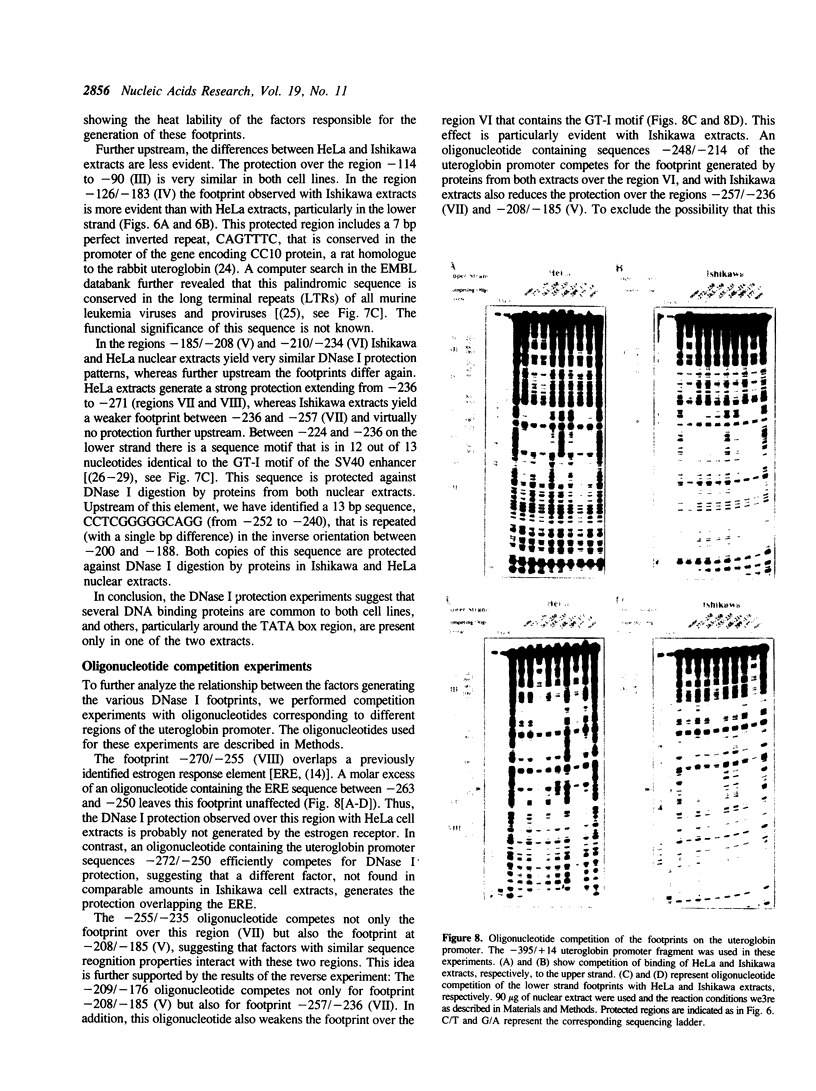
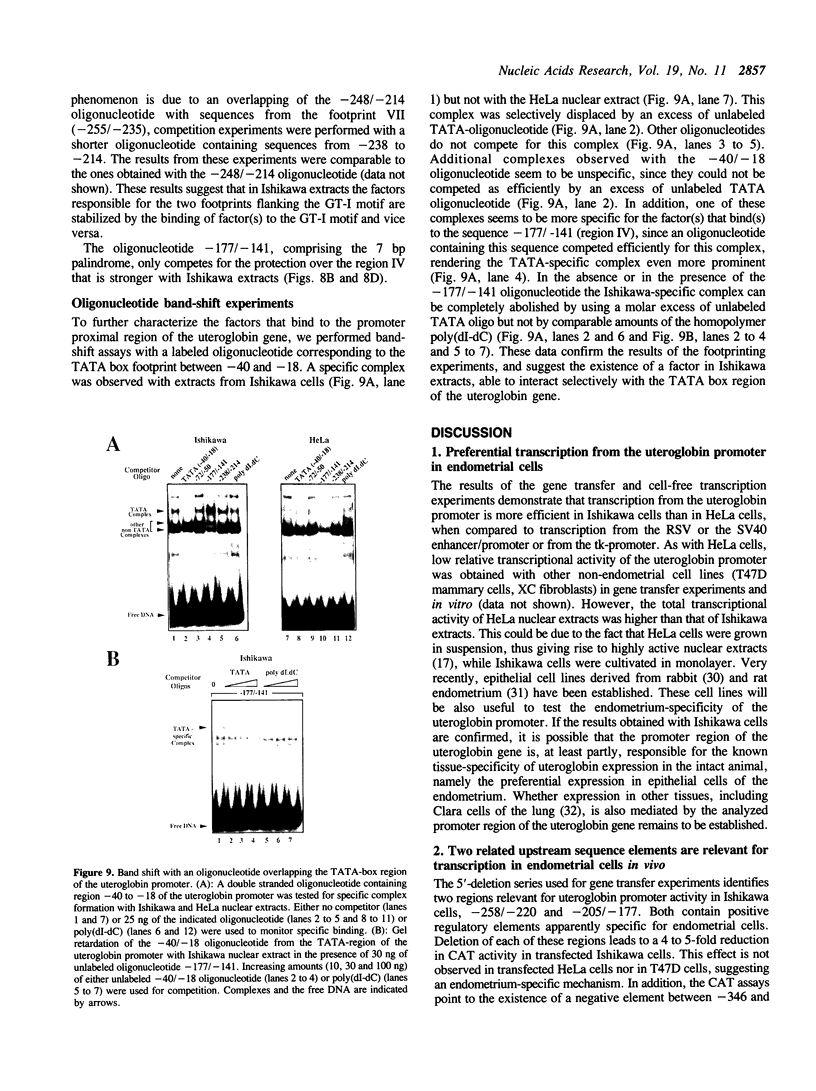
Abstract
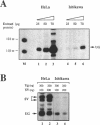
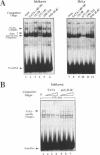
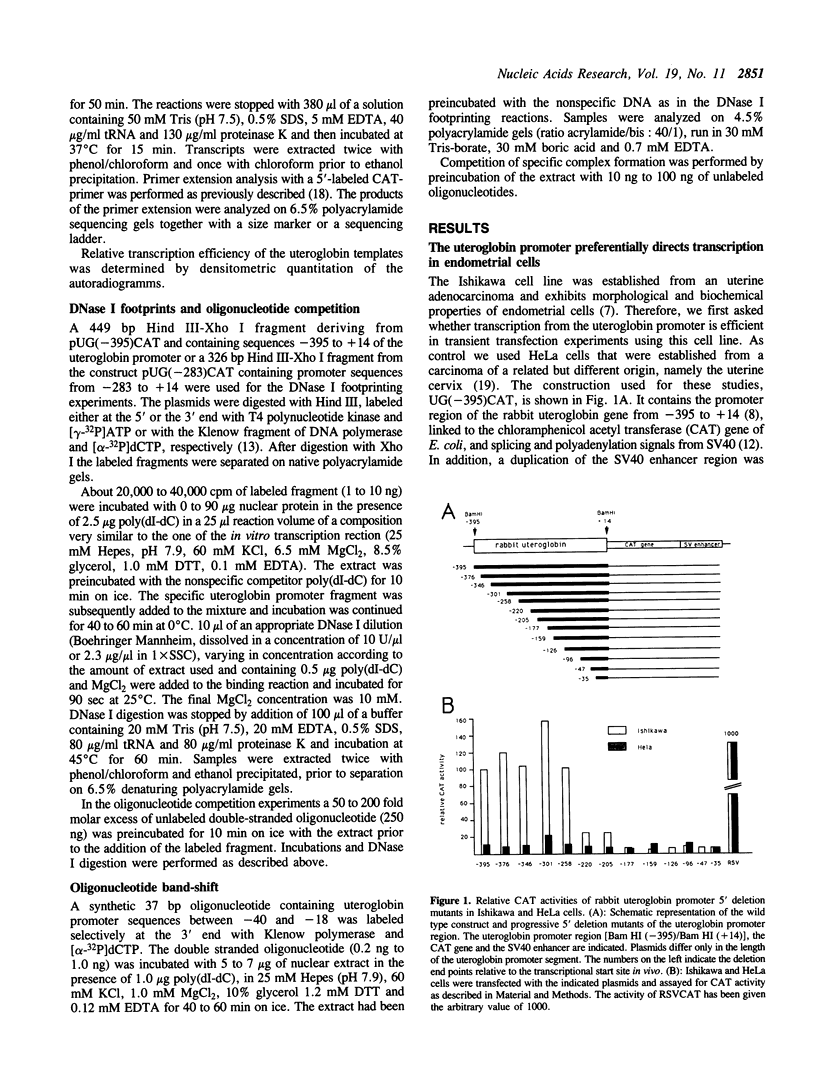
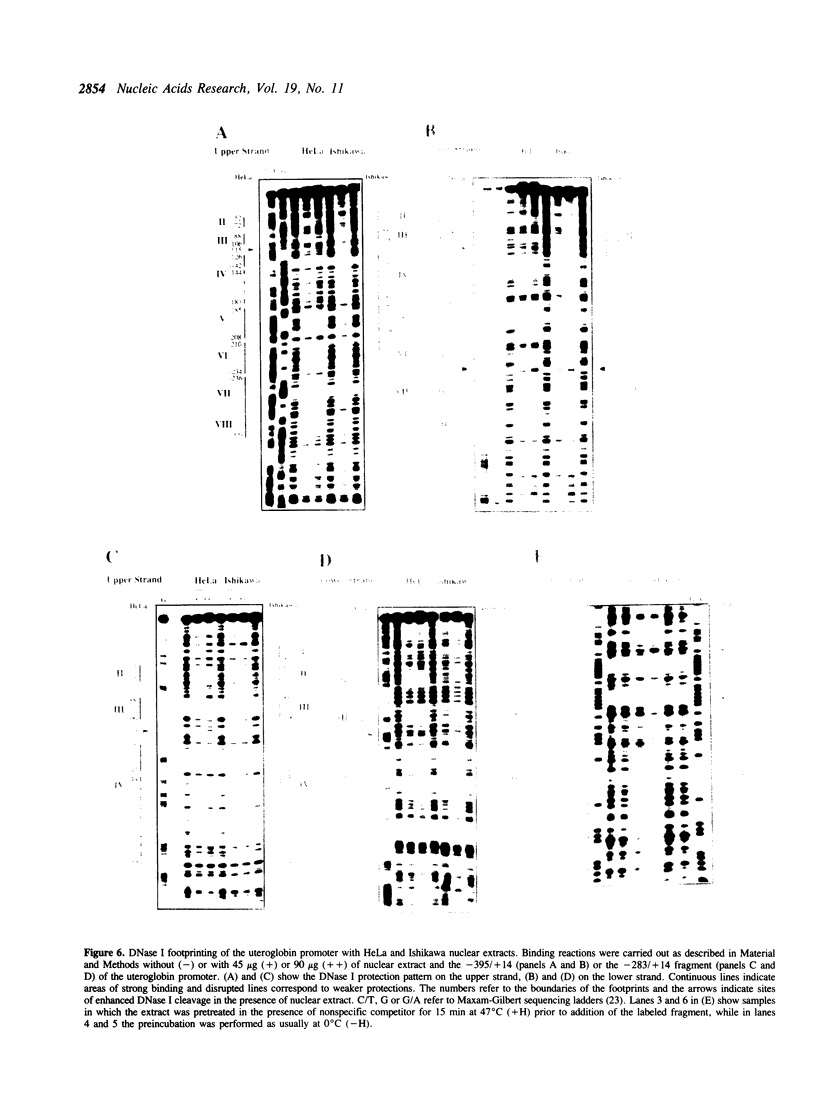
To understand the mechanisms responsible for endometrium-specific expression of the uteroglobin gene, we have compared transcription from the uteroglobin promoter in a human endometrial cell line (Ishikawa) and in HeLa cells. In transient transfection experiments and in nuclear extracts, sequences from -395 to +14 of the uteroglobin gene are able to promote transcription of a reporter gene more efficiently in Ishikawa cells than in HeLa cells relative to the RSV or the SV40 early promoter. Analysis of progressive 5'-deletion mutants identifies three promoter regions, -258/-220, -205/-177, and -96/-35, that are important for preferential transcription in endometrial cells. DNase I footprinting experiments with nuclear extracts from Ishikawa and HeLa cells reveal a series of defined protections overlapping these regions. The relative intensity of individual protections differs between the two cell lines. Oligonucleotide competition experiments suggest that similar factor(s) bind(s) to the two relevant upstream regions of the promoter that share no homology to known regulatory elements. A protection over the TATA-box is detected only with extracts from Ishikawa cells. Band shift experiments show that an Ishikawa-specific factor binds to sequences overlapping the TATA-box region that are partially conserved in other endometrium-expressed genes. We propose that novel transcription factors mediate endometrium-specific expression of the uteroglobin gene in conjunction with a tissue-specific factor that binds to the TATA-box region.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beier H. M. Uteroglobin: a hormone-sensitive endometrial protein involved in blastocyst development. Biochim Biophys Acta. 1968 Jun 26;160(2):289–291. doi: 10.1016/0005-2795(68)90108-6. [DOI] [PubMed] [Google Scholar]
