Abstract
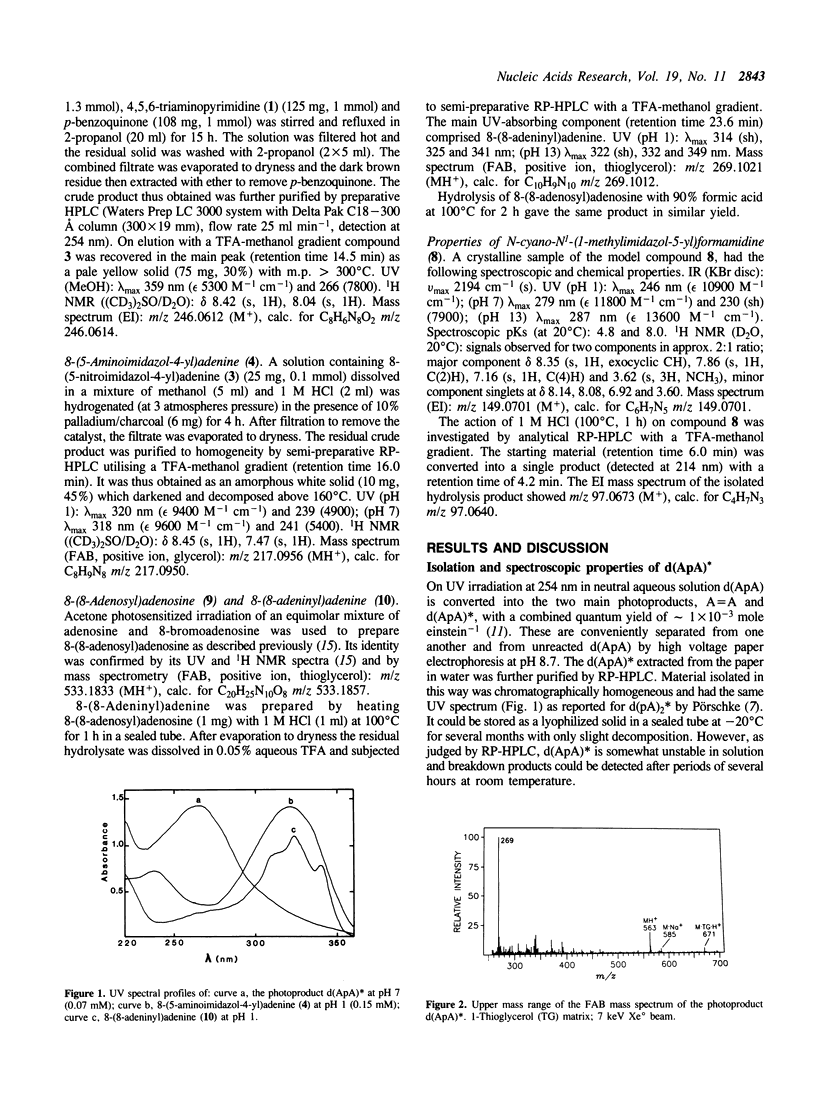
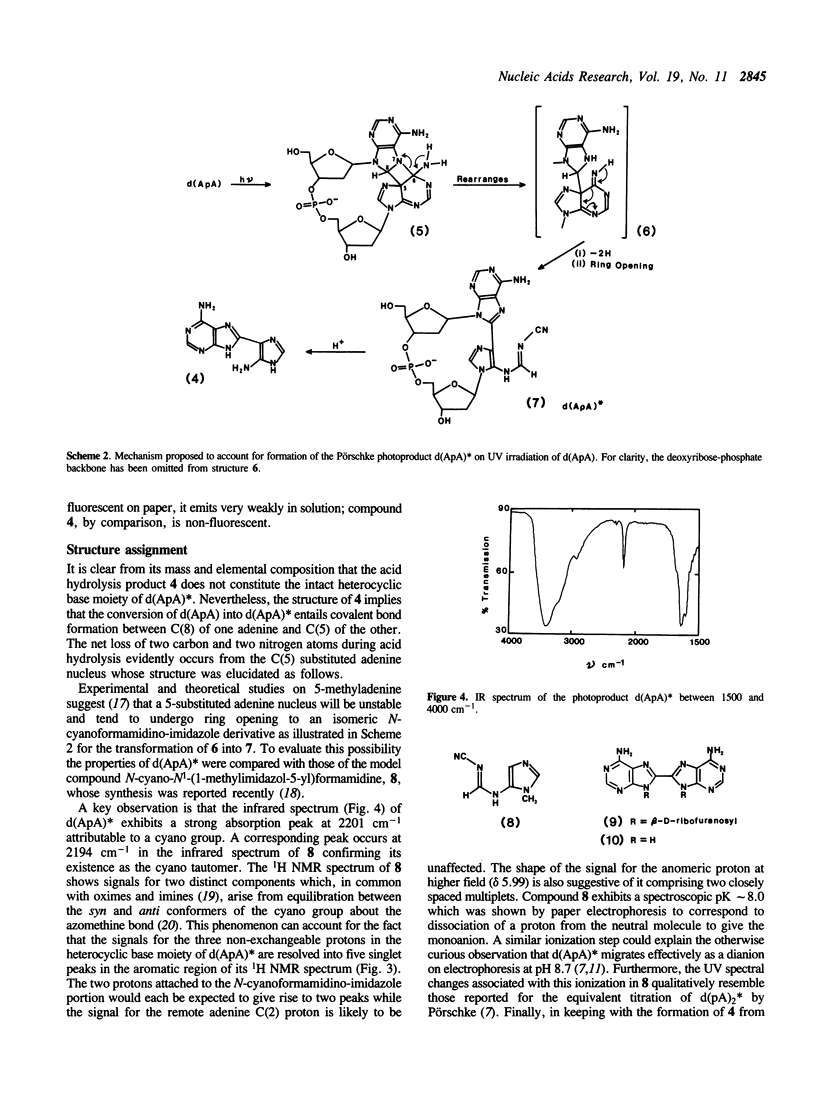
The mechanism of the photodimerization of adjacent adenine bases on the same strand of DNA has been elucidated by determining the structure of one of the two major photoproducts that are formed by UV irradiation of the deoxydinucleoside monophosphate d(ApA). The photoproduct, denoted d(ApA)*, corresponds to a species of adenine photodimer first described by Pörschke (Pörschke, D. (1973) J.Am.Chem.Soc. 95, 8440-8446). From a detailed examination of its chemical and spectroscopic properties, including comparisons with the model compound N-cyano-N1-(1-methylimidazol-5-yl)formamidine, it is deduced that d(ApA)* contains a deoxyadenosine unit covalently linked through its C(8) position to C(4) of an imidazole N(1) deoxyribonucleoside moiety bearing an N-cyanoformamidino substituent at C(5). On treatment with acid, d(ApA)* is degraded with high specificity to 8-(5-amino-imidazol-4-yl)adenine whose identity has been confirmed by independent chemical synthesis. It is concluded that the primary event in adenine photodimerization entails photoaddition of the N(7)-C(8) double bond of the 5'-adenine across the C(6) and C(5) positions of the 3'-adenine. The azetidine species thus generated acts as a common precursor to both types of d(ApA) photoproduct which are formed from it by competing modes of azetidine ring fission.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker M. M., Lesser D., Kurpiewski M., Baranger A., Jen-Jacobson L. "Ultraviolet footprinting" accurately maps sequence-specific contacts and DNA kinking in the EcoRI endonuclease-DNA complex. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6247–6251. doi: 10.1073/pnas.85.17.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M. M., Wang Z. Origin of ultraviolet damage in DNA. J Mol Biol. 1989 Dec 5;210(3):429–438. doi: 10.1016/0022-2836(89)90120-4. [DOI] [PubMed] [Google Scholar]
