.1474-919X.1948.tb01399.x) [DOI] [Google Scholar]
- 40.Hoyt D. F. 1979. Practical methods of estimating volume and fresh weight of bird eggs. Auk 96, 73–77 [Google Scholar]
- 41.Freckleton R. P. 2009. The seven deadly sins of comparative analysis. J. Evol. Biol. 22, 1367–1375 10.1111/j.1420-9101.2009.01757.x (doi:10.1111/j.1420-9101.2009.01757.x) [DOI] [PubMed] [Google Scholar]
- 42.Harvey P. H., Pagel M. D. 1991. The comparative method in evolutionary biology. Oxford, UK: Oxford University Press [Google Scholar]
- 43.Møller A. P., Birkhead T. R. 1992. A pairwise comparative method as illustrated by copulation frequency in birds. Am. Nat. 139, 644–656 [Google Scholar]
- 44.R Development Core Team 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 45.Pinheiro J., Bates D., DebRoy S., Sarkar D. & R Development Core Team 2011. nlme: linear and nonlinear mixed effects models, pp. 1–102 R package version 3; R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 46.Bates D., Maechler M. 2010. lme4: linear mixed-effects models using S4 classes. R package version 0.999375-33. See http://CRAN.R-project.org/package=lme4
- 47.Mayfield H. F. 1975. Suggestions for calculating nest success. Wilson Bulletin 87, 456–466 [Google Scholar]
- 48.Cockburn A. 2006. Prevalence of different modes of parental care in birds. Proc. R. Soc. B 273, 1375–1383 10.1098/rspb.2005.3458 (doi:10.1098/rspb.2005.3458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McNab B. K., Ellis H. I. 2006. Flightless rails endemic to islands have lower energy expenditures and clutch sizes than flighted rails on islands and continents. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 145, 295–311 10.1016/j.cbpa.2006.02.025 (doi:10.1016/j.cbpa.2006.02.025) [DOI] [PubMed] [Google Scholar]
- 50.Roff D. A. 1992. The Evolution of Life Histories. New York: Chapman and Hall [Google Scholar]
- 51.Mappes T., Grapputo A., Hakkarainen H., Huhta E., Koskela E., Saunanen R., Suorsa P. 2008. Island selection on mammalian life-histories: genetic differentiation in offspring size. BMC Evol. Biol. 8, 296. 10.1186/1471-2148-8-296 (doi:10.1186/1471-2148-8-296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fontaine J. J., Martin T. E. 2006. Reproductive responses to experimentally reduced nest predation risk among coexisting bird species. Ecol. Lett. 9, 428–434 [DOI] [PubMed] [Google Scholar]
- 53.Bosque C., Bosque M. T. 1995. Nest predation as a selective factor in the evolution of developmental rates in altricial birds. Am. Nat. 145, 234–260 [Google Scholar]
- 54.Martin T. E. 1995. Avian life history evolution in relation to nest sites, nest predation and food. Ecol. Monogr. 65, 101–127 10.2307/2937160 (doi:10.2307/2937160) [DOI] [Google Scholar]
- 55.Conway C. J., Martin T. E. 2000. Evolution of avian incubation behavior: Influence of food, temperature, and nest predation. Evolution 54, 670–685 [DOI] [PubMed] [Google Scholar]
- 56.Martin T. E. 1987. Food as a limit on breeding birds: A life-history perspective. Annu. Rev. Ecol. Syst. 18, 453–487 10.1146/annurev.es.18.110187.002321 (doi:10.1146/annurev.es.18.110187.002321) [DOI] [Google Scholar]
- 57.Remeš V., Martin T. E. 2002. Environmental influences on the evolution of growth and developmental rates in passerines. Evolution 56, 2505–2518 10.1554/0014-3820(2002)056[2505:EIOTEO]2.0.CO;2 (doi:10.1554/0014-3820(2002)056[2505:EIOTEO]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 58.Lee K. A., Wikelski M., Robinson W. D., Robinson T. R., Klasing K. C. 2008. Constitutive immune defences correlate with life-history variables in tropical birds. J. Anim. Ecol. 77, 356–363 10.1111/j.1365-2656.2007.01347.x (doi:10.1111/j.1365-2656.2007.01347.x) [DOI] [PubMed] [Google Scholar]
- 59.Covas R., Griesser M. 2007. Life history and the evolution of family living in birds. Proc. R. Soc. B 274, 1349–1357 10.1098/rspb.2007.0117 (doi:10.1098/rspb.2007.0117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ekman J., Baglione V., Eggers S., Griesser M. 2001. Delayed dispersal: living under the reign of nepotistic parents. Auk 118, 1–10 10.1642/0004-8038(2001)118[0001:DDLUTR]2.0.CO;2 (doi:10.1642/0004-8038(2001)118[0001:DDLUTR]2.0.CO;2) [DOI] [Google Scholar]
- 61.Emlen S. T. 1982. The evolution of helping. I. An ecological constraints model. Am. Nat. 119, 29–39 10.1086/283888 (doi:10.1086/283888) [DOI] [Google Scholar]
- 62.Reznick D. A., Bryga H., Endler J. A. 1990. Experimentally induced life-history evolution in a natural population. Nature 346, 357–359 [Google Scholar]