- Cato A. C., Geisse S., Wenz M., Westphal H. M., Beato M. The nucleotide sequences recognized by the glucocorticoid receptor in the rabbit uteroglobin gene region are located far upstream from the initiation of transcription. EMBO J. 1984 Dec 1;3(12):2771–2778. doi: 10.1002/j.1460-2075.1984.tb02208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato A. C., Miksicek R., Schütz G., Arnemann J., Beato M. The hormone regulatory element of mouse mammary tumour virus mediates progesterone induction. EMBO J. 1986 Sep;5(9):2237–2240. doi: 10.1002/j.1460-2075.1986.tb04490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu R., Imagawa M., Imbra R. J., Bockoven J. R., Karin M. Multiple cis- and trans-acting elements mediate the transcriptional response to phorbol esters. Nature. 1987 Oct 15;329(6140):648–651. doi: 10.1038/329648a0. [DOI] [PubMed] [Google Scholar]
- Clark L., Hay R. T. Sequence requirement for specific interaction of an enhancer binding protein (EBP1) with DNA. Nucleic Acids Res. 1989 Jan 25;17(2):499–516. doi: 10.1093/nar/17.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthésy B., Cardinaux J. R., Claret F. X., Wahli W. A nuclear factor I-like activity and a liver-specific repressor govern estrogen-regulated in vitro transcription from the Xenopus laevis vitellogenin B1 promoter. Mol Cell Biol. 1989 Dec;9(12):5548–5562. doi: 10.1128/mcb.9.12.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson I., Xiao J. H., Rosales R., Staub A., Chambon P. The HeLa cell protein TEF-1 binds specifically and cooperatively to two SV40 enhancer motifs of unrelated sequence. Cell. 1988 Sep 23;54(7):931–942. doi: 10.1016/0092-8674(88)90108-0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G., Wolf M., Katyal S. L., Singh G., Beato M., Suske G. Tissue-specific expression, hormonal regulation and 5'-flanking gene region of the rat Clara cell 10 kDa protein: comparison to rabbit uteroglobin. Nucleic Acids Res. 1990 May 25;18(10):2939–2946. doi: 10.1093/nar/18.10.