- Downs W. D., Cech T. R. An ultraviolet-inducible adenosine-adenosine cross-link reflects the catalytic structure of the Tetrahymena ribozyme. Biochemistry. 1990 Jun 12;29(23):5605–5613. doi: 10.1021/bi00475a027. [DOI] [PubMed] [Google Scholar]
- Duker N. J., Gallagher P. E. Purine photoproducts. Photochem Photobiol. 1988 Jul;48(1):35–39. [PubMed] [Google Scholar]
- Gallagher P. E., Duker N. J. Formation of purine photoproducts in a defined human DNA sequence. Photochem Photobiol. 1989 May;49(5):599–605. doi: 10.1111/j.1751-1097.1989.tb08430.x. [DOI] [PubMed] [Google Scholar]
- Gasparro F. P., Fresco J. R. Ultraviolet-induced 8,8-adenine dehydrodimers in oligo- and polynucleotides. Nucleic Acids Res. 1986 May 27;14(10):4239–4251. doi: 10.1093/nar/14.10.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning T. M., Davies R. J., Kaptein R. The solution structure of the intramolecular photoproduct of d(TpA) derived with the use of NMR and a combination of distance geometry and molecular dynamics. Nucleic Acids Res. 1990 Jan 25;18(2):277–284. doi: 10.1093/nar/18.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Sharma N. D., Davies R. J., Phillipson D. W., McCloskey J. A. The isolation and characterisation of a new type of dimeric adenine photoproduct in UV-irradiated deoxyadenylates. Nucleic Acids Res. 1987 Feb 11;15(3):1199–1216. doi: 10.1093/nar/15.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsthoorn C. S., Bostelaar L. J., Van Boom J. H., Altona C. Conformational characteristics of the trinucleoside diphosphate dApdApdA and its constituents from nuclear magnetic resonance and circular dichroism studies. Extrapolation to the stacked conformers. Eur J Biochem. 1980 Nov;112(1):95–110. doi: 10.1111/j.1432-1033.1980.tb04991.x. [DOI] [PubMed] [Google Scholar]
