Abstract
Sexually transmitted infections (STIs) are a major cause of morbidity and mortality worldwide. Although a vaccine is available for HPV, no effective vaccines exist for the HIV-1 and HSV-2 viral pathogens, and there are no cures for these infections. Furthermore, recent setbacks in clinical trials, such as the failure of the STEP trial to prevent HIV-1 infection, have emphasized the need to develop alternative approaches to interrupt transmission of these pathogens. One alternative strategy is represented by the use of topically applied microbicides, and such agents are being developed against various viruses. RNAi-based microbicides have recently been demonstrated to prevent HSV-2 transmission, and may be useful for targeting multiple STIs. In this review, microbicides that are under development for the prevention of STIs are described, with a focus on topically applied microbicidal siRNAs.
Keywords: HIV-1, HSV-2, microbicide, RNA interference, sexually transmitted infection, targeted therapy
Introduction
RNAi is a mechanism used by many species to regulate RNA expression. RNAi was originally described in plants and Caenorhabditis elegans, where it was observed that injection of small stretches of dsRNA resulted in cleavage of homologous target mRNA [1]. Subsequently, this pathway was also identified in mammalian cells [2]. Gene silencing via the RNAi pathway is mediated by small non-coding RNAs, 21 to 25 nucleotides in length, termed siRNAs. Because of their specificity and potency, siRNAs have attracted significant attention as potential therapeutic agents. Clinical trials targeting diverse diseases, such as liver cancers and respiratory syncytial virus (RSV), with siRNAs are ongoing. For example, the RSV-targeted siRNA compound ALN-RSV01 (Alnylam Pharmaceuticals Inc/Cubist Pharmaceuticals Inc/Kyowa Hakko Kirin Co Ltd) has completed phase II trials in lung transplant patients with RSV and healthy volunteers (ClinicalTrials.gov identifiers: NCT00658086 and NCT00496821, respectively).
HIV-1, HSV-2 and HPV are three major viruses that are sexually transmitted. Despite the morbidity and mortality associated with these viruses and an increased level of understanding of the diseases they cause, a prophylactic vaccine only became available for HPV in 2007 [3]. Several vaccines for HIV-1 and HSV-2 are in clinical trials, but these have demonstrated limited protection [4,5].
Recently, a clinical trial using an adenovirus serotype 5 (Ad5) vector, the STEP trial sponsored by Merck & Co Inc, was stopped because the prevention of HIV-1 infection was not observed, despite the induction of HIV-specific CD8+ T-cells [6]. Moreover, the incidence of HIV-1 was higher in uncircumcised men with pre-existing Ad5 immunity. Another vaccine trial, which combined two vaccines that had performed poorly when administered individually (ALVAC HIV, a recombinant canarypox vector vaccine, combined with AIDSVAX [Global Solutions for Infectious Diseases], a glycoprotein 120 subunit vaccine; NCT00223080), reported only modest protection from HIV-1 infection (31% fewer infections in the vaccinated group compared with the placebo-treated group) [4].
HSV-2 can facilitate HIV-1 transmission [7,8]. More than 20% of the US population is infected with HSV-2, and seroprevalence in sub-Saharan Africa has been recorded to be as high as 90% among sex workers [9]. HSV-2 is the main cause of genital ulcers, and the associated influx of inflammatory cells likely provides a permissive environment for HIV-1 transmission [10]. Subunit vaccines developed for HSV-2 have demonstrated limited efficacy, probably as a result of the inability to elicit CD8+ T-cells (reviewed in reference [5]). Live, replication-defective HSV-2 is currently being evaluated as a candidate HSV-2 vaccine [11].
With the lack of effective vaccines for HIV-1 and HSV-2, and the cost of the HPV vaccines limiting their use in developing countries, alternative strategies are required to prevent transmission of these diseases. Microbicides are topically applied compounds that inhibit viral transmission. Several microbicidal compounds targeting HIV-1 are being assessed in clinical trials, and more than 50 such agents are under investigation [12]. Moreover, RNAi-based strategies have been demonstrated to limit infection with HIV-1, HSV-2 and HPV in vitro and in vivo [3,13,14]. Most of these studies focused on systemic delivery, which would be useful as a therapeutic modality, but may not be effective for prophylaxis. siRNA-based knockdown of RSV has indicated that siRNA uptake and gene silencing in mucosal tissue is feasible [15]. Similarly, topical application of siRNAs targeting HSV-2 viral genes protected mice from lethal HSV-2 challenge [16,17].
In this review, microbicides that are under development for the prevention of STIs are described. Initial data demonstrating the feasibility of using topically applied siRNAs as microbicides and the current limitations of this approach are outlined, and potential approaches to overcome these limitations are also discussed.
The RNAi machinery
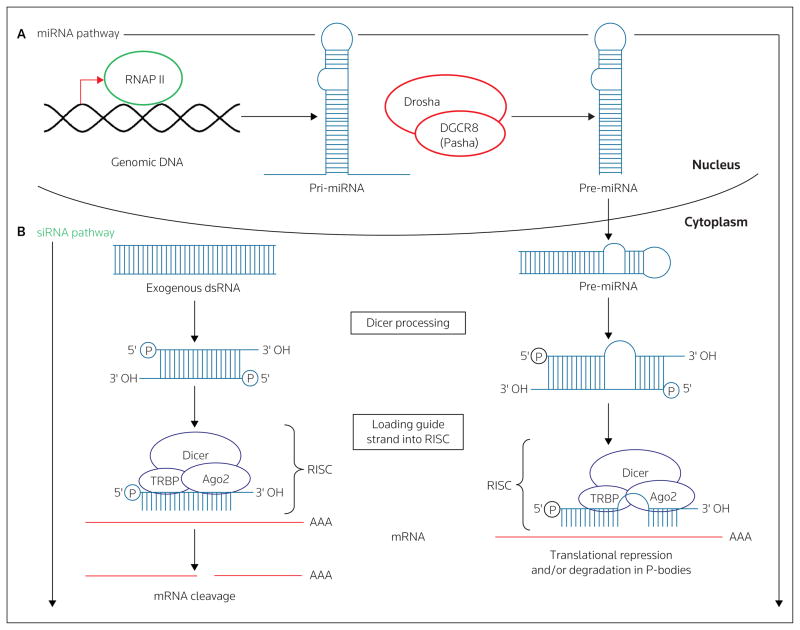
The RNAi pathway (Figure 1) can be targeted at various stages for use in therapeutic applications through an increasing number of strategies. For example, siRNAs can be derived from vector-encoding hairpin sequences (small hairpin RNA [shRNA]) [18]. Following transcription, the shRNA is cleaved by the RNase III enzyme Drosha. The shRNA is then exported into the cytoplasm via exportin 5, and is further processed by Dicer, yielding siRNAs [19]. Alternatively, synthetic siRNAs can be used, thereby circumventing the requirement for most of the RNAi pathway [2]. siRNA-mediated gene knockdown is initiated following binding of the activated RNA-induced silencing complex (RISC), which contains the siRNA guide strand, to complementary mRNA. This is followed by site-specific cleavage of the target mRNA by the RNase III enzyme Argonaute 2. Site-specific cleavage of mRNA by Argonaute 2 is a hallmark of siRNA-mediated gene knockdown, and can be verified by analysis of the target mRNA cleavage product [19].