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T. W., Horikoshi M., Roeder R. G., Green M. R. Analysis of the role of the transcription factor ATF in the assembly of a functional preinitiation complex. Cell. 1988 Sep 23;54(7):1043–1051. doi: 10.1016/0092-8674(88)90119-5. [DOI] [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Hansen U., Tenen D. G., Livingston D. M., Sharp P. A. T antigen repression of SV40 early transcription from two promoters. Cell. 1981 Dec;27(3 Pt 2):603–613. doi: 10.1016/0092-8674(81)90402-5. [DOI] [PubMed] [Google Scholar]
- Harbury P. A., Struhl K. Functional distinctions between yeast TATA elements. Mol Cell Biol. 1989 Dec;9(12):5298–5304. doi: 10.1128/mcb.9.12.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman A., Sinn E., Yamamoto T., Wang J., Roy A., Horikoshi M., Roeder R. G. Highly conserved core domain and unique N terminus with presumptive regulatory motifs in a human TATA factor (TFIID). Nature. 1990 Jul 26;346(6282):387–390. doi: 10.1038/346387a0. [DOI] [PubMed] [Google Scholar]
- Horikoshi M., Carey M. F., Kakidani H., Roeder R. G. Mechanism of action of a yeast activator: direct effect of GAL4 derivatives on mammalian TFIID-promoter interactions. Cell. 1988 Aug 26;54(5):665–669. doi: 10.1016/s0092-8674(88)80011-4. [DOI] [PubMed] [Google Scholar]
- Horikoshi M., Hai T., Lin Y. S., Green M. R., Roeder R. G. Transcription factor ATF interacts with the TATA factor to facilitate establishment of a preinitiation complex. Cell. 1988 Sep 23;54(7):1033–1042. doi: 10.1016/0092-8674(88)90118-3. [DOI] [PubMed] [Google Scholar]
- Horikoshi M., Wang C. K., Fujii H., Cromlish J. A., Weil P. A., Roeder R. G. Cloning and structure of a yeast gene encoding a general transcription initiation factor TFIID that binds to the TATA box. Nature. 1989 Sep 28;341(6240):299–303. doi: 10.1038/341299a0. [DOI] [PubMed] [Google Scholar]
- Horikoshi M., Yamamoto T., Ohkuma Y., Weil P. A., Roeder R. G. Analysis of structure-function relationships of yeast TATA box binding factor TFIID. Cell. 1990 Jun 29;61(7):1171–1178. doi: 10.1016/0092-8674(90)90681-4. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Zava D. T., Thilagar A. K., Jensen E. M., McGuire W. L. Steroid receptor analyses of nine human breast cancer cell lines. Cancer Res. 1978 Aug;38(8):2434–2437. [PubMed] [Google Scholar]