- Pörschke D. A specific photoreaction in polydeoxyadenylic acid. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2683–2686. doi: 10.1073/pnas.70.9.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pörschke D. Analysis of a specific photoreaction in oligo- and polydeoxyadenylic acids. J Am Chem Soc. 1973 Dec 12;95(25):8440–8446. doi: 10.1021/ja00806a040. [DOI] [PubMed] [Google Scholar]
- Pörschke D. Single strand conformation of adenylate chains analysed by a specific photoreaction. Determination of structure by 5' residue. Nucleic Acids Res. 1975 Sep;2(9):1621–1627. doi: 10.1093/nar/2.9.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn R. O. Search for an adenine photoproduct in DNA. Nucleic Acids Res. 1976 Apr;3(4):879–890. doi: 10.1093/nar/3.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P. J., Rodland K. D., Rachlin E. M., McCloskey J. A. Differential DNA methylation during the vegetative life cycle of Neurospora crassa. J Bacteriol. 1987 Jun;169(6):2902–2905. doi: 10.1128/jb.169.6.2902-2905.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S. K., Smith D. L., McCloskey J. A. Determination of active hydrogen content by fast atom bombardment mass spectrometry following hydrogen-deuterium exchange. Biochem Biophys Res Commun. 1983 Apr 15;112(1):126–131. doi: 10.1016/0006-291x(83)91806-5. [DOI] [PubMed] [Google Scholar]
- Sharma N. D., Davies R. J. Extent of formation of a dimeric adenine photoproduct in polynucleotides and DNA. J Photochem Photobiol B. 1989 Apr;3(2):247–258. doi: 10.1016/1011-1344(89)80066-1. [DOI] [PubMed] [Google Scholar]