Figure 1. The RNAi pathway.
(A) The micro-RNA (miRNA) pathway: The full-length RNA for the pri-miRNA is transcribed by RNA polymerase II (RNAP II) in the nucleus and assumes the canonical hairpin conformation. The longer pri-miRNA is then processed by Drosha and DGCR8 (Pasha) into the shorter pre-miRNA and exported to the cytoplasm via exportin 5. The pre-miRNA is further processed to an approximately 21-nucleotide double-stranded miRNA with two nucleotide overhangs present at the 3′ end and a monophosphate at the 5′ end. This processed miRNA is incorporated into the RNA-induced silencing comple (RISC), comprised of Dicer, Argonaute 2 (Ago2) and TAR RNA binding protein (TRBP), where a single strand is then selected as the guide strand and the non-selected or passenger strand is ejected and degraded. Incorporation of the antisense/guide strand into RISC is followed by binding to mRNA with either complete or partial complementarity, resulting in a translational block, and this can be accompanied by degradation of the target mRNA within P-bodies. (B) The siRNA pathway: Long dsRNA enters the cytoplasm of a cell (typically from an exogenous source, such as a virus). The long dsRNA is then processed into a shorter approximately 21-nucleotide siRNA with two nucleotide overhangs present at the 3′ ends and a monophosphate at the 5′ ends. The siRNA is incorporated into the RISC complex, where a single strand is then selected as the guide strand and the non-selected or passenger strand is ejected and degraded. The guide strand will bind to the complementary mRNA, resulting in sequence-specific mRNA cleavage and degradation.
Given the extensive knowledge of the RNAi mechanisms of gene knockdown, the identification of siRNAs that can optimally silence target genes can be achieved within a few months. siRNAs are initially chosen by employing algorithms to predict active sequences, using rules and guidelines based on RNA sequence and thermodynamics, such as through the use of online design tools including siDESIGN Center (Dharmacon RNAi Technologies/Thermo Scientific). Although these bioinformatics tools are useful and are continually updated, all predicted siRNAs must be evaluated carefully through extensive in vitro testing for efficacy, specificity and toxicity.
Microbicides targeting sexually transmitted infections
Microbicides can be separated into categories depending on their mode of action (see Table 1 for a summary of selected microbicides in clinical trials for STIs). First-generation microbicides, such as nonoxynol-9 (N9), act as detergents, inactivating the virus through disruption of the viral envelope. However, N9 also disrupts the vaginal epithelium, causing inflammation and disruption of the vaginal flora, and in a trial, N9 application increased HIV-1 transmission [20]. Therefore, N9 and other detergents are considered to be of limited use as microbicides for the treatment of STIs. However, a recent study demonstrated that macaques were protected from SIV when treated daily with glycerol monolaurate (GML) [21,22]. The protective effect was attributed to the ability of GML to inhibit immune activation, in particular the expression of inflammatory chemokines and cytokines such as MIP-3α and IL-8 [21,22].
Table 1.
Selected candidate microbicides in sexually transmitted infection clinical trials.
| Microbicide candidate (developer) | Method of action; preparation | Phase | Reference/ClinicalTrials.gov identifier |
|---|---|---|---|
| Truvada, a combination of FTC (Emtriva) and TDF (Viread)* | (NRTI, oral) | III | NCT00625404 |
| Tenofovir (IPM/CONRAD) | NtRTI; gel | IIb | [80] NCT00441298 |
| Dapivirine (IPM) | NNRTI; gel or ring | I/II | NCT00799058 |
| UC-781 (Biosyn Inc) | NNRTI; gel | I | NCT00385554 |
| PRO-2000 (Endo Pharmaceuticals Solutions Inc) | Entry/fusion inhibitor; gel | III** | [23,81]*** NCT00262106 |
| SPL-7013 (Starpharma Holdings Ltd/Biomecular Research Institute Ltd) | Entry/fusion inhibitor, dendrimer; gel | I** | [27] NCT00442910 |
| Acidform | Vaginal defense enhancer, pH modifier; gel | I | [82] NCT00850837 |
Compounds listed are representative of the major types (categorized by mode of action) under evaluation in clinical trials. For a comprehensive, and continuously updated list of microbicides in clinical trials, access the Microbicides website (see reference [83]).
Truvada has been launched for the treatment of HIV infection, but is in clinical trials as a microbicide.
Denotes clinical trials that have been completed.
No significant protection observed.
CONRAD Contraceptive Research and Development Organization, IPM International Partnership for Micobicides, NRTI nucleoside analog reverse transcriptase inhibitor, NtRTI nucleotide analog reverse transcriptase inhibitor, NNRTI non-nucleoside analog reverse transcriptase inhibitor, TDF tenofovir disoproxil fumarate, FTC emtricitabine
Anionic polymers prevent viral binding or entry into host cells. PRO-2000 (Endo Pharmaceuticals Solutions Inc), a naphthalene sulfonate polymer antimicrobial gel that binds CD4, reduced the risk of HIV-1 infection by 30% over a period of 3 years; however, although encouraging, this effect was not statistically significant [23]. Carraguard (PC-515; Population Council), a λ- and κ-carrageenan vaginal microbicidal gel, and cellulose sulfate (Ushercell; Polydex Pharmaceuticals Ltd) did not confer protection from HIV-1 transmission [24,25]. However, PRO-2000 and cellulose sulfate may be useful in blocking HSV-2 entry into target cells by binding glycoprotein-B [26]. Clinical trials of SPL-7013 (VivaGel; Starpharma Holdings Ltd/Biomecular Research Institute Ltd), a dendrimer reported to have both anti-HIV-1 and -HSV-2 activity, are also underway [27].
Microbicides that target specific viral proteins or host factors required for viral infection are also under development. Tenofovir (International Partnership for Microbicides [IPM]/Contraceptive Research and Development Organization [CONRAD]), a nucleotide reverse transcriptase inhibitor, is in phase II clinical trials for HIV-1 infection (Table 2). Importantly, some of these reverse transcriptase inhibitors have been demonstrated to prevent HIV-1 infection in dendritic cells and CD4+ T-cells, which are initial targets for the virus [28,29]. Other microbicidal candidates that have exhibited efficacy against STIs are entry inhibitors that block the HIV-1 coreceptor CCR5. For example, analogs of the chemokine RANTES [30], a CCR5 ligand, and maraviroc, an FDA-approved small-molecule CCR5 antagonist [31], have been reported to protect macaques from vaginal transmission of simian HIV (SHIV). mAbs targeting CCR5, HIV gp41 (2F5, 4E10) or gp120 (2G12) could also be effective as STI microbicides [32,33]. However, SHIV recovered from infected macaques that had been pretreated with the vaginal microbicide RANTES analog PSC-RANTES contained specific mutations [34]. This result highlights the possibility that microbicides can allow selection of drug-resistant variants, and this will need to be systematically monitored.
Table 2.