- Jantzen K., Fritton H. P., Igo-Kemenes T., Espel E., Janich S., Cato A. C., Mugele K., Beato M. Partial overlapping of binding sequences for steroid hormone receptors and DNaseI hypersensitive sites in the rabbit uteroglobin gene region. Nucleic Acids Res. 1987 Jun 11;15(11):4535–4552. doi: 10.1093/nar/15.11.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Kao C. C., Lieberman P. M., Schmidt M. C., Zhou Q., Pei R., Berk A. J. Cloning of a transcriptionally active human TATA binding factor. Science. 1990 Jun 29;248(4963):1646–1650. doi: 10.1126/science.2194289. [DOI] [PubMed] [Google Scholar]
- Khan A. S., Theodore T. S. Nucleotide sequence and functional analysis of the LTRs of endogenous ecotropic MuLV proviruses of AKR and BALB/c mice. Nucleic Acids Res. 1987 Sep 25;15(18):7640–7640. doi: 10.1093/nar/15.18.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan R. S., Daniel J. C., Jr "Blastokinin": inducer and regulator of blastocyst development in the rabbit uterus. Science. 1967 Oct 27;158(3800):490–492. doi: 10.1126/science.158.3800.490. [DOI] [PubMed] [Google Scholar]
- Li W. I., Chen C. L., Chou J. Y. Characterization of a temperature-sensitive beta-endorphin-secreting transformed endometrial cell line. Endocrinology. 1989 Dec;125(6):2862–2867. doi: 10.1210/endo-125-6-2862. [DOI] [PubMed] [Google Scholar]
- Lin Y. S., Carey M. F., Ptashne M., Green M. R. GAL4 derivatives function alone and synergistically with mammalian activators in vitro. Cell. 1988 Aug 26;54(5):659–664. doi: 10.1016/s0092-8674(88)80010-2. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T., Segall J., Weil P. A., Roeder R. G. Multiple factors required for accurate initiation of transcription by purified RNA polymerase II. J Biol Chem. 1980 Dec 25;255(24):11992–11996. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio F., Karin M. Transcription factors AP-3 and AP-2 interact with the SV40 enhancer in a mutually exclusive manner. EMBO J. 1989 May;8(5):1455–1460. doi: 10.1002/j.1460-2075.1989.tb03528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H., Beato M. RNA synthesis in rabbit endometrial nuclei. Hormonal regulation of transcription of the uteroglobin gene. Eur J Biochem. 1980 Nov;112(2):235–241. doi: 10.1111/j.1432-1033.1980.tb07199.x. [DOI] [PubMed] [Google Scholar]
- Nakajima N., Horikoshi M., Roeder R. G. Factors involved in specific transcription by mammalian RNA polymerase II: purification, genetic specificity, and TATA box-promoter interactions of TFIID. Mol Cell Biol. 1988 Oct;8(10):4028–4040. doi: 10.1128/mcb.8.10.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida M., Kasahara K., Kaneko M., Iwasaki H., Hayashi K. [Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors]. Nihon Sanka Fujinka Gakkai Zasshi. 1985 Jul;37(7):1103–1111. [PubMed] [Google Scholar]
- Patton S. E., Gilmore L. B., Jetten A. M., Nettesheim P., Hook G. E. Biosynthesis and release of proteins by isolated pulmonary Clara cells. Exp Lung Res. 1986;11(4):277–294. doi: 10.3109/01902148609062830. [DOI] [PubMed] [Google Scholar]
- Peterson M. G., Tanese N., Pugh B. F., Tjian R. Functional domains and upstream activation properties of cloned human TATA binding protein. Science. 1990 Jun 29;248(4963):1625–1630. doi: 10.1126/science.2363050. [DOI] [PubMed] [Google Scholar]
- Sagami I., Tsai S. Y., Wang H., Tsai M. J., O'Malley B. W. Identification of two factors required for transcription of the ovalbumin gene. Mol Cell Biol. 1986 Dec;6(12):4259–4267. doi: 10.1128/mcb.6.12.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Schmidt M. C., Kao C. C., Pei R., Berk A. J. Yeast TATA-box transcription factor gene. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7785–7789. doi: 10.1073/pnas.86.20.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen R. C., Srinivas V., Roberts R. M. cDNA sequence, gene organization, and progesterone induction of mRNA for uteroferrin, a porcine uterine iron transport protein. DNA. 1989 Oct;8(8):543–554. doi: 10.1089/dna.1989.8.543. [DOI] [PubMed] [Google Scholar]