Selected reagents used for siRNA delivery.
| Reagent | Route of administration (model used) | Reference |
|---|---|---|
| Naked siRNAs | Intranasal (mouse) | [59] |
| Intravaginal (mouse) | [16,42] | |
|
| ||
| Cholesterol-siRNA | Systemic (mouse) | [36] |
| Intravaginal (mouse) | [17] | |
|
| ||
| Positively charged protein/Peptide-antibody-siRNA | Systemic (mouse) | [47,48] |
|
| ||
| Aptamer-siRNA | Systemic (mouse) | [66,67] |
|
| ||
| SNALPs | Systemic (cynomolgus monkey) | [37] |
| Systemic (mouse) | [84] | |
|
| ||
| Nanoparticles | Intravaginal (mouse) | [35] |
| Systemic (mouse) | [85] | |
SNALP stable nucleic acid-lipid particles
siRNA as a microbicide for sexually transmitted infections
Several RNAi-based microbicides are under development for STIs. In the first study to demonstrate the feasibility of this approach, siRNAs targeting HSV-2 viral genes were demonstrated to be effective in a mouse model of HSV-2 transmission [16]. More specifically, siRNAs targeting the essential HSV-2 genes encoding for UL27, the viral envelope glycoprotein B, and UL29, a DNA-binding protein, were complexed with a cationic lipid transfection reagent. The complex was applied to vaginal mucosa, both prior to and following challenge with a lethal dose of HSV-2. siRNAs targeting viral genes, but not irrelevant control genes, conferred up to 80% protection from infection. The complexed siRNAs did not cause overt inflammation or toxicity [16].
Results from initial studies with siRNA-based microbicides, such as those described in reference [16], were encouraging. However, protection from HSV-2 was transient (siRNAs had to be administered within hours of challenge), and there were indications that the cationic lipid reagent resulted in some degree of toxicity [17,35]. In a follow-up study, higher doses of lipid reagent were demonstrated to enhance HSV-2 infection. A small, but significant, increase in the number of CD45+ cells was observed following administration of lipid reagent [17]. Therefore, to circumvent the requirement for lipid-complexed siRNAs, cholesterol-conjugated (C)-siRNAs, previously used for systemic siRNA delivery, were used [36,37]. In addition, the 3′ ends of the siRNA were further modified by replacement of a phosphodiester bond with a phosphorothioate, thereby extending siRNA half-life ([38]; see the Optimizing siRNA delivery section).
In an attempt to achieve durable protection from viral infection, C-siRNAs targeting a host-encoded entry receptor, nectin-1 [39,40], were combined with UL29-specific C-siRNAs. It had been previously demonstrated in vitro that siRNAs targeting the HIV-1 host coreceptor CCR5 persisted for longer than siRNAs targeting an HIV-1 viral gene [41]. Sustained viral suppression was associated only with siRNAs targeting CCR5, suggesting that the presence of target mRNA may determine siRNA persistence and associated gene knockdown [41]. Similarly, nectin-1 C-siRNAs protected 80% of mice when administered several days prior to HSV-2 challenge, coincident with nectin-1 down-modulation. Protection was diminished when nectin-1 C-siRNAs were administered within a few days of viral challenge [17]. As observed in the initial siRNA-based microbicide study [16], UL29 C-siRNAs conferred protection only when administered within hours of viral challenge. In sharp contrast, a combination of nectin-1- and UL29-specific C-siRNAs protected mice for at least 1 week [17].
These initial siRNA-based microbicide studies highlighted some intriguing issues regarding RNAi uptake and mechanism of action, most of which remain to be addressed. For example, topical application of lipid-complexed fluorescent siRNAs resulted in uptake throughout the epithelium and lamina propria [16], and in a separate study, a similar distribution was observed in the vaginal and rectal mucosa [42]. The mechanism by which the siRNAs gain access to these tissues has not been established. Evidence exists to suggest the uptake of either naked siRNAs or cholesterol-conjugated siRNAs through connexin-specific gap junctions or via the transmembrane protein SID-1 [43–45]. Whether connexins or SID-1 play a role in siRNA uptake across the epithelial membrane remains to be determined. For HSV-2 infection, the primary cellular targets are epithelial and neuronal cells, and topically applied siRNAs have been observed in the epithelium [16,35]. Whether neurons also take up siRNAs is unknown and, as HSV-2 establishes latency in these cells, neuronal siRNA uptake could be useful for eradicating an established HSV infection by reactivating latent virus. For example, latent virus may be reactivated by reducing the expression of the latency-associated transcript (LAT), the only viral gene abundantly expressed during latency [46]. The activated virus could then be cleared either with viral-specific siRNAs or conventional antiviral drugs.
Improving the viral protection afforded by siRNA-based microbicides
Optimizing siRNA delivery
To optimize effective intravaginal siRNA delivery, various issues have to be addressed. The formulation of the siRNA is critical; siRNA modifications may be required to protect the siRNA molecules from RNases, limit off-target effects and IFN responses. Similarly, the presence of proteases must be considered if a protein carrier is to be used. Furthermore, the pH of the vaginal lumen is low (pH 4 to 4.5), and there is a layer of protective mucus in which siRNA complexes can be trapped (Figure 2A). However, resisting degradation does not necessarily correlate with superior protection from viral transmission per se. In fact, siRNA modifications can have a deleterious effect on target gene silencing [38]. Therefore, modified siRNAs must be tested for their ability to knockdown the expression of targeted genes. Additionally, topically administered siRNAs must come into contact with, and be taken up by the appropriate cell types. Limiting siRNA uptake to cells expressing a specific receptor, such as by attaching an antibody or aptamer, is one approach being used to facilitate uptake by the desired cell population ([47,48], and discussed further in the Overcoming toxicity section). However, efficient siRNA uptake does not necessarily equate with gene knockdown. siRNAs must gain access to the cytoplasm, where RISC is located, to be effective (Figure 2B). However, after siRNAs have entered the cytoplasm, as few as 5,000 siRNA molecules can mediate effective gene knockdown [49].
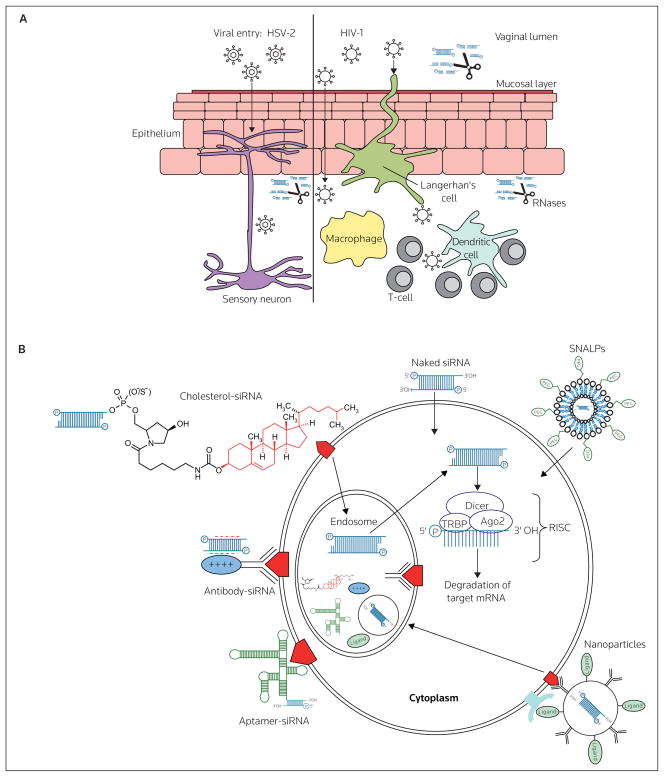
Figure 2. Routes of viral entry across vaginal mucosal epithelium and barriers to effective siRNA delivery following intravaginal administration.