- Slater E. P., Redeuihl G., Theis K., Suske G., Beato M. The uteroglobin promoter contains a noncanonical estrogen responsive element. Mol Endocrinol. 1990 Apr;4(4):604–610. doi: 10.1210/mend-4-4-604. [DOI] [PubMed] [Google Scholar]
- Strähle U., Schmid W., Schütz G. Synergistic action of the glucocorticoid receptor with transcription factors. EMBO J. 1988 Nov;7(11):3389–3395. doi: 10.1002/j.1460-2075.1988.tb03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suske G., Wenz M., Cato A. C., Beato M. The uteroglobin gene region: hormonal regulation, repetitive elements and complete nucleotide sequence of the gene. Nucleic Acids Res. 1983 Apr 25;11(8):2257–2271. doi: 10.1093/nar/11.8.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Tsai S. Y., Cook R. G., Beattie W. G., Tsai M. J., O'Malley B. W. COUP transcription factor is a member of the steroid receptor superfamily. Nature. 1989 Jul 13;340(6229):163–166. doi: 10.1038/340163a0. [DOI] [PubMed] [Google Scholar]
- Wefald F. C., Devlin B. H., Williams R. S. Functional heterogeneity of mammalian TATA-box sequences revealed by interaction with a cell-specific enhancer. Nature. 1990 Mar 15;344(6263):260–262. doi: 10.1038/344260a0. [DOI] [PubMed] [Google Scholar]
- Wiehle R. D., Helftenbein G., Land H., Neumann K., Beato M. Establishment of rat endometrial cell lines by retroviral mediated transfer of immortalizing and transforming oncogenes. Oncogene. 1990 Jun;5(6):787–794. [PubMed] [Google Scholar]
- Wobbe C. R., Struhl K. Yeast and human TATA-binding proteins have nearly identical DNA sequence requirements for transcription in vitro. Mol Cell Biol. 1990 Aug;10(8):3859–3867. doi: 10.1128/mcb.10.8.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J. H., Davidson I., Macchi M., Rosales R., Vigneron M., Staub A., Chambon P. In vitro binding of several cell-specific and ubiquitous nuclear proteins to the GT-I motif of the SV40 enhancer. Genes Dev. 1987 Oct;1(8):794–807. doi: 10.1101/gad.1.8.794. [DOI] [PubMed] [Google Scholar]