(A) Local innate immune factors, including the acidic pH of the vaginal lumen and the presence of secreted proteins and a mucous layer contribute to preventing viral transmission. HSV-2 primarily infects epithelial cells and sensory neurons, whereas the initial targets of HIV-1 are dendritic cells, macrophages and CD4+ T-cells. To be effective, siRNA-based microbicides must be stable under acidic conditions, must be resistant to protease and RNase acitivity and must be able to gain access to the appropriate cell types to prevent viral transmission. (B) Having gained access to the appropriate cells in the vaginal mucosa, the route of cellular uptake by siRNA will likely determine the efficacy of gene knockdown. siRNAs must gain access to the RNA-induced silencing complex (RISC), which is located in the cytoplasm, but siRNAs may enter cells via endosomal uptake (eg, as antibody-, aptamer- and cholesterol-siRNAs and antibody-coated nanoparticles). Alternatively, naked siRNAs (either lipid-complexed or non-complexed) and liposomal particles (eg, stable nucleic acid-lipid particles [SNALPs]), may enter the cytoplasm directly following membrane fusion, or through, as yet undetermined, channels or receptors. Ago2 Argonaute 2, TRBP TAR RNA binding protein
The delivery of siRNAs remains the biggest challenge in the development of RNAi-based therapeutics. Table 2 summarizes some of the strategies that have been used successfully. siRNAs must reach the target cells, cross the plasma membrane and gain access to the RNAi machinery in the cytoplasm. The manner in which siRNAs are delivered dictates the efficiency of the knockdown. For example, siRNAs that are not targeted to a specific cell type may not silence as efficiently as targeted siRNAs because of dilution of the siRNA. The route of administration also determines the efficacy of gene knockdown; the cell types encountered by the siRNA molecules vary and the siRNA stability is differentially affected by factors including degradation by RNases, kidney filtration and retention in the mucus present at mucosal surfaces. Several strategies have been successfully used for systemic siRNA delivery (reviewed in reference [50]), but the majority of these strategies have not been tested for topical use.
As discussed previously, siRNAs can induce undesirable responses, and these must be identified when using a new delivery strategy and/or siRNA sequence. The main problems encountered, such as off-target effects and activation of immune responses, can be overcome by using chemically modified siRNAs [51,52]. Limiting siRNA delivery to specific cell types should also minimize unwanted responses.
Despite these hurdles, siRNAs have progressed rapidly into clinical trials, with at least five siRNA agents being assessed in ongoing trials (reviewed in reference [53]). Results from a phase I trial, using lentivirally encoded shRNAs targeting HIV tat and rev genes plus two additional anti-HIV genes [54], designed to treat patients with AIDS-related lymphomas, have demonstrated that gene-modified cells with the potential to limit HIV infection were successfully delivered to patients [55].
Increasing the duration of the protective effect
Achieving durable protection from viral infection is critical for a microbicide. Most microbicides under investigation prevent transmission only if applied within hours of, or for the duration of a sex act. As a result, compliance is low, and the frequency of use is often over-reported. By combining siRNAs targeting host and viral genes, protection from viral infection may last for days or even weeks. Using a cocktail of siRNAs could also prevent the emergence of resistant viral strains, which have been reported following siRNA treatment for HIV-1 and HPV [56,57].
As discussed in the siRNA as a microbicide for STIs section, for reasons that are currently unclear, the window of protection when targeting viral genes appears to be limited, and silencing host genes may confer long-term protection. Silencing a host gene, although potentially useful in this setting, may cause toxicity. Microbicides would be used frequently, and the effect of decreased expression of genital mucosa host genes and associated immune cells must be thoroughly explored. For example, homozygous expression of a mutated nectin-1 gene during embryogenesis is associated with cleft-lip or cleft-palate syndrome [58]; however, it is not known if loss of this gene following development results in undesirable side effects. Nectin-1 is a cell-adhesion molecule, and although no changes were observed in vaginal epithelium physiology with use of an siRNA targeting both a host and a viral HSV-2 gene for 1 week [17], chronic use may alter epithelial integrity.
Data indicate that using a cocktail of siRNAs targeting only viral genes may not be effective for delivering durable gene knockdown [17,41]. Sustained release of siRNAs could overcome this problem. In a recent study, siRNA-containing nanoparticles were synthesized that released siRNAs for several weeks in vitro [35]. In vivo, vaginally applied nanoparticles were detected for at least 7 days following application and were observed in the epithelium and lamina propria. Silencing of genes (albeit endogenous genes, not viral) with the nanoparticles lasted for 14 days, the duration of the experiment. Additionally, gene knockdown was observed in the cervix, a major site for HIV-1 transmission, as well as in the uterine horns. The nanoparticles were composed of FDA-approved materials, and histological examination of vaginal tissue following nanoparticle application demonstrated no evidence of cellular infiltration or epithelial disruption [35]. It will be useful to assess whether these nanoparticles containing siRNAs targeting viral genes can provide durable protection from viral challenge.
Overcoming toxicity
To avoid the toxicity associated with delivery reagents, naked (uncomplexed) siRNAs can be administered. Naked siRNAs have been used to target the respiratory pathogens RSV and parainfluenza virus (PIV) via intranasal delivery in mice [15,59]. When administered up to 4 h prior to viral challenge, RSV-specific siRNAs reduced the viral load by up to 3 log-units in mouse lungs. Therapeutic administration of siRNAs was also effective. Analysis of the RSV mRNA cleavage product suggested the presence of an RNAi-mediated mechanism, and the use of immunostimulatory, non-RSV-specific control siRNAs could not control RSV infection. As some studies have suggested that the siRNA efficacy is mediated through immune response activation and not site-specific mRNA cleavage [60–62], these control experiments were important to confirm that the mechanism of action of the naked RSV-specific siRNAs was via RNAi-mediated mRNA cleavage. As uptake of naked siRNAs is effective in the lung mucosa, it would be reasonable to hypothesize that application of naked siRNAs might be effective for gene knockdown in the genital mucosa. However, intravaginal application of uncomplexed siRNAs has not been effective; naked siRNAs, modified for RNA stability, were unable to decrease nectin-1 expression in the vaginal mucosa [17]. However, cholesterol-conjugated siRNAs, similarly modified for RNA stability, effectively down-modulated nectin-1 expression [17].
Unwanted responses elicited by siRNAs can be limited by targeting the delivery of these molecules to specific cell types. This approach should also lower the effective siRNA dose. The transmission of HIV-1 requires the infection of dendritic cells, macrophages and T-cells [63]. Successful targeted delivery to some of these cell types has been achieved using antibody- and aptamer-directed siRNA delivery. Antibodies fused to protamine, a positively charged protein that binds nucleic acids, can bind siRNAs. In an initial study, siRNAs targeting oncogenes were incubated with an HIV-1 gp120-specific antibody fused to protamine, and injected intravenously [47]. Reduced tumor growth was observed only in tumors expressing HIV-envelope proteins. More recently, a CD7-specific single-chain antibody conjugated to nine arginines (scFvCD7-9R) was used to deliver anti-CCR5 and anti-HIV-1 siRNAs to a humanized mouse model [48]. CD7 is expressed on T-cells, and systemic administration of scFvCD7-9R-bound siRNAs protected mice from HIV-1 challenge. This treatment was also effective in controlling established HIV-1 infection, and maintaining CD4+ T-cells [48].
Nucleic acid aptamers, similar to antibodies, bind specifically to target ligands, with binding affinities that can be in the picomolar range (reviewed in reference [64]). Unlike antibodies, aptamers can be readily synthesized and are amenable to modifications, such as the inclusion of RNA modified for stability in serum. Aptamers may also lack the immunogenicity associated with antibodies. Recently, siRNAs attached to an anti-PSMA (prostate-specific membrane antigen) aptamer were demonstrated to effectively deliver siRNAs only to PSMA-expressing cells in vitro and in vivo [65–67]. Additionally, anti-gp120 aptamers with anti-HIV activity have been selected [68,69]. The aptamers were effective as inhibitors of HIV-1 infection, but conjugation of anti-tat/rev siRNA to the aptamers resulted in stronger suppression of HIV-1 (HIV p24 production in PBMCs, relative to controls, was ~35% lower in aptamer-only treated cells, compared with 35 to 50% lower in aptamer/siRNA chimeras). Using such a dual inhibitory approach may be useful for preventing the emergence of resistant viral strains.
siRNA-based therapies: A balance between efficacy and safety
siRNAs have generated considerable interest as tools that can be used to understand gene function, and as therapeutic agents. However, several studies have highlighted that RNAi-mediated gene silencing can cause unwanted side effects [52]. Furthermore, although the number of RNAi-based clinical trials is expanding rapidly, some have already been discontinued. For example, bevasiranib (OPKO Health Inc), an siRNA designed to target VEGF, thereby slowing progression of wet age-related macular degeneration (AMD), had advanced to phase III trials prior to the trial being terminated [70]. It was determined that a significant improvement in vision loss was unlikely to be achieved, because of poor efficacy of the siRNA. The use of unmodified siRNA may be a contributing factor; following intravitreal injection, the siRNA half-life is short (~minutes). Furthermore, it is unclear how amenable unmodified siRNAs are to be uptaken by endothelial cells of the choroidal vasculature.
Knockdown of untargeted genes as a result of partial mRNA sequence homology has been reported. However, this effect can be circumvented by modifications to the sense and antisense strands [51]. Additionally, overexpression of shRNAs has been demonstrated to saturate components of the RNAi machinery, such as exportin 5, leading to fatalities and brain toxicity in mice [71,72]. Saturation of the RNAi machinery may be avoidable by using siRNAs [73]; however, siRNAs, particularly at high concentrations, can overload the RNAi machinery, thereby perturbing miRNA function [74]. Administering combinations of siRNAs has also been reported to result in competition for RISC, leading to decreased silencing of one of the targeted genes [59,75]. siRNAs can induce IFN or inflammatory responses [60,76], but avoidance of certain nucleotide motifs and selective chemical modifications can abrogate these effects, without perturbing siRNA activity [77].
In addition to safety concerns directly related to the use of siRNAs in a microbicide, potential toxicity associated with a delivery vehicle must be assessed. The microbicide N9 was deemed safe in preclinical studies prior to proceeding to clinical trials, in which this agent was demonstrated to enhance HIV-1 acquisition [20]. Therefore, careful analysis of the epithelium of the vagina and ectocervix must be conducted to ensure integrity of this barrier. New models are being developed that are designed to provide more stringent assessments of microbicide safety. Mesquita et al reported that measurements of epithelial barrier disruption, following exposure to candidate microbicides, correlate with the ability of HIV to traverse a normally intact epithelium resulting in infection of PBMCs [78]. Notably, under identical culture conditions, production of proinflammatory cytokines was not significantly altered.
Conclusion
The field of RNAi has progressed at a rapid pace. The potential ability of siRNAs to target any disease for which there is an identified genetic element renders them an attractive therapeutic modality. Currently, for STIs, only one siRNA-based clinical trial has been initiated; HIV-1 and AIDS lymphomas are being targeted using a lentivirus that encodes multiple anti-HIV RNA, including shRNA [55]. Results from this trial will be useful in assessing the utility of using a combination approach, which includes shRNA to tackle HIV-1. However, the approach used in this study, lentiviral transduction of autologous CD34+ stem cells, is a form of individualized treatment, and would therefore preclude it from being used as a general therapy.
There are currently no ongoing clinical trials using siRNAs either alone or in combination with conventional drugs to inhibit the transmission of an STI. The recent termination of the phase III trial using VEGF-specific siRNAs to target wet AMD [70], combined with several studies highlighting potential safety and efficacy issues when using siRNAs [52], indicate that any data suggestive of an RNAi-specific mode of action must be stringently verified. For example, although chemical modifications and judicious sequence selection should avoid the generation of the majority of off-target and inflammatory effects, testing for the activation of IFN-related genes and microarray analysis should be used to confirm the absence of inappropriate activation. Analysis of mRNA cleavage products should confirm RNAi-mediated cleavage [36].
Methods that could limit siRNA-induced side effects potentially include the use of naked siRNAs [59], although preliminary data suggest that these molecules are not effective when applied to the vaginal mucosa [17]. Alternatively, cell-specific targeting could be useful by limiting siRNA uptake in bystander cells, thereby decreasing the effective siRNA dose and limiting unwanted responses. Recently, an antibody-based approach for the delivery of siRNAs to T-cells to suppress HIV-1 progression has been licensed by BIOO Therapeutics (a division of BIOO Scientific Corp) [79]. Additionally, as topically applied siRNAs penetrate deep into the genital mucosa [16,35,42], topically applied antibodies may gain access to T-cells, or other immune cells, present in the mucosa.
In conclusion, vaginally applied siRNAs have the ability to confer long-lasting gene silencing that equates with antiviral activity [17]. The durability of protection from viral transmission is an important factor for compliance and cost of treatment, in particular in developing countries.
Acknowledgments
The authors thank Ernest Yakob for critical reading of the manuscript. The Palliser research laboratory is supported by Albert Einstein College of Medicine start-up funds and by a Center for AIDS Research pilot project grant (AECOM/MMC 2P30AI051519) to Deborah Palliser.
References
•• of outstanding interest
• of special interest
- 1•.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–811. doi: 10.1038/35888. Describes the first study, along with reference [2], to demonstrate that dsRNA can inhibit gene expression, and that the RNAi pathway is active in mammalian cells. [DOI] [PubMed] [Google Scholar]
- 2•.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–498. doi: 10.1038/35078107. Describes the first study, along with reference [1], to demonstrate that dsRNA can inhibit gene expression, and that the RNAi pathway is active in mammalian cells. [DOI] [PubMed] [Google Scholar]
- 3.Gu W, Putral LN, Irving A, McMillan NA. The development and future of oligonucleotide-based therapies for cervical cancer. Curr Opin Mol Ther. 2007;9(2):126–131. [PubMed] [Google Scholar]
- 4.Cohen J. HIV/AIDS research. Beyond Thailand: Making sense of a qualified AIDS vaccine ‘success’. Science. 2009;326(5953):652–653. doi: 10.1126/science.326_652. [DOI] [PubMed] [Google Scholar]
- 5.Dudek T, Knipe DM. Replication-defective viruses as vaccines and vaccine vectors. Virology. 2006;344(1):230–239. doi: 10.1016/j.virol.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 6.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, Buchbinder SP, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: A case-cohort analysis. Lancet. 2008;372(9653):1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: Systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 8.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: A meta-analysis. J Infect Dis. 2002;185(1):45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 9.Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: A review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35(5):435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 10.Celum C, Levine R, Weaver M, Wald A. Genital herpes and human immunodeficiency virus: Double trouble. Bull World Health Organ. 2004;82(6):447–453. [PMC free article] [PubMed] [Google Scholar]
- 11.Hoshino Y, Dalai SK, Wang K, Pesnicak L, Lau TY, Knipe DM, Cohen JI, Straus SE. Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein, and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs. J Virol. 2005;79(1):410–418. doi: 10.1128/JVI.79.1.410-418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikolic DS, Piguet V. Vaccines and microbicides preventing HIV-1, HSV-2, and HPV mucosal transmission. J Invest Dermatol. 2010;130(2):352–361. doi: 10.1038/jid.2009.227. [DOI] [PubMed] [Google Scholar]
- 13.Berkhout B, ter Brake O. Towards a durable RNAi gene therapy for HIV-AIDS. Expert Opin Biol Ther. 2009;9(2):161–170. doi: 10.1517/14712590802653619. [DOI] [PubMed] [Google Scholar]
- 14.de Fougerolles AR. Delivery vehicles for small interfering RNA in vivo. Hum Gene Ther. 2008;19(2):125–132. doi: 10.1089/hum.2008.928. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez R, Elbashir S, Borland T, Toudjarska I, Hadwiger P, John M, Roehl I, Morskaya SS, Martinello R, Kahn J, Van Ranst M, et al. RNA interference-mediated silencing of the respiratory syncytial virus nucleocapsid defines a potent antiviral strategy. Antimicrob Agents Chemother. 2009;53(9):3952–3962. doi: 10.1128/AAC.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Palliser D, Chowdhury D, Wang QY, Lee SJ, Bronson RT, Knipe DM, Lieberman J. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature. 2006;439(7072):89–94. doi: 10.1038/nature04263. Demonstrated, for the first time, the feasibility of using siRNAs to block transmission of a viral pathogen across the genital mucosa. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Navarro F, Lal A, Basar E, Pandey RK, Manoharan M, Feng Y, Lee SJ, Lieberman J, Palliser D. Durable protection from herpes simplex virus-2 transmission following intravaginal application of siRNAs targeting both a viral and host gene. Cell Host Microbe. 2009;5(1):84–94. doi: 10.1016/j.chom.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296(5567):550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 19.Kim D, Rossi J. RNAi mechanisms and applications. Biotechniques. 2008;44(5):613–616. doi: 10.2144/000112792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, Rees H, Sirivongrangson P, Mukenge-Tshibaka L, Ettiegne-Traore V, Uaheowitchai C, Karim SS, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: A randomised controlled trial. Lancet. 2002;360(9338):971–977. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458(7241):1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlievert PM, Strandberg KL, Brosnahan AJ, Peterson ML, Pambuccian SE, Nephew KR, Brunner KG, Schultz-Darken NJ, Haase AT. Glycerol monolaurate does not alter rhesus macaque (Macaca mulatta) vaginal lactobacilli and is safe for chronic use. Antimicrob Agents Chemother. 2008;52(12):4448–4454. doi: 10.1128/AAC.00989-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdool Karim S, Coletti A, Richardson B, Ramjee G, Hoffmann I, Chirenje M, Taha T, Kapina M, Maslankowski L, Soto-Torres L. Safety and effectiveness of vaginal microbicides BufferGel and 0.5% PRO 2000/5 gel for the prevention of HIV infection in women: Results of the HPTN 035 trial. Conference on Retroviruses and Opportunistic Infections; Montreal, QC, Canada. 2009. p. Abs 48LB. [Google Scholar]
- 24.Skoler-Karpoff S, Ramjee G, Ahmed K, Altini L, Plagianos MG, Friedland B, Govender S, De Kock A, Cassim N, Palanee T, Dozier G, et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: A randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9654):1977–1987. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 25.Van Damme L, Govinden R, Mirembe FM, Guedou F, Solomon S, Becker ML, Pradeep BS, Krishnan AK, Alary M, Pande B, Ramjee G, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359(5):463–472. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 26.Cheshenko N, Keller MJ, MasCasullo V, Jarvis GA, Cheng H, John M, Li JH, Hogarty K, Anderson RA, Waller DP, Zaneveld LJ, et al. Candidate topical microbicides bind herpes simplex virus glycoprotein B and prevent viral entry and cell-to-cell spread. Antimicrob Agents Chemother. 2004;48(6):2025–2036. doi: 10.1128/AAC.48.6.2025-2036.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rupp R, Rosenthal SL, Stanberry LR. VivaGel (SPL7013 gel): A candidate dendrimer-microbicide for the prevention of HIV and HSV infection. Int J Nanomedicine. 2007;2(4):561–566. [PMC free article] [PubMed] [Google Scholar]
- 28.Balzarini J, Van Herrewege Y, Vanham G. Metabolic activation of nucleoside and nucleotide reverse transcriptase inhibitors in dendritic and Langerhans cells. AIDS. 2002;16(16):2159–2163. doi: 10.1097/00002030-200211080-00008. [DOI] [PubMed] [Google Scholar]
- 29.Van Herrewege Y, Michiels J, Van Roey J, Fransen K, Kestens L, Balzarini J, Lewi P, Vanham G, Janssen P. In vitro evaluation of nonnucleoside reverse transcriptase inhibitors UC-781 and TMC120-R147681 as human immunodeficiency virus microbicides. Antimicrob Agents Chemother. 2004;48(1):337–339. doi: 10.1128/AAC.48.1.337-339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lederman MM, Veazey RS, Offord R, Mosier DE, Dufour J, Mefford M, Piatak M, Jr, Lifson JD, Salkowitz JR, Rodriguez B, Blauvelt A, et al. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science. 2004;306(5695):485–487. doi: 10.1126/science.1099288. [DOI] [PubMed] [Google Scholar]
- 31.Veazey R, Ketas T, Dufour J, Klasse P, Moore J. Protection of rhesus macaques from vaginal infection by maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, USA. 2010. p. Abs 84LB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olson WC, Jacobson JM. CCR5 monoclonal antibodies for HIV-1 therapy. Curr Opin HIV AIDS. 2009;4(2):104–111. doi: 10.1097/COH.0b013e3283224015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9(3):343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 34.Dudley DM, Wentzel JL, Lalonde MS, Veazey RS, Arts EJ. Selection of a simian-human immunodeficiency virus strain resistant to a vaginal microbicide in macaques. J Virol. 2009;83(10):5067–5076. doi: 10.1128/JVI.00055-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Mater. 2009;8(6):526–533. doi: 10.1038/nmat2444. Demonstrated that the use of nanoparticle-encapsulated siRNAs can result in sustained siRNA release following topical application to the vaginal mucosa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36••.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432(7014):173–178. doi: 10.1038/nature03121. Demonstrated the feasilbility of C-siRNA-mediated silencing in a mouse model. [DOI] [PubMed] [Google Scholar]
- 37.Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, Judge AD, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441(7089):111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 38.Choung S, Kim YJ, Kim S, Park HO, Choi YC. Chemical modification of siRNAs to improve serum stability without loss of efficacy. Biochem Biophys Res Commun. 2006;342(3):919–927. doi: 10.1016/j.bbrc.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 39.Krummenacher C, Nicola AV, Whitbeck JC, Lou H, Hou W, Lambris JD, Geraghty RJ, Spear PG, Cohen GH, Eisenberg RJ. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J Virol. 1998;72(9):7064–7074. doi: 10.1128/jvi.72.9.7064-7074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shukla D, Dal Canto MC, Rowe CL, Spear PG. Striking similarity of murine nectin-1α to human nectin-1α (HveC) in sequence and activity as a glycoprotein D receptor for alphaherpesvirus entry. J Virol. 2000;74(24):11773–11781. doi: 10.1128/jvi.74.24.11773-11781.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song E, Lee SK, Dykxhoorn DM, Novina C, Zhang D, Crawford K, Cerny J, Sharp PA, Lieberman J, Manjunath N, Shankar P. Sustained small interfering RNA-mediated human immunodeficiency virus type 1 inhibition in primary macrophages. J Virol. 2003;77(13):7174–7181. doi: 10.1128/JVI.77.13.7174-7181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Cristofaro P, Silbermann R, Pusch O, Boden D, Konkin T, Hovanesian V, Monfils PR, Resnick M, Moss SF, Ramratnam B. Engineering mucosal RNA interference in vivo. Mol Ther. 2006;14(3):336–342. doi: 10.1016/j.ymthe.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Duxbury MS, Ashley SW, Whang EE. RNA interference: A mammalian SID-1 homologue enhances siRNA uptake and gene silencing efficacy in human cells. Biochem Biophys Res Commun. 2005;331(2):459–463. doi: 10.1016/j.bbrc.2005.03.199. [DOI] [PubMed] [Google Scholar]
- 44.Valiunas V, Polosina YY, Miller H, Potapova IA, Valiuniene L, Doronin S, Mathias RT, Robinson RB, Rosen MR, Cohen IS, Brink PR. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol. 2005;568(Pt 2):459–468. doi: 10.1113/jphysiol.2005.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, Rajeev KG, Nakayama T, Charrise K, Ndungo EM, Zimmermann T, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25(10):1149–1157. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 46.Taylor TJ, Brockman MA, McNamee EE, Knipe DM. Herpes simplex virus. Front Biosci. 2002;7:d752–d764. doi: 10.2741/taylor. [DOI] [PubMed] [Google Scholar]
- 47••.Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM, Feng Y, Palliser D, Weiner DB, Shankar P, Marasco WA, et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol. 2005;23(6):709–717. doi: 10.1038/nbt1101. Demonstrated, along with reference [48], the feasibility of using antibody-conjugated siRNAs. [DOI] [PubMed] [Google Scholar]
- 48••.Kumar P, Ban HS, Kim SS, Wu H, Pearson T, Greiner DL, Laouar A, Yao J, Haridas V, Habiro K, Yang YG, et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134(4):577–586. doi: 10.1016/j.cell.2008.06.034. Demonstrated, along with reference [47], the feasibility of using antibody-conjugated siRNAs. In this study, antibody-mediated delivery of HIV-1-specifc siRNAs inhibited HIV-1 infection in a humanized mouse model, thus demonstrating the therapeutic potential of this approach. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart SA, Dykxhoorn DM, Palliser D, Mizuno H, Yu EY, An DS, Sabatini DM, Chen IS, Hahn WC, Sharp PA, Weinberg RA, et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA. 2003;9(4):493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim DH, Rossi JJ. Strategies for silencing human disease using RNA interference. Nat Rev Genet. 2007;8(3):173–184. doi: 10.1038/nrg2006. [DOI] [PubMed] [Google Scholar]
- 51.Jackson AL, Burchard J, Leake D, Reynolds A, Schelter J, Guo J, Johnson JM, Lim L, Karpilow J, Nichols K, Marshall W, et al. Position-specific chemical modification of siRNAs reduces ‘off-target’ transcript silencing. RNA. 2006;12(7):1197–1205. doi: 10.1261/rna.30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robbins M, Judge A, MacLachlan I. siRNA and innate immunity. Oligonucleotides. 2009;19(2):89–102. doi: 10.1089/oli.2009.0180. [DOI] [PubMed] [Google Scholar]
- 53.Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference- based therapeutics. Nature. 2009;457(7228):426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anderson J, Li MJ, Palmer B, Remling L, Li S, Yam P, Yee JK, Rossi J, Zaia J, Akkina R. Safety and efficacy of a lentiviral vector containing three anti-HIV genes – CCR5 ribozyme, tat-rev siRNA, and TAR decoy – in SCID-hu mouse-derived T cells. Mol Ther. 2007;15(6):1182–1188. doi: 10.1038/sj.mt.6300157. [DOI] [PubMed] [Google Scholar]
- 55••.Benitec Ltd. Benitec and City of Hope human trial update. Press Release. 2009 May 06; The first clinical trial to use RNAi as part of a therapy for targeting an STI (AIDS lymphoma) [Google Scholar]
- 56•.Boden D, Pusch O, Lee F, Tucker L, Ramratnam B. Human immunodeficiency virus type 1 escape from RNA interference. J Virol. 2003;77(21):11531–11535. doi: 10.1128/JVI.77.21.11531-11535.2003. Demonstrated the emergence of HIV-1-resistant strains following treatment with siRNAs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang S, Tao M, McCoy JP, Jr, Zheng ZM. Short-term induction and long-term suppression of HPV16 oncogene silencing by RNA interference in cervical cancer cells. Oncogene. 2006;25(14):2094–2104. doi: 10.1038/sj.onc.1209244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki K, Hu D, Bustos T, Zlotogora J, Richieri-Costa A, Helms JA, Spritz RA. Mutations of PVRL1, encoding a cell-cell adhesion molecule/herpesvirus receptor, in cleft lip/palate-ectodermal dysplasia. Nat Genet. 2000;25(4):427–430. doi: 10.1038/78119. [DOI] [PubMed] [Google Scholar]
- 59•.Bitko V, Musiyenko A, Shulyayeva O, Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nat Med. 2005;11(1):50–55. doi: 10.1038/nm1164. Presents data on the use of naked siRNAs to prevent RSV and PIV infection. [DOI] [PubMed] [Google Scholar]
- 60.Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005;23(4):457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- 61••.Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, Appukuttan B, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452(7187):591–597. doi: 10.1038/nature06765. Demonstrated, along with reference [62], how siRNAs can mediate non-specific effects, which can be erroneously attributed to an RNAi-mediated mechanism if adequate controls are not performed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62••.Robbins M, Judge A, Ambegia E, Choi C, Yaworski E, Palmer L, McClintock K, MacLachlan I. Misinterpreting the therapeutic effects of small interfering RNA caused by immune stimulation. Hum Gene Ther. 2008;19(10):991–999. doi: 10.1089/hum.2008.131. Demonstrated, along with reference [61], how siRNAs can mediate non-specific effects, which can be erroneously attributed to an RNAi-mediated mechanism if adequate controls are not performed. [DOI] [PubMed] [Google Scholar]
- 63.Hladik F, McElrath MJ. Setting the stage: Host invasion by HIV. Nat Rev Immunol. 2008;8(6):447–457. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan AC, Levy M. Aptamers and aptamer targeted delivery. RNA Biol. 2009;6(3):316–320. doi: 10.4161/rna.6.3.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chu TC, Twu KY, Ellington AD, Levy M. Aptamer mediated siRNA delivery. Nucleic Acids Res. 2006;34(10):e73. doi: 10.1093/nar/gkl388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dassie JP, Liu XY, Thomas GS, Whitaker RM, Thiel KW, Stockdale KR, Meyerholz DK, McCaffrey AP, McNamara JO, 2nd, Giangrande PH. Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors. Nat Biotechnol. 2009;27(9):839–849. doi: 10.1038/nbt.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McNamara JO, 2nd, Andrechek ER, Wang Y, Viles KD, Rempel RE, Gilboa E, Sullenger BA, Giangrande PH. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat Biotechnol. 2006;24(8):1005–1015. doi: 10.1038/nbt1223. [DOI] [PubMed] [Google Scholar]
- 68.Zhou J, Li H, Li S, Zaia J, Rossi JJ. Novel dual inhibitory function aptamer-siRNA delivery system for HIV-1 therapy. Mol Ther. 2008;16(8):1481–1489. doi: 10.1038/mt.2008.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou J, Swiderski P, Li H, Zhang J, Neff CP, Akkina R, Rossi JJ. Selection, characterization and application of new RNA HIV gp 120 aptamers for facile delivery of Dicer substrate siRNAs into HIV infected cells. Nucleic Acids Res. 2009;37(9):3094–3109. doi: 10.1093/nar/gkp185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.OPKO Health. OPKO Health announces update on Phase III clinical trial of bevasiranib. Press release. 2009 March 06; [Google Scholar]
- 71•.Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, Marion P, Salazar F, Kay MA. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441(7092):537–541. doi: 10.1038/nature04791. Demonstrated, along with references [72], [73] and [74], the possible effects of shRNA and siRNA expression on the miRNA pathway. [DOI] [PubMed] [Google Scholar]
- 72.McBride JL, Boudreau RL, Harper SQ, Staber PD, Monteys AM, Martins I, Gilmore BL, Burstein H, Peluso RW, Polisky B, Carter BJ, et al. Artificial miRNAs mitigate shRNA-mediated toxicity in the brain: Implications for the therapeutic development of RNAi. Proc Natl Acad Sci USA. 2008;105(15):5868–5873. doi: 10.1073/pnas.0801775105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.John M, Constien R, Akinc A, Goldberg M, Moon YA, Spranger M, Hadwiger P, Soutschek J, Vornlocher HP, Manoharan M, Stoffel M, et al. Effective RNAi-mediated gene silencing without interruption of the endogenous microRNA pathway. Nature. 2007;449(7163):745–747. doi: 10.1038/nature06179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khan AA, Betel D, Miller ML, Sander C, Leslie CS, Marks DS. Transfection of small RNAs globally perturbs gene regulation by endogenous microRNAs. Nat Biotechnol. 2009;27(6):549–555. doi: 10.1038/nbt.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Castanotto D, Sakurai K, Lingeman R, Li H, Shively L, Aagaard L, Soifer H, Gatignol A, Riggs A, Rossi JJ. Combinatorial delivery of small interfering RNAs reduces RNAi efficacy by selective incorporation into RISC. Nucleic Acids Res. 2007;35(15):5154–5164. doi: 10.1093/nar/gkm543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, Noronha A, Manoharan M, Akira S, de Fougerolles A, Endres S, et al. Sequence-specific potent induction of IFN-α by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11(3):263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- 77.Robbins M, Judge A, Liang L, McClintock K, Yaworski E, MacLachlan I. 2′-O-methyl-modified RNAs act as TLR7 antagonists. Mol Ther. 2007;15(9):1663–1669. doi: 10.1038/sj.mt.6300240. [DOI] [PubMed] [Google Scholar]
- 78•.Mesquita PM, Cheshenko N, Wilson SS, Mhatre M, Guzman E, Fakioglu E, Keller MJ, Herold BC. Disruption of tight junctions by cellulose sulfate facilitates HIV infection: Model of microbicide safety. J Infect Dis. 2009;200(4):599–608. doi: 10.1086/600867. Presents a new model to predict the toxicity of microbicides under development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bioo Therapeutics: Bioo Scientific and Texas Tech collaborate to suppress the progression of HIV using targeted RNAi. Press Release. 2009 November 12; [Google Scholar]
- 80.Mayer KH, Maslankowski LA, Gai F, El-Sadr WM, Justman J, Kwiecien A, Masse B, Eshleman SH, Hendrix C, Morrow K, Rooney JF, et al. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS. 2006;20(4):543–551. doi: 10.1097/01.aids.0000210608.70762.c3. [DOI] [PubMed] [Google Scholar]
- 81.HIV ‘prevention’ gel PRO-2000 proven ineffective: Microbicides Development Programme. London, UK: 2009. www.mdp.mrc.ac.uk/archive.html. [Google Scholar]
- 82.Anderson DJ, Williams DL, Ballagh SA, Barnhart K, Creinin MD, Newman DR, Bowman FP, Politch JA, Duerr AC, Jamieson DJ. Safety analysis of the diaphragm in combination with lubricant or acidifying microbicide gels: Effects on markers of inflammation and innate immunity in cervicovaginal fluid. Am J Reprod Immunol. 2009;61(2):121–129. doi: 10.1111/j.1600-0897.2008.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Microbicides: AIDS Vaccine Advocacy Coalition (AVAC) New York, NY, USA: 2010. www.avac.org/ht/d/sp/i/178/pid/178. [Google Scholar]
- 84.Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, Hartsough K, Machemer L, Radka S, Jadhav V, Vaish N, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23(8):1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 85.Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319(5863):627–630. doi: 10.1126/science.1149859. [DOI] [PMC free article] [PubMed] [Google Scholar]